Abstract
Cell wall invertases (CINs) establish sucrose gradients between source and sink tissues, essential for the allocation of photoassimilates. Rice possesses nine CIN genes, among which OsCIN1 and OsCIN2 have been reported as key regulators of sink strength. To test whether increasing CIN activity enhances grain yield, we generated OsCIN1 overexpression lines in rice driven by the CaMV 35S promoter. Subcellular localization analysis of OsCIN1–GFP confirmed its apoplastic localization. OsCIN1 promoter::GUS analyses verified expression in vascular tissues and revealed predominant signals in the ovular vascular and lateral stylar vascular traces during seed development. Although CIN activity was markedly elevated throughout the plant, the resulting phenotypes were unexpected. Sugar profiling of flag leaves at the flowering stage showed almost complete sucrose depletion in the overexpression (OX) lines, accompanied by increased hexose and starch accumulation. Under field conditions, OsCIN1 OX plants exhibited ~50% fewer tillers and a lower 1000-grain weight relative to wild type (WT), resulting in reduced productivity. Ectopic expression of OsCIN1 disrupted the sucrose concentration gradient, weakened carbon partitioning to sink tissues, and impaired key agronomic traits. Collectively, sugar flux is governed by the spatiotemporal patterning of CINs, highlighting that precise spatial and temporal control of CIN activity is required to increase yield.
1. Introduction
Photosynthesis is the fundamental process that produces carbohydrates essential for plant growth and development [1,2]. The carbon assimilates synthesized in mesophyll cells are either temporarily stored in chloroplasts as starch for catabolic use or converted into sucrose in the cytosol for long-distance transport [3,4]. The sucrose thus produced is translocated through the phloem to sink organs such as roots, fruits, and grains, where it serves as a critical determinant of plant growth and final yield [5]. Therefore, not only the amount of carbohydrates produced by photosynthesis but also the efficiency of their partitioning from the source leaves to sink tissues such as grains has long been recognized as a key target for enhancing crop yield [6,7].
Long-distance sucrose transport is regulated by two major mechanisms: transporters and concentration gradients. Among these, sucrose transporters (SUTs) and Sugars Will Eventually be Exported Transporters (SWEETs) play particularly critical roles [8,9,10,11]. These transporters facilitate phloem loading by actively or passively mediating sucrose transport in bundle sheath cells and elements of the leaf vasculature [8]. For example, in the C4 photosynthetic system of maize (Zea mays L.), ZmSWEET13 effectively exports sucrose from bundle sheath cells into the phloem [12], whereas in wheat (Triticum aestivum L.), TaSUT1 plays an essential role in the remobilization of stored carbohydrates [13]. The regulatory control of these transporters determines the flux of sucrose, which in turn represents a critical molecular mechanism governing crop yield [14]. In fact, a study in which the Arabidopsis AtSUC2 gene was overexpressed in rice reported an increase in phloem loading efficiency, resulting in up to a 16% improvement in yield, thereby providing direct evidence that enhancing sucrose loading can substantially increase crop productivity [6].
For the efficient transport of sucrose, it is essential to maintain a concentration gradient between source and sink tissues. The enzyme that plays a pivotal role in establishing this gradient is the cell wall invertase (CIN). CINs are predominantly localized in the apoplastic space of sink organs such as developing seeds, where they rapidly hydrolyze sucrose imported through the phloem into glucose and fructose [15,16,17,18]. Because CIN is the only enzyme that hydrolyzes sucrose in the apoplast, it plays a central role in maintaining the concentration gradient and is considered the most critical enzyme that maintains the apoplastic sucrose gradient immediately before import into sink organs [19,20,21,22].
Given the pivotal role of CIN in regulating carbon allocation, numerous attempts have been made to enhance its activity to promote sink organ development [18,23]. In tomato, overexpression of CIN driven by a fruit-specific promoter resulted in increased fruit weight and overall yield [13]. Furthermore, disruption of a cell wall invertase inhibitor (INVINH) led to elevated sugar content without reducing weight in tomato [21,24]. In potato, suppression of INVINH expression was shown to increase the size of microtubers [25]. In carrot, reduced CIN expression impaired early developmental processes [26]. In maize, overexpression of CIN genes from Arabidopsis, rice, and maize consistently resulted in increased seed size [27]. In rice, expression of OsCIN2 under the control of its native promoter significantly enhanced seed size [20]. Collectively, these studies demonstrate that manipulation of CIN activity or its regulatory components has profound effects on sink organ development and final yield across diverse plant species.
Recently, we identified the function of OsCIN1. The oscin1/oscin2 double mutant exhibited a much more severe shrunken seed phenotype compared with the opaque phenotype of the oscin2 single mutant, suggesting that OsCIN1, like OsCIN2, regulates carbon partitioning from maternal to filial tissues and is likely involved in governing sugar transport and partitioning to sink organs, particularly seeds [22]. Therefore, this study aimed to reprogram source–sink sucrose partitioning through overexpression of OsCIN1 and to assess its impact on overall yield in rice.
2. Results
2.1. Generation of OsCIN1 Overexpression Lines
To analyze the function of overexpressed OsCIN1 in rice, we cloned the full-length coding sequence (CDS) of the OsCIN1 gene under the control of the 35S promoter (Figure 1a). Following transformation, regenerated plants were selected on hygromycin-containing medium. Multiple independent transgenic lines exhibiting differential OsCIN1 expression were obtained. Among these, an intermediate expresser OsCIN1 OX-1 and the highest expresser OsCIN1 OX-3 were identified by semi-quantitative RT-PCR (Figure 1b) and subsequently confirmed by quantitative RT-PCR (qRT-PCR) to quantify transcript accumulation (Figure 1c). Enzymatic assays were performed to quantify CIN activity in the two independent lines. Across both vegetative and reproductive stages, CIN activity in leaves and stem segments of the OX lines was consistently and significantly higher than that of the wild type (WT) (Figure 1d,e).
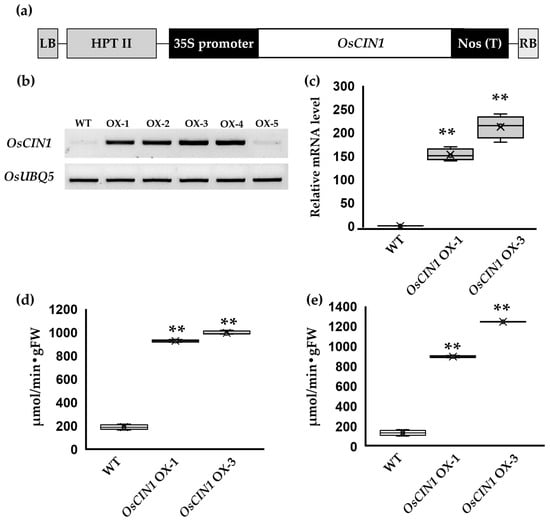
Figure 1.
OsCIN1 expression level and cell wall invertase activity in OsCIN1 OX plants. (a) Schematic representation of the vector construct used to generate OsCIN1 overexpressing rice plants. (b) RT-PCR analysis of OsCIN1 expression in overexpressing plants. OsUBQ5 was amplified as an internal control. (c) qRT-PCR analysis of OsCIN1 expression in overexpressing plants. OsACT1 was used as an internal reference gene. Relative expression levels are shown as fold change compared to WT. (d) Total cell wall invertase activity in fully expanded leaves of six-week-old plants. (e) Total cell wall invertase activity in stem base of plants at the flowering stage. Each value represents the mean ± SE of three biological replicates. Statistical analysis was performed using Student’s t-test. ** p < 0.01.
2.2. pOsCIN1::GUS Expresses Mainly in Vascular Tissues During Seed Development
To determine where OsCIN1 functions, we examined its expression pattern using a pOsCIN1-β-glucuronidase (GUS) reporter transgene (pOsCIN1::GUS). GUS activity was observed predominantly in leaf veins (Figure 2a) and in the vascular tissues of the lemma and palea (Figure 2b). Strong expression was also observed in the ovular vascular trace and lateral stylar vascular traces during early grain-filling stages (Figure 2e). At 15 days after pollination (DAP), GUS activity was specifically localized to the ovular and lateral stylar vascular traces (Figure 2d). Carbon partitioning and sucrose metabolism are recognized as central processes for grain filling in crops. During seed development, vascular traces act as conduits that deliver essential resources, including water, mineral nutrients, and sugars from the parent plant to the developing embryo and endosperm. In rice, sucrose produced in photosynthetic leaves is transported through the phloem to the developing grain, where it enters the endosperm via the dorsal vascular bundle. The sucrose then moves along the nucellar epidermis and is transferred into the endosperm and embryo by sugar transporters, after which it is converted to storage starch. This coordinated sequence of transport and conversion is essential for grain filling and determines both grain weight and overall yield. These results indicate that OsCIN1 plays an important role during the grain filling stage and seed development.

Figure 2.
Tissue-specific expression pattern of pOsCIN1::GUS in rice. (a) GUS expression in the vascular tissues of a flag leaf of heading stage. (b) GUS expression in pre-fertilization young flower. (c) GUS expression in the lemma from the 15 DAP seed. (d,e) GUS expression during seed development at 1, 3, 5, 7, and 15 days after pollination (DAP). OV: ovular vascular traces; LSV: lateral stylar vascular traces. Scale bars = 5 mm.
We examined GUS activity not only during the reproductive stage but also in young seedlings. Strong GUS activity was detected in the vascular bundles of the coleoptile and the roots, whereas in leaves the overall activity was weaker but still detectable in the vascular bundles (Supplementary Figure S1). These results indicate that OsCIN1 is expressed even in young seedlings and that its expression is preferentially localized to vascular tissues.
2.3. OsCIN1 Is Located in the Apoplast
Multiple studies have established that CINs are in the apoplast, where they hydrolyze sucrose into glucose and fructose [28,29]. They interact with cell wall matrix polysaccharides through noncovalent forces such as ionic and hydrogen bonds rather than being covalently anchored [30]. This activity shapes apoplastic sugar levels and maintains the sucrose gradient that governs source-sink carbon flux [19]. To determine whether OsCIN1, like other CINs, is localized in the apoplast, GFP-tagged OsCIN1 (OsCIN1-GFP) was expressed in transgenic callus tissue and examined by confocal fluorescence microscopy. The GFP signal confirmed that OsCIN1 localizes to the apoplast (Figure 3).

Figure 3.
Subcellular localization of OsCIN1 in the apoplast. Confocal microscopy images of OsCIN1-GFP fusion protein in transgenic rice calli. (Left) GFP fluorescence (green) showing OsCIN1 localization to the cell wall and intercellular spaces. (Middle) Bright-field image. (Right) Merged image of GFP fluorescence and bright-field. The GFP signal indicates that OsCIN1 localizes to the apoplast. Scale bar = 20 µm.
2.4. OsCIN1 Overexpression Alters Sugar Composition
Given that OsCIN catalyzes sucrose hydrolysis, we hypothesized that sugar composition would be altered in the OsCIN1 OX lines. Sucrose, the primary product of photosynthesis, is synthesized in leaves during the day, transported through the phloem, and subsequently unloaded into sink organs such as roots, young panicles, and developing grains [31]. Because a significant proportion of the carbohydrates required for grain development in rice is supplied by the flag leaf [32], we quantified soluble sugars and starch in the flag leaves of OsCIN1 OX lines at the flowering stage. Glucose, fructose, and starch levels were significantly higher in the OsCIN1 OX lines than in the WT (Figure 4a,b,d). Our experimental design does not allow compartment-specific attribution of the increased hexoses to either the cytosol or the apoplast. However, given that hexose concentrations generally exhibit much smaller diurnal fluctuations compared with sucrose and remain relatively stable [33] and that sucrose was undetectable in the OX lines whereas it was present at appreciable levels in WT flag leaves (Figure 4c), the observed increase in hexoses is most plausibly attributable to enhanced sucrose hydrolysis in the apoplast in OsCIN1 OX lines. The absence of detectable sucrose in the OX lines is likely attributable to sustained sucrose hydrolysis in the apoplast. Driven by concentration gradients, sucrose from the vacuolar pool effluxes into the cytosol and is subsequently exported across the plasma membrane into the apoplast, where it is continuously cleaved into hexoses. This sustained hydrolysis likely accelerates the depletion of the sucrose pool.

Figure 4.
Soluble sugar and starch content in flag leaves of OsCIN1 OX transgenic lines. Levels of soluble sugars: (a) glucose, (b) fructose, (c) sucrose, and (d) starch in flag leaves at the flowering stage. Two independent transgenic lines (OsCIN1 OX-1 and OsCIN1 OX-3) showed significantly altered sugar composition compared to WT plants. Each value represents the mean ± SE of three biological replicates. Statistical analysis was performed using Student’s t-test. ** p < 0.01. n.d., not detected.
2.5. Overexpression of OsCIN1 Suppresses Plant Growth and Grain Yield in Rice Paddy
To assess the impact of OsCIN1 overexpression on plant growth under field conditions, transgenic lines were cultivated in paddy fields, and their developmental characteristics were monitored. OsCIN1 OX lines exhibited significantly stunted growth compared with WT plants (Figure 5a). Tiller number in the OX lines was approximately 50% lower than that of WT plants (Figure 5a,b). Both 1000-grain weight and grain size were significantly reduced (Figure 5c–g). The depletion of sucrose in both the apoplast and cytosol of source tissues suggests that little or no sucrose was available for translocation to sink organs. Consequently, constitutively high CIN activity in both source and sink tissues likely impaired the establishment of the sucrose concentration gradient required for efficient sucrose transport. This perturbation is expected to have resulted in reductions in tiller number, seed size, and grain weight.
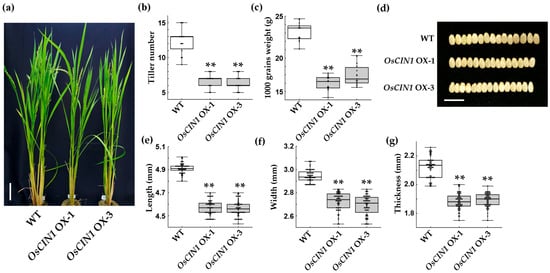
Figure 5.
Phenotypic characterization of OsCIN1 OX plants in paddy field conditions. (a) Representative plants of WT and OX lines at the flowering stage in the field. (b) Tiller number per plant. (c) 1000-grain weight. (d) Representative grain morphology showing width differences. (e–g) Grain length, width, and thickness, respectively. Scale bars, 10 cm (a); 1 cm (d). Each value represents the mean ± SE; n = eight plants (b) and n = 60 grains (e–g). Statistical analysis was performed using Student’s t-test. ** p < 0.01.
3. Discussion
3.1. OsCIN1 Functions as a Canonical Cell Wall Invertase with Tissue-Specific Expression
Understanding the activity and regulation of CINs is fundamental to elucidating how plants establish and maintain sucrose concentration gradients between source and sink tissues. These gradients, characterized by high sucrose levels in photosynthetic source tissues and low sucrose concentrations in metabolically active sinks, are generated and sustained through elevated CIN enzymatic activity in sink organs. This knowledge is crucial for developing strategies to enhance sink strength and optimize resource allocation in crop plants.
Subcellular localization analysis using GFP fusion proteins confirmed that OsCIN1 localizes to the apoplast (Figure 3). pOSCIN1::GUS line revealed that OsCIN1 is expressed in vascular tissues, with particularly expressed in the ovular vascular trace and lateral stylar vascular traces during seed development (Figure 2). This vascular-specific expression pattern establishes OsCIN1 as a canonical cell wall invertase that strategically regulates sucrose loading and unloading pathways. The strong expression in developing seed vascular tissues suggests a central role in controlling carbon flux into the grain, which determines rice yield. In addition, our recent functional genetic analysis demonstrated that the oscin1/oscin2 double mutant exhibits severe reductions in both seed starch accumulation and pollen starch synthesis. Enzyme activity assays further showed that OsCIN activity in mature anthers was approximately 500-fold higher than in leaves [22]. Taken together, these findings indicate that OsCIN1 and OsCIN2 act as the primary regulators of sink strength during reproductive development. This functional importance, combined with the role of CINs in enhancing crop productivity across multiple species [18,23,27], led us to hypothesize that constitutively overexpression of OsCIN1 would enhance sink capacity in developing seeds by maintaining high apoplastic sucrose hydrolysis activity, thereby accelerating carbon flux into grains and improving crop yield. To test this hypothesis, we generated transgenic OsCIN1-OX lines under the CaMV 35S promoter.
3.2. Constitutive OsCIN1 Overexpression Paradoxically Impairs Agronomic Traits
To examine whether the increased CIN activity could enhance productivity, we generated transgenic rice lines overexpressing OsCIN1 under the control of the constitutive CaMV 35S promoter. The overexpression lines exhibited increased CIN activity in fully expanded leaves and stem segments at both vegetative and reproductive stages (Figure 1d,e). This confirmed that the 35S promoter effectively enhances OsCIN1 expression and that the encoded enzyme retained full catalytic activity in planta. However, the phenotypic outcomes differed from our predictions. Rather than enhancing productivity, constitutive overexpression of OsCIN1 resulted in significant agronomic penalties under field conditions. Tiller number, a key yield component in rice, was reduced by approximately 50% in OX lines compared to wild type (Figure 5a,b). Furthermore, 1000-grain weight decreased significantly (Figure 5c), accompanied by reductions in grain width and thickness (Figure 5d–g). These results demonstrate that ectopic, constitutive elevation of CIN activity throughout the plant does not strengthen sink capacity as anticipated. Instead, it disrupts developmental tillering processes and seed development, ultimately diminishing overall productivity.
3.3. The Importance of Spatiotemporal Regulation of CIN Expression
The mechanistic basis for these adverse phenotypes is revealed by sugar profiling of flag leaves, where the primary photosynthetic organ supplying carbon to developing grains in rice [32]. At the flowering stage, OsCIN1 OX lines exhibited depletion of sucrose in flag leaves, while WT plants maintained suitable sucrose levels (Figure 4c). Simultaneously, glucose, fructose, and starch accumulated significantly higher levels in OsCIN1 OX flag leaves compared to wild type (Figure 4a,b,d). These metabolic perturbations indicate the impairment of source-sink carbon partitioning. In wild-type plants, sucrose synthesized in source leaves is exported to the phloem and transported to sink organs, where localized CIN activity in the apoplast establishes the concentration gradient driving continued sucrose flux. However, when CIN activity is elevated throughout the plant, sucrose is excessively hydrolyzed, leading to the collapse of the sucrose gradient between source and sink organs. The hexoses generated by CIN overexpression cannot substitute for sucrose in long-distance transport and therefore accumulate in source tissues.
The complete absence of detectable sucrose in OsCIN1 OX flag leaves indicates that sustained apoplastic sucrose hydrolysis creates a concentration gradient that continuously draws sucrose from intracellular pools (cytosol and vacuole) into the apoplast, where it is immediately cleaved. This sustained depletion eliminates the sucrose available for phloem loading and long-distance transport to sink organs. Consequently, despite high CIN activity in sink tissues, insufficient sucrose delivery results in carbon starvation of developing grains and other sink organs, explaining the observed reductions in tillering, grain size, and overall yield.
Consistent with our findings, several studies have reported that constitutive CINs overexpression disrupts normal plant development and yield traits. In cassava, overexpression of MeCWINV3 disrupted source-to-sink sugar partitioning, reduced sugar movement to storage roots, suppressed starch biosynthesis gene expression, and affected the yield [34]. Similarly, heterologous expression of yeast invertase in plant cell walls has consistently been shown to disrupt assimilate allocation, impair root development in tobacco [35,36]. These studies demonstrate that continuous expression of invertase activity perturbs normal carbon partitioning patterns and impairs plant development. Most instructive is the case of rice OsCIN2, which shares high functional similarity with OsCIN1. When OsCIN2 was overexpressed under the constitutive 35S promoter, transgenic plants exhibited shrunken seeds remarkably similar to loss-of-function mutants [20]. In contrast, when the same gene was expressed under its native promoter, grain size increased significantly [20]. These findings clearly demonstrate that the spatiotemporal pattern of CIN expression is a key determinant of phenotypic outcomes.
3.4. Functional Specialization Within the CIN Gene Family
The CIN gene family is involved in many plant species. There are six members in Arabidopsis thaliana [37], eight in tomato (Solanum lycopersicum) [38], 12 in tobacco (Nicotiana tabacum) [39], and nine in rice [40]. The large number of genes in each species suggests functional diversity rather than genetic redundancy. Different tissues and developmental stages require different metabolic pathway activation and different sucrose concentrations. The multiplicity of CIN genes enables plants to adjust sucrose hydrolysis to meet tissue-specific metabolic requirements, developmental stages, and environmental conditions (light intensity, temperature, water, and nutrients). To improve the yield by manipulating CIN activity, a comprehensive understanding is required for the tissue-specific, temporal dynamics, and expression level. Moreover, the enzyme’s catalytic properties, substrate availability, and integration with downstream pathways such as hexose transport and metabolism are also assumed. In parallel, the discovery, mechanistic characterization, and application of CIN inhibitors that enable post-translational regulation via competitive inhibition of CIN activity are required. Such a multilayered, precision-targeted strategy should circumvent the adverse outcomes of indiscriminate constitutive overexpression and, by enabling refined control of source-to-sink sucrose gradients, deliver stable and reproducible improvements in crop yield.
4. Materials and Methods
4.1. Plant Material
The japonica rice line Oryza sativa cv. Dongjin was used throughout this study. All plant materials were freshly harvested and stored at −80 °C prior to experiments. Sterilized seeds were placed on ½ Murashige and Skoog (MS) agar medium for germination at 28 °C for 7 days before being transferred to a greenhouse for cultivation under a 14 h light/10 h dark photoperiod.
4.2. Plasmid Construction
For the OsCIN1 overexpressing construct, the full-length coding sequence of OsCIN1 1191 base pairs (bp) was amplified by polymerase chain reaction (PCR) from rice (O. sativa L. ssp. japonica cv. Dongjin) cDNA using Phusion High-Fidelity DNA Polymerase (Thermo Scientific, Waltham, MA, USA) and cloned into the pCR-4-TOPO vector (Invitrogen, Waltham, MA, USA). Following sequencing verification, the OsCIN1-TOPO clone was subcloned into a Gateway entry vector and placed between the 35S promoter and Nos terminator or GFP-Nos terminator using the Multi-Round LR recombinase-mediated Gateway™ system (Invitrogen, Waltham, MA, USA), generating p35S::OsCIN1 and p35S::OsCIN1-GFP constructs. For the OsCIN1 promoter-driven GUS construct, we amplified a 2.0-kilobase promoter region of OsCIN1 from the rice genome and inserted it into plasmid pCAMBIA1305, generating pOsCIN1::GUS. All plasmids were introduced into Agrobacterium tumefaciens strain LBA4404, and rice transformation was performed as described previously [41].
4.3. Quantitative Real-Time PCR
Total RNA was extracted from rice tissues using RiboEx reagent (GeneAll Biotechnology Co., Ltd., Seoul, Republic of Korea), and 1–5 µg of total RNA was used for cDNA synthesis using the ReverTra Ace qPCR RT Kit (Toyobo, Osaka, Japan). The cDNA was diluted 10-fold and stored at −20 °C. Quantitative RT-PCR was performed as described previously with OsCIN1 primer (F: TGAGAAGCTTGATTGACCGTTC; R: ATAAGCGGCTTCTTCATTTCCC), using OsUBQ5 (F: CCTCGCCGACTACAACATC; R GCTTGTGCTTCTGCTTCTTG) as the internal reference gene for normalization with triplicate [42].
4.4. CIN Activity
Leaf and stem base samples were harvested and immediately frozen in liquid nitrogen to preserve enzyme activity. For stem base samples, 1.5-cm sections were collected from the base of the stem. CIN activity was assayed using a method adapted and modified from Roitsch and González [43].
4.5. Subcellular Localization
The p35S::OsCIN1-GFP construct was transformed into rice callus to examine subcellular localization. Confocal laser scanning microscopy was performed using a Zeiss LSM 510 META confocal microscope (Carl Zeiss, Oberkochen, Germany). GFP fluorescence was excited using a 488-nm argon laser, and emission signals were collected at 500–550 nm. Images were processed and analyzed using ImageJ 1.53e software (National Institutes of Health, Bethesda, MD, USA).
4.6. Flag Leaf Metabolite Measurement
Fresh flag leaf samples were harvested and extracted using 10% perchloric acid (prepared from 37% stock solution). The insoluble and soluble fractions were separated by centrifugation at 13,000× g for 15 min. The soluble fraction (supernatant) was neutralized with neutralization buffer (2 M KOH, 0.4 M MES) to pH 6–7. The concentrations of glucose, fructose, and sucrose were determined through an NAD(P)H-coupled enzymatic assay by sequentially adding 0.5 units of hexokinase, 0.5 units of phosphoglucose isomerase, and 2 units of invertase. The measured metabolite contents were calculated relative to sample fresh weight. The insoluble fraction was washed repeatedly with 80% ethanol to remove chlorophyll until the pellet was colorless. The pellet was resuspended in an appropriate volume of water, and starch was gelatinized at 95 °C for 15 min. The gelatinized solution was incubated with 100 mM sodium acetate buffer (pH 4.8) containing 1 unit of amyloglucosidase and 1 unit of α-amylase at 37 °C overnight. After incubation, the mixture was centrifuged at 13,000× g for 5 min, and the glucose content in the supernatant was quantified using an NAD(P)H-coupled enzymatic assay to determine starch content [44].
4.7. GUS Staining
GUS staining of seeds and seedlings of the pOsCIN1::GUS line was conducted as described previously [45].
4.8. Field Trial
Field trials were performed at the LMO field of Gyeongsang National University in Sacheon, Republic of Korea. The paddy field was flooded with 5 cm of water until the end of the active tillering stage (approximately one month after transplanting). Water management was adjusted according to the growth stage, maintaining soil moisture during the late tillering stage, then re-flooding with 10 cm of water until the milky stage. To evaluate grain weight per plant, a total of eight plants of each line were grown in the paddy field. Seeds were harvested after ripening, dried, and weighed to determine grain weight per plant.
5. Conclusions
Our results highlight a critical principle for crop improvement: manipulating CIN activity to enhance yield demands precise spatiotemporal control rather than broad constitutive expression. Each CIN gene is likely specialized to reach the metabolic demands of specific tissues, developmental stages, and environmental conditions. The tissue-specific localization of OsCIN1 in vascular tissues, particularly in ovular and lateral stylar vascular traces during seed development, demonstrates that it functions under specific spatial and temporal regulation. Future strategies to enhance sink capacity and improve crop yield must therefore be grounded in a comprehensive understanding of the spatial distribution of each CIN protein, the timing of their developmental stage–specific activation, and their functional integration with downstream hexose transporters.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms262311471/s1.
Author Contributions
S.-K.L. and J.-S.J. designed this research. C.D.N., J.-S.E., J.-I.C., S.-H.C., J.U.K., S.-C.E. and K.A.T.P. performed the experiments and analyzed data. C.D.N., J.L., S.-K.L. and J.-S.J. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by grants from the National Research Foundation (RS-2023-00210086 and RS-2025-02263262 for S.-K.L. and RS-2020-NR049590 for K.A.T.P. and 2023R1A2C1003142 for J.-S.J.) and from the Rural Development Administration (RS-2025-02223622 for J.-S.J.).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wu, W.; Chen, L.; Liang, R.; Huang, S.; Li, X.; Huang, B.; Luo, H.; Zhang, M.; Wang, X.; Zhu, H. The role of light in regulating plant growth, development and sugar metabolism: A review. Front. Plant Sci. 2024, 15, 1507628. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Yang, L.; Zhao, D.; Li, T.; Liu, Y.; Wang, X. Induced photosynthesis significantly influences biomass in Betula platyphylla seedlings compared to steady-state photosynthesis under different nitrogen forms. Plant Physiol. Biochem. PPB 2025, 228, 110225. [Google Scholar] [CrossRef] [PubMed]
- Furbank, R.T.; Kelly, S. Finding the C4 sweet spot: Cellular compartmentation of carbohydrate metabolism in C4 photosynthesis. J. Exp. Bot. 2021, 72, 6018–6026. [Google Scholar] [CrossRef]
- Funfgeld, M.; Wang, W.; Ishihara, H.; Arrivault, S.; Feil, R.; Smith, A.M.; Stitt, M.; Lunn, J.E.; Niittyla, T. Sucrose synthases are not involved in starch synthesis in Arabidopsis leaves. Nat. Plants 2022, 8, 574–582. [Google Scholar] [CrossRef]
- Lemoine, R.; La Camera, S.; Atanassova, R.; Dedaldechamp, F.; Allario, T.; Pourtau, N.; Bonnemain, J.L.; Laloi, M.; Coutos-Thevenot, P.; Maurousset, L.; et al. Source-to-sink transport of sugar and regulation by environmental factors. Front. Plant Sci. 2013, 4, 272. [Google Scholar] [CrossRef]
- Wang, L.; Lu, Q.; Wen, X.; Lu, C. Enhanced sucrose loading improves rice yield by increasing grain size. Plant Physiol. 2015, 169, 2848–2862. [Google Scholar] [CrossRef]
- Nakano, H.; Yoshinaga, S.; Takai, T.; Arai-Sanoh, Y.; Kondo, K.; Yamamoto, T.; Sakai, H.; Tokida, T.; Usui, Y.; Nakamura, H.; et al. Quantitative trait loci for large sink capacity enhance rice grain yield under free-air CO2 enrichment conditions. Sci. Rep. 2017, 7, 1827. [Google Scholar] [CrossRef]
- Hu, Z.; Tang, Z.; Zhang, Y.; Niu, L.; Yang, F.; Zhang, D.; Hu, Y. Rice SUT and SWEET Transporters. Int. J. Mol. Sci. 2021, 22, 11198. [Google Scholar] [CrossRef]
- Pegler, J.L.; Grof, C.P.; Patrick, J.W. Sugar loading of crop seeds-a partnership of phloem, plasmodesmal and membrane transport. New Phytol. 2023, 239, 1584–1602. [Google Scholar] [CrossRef]
- Lauschke, A.; Maibaum, L.; Engel, M.; Eisengraber, L.; Bayer, S.; Hackel, A.; Kuhn, C. The potato sugar transporter SWEET1g affects apoplasmic sugar ratio and phloem-mobile tuber- and flower-inducing signals. Plant Physiol. 2024, 197, kiae602. [Google Scholar] [CrossRef]
- Abuslima, E.; Kanbar, A.; Ismail, A.; Raorane, M.L.; Eiche, E.; El-Sharkawy, I.; Junker, B.H.; Riemann, M.; Nick, P. Salt stress-induced remodeling of sugar transport: A role for promoter alleles of SWEET13. Sci. Rep. 2025, 15, 7580. [Google Scholar] [CrossRef]
- Bezrutczyk, M.; Hartwig, T.; Horschman, M.; Char, S.N.; Yang, J.; Yang, B.; Frommer, W.B.; Sosso, D. Impaired phloem loading in zmsweet13a,b,c sucrose transporter triple knock-out mutants in Zea mays. New Phytol. 2018, 218, 594–603. [Google Scholar] [CrossRef]
- Albacete, A.; Cantero-Navarro, E.; Balibrea, M.E.; Grosskinsky, D.K.; de la Cruz Gonzalez, M.; Martinez-Andujar, C.; Smigocki, A.C.; Roitsch, T.; Perez-Alfocea, F. Hormonal and metabolic regulation of tomato fruit sink activity and yield under salinity. J. Exp. Bot. 2014, 65, 6081–6095. [Google Scholar] [CrossRef]
- Li, J.; He, C.; Liu, S.; Guo, Y.; Zhang, Y.; Zhang, L.; Zhou, X.; Xu, D.; Luo, X.; Liu, H.; et al. Research progress and application strategies of sugar transport mechanisms in rice. Front. Plant Sci. 2024, 15, 1454615. [Google Scholar] [CrossRef]
- Tang, Y.; Schiestl-Aalto, P.; Saurer, M.; Sahlstedt, E.; Kulmala, L.; Kolari, P.; Ryhti, K.; Salmon, Y.; Jyske, T.; Ding, Y.; et al. Tree organ growth and carbon allocation dynamics impact the magnitude and delta13C signal of stem and soil CO2 fluxes. Tree Physiol. 2022, 42, 2404–2418. [Google Scholar] [CrossRef]
- Wu, L.; Fan, S.; Li, S.; Li, J.; Zhang, Z.; Qin, Y.; Hu, G.; Zhao, J. LcINH1 as an inhibitor of cell wall invertase LcCWIN5 regulates early seed development in Litchi chinensis Sonn. Int. J. Biol. Macromol. 2024, 278, 134497. [Google Scholar] [CrossRef]
- Lu, J.; Ren, Q.; Wang, Q.; Wen, Y.; Wang, Y.; Liang, R.; Ran, D.; Jia, Y.; Zhuo, X.; Luo, J.; et al. Cell wall invertase 4 governs sucrose-hexose homeostasis in the apoplast to regulate wood development in poplar. Plants 2025, 14, 1388. [Google Scholar] [CrossRef]
- Liao, S.; Wang, L.; Li, J.; Ruan, Y.L. Cell wall invertase is essential for ovule development through sugar signaling rather than provision of carbon nutrients. Plant Physiol. 2020, 183, 1126–1144. [Google Scholar] [CrossRef]
- Kocal, N.; Sonnewald, U.; Sonnewald, S. Cell wall-bound invertase limits sucrose export and is involved in symptom development and inhibition of photosynthesis during compatible interaction between tomato and Xanthomonas campestris pv vesicatoria. Plant Physiol. 2008, 148, 1523–1536. [Google Scholar] [CrossRef]
- Wang, E.; Wang, J.; Zhu, X.; Hao, W.; Wang, L.; Li, Q.; Zhang, L.; He, W.; Lu, B.; Lin, H.; et al. Control of rice grain-filling and yield by a gene with a potential signature of domestication. Nat. Genet. 2008, 40, 1370–1374. [Google Scholar] [CrossRef]
- Kawaguchi, K.; Takei-Hoshi, R.; Yoshikawa, I.; Nishida, K.; Kobayashi, M.; Kusano, M.; Lu, Y.; Ariizumi, T.; Ezura, H.; Otagaki, S.; et al. Functional disruption of cell wall invertase inhibitor by genome editing increases sugar content of tomato fruit without decrease fruit weight. Sci. Rep. 2021, 11, 21534. [Google Scholar] [CrossRef]
- Lee, S.K.; Shim, S.H.; Eom, J.S.; Cho, J.I.; Kwak, J.U.; Eom, S.C.; Jeon, J.S. Cell wall invertases from maternal tissues modulate sucrose flux in apoplastic pathways during rice anther and seed development. Int. J. Mol. Sci. 2024, 25, 11557. [Google Scholar] [CrossRef]
- Ruan, Y.L. CWIN-sugar transporter nexus is a key component for reproductive success. J. Plant Physiol. 2022, 268, 153572. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Ni, D.A.; Ruan, Y.L. Posttranslational elevation of cell wall invertase activity by silencing its inhibitor in tomato delays leaf senescence and increases seed weight and fruit hexose level. Plant Cell 2009, 21, 2072–2089. [Google Scholar] [CrossRef]
- Liu, C.; Hu, S.; Liu, S.; Shi, W.; Xie, D.; Chen, Q.; Sun, H.; Song, L.; Li, Z.; Jiang, R.; et al. Functional characterization of a cell wall invertase inhibitor StInvInh1 revealed its involvement in potato microtuber size in vitro. Front. Plant Sci. 2022, 13, 1015815. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.Q.; Luscher, M.; Sturm, A. Antisense repression of vacuolar and cell wall invertase in transgenic carrot alters early plant development and sucrose partitioning. Plant Cell 1999, 11, 177–189. [Google Scholar] [CrossRef][Green Version]
- Li, B.; Liu, H.; Zhang, Y.; Kang, T.; Zhang, L.; Tong, J.; Xiao, L.; Zhang, H. Constitutive expression of cell wall invertase genes increases grain yield and starch content in maize. Plant Biotechnol. J. 2013, 11, 1080–1091. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.P.; Lu, Y.M.; Wang, Y.Z.; Duan, C.Q.; Yan, H.Y. Acid invertase is predominantly localized to cell walls of both the practically symplasmically isolated sieve element/companion cell complex and parenchyma cells in developing apple fruits. Plant Cell Environ. 2001, 24, 691–702. [Google Scholar] [CrossRef]
- Eschrich, W. Free space invertase, its possible role in phloem unloading. Berichte Dtsch. Bot. Ges. 1980, 93, 363–378. [Google Scholar] [CrossRef]
- Karuppiah, N.; Vadlamudi, B.; Kaufman, P.B. Purification and characterization of soluble (cytosolic) and bound (cell wall) isoforms of invertases in barley (Hordeum vulgare) elongating stem tissue. Plant Physiol. 1989, 91, 993–998. [Google Scholar] [CrossRef]
- Braun, D.M. Phloem loading and unloading of sucrose: What a long, strange trip from source to sink. Annu. Rev. Plant Biol. 2022, 73, 553–584. [Google Scholar] [CrossRef]
- Fabre, D.; Adriani, D.E.; Dingkuhn, M.; Ishimaru, T.; Punzalan, B.; Lafarge, T.; Clement-Vidal, A.; Luquet, D. The qTSN4 effect on flag leaf size, photosynthesis and panicle size, benefits to plant grain production in rice, depending on light availability. Front. Plant Sci. 2016, 7, 623. [Google Scholar] [CrossRef]
- Kim, S.W.; Lee, S.K.; Jeong, H.J.; An, G.; Jeon, J.S.; Jung, K.H. Crosstalk between diurnal rhythm and water stress reveals an altered primary carbon flux into soluble sugars in drought-treated rice leaves. Sci. Rep. 2017, 7, 8214. [Google Scholar] [CrossRef]
- Yan, W.; Wu, X.; Li, Y.; Liu, G.; Cui, Z.; Jiang, T.; Ma, Q.; Luo, L.; Zhang, P. Cell wall invertase 3 affects cassava productivity via regulating sugar allocation from source to sink. Front. Plant Sci. 2019, 10, 541. [Google Scholar] [CrossRef] [PubMed]
- von Schaewen, A.; Stitt, M.; Schmidt, R.; Sonnewald, U.; Willmitzer, L. Expression of a yeast-derived invertase in the cell wall of tobacco and Arabidopsis plants leads to accumulation of carbohydrate and inhibition of photosynthesis and strongly influences growth and phenotype of transgenic tobacco plants. EMBO J. 1990, 9, 3033–3044. [Google Scholar] [CrossRef]
- Sonnewald, U.; Brauer, M.; von Schaewen, A.; Stitt, M.; Willmitzer, L. Transgenic tobacco plants expressing yeast-derived invertase in either the cytosol, vacuole or apoplast: A powerful tool for studying sucrose metabolism and sink/source interactions. Plant J. Cell Mol. Biol. 1991, 1, 95–106. [Google Scholar] [CrossRef]
- Sherson, S.M.; Alford, H.L.; Forbes, S.M.; Wallace, G.; Smith, S.M. Roles of cell-wall invertases and monosaccharide transporters in the growth and development of Arabidopsis. J. Exp. Bot. 2003, 54, 525–531. [Google Scholar] [CrossRef]
- Godt, D.E.; Roitsch, T. Regulation and tissue-specific distribution of mRNAs for three extracellular invertase isoenzymes of tomato suggests an important function in establishing and maintaining sink metabolism. Plant Physiol. 1997, 115, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Cheng, L.; Jin, J.; He, X.; Luo, Z.; Wang, Z.; Yang, J.; Xu, X. Genome-wide identification and analysis of the invertase gene family in tobacco (Nicotiana tabacum) reveals NtNINV10 participating the sugar metabolism. Front. Plant Sci. 2023, 14, 1164296. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Van den Ende, W.; Van Laere, A.; Cheng, S.; Bennett, J. Structure, evolution, and expression of the two invertase gene families of rice. J. Mol. Evol. 2005, 60, 615–634. [Google Scholar] [CrossRef]
- Jeon, J.S.; Lee, S.; Jung, K.H.; Jun, S.H.; Jeong, D.H.; Lee, J.; Kim, C.; Jang, S.; Yang, K.; Nam, J.; et al. T-DNA insertional mutagenesis for functional genomics in rice. Plant J. Cell Mol. Biol. 2000, 22, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Jain, M.; Nijhawan, A.; Tyagi, A.K.; Khurana, J.P. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2006, 345, 646–651. [Google Scholar] [CrossRef] [PubMed]
- Roitsch, T.; Gonzalez, M.C. Function and regulation of plant invertases: Sweet sensations. Trends Plant Sci. 2004, 9, 606–613. [Google Scholar] [CrossRef]
- Stitt, M.; Lilley, R.M.; Gerhardt, R.; Heldt, H.W. Metabolite levels in specific cells and subcellular compartments of plant leaves. In Methods in Enzymology; Academic Press: Cambridge, MA, USA, 1989; Volume 174, pp. 518–552. [Google Scholar] [CrossRef]
- Chen, Y.S.; Lo, S.F.; Sun, P.K.; Lu, C.A.; Ho, T.H.; Yu, S.M. A late embryogenesis abundant protein HVA1 regulated by an inducible promoter enhances root growth and abiotic stress tolerance in rice without yield penalty. Plant Biotechnol. J. 2015, 13, 105–116. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).