Prenatal Imaging of Micrognathia, Micromelia, and Fetal Hydrops Leading to the Diagnosis of Achondrogenesis Type II with a COL2A1 Missense Mutation
Abstract
1. Introduction
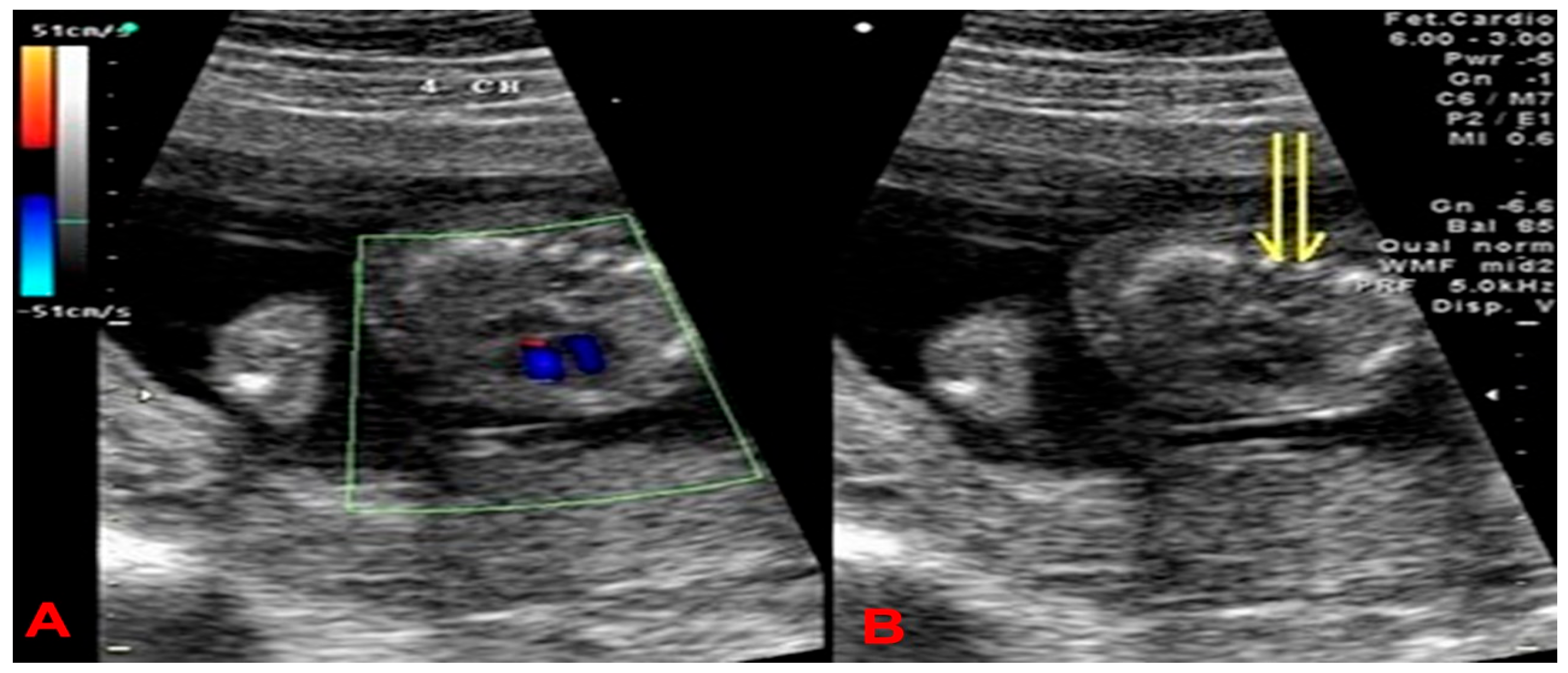
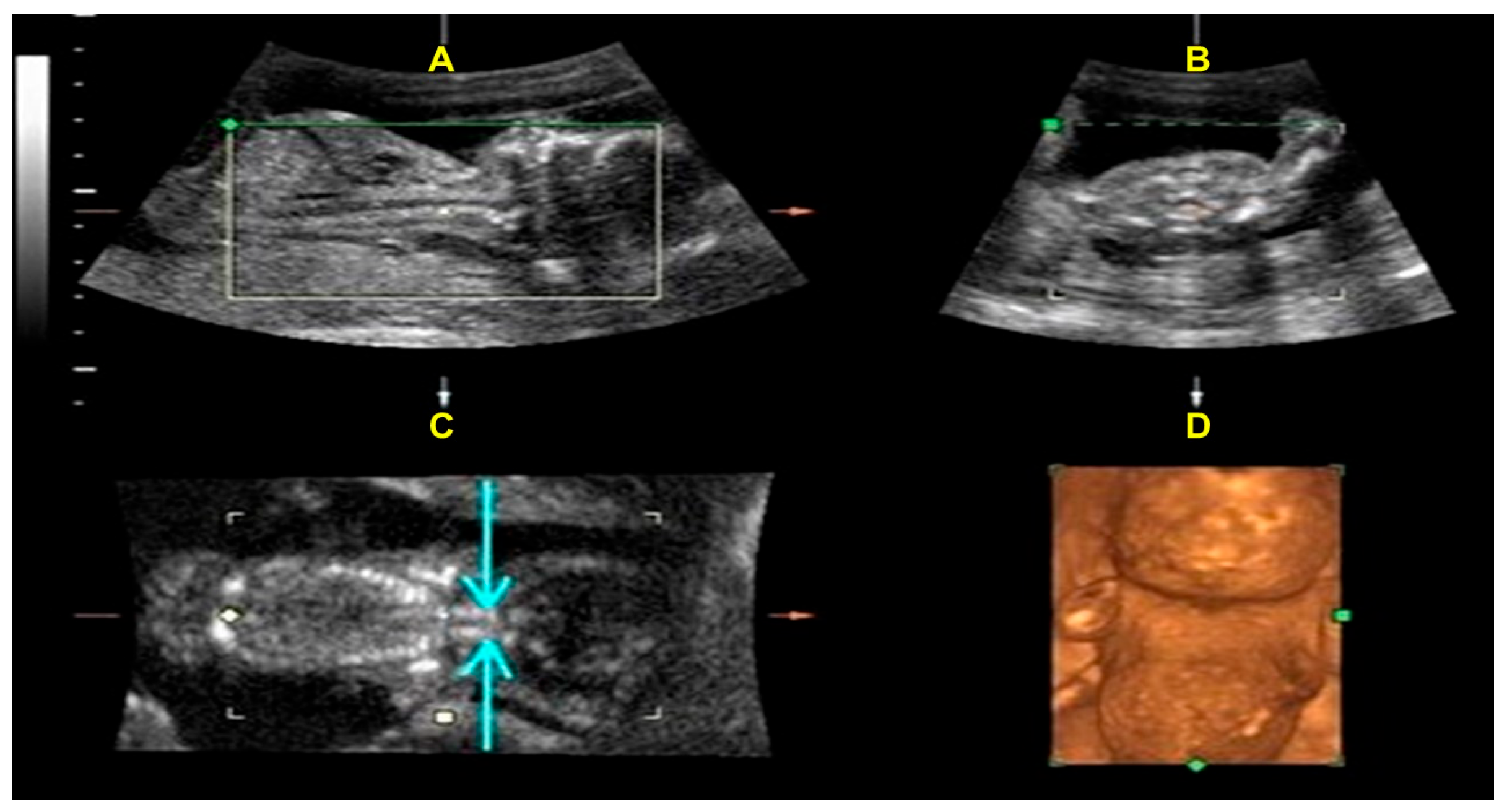
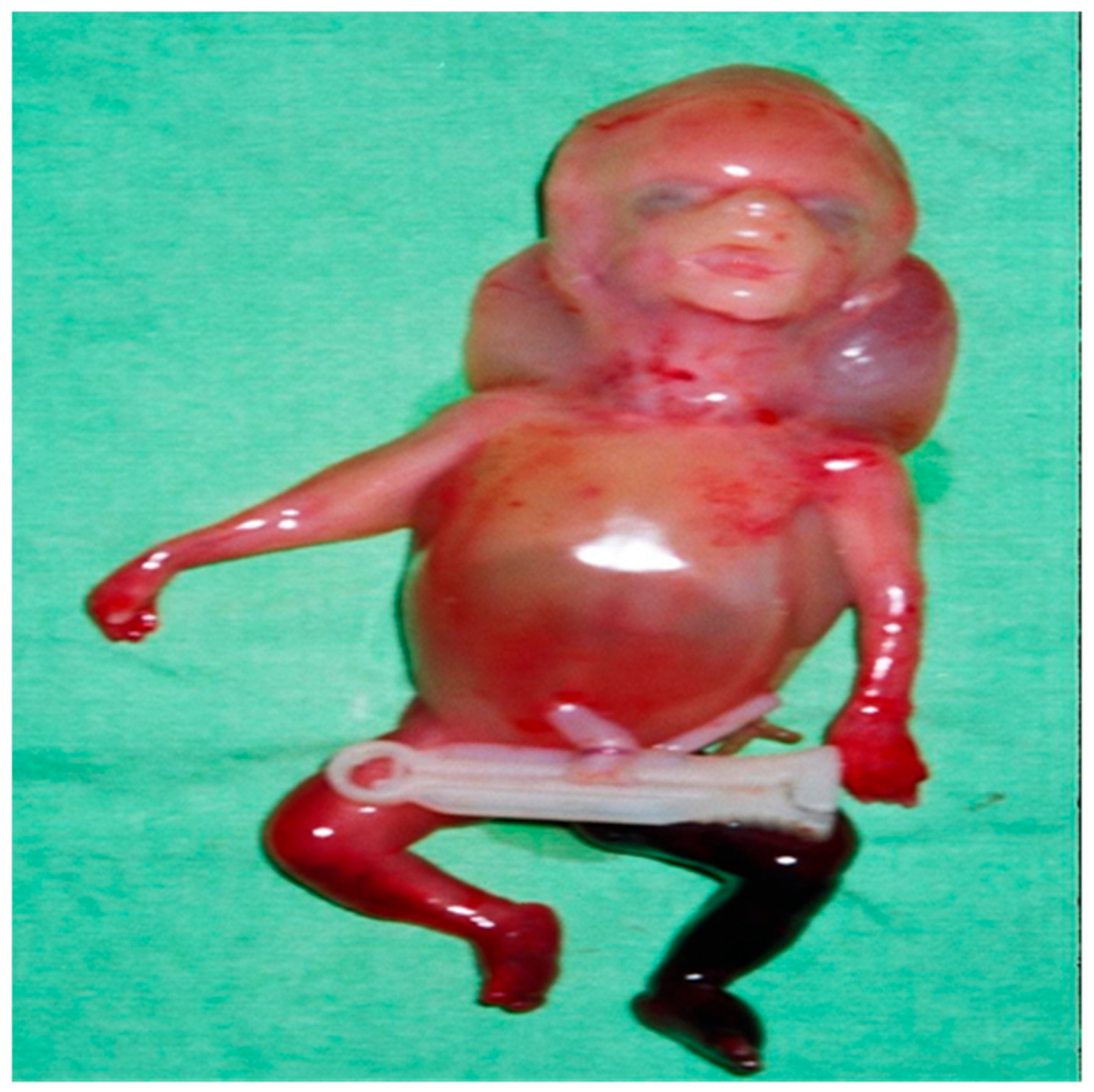
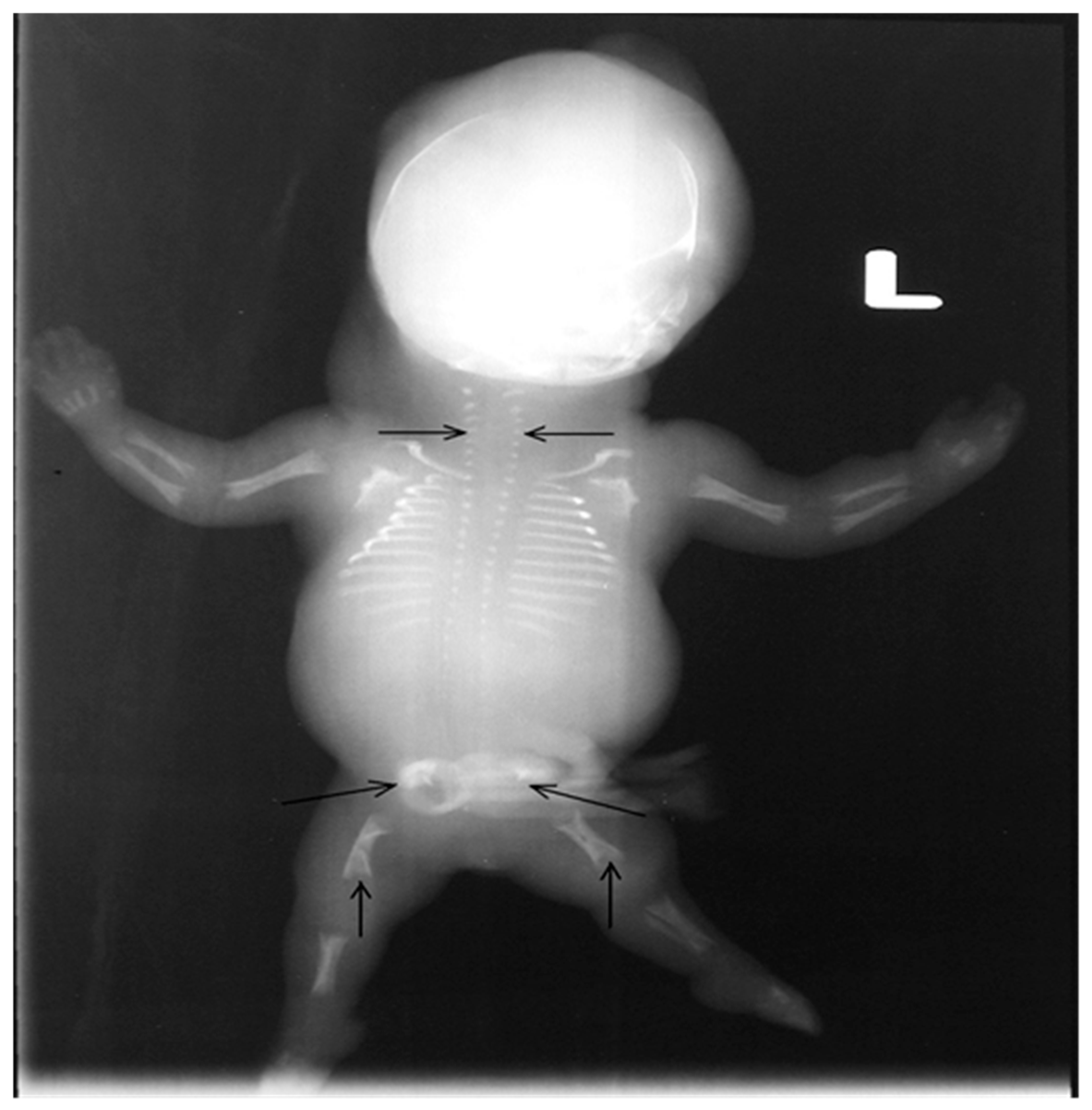
2. Case Presentation
3. Materials and Methods
Molecular Study
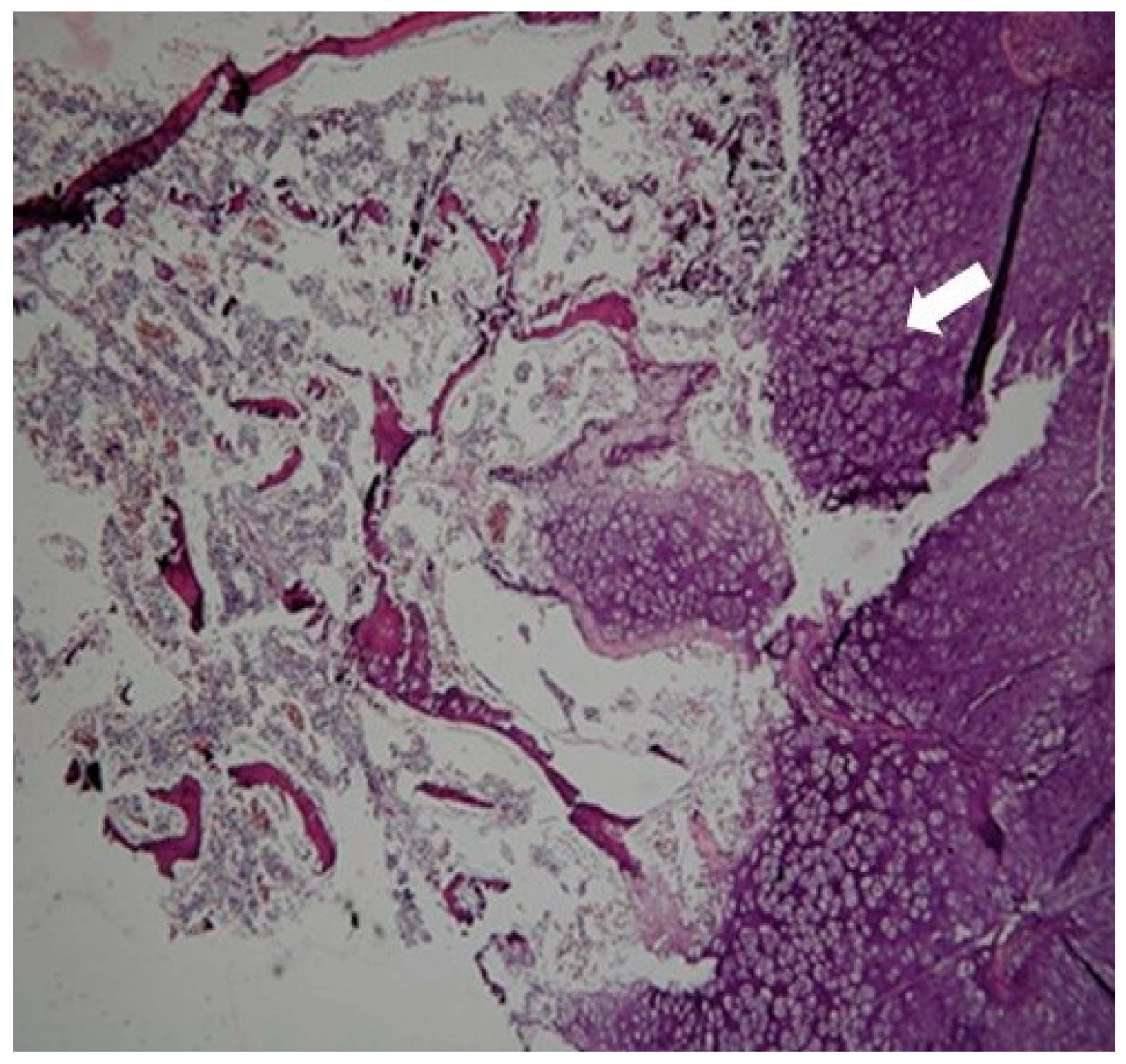
4. Results
5. Discussion
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- van der Harten, H.J.; Brons, J.T.; Dijkstra, P.F.; Niermeyer, M.F.; Meijer, C.J.; van Giejn, H.P. Arts NF. Achondrogenesis-hypochondrogenesis: The spectrum of chondrogenesis imperfecta. A radiological, ultrasonographic, and histopathologic study of 23 cases. Pediatr. Pathol. 1988, 8, 571–597. Available online: https://omim.org/entry/200600 (accessed on 16 November 2025). [PubMed]
- Borochowitz, Z.; Lachman, R.; Adomian, G.E.; Spear, G.; Jones, K.; Rimoin, D.L. Achondrogenesis type I: Delineation of further heterogeneity and identification of two distinct subgroups. J. Pediatr. 1988, 112, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.S.; Doh, J.W.; Kim, C.J.; Chi, J.G. Achondrogenesis type II (Langer-Saldino achondrogenesis): A case report. J. Korean Med. Sci. 2000, 15, 604–608. [Google Scholar] [CrossRef] [PubMed]
- Andersen, P.E., Jr. Achondrogenesis type II in twins. Br. J. Radiol. 1981, 54, 61–65. [Google Scholar] [CrossRef]
- Kannu, P.; Bateman, J.; Savarirayan, R. Clinical phenotypes associated with type II collagen mutations. J. Paediatr. Child Health 2012, 48, E38–E43. [Google Scholar] [CrossRef]
- Nishimura, G.; Nakashima, E.; Mabuchi, A.; Shimamoto, K.; Shimamoto, T.; Shimao, Y.; Nagai, T.; Yamaguchi, T.; Kosaki, R.; Ohashi, H.; et al. Identification of COL2A1 mutations in platyspondylic skeletal dysplasia, Torrance type. J. Med. Genet. 2004, 41, 75–79. [Google Scholar]
- Okamoto, T.; Nagaya, K.; Asai, H.; Tsuchida, E.; Nohara, F.; Hayashi, T.; Yamashita, A.; Nishimura, G.; Azuma, H. Platyspondylic lethal dysplasia torrance type with a heterozygous mutation in the triple helical domain of COL2A1 in two sibs from phenotypically normal parents. Am. J. Med. Genet. A 2012, 158, 1953–1956. [Google Scholar] [CrossRef]
- Désir, J.; Cassart, M.; Donner, C.; Coucke, P.; Abramowicz, M.; Mortier, G. Spondyloperipheral dysplasia as the mosaic form of platyspondylic lethal skeletal dysplasia torrance type in mother and fetus with the same COL2A1 mutation. Am. J. Med. Genet. A 2012, 158, 1948–1952. [Google Scholar]
- Gourgas, O.; Lemire, G.; Eaton, A.J.; Alshahrani, S.; Duker, A.L.; Li, J.; Carroll, R.S.; Mackenzie, S.; Nikkel, S.M.; Care4Rare Canada Consortium; et al. Specific heterozygous variants in MGP lead to endoplasmic reticulum stress and cause spondyloepiphyseal dysplasia. Nat. Commun. 2023, 14, 7054, Erratum in Nat. Commun. 2024, 15, 3655. https://doi.org/10.1038/s41467-024-47898-x. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Travessa, A.M.; Díaz-González, F.; Mirco, T.; Oliveira-Ramos, F.; Parrón-Pajares, M.; Heath, K.E.; Sousa, A.B. Spondyloepiphyseal dysplasia type Stanescu: Expanding the clinical and molecular spectrum of a very rare type II collagenopathy. Am. J. Med. Genet. A 2020, 182, 2715–2721. [Google Scholar] [CrossRef]
- Savarirayan, R.; Thompson, E.; Gécz, J. Spondyloepiphyseal dysplasia tarda (SEDL, MIM #313400). Eur. J. Hum. Genet. 2003, 11, 639–642. [Google Scholar] [CrossRef]
- Akahira-Azuma, M.; Enomoto, Y.; Nakamura, N.; Yokoi, T.; Minatogawa, M.; Harada, N.; Tsurusaki, Y.; Kurosawa, K. Novel COL2A1 variants in Japanese patients with spondyloepiphyseal dysplasia congenita. Hum. Genome Var. 2022, 9, 16. [Google Scholar] [CrossRef]
- Bisht, R.U.; Van Tassel, D.C.; Belthur, M.V. Spondyloepiphyseal dysplasia congenita: Use of complementary 3D reconstruction imaging for preoperative planning. Clin. Imaging 2022, 86, 94–97. [Google Scholar] [CrossRef] [PubMed]
- Winterpacht, A.; Hilbert, M.; Schwarze, U.; Mundlos, S.; Spranger, J.; Zabel, B.U. Kniest and Stickler dysplasia phenotypes are caused by a defect in the collagen type II gene (COL2A1). Nat. Genet. 1993, 3, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Wada, R.; Sawai, H.; Nishimura, G.; Isono, K.; Minagawa, K.; Takenobu, T.; Harada, K.; Tanaka, H.; Ishikura, R.; Komori, S. Prenatal diagnosis of Kniest dysplasia with three-dimensional helical computed tomography. J. Matern. Fetal Neonatal Med. 2011, 24, 1181–1184. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, L.; Wang, C.; Yue, H.; Zhang, H.; Gu, J.; Hu, W.; Liu, L.; Zhang, Z. Clinical and Molecular Characterization and Discovery of Novel Genetic Mutations of Chinese Patients with COL2A1-related Dysplasia. Int. J. Biol. Sci. 2020, 16, 859–868. [Google Scholar] [CrossRef]
- Zankl, A.; Zabel, B.; Hilbert, K.; Wildhardt, G.; Cuenot, S.; Xavier, B.; Ha-Vinh, R.; Bonafé, L.; Spranger, J.; Superti-Furga, A. Spondyloperipheral dysplasia is caused by truncating mutations in the C-propeptide of COL2A1. Am. J. Med. Genet. A 2004, 129, 144–148. [Google Scholar] [CrossRef]
- Bedeschi, M.F.; Bianchi, V.; Gentilin, B.; Colombo, L.; Natacci, F.; Giglio, S.; Andreucci, E.; Trespidi, L.; Acaia, B.; Furga, A.S.; et al. Prenatal manifestation and management of a mother and child affected by spondyloperipheral dysplasia with a C-propeptide mutation in COL2A1: Case report. Orphanet J. Rare Dis. 2011, 6, 7. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, S.C.; He, J.W.; Fu, W.Z.; Zhang, C.Q.; Zhang, Z.L. Identification of one novel mutation in the C-propeptide of COL2A1 in a Chinese family with spondyloperipheral dysplasia. Gene 2013, 522, 107–110. [Google Scholar] [CrossRef]
- Mortier, G.R.; Cohn, D.H.; Cormier-Daire, V.; Hall, C.; Krakow, D.; Mundlos, S.; Nishimura, G.; Robertson, S.; Sangiorgi, L.; Savarirayan, R.; et al. Nosology and classification of genetic skeletal disorders: 2019 revision. Am. J. Med. Genet. A 2019, 179, 2393–2419. [Google Scholar]
- Mortier, G. Stickler Syndrome. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2000; pp. 1993–2024. [Google Scholar]
- Shapiro, M.J.; Blair, M.P.; Solinski, M.A.; Zhang, D.L.; Jabbehdari, S. The importance of early diagnosis of Stickler syndrome: Finding opportunities for preventing blindness. Taiwan J. Ophthalmol. 2018, 8, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Lisi, V.; Guala, A.; Lopez, A.; Vitali, M.; Spadoni, E.; Olivieri, C.; Danesino, C.; Mottes, M. Linkage analysis for prenatal diagnosis in a familial case of Stickler syndrome. Genet. Couns. 2002, 13, 163–170. [Google Scholar] [PubMed]
- Gueneuc, A.; Spaggiari, E.; Millischer, A.E.; Michot, C.; O’Gorman, N.; Ville, Y. Contribution of three-dimensional ultrasound and three-dimensional helical computed tomography to prenatal diagnosis of Stickler syndrome. Ultrasound Obs. Gynecol. 2019, 54, 279–280. [Google Scholar] [CrossRef] [PubMed]
- Deborah, A. Nickerson, Vincent O. Tobe, Scott L. Taylor, PolyPhred: Automating the detection and genotyping of single nucleotide substitutions using fluorescence-based resequencing. Nucleic Acids Res. 1997, 25, 2745–2751. [Google Scholar]
- Barat-Houari, M.; Sarrabay, G.; Gatinois, V.; Fabre, A.; Dumont, B.; Genevieve, D.; Touitou, I. Mutation Update for COL2A1 Gene Variants Associated with Type II Collagenopathies. Hum. Mutat. 2016, 37, 7–15. [Google Scholar] [CrossRef]
- Liu, Y.F.; Chen, W.M.; Lin, Y.F.; Yang, R.C.; Lin, M.W.; Li, L.H.; Chang, Y.H.; Jou, Y.S.; Lin, P.Y.; Su, J.S.; et al. Type II collagen gene variants and inherited osteonecrosis of the femoral head. N. Engl. J. Med. 2005, 352, 2294–2301. [Google Scholar] [CrossRef]
- Kodandapani, S.; Ramkumar, V. Antenatal diagnosis of achondrogenesis type II. JNMA J. Nepal Med. Assoc. 2009, 48, 155–157. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wenstrom, K.D.; Williamson, R.A.; Hoover, W.W.; Grant, S.S. Achondrogenesis type II (Langer-Saldino) in association with jugular lymphatic obstruction sequence. Prenat. Diagn. 1989, 9, 527–532. [Google Scholar] [CrossRef]
- Zhang, B.; Zhang, Y.; Wu, N.; Li, J.; Liu, H.; Wang, J. Integrated analysis of COL2A1 variant data and classification of type II collagenopathies. Clin. Genet. 2020, 97, 383–395. [Google Scholar] [CrossRef]
- Unger, S.; Superti-Furga, A. Achondrogenesis Type IB. In GeneReviews® [Internet]; Adam, M.P., Feldman, J., Mirzaa, G.M., Pagon, R.A., Wallace, S.E., Amemiya, A., Eds.; University of Washington: Seattle, WA, USA, 2002; pp. 1993–2025. [Google Scholar] [PubMed]
- Stembalska, A.; Dudarewicz, L.; Śmigiel, R. Lethal and life-limiting skeletal dysplasias: Selected prenatal issues. Adv. Clin. Exp. Med. 2021, 30, 641–647. [Google Scholar] [CrossRef]
- Silveira, K.C.; Kanazawa, T.Y.; Silveira, C.; Lacarrubba-Flores, M.D.J.; Carvalho, B.S.; Cavalcanti, D.P. Molecular diagnosis in a cohort of 114 patients with rare skeletal dysplasias. Am. J. Med. Genet. C Semin. Med. Genet. 2021, 187, 396–408. [Google Scholar] [CrossRef] [PubMed]
- Dufke, A.; Hoopmann, M.; Waldmüller, S.; Prodan, N.C.; Beck-Wödl, S.; Grasshoff, U.; Heinrich, T.; Riess, A.; Kehrer, M.; Falb, R.J.; et al. A single-center experience of prenatal parent-fetus trio exome sequencing for pregnancies with congenital anomalies. Prenat. Diagn. 2022, 42, 901–910. [Google Scholar] [CrossRef] [PubMed]
- Kucińska-Chahwan, A.; Geremek, M.; Roszkowski, T.; Bijok, J.; Massalska, D.; Ciebiera, M.; Correia, H.; Pereira-Caetano, I.; Barreta, A.; Obersztyn, E.; et al. Implementation of Exome Sequencing in Prenatal Diagnosis and Impact on Genetic Counseling: The Polish Experience. Genes 2022, 13, 724. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.-C.; Chen, C.-Y.; Chen, G.-Y.; Hsiao, C.-H.; Chu, W.-C.; Huang, J.Y.-J. Prenatal Imaging of Micrognathia, Micromelia, and Fetal Hydrops Leading to the Diagnosis of Achondrogenesis Type II with a COL2A1 Missense Mutation. Int. J. Mol. Sci. 2025, 26, 11472. https://doi.org/10.3390/ijms262311472
Wu Y-C, Chen C-Y, Chen G-Y, Hsiao C-H, Chu W-C, Huang JY-J. Prenatal Imaging of Micrognathia, Micromelia, and Fetal Hydrops Leading to the Diagnosis of Achondrogenesis Type II with a COL2A1 Missense Mutation. International Journal of Molecular Sciences. 2025; 26(23):11472. https://doi.org/10.3390/ijms262311472
Chicago/Turabian StyleWu, Yi-Cheng, Chih-Yao Chen, Guan-Yeu Chen, Ching-Hua Hsiao, Woei-Chyn Chu, and Jack Yu-Jen Huang. 2025. "Prenatal Imaging of Micrognathia, Micromelia, and Fetal Hydrops Leading to the Diagnosis of Achondrogenesis Type II with a COL2A1 Missense Mutation" International Journal of Molecular Sciences 26, no. 23: 11472. https://doi.org/10.3390/ijms262311472
APA StyleWu, Y.-C., Chen, C.-Y., Chen, G.-Y., Hsiao, C.-H., Chu, W.-C., & Huang, J. Y.-J. (2025). Prenatal Imaging of Micrognathia, Micromelia, and Fetal Hydrops Leading to the Diagnosis of Achondrogenesis Type II with a COL2A1 Missense Mutation. International Journal of Molecular Sciences, 26(23), 11472. https://doi.org/10.3390/ijms262311472





