Abstract
Developing cost-effective and durable electrocatalysts with high hydrogen evolution efficiency remains a critical challenge for sustainable energy conversion. Herein, spinel-type Co2CuO4 and Co3O4 nanosheet electrodes were fabricated directly on Ni foam via a simple electrodeposition route and evaluated for the alkaline hydrogen evolution reaction (HER) in 1.0 M KOH. Structural and surface analyses confirmed the formation of phase-pure, porous, and highly interconnected nanosheet architectures, where the substitution of Cu2+ into the Co3O4 lattice induced charge-redistribution and optimized the electronic configuration. The Co2CuO4 catalyst exhibited superior activity, requiring an overpotential of 127 mV to achieve 10 mA cm−2 with a corresponding Tafel slope of 61 mV dec−1, outperforming the Co3O4 catalyst (176 mV and 95 mV dec−1). This enhancement arises from improved intrinsic kinetics, higher turnover frequency, and reduced charge-transfer resistance, reflecting an increased density of active sites and enhanced interfacial conductivity. Furthermore, the Co2CuO4 catalyst maintained excellent stability for 100 h at both 10 and 500 mA cm−2, attributed to its strong adhesion and open nanosheet framework, which facilitates efficient gas release and electrolyte diffusion. These findings establish Co2CuO4 as a promising and durable HER electrocatalyst for alkaline water electrolysis.
1. Introduction
The global pursuit of carbon-neutral energy systems has accelerated the development of sustainable and efficient hydrogen production technologies [1,2,3]. Among various strategies, water electrolysis stands out as a clean and scalable route to generating high-purity hydrogen [4,5,6]. However, the overall efficiency of water splitting is largely limited by sluggish electrode kinetics, particularly during the oxygen evolution reaction under alkaline conditions [7,8]. Although Pt-based catalysts are the benchmark for the hydrogen evolution reaction (HER) owing to the near-zero overpotential and rapid kinetics, their scarcity and high cost hinder their use in practical, large-scale applications [9]. Therefore, intensive research efforts have been devoted to exploring earth-abundant transition-metal oxides as cost-effective HER electrocatalyst alternatives to noble-metal catalysts. Among these materials, spinel-type Co3O4 has gained significant attention due to its rich redox chemistry (Co2+/Co3+), structural stability, and tunable electronic properties [10,11,12,13,14]. However, the intrinsically poor electrical conductivity and limited exposure of catalytically active sites hinder rapid charge-transfer and proton reduction, leading to moderate HER performance [15]. To address these challenges, cationic substitution has emerged as an effective strategy to modulate the electronic structure, optimize active-site distribution, and enhance catalytic activity [16].
In particular, incorporating Cu2+ into the Co3O4 lattice forms Co2CuO4, a bimetallic spinel oxide that enhances electron delocalization, promotes multiple redox transitions (Cu+/Cu2+ and Co2+/Co3+), and facilitates efficient charge-transport across the catalyst-electrolyte interface [14,17,18]. In addition to compositional optimization, morphological engineering is a key determinant of electrochemical performance, as the catalytic HER activity of transition-metal oxides depends strongly on their shape, size, and structural dimensionality [19,20]. Controlling these features allows the modulation of surface atom exposure, defect density, and charge-migration pathways. Among the various morphologies explored, such as nanoparticles, bulk materials, and nanocubes, two-dimensional (2D) interconnected nanosheet networks have demonstrated superior activity due to their high surface-to-volume ratio, short ion/electron diffusion lengths, and abundant unsaturated edge sites [20,21,22]. Their porous and mechanically robust framework, featuring an open nanosheet architecture, facilitates rapid electrolyte penetration and efficient gas release, thereby enhancing electrolyte accessibility and ensuring strong structural stability during prolonged electrolysis [23,24]. Recent studies have demonstrated that directly grown Co2CuO4 catalysts on Ni foam exhibit remarkable electrocatalytic activity. However, despite these advances, a systematic comparison of Co2CuO4 with its parent oxide for HER remains scarce, leaving an important gap in understanding how Cu incorporation modifies the electronic configuration and charge-transfer dynamics during hydrogen evolution.
In this study, we report a comparative investigation of Co2CuO4 and Co3O4 nanosheet catalysts synthesized directly on Ni foam via a simple electrodeposition approach. Comprehensive structural and electrochemical analyses reveal that the introduction of Cu2+ ions into the Co3O4 lattice modulates the cationic arrangement and electron density within the Co–O lattice, thereby enhancing the charge-transport through the lattice. The optimized Co2CuO4 catalyst exhibits an overpotential of 127 mV at a cathodic current density of 10 mA cm−2 with corresponding Tafel slope of 61 mV dec−1. The Co2CuO4 catalyst exhibit consistently smaller potential response compared to the pristine Co3O4 catalyst and even with Cu-Co-based catalyst (Table S1) when vigorously examined under various current densities and demonstrates excellent chronopotentiometric endurance even at a high current density of 500 mA cm−2. The TOF and ECSA analyses provide compelling evidence that Co2CuO4 possesses faster intrinsic reaction kinetics and superior site efficiency relative to Co3O4. The open nanosheet morphology, extensive interfacial contact with the Ni foam substrate, and well-connected conductive network collectively facilitate rapid electron/ion transport, accelerating the hydrogen evolution under alkaline conditions. This work uniquely bridges the existing gap through a comprehensive structural-electronic-electrochemical correlation, revealing how Cu substitution optimizes the spinel lattice and accelerates HER kinetics. The binder-free growth of nanosheet arrays on Ni foam offers a scalable and efficient electrode architecture. The findings not only establish a mechanistic understanding of Cu-induced electronic modulation but also propose a universal, design-guided approach for developing next-generation transition-metal oxide catalysts for sustainable hydrogen production.
2. Results
2.1. Morphological and Compositional Properties of Co2CuO4 and Co3O4 Films
The formation of Co2CuO4 and Co3O4 films on Ni foam follows a sequential electrochemical deposition and thermally induced transformation process. During the electrodeposition stage, the applied cathodic potential drives the reduction in metal salt ions and induces local water hydrolysis, generating the hydroxide ions near the substrate surface. These hydroxide ions react with Co2+ and Cu2+ ions in the electrolyte to form a mixed metal hydroxide intermediate molecule, which progressively nucleates and grows into nanosheet assemblies, as described in Equations (1) and (2):
Co2+ + Cu2+ + 4 (OH)− → Co(OH)2↓ + Cu(OH)2↓,
2 Co(OH)2 + Cu(OH)2 → Co2CuO4↓,
Figure 1 shows the field emission scanning electron microscope (FE-SEM) images of Co2CuO4 and Co3O4 nanosheet films. The pure Co3O4 film (Figure 1a) exhibits a dense array of ultrathin crumpled nanosheets, which uniformly cover the surface of the Ni foam substrate. These nanosheets are vertically aligned and interlinked, forming a three-dimensional porous network with open inter-sheet voids ranging from approximately 50 to 600 nm. This highly interconnected wrinkled nanosheet framework facilitates rapid electrolyte penetration and exposes a large number of electrochemically active edge sites, which are beneficial for catalytic reactions. The wrinkled sheet-like morphology indicates a high degree of structural flexibility and mechanical stability [25]. The smooth surface of the nanosheets (Figure 1b) and uniform coverage across the substrate suggest a controlled nucleation-growth balance during the electrodeposition process. The Co2CuO4 film (Figure 1c) demonstrates a distinctly evolved nanosheet structure. The thickness of these nanosheets ranges from approximately 17 to 35 nm, and more distinct voids are observed between these nanosheets (Figure 1d), exhibiting well-developed wrinkles and interconnected edges that create a 3D honeycomb-like architecture. The larger lateral growth of nanosheets and the wider inter-sheet voids between observed in the Co2CuO4 film compared to the Co3O4 film is a result of altered nucleation kinetics and the surface energy distribution during the electrodeposition. The inter-sheet spacing increases to several hundred nanometers, forming visible open channels that may serve as efficient pathways for electrolyte diffusion and gas bubble release during electrocatalytic HERs.
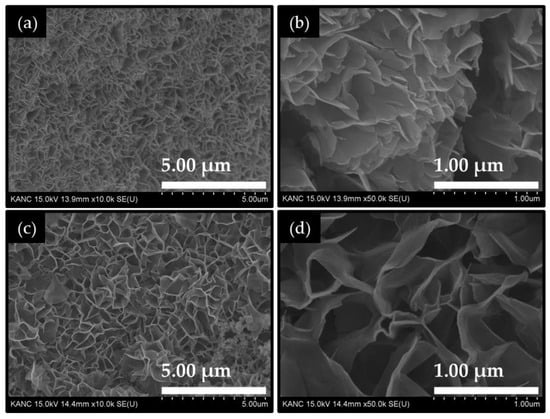
Figure 1.
FE-SEM images of the electrodeposited (a,b) Co3O4 and (c,d) Co2CuO4 films recorded at different magnifications.
Thereafter, to confirm the elemental composition and spatial distribution of the nanosheet films, energy-dispersive X-ray spectroscopy (EDX) coupled with FE-SEM was employed for both Co2CuO4 and Co3O4 samples (Figure S1). The Co2CuO4 nanosheet film displays distinct EDX peaks corresponding to Co, Cu, and O constituents, validating the successful formation of the desired material phase. The quantitative analysis reveals an approximate atomic ratio of Co:Cu ≈ 2:1, which aligns closely with the expected stoichiometry of the spinel Co2CuO4 structure. The oxygen signal remains consistent with that of a well-oxidized phase, indicating complete conversion of the hydroxide precursor during annealing. Whereas the EDX spectrum of the Co3O4 nanosheet film reveals the prominent presence of Co and oxygen O peaks, verifying the successful formation of the binary cobalt oxide phase. The absence of any extraneous signals indicates the high purity of the films, as no residual precursor elements or substrate contamination were detected. The relatively high oxygen content corresponds well to the stoichiometric ratio of Co:O ≈ 3:4, consistent with the formation of the spinel Co3O4 phase.
2.2. Crystallographic and Bonding Properties of Co2CuO4 and Co3O4 Films
The structural characteristics of the electrodeposited Co2CuO4 and Co3O4 films were investigated by X-ray diffraction (XRD) technique to identify phase formation and crystallinity of the Co-based lattices, and the corresponding patterns are presented in Figure 1a. The XRD spectra of the films exhibit three intense and well-defined diffraction peaks marked with a circle symbol corresponding to the (111), (200), and (220) planes of the nickel foam substrate along with a series of additional diffraction peaks. These substrate reflections are unavoidable due to the high porosity and metallic conductivity of Ni foam, which serves as both the structural backbone and the current collector during the electrodeposition. For the Co2CuO4 film, the major diffraction peaks appear at 2θ = 31.15°, 36.76°, 38.83°, 59.31°, and 65.19°, which correspond to the (220), (311), (222), (511), and (440) lattice planes of cubic Co2CuO4 (JCPDS No. 01-1155) [26]. The sharpness and intensity of these reflections confirm the high degree of crystallinity of the electrodeposited film and the successful formation of a single-phase spinel structure. Whereas when the copper precursor was omitted during electrodeposition, the diffraction pattern of the film changed distinctly, producing the typical reflections of cubic Co3O4 (JCPDS No. 76-1802) [27]. For the Co3O4 film, the diffraction peaks are located at 2θ = 31.17°, 36.83°, 59.42°, and 65.07°, which can be indexed to the (220), (311), (511), and (440) planes of the spinel Co3O4 phase. Furthermore, the spinel structure of both Co2CuO4 and Co3O4 belongs to the Fd m (227) space group, which offers structural robustness and isotropic electronic pathways. The absence of any extraneous peaks associated with Cu/Co(OH)2 or other sub-oxides indicates that Cu is homogeneously incorporated into the cobaltite lattice, confirming the phase purity of the electrodeposited Co2CuO4 and Co3O4 films. The diffraction peaks of the Co2CuO4 are slightly broadened compared to the Co3O4, which is a result of localized lattice strain arising from the homogeneous substitution of Cu into the Co spinel network. The average crystallite sizes were estimated to be approximately 22.27 nm for Co2CuO4 and 27.65 nm for Co3O4 with the help of the (311) diffraction peak using the Debye-Scherrer equation. The slight reduction in crystallite size in Co2CuO4 indicates that Cu substitution influences nucleation and growth, leading to limited crystal coarsening and subtle structural distortion within the spinel lattice. The structural contrast between Co2CuO4 and Co3O4 films demonstrates the vital role of cation composition in dictating the final spinel phase.
Raman spectroscopy was employed to further confirm the crystalline phases and probe structural and vibrational characteristics of the electrodeposited Co2CuO4 and Co3O4 films (Figure 2b). The Raman spectrum of Co2CuO4 displays five characteristic active modes consistent with the cubic spinel structure, including three F2g, one Eg, and one A1g modes [28]. The Raman peaks are centered at 189, 472, 515, 606, and 677 cm−1 corresponding to the F2g(3), Eg, F2g(2), F2g(1), and A1g(1) vibrational modes, respectively [28,29]. The intense A1g mode arises from the symmetric stretching of Co–O bonds involving octahedrally coordinated Co3+ cations, while the F2g and Eg modes originate from Co2+ ions in tetrahedral sites, confirming the dual-valence nature of the spinel framework. The Raman spectrum of the Co3O4 film also showcases the similar vibrational Raman modes compared to the Co2CuO4; however, the Raman peaks were slightly blue shifted accompanied by the narrowed Co2CuO4 Raman bands [30,31]. A comparative broadened band of Co2CuO4 than Co3O4 suggest the variation of Co–O bond lengths and symmetry due to the partial substitution of Cu2+ within the spinel network, which introduces the local strain, and modifies the electronic environment within the lattice. Both of which are beneficial for improving surface reactivity and charge-transfer efficiency during the catalytic HER. Therefore, together the XRD and the Raman analysis verify that the electrodeposition and subsequent annealing treatments successfully yield phase-pure spinel Co2CuO4 and Co3O4 with well-preserved crystallinity.
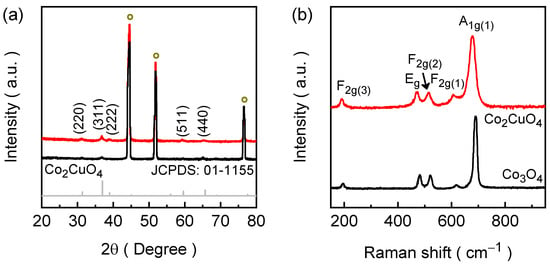
Figure 2.
(a) XRD spectra along with the standard JCPDS reference patter of Co2CuO4 and (b) Raman spectra of electrodeposited Co3O4 (black) and Co2CuO4 (red) films.
2.3. Chemical Bonding States of Co2CuO4 than Co3O4 Films
The survey spectra of Co2CuO4 and Co3O4 films (Figure 3a) reveal the presence of Co, Cu, and O without any additional peaks other than C, indicating the phase purity of the spinel oxide. The Gaussian curve fitting model was used to fit the narrow-scan emission spectra of the constituent elements. The Co 2p spectrum of Co2CuO4 (Figure 3b) shows two main peaks centered at 779.36 eV (Co 2p3/2) and 794.87 eV (Co 2p1/2) accompanied by characteristic satellite (Sat.) peaks at 788.37 and 804.62 eV [32,33]. After deconvolution, the Co 2p3/2 and Co 2p1/2 doublets were converted into the spin–orbit components arising from both Co3+ (778.98 and 794.16 eV) and Co2+ (780.57 and 795.91 eV) species with the spin energy separation of 15.18 eV, confirming the dual-valence Co state consistent with the spinel structure [32,34,35]. In the Cu 2p region (Figure 3c), two major peaks are observed at 932.31 and 952.35 eV, corresponding to Cu 2p3/2 and Cu 2p1/2 spin–orbit components, respectively, accompanied by two distinct shake-up satellite peaks located at approximately 943.75 and 963.59 eV, which are characteristic of Cu2+ species in octahedral coordination [36]. The slight asymmetry and broadening of the Cu 2p3/2 peak suggest the coexistence of a minor Cu+ component, indicating a mixed-valence Cu+/Cu2+ configuration that promotes redox reversibility and electronic conductivity within the spinel framework [37]. Figure 3d shows the O 1s spectrum of Co2CuO4, which was deconvoluted into three distinct components. The O1, O2, and O3 assigned peaks corresponds to the lattice oxygen (OL) at 529.67 eV, oxygen vacancy sites (Ov) at 530.52 eV, and chemisorbed water or oxygen associated with surface hydroxyl species (Oc) at 531.79 eV [38]. The intensity and spin–orbit separation of the Co 2p3/2 and Co 2p1/2 components along with the O 1s peak feature of pristine Co3O4 films are almost identical to those of the Co2CuO4 film. However, compared to the Co2CuO4, the Co 2p peaks of the Co3O4 exhibit a slight positive binding energy shift, indicating a redistribution of electron density around Co sites in Co2CuO4 due to the substitutional incorporation of Cu2+ within the spinel lattice [39]. This electronic modification facilitates charge redistribution and strengthens metal-oxygen covalency, ultimately contributing to improved electrocatalytic kinetics.
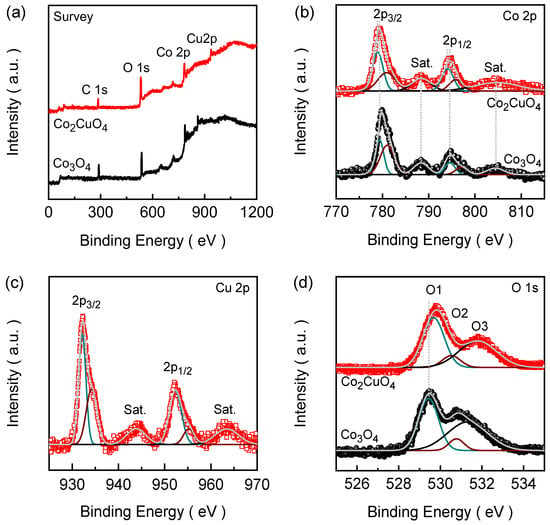
Figure 3.
XPS (a) Survey spectra; High-resolution (b) Co 2p; (c) Cu 2p; and (d) O 1s emission spectra. All high-resolution spectra were deconvoluted using Gaussian fitting to precisely evaluate the binding energies and corresponding oxidation states.
2.4. Electrochemical Properties of Co2CuO4 than Co3O4 Electrode Films
The HER activities of the Co2CuO4 and Co3O4 catalysts were assessed using linear sweep voltammetry (LSV) in 1.0 M KOH to elucidate the effect of the binary metal oxide catalysts on catalytic efficiency and durability in an alkaline KOH medium. As shown in Figure 4a, the iR-compensated polarization curves reveal a pronounced improvement in activity of the Co2CuO4 compared with the Co3O4 catalyst with a lower overpotential and a steeper current response. At a benchmark current density of 10 mA cm−2, the Co2CuO4 catalyst exhibits an overpotential of 127 mV, notably lower than that of pristine Co3O4 (176 mV). Although both catalysts display similar reaction features, the reduced potential in Co2CuO4 suggests improved charge-transport efficiency and enhanced accessibility of surface-active sites. The performance distinction between the Co2CuO4 and Co3O4 catalysts becomes more evident at higher current densities (Figure 4b), indicating differences in the charge-transfer kinetics and structural stability under the dynamic electrolysis conditions, which correlates well with their morphological features observed in the FE-SEM images (Figure 1). The Co2CuO4 achieves the current densities of 50, 100, and 500 mA cm−2 at an overpotentials of 181, 213, and 304 mV, respectively, while Co3O4 catalyst requires 252, 292, and 439 mV to drive the same current densities. The smaller potential increment of Co2CuO4 under dynamic load conditions indicates the better endurance during high-current operation and stronger electrode stability. This enhancement is associated with a comparatively open and interconnected nanosheet framework of Co2CuO4, which facilitates both electrolyte diffusion and charge transport. The intimate interface between the catalyst layer and the Ni foam substrate further ensures efficient electron/ion migration and mechanical integrity during sustained electrolysis.
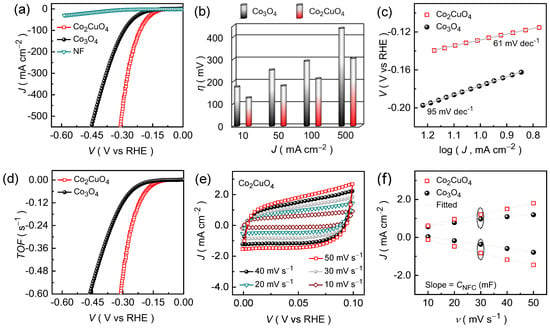
Figure 4.
Electrochemical HER activities of Co2CuO4 and Co3O4 catalysts evaluated in an alkaline 1.0 M KOH electrolyte medium. (a) LSV curves; (b) Overpotential as a function of current density; (c) Tafel slopes; and (d) TOF curves for Co2CuO4 and Co3O4 catalysts; (e) Non-Faradaic CV curves for Co2CuO4 and Co3O4 catalysts; and (f) CNFC plots for the estimation of ECSA of Co2CuO4 and Co3O4 catalysts.
To elucidate the underlying HER kinetics, the Tafel slope analysis was performed using the polarization curves. Figure 4c shows the obtained Tafel curves of the Co2CuO4 and Co3O4 catalysts. The measured Tafel slopes are 61 mV dec−1 for the Co2CuO4 catalyst and 95 mV dec−1 for Co3O4 catalyst, confirming the faster charge-transfer kinetics in the former Co2CuO4 catalyst. In alkaline media, the HER typically proceeds through a two-step sequence involving the adsorption of hydrogen intermediates followed by their subsequent desorption as molecular hydrogen from the catalyst surface. The reaction initiates with the Volmer step (theoretical Tafel slope = 120 mV dec−1), wherein water molecules are reduced at the active metal sites (M) to form hydroxide ions (OH−) and adsorbed hydrogen species (MHads):
M + H2O + e− → MHads + OH−,
The subsequent hydrogen evolution occurs through the electrochemical desorption process (Heyrovsky reaction, theoretical Tafel slope = 40 mV dec−1):
or through the chemical recombination (Tafel reaction, theoretical Tafel slope = 30 mV dec−1) step:
MHads + H2O + e− → M + OH− + H2,
2 MHads → 2 M + H2,
The experimentally measured Tafel slopes (61 and 95 mV dec−1) lie between the theoretical limits of the Volmer (120 mV dec−1) and Heyrovsky (40 mV dec−1) processes, suggesting that the HER kinetics on both catalysts predominantly follow the Volmer-Heyrovsky mechanism, where water dissociation and electrochemical desorption occurs sequentially [40]. The smaller Tafel slope of Co2CuO4 signifies a faster charge-transfer rate and a lower kinetic barrier for hydrogen release, implying that the electrochemical desorption (Heyrovsky step) acts as the rate-determining step. These kinetic trends are consistent with the LSV results and demonstrate the strong correlation between catalytic structure and HER activity, which is further supported by turnover frequency (TOF) analysis.
The intrinsic reaction kinetics of the Co2CuO4 and Co3O4 catalysts were further evaluated by estimating the TOF values, which reflect the activity of individual catalytic sites. The TOF values were calculated using the relation [41]:
where J represents the current density (A cm−2), A denotes the geometric area of the electrode (1 × 1 cm2), F signifies the Faraday constant (96,485 C mol−1), n designates the number of electrons transferred per hydrogen molecule (n = 2 for HER), and N corresponds to the number of moles of active catalyst loaded on the electrode. As shown in Figure 4d, the “V vs. TOF” plots clearly demonstrate that Co2CuO4 catalyst exhibits significantly higher TOF values across the entire potential range compared with the Co3O4 catalyst, indicating superior intrinsic activity per active site. The TOF values at an overpotential of 300 mV for Co2CuO4 was calculated to be approximately 0.504 s−1, which is nearly five-fold higher than that of pure Co3O4 (0.116 s−1) at the same potential. Moreover, the Co2CuO4 catalyst achieves a mass activity of 0.641 A mg−1, which is nearly 3.8-fold higher compared to the Co3O4 (0.169 A mg−1) catalyst at the same reference potential. This pronounced enhancement highlights the accelerated catalytic turnover capability of Co2CuO4, reflecting its improved charge-transport efficiency and higher density of accessible active sites, which are strongly linked with the open nanosheet architecture. The interconnected nanosheet framework offers numerous catalytically exposed sites and shorter ion diffusion pathways, while ensuring efficient electron transport through the continuous conductive network (Figure S2a and Table S2).
TOF = (J · A)/(n · N · F),
Thereafter, the electrochemically active surface area of the Co2CuO4 and Co3O4 catalysts was estimated from the non-faradaic capacitance (CNFC) values, obtained from cyclic voltammetry (CV, Figure 4e and Figure S2b) curves recorded at various scan rates within the non-faradaic potential window using the following relation:
where JNFC, v, CE represents the non-faradaic current density, scan rate, and KOH electrolyte capacitance [42]. The anodic and cathodic current densities were plotted as a function of scan rate (Figure 4f) and the slope of the resulting linear fit correspond to CNFC. The Co2CuO4 catalyst exhibits a considerably steeper slope compared to the Co3O4 catalyst, demonstrating the higher CNFC value of 31.33 mF cm−2 compared to the Co3O4 (~18.17 mF cm−2). Since ECSA is directly proportional to the non-faradaic capacitance, the larger CNFC of Co2CuO4 indicates a greater density of electrochemically accessible sites for catalytic HERs, attributed to its highly interconnected nanosheet architecture, which provides abundant active edges and open channels that promote rapid electrolyte infiltration and charge exchange. The higher ECSA of the Co2CuO4 catalyst facilitates the enhanced water molecule adsorption and the efficient charge accumulation at the electrode-electrolyte interface, thereby accelerating HER kinetics.
ECSA = CNFC/CE,
CNFC = JNFC/v,
Chronopotentiometric curves were recorded as a function of current density for the Co2CuO4 and Co3O4 catalysts (Figure 5a) to further assess their catalytic robustness and dynamic stability under an alkaline KOH medium. The applied cathodic current density was systematically increased from 10 to 50 mA cm−2 with a step of 10 mA cm−2 and subsequently raised in steps of 100 mA cm−2 up to 500 mA cm−2. Interestingly, both catalysts display steady potential response during continuous operation, demonstrating excellent stability under varying current loads. The Co2CuO4 catalyst consistently sustains lower potential values throughout the entire current range than the Co3O4 catalysts, confirming the enhanced catalytic efficiency and superior endurance even at a high-current HER evaluation. The nearly static voltage response at each step without noticeable degradation highlights the strong interfacial adhesion between the catalyst layer and the Ni foam substrate, ensuring mechanical and electrochemical stability under vigorous hydrogen gas evolution conditions. The linear relationship between potential and current density further confirms efficient charge-transfer during the stepped electrolysis, indicating high electrode reliability and effective ionic transport within the porous 2D nanosheet structure. Furthermore, the long-term durability of Co2CuO4 catalyst was then evaluated through extended chronopotentiometric testing at fixed current densities of 10 and 500 mA cm−2 for a continuous duration of 100 h (Figure 5b). The Co2CuO4 catalyst maintains a remarkably stable potential response throughout the entire stability test without significant alteration in the potential value. The sustained voltage stability of Co2CuO4 catalyst demonstrated outstanding structural integrity and robust charge-transport pathways during the extended hydrogen evolution. The post-stability LSV curves (Figure 5c) and EIS spectra (Figure 5d) exhibit nearly identical polarization profiles and minimal variation in the charge-transfer resistance for the Co2CuO4 catalyst relative to the initial measurement, verifying that the catalyst retains its intrinsic activity and electrode integrity after prolonged HER operation. Nonetheless, the reliability test (Figure 5e and Figure S3) was conducted using five independently fabricated Co2CuO4 and Co3O4 catalyst, which reveals an almost identical electrochemical responses, indicating the excellent reproducibility of the catalytic performance under alkaline HER conditions.
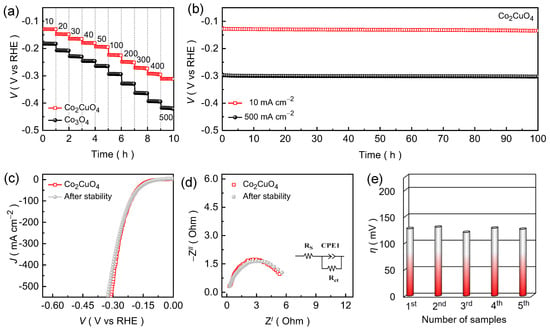
Figure 5.
(a) Voltage step profile being function of scan rate for the Co2CuO4 and Co3O4 catalysts evaluated in an alkaline 1.0 M KOH electrolyte medium; (b) Chronopotentiometric stability curves recorded over 100 h at 10 and 500 mA cm−2; Post stability measured (c) LSV and (d) EIS curves for the Co2CuO4 catalyst; (e) Reliability of the HER performance for Co2CuO4 catalysts evaluated through series of catalyst testing.
3. Materials and Methods
3.1. Materials
Analytical-grade reagents purchased from Sigma-Aldrich (St. Louis, MO, USA) were used in all experiments without any further purification. Cobalt(II) chloride hexahydrate (CoCl2·6H2O, ≥98%), copper(II) chloride dihydrate (CuCl2·2H2O, ≥98%), ammonium hydroxide solution (NH4OH, ≥99.99%), and potassium hydroxide (KOH, ≥85%) were used for material fabrication and testing. Hydrochloric acid (HCl, 37%), ethanol (CH3CH2OH, ≥95%), and Acetone (CH3COCH3, ≥99.5%) were employed as cleaning and processing agents. Three macroporous nickel foam used as the conductive substrate for electrode preparation. Before electrodeposition, the Ni foam pieces were sequentially ultrasonicated in ethanol, diluted HCl, deionized water, and acetone to eliminate surface contaminants and ensure good adhesion of the deposited films.
3.2. Synthesis of Co3O4 and Co2CuO4 Electrodes
The Co2CuO4 nanosheet film was synthesized on Ni foam through a facile electrodeposition technique. In a typical procedure, an aqueous electrolyte bath 50 mL was prepared in a glass beaker using CuCl2·2H2O and CoCl2·6H2O under continuous stirring at room temperature, and a pre-cleaned Ni foam was subjected to an electrodeposition using VersaSTAT instrument (Ametek Scientific Instruments, Berwyn, PA, USA). The Ni foam, Pt wire, and saturated calomel electrode (SCE) served as the working, counter, and reference electrode, respectively. The electrodeposition process was performed for 300 s at a biasing potential of −1.0 V (vs. SCE). The (Co-Cu) hydroxide precursor film was dried overnight followed by air annealing for 2 h at 350 °C to obtain the desired Co2CuO4 film. For comparison, the Co3O4 film was also synthesized using a similar electrodeposition process, except the utilization of CuCl2·2H2O in the electrolyte solution bath. The experimental conditions were optimized based on the preliminary experimental results. The detailed FE-SEM description is presented in the Supporting Information section (Figure S4).
3.3. Material Characterization
The structural, morphological, and chemical characteristics of the prepared Co3O4 and Co2CuO4 were comprehensively analyzed using various advanced techniques. The crystal structure and phase purity were examined by high-resolution X-ray diffraction (XRD) using Cu Kα radiation (λ = 1.5406 Å). The XRD spectra were recorded at spectral angle ranging between 20° and 80°. The surface chemical composition and oxidation states of the constituent elements were investigated using X-ray photoelectron spectroscopy (XPS) on a Phi 5000 VersaProbe Scanning Microprobe (ULVAC-Phi, Chigasaki, Japan). All binding energies were calibrated using the carbon C 1s peak situated at 286.53 eV as a reference. The surface morphology and microstructural features were observed using field-emission scanning electron microscopy (FE-SEM) and the elemental composition and spatial distribution were determined through energy-dispersive X-ray spectroscopy (EDX) integrated into the SEM system. Raman spectroscopy was performed on a LabRam Aramis spectrometer (Horiba Jobin Yvon, Yongin, Republic of Korea) using a 514 nm Ar-ion laser as the excitation source to analyze the vibrational characteristics and structural features of the film samples.
3.4. Electrochemical Test
The hydrogen evolution reaction (HER) characteristics of the fabricated Co2CuO4 and Co3O4 electrodes were assessed on a VersaSTAT electrochemical workstation using a conventional three-electrode setup with 1.0 M KOH as the alkaline electrolyte. The catalyst-coated nickel foam served as the working electrode, while a graphite rod and a SCE served as the counter and reference electrodes, respectively. All electrochemical tests were conducted at 25 ± 2 °C in freshly prepared 1.0 M KOH solution purged with high-purity argon for at least 30 min prior to measurement to remove dissolved oxygen. Linear sweep voltammetry (LSV) experiments were performed in the potential window range between 0.0 to −1.6 V (vs. SCE) at a scan rate of 1.0 mV s−1 to determine the polarization behavior. The measured SCE potential scale (i.e., ESCE) was changed to the reversible hydrogen electrode (RHE) scale (i.e., ERHE) and the internal ohmic losses were normalized using iR-compensation (90%), where the series resistance (Rs) was obtained from the high-frequency intercept of the Nyquist plot in EIS measurements, as shown in the following equations [43]:
where pH and ESCE° are the hydrogen-ion concentration in the electrolyte solution and the standard potential of SCE at room temperature. The reaction kinetics were then analyzed by plotting Tafel curves, which were derived from the linear portion of the polarization data and fitted according to the following equation [6]:
where a and b are the arbitrary constant and Tafel slope, respectively. The stability and durability of the electrode were examined through chronopotentiometric testing at constant current density over extended operation periods. The accessible electrochemically active surface area (ECSA) for the Co3O4 and Co2CuO4 electrodes was estimated through non-Faradaic cyclic voltammetry (CV) curves. The non-Faradaic CV curves were recorded within the potential range between 0.00 and 0.10 V (vs. RHE) at various scan rates and the non-Faradaic capacitance (CNFC) was estimated to approximate the density of accessible active sites. The electrochemical impedance spectroscopy (EIS) measurements were conducted at a fixed overpotential with a 10 mV AC amplitude between the frequency range of 0.01 and 10 kHz to probe the charge-transfer resistance (Rct).
ERHE = ESCE + (0.059 × pH) + ESCE°,
η = ERHE − (J × Rs),
η = a + (b × log(J)),
4. Conclusions
In summary, a systematic comparison of Co2CuO4 and Co3O4 nanosheet catalysts demonstrates how cationic substitution and electronic modulation significantly influence hydrogen evolution behavior under alkaline conditions. The incorporation of Cu2+ into the Co3O4 spinel framework tailors the electronic structure, enhances conductivity, and introduces a mixed-valence configuration of copper and cobalt, all of which collectively promote faster reaction kinetics and efficient charge-transportation. The Co2CuO4 catalyst exhibits notably lower overpotential of 127 mV, smaller Tafel slopes of 61 mV dec−1, and higher TOF values than pristine Co3O4 catalyst, corroborating its superior intrinsic catalytic activity. The enlarged electrochemically active surface area and reduced interfacial resistance further ensure rapid charge-transfer and efficient mass transport. The long-term chronopotentiometric stability tests up to 100 h at 10 mA cm−2 validate its mechanical robustness and excellent durability, maintaining steady performance even under high current density of 500 mA cm−2. Importantly, this work provides mechanistic insight into how Cu incorporation modulates the Co–O bonding environment and electronic configuration within the spinel lattice, resulting in enhanced orbital hybridization between Cu 3d, Co 3d, and O 2p states. This electronic reconstruction effectively lowers the energy barrier for intermediate adsorption and accelerates the Volmer-Heyrovsky process during HER. Overall, these findings establish a rational Cu-substitution strategy that couples structural and electronic tuning, offering a deeper understanding of activity enhancement mechanisms and a scalable pathway toward high-efficiency transition-metal oxide catalysts for sustainable hydrogen production.
Author Contributions
Methodology, Conceptualization, visualization, software, investigation, writing—original draft preparation, writing—review and editing, S.S. and A.T.A.A.; data curation, software, M.M.M. and A.S.A.; formal analysis, Y.L.; resources, supervision, software, visualization, funding acquisition, writing—review and editing, project administration, S.L. and S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Research Foundation (NRF) of Korea through the basic science research program (RS-2023-00236798 and RS-2023-NR076644) funded by the Korean Government.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Acknowledgments
This research was supported by the NRF of Korea grant funded by the Korean government. The authors gratefully acknowledge the research facilities and support provided by Dongguk University, Republic of Korea.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| HER | Hydrogen evolution reaction |
| Pt | Platinum |
| FE-SEM | Field-emission scanning electron microscopy |
| EDX | Energy-dispersive X-ray spectroscopy |
| XRD | X-ray diffraction |
| XPS | X-ray photoelectron spectroscopy |
| SCE | Saturated calomel electrode |
| RHE | Reversible hydrogen electrode |
| EIS | Electrochemical impedance spectroscopy |
| JNFC | Non-faradaic current density |
| CNFC | Non-faradaic capacitance |
| ECSA | electrochemically active surface area |
| CV | Cyclic voltammetry |
| LSV | Linear sweep voltammetry |
| TOF | Turnover frequency |
| η | Overpotential |
| Rct | Charge-transfer resistance |
| F | Faraday’s constant |
| A | Geometric area of the electrode |
| N | Number of moles of active catalyst |
| n | Number of electrons transferred per hydrogen molecule |
References
- Evro, S.; Oni, B.A.; Tomomewo, O.S. Carbon neutrality and hydrogen energy systems. Int. J. Hydrogen Energy 2024, 78, 1449–1467. [Google Scholar] [CrossRef]
- Boretti, A.; Pollet, B.G. Hydrogen economy: Paving the path to a sustainable, low-carbon future. Int. J. Hydrogen Energy 2024, 93, 307–319. [Google Scholar] [CrossRef]
- Barrientos, C.; Moris, S.; Arias, D.; Pecchi, G.; Ibarra, J.; Ramírez, G.; Gidi, L. New Paste Electrode Based on Copper and Gallium Mixed Metal Oxides-Decorated CNT for Highly Electrocatalyzed Hydrogen Evolution Reaction. Int. J. Mol. Sci. 2025, 26, 9057. [Google Scholar] [CrossRef]
- Vedrtnam, A.; Kalauni, K.; Pahwa, R. A review of water electrolysis technologies with insights into optimization and numerical simulations. Int. J. Hydrogen Energy 2025, 140, 694–727. [Google Scholar] [CrossRef]
- Vedrtnam, A.; Kalauni, K.; Pahwa, R. Water Electrolysis Technologies and Their Modeling Approaches: A Comprehensive Review. Eng 2025, 6, 81. [Google Scholar] [CrossRef]
- Ahmed, A.T.A.; Cho, S.; Im, H.; Jana, A. Enhanced Catalytic Activity of CuO@CuS Core–Shell Structure for Highly Efficient HER Application. Nanomaterials 2024, 14, 1941. [Google Scholar] [CrossRef] [PubMed]
- Irshad, H.; Zia, M.; Al-Hajri, R.; Khattak, Z.A.K.; Al-Abri, M.; Ahmad, N.; Younus, H.A. Electrocatalysts for hydrogen and oxygen evolution reactions under neutral/near-neutral conditions: Summary and challenges. Int. J. Hydrogen Energy 2025, 137, 1009–1041. [Google Scholar] [CrossRef]
- Thalji, M.R.; Mahmoudi, F.; Bachas, L.G.; Park, C. MXene-Based Electrocatalysts for Water Splitting: Material Design, Surface Modulation, and Catalytic Performance. Int. J. Mol. Sci. 2025, 26, 8019. [Google Scholar] [CrossRef]
- Jarząbek-Karnas, M.; Bojarska, Z.; Klemczak, P.; Werner, Ł.; Makowski, Ł. Advanced Hybrid Nanocatalysts for Green Hydrogen: Carbon-Supported MoS2 and ReS2 as Noble Metal Alternatives. Int. J. Mol. Sci. 2025, 26, 6640. [Google Scholar] [CrossRef]
- Bergedahl, M.; Narea, P.; Llanos, J.; Pulido, R.; Naveas, N.; Amo-Ochoa, P.; Zamora, F.; Delgado, G.E.; Madrid, F.M.G.; León, Y.; et al. Synthesis, Crystal Structures, Hirshfeld Surface Analysis, Computational Investigations, Thermal Properties, and Electrochemical Analysis of Two New Cu(II) and Co(II) Coordination Polymers with the Ligand 5-Methyl-1-(pyridine-4-yl-methyl)-1H-1,2,3-triazole-4-carboxylate. Int. J. Mol. Sci. 2025, 26, 1671. [Google Scholar]
- Gupta, S.; Fernandes, R.; Patel, R.; Spreitzer, M.; Patel, N. A review of cobalt-based catalysts for sustainable energy and environmental applications. Appl. Catal. A Gen. 2023, 661, 119254. [Google Scholar] [CrossRef]
- Chen, X.H.; Li, T.; Li, X.; Lei, J.; Li, N.B.; Luo, H.Q. Oxyanion-Coordinated Co-Based Catalysts for Optimized Hydrogen Evolution: The Feedback of Adsorbed Anions to the Catalytic Activity and Mechanism. ACS Catal. 2023, 13, 6721–6729. [Google Scholar] [CrossRef]
- Huang, C.; Qin, P.; Luo, Y.; Ruan, Q.; Liu, L.; Wu, Y.; Li, Q.; Xu, Y.; Liu, R.; Chu, P.K. Recent progress and perspective of cobalt-based catalysts for water splitting: Design and nanoarchitectonics. Mater. Today Energy 2022, 23, 100911. [Google Scholar] [CrossRef]
- Li, S.; Hao, X.; Abudula, A.; Guan, G. Nanostructured Co-based bifunctional electrocatalysts for energy conversion and storage: Current status and perspectives. J. Mater. Chem. A 2019, 7, 18674–18707. [Google Scholar] [CrossRef]
- Chen, G.-Y.; Zhu, S.-L.; Han, X.-Q.; Wang, D.-C.; Zhang, J.-C.; Huai, X.-D.; Li, X.; Zhang, F.-Y.; Xiang, Z.; Zhang, W.-Z. Engineering Cationic Vacancies in Octahedral Sites of Co3O4 for High-Efficiency Oxygen Evolution. Energy Fuels 2023, 37, 8523–8530. [Google Scholar] [CrossRef]
- Ibarra, J.; Aguirre, M.J.; del Río, R.; Henriquez, R.; Faccio, R.; Dalchiele, E.A.; Arce, R.; Ramírez, G. α-Fe2O3/, Co3O4/, and CoFe2O4/MWCNTs/Ionic Liquid Nanocomposites as High-Performance Electrocatalysts for the Electrocatalytic Hydrogen Evolution Reaction in a Neutral Medium. Int. J. Mol. Sci. 2024, 25, 7043. [Google Scholar] [CrossRef]
- Li, G.; Liu, F.; Ma, W.; Li, H.; Li, S. Surface Modification of a Lignin-Derived Carbon-Supported Co-Based Metal/Oxide Nanostructure for Alkaline Water Splitting. Molecules 2023, 28, 5648. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, K.A.T.; Mujtaba, M.M.; Thakre, K.; Momin, Z.H.; Ansari, A.S.; Cho, S.; Ahmed, A.T.A. From granules to nanosheets: Unlocking the energy potential of CoFe2O4 for highly-efficient asymmetric energy storage applications. J. Energy Storage 2025, 140, 119168. [Google Scholar] [CrossRef]
- Aqueel Ahmed, A.T.; Hou, B.; Chavan, H.S.; Jo, Y.; Cho, S.; Kim, J.; Pawar, S.M.; Cha, S.; Inamdar, A.I.; Kim, H.; et al. Self-Assembled Nanostructured CuCo2O4 for Electrochemical Energy Storage and the Oxygen Evolution Reaction via Morphology Engineering. Small 2018, 14, 1800742. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ji, L.; Liu, R.; Zhang, C.; Mak, C.H.; Zou, X.; Shen, H.-H.; Leu, S.-Y.; Hsu, H.-Y. A review on morphology engineering for highly efficient and stable hybrid perovskite solar cells. J. Mater. Chem. A 2018, 6, 12842–12875. [Google Scholar] [CrossRef]
- Chen, K.; Sun, C.; Xue, D. Morphology engineering of high performance binary oxide electrodes. Phys. Chem. Chem. Phys. 2015, 17, 732–750. [Google Scholar] [CrossRef]
- Tan, F.; Dong, N.; He, J.; Yuan, L.; Lin, Z.; Liu, X.; Yang, X.; Li, B. Morphology Engineering of Aggregation-Induced Emission-Based Metal–Organic Frameworks Templated with Cellulose Nanocrystals for Lactic Acid Detection. ACS Mater. Lett. 2025, 7, 2041–2048. [Google Scholar] [CrossRef]
- Timmerman, M.A.; Xia, R.; Le, P.T.P.; Wang, Y.; ten Elshof, J.E. Metal Oxide Nanosheets as 2D Building Blocks for the Design of Novel Materials. Chem. Eur. J. 2020, 26, 9084–9098. [Google Scholar] [CrossRef]
- Yang, J.; Zeng, Z.; Kang, J.; Betzler, S.; Czarnik, C.; Zhang, X.; Ophus, C.; Yu, C.; Bustillo, K.; Pan, M.; et al. Formation of two-dimensional transition metal oxide nanosheets with nanoparticles as intermediates. Nat. Mater. 2019, 18, 970–976. [Google Scholar] [CrossRef]
- Baimova, J.; Polyakova, P.; Shcherbinin, S. Effect of the Structure Morphology on the Mechanical Properties of Crumpled Graphene Fiber. Fibers 2021, 9, 85. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, J.; Zheng, Y.; Zhang, Y.; Hu, X.; Xu, T. Construction of CuCo2O4@CuCo2O4 hierarchical nanowire arrays grown on Ni foam for high-performance supercapacitors. RSC Adv. 2017, 7, 3983–3991. [Google Scholar] [CrossRef]
- Lin, D.; Zheng, W.; Lin, S.; Zhang, L.; Li, W.; Zhuo, Q.; Yang, W.; Luo, Y.; Qian, Q.; Chen, Q. Morphology effect of In2O3/Co3O4 on Co–In2O3 interface formation to drive the hydrogenation of CO2 to methanol. Int. J. Hydrogen Energy 2024, 90, 42–51. [Google Scholar] [CrossRef]
- Madadi, M.; Salarizadeh, P.; Rohani Moghadam, M.; Bazmandegan-Shamili, A. CuCo2O4 supported graphene quantum dots as a new and promising catalyst for methanol oxidation reaction. J. Electroanal. Chem. 2023, 941, 117532. [Google Scholar] [CrossRef]
- Lin, X.; Xue, X.; Du, J. Electrochemical glucose-to-formic acid conversion coupled with alkaline hydrogen production over nanostructured CuCo2O4 catalysts. J. Mater. Chem. A 2024, 12, 32095–32103. [Google Scholar] [CrossRef]
- Koniakhin, S.V.; Utesov, O.I.; Yashenkin, A.G. Raman peak shift and broadening in crystalline nanoparticles with lattice impurities. Diam. Relat. Mater. 2024, 146, 111182. [Google Scholar] [CrossRef]
- UmaSudharshini, A.; Bououdina, M.; Venkateshwarlu, M.; Dhamodharan, P.; Manoharan, C. Solvothermal synthesis of Cu-doped Co3O4 nanosheets at low reaction temperature for potential supercapacitor applications. Appl. Phys. A 2021, 127, 353. [Google Scholar] [CrossRef]
- Mayer, B.; Uhlenbrock, S.; Neumann, M. XPS satellites in transition metal oxides. J. Electron Spectrosc. Relat. Phenom. 1996, 81, 63–67. [Google Scholar] [CrossRef]
- Ho, C.-T.; Weng, T.-H.; Wang, C.-Y.; Yen, S.-J.; Yew, T.-R. Tunable band gaps of Co3−xCuxO4 nanorods with various Cu doping concentrations. RSC Adv. 2014, 4, 20053–20057. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, S.; Zhu, J.; Wang, Z.; Chen, S.; Qi, J.; Wang, H. Dual-Engineering Tailored Co3O4 Hollow Microspheres Assembled by Nanosheets for Boosting Oxygen Evolution Reaction. Molecules 2025, 30, 2181. [Google Scholar] [CrossRef]
- Kalasina, S.; Kongsawatvoragul, K.; Phattharasupakun, N.; Phattharaphuti, P.; Sawangphruk, M. Cobalt oxysulphide/hydroxide nanosheets with dual properties based on electrochromism and a charge storage mechanism. RSC Adv. 2020, 10, 14154–14160. [Google Scholar] [CrossRef]
- Sun, J.; Tian, X.; Xu, C.; Chen, H. Porous CuCo2O4 microtubes as a promising battery-type electrode material for high-performance hybrid supercapacitors. J. Mater. 2021, 7, 1358–1368. [Google Scholar] [CrossRef]
- Tang, J.; Ge, Y.; Shen, J.; Ye, M. Facile synthesis of CuCo2S4 as a novel electrode material for ultrahigh supercapacitor performance. Chem. Commun. 2016, 52, 1509–1512. [Google Scholar] [CrossRef]
- Wang, H.; Wang, D.; Sun, C.; Zhao, X.; Xu, C.; Li, Z.; Hou, Y.; Lei, L.; Yang, B.; Duan, X. Oriented generation of 1O2 from peroxymonosulfate via Co3O4 facet engineering. Appl. Catal. B Environ. 2025, 364, 124854. [Google Scholar] [CrossRef]
- Behera, A.; Seth, D.; Agarwal, M.; Haider, M.A.; Bhattacharyya, A.J. Exploring Cu-Doped Co3O4 Bifunctional Oxygen Electrocatalysts for Aqueous Zn–Air Batteries. ACS Appl. Mater. Interfaces 2024, 16, 17574–17586. [Google Scholar] [CrossRef]
- Talha Aqueel Ahmed, A.; Ho Lee, C.; Saad Ansari, A.; Pawar, S.M.; Han, J.; Park, S.; Shin, G.; Yeon, S.; Cho, S.; Seol, J.; et al. Hybridized heterostructure of CoS and MoS2 nanoparticles for highly-efficient and robust bifunctional water electrolysis. Appl. Surf. Sci. 2022, 592, 153196. [Google Scholar] [CrossRef]
- Ahmed, A.T.A.; Seol, J.H.; Seok, J.H.; Jana, A.; Meena, A.; Cho, S.; Sree, V.G.; Park, Y.; Lee, S.U.; Im, H. Boosting the electrocatalytic performance of CuCo2S4 via surface-state engineering for ampere current water electrolysis applications. Appl. Surf. Sci. 2025, 680, 161353. [Google Scholar] [CrossRef]
- McCrory, C.C.; Jung, S.; Peters, J.C.; Jaramillo, T.F. Benchmarking heterogeneous electrocatalysts for the oxygen evolution reaction. J. Am. Chem. Soc. 2013, 135, 16977–16987. [Google Scholar] [CrossRef]
- Ahmed, A.T.A.; Sree, V.G.; Meena, A.; Inamdar, A.I.; Im, H.; Cho, S. In Situ Transformed CoOOH@Co3S4 Heterostructured Catalyst for Highly Efficient Catalytic OER Application. Nanomaterials 2024, 14, 1732. [Google Scholar] [CrossRef]
- Hanan, A.; Lakhan, M.N.; Shu, D.; Hussain, A.; Ahmed, M.; Soomro, I.A.; Kumar, V.; Cao, D. An efficient and durable bifunctional electrocatalyst based on PdO and Co2FeO4 for HER and OER. Int. J. Hydrogen Energy 2023, 48, 19494–19508. [Google Scholar] [CrossRef]
- Aqueel Ahmed, A.T.; Pawar, S.M.; Inamdar, A.I.; Kim, H.; Im, H. A Morphologically Engineered Robust Bifunctional CuCo2O4 Nanosheet Catalyst for Highly Efficient Overall Water Splitting. Adv. Mater. Interfaces 2020, 7, 1901515. [Google Scholar] [CrossRef]
- Tan, S.; Ji, Y.; Ren, F.; Chen, F.; Ouyang, W. Improved energy conversion and storage performance enabled by hierarchical zigzag-like P-doped CuCo2O4 nanosheets based 3D electrode materials. Int. J. Hydrogen Energy 2022, 47, 9248–9260. [Google Scholar] [CrossRef]
- Guo, Y.; Tang, J.; Wang, Z.; Kang, Y.-M.; Bando, Y.; Yamauchi, Y. Elaborately assembled core-shell structured metal sulfides as a bifunctional catalyst for highly efficient electrochemical overall water splitting. Nano Energy 2018, 47, 494–502. [Google Scholar] [CrossRef]
- Wang, K.; Lin, Z.; Tang, Y.; Tang, Z.; Tao, C.-L.; Qin, D.-D.; Tian, Y. Selenide/sulfide heterostructured NiCo2Se4/NiCoS4 for oxygen evolution reaction, hydrogen evolution reaction, water splitting and Zn-air batteries. Electrochim. Acta 2021, 368, 137584. [Google Scholar] [CrossRef]
- Wang, C.; Jiu, H.; Zhang, L.; Song, W.; Zhang, Y.; Wei, H.; Xu, Q.; Che, S.; Guo, Z.; Qin, Y. Bifunctional CuCo2O4/CoOOH as a synergistic catalyst supported on nickel foam for alkaline overall water splitting. J. Alloys Compd. 2022, 929, 167367. [Google Scholar] [CrossRef]
- Zequine, C.; Wang, F.; Li, X.; Guragain, D.; Mishra, S.R.; Siam, K.; Kahol, P.K.; Gupta, R.K. Nanosheets of CuCo2O4 As a High-Performance Electrocatalyst in Urea Oxidation. Appl. Sci. 2019, 9, 793. [Google Scholar] [CrossRef]
- Ahmed, A.T.A.; Sekar, S.; Khadtare, S.S.; Rochman, N.T.; Chinna, B.; Ansari, A.S. Anion-exchange synthesis of an MnCo2S4 electrocatalyst towards facilitated ultralong hydrogen evolution reaction in acidic and alkaline media. CrystEngComm 2024, 26, 215–222. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).