Combination of 20(R)-Rg3 and HUCMSCs Alleviates Type 2 Diabetes Mellitus in C57BL/6 Mice by Activating the PI3K/Akt Signaling Pathway
Abstract
1. Introduction
2. Results
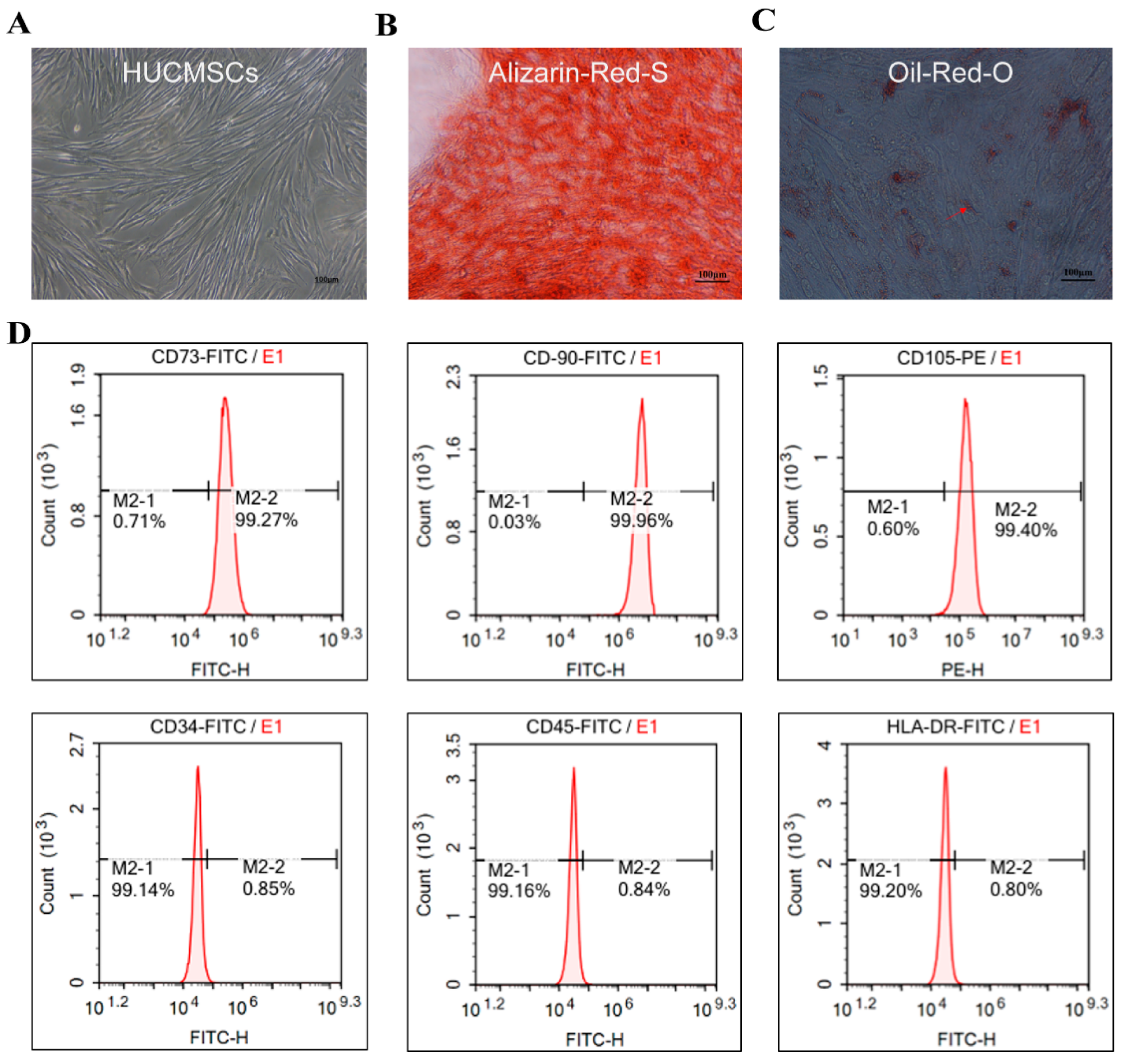
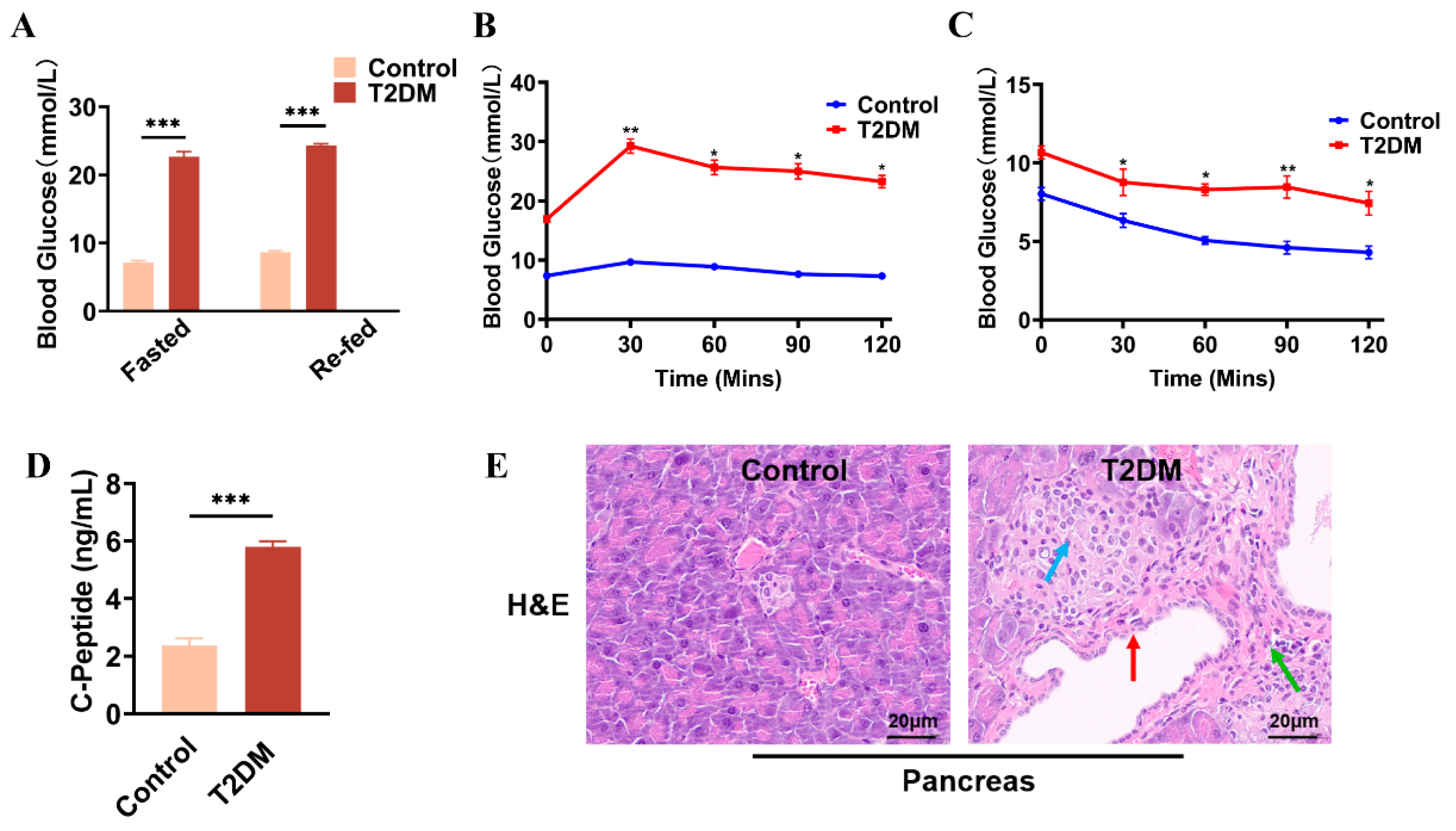
2.1. Identification of HUCMSCs and HFD/STZ-Induced T2DM Mouse Model
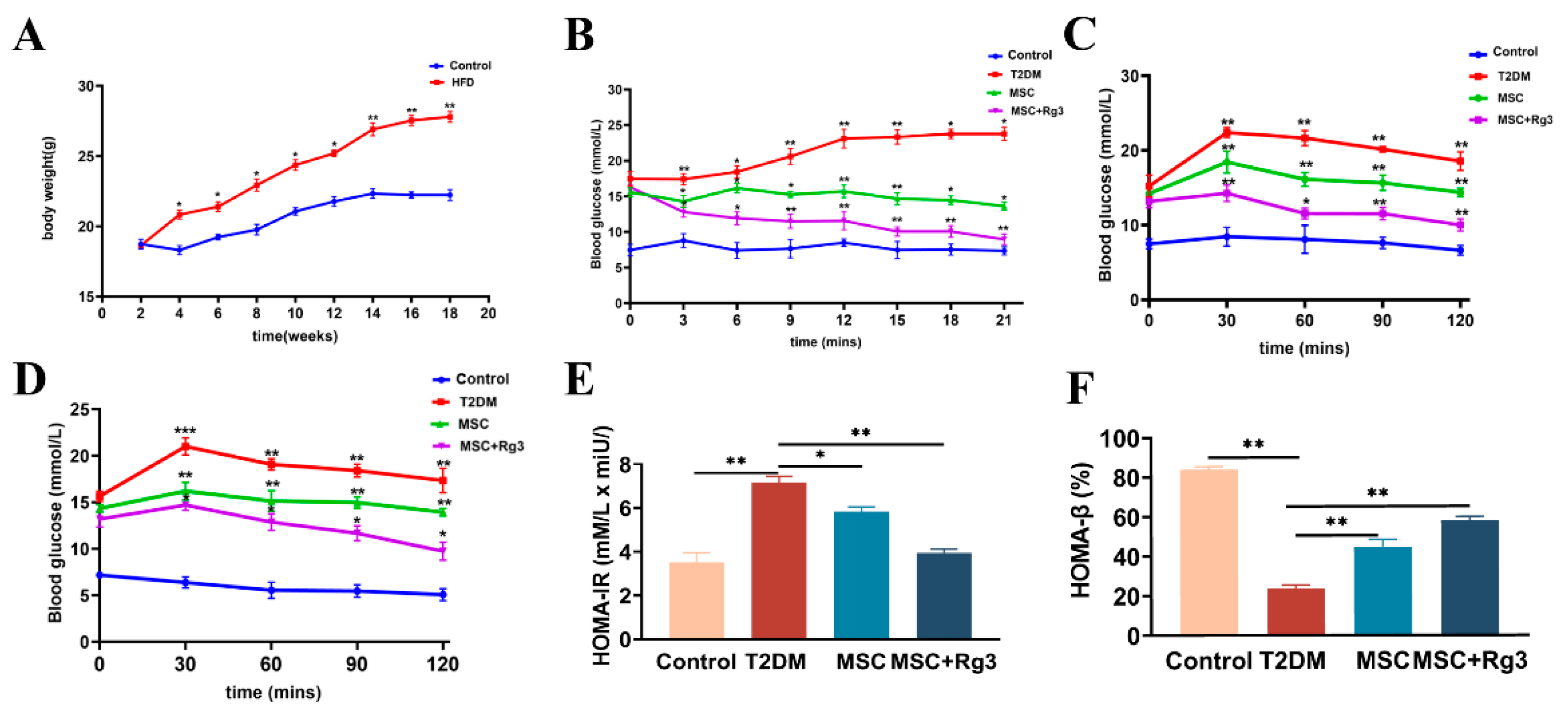
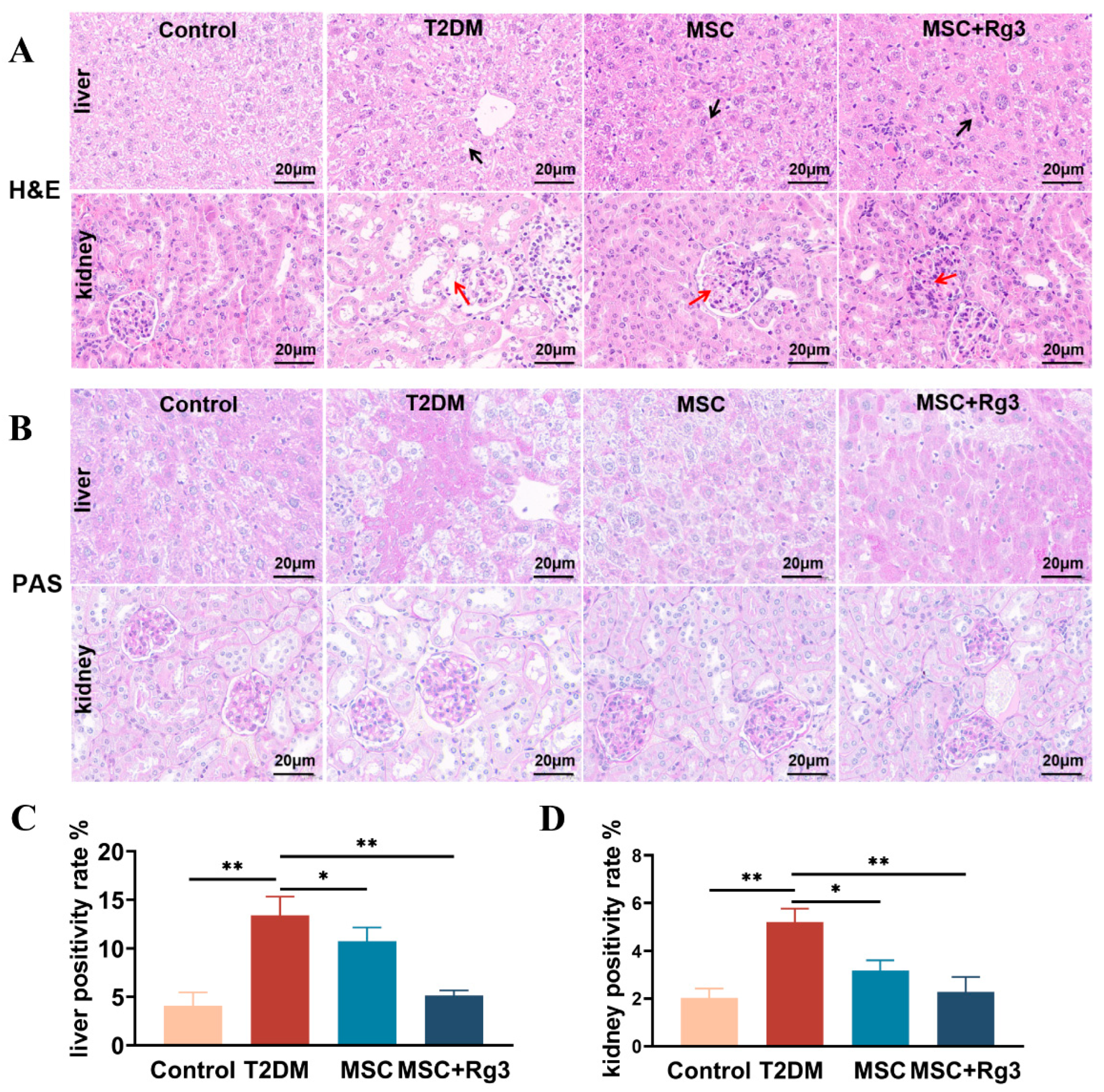
2.2. Histological Evidence That HUCMSCs Combined with 20(R)-Rg3 Reverses Insulin Resistance in T2DM
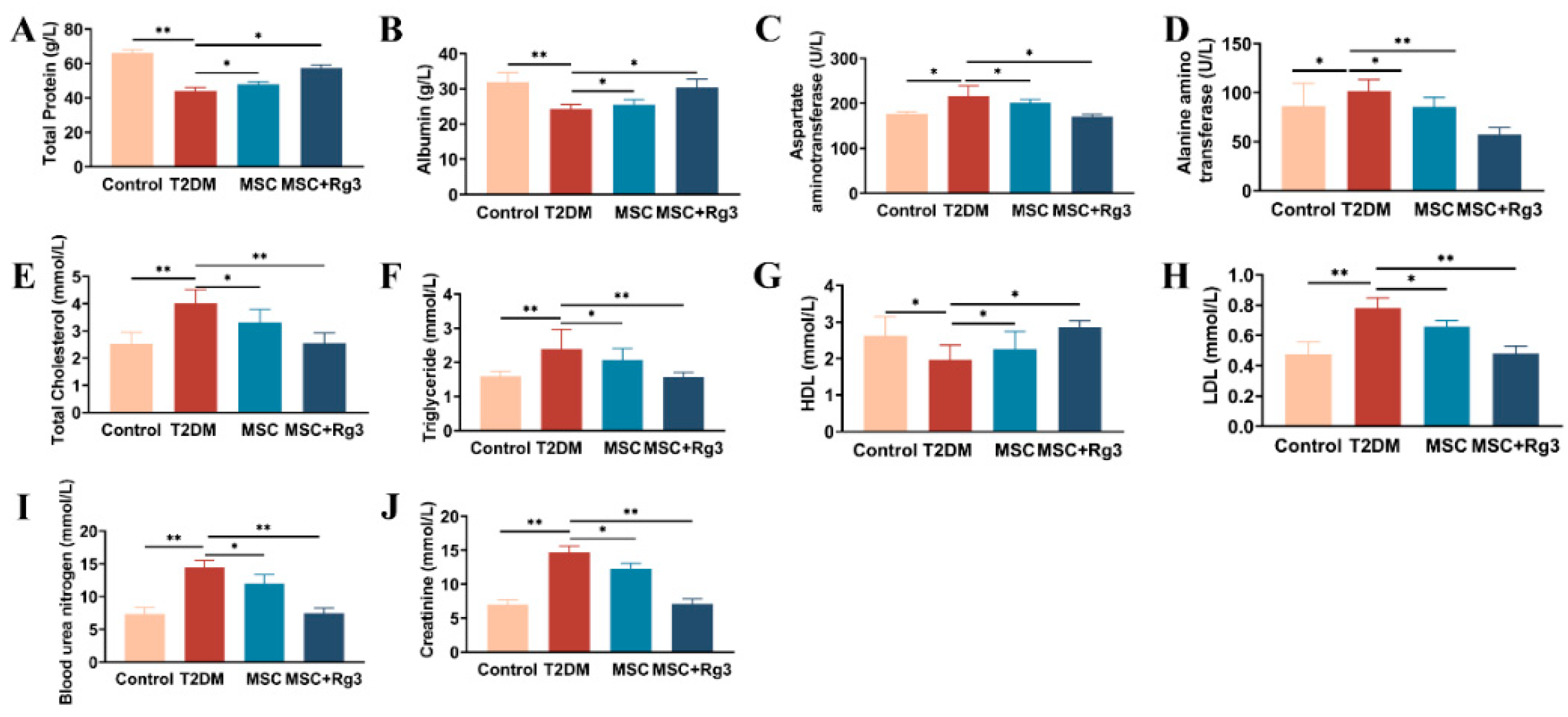
2.3. Serological Insights into the Attenuation of Insulin Resistance by HUCMSCs Combined with 20(R)-Rg3 in T2DM
2.4. In Vivo Imaging and Homing of HUCMSCs After Tail-Vein Injection
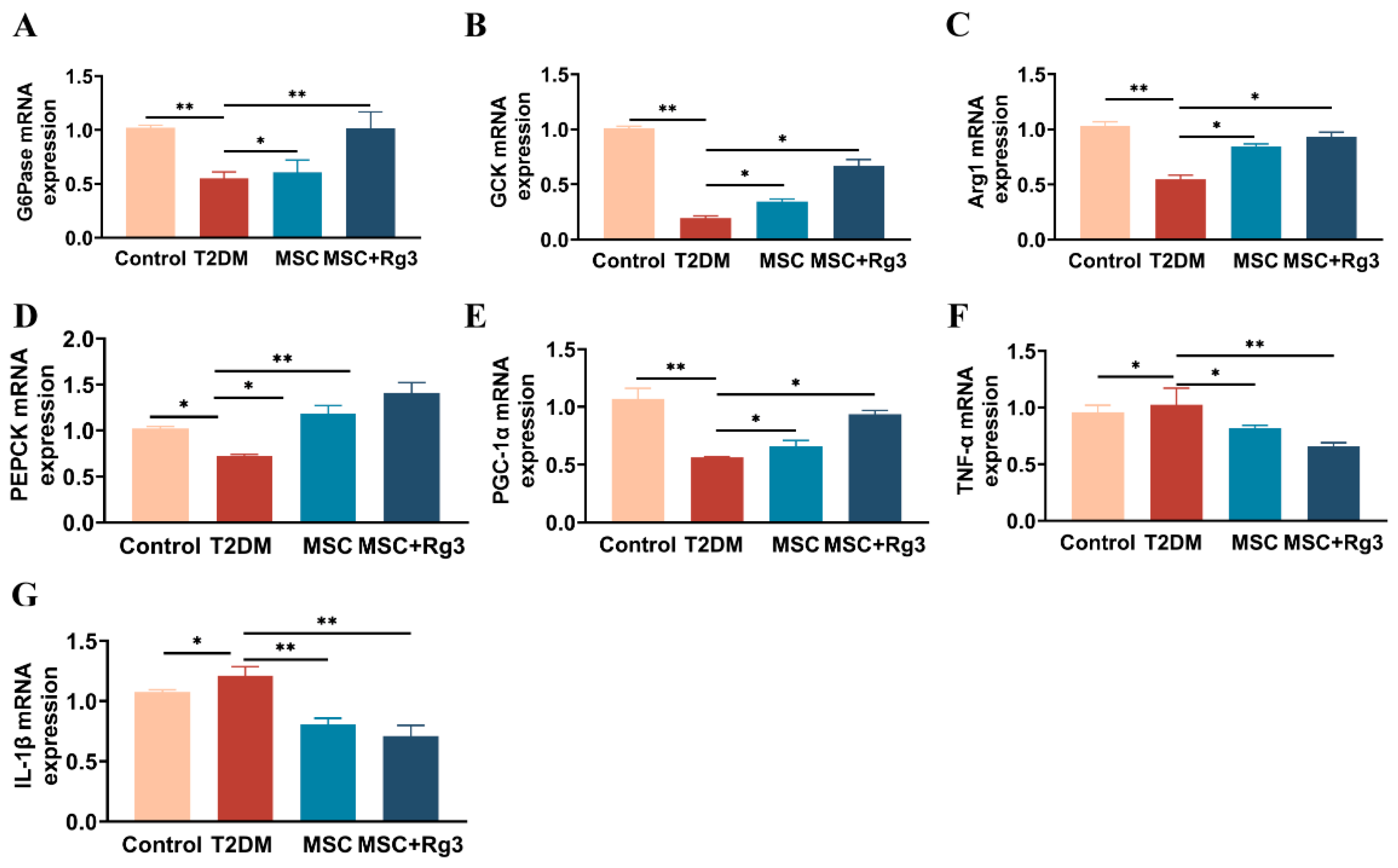
2.5. HUCMSCs Combined with 20(R)-Rg3 Promote Glycogen Storage in T2DM Mice
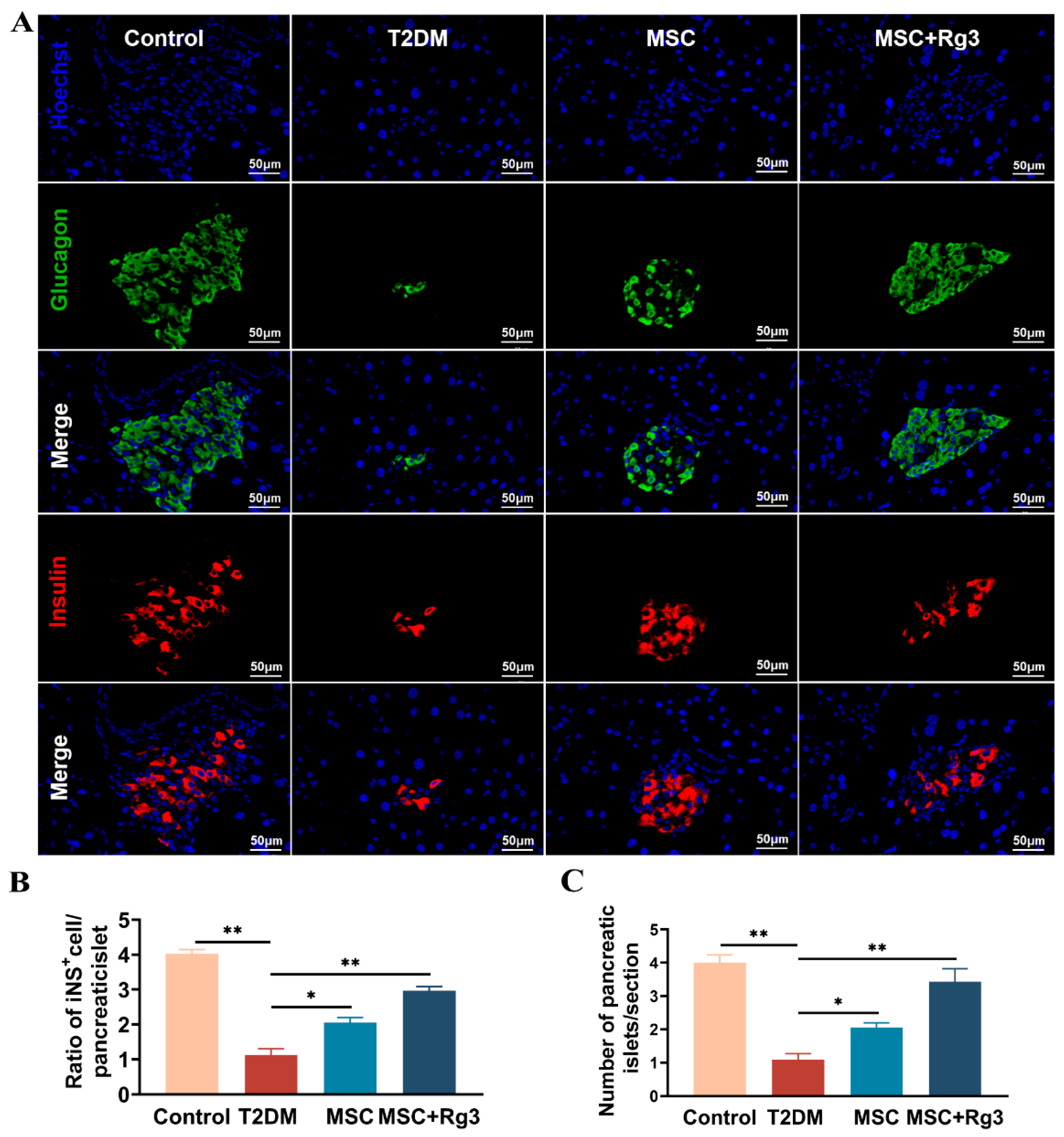
2.6. HUCMSCs Combined with 20(R)-Rg3 Promote Insulin Secretion and Islet “Regeneration”
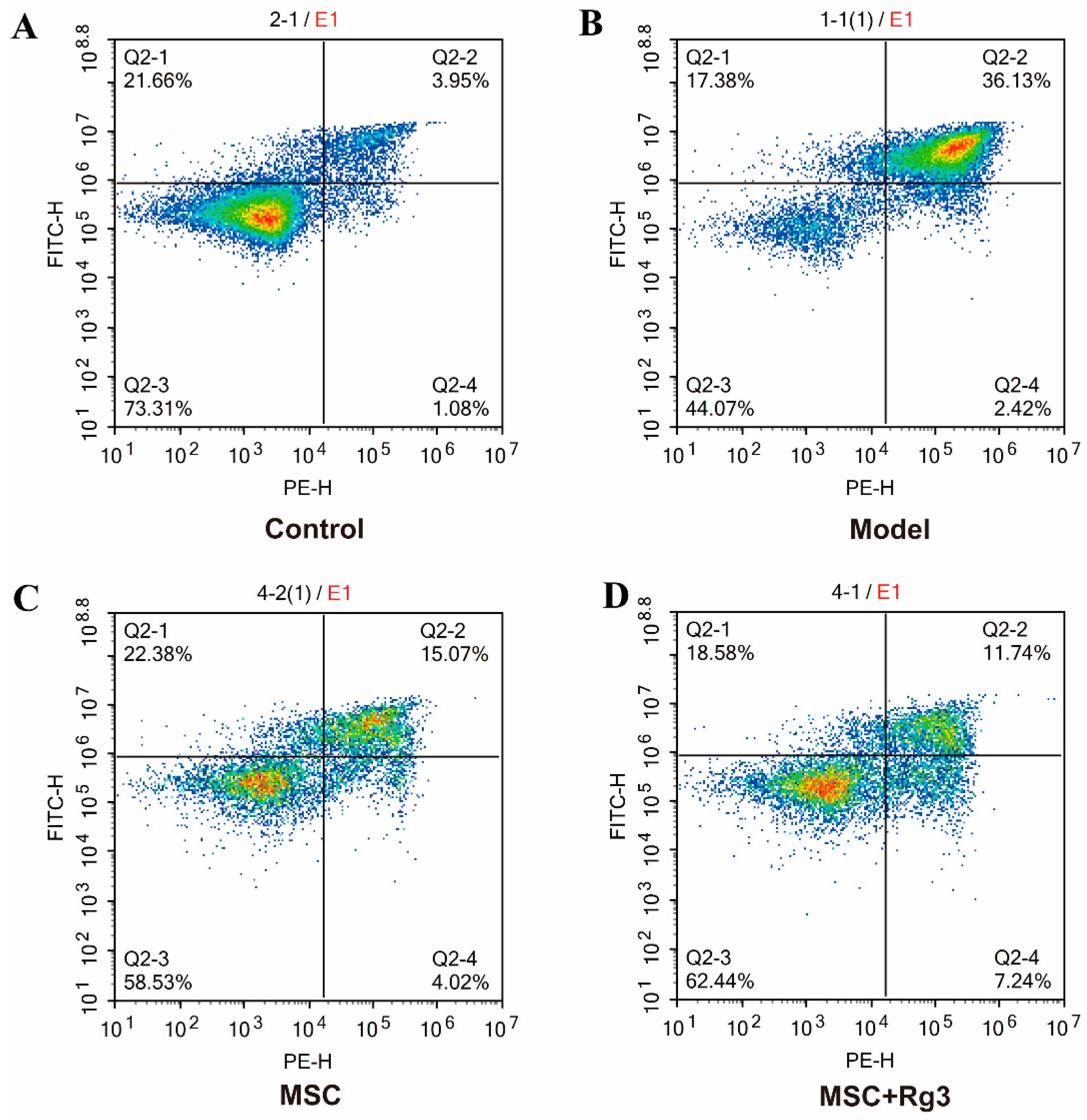
2.7. Rg3 Exerts a Protective Effect Against Spontaneous Apoptosis in HUCMSCs
2.8. Transcriptome Differential Expression Analysis
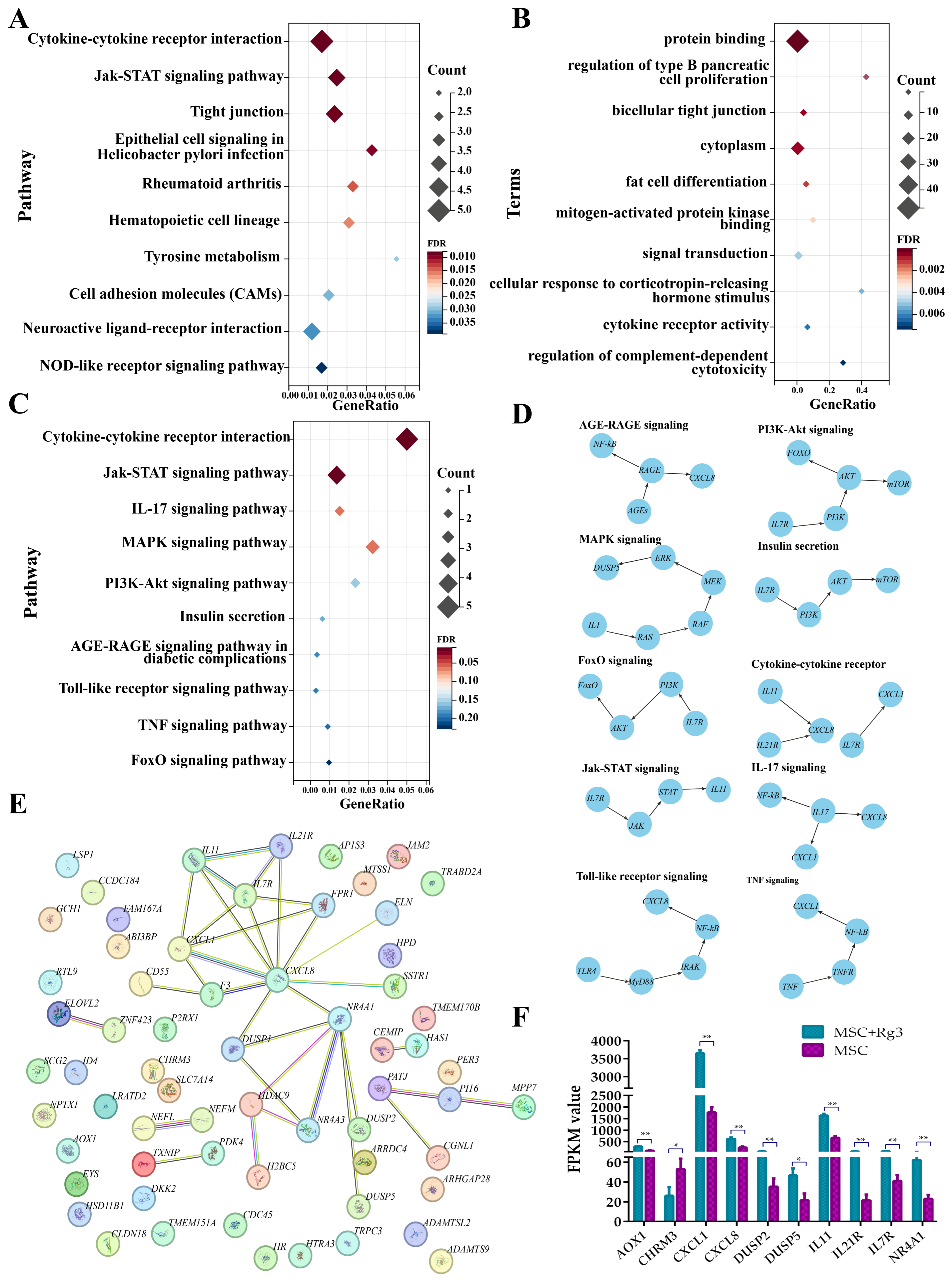
2.9. Functional Enrichment Analysis of Differentially Expressed Genes
3. Discussion
Synergistic Mechanisms of HUCMSCs and 20(R)-Rg3: Toward a “1 + 1 > 2” Effect
4. Materials and Methods
4.1. Animals and Treatment
4.2. Oral Glucose Tolerance Tests (OGTTs)
4.3. Intraperitoneal Insulin Tolerance Tests (IPITTs)
4.4. Culture for the Identification of HUCMSCs
4.5. Osteogenic Differentiation Assay
4.6. Adipogenic Differentiation Assay
4.7. Biochemical Sampling and Analysis
4.8. Real-Time Quantitative Polymerase Chain Reaction (RT-qPCR)
4.9. Histological Analysis
4.10. Immunofluorescence Analysis
4.11. Flow Cytometry Protocol for Apoptosis Detection (Annexin V-FITC/PI Staining)
4.12. RNA Sequencing (RNA-Seq)
4.12.1. Sample Quality Control
4.12.2. Library Preparation for Transcriptome Sequencing
4.12.3. Clustering and Sequencing (Novogene Experimental Department)
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HUCMSCs | human umbilical cord mesenchymal stem cells |
| T2DM | type 2 diabetes mellitus |
| HFD | high-fat diet |
| STZ | streptozotocin |
| TG | triglycerides |
| TC | total cholesterol |
| LDL | low-density lipoprotein |
| HDL | high-density lipoprotein |
| DM | diabetes mellitus |
| IR | insulin resistance |
| AMPK | adenosine monophosphate-activated protein kinase |
| PPARγ | peroxisome proliferator-activated receptor γ |
| IRS-1 | insulin receptor substrate |
| OGTTs | oral glucose tolerance tests |
| IPITTs | intraperitoneal insulin tolerance tests |
| TP | total protein |
| ALB | albumin |
| AST | aspartate aminotransferase |
| ALT | alanine aminotransferase |
| Cr | creatinine |
| BUN | blood urea nitrogen |
| C-P | C-peptide |
| VEGF | vascular endothelial growth factor |
| PGE2 | prostaglandin E2 |
| HbA1c | glycated hemoglobin |
| INS | insulin concentration |
| ELISA | enzyme-linked immunosorbent assay |
| GO | gene ontology |
| KEGG | kyoto encyclopedia of genes and genomes |
| cDNA | complementary DNA |
| PPI | protein–protein interaction |
| RT-qPCR | Real-time quantitative polymerase chain reaction |
| H&E | hematoxylin-eosin |
| PAS | periodate-Schiff staining |
| scRNA-seq | single-cell RNA sequencing |
| IDF | international diabetes federation |
| ISCT | identification criteria established by the International Society for Cell and Gene Therapy |
| GCK | glucokinase |
| PEPCK | phosphoenolpyruvate carboxykinase |
| G6Pase | glucose-6-phosphatase |
| PGC-1α | peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| TNF-α | tumor necrosis factor-α |
| IL-1β | interleukin-1β |
| PCA | principal component analysis |
| DEGs | differentially expressed genes |
| HGF | hepatocyte growth factor |
| SOD | superoxide dismutase |
| NF-κB | nuclear factor kappa B |
| TGF-β | transforming growth factor-β |
References
- Zhang, H.; Wang, X.; Hu, B.; Li, P.; Abuduaini, Y.; Zhao, H.; Jieensihan, A.; Chen, X.; Wang, S.; Guo, N.; et al. Human umbilical cord mesenchymal stem cells attenuate diabetic nephropathy through the IGF1R-CHK2-p53 signalling axis in male rats with type 2 diabetes mellitus. J. Zhejiang Univ. Sci. B 2024, 25, 568–580. [Google Scholar] [CrossRef]
- Wang, L.; Liu, T.; Liang, R.; Wang, G.; Liu, Y.; Zou, J.; Liu, N.; Zhang, B.; Liu, Y.; Ding, X.; et al. Mesenchymal stem cells ameliorate β cell dysfunction of human type 2 diabetic islets by reversing β cell dedifferentiation. EBioMedicine 2020, 51, 102615. [Google Scholar] [CrossRef]
- Lu, L.-L.; Liu, L.-Z.; Li, L.; Hu, Y.-Y.; Xian, X.-H.; Li, W.-B. Sodium butyrate improves cognitive dysfunction in high-fat diet/streptozotocin-induced type 2 diabetic mice by ameliorating hippocampal mitochondrial damage through regulating AMPK/PGC-1α pathway. Neuropharmacology 2024, 261, 110139. [Google Scholar] [CrossRef]
- Raz, I.; Riddle, M.C.; Rosenstock, J.; Buse, J.B.; Inzucchi, S.E.; Home, P.D.; Del Prato, S.; Ferrannini, E.; Chan, J.C.N.; Leiter, L.A.; et al. Personalized management of hyperglycemia in type 2 diabetes: Reflections from a Diabetes Care Editors’ Expert Forum. Diabetes Care 2013, 36, 1779–1788. [Google Scholar] [CrossRef] [PubMed]
- Nyenwe, E.A.; Jerkins, T.W.; Umpierrez, G.E.; Kitabchi, A.E. Management of type 2 diabetes: Evolving strategies for the treatment of patients with type 2 diabetes. Metabolism 2011, 60, 1–23. [Google Scholar] [CrossRef]
- Boura-Halfon, S.; Zick, Y. Phosphorylation of IRS proteins, insulin action, and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2009, 296, E581–E591. [Google Scholar] [CrossRef]
- Wang, M.; Song, L.; Strange, C.; Dong, X.; Wang, H. Therapeutic Effects of Adipose Stem Cells from Diabetic Mice for the Treatment of Type 2 Diabetes. Mol. Ther. 2018, 26, 1921–1930. [Google Scholar] [CrossRef]
- Petersen, M.C.; Shulman, G.I. Mechanisms of Insulin Action and Insulin Resistance. Physiol. Rev. 2018, 98, 2133–2223. [Google Scholar] [CrossRef]
- Oh, K.-K.; Yoon, S.-J.; Song, S.H.; Park, J.H.; Kim, J.S.; Kim, D.J.; Suk, K.-T. The synchronized feature of Saururus chinensis and gut microbiota against T2DM, NAFLD, obesity and hypertension via integrated pharmacology. Artif. Cells Nanomed. Biotechnol. 2024, 52, 278–290. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Qiu, F.-S.; Xie, W.; Yu, W.-Y.; Su, Z.-A.; Qin, G.-M.; Kang, Y.-K.; Jiang, S.-L.; Yu, C.-H. Gypenoside A-loaded mPEG-PLGA nanoparticles ameliorate high-glucose-induced retinal microvasculopathy by inhibiting ferroptosis. Int. J. Pharm. 2024, 666, 124758. [Google Scholar] [CrossRef] [PubMed]
- Vikram, A.; Tripathi, D.N.; Kumar, A.; Singh, S. Oxidative stress and inflammation in diabetic complications. Int. J. Endocrinol. 2014, 2014, 679754. [Google Scholar] [CrossRef]
- Ye, C.; Li, Y.; Shi, J.; He, L.; Shi, X.; Yang, W.; Lei, W.; Quan, S.; Lan, X.; Liu, S. Network pharmacology analysis revealed the mechanism and active compounds of jiao tai wan in the treatment of type 2 diabetes mellitus via SRC/PI3K/AKT signaling. J. Ethnopharmacol. 2025, 337, 118898. [Google Scholar] [CrossRef]
- Weinberg Sibony, R.; Segev, O.; Dor, S.; Raz, I. Drug Therapies for Diabetes. Int. J. Mol. Sci. 2023, 24, 17147. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.A.; Wefers, J.; Meier, J.J. Treatment of type 2 diabetes: Challenges, hopes, and anticipated successes. Lancet Diabetes Endocrinol. 2021, 9, 525–544. [Google Scholar] [CrossRef] [PubMed]
- Abu-Rmeileh, N.M.E.; Husseini, A.; Capewell, S.; O’Flaherty, M. Preventing type 2 diabetes among Palestinians: Comparing five future policy scenarios. BMJ Open 2013, 3, e003558. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Shi, H.; Yin, S.; Ji, C.; Zhang, X.; Zhang, B.; Wu, P.; Shi, Y.; Mao, F.; Yan, Y.; et al. Human Mesenchymal Stem Cell Derived Exosomes Alleviate Type 2 Diabetes Mellitus by Reversing Peripheral Insulin Resistance and Relieving β-Cell Destruction. ACS Nano 2018, 12, 7613–7628. [Google Scholar] [CrossRef]
- Song, J.; Liu, J.; Cui, C.; Hu, H.; Zang, N.; Yang, M.; Yang, J.; Zou, Y.; Li, J.; Wang, L.; et al. Mesenchymal stromal cells ameliorate diabetes-induced muscle atrophy through exosomes by enhancing AMPK/ULK1-mediated autophagy. J. Cachexia Sarcopenia Muscle 2023, 14, 915–929. [Google Scholar] [CrossRef]
- Yin, Y.; Hao, H.; Cheng, Y.; Gao, J.; Liu, J.; Xie, Z.; Zhang, Q.; Zang, L.; Han, W.; Mu, Y. The homing of human umbilical cord-derived mesenchymal stem cells and the subsequent modulation of macrophage polarization in type 2 diabetic mice. Int. Immunopharmacol. 2018, 60, 235–245. [Google Scholar] [CrossRef]
- Aierken, A.; Li, B.; Liu, P.; Cheng, X.; Kou, Z.; Tan, N.; Zhang, M.; Yu, S.; Shen, Q.; Du, X.; et al. Melatonin treatment improves human umbilical cord mesenchymal stem cell therapy in a mouse model of type II diabetes mellitus via the PI3K/AKT signaling pathway. Stem Cell Res. Ther. 2022, 13, 164. [Google Scholar] [CrossRef]
- De Souza, L.R.; Jenkins, A.L.; Jovanovski, E.; Rahelić, D.; Vuksan, V. Ethanol extraction preparation of American ginseng (Panax quinquefolius L) and Korean red ginseng (Panax ginseng C.A. Meyer): Differential effects on postprandial insulinemia in healthy individuals. J. Ethnopharmacol. 2015, 159, 55–61. [Google Scholar] [CrossRef]
- Smith, I.; Williamson, E.M.; Putnam, S.; Farrimond, J.; Whalley, B.J. Effects and mechanisms of ginseng and ginsenosides on cognition. Nutr. Rev. 2014, 72, 319–333. [Google Scholar] [CrossRef]
- Wong, A.S.T.; Che, C.-M.; Leung, K.-W. Recent advances in ginseng as cancer therapeutics: A functional and mechanistic overview. Nat. Prod. Rep. 2015, 32, 256–272. [Google Scholar] [CrossRef]
- Yuan, H.-D.; Kim, J.T.; Kim, S.H.; Chung, S.H. Ginseng and diabetes: The evidences from in vitro, animal and human studies. J. Ginseng Res. 2012, 36, 27–39. [Google Scholar] [CrossRef]
- Mohanan, P.; Subramaniyam, S.; Mathiyalagan, R.; Yang, D.-C. Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. J. Ginseng Res. 2018, 42, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.H.; Kim, J.-H. A review on the medicinal potentials of ginseng and ginsenosides on cardiovascular diseases. J. Ginseng Res. 2014, 38, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-T.; Lee, M.-S.; Kim, H.-J.; Sung, M.-J.; Kim, H.Y.; Kim, M.S.; Kwon, D.Y. Antiobesity effect of ginsenoside Rg3 involves the AMPK and PPAR-gamma signal pathways. Phytother. Res. 2009, 23, 262–266. [Google Scholar] [CrossRef]
- Ginsberg, H.N.; Maccallum, P.R. The obesity, metabolic syndrome, and type 2 diabetes mellitus pandemic: II. Therapeutic management of atherogenic dyslipidemia. J. Clin. Hypertens. 2009, 11, 520–527. [Google Scholar] [CrossRef] [PubMed]
- Smith, B.K.; Steinberg, G.R. AMP-activated protein kinase, fatty acid metabolism, and insulin sensitivity. Curr. Opin. Clin. Nutr. Metab. Care 2017, 20, 248–253. [Google Scholar] [CrossRef]
- Dong, X.; Kong, L.; Huang, L.; Su, Y.; Li, X.; Yang, L.; Ji, P.; Li, W.; Li, W. Ginsenoside Rg1 treatment protects against cognitive dysfunction via inhibiting PLC-CN-NFAT1 signaling in T2DM mice. J. Ginseng Res. 2023, 47, 458–468. [Google Scholar] [CrossRef]
- Cai, Y.; Li, Y.; Xiong, Y.; Geng, X.; Kang, Y.; Yang, Y. Diabetic foot exacerbates gut mycobiome dysbiosis in adult patients with type 2 diabetes mellitus: Revealing diagnostic markers. Nutr. Diabetes 2024, 14, 71. [Google Scholar] [CrossRef]
- Kowalska, K.; Wilczopolski, P.; Buławska, D.; Młynarska, E.; Rysz, J.; Franczyk, B. The Importance of SGLT-2 Inhibitors as Both the Prevention and the Treatment of Diabetic Cardiomyopathy. Antioxidants 2022, 11, 2500. [Google Scholar] [CrossRef]
- Perreault, L.; Skyler, J.S.; Rosenstock, J. Novel therapies with precision mechanisms for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2021, 17, 364–377. [Google Scholar] [CrossRef] [PubMed]
- Mohsin, F.; Javaid, S.; Tariq, M.; Mustafa, M. Molecular immunological mechanisms of impaired wound healing in diabetic foot ulcers (DFU), current therapeutic strategies and future directions. Int. Immunopharmacol. 2024, 139, 112713. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Whitehead, P. Active immunotherapy for chronic diseases. Vaccine 2013, 31, 1777–1784. [Google Scholar] [CrossRef] [PubMed]
- Scheurlen, K.M.; Parks, M.A.; Macleod, A.; Galandiuk, S. Unmet Challenges in Patients with Crohn’s Disease. J. Clin. Med. 2023, 12, 5595. [Google Scholar] [CrossRef]
- Davis, N.E.; Hamilton, D.; Fontaine, M.J. Harnessing the immunomodulatory and tissue repair properties of mesenchymal stem cells to restore β cell function. Curr. Diabetes Rep. 2012, 12, 612–622. [Google Scholar] [CrossRef]
- Mikłosz, A.; Chabowski, A. Adipose-derived Mesenchymal Stem Cells Therapy as a new Treatment Option for Diabetes Mellitus. J. Clin. Endocrinol. Metab. 2023, 108, 1889–1897. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Liao, Q.; Wong, Y.K.; Chen, X.; Yang, C.; Xu, C.; Sun, J.; Wang, J. The role of melatonin in the treatment of type 2 diabetes mellitus and Alzheimer’s disease. Int. J. Biol. Sci. 2022, 18, 983–994. [Google Scholar] [CrossRef]
- Ni, J.; Liu, Z.; Jiang, M.; Li, L.; Deng, J.; Wang, X.; Su, J.; Zhu, Y.; He, F.; Mao, J.; et al. Ginsenoside Rg3 ameliorates myocardial glucose metabolism and insulin resistance via activating the AMPK signaling pathway. J. Ginseng Res. 2022, 46, 235–247. [Google Scholar] [CrossRef]
- Han, S.; Zhang, X.; Li, Z.; Cui, G.; Xue, B.; Yu, Y.; Guo, J.; Zhang, H.; Yang, J.; Teng, L. A ginsenoside G-Rg3 PEGylated long-circulating liposome for hyperglycemia and insulin resistance therapy in streptozotocin-induced type 2 diabetes mice. Eur. J. Pharm. Biopharm. 2024, 201, 114350. [Google Scholar] [CrossRef]
- Abu-El-Rub, E.; Almahasneh, F.; Khasawneh, R.R.; Alzu’bi, A.; Ghorab, D.; Almazari, R.; Magableh, H.; Sanajleh, A.; Shlool, H.; Mazari, M.; et al. Human mesenchymal stem cells exhibit altered mitochondrial dynamics and poor survival in high glucose microenvironment. World J. Stem Cells 2023, 15, 1093–1103. [Google Scholar] [CrossRef] [PubMed]
- Mateen, M.A.; Alaagib, N.; Haider, K.H. High glucose microenvironment and human mesenchymal stem cell behavior. World J. Stem Cells 2024, 16, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-J.; Deng, Y.-X.; Shi, Q.-Z.; He, M.-Y.; Chen, B.; Qiu, X.-M. Hypolipidemic effect of the Chinese polyherbal Huanglian Jiedu decoction in type 2 diabetic rats and its possible mechanism. Phytomedicine 2014, 21, 615–623. [Google Scholar] [CrossRef] [PubMed]



| Primer Name | Primer Sequence |
|---|---|
| GAPDH Forward Primer | GGTGAAGGTCGGTGTGAACG |
| GAPDH Reverse Primer | CTCGCTCCTGGAAGATGGTG |
| G6Pase Forward Primer | CGACTCGCTATCTCCAAGTGA |
| G6Pase Reverse Primer | GGGCGTTGTCCAAACAGAAT |
| PEPCK Forward Primer | CTGCATAACGGTCTGGACTTC |
| PEPCK Reverse Primer | GCCTTCCACGAACTTCCTCAC |
| GCk Forward Primer | AGGAGGCCAGTGTAAAGATGT |
| GCk Reverse Primer | CTCCCAGGTCTAAGGAGAGAAA |
| IL-1β Forward Primer | GAAATGCCACCTTTTGACAGTG |
| IL-1β Reverse Primer | TGGATGCTCTCATCAGGACAG |
| TNF-α Forward Primer | CAGGCGGTGCCTATGTCTC |
| TNF-α Reverse Primer | CGATCACCCCGAAGTTCAGTAG |
| Arg1 Forward Primer | CTCCAAGCCAAAGTCCTTAGAG |
| Arg1 Reverse Primer | GGAGCTGTCATTAGGGACATCA |
| PGC-1 Forward Primer | TATGGAGTGACATAGAGTGTGCT |
| PGC-1 Reverse Primer | GTCGCTACACCACTTCAATCC |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Zheng, J.; Guo, X.; Wang, G.; Wang, F.; Meng, X. Combination of 20(R)-Rg3 and HUCMSCs Alleviates Type 2 Diabetes Mellitus in C57BL/6 Mice by Activating the PI3K/Akt Signaling Pathway. Int. J. Mol. Sci. 2025, 26, 11469. https://doi.org/10.3390/ijms262311469
Zhou Z, Zheng J, Guo X, Wang G, Wang F, Meng X. Combination of 20(R)-Rg3 and HUCMSCs Alleviates Type 2 Diabetes Mellitus in C57BL/6 Mice by Activating the PI3K/Akt Signaling Pathway. International Journal of Molecular Sciences. 2025; 26(23):11469. https://doi.org/10.3390/ijms262311469
Chicago/Turabian StyleZhou, Zhengjie, Jingtong Zheng, Xiaoping Guo, Guoqiang Wang, Fang Wang, and Xiaoting Meng. 2025. "Combination of 20(R)-Rg3 and HUCMSCs Alleviates Type 2 Diabetes Mellitus in C57BL/6 Mice by Activating the PI3K/Akt Signaling Pathway" International Journal of Molecular Sciences 26, no. 23: 11469. https://doi.org/10.3390/ijms262311469
APA StyleZhou, Z., Zheng, J., Guo, X., Wang, G., Wang, F., & Meng, X. (2025). Combination of 20(R)-Rg3 and HUCMSCs Alleviates Type 2 Diabetes Mellitus in C57BL/6 Mice by Activating the PI3K/Akt Signaling Pathway. International Journal of Molecular Sciences, 26(23), 11469. https://doi.org/10.3390/ijms262311469






