Aging and Thymosin Alpha-1
Abstract
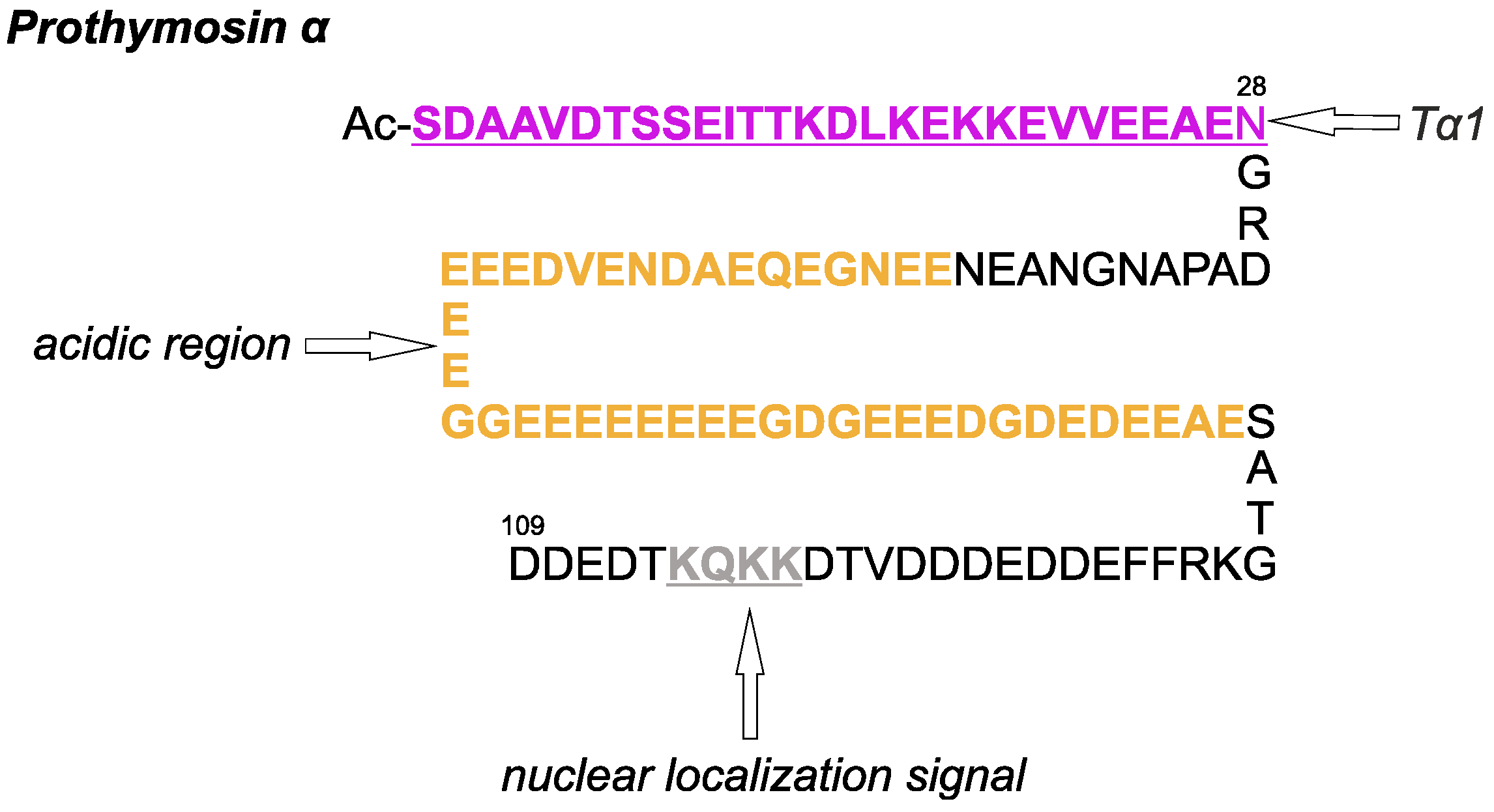
1. Introduction
2. The Role of the Thymus in Aging
3. The Role of Thymosin-α1 in the Aging Process
3.1. Immunomodulatory Activity
- Reduces age-related inflammation by increasing IL-10 levels and suppressing immune hyperreactivity;
- Maintains immune homeostasis, preventing both excessive activation (autoimmune reactions) and immune deficiency;
- Improves T-cell function, compensating for the age-related decline in their activity;
- Corrects the imbalance between immune activation and tolerance, which is especially important in atherosclerosis, neurodegeneration, and metabolic disorders.
3.2. Antioxidant Activity
3.3. Activity in the Nervous System
3.4. Activity in the Cardiovascular System
3.5. Age-Related Changes in Tα1 Production and Their Consequences
- Decreased thymic function;
- Impaired immune surveillance;
- Imbalance between immune activation and tolerance;
- Chronic inflammation;
- Decreased regulatory influence on the hypothalamic–pituitary system;
- Decreased neuroprotective potential;
- Oxidative stress.
3.6. Biological Activity of Tα1 and Aging: Research Data
4. Potential Applications of Refnot for Addressing Age-Related Changes
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| ACE | Angiotensin-converting enzyme |
| AP-1 | Activating protein-1 |
| CT | Computed tomography |
| DN | Double negative precursor |
| DP | Double positive precursor |
| FGF | Fibroblast growth factor |
| FSH | Follicle-stimulating hormone |
| Gal-1 | Galectin-1 |
| GCs | Glucocorticoids |
| GCSF | Granulocyte colony-stimulating factor |
| GH | Growth hormone |
| GHSR | Growth hormone secretagogue receptor |
| GMCSF | Granulocyte-macrophage colony-stimulating factor |
| HIV | Human immunodeficiency virus |
| HLA-DR | Human leukocyte antigen, isotype DR |
| IDO-1 | Indolylamine 2,3-deoxygenase-1 |
| IGF | Insulin-like growth factor |
| IFN | Interferon |
| IFN-αβR | Interferon-alpha/beta receptor |
| IL | Interleukin |
| JNK | c-Jun N-terminal kinase |
| KGF | Keratinocyte growth factor |
| LIF | Leukemia inhibitory factor |
| LPS | Lipopolysaccharide |
| MCSF | Macrophage colony-stimulating factor |
| MHC | Major histocompatibility complex |
| MRI | Magnetic resonance imaging |
| NF-κB | Nuclear factor kappa-light-chain-enhancer of activated B cells |
| NGF | Nerve growth factor |
| NK | Natural killer |
| Nrf-2 | Nuclear factor erythroid 2–related factor 2 |
| OSM | Oncostatin M |
| p75NGFr | Low-affinity nerve growth factor receptor |
| PET-CT | Positron emission tomography-computed tomography |
| PD-1L | Programmed death-ligand 1 |
| PKC | Protein kinase C |
| PTMA | Prothymosin-α |
| RE | Reticuloepithelial |
| SAPK | Stress-activated protein kinase |
| SARS-CoV-2 | Severe acute respiratory syndrome coronavirus 2 |
| SCF | Stem cell factor |
| COVID-19 | Coronavirus disease 2019 |
| SP | Single positive T cell |
| Tα1 | Thymosin alpha-1 |
| TCR | T-cell receptor |
| TEC | Thymic epithelial cell |
| TIM | T-cell immunoglobulin |
| TLR | Toll-like receptor |
| TNFα | Tumor necrosis factor alpha |
| TNF-T | Fusion protein of tumor necrosis factor alpha and thymosin alpha-1 |
| TREC | T-cell receptor excision circles |
| Treg | Regulatory T cell |
| VSV | Vesicular stomatitis virus |
References
- Csaba, G. The Immunoendocrine Thymus as a Pacemaker of Lifespan. Acta Microbiol. Immunol. Hung. 2016, 63, 139–158. [Google Scholar] [CrossRef]
- Rezzani, R.; Franco, C.; Hardeland, R.; Rodella, L.F. Thymus-Pineal Gland Axis: Revisiting Its Role in Human Life and Ageing. Int. J. Mol. Sci. 2020, 21, e8806. [Google Scholar] [CrossRef]
- Rensing, L.; Rippe, V. Das Immunsystem. In Altern; Springer Spektrum: Berlin/Heidelberg, Germany, 2014; pp. 141–157. [Google Scholar]
- Zhang, Q.; Yang, K.; Yangyang, P.; He, J.; Yu, S.; Cui, Y. Age-Related Changes in the Morphology and Protein Expression of the Thymus of Healthy Yaks (Bos grunniens). Am. J. Vet. Res. 2016, 77, 567–574. [Google Scholar] [CrossRef]
- Rezzani, R.; Nardo, L.; Favero, G.; Peroni, M.; Rodella, L.F. Thymus and Aging: Morphological, Radiological, and Functional Overview. Age (Dordr) 2014, 36, 313–351. [Google Scholar] [CrossRef] [PubMed]
- Taub, D.D.; Longo, D.L. Insights into Thymic Aging and Regeneration. Immunol. Rev. 2005, 205, 72–93. [Google Scholar] [CrossRef] [PubMed]
- Bodey, B.; Siegel, S.E.; Kaiser, S.E. Involution of the Mammalian Thymus and Its Role in the Overall Aging Process. In Immunological Aspects of Neoplasia—The Role of the Thymus. Cancer Growth and Progression; Springer: Dordrecht, The Netherlands, 2004; Volume 17, pp. 147–165. [Google Scholar]
- Zhao, C.; Davies, J.D. A Peripheral CD4+ T Cell Precursor for Naive, Memory, and Regulatory T Cells. J. Exp. Med. 2010, 207, 2883–2894. [Google Scholar] [CrossRef]
- Chen, W.; Jin, W.; Hardegen, N.; Lei, K.J.; Li, L.; Marinos, N.; McGrady, G.; Wahl, S.M. Conversion of Peripheral CD4+CD25− Naive T Cells to CD4+CD25+ Regulatory T Cells by TGF-beta induction of Transcription factor Foxp3. J. Exp. Med. 2003, 198, 1875–1886. [Google Scholar] [CrossRef]
- Berard, M.; Tough, D.F. Qualitative Differences between Naïve and Memory T Cells. Immunology 2002, 106, 127–138. [Google Scholar] [CrossRef] [PubMed]
- Levings, M.K.; Sangregorio, R.; Roncarolo, M.G. Human CD25(+)CD4(+) T Regulatory Cells Suppress Naive and Memory T Cell Proliferation and Can Be Expanded In Vitro without Loss of Function. J. Exp. Med. 2001, 193, 1295–1302. [Google Scholar] [CrossRef]
- Liang, Z.; Dong, X.; Zhang, Z.; Zhang, Q.; Zhao, Y. Age-Related Thymic Involution: Mechanisms and Functional Impact. Aging Cell 2022, 21, e13671. [Google Scholar] [CrossRef]
- Gubbels Bupp, M.R.; Jorgensen, T.N. Androgen-Induced Immunosuppression. Front. Immunol. 2018, 9, 794. [Google Scholar] [CrossRef]
- Guevara Patiño, J.A.; Ivanov, V.N.; Lacy, E.; Elkon, K.B.; Marino, M.W.; Nikolić-Žugić, J. TNF-α Is the Critical Mediator of the Cyclic AMP-Induced Apoptosis of CD8+4+ Double-Positive Thymocytes. J. Immunol. 2000, 164, 1689–1694. [Google Scholar] [PubMed]
- Jondal, M.; Pazirandeh, A.; Okret, S. Different Roles for Glucocorticoids in Thymocyte Homeostasis? Trends Immunol. 2004, 25, 595–600. [Google Scholar] [CrossRef]
- Talabér, G.; Jondal, M.; Okret, S. Extra-Adrenal Glucocorticoid Synthesis: Immune Regulation and Aspects on Local Organ Homeostasis. Mol. Cell Endocrinol. 2013, 380, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Hasan, K.M.; Rahman, M.S.; Arif, K.M.; Sobhani, M.E. Psychological Stress and Aging: Role of Glucocorticoids (GCs). Age (Dordr) 2012, 34, 1421–1433. [Google Scholar] [CrossRef]
- Ferone, D.; Pivonello, R.; Van Hagen, P.M.; Dalm, V.A.; Lichtenauer-Kaligis, E.G.; Waaijers, M.; Van Koetsveld, P.M.; Mooy, D.M.; Colao, A.; Minuto, F.; et al. Quantitative and functional expression of somatostatin receptor subtypes in human thymocytes. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E1056–E1066. [Google Scholar] [CrossRef]
- Sandoval, K.E.; Witt, K.A. Somatostatin: Linking Cognition and Alzheimer Disease to Therapeutic Targeting. Pharmacol. Rev. 2024, 76, 1291–1325. [Google Scholar] [CrossRef]
- Dimri, R.; Sharabi, Y.; Shoham, J. Specific Inhibition of Glucocorticoid-Induced Thymocyte Apoptosis by Substance P. J. Immunol. 2000, 164, 2479–2486. [Google Scholar] [CrossRef]
- Kim, D.J.; Chang, S.S.; Lee, J. Anti-Aging Potential of Substance P-Based Hydrogel for Human Skin Longevity. Int. J. Mol. Sci. 2019, 20, 4453. [Google Scholar] [CrossRef]
- Delgado, M.; Garrido, E.; Martinez, C.; Leceta, J.; Gomariz, R.P. Vasoactive Intestinal Peptide and Pituitary Adenylate Cyclase-Activating Polypeptides (PACAP27) and PACAP38) Protect CD4+CD8+ Thymocytes from Glucocorticoid-Induced Apoptosis. Blood 1996, 87, 5152–5161. [Google Scholar] [CrossRef] [PubMed]
- Pankhaniya, R.; Jabrane-Ferrat, N.; Gaufo, G.O.; Sreedharan, S.P.; Dazin, P.; Kaye, J.; Goetzl, E.J. Vasoactive Intestinal Peptide Enhancement of Antigen-Induced Differentiation of a Cultured Line of Mouse Thymocytes. FASEB J. 1998, 12, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Deng, G.; Jin, L. The Effects of Vasoactive Intestinal Peptide in Neurodegenerative Disorders. Neurol. Res. 2017, 39, 65–72. [Google Scholar] [CrossRef]
- Jiang, W.; Wang, H.; Li, Y.S.; Luo, W. Role of vasoactive intestinal peptide in osteoarthritis. J. Biomed. Sci. 2016, 23, 63. [Google Scholar] [CrossRef]
- Silva, A.B.; Palmer, D.B. Evidence of Conserved Neuroendocrine Interactions in the Thymus: Intrathymic Expression of Neuropeptides in Mammalian and Non-Mammalian Vertebrates. Neuroimmunomodulation 2011, 18, 264–270. [Google Scholar] [CrossRef]
- Wu, H.; Lin, X.Q.; Long, Y.; Wang, J. Calcitonin Gene-Related Peptide Is Potential Therapeutic Target of Osteoporosis. Heliyon 2022, 8, e12288. [Google Scholar] [CrossRef]
- Medina, S.; Del Río, M.; Hernanz, A.; De la Fuente, M. Age-Related Changes in the Neuropeptide Y Effects on Murine Lymphoproliferation and Interleukin-2 Production. Peptides 2000, 21, 1403–1409. [Google Scholar] [CrossRef]
- Botelho, M.; Cavadas, C. Neuropeptide Y: An Anti-Aging Player? Trends Neurosci. 2015, 38, 701–711. [Google Scholar] [CrossRef] [PubMed]
- Savino, W. Neuroendocrine Control of T Cell Development in Mammals: Role of Growth Hormone in Modulating Thymocyte Migration. Exp. Physiol. 2007, 92, 813–817. [Google Scholar] [CrossRef]
- Fernández-Garza, L.E.; Guillen-Silva, F.; Sotelo-Ibarra, M.A.; Domínguez-Mendoza, A.E.; Barrera-Barrera, S.A.; Barrera-Saldaña, H.A. Growth Hormone and Aging: A Clinical Review. Front. Aging 2025, 6, 1549453. [Google Scholar] [CrossRef] [PubMed]
- Arnold, E.; Thébault, S.; Aroña, R.M.; Martínez de la Escalera, G.; Clapp, C. Prolactin Mitigates Deficiencies of Retinal Function Associated with Aging. Neurobiol. Aging 2020, 85, 38–48. [Google Scholar] [CrossRef]
- Carretero-Hernández, M.; Herráez, E.; Catalano-Iniesta, L.; Hernández-González, D.; Díez-Castro, D.; Rodríguez-Vicente, A.E.; García-Barrado, J.; Vicente-García, T.; Robles-García, M.; Blanco, E.J.; et al. Lifetime Variations in Prolactin Expression in the Hippocampus and Dentate Gyrus of the Rat. Int. J. Mol. Sci. 2025, 26, 7299. [Google Scholar] [CrossRef] [PubMed]
- Burnham, V.L.; Thornton, J.E. Luteinizing Hormone as a Key Player in the Cognitive Decline of Alzheimer’s Disease. Horm. Behav. 2015, 76, 48–56. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, M.; Lizneva, D.; Kim, S.M.; Sun, L.; Iqbal, J.; New, M.I.; Rosen, C.J.; Yuen, T. FSH, Bone Mass, Body Fat, and Biological Aging. Endocrinology 2018, 159, 3503–3514. [Google Scholar] [CrossRef]
- Tenk, J.; Rostás, I.; Füredi, N.; Mikó, A.; Solymár, M.; Soós, S.; Gaszner, B.; Feller, D.; Székely, M.; Pétervári, E.; et al. Age-Related Changes in Central Effects of Corticotropin-Releasing Factor (CRF) Suggest a Role for This Mediator in Aging Anorexia and Cachexia. Geroscience 2017, 39, 61–72. [Google Scholar] [CrossRef]
- Elewa, R.M.; Abdallah, M.; Youssef, N.; Zouboulis, C.C. Aging-related changes in cutaneous corticotropin-releasing hormone system reflect a defective neuroendocrine-stress response in aging. Rejuvenation Res. 2012, 15, 366–373. [Google Scholar] [CrossRef]
- Chaves, V.E.; Tilelli, C.Q.; Brito, N.A.; Brito, M.N. Role of Oxytocin in Energy Metabolism. Peptides 2013, 45, 9–14. [Google Scholar] [CrossRef]
- Elabd, C.; Cousin, W.; Upadhyayula, P.; Chen, R.Y.; Chooljian, M.S.; Li, J.; Kung, S.; Jiang, K.P.; Conboy, I.M. Oxytocin Is an Age-Specific Circulating Hormone That Is Necessary for Muscle Maintenance and Regeneration. Nat. Commun. 2014, 5, 4082. [Google Scholar] [CrossRef]
- Orihashi, R.; Mizoguchi, Y. Oxytocin for Maintaining Mental Health in Older Adults. Arch. Gerontol. Geriatr. Plus 2024, 1, 100090. [Google Scholar] [CrossRef]
- Mutig, K.; Lebedeva, S.; Singh, P.B. Inflammation and Vasopressin Hypersecretion in Aging. Front. Endocrinol. 2025, 16, 1689787, Correction in Front. Endocrinol. 2025, 16, 1724342. [Google Scholar] [CrossRef]
- Oner, H.; Kus, I.; Oner, J.; Ogetürk, M.; Ozan, E.; Ayar, A. Possible Effects of Melatonin on Thymus Gland after Pinealectomy in Rats. Neuro Endocrinol. Lett. 2004, 25, 115–118. [Google Scholar]
- Molinero, P.; Soutto, M.; Benot, S.; Hmadcha, A.; Guerrero, J.M. Melatonin Is Responsible for the Nocturnal Increase Observed in Serum and Thymus of Thymosin Alpha1 and Thymulin Concentrations: Observations in Rats and Humans. J. Neuroimmunol. 2000, 103, 180–188. [Google Scholar] [CrossRef]
- Karasek, M. Melatonin, Human Aging, and Age-Related Diseases. Exp. Gerontol. 2004, 39, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Rudert, W.A.; Grupillo, M.; He, J.; Sisino, G.; Trucco, M. Thymus-Specific Deletion of Insulin Induces Autoimmune Diabetes. EMBO J. 2009, 28, 2812–2824. [Google Scholar] [CrossRef] [PubMed]
- Csaba, G.; Kovács, P.; Pállinger, E. Influence of In Vitro and In Vivo Insulin Treatment on the Hormone (Histamine, Serotonin, Endorphin and Triiodothyronine) Content of Thymus and Spleen Cells. Life Sci. 2006, 78, 1034–1037. [Google Scholar] [CrossRef] [PubMed]
- Kolb, H.; Kempf, K.; Martin, S. Insulin and Aging—A Disappointing Relationship. Front. Endocrinol. 2023, 14, 1261298. [Google Scholar] [CrossRef]
- Sonntag, W.E.; Deak, F.; Ashpole, N.; Toth, P.; Csiszar, A.; Freeman, W.; Ungvari, Z. Insulin-Like Growth Factor-1 in CNS and Cerebrovascular Aging. Front. Aging Neurosci. 2013, 5, 27. [Google Scholar] [CrossRef]
- Werner, H.; Laron, Z. Insulin-Like Growth Factors and Aging: Lessons from Laron Syndrome. Front. Endocrinol. 2023, 14, 1291812. [Google Scholar] [CrossRef]
- Shamonki, I.M.; Frumar, A.M.; Tataryn, I.V.; Meldrum, D.R.; Davidson, B.H.; Parthemore, J.G.; Judd, H.L.; Deftos, L.J. Age-Related Changes of Calcitonin Secretion in Females. J. Clin. Endocrinol. Metab. 1980, 50, 437–439. [Google Scholar] [CrossRef]
- Malicet, C.; Giroux, V.; Vasseur, S.; Dagorn, J.C.; Neira, J.L.; Iovanna, J.L. Regulation of Apoptosis by the P8/Prothymosin Alpha Complex. Proc. Natl. Acad. Sci. USA 2006, 103, 2671–2676. [Google Scholar] [CrossRef]
- Karapetian, R.N.; Evstafieva, A.G.; Abaeva, I.S.; Chichkova, N.V.; Filonov, G.S.; Rubtsov, Y.P.; Sukhacheva, E.A.; Melnikov, S.V.; Schneider, U.; Wanker, E.E.; et al. Nuclear Oncoprotein Prothymosin Alpha Is a Partner of Keap1: Implications for Expression of Oxidative Stress-Protecting Genes. Mol. Cell Biol. 2005, 25, 1089–1099. [Google Scholar] [CrossRef]
- Moreira, D.; Díaz-Jullien, C.; Sarandeses, C.S.; Covelo, G.; Barbeito, P.; Freire, M. The Influence of Phosphorylation of Prothymosin Alpha on Its Nuclear Import and Antiapoptotic Activity. Biochem. Cell Biol. 2013, 91, 265–269. [Google Scholar] [CrossRef]
- Freire, M.; Barbeito, P.; Sarandeses, C.S.; Díaz-Jullien, C.; Muras, J.; Covelo, G.; Moreira, D.; Freire-Cobo, C. Prothymosin α (ProTα) and Its Protein Interactions: A Nuclear and Cytoplasmic Multifunctional Protein. J. Immunol. Sci. 2018, 2, 19–25. [Google Scholar] [CrossRef]
- Romani, L.; Moretti, S.; Fallarino, F.; Bozza, S.; Ruggeri, L.; Casagrande, A.; Aversa, F.; Bistoni, F.; Velardi, A.; Garaci, E. Jack of All Trades: Thymosin α1 and Its Pleiotropy. Ann. N. Y. Acad. Sci. 2012, 1269, 1–6. [Google Scholar] [CrossRef]
- Garaci, E.; Paci, M.; Matteucci, C.; Costantini, C.; Puccetti, P.; Romani, L. Phenotypic Drug Discovery: A Case for Thymosin Alpha-1. Front. Med. 2024, 11, 1388959. [Google Scholar] [CrossRef] [PubMed]
- Esipov, R.S.; Stepanenko, V.N.; Beyrakhova, K.A.; Muravjeva, T.I.; Miroshnikov, A.I. Production of Thymosin Alpha1 via Non-Enzymatic Acetylation of the Recombinant Precursor. Biotechnol. Appl. Biochem. 2010, 56, 17–25. [Google Scholar] [CrossRef]
- Dominari, A.; Hathaway Iii, D.; Pandav, K.; Matos, W.; Biswas, S.; Reddy, G.; Thevuthasan, S.; Khan, M.A.; Mathew, A.; Makkar, S.S.; et al. Thymosin Alpha 1: A Comprehensive Review of the Literature. World J. Virol. 2020, 9, 67–78. [Google Scholar] [CrossRef]
- Dinetz, E.; Lee, E. Comprehensive Review of the Safety and Efficacy of Thymosin Alpha 1 in Human Clinical Trials. Altern. Ther. Health Med. 2024, 30, 6–12. [Google Scholar] [PubMed]
- Matteucci, C.; Minutolo, A.; Sinibaldi-Vallebona, P.; Palamara, A.T.; Rasi, G.; Mastino, A.; Garaci, E. Transcription Profile of Human Lymphocytes Following In Vitro Treatment with Thymosin Alpha-1. Ann. N. Y. Acad. Sci. 2010, 1194, 6–19. [Google Scholar] [CrossRef]
- Snyder-Mackler, N.; Somel, M.; Tung, J. Shared Signatures of Social Stress and Aging in Peripheral Blood Mononuclear Cell Gene Expression Profiles. Aging Cell 2014, 13, 954–957. [Google Scholar] [CrossRef]
- Palmer, D.; Fabris, F.; Doherty, A.; Freitas, A.A.; de Magalhães, J.P. Ageing Transcriptome Meta-Analysis Reveals Similarities and Differences between Key Mammalian Tissues. Aging 2021, 13, 3313–3341. [Google Scholar] [CrossRef]
- Ventevogel, M.S.; Sempowski, G.D. Thymic Rejuvenation and Aging. Curr. Opin. Immunol. 2013, 25, 516–522. [Google Scholar] [CrossRef]
- Knutsen, A.P.; Freeman, J.J.; Mueller, K.R.; Roodman, S.T.; Bouhasin, J.D. Thymosin-Alpha1 Stimulates Maturation of CD34+ Stem Cells Into CD3+4+ Cells in an In Vitro Thymic Epithelia Organ Coculture Model. Int. J. Immunopharmacol. 1999, 21, 15–26. [Google Scholar] [CrossRef]
- Baumann, C.A.; Badamchian, M.; Goldstein, A.L. Thymosin Alpha 1 Antagonizes Dexamethasone and CD3-Induced Apoptosis of CD4+ CD8+ Thymocytes through the Activation of cAMP and Protein Kinase C Dependent Second Messenger Pathways. Mech. Ageing Dev. 1997, 94, 85–101. [Google Scholar] [CrossRef]
- Roy, R.; Singh, S.M.; Shanker, A.; Sodhi, A. Mechanism of Thymocyte Apoptosis Induced by Serum of Tumor-Bearing Host: The Molecular Events Involved and Their Inhibition by Thymosin Alpha-1. Int. J. Immunopharmacol. 2000, 22, 309–321, Erratum in Int. J. Immunopharmacol. 2000, 22, 833.. [Google Scholar] [CrossRef] [PubMed]
- Rabinovich, G.A.; Riera, C.M.; Sotomayor, C.E. Galectin-1, an Alternative Signal for T Cell Death, Is Increased in Activated Macrophages. Braz. J. Med. Biol. Res. 1999, 32, 557–567. [Google Scholar] [CrossRef]
- Nguyen, J.T.; Evans, D.P.; Galvan, M.; Pace, K.E.; Leitenberg, D.; Bui, T.N.; Baum, L.G. CD45 Modulates Galectin-1-Induced T Cell Death: Regulation by Expression of Core 2 O-Glycans. J. Immunol. 2001, 167, 5697–6707. [Google Scholar] [CrossRef] [PubMed]
- Bi, S.; Earl, L.A.; Jacobs, L.; Baum, L.G. Structural Features of Galectin-9 and Galectin-1 That Determine Distinct T Cell Death Pathways. J. Biol. Chem. 2008, 283, 12248–12258. [Google Scholar] [CrossRef] [PubMed]
- Matteucci, C.; Nepravishta, R.; Argaw-Denboba, A.; Mandaliti, W.; Giovinazzo, A.; Petrone, V.; Balestrieri, E.; Sinibaldi-Vallebona, P.; Pica, F.; Paci, M.; et al. Thymosin α1 Interacts with Galectin-1 Modulating the Β-Galactosides Affinity and Inducing Alteration in The Biological Activity. Int. Immunopharmacol. 2023, 118, 110113. [Google Scholar] [CrossRef]
- Paul, S.; Sodhi, A. Modulatory Role of Thymosin-Alpha-1 in Normal Bone-Marrow Haematopoiesis and Its Effect on Myelosuppression in T-Cell Lymphoma Bearing Mice. Immunol. Lett. 2002, 82, 171–182. [Google Scholar] [CrossRef]
- Romani, L.; Bistoni, F.; Gaziano, R.; Bozza, S.; Montagnoli, C.; Perruccio, K.; Pitzurra, L.; Bellocchio, S.; Velardi, A.; Rasi, G.; et al. Thymosin Alpha 1 Activates Dendritic Cells for Antifungal Th1 Resistance through Toll-Like Receptor Signaling. Blood 2004, 103, 4232–4239. [Google Scholar] [CrossRef]
- Espinar-Buitrago, M.S.; Tarancon-Diez, L.; Vazquez-Alejo, E.; Magro-Lopez, E.; Genebat, M.; Romero-Candau, F.; Leal, M.; Muñoz-Fernandez, M.A. The Use of Alpha 1 Thymosin as an Immunomodulator of the Response against SARS-CoV-2. Immun. Ageing 2023, 20, 32. [Google Scholar] [CrossRef]
- Espinar-Buitrago, M.S.; Vazquez-Alejo, E.; Magro-Lopez, E.; Tarancon-Diez, L.; Leal, M.; Muñoz-Fernandez, M.A. Immune Modulation via Dendritic Cells by the Effect of Thymosin-Alpha-1 on Immune Synapse in HCMV Infection. Int. Immunopharmacol. 2023, 125, 111103. [Google Scholar] [CrossRef]
- Espinar-Buitrago, M.S.; Magro-López, E.; Vázquez-Alejo, E.; Muñoz-Fernández, M.Á. Enhanced Immunomodulatory Effects of Thymosin-Alpha-1 in Combination with Polyanionic Carbosilane Dendrimers against HCMV Infection. Int. J. Mol. Sci. 2024, 25, 1952. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, Z.; Zhou, C.; Yao, H.; Li, M.; Xu, C. The Modulation of Thymosin Alpha 1 in the Maturation, Differentiation and Function of Murine Bone Marrow-Derived Dendritic Cells in the Absence or Presence of Tumor Necrosis Factor-Alpha. Int. Immunopharmacol. 2004, 4, 539–546. [Google Scholar] [CrossRef] [PubMed]
- Romani, L.; Bistoni, F.; Perruccio, K.; Montagnoli, C.; Gaziano, R.; Bozza, S.; Bonifazi, P.; Bistoni, G.; Rasi, G.; Velardi, A.; et al. Thymosin Alpha1 Activates Dendritic Cell Tryptophan Catabolism and Establishes a Regulatory Environment for Balance of Inflammation and Tolerance. Blood 2006, 108, 2265–2274. [Google Scholar] [CrossRef]
- Yang, X.; Qian, F.; He, H.Y.; Liu, K.J.; Lan, Y.Z.; Ni, B.; Tian, Y.; Fu, X.L.; Zhang, J.; Shen, Z.G.; et al. Effect of Thymosin Alpha-1 on Subpopulations of Th1, Th2, Th17, and Regulatory T Cells (Tregs) In Vitro. Braz. J. Med. Biol. Res. 2012, 45, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Bozza, S.; Gaziano, R.; Bonifazi, P.; Zelante, T.; Pitzurra, L.; Montagnoli, C.; Moretti, S.; Castronari, R.; Sinibaldi, P.; Rasi, G.; et al. Thymosin Alpha1 Activates the TLR9/Myd88/IRF7-Dependent Murine Cytomegalovirus Sensing for Induction of Anti-Viral Responses In Vivo. Int. Immunol. 2007, 19, 1261–1270. [Google Scholar] [CrossRef]
- Renga, G.; Bellet, M.M.; Pariano, M.; Gargaro, M.; Stincardini, C.; D’Onofrio, F.; Mosci, P.; Brancorsini, S.; Bartoli, A.; Goldstein, A.L.; et al. Thymosin α1 Protects from CTLA-4 Intestinal Immuno-Pathology. Life Sci. Alliance 2020, 3, e202000662. [Google Scholar] [CrossRef] [PubMed]
- Serafino, A.; Pica, F.; Andreola, F.; Gaziano, R.; Moroni, N.; Moroni, G.; Zonfrillo, M.; Pierimarchi, P.; Sinibaldi-Vallebona, P.; Garaci, E. Thymosin α1 Activates Complement Receptor-Mediated Phagocytosis in Human Monocyte-Derived Macrophages. J. Innate Immun. 2014, 6, 72–88. [Google Scholar] [CrossRef]
- Novoselova, E.G.; Glushkova, O.V.; Khrenov, M.O.; Lunin, S.M.; Sharapov, M.G.; Goncharov, R.G.; Mubarakshina, E.K.; Novoselova, T.V.; Parfenyuk, S.B. The Thymic Hormone Thymosin-1 α Reduces the Pro-Inflammatory Response of RAW 264.7 Cells Induced by Endotoxin. Mol. Biol. 2023, 57, 1006–1016. [Google Scholar] [CrossRef]
- Peng, X.; Zhang, P.; Wang, X.; Chan, J.; Zhu, M.; Jiang, M.; Tuthill, C.; Wan, Y.; Dragoi, A.M.; Chu, W.M. Signaling Pathways Leading to the Activation of IKK and MAPK by Thymosin Alpha1. Ann. N. Y. Acad. Sci. 2007, 1112, 339–350. [Google Scholar] [CrossRef]
- Ricci, D.; Etna, M.P.; Severa, M.; Fiore, S.; Rizzo, F.; Iannetta, M.; Andreoni, M.; Balducci, S.; Stefanelli, P.; Palamara, A.T.; et al. Novel Evidence of Thymosin α1 Immunomodulatory Properties in SARS-CoV-2 Infection: Effect on Innate Inflammatory Response in a Peripheral Blood Mononuclear Cell-Based In Vitro Model. Int. Immunopharmacol. 2023, 117, 109996. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Chen, F.D.; Zhou, L.; Gong, X.G.; Han, Q.F. Proliferative and Anti-Proliferative Effects of Thymosin Alpha1 on Cells Are Associated with Manipulation of Cellular ROS Levels. Chem. Biol. Interact. 2009, 180, 383–388. [Google Scholar] [CrossRef]
- Kharazmi-Khorassani, J.; Asoodeh, A.; Tanzadehpanah, H. Antioxidant and Angiotensin-Converting Enzyme (ACE) Inhibitory Activity of Thymosin Alpha-1 (Thα1) Peptide. Bioorg Chem. 2019, 87, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Armutcu, F.; Coskun, O.; Gürel, A.; Kanter, M.; Can, M.; Ucar, F.; Unalacak, M. Thymosin Alpha 1 Attenuates Lipid Peroxidation and Improves Fructose-Induced Steatohepatitis in Rats. Clin. Biochem. 2005, 38, 540–547. [Google Scholar] [CrossRef]
- Gökkusu, C.; Ademoğlu, E.; Türkoğlu, U.M.; Oz, H.; Oz, F. Thymosin Alpha 1 Protects Liver and Aorta from Oxidative Damage in Atherosclerotic Rabbits. Life Sci. 1996, 59, 1059–1067. [Google Scholar] [CrossRef]
- Aldwin, C.M.; Spiro, A., 3rd; Clark, G.; Hall, N. Thymic Peptides, Stress, and Depressive Symptoms in Older Men: A Comparison of Different Statistical Techniques for Small Samples. Brain Behav. Immun. 1991, 5, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Coe, C.L.; Hall, N.R. Psychological Disturbance Alters Thymic and Adrenal Hormone Secretion in a Parallel but Independent Manner. Psychoneuroendocrinology 1996, 21, 237–247. [Google Scholar] [CrossRef]
- Turrini, P.; Aloe, L. Evidence that Endogenous Thymosin Alpha-1 is Present in the Rat Central Nervous System. Neurochem. Int. 1999, 35, 463–470. [Google Scholar] [CrossRef]
- Su, Y.L.; Ho, K.L.; Dalakas, M.C.; Mutchnick, M.G. Localization of Immunoreactive Thymosin Alpha 1 in Astrocytes of Normal Human Brain. Ann. Neurol. 1989, 26, 277–280. [Google Scholar] [CrossRef]
- Milenkovic, L.; McCann, S.M. Effects of Thymosin Alpha-1 on Pituitary Hormone Release. Neuroendocrinology 1992, 55, 14–19. [Google Scholar] [CrossRef]
- Milenkovic, L.; Lyson, K.; Aguila, M.C.; McCann, S.M. Effect of Thymosin Alpha 1 on Hypothalamic Hormone Release. Neuroendocrinology 1992, 56, 674–679. [Google Scholar] [CrossRef]
- Huang, J.; Jiang, H.; Pan, M.; Jiang, Y.; Xie, L. Immunopotentiator Thymosin Alpha-1 Attenuates Inflammatory Pain by Modulating the Wnt3a/β-Catenin Pathway in Spinal Cord. Neuroreport 2020, 31, 69–75. [Google Scholar] [CrossRef]
- Walker, D.G.; Lue, L.F.; Beach, T.G. Gene Expression Profiling of Amyloid Beta Peptide-Stimulated Human Post-Mortem Brain Microglia. Neurobiol. Aging 2001, 22, 957–966. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; He, F.; Xu, Y.; Zhang, Y.; Wang, X.; Zhou, C.; Huang, Y.; Zou, J. Immunopotentiator Thymosin Alpha-1 Promotes Neurogenesis and Cognition in the Developing Mouse via a Systemic Th1 Bias. Neurosci. Bull. 2017, 33, 675–684. [Google Scholar] [CrossRef]
- Turrini, P.; Tirassa, P.; Vigneti, E.; Aloe, L. A Role of the Thymus and Thymosin-Alpha1 in Brain NGF Levels and NGF Receptor Expression. J. Neuroimmunol. 1998, 82, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, Z.W.; Zhou, W.X.; Zhang, Y.X. Thymosin Alpha-1 Modulates Excitatory Synaptic Transmission in Cultured Hippocampal Neurons in Rats. Neurosci. Lett. 2003, 350, 81–84. [Google Scholar] [CrossRef]
- Aynekulu Mersha, D.G.; Fromme, S.E.; van Boven, F.; Arteaga-Henríquez, G.; Wijkhuijs, A.; van der Ent, M.; Bergmans, R.; Baune, B.T.; Drexhage, H.A.; Dalm, V. Indications for an Antidepressive Effect of Thymosin Alpha-1 in a Small Open-Label Proof of Concept Study in Common Variable Immune Deficiency Patients with Depression. Brain Behav. Immun. Health 2025, 43, 100934. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Wang, W.Y.; Pang, X.C.; Wang, Z.; Wang, C.Z.; Zhou, H.; Zheng, B.; Cui, Y.M. Thymosin-α1 Binds with ACE and Downregulates the Expression of ACE2 in Human Respiratory Epithelia. Front. Biosci. (Landmark Ed.) 2022, 27, 48. [Google Scholar] [CrossRef]
- Gökkuşu, C.; Ademoğlu, E.; Oz, H.; Türkoğlu, U.M. Effects of Thymosin Alpha-1 on Erythrocyte Lipid Levels and Erythrocyte Membrane (Na+-K+)-ATPase Activity in Experimental Hypercholesterolemia. Jpn. J. Med. Sci. Biol. 1997, 50, 45–53. [Google Scholar] [PubMed]
- McFarland, E.J.; Scearce, R.M.; Haynes, B.F. The Human Thymic Microenvironment: Cortical Thymic Epithelium is an Antigenically Distinct Region of the Thymic Microenvironment. J. Immunol. 1984, 133, 1241–1249. [Google Scholar] [CrossRef]
- Haynes, B.F.; Shimizu, K.; Eisenbarth, G.S. Identification of Human and Rodent Thymic Epithelium Using Tetanus Toxin and Monoclonal Antibody A2B5. J. Clin. Investig. 1983, 71, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, M.C.; Engel, W.K.; McClure, J.E.; Goldstein, A.L.; Askanas, V. Immunocytochemical Localization of Thymosin-Alpha 1 in Thymic Epithelial Cells of Normal and Myasthenia Gravis Patients and in Thymic Cultures. J. Neurol. Sci. 1981, 50, 239–247. [Google Scholar] [CrossRef]
- Hirokawa, K.; McClure, J.E.; Goldstein, A.L. Age-Related Changes in Localization of Thymosin in the Human Thymus. Thymus 1982, 4, 19–29. [Google Scholar]
- Oates, K.K.; Naylor, P.H.; Goldstein, A.L. Localization of Thymosin Alpha 1 Production to Thymus Medullary Epithelial Cells by Use of Monoclonal Antibodies. Hybridoma 1987, 6, 47–59. [Google Scholar] [CrossRef]
- Naruse, H.; Hashimoto, T.; Yamakawa, Y.; Iizuka, M.; Yamada, T.; Masaoka, A. Immunoreactive Thymosin Alpha 1 in Human Thymus and Thymoma. J. Thorac. Cardiovasc. Surg. 1993, 106, 1065–1071. [Google Scholar] [CrossRef] [PubMed]
- Tsitsiloni, O.E.; Stiakakis, J.; Koutselinis, A.; Gogas, J.; Markopoulos, C.; Yialouris, P.; Bekris, S.; Panoussopoulos, D.; Kiortsis, V.; Voelter, W.; et al. Expression of Alpha-Thymosins in Human Tissues in Normal and Abnormal Growth. Proc. Natl. Acad. Sci. USA 1993, 90, 9504–9507. [Google Scholar] [CrossRef]
- Wu, M.H.; Low, T.L. Distribution of Thymic Hormones in Thymic Tumors and Myasthenic Thymus. Proc. Natl. Sci. Counc. Repub. China B 1996, 20, 1–5. [Google Scholar] [PubMed]
- van Baarlen, J.; Schuurman, H.J.; Reitsma, R.; Huber, J. Acute Thymus Involution During Infancy and Childhood: Immunohistology of the Thymus and Peripheral Lymphoid Tissues after Acute Illness. Pediatr. Pathol. 1989, 9, 261–275. [Google Scholar] [CrossRef]
- Goya, R.G.; Naylor, P.H.; Goldstein, A.L.; Meites, J. Changes in Circulating Levels of Neuroendocrine and Thymic Hormones During Aging in Rats: A Correlation Study. Exp. Gerontol. 1990, 25, 149–157. [Google Scholar] [CrossRef]
- Weller, F.E.; Shah, U.; Cummings, G.D.; Chretien, P.B.; Mutchnick, M.G. Serum Levels of Immunoreactive Thymosin Alpha 1 and Thymosin Beta 4 in Large Cohorts of Healthy Adults. Thymus 1992, 19, 45–52. [Google Scholar]
- Pica, F.; Gaziano, R.; Casalinuovo, I.A.; Moroni, G.; Buè, C.; Limongi, D.; D’Agostini, C.; Tomino, C.; Perricone, R.; Palamara, A.T.; et al. Serum Thymosin Alpha 1 Levels in Normal and Pathological Conditions. Expert. Opin. Biol. Ther. 2018, 18, 13–21. [Google Scholar] [CrossRef]
- Frasca, D.; Adorini, L.; Doria, G. Enhancement of Helper and Suppressor T Cell Activities by Thymosin Alpha 1 Injection in Old Mice. Immunopharmacology 1985, 10, 41–49. [Google Scholar] [CrossRef]
- Frasca, D.; Adorini, L.; Mancini, C.; Doria, G. Reconstitution of T Cell Functions in Aging Mice by Thymosin Alpha 1. Immunopharmacology 1986, 11, 155–163. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Garavini, M.; Doria, G. Recovery of T-Cell Functions in Aged Mice Injected with Synthetic Thymosin-Alpha 1. Cell. Immunol. 1982, 72, 384–391. [Google Scholar] [CrossRef] [PubMed]
- Frasca, D.; Adorini, L.; Doria, G. Enhanced Frequency of Mitogen-Responsive T Cell Precursors in Old Mice Injected with Thymosin Alpha 1. Eur. J. Immunol. 1987, 17, 727–730. [Google Scholar] [CrossRef]
- Ershler, W.B.; Hebert, J.C.; Blow, A.J.; Granter, S.R.; Lynch, J. Effect of Thymosin Alpha One on Specific Antibody Response and Susceptibility to Infection in Young and Aged Mice. Int. J. Immunopharmacol. 1985, 7, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Twomey, J.J.; Kouttab, N.M. Selected Phenotypic Induction of Null Lymphocytes from Mice with Thymic and Nonthymic Agents. Cell. Immunol. 1982, 72, 186–194. [Google Scholar] [CrossRef]
- Hadden, J.W.; Saha, A.; Sosa, M.; Hadden, E.M. Immunotherapy with Natural Interleukins and/or Thymosin Alpha 1 Potently Augments T-Lymphocyte Responses of Hydrocortisone-Treated Aged Mice. Int. J. Immunopharmacol. 1995, 17, 821–828. [Google Scholar] [CrossRef]
- Ershler, W.B.; Coe, C.L.; Laughlin, N.; Klopp, R.G.; Gravenstein, S.; Roecker, E.B.; Schultz, K.T. Aging and Immunity in Non-Human Primates. II. Lymphocyte Response in Thymosin Treated Middle-Aged Monkeys. J. Gerontol. 1988, 43, B142–B146. [Google Scholar] [CrossRef]
- Ershler, W.B.; Moore, A.L.; Socinski, M.A. Influenza and Aging: Age-Related Changes and the Effects of Thymosin on the Antibody Response to Influenza Vaccine. J. Clin. Immunol. 1984, 4, 445–454. [Google Scholar] [CrossRef]
- Ershler, W.B.; Gravenstein, S.; Geloo, Z.S. Thymosin Alpha 1 as an Adjunct to Influenza Vaccination in the Elderly: Rationale and Trial Summaries. Ann. N. Y. Acad. Sci. 2007, 1112, 375–384. [Google Scholar] [CrossRef]
- Pozo-Balado, M.D.M.; Bulnes-Ramos, Á.; Olivas-Martínez, I.; Garrido-Rodríguez, V.; Lozano, C.; Álvarez-Ríos, A.I.; Sánchez-Sánchez, B.; Sánchez-Bejarano, E.; Maldonado-Calzado, I.; Martín-Lara, J.M.; et al. Higher Plasma Levels of Thymosin-α1 Are Associated with a Lower Waning of Humoral Response after COVID-19 Vaccination: An Eight Months Follow-Up Study in a Nursing Home. Immun. Ageing 2023, 20, 9. [Google Scholar] [CrossRef]
- Shmeljov, V.A. REFNOT. Rekombinantnyj Faktor Nekroza Opuholej-Timozin-α1, Preparat s Nizkoj Sistemnoj Toksichnost’ju dlja Lechenija Onkologicheskih Zabolevanij [REFNOT. Recombinant Tumor Necrosis Factor-Thymosin-α1, a Medicine with Low Systemic Toxicity for the Treatment of Oncological Diseases]; Refnot-Farm: Moscow, Russia, 2010. [Google Scholar]
- Vladimirova, L.Y.; Podzorova, N.A.; Zlatnik, E.Y.; Zakora, G.I.; Bakhtin, A.V.; Myagkova, V.S. Opyt Peritumoral’nogo Primenenija Rekombinantnogo Faktora Nekroza Opuholi—Timozina-α1 pri Mestno-Rasprostranennom Rake Molochnoj Zhelezy IIB-IIIB Stadii [Experience of Peritumoral Application of Recombinant Hybrid Protein of Tumor Necrosis Factor-Alpha-Thymosin-Alpha1 in Stage IIB-IIIB Locally Advanced Breast Cancer]. Fundam. Issled. 2014, 7, 921–926. [Google Scholar]
- Bryuzgin, V.V.; Platinskij, L.N. Rol’ Citokinov v Himioterapii Zlokachestvennyh Opuholej: Praktika Primenenija Citokinovyh Preparatov Refnot® i Ingaron® pri Rasprostranennyh Opuholevyh Processah s Mnozhestven-Nymi Metastazami [The Role of Cytokines in the Chemotherapy of Malignant Tumors: The Practice of Cytokines Refnot® and Ingaron® Administration in Advanced Cancer with Multiple Metastases]. Sovrem. Onkol. 2014, 16, 70–75. [Google Scholar]
- Vladimirova, L.Y.; Nepomnyashchaya, E.M.; Podzorova, N.A.; Novikova, I.A.; Uliyanova, E.P.; Abramova, N.A. Rekombinantnyj Faktor Nekroza Opuholi-Timozin-α-1: Vlijanie na Jeffektivnost’ Neoadjuvantnoj Himioterapii i Neoangiogenez pri Rake Molochnoj Zhelezy [Recombinant Tumor Necrosis Factor-Thymosin-α1: The Impact on the Effectiveness of Neoadjuvant Chemotherapy and Angiogenesis in Breast Cancer]. Vopr. Onkol. 2017, 63, 76–81. [Google Scholar]
- Shmelev, V.A.; Grigor’ev, B.V.; Mozharova, T.I.; Popov, S.G. Timozin Al’fa-1 i Gibridnye Belki, Sostoiashchie iz Faktora Nekroza Opukholeĭ Al’fa i Timozina Al’fa-1, Uvelichivaiut Éffektivnost’ Vaktsinatsii protiv Vozbuditelia Chumy [Thymosin Alpha-1 and Hybrid Proteins Consisting of Tumor Necrosis Factor-Alpha and Thymosin Alpha-1 Enhance the Efficacy of Vaccination against the Causative Agent of Plague]. Zh Mikrobiol. Epidemiol. Immunobiol. 1994, 4, 85–89. [Google Scholar]
- Kadagidze, Z.G.; Slavina, E.G.; Chertkova, A.I.; Abramov, M.E. Vlijanie Refnota na Immunitet u Onkologicheskih Bol’nyh [The Effect of Refnot on Immunity in Cancer Patients]. Pharmateka 2015, 8, 16–20. [Google Scholar]
- Slavina, E.G.; Chertkova, A.I.; Zabotina, T.N.; Borunova, A.A.; Nurtdinova, V.A.; Abramov, M.E.; Kadagidze, Z.G. Refnot—Novyj Otechestvennyj Immunomoduljator dlja Lechenija Melanomy [Refnot—The New Russian Immunomodifter for Melanoma Treatment]. Ross. Allergol. Zhurnal 2012, 5, 248–249. [Google Scholar]
- Zahn, G.; Greischel, A. Pharmacokinetics of Tumor Necrosis Factor Alpha after Intravenous Administration in Rats. Dose Dependence and Influence of Tumor Necrosis Factor Beta. Arzneimittelforschung 1989, 39, 1180–1182. [Google Scholar]
- Creaven, P.J.; Plager, J.E.; Dupere, S.; Huben, R.P.; Takita, H.; Mittelman, A.; Proefrock, A. Phase I Clinical Trial of Recombinant Human Tumor Necrosis Factor. Cancer Chemother. Pharmacol. 1987, 20, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Thymalfasin: Uses, Interactions, Mechanism of Action|DrugBank Online. Available online: https://go.drugbank.com/drugs/DB04900 (accessed on 18 September 2025).
- Lyamina, S.; Baranovskii, D.; Kozhevnikova, E.; Ivanova, T.; Kalish, S.; Sadekov, T.; Klabukov, I.; Maev, I.; Govorun, V. Mesenchymal Stromal Cells as a Driver of Inflammaging. Int. J. Mol. Sci. 2023, 24, 6372. [Google Scholar] [CrossRef] [PubMed]
- Raghuvanshi, K.; Raghuvanshi, D.; Kumar, D.; Nepovimova, E.; Valko, M.; Kuca, K.; Verma, R. Exploring the Role of mTOR Pathway in Aging and Age-Related Disorders. EXCLI J. 2025, 24, 992–1015. [Google Scholar]
- Raffaele, M.; Vinciguerra, M. The Costs and Benefits of Senotherapeutics for Human Health. Lancet Healthy Longev. 2022, 3, e67–e77. [Google Scholar] [CrossRef]
- Cruickshank-Taylor, A.B.; Kozora, J.S.; Carew, J.S.; Nawrocki, S.T.; Wang, W. Proteolysis Targeting Chimeras as Senolytics: An Emerging Senotherapy for Combating Aging. J. Pharmacol. Exp. Ther. 2025, 392, 103752. [Google Scholar] [CrossRef] [PubMed]

| Hormone | Role in Aging | Activities in Thymus | Refs. |
|---|---|---|---|
| Glucocorticoids (GCs) | Accelerate immunosenescence; cause bone and muscle loss | Dual role: induce apoptosis and support thymocyte survival | [15,16,17] |
| Somatostatin | Decline linked to cognitive impairment and sleep disturbances | Decreased receptor levels implicated in thymic involution | [18,19] |
| Substance P | Decline impairs tissue repair and nerve function; anti-inflammatory | Protects thymocytes from apoptosis | [20,21] |
| Vasointestinal peptide | Regulates inflammation, cell survival, and circadian rhythm | Protects thymocytes from apoptosis; promotes T-cell differentiation | [22,23,24,25] |
| Calcitonin gene-related peptide | Decline impairs bone and vascular health | Solely inhibitory: induces apoptosis and blocks T-cell development | [26,27] |
| Neuropeptide Y | Promotes autophagy, which declines with age | Promotes thymocyte proliferation (in young animals) | [28,29] |
| Growth hormone (GH) | Maintains muscle mass, bone density, and metabolism | Stimulates thymocyte/TEC proliferation, thymic secretion, and T-cell export | [30,31] |
| Prolactin | Mitigates retinal dysfunction and neuronal injury | Induces thymulin production in TECs | [1,32,33] |
| Luteinizing hormone | Increased levels affect the brain | Enhances the proliferation of thymocytes | [1,34] |
| Follicle-stimulating hormone (FSH) | Contributes to fat accumulation and bone loss | Unclear activity in thymus | [35] |
| Corticotropin-releasing factor | Contributes to age-related decline | Unclear activity in thymus | [36,37] |
| Oxytocin | Improves muscle, bone, and brain function | Unclear activity in thymus | [38,39,40] |
| Vasopressin | Altered secretion leads to cardiovascular and kidney issues | Unclear activity in thymus | [41] |
| Melatonin | Antioxidant; regulates sleep–wake cycle | Increases thymus weight and elevates thymic hormone levels | [42,43,44] |
| Insulin | High levels may accelerate aging; crucial for brain function | Alters thymocyte synthesis; absence leads to autoimmune diabetes | [45,46,47] |
| Insulin-like growth factor (IGF) | Decline associated with aging; disrupted signaling extends lifespan | Unclear activity in thymus | [48,49] |
| Calcitonin | Decline contributes to bone loss (post-menopause) | Unclear activity in thymus | [50] |
| Species, Age | Condition or Pathology | Tα1 Effect | Ref. |
|---|---|---|---|
| Mice 15–24 months | Immunodeficiency, aging | Stimulation of T-helper activity | [115,116,117] |
| Mice 9–20 months | Aging | Increased T-cell precursor counts | [118] |
| Mice 23 months | Aging | Enhanced antibody production following immunization with T-dependent (tetanus toxoid) but not T-independent antigen (pneumococcal capsular polysaccharide) | [119] |
| Athymic mice | Athymic | Induction of CD90.2 expression (a marker of mature T cells) on null lymphocytes (T or B cell markers) | [120] |
| Mice a model of thymus involution under the influence of hydrocortisone | Thymus involution | Enhancement of the proliferative response of splenocytes and thymocytes to IL-1, IL-2, a mixture of cytokines, concavalin A and phytohemagglutinin | [121] |
| Rhesus macaques 18–25 years old | Aging | Increased activity of natural killer cells | [122] |
| Humans 65–92 years old | Aging, influenza vaccination | Increased synthesis of specific antibodies by peripheral blood mononuclear cells after influenza vaccination | [123] |
| Humans 65–99 years old | Aging, influenza vaccination | Restoration of the humoral immune response to influenza vaccine | [124] |
| Humans 65 years and older | Aging, influenza vaccination | Increase in specific antibody titer after influenza vaccination and mitigation of side effects | [124] |
| Humans 65 years and older | Aging, COVID-19 vaccination | Correlation between plasma Tα1 level and the presence of IgG antibodies against SARS-CoV-2 S protein after COVID-19 vaccination: the duration of protection depends on the Tα1 concentration | [125] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simonova, M.A.; Ivanov, I.; Shoshina, N.S.; Komyakova, A.M.; Makarov, D.A.; Baranovskii, D.S.; Klabukov, I.D.; Telepenina, K.P.; Atiakshin, D.A.; Shegay, P.V.; et al. Aging and Thymosin Alpha-1. Int. J. Mol. Sci. 2025, 26, 11470. https://doi.org/10.3390/ijms262311470
Simonova MA, Ivanov I, Shoshina NS, Komyakova AM, Makarov DA, Baranovskii DS, Klabukov ID, Telepenina KP, Atiakshin DA, Shegay PV, et al. Aging and Thymosin Alpha-1. International Journal of Molecular Sciences. 2025; 26(23):11470. https://doi.org/10.3390/ijms262311470
Chicago/Turabian StyleSimonova, Maria A., Igor Ivanov, Natalia S. Shoshina, Alina M. Komyakova, Dmitry A. Makarov, Denis S. Baranovskii, Ilya D. Klabukov, Kristina P. Telepenina, Dmitrii A. Atiakshin, Peter V. Shegay, and et al. 2025. "Aging and Thymosin Alpha-1" International Journal of Molecular Sciences 26, no. 23: 11470. https://doi.org/10.3390/ijms262311470
APA StyleSimonova, M. A., Ivanov, I., Shoshina, N. S., Komyakova, A. M., Makarov, D. A., Baranovskii, D. S., Klabukov, I. D., Telepenina, K. P., Atiakshin, D. A., Shegay, P. V., Kaprin, A. D., & Stepanenko, V. N. (2025). Aging and Thymosin Alpha-1. International Journal of Molecular Sciences, 26(23), 11470. https://doi.org/10.3390/ijms262311470









