Assessing Peripheral Blood Biomarkers and Predictive Patterns in Multiple Sclerosis Using Cytokines and Immune Gene Expression Profiles in Ocrelizumab-Treated Patients: Tracking Tumor Necrosis Factor
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Isolation of PBMCs
4.3. RNA Extraction and RT-qPCR Analysis
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MS | multiple sclerosis |
| RRMS | relapsing–remitting multiple sclerosis |
| PBMCs | peripheral blood mononuclear cells |
| IL | interleukin |
| TNF | tumor necrosis factor |
| EDA | evidence of disease activity |
| CNS | central nervous system |
| DMT | disease-modifying therapies |
| CD20 | cluster of differentiation 20 |
| MRI | magnetic resonance imaging |
| ARR | annualized relapse rate |
| CDP | confirmed disability progression |
| HC | healthy control |
| BL | baseline |
| EDSS | Expanded Disability Status Scale |
| SD | standard deviation |
| SEM | standard error of measurement |
| IQR | interquartile range |
| Breg | regulatory B cells |
| GM-CSF | granulocyte–macrophage colony-stimulating factor |
References
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple sclerosis. Nat. Rev. Dis. Primers 2018, 4, 43. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B.; Barkhof, F.; et al. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef]
- He, A.; Merkel, B.; Brown, J.W.L.; Zhovits, R.L.; Kister, I.; Malpas, C.B.; Sharmin, S.; Horakova, D.; Kubala, H.E.; Spelman, T.; et al. Timing of high-efficacy therapy for multiple sclerosis: A retrospective observational cohort study. Lancet Neurol. 2020, 19, 307–316. [Google Scholar] [CrossRef]
- Guger, M.; Enzinger, C.; Leutmezer, F.; Di Pauli, F.; Kraus, J.; Kalcher, S.; Kvas, E.; Berger, T. Early intensive versus escalation treatment in patients with relapsing-remitting multiple sclerosis in Austria. J. Neurol. 2024, 271, 3142–3152. [Google Scholar] [CrossRef]
- Freeman, L.; Longbrake, E.E.; Coyle, P.K.; Hendin, B.; Vollmer, T. High-efficacy therapies for treatment-naïve individuals with relapsing-remitting multiple sclerosis. CNS Drugs 2022, 36, 1285–1299. [Google Scholar] [CrossRef]
- de Sèze, J.; Maillart, E.; Gueguen, A.; Laplaud, D.A.; Michel, L.; Thouvenot, E.; Zephir, H.; Zimmer, L.; Biotti, D.; Liblau, R. Anti-CD20 therapies in multiple sclerosis: From pathology to the clinic. Front. Immunol. 2023, 14, 1004795. [Google Scholar] [CrossRef] [PubMed]
- Selmaj, K.; Cree, B.A.C.; Barnett, M.; Thompson, A.; Hartung, H.P. Multiple sclerosis: Time for early treatment with high-efficacy drugs. J. Neurol. 2024, 271, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Montalban, X.; Gold, R.; Thompson, A.J.; Otero-Romero, S.; Amato, M.P.; Chandraratna, D.; Clanet, M.; Comi, G.; Derfuss, T.; Fazekas, F.; et al. ECTRIMS/EAN guideline on the pharmacological treatment of people with multiple sclerosis. Mult. Scler. 2018, 24, 96–120, Erratum in Mult. Scler. 2020, 26, 517. [Google Scholar] [CrossRef]
- Filippi, M.; Amato, M.P.; Centonze, D.; Gallo, P.; Gasperini, C.; Inglese, M.; Patti, F.; Pozzilli, C.; Preziosa, P.; Trojano, M. Early use of high-efficacy disease-modifying therapies makes the difference in people with multiple sclerosis: An expert opinion. J. Neurol. 2022, 269, 5382–5394, Erratum in J. Neurol. 2022, 269, 6690–6691. [Google Scholar] [CrossRef] [PubMed]
- Hauser, S.L.; Bar-Or, A.; Comi, G.; Giovannoni, G.; Hartung, H.P.; Hemmer, B.; Lublin, F.; Montalban, X.; Rammohan, K.W.; Selmaj, K.; et al. Ocrelizumab versus interferon beta-1a in relapsing multiple sclerosis. N. Engl. J. Med. 2017, 376, 221–234. [Google Scholar] [CrossRef]
- Kaskow, B.J.; Baecher-Allan, C. Effector T cells in multiple sclerosis. Cold Spring Harb. Perspect. Med. 2018, 8, a029025. [Google Scholar] [CrossRef]
- Cencioni, M.T.; Mattoscio, M.; Magliozzi, R.; Bar-Or, A.; Muraro, P.A. B cells in multiple sclerosis: From targeted depletion to immune reconstitution therapies. Nat. Rev. Neurol. 2021, 17, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Gharibi, T.; Babaloo, Z.; Hosseini, A.; Marofi, F.; Ebrahimi-Kalan, A.; Jahandideh, S.; Baradaran, B. The role of B cells in the immunopathogenesis of multiple sclerosis. Immunology 2020, 160, 325–335. [Google Scholar] [CrossRef]
- Arneth, B.M. Impact of B cells to the pathophysiology of multiple sclerosis. J. Neuroinflam. 2019, 16, 128. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Zhang, H.; Liu, Y.; Sun, B.; Mu, G. Global perspectives on the contribution of B cells to multiple sclerosis. Front. Immunol. 2024, 15, 1442694. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Patterson, K.R.; Bar-Or, A. Reassessing B cell contributions in multiple sclerosis. Nat. Immunol. 2018, 19, 696–707. [Google Scholar] [CrossRef]
- Baecher-Allan, C.; Kaskow, B.J.; Weiner, H.L. Multiple sclerosis: Mechanisms and immunotherapy. Neuron 2018, 97, 742–768. [Google Scholar] [CrossRef]
- Margoni, M.; Preziosa, P.; Filippi, M.; Rocca, M.A. Anti-CD20 therapies for multiple sclerosis: Current status and future perspectives. J. Neurol. 2022, 269, 1316–1334. [Google Scholar] [CrossRef]
- Meyer, S.; Evers, M.; Jansen, J.H.M.; Buijs, J.; Broek, B.; Reitsma, S.E.; Moerer, P.; Amini, M.; Kretschmer, A.; Ten Broeke, T.; et al. New insights in type I and II CD20 antibody mechanisms-ofaction with a panel of novel CD20 antibodies. Br. J. Haematol. 2018, 180, 808–820. [Google Scholar] [CrossRef]
- Montalvao, F.; Garcia, Z.; Celli, S.; Breart, B.; Deguine, J.; Van Rooijen, N.; Bousso, P. The mechanism of anti-CD20-mediatedB cell depletion revealed by intravital imaging. J. Clin. Investig. 2013, 123, 5098–5103. [Google Scholar] [CrossRef]
- Yang, C.S.; Yang, L.; Li, T.; Zhang, D.Q.; Jin, W.N.; Li, M.S.; Su, N.; Zhangning, N.; Liu, Q.; Shao, Z.H.; et al. Responsiveness to reduced dosage of rituximab in Chinese patients with neuromyelitis optica. Neurology 2013, 81, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Wolinsky, J.S.; Montalban, X.; Hauser, S.L.; Kappos, L.; Julian, L.; Manfrini, M.; Belachew, S.; Model, F.; Hubeaux, S.; Bar-Or, A. Prespecified subgroup analyses of ocrelizumab efficacy in primary progressive multiple sclerosis. CMSC 2018, DX42. [Google Scholar]
- Hauser, S.L.; Cree, B.A.C. Treatment of multiple sclerosis: A review. Am. J. Med. 2020, 133, 1380–1392.e2. [Google Scholar] [CrossRef] [PubMed]
- Montalban, X.; Hauser, S.L.; Kappos, L.; Arnold, D.L.; Bar-Or, A.; Comi, G.; de Seze, J.; Giovannoni, G.; Hartung, H.P.; Hemmer, B.; et al. Ocrelizumab versus placebo in primary progressive multiple sclerosis. N. Engl. J. Med. 2017, 376, 209–220. [Google Scholar] [CrossRef]
- Frampton, J.E. Ocrelizumab: First global approval. Drugs 2017, 77, 1035–1041. [Google Scholar] [CrossRef] [PubMed]
- Frisch, E.S.; Pretzsch, R.; Weber, M.S. A milestone in multiple sclerosis therapy: Monoclonal antibodies against CD20. Neurotherapeutics 2021, 18, 1602–1622. [Google Scholar] [CrossRef]
- Butzkueven, H.; Arkelsten, S.; Comi, G.; Costello, K.; Devlin, M.; Drulovic, J.; Gray, E.; Haartsen, J.; Helme, A.; Hlavácová, J.; et al. Brain health-time matters: Multiple sclerosis, neuromyelitis optica spectrum disorder (NMOSD), myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) and related conditions. 2024 report. Mult. Scler. Relat. Disord. 2025, 101, 106456. [Google Scholar] [CrossRef]
- Ziemssen, T.; Derfuss, T.; de Stefano, N.; Giovannoni, G.; Palavra, F.; Tomic, D.; Wollmer, T.; Schippling, S. Optimizing treatment success in multiple sclerosis. J. Neurol. 2016, 263, 1053–1065. [Google Scholar] [CrossRef]
- Petro, T.M.; Chen, S.S.; Panther, R.B. Effect of CD80 and CD86 on T cell cytokine production. Immunol. Investig. 1995, 24, 965–976. [Google Scholar] [CrossRef]
- Lim, T.S.; Goh, J.K.; Mortellaro, A.; Lim, C.T.; Hämmerling, G.J.; Ricciardi-Castagnoli, P. CD80 and CD86 differentially regulate mechanical interactions of T-cells. PLoS ONE 2012, 7, e45185. [Google Scholar] [CrossRef]
- Jeannin, P.; Magistrelli, G.; Aubry, J.P.; Caron, G.; Gauchat, J.F.; Renno, T.; Herbault, N.; Goetsch, L.; Blaecke, A.; Dietrich, P.Y.; et al. Soluble CD86 is a costimulatory molecule for human T lymphocytes. Immunity 2000, 13, 303–312. [Google Scholar] [CrossRef]
- Shang, Z.; Sun, W.; Zhang, M.; Xu, L.; Jia, X.; Zhang, R.; Fu, S. Identification of key genes associated with multiple sclerosis. PeerJ 2020, 8, e8357. [Google Scholar] [CrossRef] [PubMed]
- Duddy, M.; Niino, M.; Adatia, F.; Hebert, S.; Freedman, M.; Atkins, H.; Kim, H.J.; Bar-Or, A. Distinct effector cytokine profiles of memory and naive B cell subsets. J. Immunol. 2007, 178, 6092–6099. [Google Scholar] [CrossRef] [PubMed]
- Knippenberg, S.; Peelen, E.; Smolders, J.; Thewissen, M.; Menheere, P.; Cohen Tervaert, J.W.; Hupperts, R.; Damoiseaux, J. Reduction in IL-10 producing B cells in multiple sclerosis. J. Neuroimmunol. 2011, 239, 80–86. [Google Scholar] [CrossRef]
- Fernández-Velasco, J.I.; Kuhle, J.; Monreal, E.; Meca-Lallana, V.; Meca-Lallana, J.; Izquierdo, G.; Gascón-Giménez, F.; de la Maza, S.S.; Walo-Delgado, P.E.; Maceski, A.; et al. Effect of ocrelizumab in blood leukocytes of patients with primary progressive MS. Neurol. Neuroimmunol. Neuroinflamm. 2021, 8, e940. [Google Scholar] [CrossRef] [PubMed]
- Andretto, V.; Dusi, S.; Zilio, S.; Repellin, M.; Kryza, D.; Ugel, S.; Lollo, G. Tackling TNF-α in autoinflammatory and autoimmune diseases. Adv. Drug Deliv. Rev. 2023, 201, 115080. [Google Scholar] [CrossRef]
- Mazziotti, V.; Crescenzo, F.; Turano, E.; Guandalini, M.; Bertolazzo, M.; Ziccardi, S.; Virla, F.; Camera, V.; Marastoni, D.; Tamanti, A.; et al. Contribution of tumor necrosis factor to multiple sclerosis progression. J. Neuroinflam. 2024, 21, 209. [Google Scholar] [CrossRef]
- Gold, R.; Linington, C.; Lassmann, H. Pathogenesis and therapy via animal models of MS. Brain 2006, 129, 1953–1971. [Google Scholar] [CrossRef]
- van Oosten, B.W.; Barkhof, F.; Truyen, L.; Boringa, J.B.; Bertelsmann, F.W.; von Blomberg, B.M.; Woody, J.N.; Hartung, H.P.; Polman, C.H. Increased MRI activity in MS patients treated with anti-TNF antibody. Neurology 1996, 47, 1531–1534. [Google Scholar] [CrossRef]
- Wiendl, H.; Neuhaus, O.; Kappos, L.; Hohlfeld, R. Failed and discontinued clinical trials in MS therapy. Nervenarzt 2000, 71, 597–610. [Google Scholar] [CrossRef]
- Selmaj, K.; Raine, C.S.; Cannella, B.; Brosnan, C.F. Identification of lymphotoxin and TNF in MS lesions. J. Clin. Investig. 1991, 87, 949–954. [Google Scholar] [CrossRef]
- Domingues, R.B.; Fernandes, G.B.P.; Leite, F.B.V.M.; Tilbery, C.P.; Thomaz, R.B.; Silva, G.S.; Mangueira, C.L.P.; Soares, C.A.S. The cerebrospinal fluid in multiple sclerosis. Einstein 2017, 15, 100–104. [Google Scholar] [CrossRef] [PubMed]
- Drulovic, J.; Mostarica-Stojkovic, M.; Levic, Z.; Stojsavljevic, N.; Pravica, V.; Mesaros, S. Interleukin-12 and tumor necrosis factor-alpha levels in cerebrospinal fluid of multiple sclerosis patients. J. Neurol. Sci. 1997, 147, 145–150. [Google Scholar] [CrossRef]
- Ribeiro, C.M.; Oliveira, S.R.; Alfieri, D.F.; Flauzino, T.; Kaimen-Maciel, D.R.; Simão, A.N.C.; Maes, M.; Reiche, E.M.V. TNF-α and soluble receptors in multiple sclerosis. Inflamm. Res. 2019, 68, 1049–1059. [Google Scholar] [CrossRef]
- Welser-Alves, J.V.; Milner, R. Microglia are the major source of TNF-α and TGF-β1. Neurochem. Int. 2013, 63, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Liese, A.M.; Siddiqi, M.Q.; Siegel, J.H.; Denny, T.; Spolarics, Z. Augmented TNF-α and IL-10 production by primed monocytes. J. Leukoc. Biol. 2001, 70, 289–296. [Google Scholar] [CrossRef]
- Lassmann, H.; Brück, W.; Lucchinetti, C.F. The immunopathology of multiple sclerosis. Brain Pathol. 2007, 17, 210–218. [Google Scholar] [CrossRef]
- Amin, M.; Hersh, C.M. Updates and advances in MS neurotherapeutics. Neurodegener. Dis. Manag. 2023, 13, 47–70. [Google Scholar] [CrossRef] [PubMed]
- Stampanoni Bassi, M.; Drulovic, J.; Pekmezovic, T.; Iezzi, E.; Sica, F.; Gilio, L.; Gentile, A.; Musella, A.; Mandolesi, G.; Furlan, R.; et al. Cerebrospinal fluid inflammatory biomarkers predicting interferon-beta response in MS patients. Ther. Adv. Neurol. Disord. 2020, 13, 1756286420970833. [Google Scholar] [CrossRef]
- Mathey, G.; Epstein, J.; Alix, T.; Julien, M.; Nisse, E.; Pittion-Vouyovitch, S.; Prunis, C.; Malaplate, C. Cerebrospinal fluid levels of chitinase 3-like 1 and interleukin-6 can predict response to platform therapies in relapsing multiple sclerosis. J. Neurol. 2025, 272, 592. [Google Scholar] [CrossRef]
- Vilaseca, A.; Urcelay, E.; Malhotra, S.; Castillo, M.; Aroca, M.; Vidal-Jordana, A.; Pappolla, A.; Carvajal, R.; Arrambide, G.; González-Jiménez, A.; et al. The Variant rs7665090 Is Associated with Interferon-Beta Response in Multiple Sclerosis Patients. Eur. J. Neurol. 2025, 32, e70227. [Google Scholar] [CrossRef]
- Zhang, B.C.; Schneider-Hohendorf, T.; Elyanow, R.; Pignolet, B.; Falk, S.; Wünsch, C.; Deffner, M.; Yusko, E.; May, D.; Mattox, D.; et al. HLA-A∗03:01 as predictive genetic biomarker for glatiramer acetate treatment response in multiple sclerosis: A retrospective cohort analysis. EBioMedicine 2025, 118, 105873. [Google Scholar] [CrossRef]
- Ocrevus 300 mg Concentrate for Solution for Infusion: Summary of Product Characteristics. Available online: https://www.ema.europa.eu/en/documents/product-information/ocrevus-epar-product-information_en.pdf (accessed on 1 June 2022).
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: EDSS. Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.J.; Banwell, B.L.; Barkhof, F.; Carroll, W.M.; Coetzee, T.; Comi, G.; Correale, J.; Fazekas, F.; Filippi, M.; Freedman, M.S.; et al. Diagnosis of multiple sclerosis: 2017 revisions. Lancet Neurol. 2018, 17, 162–173. [Google Scholar] [CrossRef] [PubMed]
- Giovannoni, G.; Tomic, D.; Bright, J.R.; Havrdová, E. “No evident disease activity” in MS management. Mult. Scler. 2017, 23, 1179–1187. [Google Scholar] [CrossRef] [PubMed]
- Stegnjaic, G.; Tsiailanis, A.D.; Lazarevic, M.; Gkalpinos, V.K.; Djedovic, N.; Antoniou, T.; Stanisavljevic, S.; Dimitrijevic, M.; Momcilovic, M.; Miljkovic, D.J.; et al. Phenethyl ester of gallic acid ameliorates EAE. Molecules 2022, 27, 8770. [Google Scholar] [CrossRef]
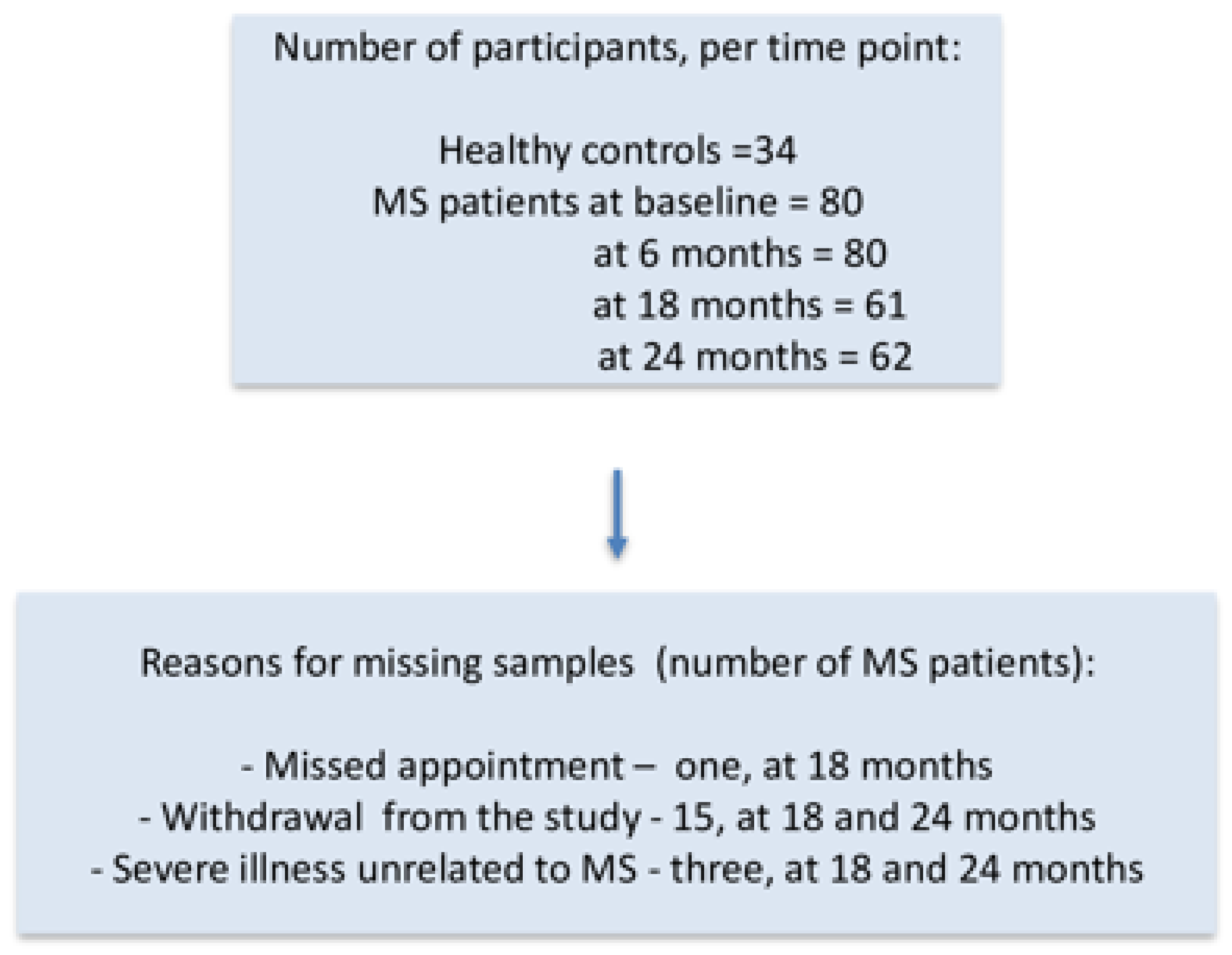
| Variables | RRMS (n = 35) | PPMS (n = 45) |
|---|---|---|
| Sex (number, %) | ||
| -Male | 11 (31.4%) | 9 (20%) |
| -Female | 24 (68.6%) | 36 (80%) |
| Age at the beginning of the ocrelizumab treatment (years) (mean ± SD) | 40.9 ± 8.7 | 44.7 ± 10.0 |
| Duration of disease (years) (mean ± SD) | 11.7 ± 7.2 | 8.6 ± 5.7 |
| EDSS score at the beginning of the ocrelizumab treatment (median, IQR) | 4.0 (2.5) | 6.0 (2.0) |
| Relapses in the year prior to the ocrelizumab treatment (number, %) | ||
| -Yes | 30 (85.7%) | / |
| -No | 5 (14.3%) | / |
| Active MRI lesions at the beginning of the ocrelizumab treatment (number, %) | ||
| -Yes | 10 (28.6%) | 5 (11.1%) |
| -No | 25 (71.4%) | 40 (88.9%) |
| Molecules | MS Patients Treated with Ocrelizumab | Healthy Controls | p | ||||
|---|---|---|---|---|---|---|---|
| Baseline | After 6 Months | After 12 Months | After 18 Months | After 24 Months | |||
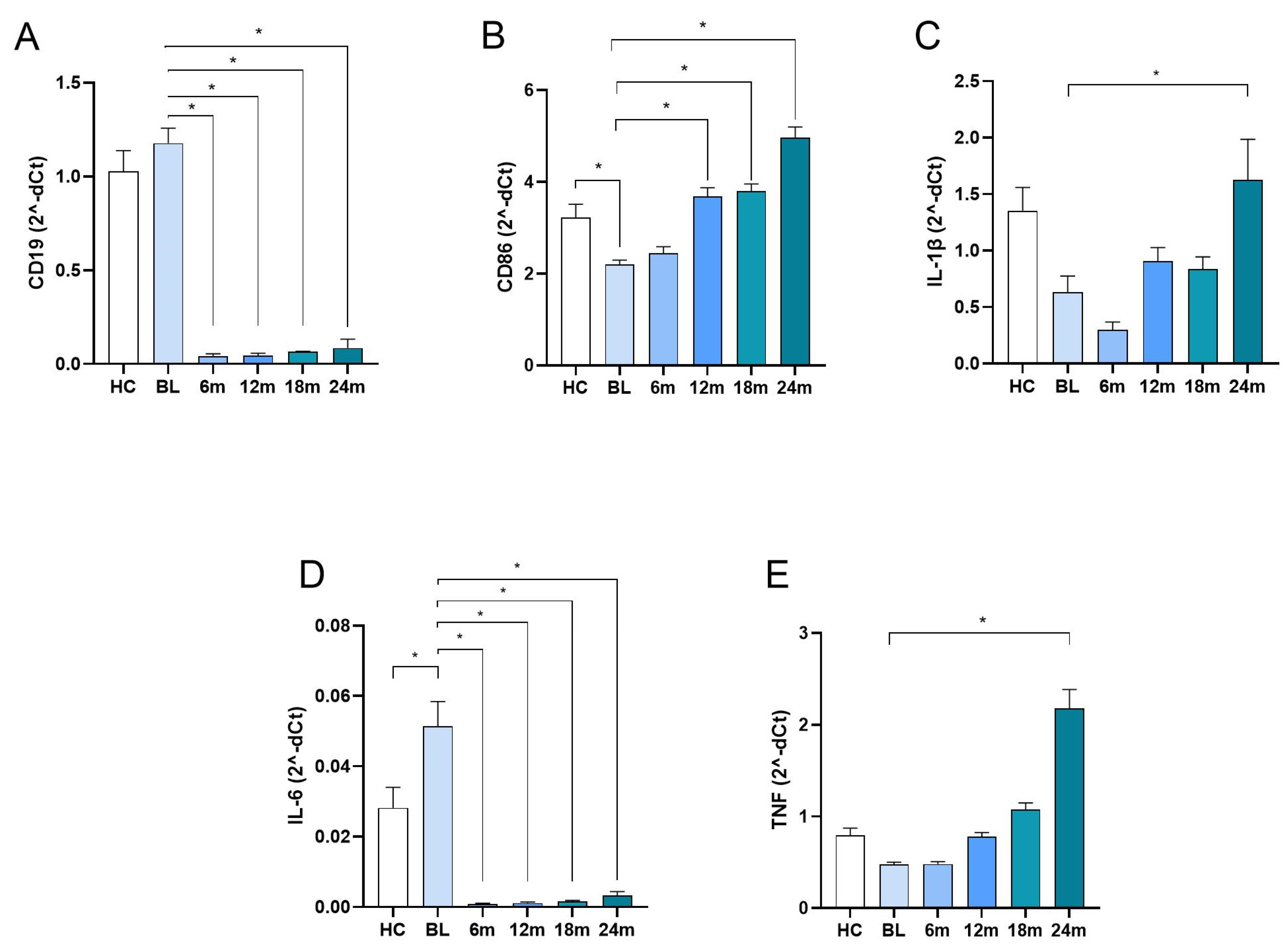
| CD19 | 1.177 ± 0.716 | 0.042 ± 0.107 | 0.044 ± 0.103 | 0.066 ± 0.016 | 0.085 ± 0.375 | 1.026 ± 0.649 | <0.001 1 |
| CD86 | 2.199 ± 0.852 | 2.449 ± 1.237 | 3.686 ± 1.542 | 3.798 ± 1.206 | 4.959 ± 1.876 | 3.227 ± 1.656 | <0.001 2 |
| IL1B | 0.631 ± 1.225 | 0.298 ± 0.615 | 0.905 ± 0.998 | 0.834 ± 0.851 | 1.625 ± 2.838 | 1.353 ± 1.196 | <0.001 3 |
| IL6 | 0.051 ± 0.063 | 0.001 ± 0.001 | 0.001 ± 0.295 | 0.002 ± 0.009 | 0.003 ± 0.368 | 0.028 ± 0.034 | <0.001 1 |
| TNF | 0.478 ± 0.203 | 0.480 ± 0.234 | 0.780 ± 0.365 | 1.0759 ± 0.560 | 2.178 ± 1.635 | 0.792 ± 0.472 | <0.001 4 |
| Variable | All Patients (Mean ± SD) | RRMS (Mean ± SD) | PPMS (Mean ± SD) | p |
|---|---|---|---|---|
| CD19 | ||||
| BL | 1.1170 ± 0.7163 | 1.2414 ± 0.9411 | 1.0200 ± 0.4637 | 0.172 |
| after 6 months | 0.0416 ± 0.1073 | 0.0399 ± 0.0726 | 0.0429 ± 0.1287 | 0.904 |
| after 12 months | 0.0440 ± 0.1031 | 0.0240 ± 0.4110 | 0.0589 ± 0.1302 | 0.193 |
| after 18 months | 0.0660 ± 0.1249 | 0.0389 ± 0.1016 | 0.0863 ± 0.1373 | 0.144 |
| after 24 months | 0.0851 ± 0.3745 | 0.0311 ± 0.0840 | 0.1296 ± 0.4990 | 0.307 |
| CD86 | ||||
| BL | 2.1949 ± 0.8591 | 2.1770 ± 0.9535 | 2.2082 ± 0.7887 | 0.876 |
| after 6 months | 2.4486 ± 1.2369 | 2.3634 ± 1.1103 | 2.5147 ± 1.3356 | 0.591 |
| after 12 months | 3.6857 ± 1.5417 | 3.9643 ± 1.6941 | 3.4458 ± 1.3763 | 0.172 |
| after 18 months | 3.7978 ± 1.2063 | 3.4866 ± 0.9775 | 4.0290 ± 1.3177 | 0.082 |
| after 24 months | 4.9589 ± 2.7263 | 4.4361 ± 2.4722 | 5.3893 ± 2.8838 | 0.173 |
| IL1B | ||||
| BL | 0.6309 ± 1.2254 | 0.5105 ± 1.1409 | 0.7149 ± 1.2876 | 0.487 |
| after 6 months | 0.2980 ± 0.6145 | 0.3211 ± 0.6191 | 0.2800 ± 0.6174 | 0.769 |
| after 12 months | 0.9048 ± 0.9982 | 0.8922 ± 0.9611 | 0.9156 ± 1.0426 | 0.925 |
| after 18 months | 0.8341 ± 0.8518 | 0.8865 ± 0.9931 | 0.7951 ± 0.7426 | 0.682 |
| after 24 months | 1.6249 ± 2.8376 | 1.3832 ± 1.2565 | 1.8239 ± 3.6746 | 0.547 |
| IL6 | ||||
| BL | 0.0514 ± 0.0629 | 0.0584 ± 0.0743 | 0.0459 ± 0.0526 | 0.382 |
| after 6 months | 0.0009 ± 0.0013 | 0.0011 ± 0.0015 | 0.0008 ± 0.0011 | 0.343 |
| after 12 months | 0.0011 ± 0.0023 | 0.0007 ± 0.0017 | 0.0015 ± 0.0027 | 0.187 |
| after 18 months | 0.0015 ± 0.0029 | 0.0014 ± 0.0032 | 0.0016 ± 0.0028 | 0.809 |
| after 24 months | 0.0032 ± 0.0093 | 0.0024 ± 0.0073 | 0.0039 ± 0.0107 | 0.52 |
| TNF | ||||
| BL | 0.4776 ± 0.2027 | 0.4563 ± 0.1802 | 0.4862 ± 0.2188 | 0.663 |
| after 6 months | 0.4801 ± 0.2338 | 0.4880 ± 0.2369 | 0.4741 ± 0.2339 | 0.793 |
| after 12 months | 0.7801 ± 0.3648 | 0.8124 ± 0.3861 | 0.7522 ± 0.3485 | 0.505 |
| after 18 months | 1.0749 ± 0.5595 | 1.0015 ± 0.4972 | 2.3645 ± 1.7958 | 0.381 |
| after 24 months | 2.1783 ± 1.6354 | 1.9522 ± 1.4157 | 2.3645 ± 1.7958 | 0.327 |
| Variable | RRMS (n = 35) | PPMS (n = 45) | ||
|---|---|---|---|---|
| During First Year | During Second Year | During First Year | During Second Year | |
| Patients with relapses (no, %) | 2 (5.7%) | 3 (9.1%) | / | / |
| EDSS (median, IQR) | 4.0 (2.0) | 4.0 (3.0) | 6.0 (2.5) | 6.0 (2.5) |
| Patients with active, Gd+ MRI lesions (no, %) | 1 (2.9%) | 1 (3.1%) | 1 (2.4%) | 1 (2.6%) |
| Patients with new/enlarging T2w MRI lesions (no, %) | 3 (8.6%) | 4 (11.4%) | 2 (4.7%) | 2 (5.3%) |
| IL1B | TNF | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | p | OR | 95% CI | p | |
| Relapses | 4.20 | 0.62–41.62 | 0.999 | 1.29 | 0.07–22.62 | 0.618 |
| Disability progression | 0.94 | 0.36–2.51 | 0.908 | 4.93 | 1.70–14.26 | 0.003 |
| Gadolinium-enhancing lesions | 4.89 | 0.45–35.23 | 0.315 | 1.08 | 0.32–3.72 | 0.900 |
| New or enlarging T2 lesions | 6.06 | 1.44–25.56 | 0.014 | 1.35 | 0.46–4.28 | 0.799 |
| Combined unique active lesions | 5.13 | 1.21–21.84 | 0.027 | 1.25 | 0.36–4.33 | 0.722 |
| Evidence of disease activity | 1.76 | 0.67–4.67 | 0.253 | 3.34 | 1.27–8.80 | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jevtić, B.; Momcilovic, N.; Stegnjaić, G.; Lazarević, M.; Stanisavljević, S.; Tamas, O.; Veselinovic, N.; Budimkic, M.; Mesaros, S.; Miljković, Đ.; et al. Assessing Peripheral Blood Biomarkers and Predictive Patterns in Multiple Sclerosis Using Cytokines and Immune Gene Expression Profiles in Ocrelizumab-Treated Patients: Tracking Tumor Necrosis Factor. Int. J. Mol. Sci. 2025, 26, 11295. https://doi.org/10.3390/ijms262311295
Jevtić B, Momcilovic N, Stegnjaić G, Lazarević M, Stanisavljević S, Tamas O, Veselinovic N, Budimkic M, Mesaros S, Miljković Đ, et al. Assessing Peripheral Blood Biomarkers and Predictive Patterns in Multiple Sclerosis Using Cytokines and Immune Gene Expression Profiles in Ocrelizumab-Treated Patients: Tracking Tumor Necrosis Factor. International Journal of Molecular Sciences. 2025; 26(23):11295. https://doi.org/10.3390/ijms262311295
Chicago/Turabian StyleJevtić, Bojan, Nikola Momcilovic, Goran Stegnjaić, Milica Lazarević, Suzana Stanisavljević, Olivera Tamas, Nikola Veselinovic, Maja Budimkic, Sarlota Mesaros, Đorđe Miljković, and et al. 2025. "Assessing Peripheral Blood Biomarkers and Predictive Patterns in Multiple Sclerosis Using Cytokines and Immune Gene Expression Profiles in Ocrelizumab-Treated Patients: Tracking Tumor Necrosis Factor" International Journal of Molecular Sciences 26, no. 23: 11295. https://doi.org/10.3390/ijms262311295
APA StyleJevtić, B., Momcilovic, N., Stegnjaić, G., Lazarević, M., Stanisavljević, S., Tamas, O., Veselinovic, N., Budimkic, M., Mesaros, S., Miljković, Đ., Pekmezovic, T., Drulovic, J., & Nikolovski, N. (2025). Assessing Peripheral Blood Biomarkers and Predictive Patterns in Multiple Sclerosis Using Cytokines and Immune Gene Expression Profiles in Ocrelizumab-Treated Patients: Tracking Tumor Necrosis Factor. International Journal of Molecular Sciences, 26(23), 11295. https://doi.org/10.3390/ijms262311295









