Abstract
This study aimed to evaluate the antibacterial activity of Cistus salviifolius L. and Helichrysum stoechas (L.) DC extracts against S. aureus, including methicillin-resistant S. aureus (MRSA) strains. To this end, assays were conducted to assess killing kinetics, antibiotic combination effects, modulatory effects on ethidium bromide, inhibition of quorum sensing, and biofilm formation. H. stoechas extract demonstrated the strongest activity, with MIC values ranging from 7.8 to 62.5 µg/mL. When combined with antibiotics such as ampicillin, ciprofloxacin, or vancomycin, the extracts of C. salviifolius and H. stoechas predominantly exhibited synergistic (FICI value ≤ 0.5) or additive effects (0.5 < FICI ≤ 1), with some combinations resensitizing resistant strains. The aerial parts of C. salviifolius displayed modulatory effects on ethidium bromide MIC, reducing the concentration from 32 to 8 µg/mL, suggesting efflux pump inhibitory activity. In addition, this extract displayed slight quorum-sensing inhibition at a concentration of 125 µg/mL. Moreover, C. salviifolius and H. stoechas extracts inhibit the formation of biofilm by S. aureus strains, even at subinhibitory concentrations (0.5× and 0.25× MIC). The presence of compounds such as myricetin 3 O-galactoside, catechin derivatives, gallic acid, kaempferol, and chlorogenic acid in the extracts may contribute to their anti-Staphylococcus activity. These results demonstrated the dual antimicrobial and antivirulence potential of C. salviifolius and H. stoechas extracts, highlighting their promise as therapeutic agents or adjuvants against S. aureus. These extracts can be promising candidates for further studies on the development of novel strategies targeting multiple pathogenic pathways.
1. Introduction
Staphylococcus aureus is a Gram-positive bacterium with a spherical shape that typically forms clusters []. Typically a commensal organism, S. aureus can become an opportunistic pathogen capable of causing diverse and severe infections. It commonly colonizes humans, most frequently affecting the skin and soft tissues; however, it is also one of the leading opportunistic bacterial pathogens, responsible for significant morbidity and mortality worldwide [,,,,].
The development and use of antibiotic therapies have markedly reduced mortality associated with S. aureus infections; however, this has been accompanied by the emergence and proliferation of multidrug-resistant strains []. The acquisition of resistance has driven the worldwide spread of diverse methicillin-resistant S. aureus (MRSA) lineages []. The rapid adaptive capacity of MRSA, coupled with the accumulation of additional acquired resistance mechanisms, further exacerbates the challenges in the effective management and therapeutic control of S. aureus infections []. In such a manner, MRSA antibiotic resistance poses a serious clinical challenge, and it is among the most pressing and urgent threats to global public health nowadays [,,,].
The emergence of antibiotic resistance currently exceeds the rate at which new drugs are developed for treating infections [,]. The development of new antibiotics is often not cost-effective, leading to a shortage of novel drugs and further aggravating the problem of resistance management [,,]. Among different strategies, antibiotic combination has been shown to substantially enhance antibacterial efficacy in the treatment of multi-drug-resistant S. aureus infection [,,,]. In the context of increasing antimicrobial resistance, novel strategies are being explored, including the use of plant-derived compounds as antimicrobial agents, anti-virulence modulators, or adjuvants that enhance the efficacy of conventional antibiotics. Among these approaches, antibiotic adjuvants represent a particularly promising and complementary strategy to combat antibiotic resistance by either directly targeting resistance mechanisms or enhancing the activity of existing antibiotics, thereby restoring or improving their antimicrobial effectiveness [,]. Commonly used antibiotic adjuvants include β-lactamase inhibitors, efflux pump inhibitors, and outer membrane permeabilizers, each targeting specific bacterial resistance mechanisms []. Another promising approach is the use of anti-virulence strategies as adjuvants to antibiotic therapy []. Anti-virulence compounds can be particularly advantageous, as they may attenuate pathogenicity without compromising bacterial viability, thereby reducing selective pressure and minimizing the risk of resistance development [].
Herbal medicines have been used for centuries to treat infectious diseases, and growing evidence indicates that plant extracts exhibit notable activity against microorganisms, including drug-resistant strains such as MRSA strains []. Consequently, plant-derived compounds are being recognized as promising candidates for the development of novel antimicrobial strategies [,,]. Plants have evolved sophisticated defense mechanisms against microbial pathogens, including production of enzymes and secondary metabolites, reinforcement of cellular structures, and other protective strategies against environmental stressors and predators [,]. These natural defenses have provided considerable therapeutic potential. While traditional remedies relied on crude extracts or powdered preparations, modern approaches have refined plant-based drugs into purified phytochemicals with significant pharmacological properties []. Among these, polyphenol-rich plant extracts have demonstrated inhibitory effects against pathogenic bacteria and fungi, with both whole extracts and isolated compounds, showing activity against S. aureus [,,,,,].
Plant species of the genus Cistus L. (family Cistaceae) are evergreen flowering shrubs, commonly known as rock-roses, primarily distributed across the Mediterranean region. They thrive in harsh environments, including rocky, dry, semi-arid, or nutrient-poor soils, and have a long history of use in traditional folk medicine for various purposes [,,,]. Most species of this family, characterized by their fragrance and sweet scent, are also highly valued in the perfume industry []. Cistus salviifolius L. is a perennial shrub reaching up to 60 cm in height, with ovate-elliptic, small, simple, and rough-textured leaves, and actinomorphic white flowers. Its flowering period typically spans from March to May [,,]. Extracts of C. salviifolius have been shown to exhibit various bioactive properties, including antioxidant, anti-inflammatory, anti-acetylcholinesterase, anti-xanthine oxidase, antidiabetic, antihypertensive, anticancer, antiproliferative, and antimicrobial activity [,,,,,,,,,,,,,,,].
The genus Helichrysum Mill., belonging to the Asteraceae family, includes up to 600 species of flowering plants widely distributed across the southern regions of the world. Species within this genus may be annuals, herbaceous perennials, or shrubs, with some reaching heights of up to 90 cm [,,,]. Helichrysum stoechas (L.) DC, commonly known as Mediterranean strawflower, curry plant, or yellow amaranth, is a fragrant, thermophilic halophyte native to southern Europe. It is an evergreen aromatic subshrub, either perennial or annual, that thrives in dry, rocky, and sandy soils. The plant is hermaphroditic, with grayish-green foliage and small, spherical yellow inflorescences [,]. Extracts of H. stoechas also display bioactive properties, particularly antioxidant, antiviral, antiproliferative, antidiabetic, neuroprotective, anti-inflammatory, antihypertensive, analgesic, anticancer, and antimicrobial activity [,,,,,,,,,].
Despite previous studies reporting the biological activities of C. salviifolius and H. stoechas, limited information is available regarding their antibacterial and antivirulence properties against S. aureus, including MRSA. Moreover, the potential synergistic interactions of these extracts with conventional antibiotics and their effects on key virulence mechanisms, such as quorum sensing and biofilm formation, remain poorly understood. Therefore, this study aimed to address these knowledge gaps by evaluating the antimicrobial of C. salviifolius and H. stoechas and assessing their impact on the bacterium’s virulence and investigate their combined effect with conventional antibiotics.
2. Results and Discussion
In our previous study [], we conducted a broad screening of extracts from seven different plant species to evaluate their bioactive potential, including antioxidant capacity, in vitro anti-inflammatory effects, antimicrobial activity, biocompatibility, and chemical composition. From this initial assessment, C. salviifolius and H. stoechas emerged as the most promising candidates, particularly due to their notable antimicrobial effects. Since the strongest activity was observed against Gram-positive bacteria—most prominently S. aureus—we selected S. aureus for a more detailed investigation.
2.1. Phytochemical Characterization
The preparation of the hydroethanolic extracts of C. salviifolius aerial parts and stems, and of H. stoechas, as well as their phytochemical analysis, has been described in previous work []. Briefly, C. salviifolius extracts were rich in flavonoids and phenolic acids, particularly neochlorogenic acid, gallic acid, gallocatechin 3-O-gallate, and rutin and arabinoside derivatives as quercetin glycosides. They are also characterized by the presence of kaempferol 3-O-glucosyl-rhamnosyl-galactoside and additional coumarins, including scopoletin and coumarin. H. stoechas extract showed the presence of hydroxycinnamic acid esters, particularly 3-feruloylquinic acid and 3,4-dicaffeoylquinic acid, as well as chlorogenic and neochlorogenic acids. In the case of 3,4-dicaffeoylquinic acid, this compound was identified exclusively in the extract of H. stoechas. A range of flavonoids, including myricetin, quercetin, and luteolin glycosides, were also detected, along with scopoletin and usnic acid [].
2.2. Activity of Cistus salviifolius and Helichrysum stoechas Extracts Against S. aureus Strains
Considering the documented antimicrobial potential of the C. salviifolius and H. stoechas extracts and their profile as polyphenol-rich extracts, their activity against S. aureus was further explored. The evaluation was performed using a reference strain (S. aureus ATCC 25923) and two methicillin-resistant clinical isolates (MRSA 12/08 and MRSA 05/15), by first determining the minimum inhibitory concentrations (MICs) of the extracts (Table 1).

Table 1.
Minimum inhibitory concentration (MIC, µg/mL) and minimum bactericidal concentration (MBC, µg/mL) of extracts and antibiotics on S. aureus strains presented as modal values.
The extract with the most promising anti-staphylococcal activity was from H. stoechas, exhibiting similar antimicrobial activity to the antibiotic vancomycin. Regarding the MRSA 12/08, the MIC values obtained for the extracts increased compared to the other strains, with the extract obtained from the stems of C. salviifolius showing no activity in the tested range of concentrations and thus showing the influence of plant parts on the antibacterial activity.
The antibacterial activity of C. salviifolius extracts against S. aureus strains aligns with previous studies [,,,,,,,,,,], though reported efficacy varies due to plant growth conditions, extraction methods, and experimental conditions [,]. C. salviifolius aqueous extracts activity has been linked to antibiotic resistance profiles of S. aureus clinical isolates, with β-lactams and quinolones resistance strains showing higher susceptibility. Mechanistically, disruption of the bacterial plasma membrane or cell wall has been proposed as a possible mode of action []. Polyphenols appear to play a central role in the observed effects, as in MRSA, molecules bearing COOH and OH groups in ortho and para positions or an O–CH3 group in the meta position have been associated with enhanced anti-MRSA activity [], a feature consistent with the flavonoids identified in our extracts. Synergistic interactions among major polyphenols of C. salviifolius can enhance activity (up to 20-fold) compared to individual compounds, in some cases approaching that of standard antibiotics. Myricetin was identified as the main contributor to these effects [], since our extracts contain myricetin-3-O-galactoside, a glycosylated form of myricetin, this compound may significantly contribute to the observed anti-staphylococcal activity.
For H. stoechas extracts, evidence suggests strong activity against Gram-positive bacteria, particularly S. aureus, with its efficacy being influenced by extraction solvents, with MICs ranging from 8 to 250 µg/mL in Gram-positive bacteria [,,,,,]. The chemical basis for this activity appears to be related to phenolic compounds, with dimethyl phthalate identified as an active constituent that disrupts S. aureus cellular structures, membrane integrity, and energy metabolism, ultimately leading to cell death []. Collectively, these findings indicate that H. stoechas possesses promising anti-staphylococcal potential.
To further characterize the antimicrobial properties of the plant extracts and capture their dynamics of bacterial killing, time-kill assays were performed (Figure 1). For the MRSA 12/08 strain, the stem extract of C. salviifolius was not included in subsequent assays, as its MIC could not be determined, thereby precluding the selection of appropriate concentrations for further methodological evaluations.
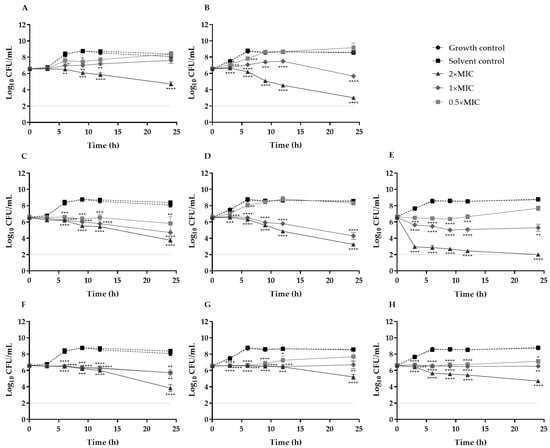
Figure 1.
Time–kill curves for S. aureus ATCC 25923 (A,C,F), MRSA 05/15 (B,D,G) and MRSA 12/08 (E,H) strains incubated with C. salviifolius stems (A,B), C. salviifolius aerial parts (C,D,E) and H. stoechas (F,G,H) extracts from 0.5× MIC to 2× MIC at 37 °C. The light gray horizontal line at 2 Log10 CFU/mL represents the detection limit of the method. * (p < 0.05) ** (p < 0.01); *** (p < 0.001); **** (p < 0.0001).
According to the classification of Ishak et al. [], these extracts exhibited predominantly bactericidal activity (MBC/MIC ratio ≤ 4), supported by time-kill assays that revealed significant reductions in cell viability, particularly at 2× MIC, with the effect being more pronounced for C. salviifolius.
To our knowledge, no prior studies have evaluated the time-dependent effects of these extracts. However, in a related work with another Helichrysum species, exposure of S. aureus ATCC 6538P to H. italicum diethyl ether extract decreased viable counts by 3 log10 within 30 min at four- and ten-fold the MIC (1000 and 2500 µg/mL, respectively), with a slower killing rate after, reaching approximately ≤2 log CFU/mL after 24–48 h [], thus underscoring the antimicrobial potential of the genus Helichrysum []. Regarding isolated compounds, gallic acid, which is present in the C. salviifolius and H. stoechas extracts, has shown notable anti-MRSA activity with a MIC value of 32 μg/mL. Growth curve analysis further revealed that at 32 and 64 μg/mL, bacterial populations remained unchanged throughout incubation, while at lower concentrations (8 and 16 μg/mL), gallic acid effectively suppressed MRSA proliferation and prolonged its growth cycle [].
2.3. Combined Effects of C. salviifolius and H. stoechas Extracts and Antibiotics, and Evaluation of Modulatory Activity of These Extracts
The emergence of multidrug-resistant (MDR) pathogens has complicated the effective treatment of numerous infectious diseases [,]. In this context, the combination of plant-derived extracts and antibiotics has gained attention, as it can modulate efflux pump expression, disrupt cellular structures, and interfere with other resistance mechanisms in MRSA, thereby enhancing antibiotic efficacy [,,]. This synergistic approach may restore antibiotic susceptibility, enable dose reduction, minimize associated toxicity, and potentially help to counteract antimicrobial resistance [,,,,].
Accordingly, this work explored the potential synergistic interactions between C. salviifolius and H. stoechas extracts and the antibiotics ampicillin, ciprofloxacin, and vancomycin (Table 2).

Table 2.
Effect of interaction between extracts and antibiotics based on the fractional inhibitory concentration index (FICI) values. The range of FICI values obtained is presented in parentheses.
The C. salviifolius and H. stoechas extracts enhanced the activity of ampicillin, ciprofloxacin, and vancomycin against the tested S. aureus strains, demonstrating predominantly synergistic (FICI ≤ 0.5) or additive (0.5 < FICI ≤ 1) effects. Notably, these interactions resulted in the resensitization of the resistant MRSA 12/08 strain to ampicillin or ciprofloxacin, reducing their MICs from 32 µg/mL to ≤2 µg/mL, a decrease of more than 26-fold when combined with the extracts.
The observed effects are consistent with previous reports describing the ability of catechin derivatives to act synergistically with β-lactam antibiotics [,,]. Mechanistically, these compounds may enhance the activity of β-lactams by disrupting cell wall integrity, through direct binding to peptidoglycan, and by penicillinase inhibition []. They may also interfere with the synthesis of the penicillin-binding protein PBP2a, suppress β-lactamase secretion [,] and increase intracellular antibiotic accumulation potentially through the downregulation of key efflux pump genes []. Phytochemical analysis of the extracts further revealed that the stem-derived extract contained both (+)-gallocatechin and (+)-gallocatechin 3-O-gallate, whereas the C. salviifolius extract obtained from the aerial parts contained only (+)-gallocatechin 3-O-gallate. Overall, these findings highlight the potential of C. salviifolius extracts, particularly their (+)-gallocatechin derivatives, as adjuvants capable of restoring antibiotic efficacy and counteracting resistance mechanisms in MRSA. Concerning the compounds only present in the H. stoechas extract, 4′,5′-O-dicaffeoylquinic acid (4′,5′-ODCQA, an isomer of dicaffeoliquinic acid) was shown to act synergistically with fluoroquinolones against S. aureus []. Beyond these specific compounds, several compounds identified across the three extracts and their derivates also displayed synergistic interactions with β-lactam and fluroquinolones, such as quercetin, gallic acid, caffeic acid, p-coumaric acid, and other coumarinic compounds, and usnic acid [,,,,,]. Notably, ciprofloxacin–usnic acid combination exhibited synergistic or additive effects against MRSA clinical isolates, whereas the vancomycin–usnic acid combination consistently demonstrated synergy against all MRSA isolates tested []. Taken together, the synergistic effects observed for multiple compounds strongly suggest that the bioactive constituents of C. salviifolius and H. stoechas extracts contribute to the restoration of antibiotic activity against resistant S. aureus strains. These interactions are particularly relevant given the diverse resistance mechanisms of S. aureus [,,,,,].
Thus, efflux pump inhibitors (EPIs) have emerged as important modulators of antimicrobial resistance, targeting these transporters to prevent extrusion of toxic compounds, including antibiotics [,,]. Medicinal plants may provide a rich source of promising EPIs, owing to their chemically and structurally diverse secondary metabolites that exhibit multiple pharmacological properties [,,]. Here, the experimental approach to detect EPI activity of the extracts was to test the combined action of ethidium bromide with C. salviifolius and H. stoechas extracts added at a sub inhibitory concentration (Table 3).

Table 3.
Minimum inhibitory concentration (MIC) of ethidium bromide against S. aureus strains in the absence (negative control) and presence of the extracts (1/4× MIC) or reserpine (positive control, 20 µg/mL).
A positive modulating effect was demonstrated when in the presence of aerial parts of C. salviifolius extract, decreasing the ethidium bromide MIC to similar levels of those observed for the positive control, reserpine, a well-known efflux pump inhibitor [,]. This reduction in MIC of ethidium bromide can indicate a potential inhibition of efflux pumps by the aerial parts of C. salviifolius. This activity may be consistent with the presence of compounds such as kaempferol glycosides, which have demonstrated potent NorA inhibition in S. aureus, reducing the MIC of ethidium bromide and norfloxacin by up to 64-fold and 32-fold, respectively [,,]. Apigenin derivatives present in C. salviifolius extracts may also support these modulatory effects, as reported apigenin as an EPI []. Other compounds that also support efflux inhibition, are quercetin and coumarinic compounds, which by in silico analysis demonstrated to form a hydrogen bond with NorA and hydrophobic interactions, suggesting inhibition of NorA and MepA in S. aureus, potentially interfering with the efflux of toxic compounds like ethidium bromide [,,].
Taken together, these findings indicate that the diverse mechanisms of action of compounds present in C. salviifolius and H. stoechas likely contribute, at least partially, to the enhanced anti-Staphylococcal activity of the antibiotics observed in this study beside efflux pump inhibition [,,,,,].
2.4. Inhibitory Effect of C. salviifolius and H. stoechas Extracts on Quorum Sensing
Quorum sensing (QS) is a density-dependent communication mechanism that regulates bacterial gene expression, including virulence, biofilm formation, and bacterial adaptation [,,,,,]. Targeting QS offers a promising anti-virulence strategy, thus Chromobacterium violaceum, whose violacein pigment is regulated by QS [], has been used as a biosensor to evaluate the potential of the plant extracts as QS inhibitors (Figure 2).
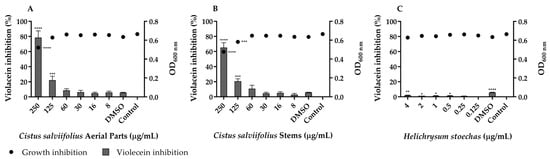
Figure 2.
Quorum-sensing inhibition by C. salviifolius aerial parts (A), C. salviifolius stems (B) and H. stoechas (C) extracts against Chromobacterium violaceum ATCC 12472. The solvent control dimethyl sulfoxide (DMSO) at a final concentration of 0.5% was also tested. Percentage of violacein inhibition (%) by different concentrations of extracts and evaluation of microbial viability (OD600 nm) after 48 h of incubation. * (p < 0.05); ** (p < 0.01); *** (p < 0.001); **** (p < 0.0001).
The extract from the aerial parts of C. salviifolius exhibited QS inhibitory activity at a concentration of 125 µg/mL, leading to a significant reduction in violacein production (p < 0.001) without affecting the growth of C. violaceum. In turn, the tested concentrations of H. stoechas did not inhibit violacein production, suggesting that this extract may not interfere with QS.
Several molecules have been identified that interfere with S. aureus QS. Among them, luteolin acts as a concentration-dependent antibiofilm agent against MDR S. aureus by inhibiting the agr QS system [].
Simultaneously, QS signal degradation may limit their distribution within a biofilm, emphasizing the key role of efflux pumps in modulating bacterial QS responses []. In fact, efflux pumps can mediate the export of polymeric substances involved in biofilm production, as well as QS molecules that regulate biofilm formation, thereby influencing bacterial adhesion and aggregation on solid surfaces [,].
2.5. Inhibition of S. aureus Biofilm Formation by C. salviifolius and H. stoechas Extracts
Bacteria predominantly exist as surface-attached communities, forming biofilms on diverse biotic or abiotic surfaces [,,,]. Within biofilms, cells are embedded in a protective extracellular polymeric substance (EPS) that enhances survival, stress tolerance, and antimicrobial resistance [,,,,,]. Compared with planktonic cells, biofilm-associated bacteria exhibit 10–1000-fold higher tolerance to antimicrobials due to multiple factors, including restricted drug penetration, metabolic alterations, efflux pump activation, and the presence of persistent or dormant cells [,,,,,].
Conventional physical, chemical, and antibiotic-based treatments are often ineffective or costly, underscoring the need for targeted anti-biofilm strategies, considering not only their eradication but also their formation [,,]. Natural products, particularly plant-derived compounds, have emerged as promising candidates with anti-virulence and antibiofilm activity [,,,,,]. Accordingly, the ability of C. salviifolius and H. stoechas extracts to inhibit biofilm formation was evaluated against S. aureus strains (Figure 3).
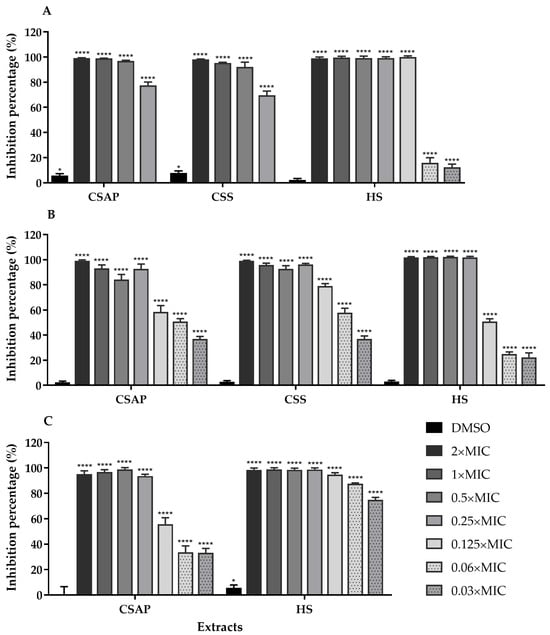
Figure 3.
Effects of different concentrations of C. salviifolius aerial parts (CSAP), C. salviifolius stems (CSS), and H. stoechas (HS) extracts on the formation of biofilms of S. aureus ATCC 25923 (A), MRSA 05/15 (B), and MRSA 12/08 (C) strains. Biofilm formation was estimated by the crystal-violet assay, and results are expressed as % of biofilm biomass inhibition. Dimethyl sulfoxide (DMSO) was used as a solvent control. * (p < 0.05); **** (p < 0.0001).
The extracts of C. salviifolius and H. stoechas exhibited potent anti-virulence properties at concentrations corresponding to the MIC and 2× MIC, completely inhibiting biofilm formation in S. aureus strains. Remarkably, even at subinhibitory concentrations, these extracts demonstrated strong anti-biofilm activity.
The ability of C. salviifolius extracts to inhibit biofilm formation by S. aureus strains has also been demonstrated in other studies. For instance, Onal et al. [] reported that the hydroethanolic extract of C. salviifolius inhibited the biofilm production of several S. aureus strains. A more detailed focus on compounds of the C. salviifolius extracts shows that (+)-gallocatechin and its derivates not only exert bactericidal effects, but also inhibit key enzymes required for S. aureus survival and virulence [,]. Similarly, quercetin and myricetin (derivatives of these compounds were identified in the three extracts) reduced the biofilm formed by different staphylococcal species and affected the formation of aggregates []. Other compounds also present in H. stoechas extracts, such as gallic acid, luteolin, myricetin, kaempferol, neochlorogenic acid, chlorogenic acid, and rutin, exhibit strong anti-biofilm activity against S. aureus, including MRSA, reducing the biofilm biomass and metabolic activity by impairing adhesion, reduction in surface hydrophobicity, disrupting the bacterial cell wall and membrane integrity, inhibiting extracellular polysaccharide production [,,], enhancing antibiotic penetration, killing biofilm-associated cells [], and altering bacterial morphology []. Given these reported mechanisms, derivatives of these compounds may exert comparable or complementary anti-biofilm effects, providing a rationale for the observed activity in the investigation of the present study. Taken together, these findings suggest that C. salviifolius extracts exert their antibacterial activity against S. aureus, including MRSA, through a multifactorial mode of action involving biofilm inhibition, partial interference with quorum sensing, and modulatory effects on efflux pump activity.
In contrast, evidence on H. stoechas is less established, but related studies on H. italicum indicate a strong antibiofilm potential, with effects associated with morphological alterations, membrane disruption, and bacterial lysis, confirming that the mode of action is likely linked to interference with adhesion and membrane integrity [,,,,]. In fact, the H. stoechas extract neither inhibited violacein production in C. violaceum nor showed modulatory activity against efflux pumps in S. aureus. These results suggest that the anti-biofilm effect of H. stoechas extract may not be mediated by interference with bacterial communication or as EPI, instead, it probably acts via alternative antimicrobial mechanisms, such as interference with S. aureus adhesion.
Together, these observations highlight that multiple bioactive compounds in the extracts may be acting through complementary mechanisms to effectively prevent and disrupt S. aureus biofilms. Further studies are required to confirm that these are potential mechanisms underlying the antimicrobial activity of our extracts, particularly of H. stoechas extract, regarding the inhibition of biofilm formation.
3. Materials and Methods
3.1. Cistus salviifolius and Helichrysum stoechas Extracts
The wild plants C. salviifolius (aerial parts or stems), and H. stoechas (leaves and stems), were collected in the northern area of Serra da Gardunha, Portugal, during spring of 2023 (collection coordinates: 40°07′27.5″ N 7°30′22.9″ W). The plant species were identified by technicians at the Biotech Plant Lab of Beira Interior, Castelo Branco, Portugal, and a voucher specimen was deposited in our laboratory. These plants were extracted and subjected to phytochemical analysis using Ultra-High Performance Liquid Chromatography coupled with trapped ion mobility spectrometry time-of-flight mass spectrometry (UHPLC timsTOF-MS), as described previously by Coimbra et al. [] and further characterized regarding anti-Staphylococcus aureus activity (Figure 4).
Figure 4.
Schematic diagram illustrating the experimental design of the present work.
3.2. Plant Extracts, Microorganisms and Culture Media
Extracts were dissolved in dimethyl sulfoxide (DMSO, Fisher Chemical, UK) at a final concentration of 200 mg/mL and stored at −20 °C until use.
S. aureus reference strain ATCC 25923 and the clinical isolates MRSA 12/08 and MRSA 05/15 were grown in Tryptone Soy Broth and Tryptone Soy agar (TSB or TSA, VWR, Leuven, Belgium). The cultures were subcultured onto solid medium and incubated at 37 °C for 24 h.
3.3. Determination of the Minimum Inhibitory Concentration (MIC)
The susceptibility of the S. aureus strains to the extracts was evaluated through the microdilution method accordingly to Coimbra et al. []. Briefly, the inoculum, prepared by directly suspending cells in an isotonic solution of NaCl (0.85% w/v), was adjusted to a 0.5 McFarland and subsequently diluted in the TSB medium to achieve an approximate final concentration of 5 × 105 colony-forming units (CFU)/mL per well. The assays were conducted using the extracts at a maximum concentration of 2000 µg/mL in a total volume of 100 µL per well. MICs for ampicillin (NzyTech, Lisboa, Portugal), ciprofloxacin (Sigma-Aldrich, St. Louis, MO, USA), and vancomycin (Thermo Scientific, Waltham, MA, USA) were also performed. After incubating at 37 °C for 24 h, 30 μL of a 0.01% resazurin solution (Sigma-Aldrich, USA) was added to each well, followed by an additional 2 h incubation at 37 °C. Inhibition of the test microorganism was indicated by the resazurin remaining blue in color. A minimum of three independent experiments, each performed in duplicate, were conducted, and the results are presented as modal values.
3.4. Time–Kill Curves
The time–kill curve assay with the extracts was performed based on Coimbra et al. [] with minor modifications. Briefly, S. aureus strains obtained from an overnight culture were used to prepare a cell suspension with a final concentration of 106 CFU/mL, and it was exposed to 0.5×, 1×, and 2× MIC of extracts. Solvent (DMSO, 1% v/v) and growth controls were also performed. Viable cell counts were determined using the drop-plate method at 0, 3, 6, 9, 12, and 24 h from the centrifuge tubes incubated at 37 °C. Each experiment was independently performed at least three times.
3.5. Checkerboard Assay
The checkerboard method was used to test the combined effect of the extracts and antibiotics, according to Coimbra et al. [], with modifications. Two microplates were prepared: one in which the extract was serially diluted vertically with TSB to a final volume of 50 µL per well, and another in which antibiotics (ampicillin, ciprofloxacin, or vancomycin) were serially diluted horizontally with TSB. Subsequently, 50 µL from each well of the antibiotic plate was transferred to the corresponding well of the extract plate using a multichannel pipette. The inoculum was prepared as described in Section 3.3, and the suspension was then diluted at 1:67 with TSB, and 50 µL were added to obtain a final volume of 150 µL per well. The concentrations of the extracts and antibiotics were selected based on previously determined MIC values. The microplates were incubated at 37 °C for a period of 24 h, and after incubating, 45 μL of a 0.01% resazurin solution was added to each well, followed by an additional 2 h incubation at 37 °C. The combined effects of extracts and antibiotics were evaluated using the fractional inhibitory concentration index (FICI), calculated as the sum of the fractional inhibitory concentrations (FICs) of the extracts and the antibiotics. The FIC was defined as the MIC of the extract or antibiotic in combination divided by its MIC when used alone. A FICI value ≤ 0.5 was interpreted as indicative of synergy; 0.5 < FICI ≤ 1 as an additive effect; 1 < FICI < 4 as indifference; and FICI ≥ 4 as antagonism [].
3.6. Evaluation of the Modulatory Effect of Extracts on Ethidium Bromide Activity
The modulatory effect of the extracts was evaluated using the microdilution method as previously described for ethidium bromide (Fisher Scientific, Brussels, Belgium) in the presence or absence of subinhibitory concentration (1/4× MIC) of each extract or reserpine (20 µg/mL, Sigma-Aldrich, USA), according to []. A positive modulatory effect was demonstrated when the presence of the extract decreased the baseline MIC of ethidium bromide. All assays were performed in duplicate across at least three independent experiments and presented as modal values.
3.7. Inhibition of Quorum Sensing
The inhibition of the quorum sensing by the extracts was evaluated with the biosensor strain Chromobacterium violaceum ATCC 12472 and performed based on Asensio et al. []. A bacterial suspension of C. violaceum was prepared from an overnight culture grown at 30 °C and 250 rpm in Luria–Bertani (LB, Liofilchem, Italy) broth and subsequently diluted in fresh LB to an optical density at 600 nm of 0.02. The extracts were serially two-fold diluted in LB, and 500 µL of each dilution was added to 48-well flat-bottom plates. DMSO at a final concentration of 0.5% was used as the solvent control. 500 µL of the bacterial suspension was added to each well, and the plates were incubated for 48 h at 30 °C. Following incubation, 750 µL were centrifuged at 5000× g for 3 min. The supernatants were discarded, and the pellets were vortexed with 750 µL of DMSO to solubilize the pigment violacein. Subsequently, 200 µL of the violacein-containing supernatant was transferred in triplicate to a 96-well microplate, and the absorbance was measured at 585 nm using a microplate spectrophotometer (Bio-Rad xMark, Hercules, CA, USA). After a centrifugation at 6000× g for 5 min, the cellular pellet was suspended in 750 µL of distilled water, and 200 µL of the suspension was transferred in triplicate to a 96-well microplate, and the optical density was measured at 600 nm.
Subsequently, the percentage of violacein inhibition (%) was then calculated using the following equation:
where Abssample represents the absorbance of each sample measured at 585 nm, and Absgrowth control corresponds to the absorbance of the control.
3.8. Antibiofilm Activity
The analysis of the effect of the extracts in biofilm formation by S. aureus strains was based on the previously described method of [,] with modifications. In brief, serial two-fold dilutions of the extracts (ranging from 0.25 to 2× MIC or 0.03 to 2× MIC, depending on the extract or strain) were prepared in TSB supplemented with 0.5% glucose in 96-well flat-bottom plates. S. aureus strains were cultured overnight in TSB at 37 °C with agitation at 250 rpm and suspensions diluted and adjusted to obtain a final inoculum of 1 × 107 CFU/mL in each well. Subsequently, 100 µL of each bacterial suspension was added to the wells, yielding a final volume of 200 µL. The bacterial suspension in medium served as the positive control, while culture medium alone was used as the negative control. A solvent control containing DMSO 1% was also included. The plates were incubated at 37 °C for 24 h. Following incubation, the plate content was discarded, and each well was washed twice with 200 µL of distilled water to remove loosely attached cells. The adherent bacteria were then fixed with 200 µL of methanol (VWR, Rosny-sous-Bois, France) for 20 min. After removing the methanol, the plates were air-dried and subsequently stained with 0.1% (w/v) crystal violet (200 µL per well) for 10 min. Excess dye was discarded, and the wells were washed three times with 300 µL of distilled water. The bound crystal violet was then solubilized with 200 µL of 33% (v/v) glacial acetic acid, and absorbance was measured at 570 nm using a microplate reader. All assays were carried out with at least five replicates in each of three independent experiments.
3.9. Statistical Analysis
The statistical analysis of the results was performed using the one-way ANOVA and Dunnett test using the GraphPad Prism v8.01 software, with a 95% confidence interval, considering the values of p < 0.05 as statistically significant.
4. Conclusions
This study evaluated extracts of C. salviifolius and H. stoechas against Staphylococcus aureus. H. stoechas showed the strongest activity (MIC 7.8–62.5 µg/mL). In combination with ampicillin, ciprofloxacin, or vancomycin, C. salviifolius and H. stoechas extracts enhanced antibiotic efficacy, displaying synergistic or additive effects and restoring susceptibility of a MRSA strain. The aerial parts of C. salviifolius displayed modulatory effects on ethidium bromide and exhibited slight quorum sensing inhibition in C. violaceum, while all extracts inhibited biofilm formation even at subinhibitory concentrations. These findings highlight the dual potential of these extracts as direct antimicrobial agents and as adjuvants. Further studies are needed to elucidate mechanisms of action, specific targets, and key pharmaceutical properties such as stability and bioavailability.
Author Contributions
Conceptualization, Â.L., S.F., and A.P.D.; methodology, A.C.; formal analysis, A.C., and S.F.; writing—original draft preparation, A.C.; writing—review and editing, A.C., Â.L., P.D.G., S.F., and A.P.D.; supervision, Â.L., S.F. and A.P.D.; funding acquisition, Â.L., P.D.G., S.F. and A.P.D.; project administration, P.D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research is within the activities of the project Montanha Viva-Sistema Previsional Inteligente de Suporte à Decisão em Sustentabilidade”, project PD21-00009, promoted by PROMOVE program funded by Fundação La Caixa and supported by Fundação para a Ciência e a Tecnologia and BPI. This work was developed within the scope of the CICS-UBI projects UIDB/00709/2020 and UIDP/00709/2020, financed by national funds through the Portuguese Foundation for Science and Technology/MCTES. Pedro Dinis Gaspar acknowledges the support of FCT—Fundação para a Ciência e a Tecnologia, I.P. and Centre for Mechanical and Aerospace Science and Technologies (C-MAST), under the project UIDB/00151/2020 (https://doi.org/10.54499/UIDB/00151/2020; https://doi.org/10.54499/UIDP/00151/2020) (accessed on 28/07/2025).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the text.
Acknowledgments
Alexandra Coimbra is recipient of a research fellowship within the Research project titled “Montanha Viva-Sistema Previsional Inteligente de Suporte à Decisão em Sustentabilidade” (Ref. PD21-00009), funded by Fundação La Caixa.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Nandhini, P.; Kumar, P.; Mickymaray, S.; Alothaim, A.S.; Somasundaram, J.; Rajan, M. Recent Developments in Methicillin-Resistant Staphylococcus aureus (MRSA) Treatment: A Review. Antibiotics 2022, 11, 606. [Google Scholar] [CrossRef]
- Howden, B.P.; Giulieri, S.G.; Lung, T.W.F.; Baines, S.L.; Sharkey, L.K.; Lee, J.Y.H.; Hachani, A.; Monk, I.R.; Stinear, T.P. Staphylococcus aureus Host Interactions and Adaptation. Nat. Rev. Microbiol. 2023, 21, 380–395. [Google Scholar] [CrossRef]
- Peacock, S.J.; Paterson, G.K. Mechanisms of Methicillin Resistance in Staphylococcus aureus. Annu. Rev. Biochem. 2015, 84, 577–601. [Google Scholar] [CrossRef]
- Idrees, M.; Sawant, S.; Karodia, N.; Rahman, A. Staphylococcus aureus Biofilm: Morphology, Genetics, Pathogenesis and Treatment Strategies. Int. J. Environ. Res. Public Health 2021, 18, 7602. [Google Scholar] [CrossRef]
- Tuon, F.F.; Suss, P.H.; Telles, J.P.; Dantas, L.R.; Borges, N.H.; Ribeiro, V.S.T. Antimicrobial Treatment of Staphylococcus aureus Biofilms. Antibiotics 2023, 12, 87. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, H.; Hu, G.; Liu, J.; Lian, S.; Pang, S.; Zhu, G.; Ding, X. Unmasking MRSA’s Armor: Molecular Mechanisms of Resistance and Pioneering Therapeutic Countermeasures. Microorganisms 2025, 13, 1928. [Google Scholar] [CrossRef]
- Li, J.; Cheng, F.; Wei, X.; Bai, Y.; Wang, Q.; Li, B.; Zhou, Y.; Zhai, B.; Zhou, X.; Wang, W.; et al. Methicillin-Resistant Staphylococcus aureus (MRSA): Resistance, Prevalence, and Coping Strategies. Antibiotics 2025, 14, 771. [Google Scholar] [CrossRef]
- Bowler, P.; Murphy, C.; Wolcott, R. Biofilm Exacerbates Antibiotic Resistance: Is This a Current Oversight in Antimicrobial Stewardship? Antimicrob. Resist. Infect. Control 2020, 9, 162. [Google Scholar] [CrossRef]
- Nwobodo, D.C.; Ugwu, M.C.; Anie, C.O.; Al-Ouqaili, M.T.S.; Ikem, J.C.; Chigozie, U.V.; Saki, M. Antibiotic Resistance: The Challenges and Some Emerging Strategies for Tackling a Global Menace. J. Clin. Lab. Anal. 2022, 36, e24655. [Google Scholar] [CrossRef] [PubMed]
- Nikolic, P.; Mudgil, P. The Cell Wall, Cell Membrane and Virulence Factors of Staphylococcus aureus and Their Role in Antibiotic Resistance. Microorganisms 2023, 11, 259. [Google Scholar] [CrossRef] [PubMed]
- Frieri, M.; Kumar, K.; Boutin, A. Antibiotic Resistance. J. Infect. Public Health 2017, 10, 369–378. [Google Scholar] [CrossRef]
- Ding, D.; Wang, B.; Zhang, X.; Zhang, J.; Zhang, H.; Liu, X.; Gao, Z.; Yu, Z. The Spread of Antibiotic Resistance to Humans and Potential Protection Strategies. Ecotoxicol. Environ. Saf. 2023, 254, 114734. [Google Scholar] [CrossRef]
- Halawa, E.M.; Fadel, M.; Al-Rabia, M.W.; Behairy, A.; Nouh, N.A.; Abdo, M.; Olga, R.; Fericean, L.; Atwa, A.M.; El-Nablaway, M.; et al. Antibiotic Action and Resistance: Updated Review of Mechanisms, Spread, Influencing Factors, and Alternative Approaches for Combating Resistance. Front. Pharmacol. 2024, 14, 1305294. [Google Scholar] [CrossRef]
- Manso, T.; Lores, M.; de Miguel, T. Antimicrobial Activity of Polyphenols and Natural Polyphenolic Extracts on Clinical Isolates. Antibiotics 2022, 11, 46. [Google Scholar] [CrossRef]
- Bao, M.; Zhang, L.; Liu, B.; Li, L.; Zhang, Y.; Zhao, H.; Ji, X.; Chen, Q.; Hu, M.; Bai, J.; et al. Synergistic Effects of Anti-MRSA Herbal Extracts Combined with Antibiotics. Future Microbiol. 2020, 15, 1265–1276. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, N.A.; Rathore, D.; Janiyani, K.; Gupta, A.; Sulieman, A.M.E.; Tahir, H.E.; Mir, R.H.; Fatima, S.B.; Adnan, M.; Surti, M. A Comprehensive Review on Plant-Derived Bioactive Saponins as Promising Antimicrobial Agents: From Bioavailability Challenges, Molecular Mechanistic Insights to Therapeutic Applications. Naunyn Schmiedebergs Arch. Pharmacol. 2025, 1–31. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Gupta, N.; Dutta, A.; Bonomo, M.G.; Milella, L.; Sarker, S.D.; Nahar, L. Mitigating ROS Signalling Pathway-Mediated Defence Mechanism: A Novel Approach to Counteract Bacterial Resistance Using Natural Antioxidant-Based Antibiotics. Phytochem. Rev. 2025, 1–33. [Google Scholar] [CrossRef]
- Morguette, A.E.B.; Bartolomeu-Gonçalves, G.; Andriani, G.M.; Bertoncini, G.E.S.; de Castro, I.M.; Spoladori, L.F.d.A.; Bertão, A.M.S.; Tavares, E.R.; Yamauchi, L.M.; Yamada-Ogatta, S.F. The Antibacterial and Wound Healing Properties of Natural Products: A Review on Plant Species with Therapeutic Potential against Staphylococcus aureus Wound Infections. Plants 2023, 12, 2147. [Google Scholar] [CrossRef]
- Khare, T.; Anand, U.; Dey, A.; Assaraf, Y.G.; Chen, Z.S.; Liu, Z.; Kumar, V. Exploring Phytochemicals for Combating Antibiotic Resistance in Microbial Pathogens. Front. Pharmacol. 2021, 12, 720726. [Google Scholar] [CrossRef]
- Barbieri, R.; Coppo, E.; Marchese, A.; Daglia, M.; Sobarzo-Sánchez, E.; Nabavi, S.F.; Nabavi, S.M. Phytochemicals for Human Disease: An Update on Plant-Derived Compounds Antibacterial Activity. Microbiol. Res. 2017, 196, 44–68. [Google Scholar] [CrossRef] [PubMed]
- Górniak, I.; Bartoszewski, R.; Króliczewski, J. Comprehensive Review of Antimicrobial Activities of Plant Flavonoids. Phytochem. Rev. 2019, 18, 241–272. [Google Scholar] [CrossRef]
- Khameneh, B.; Iranshahy, M.; Soheili, V.; Bazzaz, B.S.F. Review on Plant Antimicrobials: A Mechanistic Viewpoint. Antimicrob. Resist. Infect. Control 2019, 8, 118. [Google Scholar] [CrossRef] [PubMed]
- Morales-Soto, A.; Oruna-Concha, M.J.; Elmore, J.S.; Barrajón-Catalán, E.; Micol, V.; Roldán, C.; Segura-Carretero, A. Volatile Profile of Spanish Cistus Plants as Sources of Antimicrobials for Industrial Applications. Ind. Crops Prod. 2015, 74, 425–433. [Google Scholar] [CrossRef]
- Alamami, A.; Elshibani, F.; Alshalmani, S.; Sharkasi, M.A.; Elremali, N.; Daboub, A. High-Performance Liquid Chromatography Analysis and Antimicrobial Activities of Libyan Cistus salviifolius Extract. J. Pharm. Res. Int. 2021, 33, 32–40. [Google Scholar] [CrossRef]
- Bouabidi, M.; Salamone, F.L.; Gadhi, C.; Bouamama, H.; Speciale, A.; Ginestra, G.; Pulvirenti, L.; Siracusa, L.; Nostro, A.; Cristani, M. Efficacy of Two Moroccan Cistus Species Extracts against Acne Vulgaris: Phytochemical Profile, Antioxidant, Anti-Inflammatory and Antimicrobial Activities. Molecules 2023, 28, 2797. [Google Scholar] [CrossRef]
- El Euch, S.K.; Bouajila, J.; Bouzouita, N. Chemical Composition, Biological and Cytotoxic Activities of Cistus salviifolius Flower Buds and Leaves Extracts. Ind. Crops Prod. 2015, 76, 1100–1105. [Google Scholar] [CrossRef]
- Christou, A.; Nikola, F.; Goulas, V. Optimization of the Ultrasound-Assisted Extraction of Phenolic Antioxidants from Cistus salvifolius L. Using Response Surface Methodology. Chem. Biodivers. 2025, 22, e202401337. [Google Scholar] [CrossRef]
- Hitl, M.; Bijelić, K.; Stilinović, N.; Božin, B.; Srđenović-Čonić, B.; Torović, L.; Kladar, N. Phytochemistry and Antihyperglycemic Potential of Cistus salviifolius L., Cistaceae. Molecules 2022, 27, 8003. [Google Scholar] [CrossRef]
- Boy, F.R.; Casquete, R.; Martínez, A.; Córdoba, M.d.G.; Ruíz-Moyano, S.; Benito, M.J. Antioxidant, Antihypertensive and Antimicrobial Properties of Phenolic Compounds Obtained from Native Plants by Different Extraction Methods. Int. J. Environ. Res. Public Health 2021, 18, 2475. [Google Scholar] [CrossRef]
- Álvarez-Martínez, F.J.; Rodríguez, J.C.; Borrás-Rocher, F.; Barrajón-Catalán, E.; Micol, V. The Antimicrobial Capacity of Cistus salviifolius and Punica granatum Plant Extracts against Clinical Pathogens Is Related to Their Polyphenolic Composition. Sci. Rep. 2021, 11, 588. [Google Scholar] [CrossRef]
- Zalegh, I.; Bourhia, M.; Zerouali, K.; Katfy, K.; Nayme, K.; Khallouki, F.; Benzaarate, I.; Salamatullah, A.M.; Alzahrani, A.; Nafidi, H.A.; et al. Molecular Characterization of Gene-Mediated Resistance and Susceptibility of ESKAPE Clinical Isolates to Cistus monspeliensis L. and Cistus salviifolius L. Extracts. J. Evid.-Based Complement. Altern. Med. 2022, 2022, 7467279. [Google Scholar] [CrossRef]
- Carev, I.; Maravić, A.; Ilić, N.; Čulić, V.Č.; Politeo, O.; Zorić, Z.; Radan, M. UPLC-MS/MS Phytochemical Analysis of Two Croatian Cistus Species and Their Biological Activity. Life 2020, 10, 112. [Google Scholar] [CrossRef]
- Boubekeur, S.; Messaoudi, M.; Awuchi, C.G.; Otekunrin, O.A.; Sawicka, B.; Idjeri-Mecherara, S.; Bouchareb, S.; Hassani, A.; Sharifi-Rad, M.; Begaa, S.; et al. Biological Properties and Polyphenols Content of Algerian Cistus salviifolius L. Aerial Parts. Eur. J. Biol. Res. 2022, 12, 163–180. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Aouadhi, C.; Kaddour, R.; Gruber, M.; Zargouni, H.; Zaouali, W.; Ben Hamida, N.; Ben Nasri, M.; Ouerghi, Z.; Hosni, K. Comparison of Antioxidant and Antimicrobial activities of Two Cultivated Cistus Species from Tunisia. Biosci. J. 2016, 32, 226–237. [Google Scholar] [CrossRef]
- Mastino, P.M.; Marchetti, M.; Costa, J.; Juliano, C.; Usai, M. Analytical Profiling of Phenolic Compounds in Extracts of Three Cistus Species from Sardinia and Their Potential Antimicrobial and Antioxidant Activity. Chem. Biodivers. 2021, 18, e2100053. [Google Scholar] [CrossRef] [PubMed]
- Oyardi, Ö.; Hacioğlu, M.; Özdemir, E.; Erbay, M.Ş.; Kültür, Ş.; Bozkurt Güzel, Ç. Screening of Antimicrobial, Antibiofilm, and Cytotoxic Activities of Some Medicinal Plants from Balıkesir Province, Türkiye: Potential Effects of Allium paniculatum Flower. Turk. J. Pharm. Sci. 2024, 21, 252–258. [Google Scholar] [CrossRef]
- Onal, F.N.; Ozturk, I.; Kose, F.A.; Der, G.; Kilinc, E.; Baykan, S. Comparative Evaluation of Polyphenol Contents and Biological Activities of Five Cistus L. Species Native to Turkey. Chem. Biodivers. 2023, 20, e202200915. [Google Scholar] [CrossRef]
- Sánchez-Hernández, E.; Álvarez-Martínez, J.; González-García, V.; Casanova-Gascón, J.; Martín-Gil, J.; Martín-Ramos, P. Helichrysum stoechas (L.) Moench Inflorescence Extract for Tomato Disease Management. Molecules 2023, 28, 5861. [Google Scholar] [CrossRef]
- Rebaya, A.; Belghith, S.I.; Cherif, J.K.; Ayadi, M.T. Total Phenolic Compounds and Antioxidant Potential of Rokrose (Cistus salviifolius) Leaves and Flowers Grown in Tunisia. Int. J. Pharm. Phytochem. Res. 2016, 8, 327–331. [Google Scholar]
- Kühn, C.; Arapogianni, N.E.; Halabalaki, M.; Hempel, J.; Hunger, N.; Wober, J.; Skaltsounis, A.L.; Vollmer, G. Constituents from Cistus salvifolius (Cistaceae) Activate Peroxisome Proliferator-Activated Receptor-γ but Not -δ And Stimulate Glucose Uptake by Adipocytes. Planta Med. 2011, 77, 346–353. [Google Scholar] [CrossRef]
- Coimbra, A.; Gallardo, E.; Luís, Â.; Gaspar, P.D.; Ferreira, S.; Duarte, A.P. Bioactive Potential of Wild Plants from Gardunha Mountain: Phytochemical Characterization and Biological Activities. Molecules 2025, 30, 3876. [Google Scholar] [CrossRef] [PubMed]
- Kutluk, I.; Aslan, M.; Orhan, I.E.; Özçelik, B. Antibacterial, Antifungal and Antiviral Bioactivities of Selected Helichrysum Species. S Afr. J. Bot. 2018, 119, 252–257. [Google Scholar] [CrossRef]
- Boubakeur, H.; Rebbas, K.; Belhattab, R. Activités Antioxydante et Antibactérienne Des Extraits d’Helichrysum stoechas (L.) Moench. Phytothérapie 2017, 16, 122–132. [Google Scholar] [CrossRef]
- Zengin, G.; Cvetanović, A.; Gašić, U.; Tešić, Ž.; Stupar, A.; Bulut, G.; Sinan, K.I.; Uysal, S.; Picot-Allain, M.C.N.; Mahomoodally, M.F. A Comparative Exploration of the Phytochemical Profiles and Bio-Pharmaceutical Potential of Helichrysum stoechas Subsp. barrelieri Extracts Obtained via Five Extraction Techniques. Process Biochem. 2020, 91, 113–125. [Google Scholar] [CrossRef]
- Kherbache, A.; Senator, A.; Laouicha, S.; Al-Zoubi, R.M.; Bouriche, H. Phytochemical Analysis, Antioxidant and Anti-Inflammatory Activities of Helichrysum stoechas (L.) Moench Extracts. Biocatal. Agric. Biotechnol. 2020, 29, 101826. [Google Scholar] [CrossRef]
- Albayrak, S.; Aksoy, A.; Sagdic, O.; Hamzaoglu, E. Compositions, Antioxidant and Antimicrobial Activities of Helichrysum (Asteraceae) Species Collected from Turkey. Food Chem. 2010, 119, 114–122. [Google Scholar] [CrossRef]
- Bogdadi, H.A.A.; Kokoska, L.; Havlik, J.; Kloucek, P.; Rada, V.; Vorisek, K. In Vitro Antimicrobial Activity of Some Libyan Medicinal Plant Extracts. Pharm. Biol. 2007, 45, 386–391. [Google Scholar] [CrossRef]
- Les, F.; Venditti, A.; Cásedas, G.; Frezza, C.; Guiso, M.; Sciubba, F.; Serafini, M.; Bianco, A.; Valero, M.S.; López, V. Everlasting Flower (Helichrysum stoechas Moench) as a Potential Source of Bioactive Molecules with Antiproliferative, Antioxidant, Antidiabetic and Neuroprotective Properties. Ind. Crops Prod. 2017, 108, 295–302. [Google Scholar] [CrossRef]
- Hussain, M.S.; Azam, F.; Eldarrat, H.A.; Haque, A.; Khalid, M.; Hassan, M.Z.; Ali, M.; Arif, M.; Ahmad, I.; Zaman, G.; et al. Structural, Functional, Molecular, and Biological Evaluation of Novel Triterpenoids Isolated from Helichrysum stoechas (L.) Moench. Collected from Mediterranean Sea Bank: Misurata- Libya. Arabian J. Chem. 2022, 15, 103818. [Google Scholar] [CrossRef]
- Hussain, M.S.; Azam, F.; Eldarrat, H.A.; Alkskas, I.; Mayoof, J.A.; Dammona, J.M.; Ismail, H.; Ali, M.; Arif, M.; Haque, A. Anti-Inflammatory, Analgesic and Molecular Docking Studies of Lanostanoic Acid 3-O-α-D-Glycopyranoside Isolated from Helichrysum stoechas. Arab. J. Chem. 2020, 13, 9196–9206. [Google Scholar] [CrossRef]
- Valero, M.S.; Nuñez, S.; Les, F.; Castro, M.; Gómez-Rincón, C.; Arruebo, M.P.; Plaza, M.Á.; Köhler, R.; López, V. The Potential Role of Everlasting Flower (Helichrysum stoechas Moench) as an Antihypertensive Agent: Vasorelaxant Effects in the Rat Aorta. Antioxidants 2022, 11, 1092. [Google Scholar] [CrossRef]
- Silva, L.; Rodrigues, A.M.; Ciriani, M.; Falé, P.L.V.; Teixeira, V.; Madeira, P.; Machuqueiro, M.; Pacheco, R.; Florêncio, M.H.; Ascensão, L.; et al. Antiacetylcholinesterase Activity and Docking Studies with Chlorogenic Acid, Cynarin and Arzanol from Helichrysum stoechas (Asteraceae). Med. Chem. Res. 2017, 26, 2942–2950. [Google Scholar] [CrossRef]
- Abouzeed, Y.M.; Elfahem, A.; Zgheel, F.; Ahmed, M.O. Antibacterial in Vitro Activities of Selected Medicinal Plants against Methicillin Resistant Staphylococcus aureus from Libyan Environment. J. Environ. Anal. Toxicol. 2013, 3, 1000194. [Google Scholar] [CrossRef]
- Tomás-Menor, L.; Morales-Soto, A.; Barrajón-Catalán, E.; Roldán-Segura, C.; Segura-Carretero, A.; Micol, V. Correlation between the Antibacterial Activity and the Composition of Extracts Derived from Various Spanish Cistus Species. Food Chem. Toxicol. 2013, 55, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Friedman, M. Antibiotic-Resistant Bacteria: Prevalence in Food and Inactivation by Food-Compatible Compounds and Plant Extracts. J. Agric. Food Chem. 2015, 63, 3805–3822. [Google Scholar] [CrossRef]
- Tomás-Menor, L.; Barrajón-Catalán, E.; Segura-Carretero, A.; Martí, N.; Saura, D.; Menéndez, J.A.; Joven, J.; Micol, V. The Promiscuous and Synergic Molecular Interaction of Polyphenols in Bactericidal Activity: An Opportunity to Improve the Performance of Antibiotics? Phytother. Res. 2015, 29, 466–473. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C.; Villar, A. Antimicrobial Activities of Helichrysum stoechas. Planta Med. 1990, 56, 646. [Google Scholar] [CrossRef]
- Ríos, J.L.; Recio, M.C.; Villar, A.A. Isolation and Identification of the Antibacterial Compounds from Helichrysum stoechas. J. Ethnopharmacol. 1991, 33, 51–55. [Google Scholar] [CrossRef]
- Zhu, X.; Liu, H.; Wang, Z.; Tian, R.; Li, S. Dimethyl Phthalate Damages Staphylococcus aureus by Changing the Cell Structure, Inducing Oxidative Stress and Inhibiting Energy Metabolism. J. Environ. Sci. 2021, 107, 171–183. [Google Scholar] [CrossRef]
- Ishak, A.; Mazonakis, N.; Spernovasilis, N.; Akinosoglou, K.; Tsioutis, C. Bactericidal versus Bacteriostatic Antibacterials: Clinical Significance, Differences and Synergistic Potential in Clinical Practice. J. Antimicrob. Chemother. 2025, 80, 1–17. [Google Scholar] [CrossRef]
- Nostro, A.; Bisignano, G.; Cannatelli, M.A.; Crisafi, G.; Germanò, M.P.; Alonzo, V. Effects of Helichrysum italicum Extract on Growth and Enzymatic Activity of Staphylococcus aureus. Int. J. Antimicrob. Agents 2001, 17, 517–520. [Google Scholar] [CrossRef]
- Sang, H.; Jin, H.; Song, P.; Xu, W.; Wang, F. Gallic Acid Exerts Antibiofilm Activity by Inhibiting Methicillin-Resistant Staphylococcus aureus Adhesion. Sci. Rep. 2024, 14, 17220. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, M.; Ullah, F.; Sadiq, A.; Ullah, F.; Ovais, M.; Ahmed, J.; Devkota, H.P. Synergistic Interactions of Phytochemicals with Antimicrobial Agents: Potential Strategy to Counteract Drug Resistance. Chem. Biol. Interact. 2019, 308, 294–303. [Google Scholar] [CrossRef]
- Singh, K.; Coopoosamy, R.M.; Gumede, N.J.; Sabiu, S. Computational Insights and in Vitro Validation of Antibacterial Potential of Shikimate Pathway-Derived Phenolic Acids as NorA Efflux Pump Inhibitors. Molecules 2022, 27, 2601. [Google Scholar] [CrossRef]
- Buchmann, D.; Schultze, N.; Borchardt, J.; Böttcher, I.; Schaufler, K.; Guenther, S. Synergistic Antimicrobial Activities of Epigallocatechin Gallate, Myricetin, Daidzein, Gallic Acid, Epicatechin, 3-Hydroxy-6-Methoxyflavone and Genistein Combined with Antibiotics against ESKAPE Pathogens. J. Appl. Microbiol. 2022, 132, 949–963. [Google Scholar] [CrossRef]
- Pulingam, T.; Parumasivam, T.; Gazzali, A.M.; Sulaiman, A.M.; Chee, J.Y.; Lakshmanan, M.; Chin, C.F.; Sudesh, K. Antimicrobial Resistance: Prevalence, Economic Burden, Mechanisms of Resistance and Strategies to Overcome. Eur. J. Pharm. Sci. 2022, 170, 106103. [Google Scholar] [CrossRef] [PubMed]
- Roudashti, S.; Zeighami, H.; Mirshahabi, H.; Bahari, S.; Soltani, A.; Haghi, F. Synergistic Activity of Sub-Inhibitory Concentrations of Curcumin with Ceftazidime and Ciprofloxacin against Pseudomonas aeruginosa Quorum Sensing Related Genes and Virulence Traits. World J. Microbiol. Biotechnol. 2017, 33, 50. [Google Scholar] [CrossRef]
- Hu, Z.-Q.; Zhao, W.-H.; Hara, Y.; Shimamura, T. Epigallocatechin Gallate Synergy with Ampicillin/Sulbactam against 28 Clinical Isolates of Methicillin-Resistant Staphylococcus aureus. J. Antimicrob. Chemother. 2001, 48, 361–364. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.H.; Hu, Z.Q.; Okubo, S.; Hara, Y.; Shimamura, T. Mechanism of Synergy between Epigallocatechin Gallate and β-Lactams against Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2001, 45, 1737–1742. [Google Scholar] [CrossRef]
- Zhao, W.H.; Hu, Z.Q.; Hara, Y.; Shimamura, T. Inhibition of Penicillinase by Epigallocatechin Gallate Resulting in Restoration of Antibacterial Activity of Penicillin against Penicillinase-Producing Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 2266–2268. [Google Scholar] [CrossRef]
- Hu, Z.Q.; Zhao, W.H.; Asano, N.; Yoda, Y.; Hara, Y.; Shimamura, T. Epigallocatechin Gallate Synergistically Enhances the Activity of Carbapenems against Methicillin-Resistant Staphylococcus aureus. Antimicrob. Agents Chemother. 2002, 46, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Abreu, A.C.; McBain, A.J.; Simões, M. Plants as Sources of New Antimicrobials and Resistance-Modifying Agents. Nat. Prod. Rep. 2012, 29, 1007–1021. [Google Scholar] [CrossRef]
- Qin, R.; Xiao, K.; Li, B.; Jiang, W.; Peng, W.; Zheng, J.; Zhou, H. The Combination of Catechin and Epicatechin Gallate from Fructus Crataegi Potentiates β-Lactam Antibiotics against Methicillin-Resistant Staphylococcus aureus (MRSA) in Vitro and in Vivo. Int. J. Mol. Sci. 2013, 14, 1802–1821. [Google Scholar] [CrossRef]
- Fiamegos, Y.C.; Kastritis, P.L.; Exarchou, V.; Han, H.; Bonvin, A.M.J.J.; Vervoort, J.; Lewis, K.; Hamblin, M.R.; Tegos, G.P. Antimicrobial and Efflux Pump Inhibitory Activity of Caffeoylquinic Acids from Artemisia absinthium against Gram-Positive Pathogenic Bacteria. PLoS ONE 2011, 6, e18127. [Google Scholar] [CrossRef]
- Lima, V.N.; Oliveira-Tintino, C.D.M.; Santos, E.S.; Morais, L.P.; Tintino, S.R.; Freitas, T.S.; Geraldo, Y.S.; Pereira, R.L.S.; Cruz, R.P.; Menezes, I.R.A.; et al. Antimicrobial and Enhancement of the Antibiotic Activity by Phenolic Compounds: Gallic Acid, Caffeic Acid and Pyrogallol. Microb. Pathog. 2016, 99, 56–61. [Google Scholar] [CrossRef]
- Sharma, H.K.; Gupta, P.; Nagpal, D.; Mukherjee, M.; Parmar, V.S.; Lather, V. Virtual Screening and Antimicrobial Evaluation for Identification of Natural Compounds as the Prospective Inhibitors of Antibacterial Drug Resistance Targets in Staphylococcus aureus. Fitoterapia 2023, 168, 105554. [Google Scholar] [CrossRef]
- Gangwar, B.; Kumar, S.; Kumar, P.; Pal, A.; Darokar, M.P. A Mechanistic Insight into the Anti-Staphylococcal Mode of Action of (+)-Usnic Acid and Its Synergy with Norfloxacin Against Methicillin-Resistant Staphylococcus aureus. Biomolecules 2025, 15, 750. [Google Scholar] [CrossRef]
- Martin, A.L.A.R.; Pereira, R.L.S.; Rocha, J.E.; Farias, P.A.M.; Freitas, T.S.; Caldas, F.R.d.L.; Figueredo, F.G.; Sampaio, N.F.L.; Oliveira-Tintino, C.D.d.M.; Tintino, S.R.; et al. Unlocking Bacterial Defense: Exploring the Potent Inhibition of NorA Efflux Pump by Coumarin Derivatives in Staphylococcus aureus. Microb. Pathog. 2024, 190, 106608. [Google Scholar] [CrossRef]
- Araújo-Neto, J.B.d.; Oliveira-Tintino, C.D.d.M.; de Araújo, G.A.; Alves, D.S.; Ribeiro, F.R.; Brancaglion, G.A.; Carvalho, D.T.; Lima, C.M.G.; Ali, H.S.H.M.; Rather, I.A.; et al. 3-Substituted Coumarins Inhibit NorA and MepA Efflux Pumps of Staphylococcus aureus. Antibiotics 2023, 12, 1739. [Google Scholar] [CrossRef] [PubMed]
- Rampacci, E.; Felicetti, T.; Cernicchi, G.; Stefanetti, V.; Sabatini, S.; Passamonti, F. Inhibition of Staphylococcus pseudintermedius Efflux Pumps by Using Staphylococcus aureus NorA Efflux Pump Inhibitors. Antibiotics 2023, 12, 806. [Google Scholar] [CrossRef] [PubMed]
- Sionov, R.V.; Steinberg, D. Targeting the Holy Triangle of Quorum Sensing, Biofilm Formation, and Antibiotic Resistance in Pathogenic Bacteria. Microorganisms 2022, 10, 1239. [Google Scholar] [CrossRef] [PubMed]
- Gaurav, A.; Bakht, P.; Saini, M.; Pandey, S.; Pathania, R. Role of Bacterial Efflux Pumps in Antibiotic Resistance, Virulence, and Strategies to Discover Novel Efflux Pump Inhibitors. Microbiology 2023, 169, 001333. [Google Scholar] [CrossRef]
- Santos, M.; Santos, R.; Soeiro, P.; Silvestre, S.; Ferreira, S. Resveratrol as an Inhibitor of the NorA Efflux Pump and Resistance Modulator in Staphylococcus aureus. Antibiotics 2023, 12, 1168. [Google Scholar] [CrossRef]
- Seukep, A.J.; Kuete, V.; Nahar, L.; Sarker, S.D.; Guo, M. Plant-Derived Secondary Metabolites as the Main Source of Efflux Pump Inhibitors and Methods for Identification. J. Pharm. Anal. 2020, 10, 277–290. [Google Scholar] [CrossRef]
- Cernicchi, G.; Felicetti, T.; Sabatini, S. Microbial Efflux Pump Inhibitors: A Journey around Quinoline and Indole Derivatives. Molecules 2021, 26, 6996. [Google Scholar] [CrossRef]
- Shaheen, A.; Afridi, W.A.; Mahboob, S.; Sana, M.; Zeeshan, N.; Ismat, F.; Mirza, O.; Iqbal, M.; Rahman, M. Reserpine Is the New Addition into the Repertoire of AcrB Efflux Pump Inhibitors. Mol. Biol. 2019, 53, 596–605. [Google Scholar] [CrossRef]
- Randhawa, H.K.; Hundal, K.K.; Ahirrao, P.N.; Jachak, S.M.; Nandanwar, H.S. Efflux Pump Inhibitory Activity of Flavonoids Isolated from Alpinia calcarata against Methicillin-Resistant Staphylococcus aureus. Biologia 2016, 71, 484–493. [Google Scholar] [CrossRef]
- Holler, J.G.; Christensen, S.B.; Slotved, H.C.; Rasmussen, H.B.; Gúzman, A.; Olsen, C.E.; Petersen, B.; Mølgaard, P. Novel Inhibitory Activity of the Staphylococcus aureus NorA Efflux Pump by a Kaempferol Rhamnoside Isolated from Persea lingue Nees. J. Antimicrob. Chemother. 2012, 67, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Falcão-Silva, V.S.; Silva, D.A.; De Fátima, M.; Souza, V.; Siqueira-Junior, J.P. Modulation of Drug Resistance in Staphylococcus aureus by a Kaempferol Glycoside from Herissantia tiubae (Malvaceae). Phytother. Res. 2009, 23, 1367–1370. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.R.; Ettefagh, K.A.; Todd, D.; Cole, P.S.; Egan, J.M.; Foil, D.H.; Graf, T.N.; Schindler, B.D.; Kaatz, G.W.; Cech, N.B. A Mass Spectrometry-Based Assay for Improved Quantitative Measurements of Efflux Pump Inhibition. PLoS ONE 2015, 10, e0124814. [Google Scholar] [CrossRef]
- Dos Santos, J.F.S.; Tintino, S.R.; Da Silva, A.R.P.; Barbosa, C.R.d.S.; Scherf, J.R.; Silveira, Z.d.S.; de Freitas, T.S.; Neto, L.J.d.L.; Barros, L.M.; Menezes, I.R.d.A.; et al. Enhancement of the Antibiotic Activity by Quercetin against Staphylococcus aureus Efflux Pumps. J. Bioenerg. Biomembr. 2021, 53, 157–167. [Google Scholar] [CrossRef]
- Lade, H.; Kim, J.S. Molecular Determinants of β-Lactam Resistance in Methicillin-Resistant Staphylococcus aureus (MRSA): An Updated Review. Antibiotics 2023, 12, 1362. [Google Scholar] [CrossRef]
- Ealand, C.S.; Machowski, E.E.; Kana, B.D. β-Lactam Resistance: The Role of Low Molecular Weight Penicillin Binding Proteins, β-Lactamases and LD-Transpeptidases in Bacteria Associated with Respiratory Tract Infections. IUBMB Life 2018, 70, 855–868. [Google Scholar] [CrossRef]
- Belay, W.Y.; Getachew, M.; Tegegne, B.A.; Teffera, Z.H.; Dagne, A.; Zeleke, T.K.; Abebe, R.B.; Gedif, A.A.; Fenta, A.; Yirdaw, G.; et al. Mechanism of Antibacterial Resistance, Strategies and next-Generation Antimicrobials to Contain Antimicrobial Resistance: A Review. Front. Pharmacol. 2024, 15, 1444781. [Google Scholar] [CrossRef]
- Darby, E.M.; Trampari, E.; Siasat, P.; Gaya, M.S.; Alav, I.; Webber, M.A.; Blair, J.M.A. Molecular Mechanisms of Antibiotic Resistance Revisited. Nat. Rev. Microbiol. 2023, 21, 280–295. [Google Scholar] [CrossRef]
- Cong, Y.; Yang, S.; Rao, X. Vancomycin Resistant Staphylococcus aureus Infections: A Review of Case Updating and Clinical Features. J. Adv. Res. 2020, 21, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Stogios, P.J.; Savchenko, A. Molecular Mechanisms of Vancomycin Resistance. Protein Sci. 2020, 29, 654–669. [Google Scholar] [CrossRef]
- Kitpipit, W.; Scholfield, C.N.; Sangkanu, S.; Nissapatorn, V.; Pereira, M.L.; Paul, A.K.; Mitsuwan, W. Virulence Factors and Quorum Sensing as Targets of New Therapeutic Options by Plant-Derived Compounds against Bacterial Infections Caused by Human and Animal Pathogens. Vet. World 2023, 16, 1346–1355. [Google Scholar] [CrossRef]
- Díaz-Nuñez, J.L.; García-Contreras, R.; Castillo-Juárez, I. The New Antibacterial Properties of the Plants: Quo vadis Studies of Anti-Virulence Phytochemicals? Front. Microbiol. 2021, 12, 667126. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Song, G.; Sun, M.; Wang, J.; Wang, Y. Prevalence and Therapies of Antibiotic-Resistance in Staphylococcus aureus. Front. Cell Infect. Microbiol. 2020, 10, 107. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zuo, J.; Teng, J.; Yang, L.; Guo, J.; Liu, L.; Li, P. Antibiofilm Potential of Luteolin against Multidrug-Resistant Staphylococcus aureus Isolated from Dairy Goats and Farm Environments. Environ. Pollut. 2023, 335, 122274. [Google Scholar] [CrossRef]
- Neves, A.R.; Durães, F.; Freitas-Silva, J.; Szemerédi, N.; Martins-da-Costa, P.; Pinto, E.; Correia-da-Silva, M.; Spengler, G.; Sousa, E. Derivatives of Trimethoxybenzoic Acid and Gallic Acid as Potential Efflux Pump Inhibitors: In Silico and in Vitro Studies. Int. J. Mol. Sci. 2022, 23, 14468. [Google Scholar] [CrossRef]
- Alav, I.; Sutton, J.M.; Rahman, K.M. Role of Bacterial Efflux Pumps in Biofilm Formation. J. Antimicrob. Chemother. 2018, 73, 2003–2020. [Google Scholar] [CrossRef]
- Balcázar, J.L.; Subirats, J.; Borrego, C.M. The Role of Biofilms as Environmental Reservoirs of Antibiotic Resistance. Front. Microbiol. 2015, 6, 1216. [Google Scholar] [CrossRef]
- Kaushik, A.; Kest, H.; Sood, M.; Steussy, B.W.; Thieman, C.; Gupta, S. Biofilm Producing Methicillin-Resistant Staphylococcus aureus (MRSA) Infections in Humans: Clinical Implications and Management. Pathogens 2024, 13, 76. [Google Scholar] [CrossRef]
- Rather, M.A.; Gupta, K.; Mandal, M. Microbial Biofilm: Formation, Architecture, Antibiotic Resistance, and Control Strategies. Braz. J. Microbiol. 2021, 52, 1701–1718. [Google Scholar] [CrossRef]
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33, e00181. [Google Scholar] [CrossRef] [PubMed]
- Craft, K.M.; Nguyen, J.M.; Berg, L.J.; Townsend, S.D. Methicillin-Resistant: Staphylococcus aureus (MRSA): Antibiotic-Resistance and the Biofilm Phenotype. MedChemComm 2019, 10, 1231–1241. [Google Scholar] [CrossRef] [PubMed]
- Rather, M.A.; Gupta, K.; Bardhan, P.; Borah, M.; Sarkar, A.; Eldiehy, K.S.H.; Bhuyan, S.; Mandal, M. Microbial Biofilm: A Matter of Grave Concern for Human Health and Food Industry. J. Basic Microbiol. 2021, 61, 380–395. [Google Scholar] [CrossRef]
- Abdallah, M.; Benoliel, C.; Drider, D.; Dhulster, P.; Chihib, N.E. Biofilm Formation and Persistence on Abiotic Surfaces in the Context of Food and Medical Environments. Arch. Microbiol. 2014, 196, 453–472. [Google Scholar] [CrossRef] [PubMed]
- Chadha, J.; Harjai, K.; Chhibber, S. Repurposing Phytochemicals as Anti-Virulent Agents to Attenuate Quorum Sensing-Regulated Virulence Factors and Biofilm Formation in Pseudomonas aeruginosa. Microb. Biotechnol. 2022, 15, 1695–1718. [Google Scholar] [CrossRef]
- Kincses, A.; Ghazal, T.S.A.; Veres, K.; Spengler, G.; Hohmann, J. Phenolic Compounds from Origanum majorana with Biofilm-Inhibitory Activity against Methicillin-Resistant Staphylococcus aureus and Escherichia coli Strains. Pharm. Biol. 2025, 63, 402–410. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, C.; Zhou, Y.; Hu, T.; Zhang, Y.; Lv, X.; Li, J.; Zhou, Y. Discovery of Potential Anti-Microbial Molecules and Spectrum Correlation Effect of Ardisia crenata Sims via High-Performance Liquid Chromatography Fingerprints and Molecular Docking. Molecules 2024, 29, 1178. [Google Scholar] [CrossRef]
- Miyamoto, T.; Zhang, X.; Ueyama, Y.; Apisada, K.; Nakayama, M.; Suzuki, Y.; Ozawa, T.; Mitani, A.; Shigemune, N.; Shimatani, K.; et al. Development of Novel Monoclonal Antibodies Directed against Catechins for Investigation of Antibacterial Mechanism of Catechins. J. Microbiol. Methods 2017, 137, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Matilla-Cuenca, L.; Gil, C.; Cuesta, S.; Rapún-Araiz, B.; Žiemytė, M.; Mira, A.; Lasa, I.; Valle, J. Antibiofilm Activity of Flavonoids on Staphylococcal Biofilms through Targeting BAP Amyloids. Sci. Rep. 2020, 10, 18968. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.-Y.; Xiao, K.; Zhang, J.-Q.; Zhong, K.; Grosu, E.; Gao, Z.; Wu, Y.-P.; Gao, H. Antibacterial and Antibiofilm Effects of Zanthoxylum bungeanum Leaves against Staphylococcus aureus. Nat. Prod. Commun. 2018, 13, 1001–1006. [Google Scholar] [CrossRef]
- Silva, L.N.; Da Hora, G.C.A.; Soares, T.A.; Bojer, M.S.; Ingmer, H.; Macedo, A.J.; Trentin, D.S. Myricetin Protects Galleria mellonella against Staphylococcus aureus Infection and Inhibits Multiple Virulence Factors. Sci. Rep. 2017, 7, 2823. [Google Scholar] [CrossRef]
- Qian, W.; Liu, M.; Fu, Y.; Zhang, J.; Liu, W.; Li, J.; Li, X.; Li, Y.; Wang, T. Antimicrobial Mechanism of Luteolin against Staphylococcus aureus and Listeria monocytogenes and Its Antibiofilm Properties. Microb. Pathog. 2020, 142, 104056. [Google Scholar] [CrossRef]
- Onmaz, N.E.; Yilmaz, D.D.; Imre, K.; Morar, A.; Gungor, C.; Yilmaz, S.; Gundog, D.A.; Dishan, A.; Herman, V.; Gungor, G. Green Synthesis of Gold Nanoflowers Using Rosmarinus officinalis and Helichrysum italicum Extracts: Comparative Studies of Their Antimicrobial and Antibiofilm Activities. Antibiotics 2022, 11, 1466. [Google Scholar] [CrossRef]
- Bezek, K.; Kramberger, K.; Barlič-Maganja, D. Antioxidant and Antimicrobial Properties of Helichrysum italicum (Roth) G. Don Hydrosol. Antibiotics 2022, 11, 1017. [Google Scholar] [CrossRef]
- Soltanbeigi, E.; Isitez, N.; Erdogmus, S.F.; Ozliman, S. Helichrysum italicum (Roth) G. Don Essential Oil: Composition and Potential Anti-Tumour, Anti-Inflammatory, Antimicrobial, and Antibiofilm Effects. J. Biol. Act. Prod. Nat. 2025, 15, 353–368. [Google Scholar] [CrossRef]
- Willdigg, J.R.; Helmann, J.D. Mini Review: Bacterial Membrane Composition and Its Modulation in Response to Stress. Front. Mol. Biosci. 2021, 8, 634438. [Google Scholar] [CrossRef] [PubMed]
- Cui, H.; Li, W.; Li, C.; Lin, L. Synergistic Effect between Helichrysum italicum Essential Oil and Cold Nitrogen Plasma against Staphylococcus aureus Biofilms on Different Food-Contact Surfaces. Int. J. Food Sci. Technol. 2016, 51, 2493–2501. [Google Scholar] [CrossRef]
- Coimbra, A.; Miguel, S.; Ribeiro, M.; Coutinho, P.; Silva, L.; Duarte, A.P.; Ferreira, S. Thymus zygis Essential Oil: Phytochemical Characterization, Bioactivity Evaluation and Synergistic Effect with Antibiotics against Staphylococcus aureus. Antibiotics 2022, 11, 146. [Google Scholar] [CrossRef] [PubMed]
- Asensio, C.M.; Quiroga, P.R.; Al-Gburi, A.; Huang, Q.; Grosso, N.R. Rheological Behavior, Antimicrobial and Quorum Sensig Inhibition Study of an Argentinean Oregano Essential Oil Nanoemulsion. Front. Nutr. 2020, 7, 569913. [Google Scholar] [CrossRef]
- Stepanović, S.; Ćirković, I.; Ranin, L.; Švabić-Vlahović, M. Biofilm Formation by Salmonella spp. and Listeria monocytogenes on Plastic Surface. Lett. Appl. Microbiol. 2004, 38, 428–432. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).