Assessing Molecular Mechanisms of Stress Induced Salinity Adaptation in the Juvenile Ornate Spiny Lobster, Panulirus ornatus
Abstract
1. Introduction
2. Results
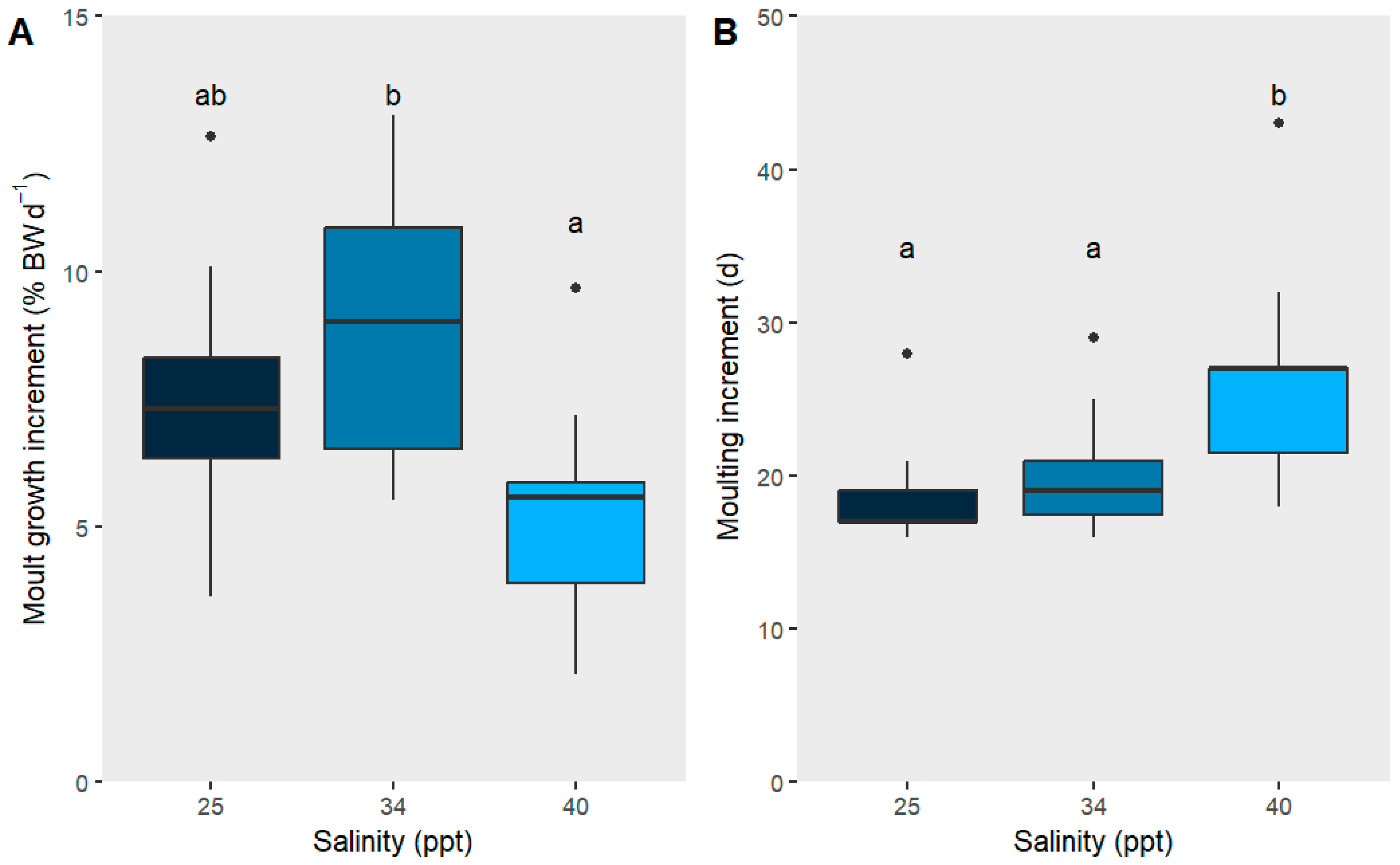
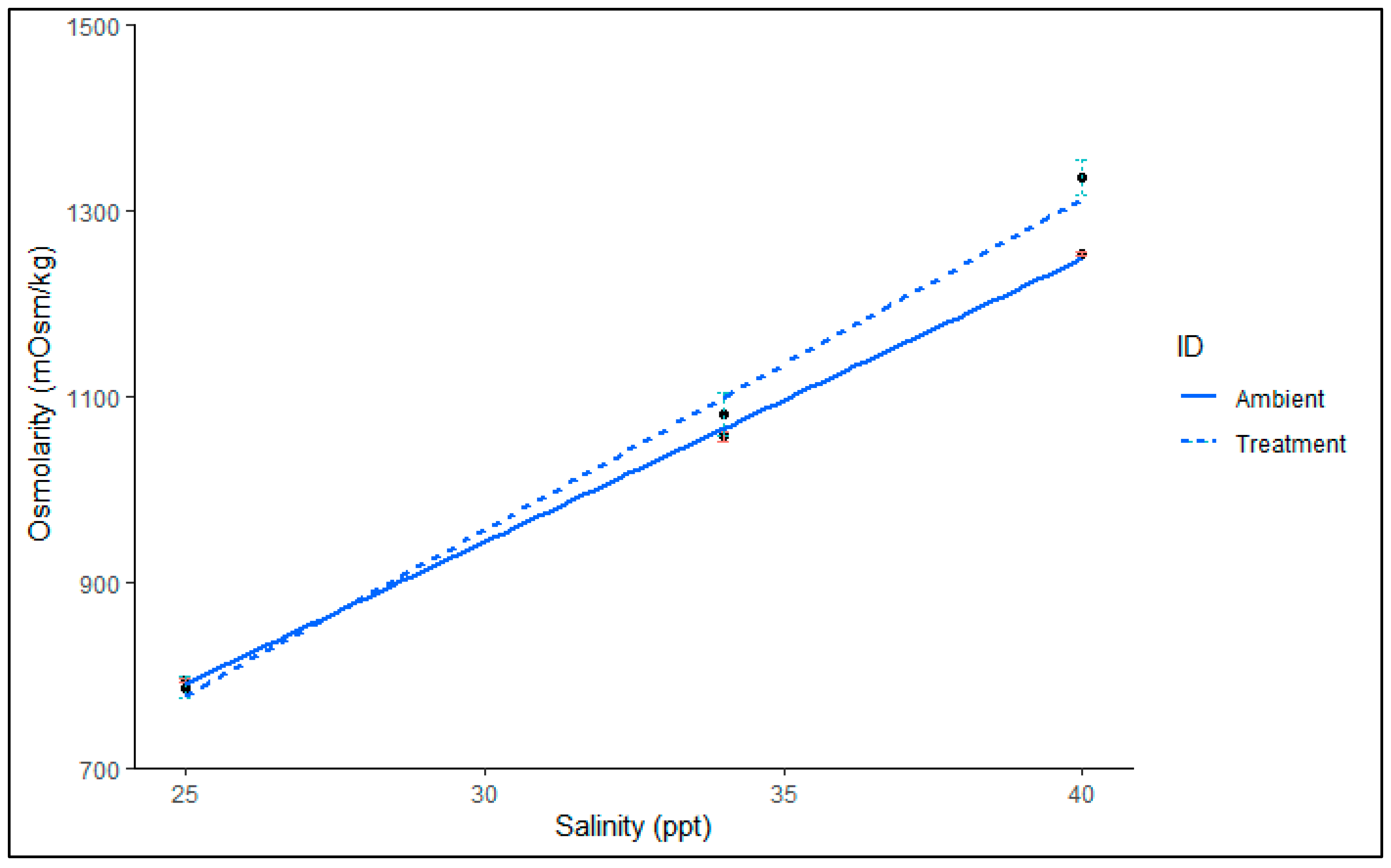
2.1. Chronic Salinity Parameters
2.2. Transcriptome Sequencing of Panulirus ornatus Acutely Exposed Gills
2.2.1. Differential Expression of Genes That Occurred Following Acute Salinity Acclimation: Preliminary Analysis
2.2.2. Transcriptomic Expression of Genes of Interest
3. Discussion
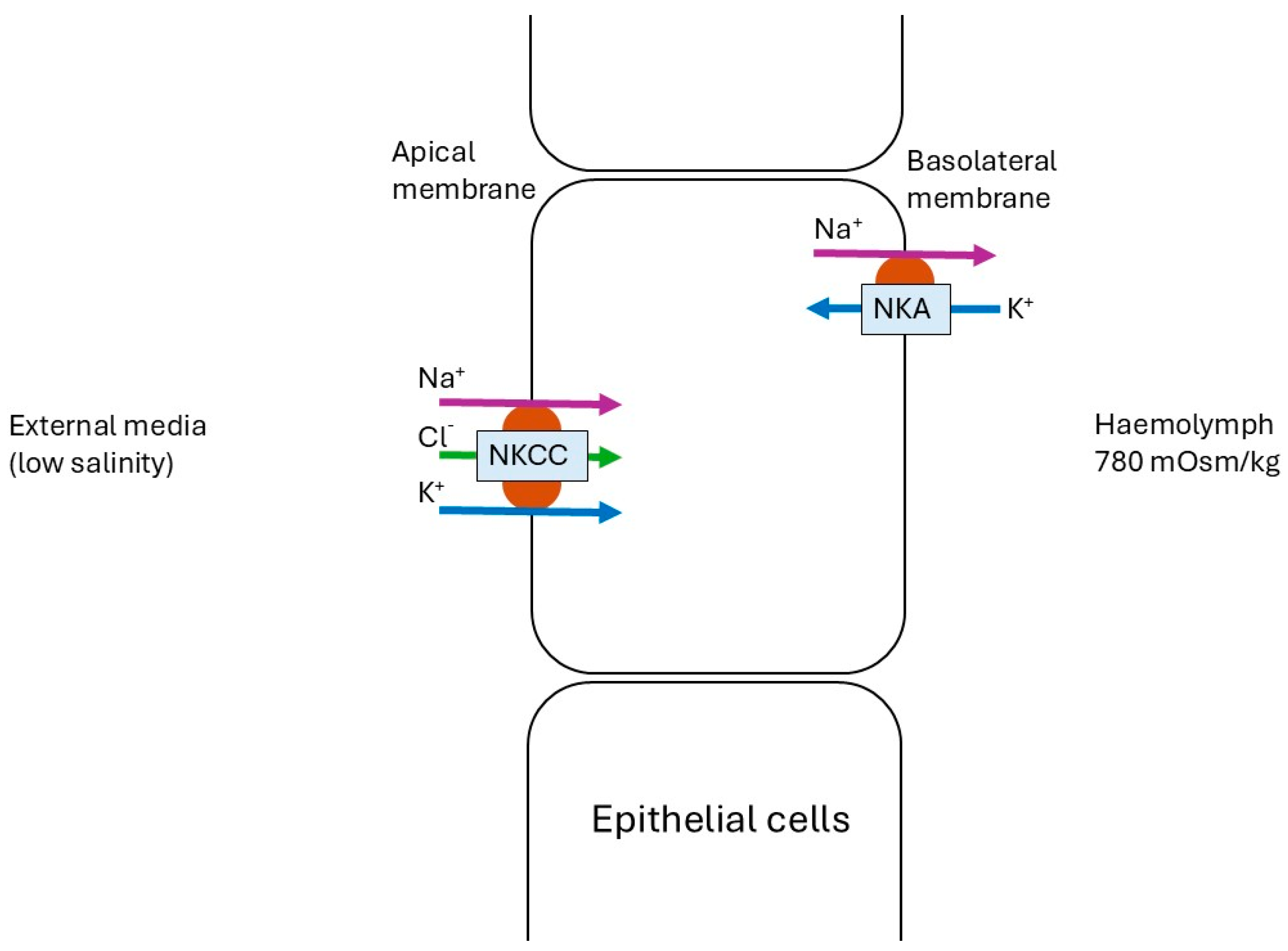
3.1. Low- vs. Control Salinity Exposure
3.2. High vs. Control Salinity Exposure
3.3. High- vs. Low-Salinity Exposure
3.4. Acute vs. Chronic Exposure Times
3.5. Implication on Aquaculture
3.6. Study Limitation and Future Prospects
4. Methods
4.1. Acute Exposure
4.2. Chronic Exposure
4.2.1. Experimental System and Protocol
4.2.2. Acclimation Period
4.2.3. RAS System
4.2.4. Sampling
4.3. RNA Extraction
4.4. Gill Transcriptome Sequencing, Assembly and Analysis: Acute Salinity Exposure
4.5. Quantitative PCR (qPCR) Analysis of Osmoregulatory Gene Expression: Chronic Salinity Exposure
4.6. Osmolarity
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Infante, V.; Blaire, J. Pioneering Tropical Rock Lobster Raft Grow-out for Northern Australia. In Final Report CRCNA Project A.3.2021116; Ornatas Research & Development, CRCNA: Townsville, Australia, 2024; p. 27. [Google Scholar]
- Roy, L.A.; Davis, D.A.; Saoud, I.P.; Boyd, C.A.; Pine, H.J.; Boyd, C.E. Shrimp culture in inland low salinity waters. Rev. Aquac. 2010, 2, 191–208. [Google Scholar] [CrossRef]
- Charmantier, G.; Haond, C.; Lignot, J.; Charmantier-Daures, M. Ecophysiological adaptation to salinity throughout a life cycle: A review in homarid lobsters. J. Exp. Biol. 2001, 204, 967–977. [Google Scholar] [CrossRef]
- Spencer, E.L.; Fitzgibbon, Q.P.; Day, R.D.; Trotter, A.J.; Smith, G.G. Juvenile Panulirus ornatus physiological constraints to salinity change. Aquaculture 2025, 599, 742161. [Google Scholar] [CrossRef]
- Jeffs, A. Status and challenges for advancing lobster aquaculture. J. Mar. Biol. Assoc. India 2010, 52, 320–326. [Google Scholar]
- Nankervis, L.; Jones, C. Recent advances and future directions in practical diet formulation and adoption in tropical Palinurid lobster aquaculture. Rev. Aquac. 2022, 14, 1830–1842. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2020. Sustainablity in Action; FAO: Rome, Italy, 2020. [Google Scholar]
- Jones, C.M. Tropical spiny lobster aquaculture development in Vietnam, Indonesia and Australia. J. Marion Biol. Assoc. India 2010, 52, 304–315. [Google Scholar]
- Jones, C.M.; Anh, T.L.; Priyambodo, B. Lobster aquaculture development in Vietnam and Indonesia. In Lobsters: Biology, Fisheries and Aquaculture; Springer: Singapore, 2019; pp. 541–570. [Google Scholar]
- Blaire, J.; Infante, V. Ornatas Innovation & Development, Pty Ltd., Townsville, Queensland, Australia, Unpublished salinity and temperature data, 2023.
- Dennis, D.M.; Pitcher, C.R.; Skewes, T.D. Distribution and transport pathways of Panulirus ornatus (Fabricius, 1776) and Panulirus spp. larvae in the Coral Sea, Australia. Mar. Freshw. Res. 2001, 52, 1175–1185. [Google Scholar]
- Skewes, T.; Dennis, D.; Pitcher, C.; Long, B. Age structure of Panulirus ornatus in two habitats in Torres Strait, Australia. Mar. Freshw. Res. 1997, 48, 745–750. [Google Scholar]
- Jones, C.M. Temperature and salinity tolerances of the tropical spiny lobster, Panulirus ornatus. J. World Aquac. Soc. 2009, 40, 744–752. [Google Scholar] [CrossRef]
- Ye, L.; Jiang, S.; Zhu, X.; Yang, Q.; Wen, W.; Wu, K. Effects of salinity on growth and energy budget of juvenile Penaeus monodon. Aquaculture 2009, 290, 140–144. [Google Scholar] [CrossRef]
- Spencer, E.L.; Fitzgibbon, Q.P.; Day, R.D.; Trotter, A.J.; Smith, G.G. Effects of acute salinity stress on the survival and haemolymph biochemistry of juvenile tropical rock lobster, Panulirus ornatus, at different moult stages. Aquaculture 2023, 573, 739597. [Google Scholar] [CrossRef]
- Thabet, R.; Ayadi, H.; Koken, M.; Leignel, V. Homeostatic responses of crustaceans to salinity changes. Hydrobiologia 2017, 799, 1–20. [Google Scholar] [CrossRef]
- Henry, R.P.; Lucu, Č.; Onken, H.; Weihrauch, D. Multiple functions of the crustacean gill: Osmotic/ionic regulation, acid-base balance, ammonia excretion, and bioaccumulation of toxic metals. Front. Physiol. 2012, 3, 431. [Google Scholar] [CrossRef]
- Lignot, J.-H.; Charmantier, G. Osmoregulation and Excretion. Nat. Hist. Crustac. 2015, 4, 249–285. [Google Scholar]
- Lucu, Č.; Towle, D.W. Na+, K+-ATPase in gills of aquatic crustacea. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2003, 135, 195–214. [Google Scholar] [CrossRef]
- Romano, N.; Zeng, C. Osmoregulation in decapod crustaceans: Implications to aquaculture productivity, methods for potential improvement and interactions with elevated ammonia exposure. Aquaculture 2012, 334, 12–23. [Google Scholar] [CrossRef]
- Henry, R.P.; Havird, J.C. Osmotic and Ionic Regulation. In Frontiers in Invertebrate Physiology: A Collection of Reviews; Apple Academic Press: Palm Bay, FL, USA, 2024; pp. 117–174. [Google Scholar]
- Towle, D.W.; Weihrauch, D. Osmoregulation by gills of euryhaline crabs: Molecular analysis of transporters. Am. Zool. 2001, 41, 770–780. [Google Scholar]
- McNamara, J.C.; Faria, S.C. Evolution of osmoregulatory patterns and gill ion transport mechanisms in the decapod Crustacea: A review. J. Comp. Physiol. B 2012, 182, 997–1014. [Google Scholar] [CrossRef]
- Luo, L.; Yang, L.-S.; Huang, J.-H.; Jiang, S.-G.; Zhou, F.-L.; Li, Y.-D.; Jiang, S.; Yang, Q.-B. Effects of Different Salinity Stress on the Transcriptomic Responses of Freshwater Crayfish (Procambarus clarkii, Girard, 1852). Biology 2024, 13, 530. [Google Scholar] [CrossRef]
- Faleiros, R.O.; Garçon, D.P.; Lucena, M.N.; McNamara, J.C.; Leone, F.A. Short-and long-term salinity challenge, osmoregulatory ability, and (Na+, K+)-ATPase kinetics and α-subunit mRNA expression in the gills of the thinstripe hermit crab Clibanarius symmetricus (Anomura, Diogenidae). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2018, 225, 16–25. [Google Scholar] [CrossRef]
- Evans, D.H.; Claiborne, J.B. Osmotic and ionic regulation in fishes. In Osmotic and Ronic Regulation; CRC Press: Boca Raton, FL, USA, 2008; pp. 295–366. [Google Scholar]
- Pequeux, A. Osmotic regulation in crustaceans. J. Crustac. Biol. 1995, 15, 1–60. [Google Scholar] [CrossRef]
- Kirschner, L.B. The mechanism of sodium chloride uptake in hyperregulating aquatic animals. J. Exp. Biol. 2004, 207, 1439–1452. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freire, C.A.; Onken, H.; McNamara, J.C. A structure–function analysis of ion transport in crustacean gills and excretory organs. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 151, 272–304. [Google Scholar] [CrossRef]
- Towle, D.W.; Kays, W.T. Basolateral localization of Na+ + K+-ATPase in gill epithelium of two osmoregulating crabs, Callinectes sapidus and Carcinus maenas. J. Exp. Zool. 1986, 239, 311–318. [Google Scholar] [CrossRef]
- Ip, Y.K.; Loong, A.M.; Kuah, J.S.; Sim, E.W.; Chen, X.L.; Wong, W.P.; Lam, S.H.; Delgado, I.L.; Wilson, J.M.; Chew, S.F. Roles of three branchial Na+-K+-ATPase α-subunit isoforms in freshwater adaptation, seawater acclimation, and active ammonia excretion in Anabas testudineus. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2012, 303, R112–R125. [Google Scholar] [CrossRef]
- Towle, D.W.; Holleland, T. Ammonium ion substitutes for K+ in ATP-dependent Na+ transport by basolateral membrane vesicles. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1987, 252, R479–R489. [Google Scholar] [CrossRef]
- Masui, D.C.; Furriel, R.d.P.M.; McNamara, J.C.; Mantelatto, F.L.M.; Leone, F.d.A. Modulation by ammonium ions of gill microsomal (Na+, K+)-ATPase in the swimming crab Callinectes danae: A possible mechanism for regulation of ammonia excretion. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2002, 132, 471–482. [Google Scholar] [CrossRef]
- Chung, K.-F.; Lin, H.-C. Osmoregulation and Na, K-ATPase expression in osmoregulatory organs of Scylla paramamosain. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 144, 48–57. [Google Scholar] [CrossRef]
- Haas, M.; Forbush, B., III. The Na-K-Cl cotransporter of secretory epithelia. Annu. Rev. Physiol. 2000, 62, 515–534. [Google Scholar] [CrossRef]
- Gao, X.; Li, Y.; Li, X.; Wu, F.; Song, C.; Liu, Y. The response and osmotic pressure regulation mechanism of Haliotis discus hannai (Mollusca, Gastropoda) to sudden salinity changes. Hydrobiologia 2017, 795, 181–198. [Google Scholar] [CrossRef]
- Lv, J.; Zhang, D.; Liu, P.; Li, J. Effects of salinity acclimation and eyestalk ablation on Na+, K+, 2Cl− cotransporter gene expression in the gill of Portunus trituberculatus: A molecular correlate for salt-tolerant trait. Cell Stress Chaperones 2016, 21, 829–836. [Google Scholar] [CrossRef]
- Xu, B.-P.; Tu, D.-D.; Yan, M.-C.; Shu, M.-A.; Shao, Q.-J. Molecular characterization of a cDNA encoding Na+/K+/2Cl− cotransporter in the gill of mud crab (Scylla paramamosain) during the molt cycle: Implication of its function in osmoregulation. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2017, 203, 115–125. [Google Scholar] [CrossRef]
- Haas, M.; Forbush, B. The na-k-cl cotransporters. J. Bioenerg. Biomembr. 1998, 30, 161–172. [Google Scholar] [CrossRef]
- Truchot, J. Temperature and acid-base regulation in the shore crab Carcinus maenas (L.). Respir. Physiol. 1973, 17, 11–20. [Google Scholar] [CrossRef]
- Truchot, J.-P. The effect of water salinity and acid-base state on the blood acid-base balance in the euryhaline crab, Carcinus maenas (L.). Comp. Biochem. Physiol. Part A Physiol. 1981, 68, 555–561. [Google Scholar] [CrossRef]
- Fehsenfeld, S.; Kiko, R.; Appelhans, Y.; Towle, D.W.; Zimmer, M.; Melzner, F. Effects of elevated seawater p CO2 on gene expression patterns in the gills of the green crab, Carcinus maenas. BMC Genom. 2011, 12, 488. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.-R.; Lin, H.-C. V-type H+-ATPase and Na+, K+-ATPase in the gills of 13 euryhaline crabs during salinity acclimation. J. Exp. Biol. 2007, 210, 620–627. [Google Scholar] [CrossRef]
- Weihrauch, D.; Ziegler, A.; Siebers, D.; Towle, D.W. Molecular characterization of V-type H+-ATPase (B-subunit) in gills of euryhaline crabs and its physiological role in osmoregulatory ion uptake. J. Exp. Biol. 2001, 204, 25–37. [Google Scholar] [CrossRef]
- Zare, S.; Greenaway, P. The effect of moulting and sodium depletion on sodium transport and the activities of Na+ K+-ATPase, and V-ATPase in the freshwater crayfish Cherax destructor (Crustacea: Parastacidae). Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 119, 739–745. [Google Scholar]
- Towle, D.W.; Paulsen, R.S.; Weihrauch, D.; Kordylewski, M.; Salvador, C.; Lignot, J.-H.; Spanings-Pierrot, C. Na+ + K+-ATPase in gills of the blue crab Callinectes sapidus: cDNA sequencing and salinity-related expression of α-subunit mRNA and protein. J. Exp. Biol. 2001, 204, 4005–4012. [Google Scholar]
- Henry, R.P. Multiple functions of carbonic anhydrase in the crustacean gill. J. Exp. Zool. 1988, 248, 19–24. [Google Scholar] [CrossRef]
- Serrano, L.; Henry, R.P. Differential expression and induction of two carbonic anhydrase isoforms in the gills of the euryhaline green crab, Carcinus maenas, in response to low salinity. Comp. Biochem. Physiol. Part D Genom. Proteom. 2008, 3, 186–193. [Google Scholar] [CrossRef]
- Tresguerres, M.; Parks, S.K.; Sabatini, S.E.; Goss, G.G.; Luquet, C.M. Regulation of ion transport by pH and [HCO3−] in isolated gills of the crab Neohelice (Chasmagnathus) granulata. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2008, 294, R1033–R1043. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Pavasovic, A.; Amin, S.; Mather, P.B.; Prentis, P.J. Comparative analysis of gill transcriptomes of two freshwater crayfish, Cherax cainii and C. destructor. Mar. Genom. 2015, 22, 11–13. [Google Scholar] [CrossRef]
- Mitchell, R.T. Transcriptional Adaptations to Low Salinity in Euryhaline Crustaceans. Ph.D. Thesis, Auburn University, Auburn, AL, USA, 2014. [Google Scholar]
- Donowitz, M.; Tse, C.M.; Fuster, D. SLC9/NHE gene family, a plasma membrane and organellar family of Na+/H+ exchangers. Mol. Asp. Med. 2013, 34, 236–251. [Google Scholar] [CrossRef]
- Li, H.; Ren, C.; Jiang, X.; Cheng, C.; Ruan, Y.; Zhang, X.; Huang, W.; Chen, T.; Hu, C. Na+/H+ exchanger (NHE) in Pacific white shrimp (Litopenaeus vannamei): Molecular cloning, transcriptional response to acidity stress, and physiological roles in pH homeostasis. PLoS ONE 2019, 14, e0212887. [Google Scholar] [CrossRef]
- Romero, M.F.; Chen, A.-P.; Parker, M.D.; Boron, W.F. The SLC4 family of bicarbonate (HCO3−) transporters. Mol. Asp. Med. 2013, 34, 159–182. [Google Scholar] [CrossRef]
- Cai, Y.-M.; Chen, T.; Ren, C.-H.; Huang, W.; Jiang, X.; Gao, Y.; Huo, D.; Hu, C.-Q. Molecular characterization of Pacific white shrimp (Litopenaeus vannamei) sodium bicarbonate cotransporter (NBC) and its role in response to pH stress. Fish Shellfish Immunol. 2017, 64, 226–233. [Google Scholar] [CrossRef]
- Foguesatto, K.; Bastos, C.L.Q.; Boyle, R.T.; Nery, L.E.M.; Souza, M.M. Participation of Na+/K+-ATPase and aquaporins in the uptake of water during moult processes in the shrimp Palaemon argentinus (Nobili, 1901). J. Comp. Physiol. Biol. 2019, 189, 523–535. [Google Scholar] [CrossRef]
- Williams, K.C. Spiny Lobster Ecology and Exploitation in the South China Sea Region. In Proceedings of the Workshop Held at the Institute of Oceanography, Nha Trang, Vietnam, 20–21 July 2004; Australian Centre for International Agricultural Research: Canberra, Austrilia, 2004. [Google Scholar]
- Lovett, D.L.; Verzi, M.P.; Burgents, J.E.; Tanner, C.A.; Glomski, K.; Lee, J.J.; Towle, D.W. Expression profiles of Na+, K+-ATPase during acute and chronic hypo-osmotic stress in the blue crab Callinectes sapidus. Biol. Bull. 2006, 211, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.-K.; Tsai, H.-J.; Liu, C.-C.; Lee, T.-H.; Hwang, P.-P. Salinity-dependent expression of a Na+, K+, 2Cl− cotransporter in gills of the brackish medaka Oryzias dancena: A molecular correlate for hyposmoregulatory endurance. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2010, 157, 7–18. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Wu, Q.; Fang, S.; Li, S.; Zheng, H.; Zhang, Y.; Ikhwanuddin, M.; Ma, H. mRNA profile provides novel insights into stress adaptation in mud crab megalopa, Scylla paramamosain after salinity stress. BMC Genom. 2020, 21, 559. [Google Scholar] [CrossRef]
- Lv, J.; Liu, P.; Wang, Y.; Gao, B.; Chen, P.; Li, J. Transcriptome analysis of Portunus trituberculatus in response to salinity stress provides insights into the molecular basis of osmoregulation. PLoS ONE 2013, 8, e82155. [Google Scholar] [CrossRef]
- Riestenpatt, S.; Onken, H.; Siebers, D. Active absorption of Na+ and Cl− across the gill epithelium of the shore crab Carcinus maenas: Voltage-clamp and ion-flux studies. J. Exp. Biol. 1996, 199, 1545–1554. [Google Scholar] [CrossRef]
- Henry, R.P.; Campoverde, M. Neuroendocrine regulation of carbonic anhydrase expression in the gills of the euryhaline green crab, Carcinus maenas. J. Exp. Zool. Part A Comp. Exp. Biol. 2006, 305, 663–668. [Google Scholar] [CrossRef]
- Spencer, A.M.; Fielding, A.H.; Kamemoto, F.I. The relationship between gill NaK-ATPase activity and osmoregulatory capacity in various crabs. Physiol. Zool. 1979, 52, 1–10. [Google Scholar] [CrossRef]
- Henry, R.P.; Cameron, J.N. The distribution and partial characterization of carbonic anhydrase in selected aquatic and terrestrial decapod crustaceans. J. Exp. Zool. 1982, 221, 309–321. [Google Scholar] [CrossRef]
- Winkler, A. Effects of inorganic sea water constituents on branchial Na-K-ATPase activity in the shore crab Carcinus maenas. Mar. Biol. 1986, 92, 537–544. [Google Scholar] [CrossRef]
- Harris, R.; Bayliss, D. Gill (Na+ + K+)-ATPases in decapod crustaceans: Distribution and characteristics in relation to Na+ regulation. Comp. Biochem. Physiol. Part A Physiol. 1988, 90, 303–308. [Google Scholar] [CrossRef]
- Onken, H.; Riestenpatt, S. NaCl absorption across split gill lamellae of hyperregulating crabs: Transport mechanisms and their regulation. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 1998, 119, 883–893. [Google Scholar] [CrossRef]
- Barman, H.K.; Patra, S.K.; Das, V.; Mohapatra, S.D.; Jayasankar, P.; Mohapatra, C.; Mohanta, R.; Panda, R.P.; Rath, S.N. Identification and characterization of differentially expressed transcripts in the gills of freshwater prawn (Macrobrachium rosenbergii) under salt stress. Sci. World J. 2012, 2012, 149361. [Google Scholar] [CrossRef] [PubMed]
- Jaffer, Y.D.; Bhat, I.A.; Mir, I.N.; Bhat, R.A.H.; Sidiq, M.J.; Jana, P. Adaptation of cultured decapod crustaceans to changing salinities: Physiological responses, molecular mechanisms and disease implications. Rev. Aquac. 2024, 16, 1520–1543. [Google Scholar] [CrossRef]
- Firmino, K.C.S.; Faleiros, R.O.; Masui, D.C.; McNamara, J.C.; Furriel, R.P.M. Short-and long-term, salinity-induced modulation of V-ATPase activity in the posterior gills of the true freshwater crab, Dilocarcinus pagei (Brachyura, Trichodactylidae). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2011, 160, 24–31. [Google Scholar] [CrossRef]
- Faleiros, R.O.; Goldman, M.H.S.; Furriel, R.P.; McNamara, J.C. Differential adjustment in gill Na+/K+-and V-ATPase activities and transporter mRNA expression during osmoregulatory acclimation in the cinnamon shrimp Macrobrachium amazonicum (Decapoda, Palaemonidae). J. Exp. Biol. 2010, 213, 3894–3905. [Google Scholar] [CrossRef] [PubMed]
- Bozza, D.C.; Freire, C.A.; Prodocimo, V. Osmo-ionic regulation and carbonic anhydrase, Na+/K+-ATPase and V-H+-ATPase activities in gills of the ancient freshwater crustacean Aegla schmitti (Anomura) exposed to high salinities. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2019, 231, 201–208. [Google Scholar] [CrossRef]
- Boudour-Boucheker, N.; Boulo, V.; Charmantier-Daures, M.; Anger, K.; Charmantier, G.; Lorin-Nebel, C. Osmoregulation in larvae and juveniles of two recently separated Macrobrachium species: Expression patterns of ion transporter genes. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2016, 195, 39–45. [Google Scholar] [CrossRef]
- Gilmour, K.; Perry, S. Carbonic anhydrase and acid–base regulation in fish. J. Exp. Biol. 2009, 212, 1647–1661. [Google Scholar] [CrossRef]
- Havird, J.C.; Henry, R.P.; Wilson, A.E. Altered expression of Na+/K+–ATPase and other osmoregulatory genes in the gills of euryhaline animals in response to salinity transfer: A meta-analysis of 59 quantitative PCR studies over 10 years. Comp. Biochem. Physiol. Part D Genom. Proteom. 2013, 8, 131–140. [Google Scholar] [CrossRef]
- Luquet, C.M.; Weihrauch, D.; Senek, M.; Towle, D.W. Induction of branchial ion transporter mRNA expression during acclimation to salinity change in the euryhaline crab Chasmagnathus granulatus. J. Exp. Biol. 2005, 208, 3627–3636. [Google Scholar] [CrossRef]
- Serrano, L.; Halanych, K.M.; Henry, R.P. Salinity-stimulated changes in expression and activity of two carbonic anhydrase isoforms in the blue crab Callinectes sapidus. J. Exp. Biol. 2007, 210, 2320–2332. [Google Scholar] [CrossRef]
- Towle, D.W.; Palmer, G.E.; Harris, J.L., III. Role of gill Na+ + K+-dependent ATPase in acclimation of blue crabs (Callinectes sapidus) to low salinity. J. Exp. Zool. 1976, 196, 315–321. [Google Scholar] [CrossRef]
- Vargas, J.; Herpers, B.; McKie, A.; Gledhill, S.; McDonnell, J.; Van Den Heuvel, M.; Davies, K.; Ponting, C. Stromal cell-derived receptor 2 and cytochrome b561 are functional ferric reductases. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2003, 1651, 116–123. [Google Scholar] [CrossRef]
- Abbaspour, N.; Hurrell, R.; Kelishadi, R. Review on iron and its importance for human health. J. Res. Med. Sci. Off. J. Isfahan Univ. Med. Sci. 2014, 19, 164. [Google Scholar]
- Dallman, P.R. Biochemical Basis for the Manifestations of Iron Deficiency; Springer: New York, NY, USA, 1986. [Google Scholar]
- Puig, S.; Ramos-Alonso, L.; Romero, A.M.; Martínez-Pastor, M.T. The elemental role of iron in DNA synthesis and repair. Metallomics 2017, 9, 1483–1500. [Google Scholar] [CrossRef]
- Tom, M.; Manfrin, C.; Giulianini, P.G.; Pallavicini, A. Crustacean oxi-reductases protein sequences derived from a functional genomic project potentially involved in ecdysteroid hormones metabolism–A starting point for function examination. Gen. Comp. Endocrinol. 2013, 194, 71–80. [Google Scholar] [CrossRef]
- McNamara, J.C.; Freire, C.A.; Torres, A.H., Jr.; Faria, S.C. The conquest of fresh water by the palaemonid shrimps: An evolutionary history scripted in the osmoregulatory epithelia of the gills and antennal glands. Biol. J. Linn. Soc. 2015, 114, 673–688. [Google Scholar] [CrossRef]
- Havird, J.C.; Mitchell, R.T.; Henry, R.P.; Santos, S.R. Salinity-induced changes in gene expression from anterior and posterior gills of Callinectes sapidus (Crustacea: Portunidae) with implications for crustacean ecological genomics. Comp. Biochem. Physiol. Part D Genom. Proteom. 2016, 19, 34–44. [Google Scholar] [CrossRef]
- Leone, F.A.; Garçon, D.P.; Lucena, M.N.; Faleiros, R.O.; Azevedo, S.V.; Pinto, M.R.; McNamara, J.C. Gill-specific (Na+, K+)-ATPase activity and α-subunit mRNA expression during low-salinity acclimation of the ornate blue crab Callinectes ornatus (Decapoda, Brachyura). Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2015, 186, 59–67. [Google Scholar] [CrossRef]
- Ran, H.; Li, Z.; Yang, F.; Fan, Z.; Xu, C.; Han, F.; Farhadi, A.; Li, E.; Chen, H. Molecular pathways of osmoregulation in response to salinity stress in the gills of the scalloped spiny lobster (Panulirus homarus) within survival salinity. Comp. Biochem. Physiol. Part D Genom. Proteom. 2024, 52, 101308. [Google Scholar] [CrossRef]
- Ren, X.; Jia, S.; Gao, B.; Zhou, Q.; Xu, Y.; Liu, P.; Li, J. Application of proteomics and metabolomics to assess ammonia stress response and tolerance mechanisms of juvenile ornate rock lobster Panulirus ornatus. Sci. Total Environ. 2022, 837, 155751. [Google Scholar] [CrossRef]
- Wang, H.; Wei, H.; Tang, L.; Lu, J.; Mu, C.; Wang, C. A proteomics of gills approach to understanding salinity adaptation of Scylla paramamosain. Gene 2018, 677, 119–131. [Google Scholar] [CrossRef]
- Chen, J.-C.; Chen, C.-T.; Cheng, S.-Y. Nitrogen excretion and changes of hemocyanin, protein and free amino acid levels in the hemolymph of Penaeus monodon exposed to different concentrations of ambient ammonia-N at different salinity levels. Mar. Ecol.-Prog. Ser. 1994, 110, 85. [Google Scholar] [CrossRef]
- Chen, J.-C.; Lin, J.-N.; Chen, C.-T.; Lin, M.-N. Survival, growth and intermolt period of juvenile Penaeus chinensis (Osbeck) reared at different combinations of salinity and temperature. J. Exp. Mar. Biol. Ecol. 1996, 204, 169–178. [Google Scholar] [CrossRef]
- Haberfield, E.C.; Haas, L.; Hammen, C. Early ammonia release by a polychaete Nereis virens and a crab Carcinus maenas in diluted sea water. Comp. Biochem. Physiol. Part A Physiol. 1975, 52, 501–503. [Google Scholar] [CrossRef]
- Mangum, C.; Silverthorn, S.; Harris, J.; Towle, D.; Krall, A. The relationship between blood pH, ammonia excretion and adaptation to low salinity in the blue crab Callinectes sapidus. J. Exp. Zool. 1976, 195, 129–136. [Google Scholar] [CrossRef]
- Lee, W.-C.; Chen, J.-C. Hemolymph ammonia, urea and uric acid levels and nitrogenous excretion of Marsupenaeus japonicus at different salinity levels. J. Exp. Mar. Biol. Ecol. 2003, 288, 39–49. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, H.; Li, H.; Chen, T.; Ruan, Y.; Ren, C.; Luo, P.; Wang, Y.; Liu, B.; Li, H. Molecular identification of anion exchange protein 3 in Pacific white shrimp (Litopenaeus vannamei): mRNA profiles for tissues, ontogeny, molting, and ovarian development and its potential role in stress-induced gill damage. Front. Physiol. 2021, 12, 726600. [Google Scholar] [CrossRef] [PubMed]
- FAO. Cultured Aquatic Species Information Programme: Litopenaeus vannamei. Available online: https://www.fao.org/fishery/en/culturedspecies/litopenaeus_vannamei (accessed on 11 October 2025).
- De Silva, S.S.; Soto, D. Climate change and aquaculture: Potential impacts, adaptation and mitigation. In Climate Change Implications for Fisheries and Aquaculture: Overview of Current Scientific Knowledge; FAO Fisheries and Aquaculture Technical Paper: Rome, Italy, 2009; Volume 530, pp. 151–212. [Google Scholar]
- Fitzgibbon, Q.P.; Battaglene, S.C. Effect of photoperiod on the culture of early-stage phyllosoma and metamorphosis of spiny lobster (Sagmariasus verreauxi). Aquaculture 2012, 368, 48–54. [Google Scholar] [CrossRef]
- Smith, G.; Salmon, M.; Kenway, M.; Hall, M. Description of the larval morphology of captive reared Panulirus ornatus spiny lobsters, benchmarked against wild-caught specimens. Aquaculture 2009, 295, 76–88. [Google Scholar] [CrossRef]
- Aiken, D. Proecdysis, setal development, and molt prediction in the American lobster (Homarus americanus). J. Fish. Board Can. 1973, 30, 1337–1344. [Google Scholar] [CrossRef]
- Landman, M.J.; Fitzgibbon, Q.P.; Wirtz, A.; Codabaccus, B.M.; Ventura, T.; Smith, G.G.; Carter, C.G. Physiological status and nutritional condition of cultured juvenile Thenus australiensis over the moult cycle. Comp. Biochem. Physiol. Part B Biochem. Mol. Biol. 2020, 250, 110504. [Google Scholar] [CrossRef]
- Lewis, C.L.; Fitzgibbon, Q.P.; Smith, G.G.; Elizur, A.; Ventura, T. Transcriptomic analysis and time to hatch visual prediction of embryo development in the ornate spiny lobster (Panulirus ornatus). Front. Mar. Sci. 2022, 9, 1009. [Google Scholar] [CrossRef]
- Havird, J.C.; Santos, S.R.; Henry, R.P. Osmoregulation in the Hawaiian anchialine shrimp Halocaridina rubra (Crustacea: Atyidae): Expression of ion transporters, mitochondria-rich cell proliferation and hemolymph osmolality during salinity transfers. J. Exp. Biol. 2014, 217, 2309–2320. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, S.; Hu, Y.; Pan, L. Cloning and expression analysis of two carbonic anhydrase genes in white shrimp Litopenaeus vannamei, induced by pH and salinity stresses. Aquaculture 2015, 448, 391–400. [Google Scholar] [CrossRef]
- Chen, X.L.; Zhang, B.; Chng, Y.R.; Ong, J.L.; Chew, S.F.; Wong, W.P.; Lam, S.H.; Ip, Y.K. Na+/H+ exchanger 3 is expressed in two distinct types of ionocyte, and probably augments ammonia excretion in one of them, in the gills of the climbing perch exposed to seawater. Front. Physiol. 2017, 8, 880. [Google Scholar] [CrossRef]
- Ertl, N.G.; O’Connor, W.A.; Elizur, A. Molecular effects of a variable environment on Sydney rock oysters, Saccostrea glomerata: Thermal and low salinity stress, and their synergistic effect. Mar. Genom. 2019, 43, 19–32. [Google Scholar] [CrossRef]
- Herrera, V.; Emanuel, J.R.; Ruiz-Opazo, N.; Levenson, R.; Nadal-Ginard, B. Three differentially expressed Na, K-ATPase alpha subunit isoforms: Structural and functional implications. J. Cell Biol. 1987, 105, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Isenring, P.; Jacoby, S.C.; Chang, J.; Forbush, B., III. Mutagenic mapping of the Na-K-Cl cotransporter for domains involved in ion transport and bumetanide binding. J. Gen. Physiol. 1998, 112, 549–558. [Google Scholar] [CrossRef]
- Ali, M.Y.; Pavasovic, A.; Dammannagoda, L.K.; Mather, P.B.; Prentis, P.J. Comparative molecular analyses of select pH-and osmoregulatory genes in three freshwater crayfish Cherax quadricarinatus, C. destructor and C. cainii. PeerJ 2017, 5, e3623. [Google Scholar] [CrossRef]
- Angst, P.; Dexter, E.; Stillman, J.H. Genome assemblies of two species of porcelain crab, Petrolisthes cinctipes and Petrolisthes manimaculis (Anomura: Porcellanidae). G3 Genes Genomes Genet. 2024, 14, jkad281, Correction in G3 Genes Genomes Genet. 2024, 14, jkae027. [Google Scholar] [CrossRef]
- Towle, D.W.; Rushton, M.E.; Heidysch, D.; Magnani, J.J.; Rose, M.J.; Amstutz, A.; Jordan, M.K.; Shearer, D.W.; Wu, W.-S. Sodium/proton antiporter in the euryhaline crab Carcinus maenas: Molecular cloning, expression and tissue distribution. J. Exp. Biol. 1997, 200, 1003–1014. [Google Scholar] [CrossRef]
- Wang, Y.; Li, E.; Chen, L.; Wang, X.; Zhang, F.; Gao, L.; Long, L. Effect of acute salinity stress on soluble protein, hemocyanin, haemolymph glucose and hepatopancreas glycogen of Eriocheir sinensis. Acta Hydrobiol. Sin. 2012, 36, 1056–1062. [Google Scholar] [CrossRef]
- Polinski, J.M.; Zimin, A.V.; Clark, K.F.; Kohn, A.B.; Sadowski, N.; Timp, W.; Ptitsyn, A.; Khanna, P.; Romanova, D.Y.; Williams, P. The American lobster genome reveals insights on longevity, neural, and immune adaptations. Sci. Adv. 2021, 7, eabe8290. [Google Scholar] [CrossRef] [PubMed]
- Hyde, C.J.; Fitzgibbon, Q.P.; Elizur, A.; Smith, G.G.; Ventura, T. Transcriptional profiling of spiny lobster metamorphosis reveals three new additions to the nuclear receptor superfamily. BMC Genom. 2019, 20, 531, Correction in BMC Genom. 2019, 20, 622. [Google Scholar]
- Ventura, T.; Chandler, J.C.; Nguyen, T.V.; Hyde, C.J.; Elizur, A.; Fitzgibbon, Q.P.; Smith, G.G. Multi-tissue transcriptome analysis identifies key sexual development-related genes of the ornate spiny lobster (Panulirus ornatus). Genes 2020, 11, 1150. [Google Scholar] [CrossRef] [PubMed]
- Letunic, I.; Doerks, T.; Bork, P. SMART: Recent updates, new developments and status in 2015. Nucleic Acids Res. 2015, 43, D257–D260. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Community, T.G. The Galaxy platform for accessible, reproducible, and collaborative data analyses: 2024 update. Nucleic Acids Res. 2024, 52, W83–W94. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



| Salinity (ppt) | 25 | 34 | 40 | df | F or x2 | P | Test |
|---|---|---|---|---|---|---|---|
| Survival (%) | 83 | 100 | 91 | ||||
| Initial WW (g) | 2.88 ± 0.89 | 3.20 ± 0.91 | 2.84 ± 0.45 | 1, 40 | 0.015 | 0.91 | ANOVA |
| Initial CL (mm) | 11.5 ± 1.98 | 11.3 ± 1.88 | 12.0 ± 2.47 | 3 | 1.14 | 0.77 | Kruskal–Wallis |
| Final WW (g) | 5.90 ± 1.028 | 7.02 ± 1.83 | 5.79 ± 1.34 | 1, 33 | 0.012 | 0.91 | ANOVA |
| Final CL (mm) | 21.58 ± 1.93 | 22.22 ± 2.11 | 20.22 ± 2.73 | 1, 33 | 1.331 | 0.257 | ANOVA |
| WW gain (g) | 3.57 ± 1.06 | 3.82 ± 0.99 | 2.95 ± 1.38 | 1, 33 | 1.16 | 0.29 | ANOVA |
| CL gain (mm) | 18.1 ± 5.71 | 14.0 ± 1.42 | 8.98 ± 8.03 | 3, 14 | 2.19 | 0.15 | ANOVA |
| Moult growth increment (%BWd−1) (J8–J9) | 7.65 ab ± 2.43 | 8.89 b ± 2.67 | 5.19 a ± 2.16 | 2, 33 | 7.267 | 0.01 * | ANOVA |
| Moult increment (days) (J8–J9) | 18.7 a ± 3.62 | 19.93 a ± 3.75 | 26.0 b ± 7.09 | 2, 33 | 6.823 | 0.01 * | ANOVA |
| Salinity (ppt) | 25 | 34 | 40 | df | x2 | P | Test |
|---|---|---|---|---|---|---|---|
| NKA (RPKM) | 481.9 ± 144.35 | 248.8 ± 129.30 | 233.65 ± 46.46 | 2 | 4.03 | 0.39 | Kruskal–Wallis |
| CA (RPKM) | 803.37 ± 223.67 | 811.30 ± 294.08 | 608.55 ± 185.47 | 2 | 0.47 | 0.88 | Kruskal–Wallis |
| VHE (RPKM) | 31.13 ± 5.85 | 33.66 ± 6.21 | 33.45 ± 12.94 | 2 | 0.25 | 0.88 | Kruskal–Wallis |
| NKCC (RPKM) | 235.43 ± 183.12 | 7.03 ± 5.51 | 0.60 ± 0.45 | 2 | 6.33 | 0.15 | Kruskal–Wallis |
| NBC (RPKM) | 107.03 ± 28.02 | 70.90 ± 26.76 | 48.40± 10.04 | 2 | 3.14 | 0.42 | Kruskal–Wallis |
| NHE (RPKM) | 11.90 ± 1.32 | 10.76 ± 4.83 | 8.60 ± 0.28 | 2 | 2.47 | 0.43 | Kruskal–Wallis |
| Salinity (ppt) | 25 | 34 | 40 | df | x2 | P | Test |
|---|---|---|---|---|---|---|---|
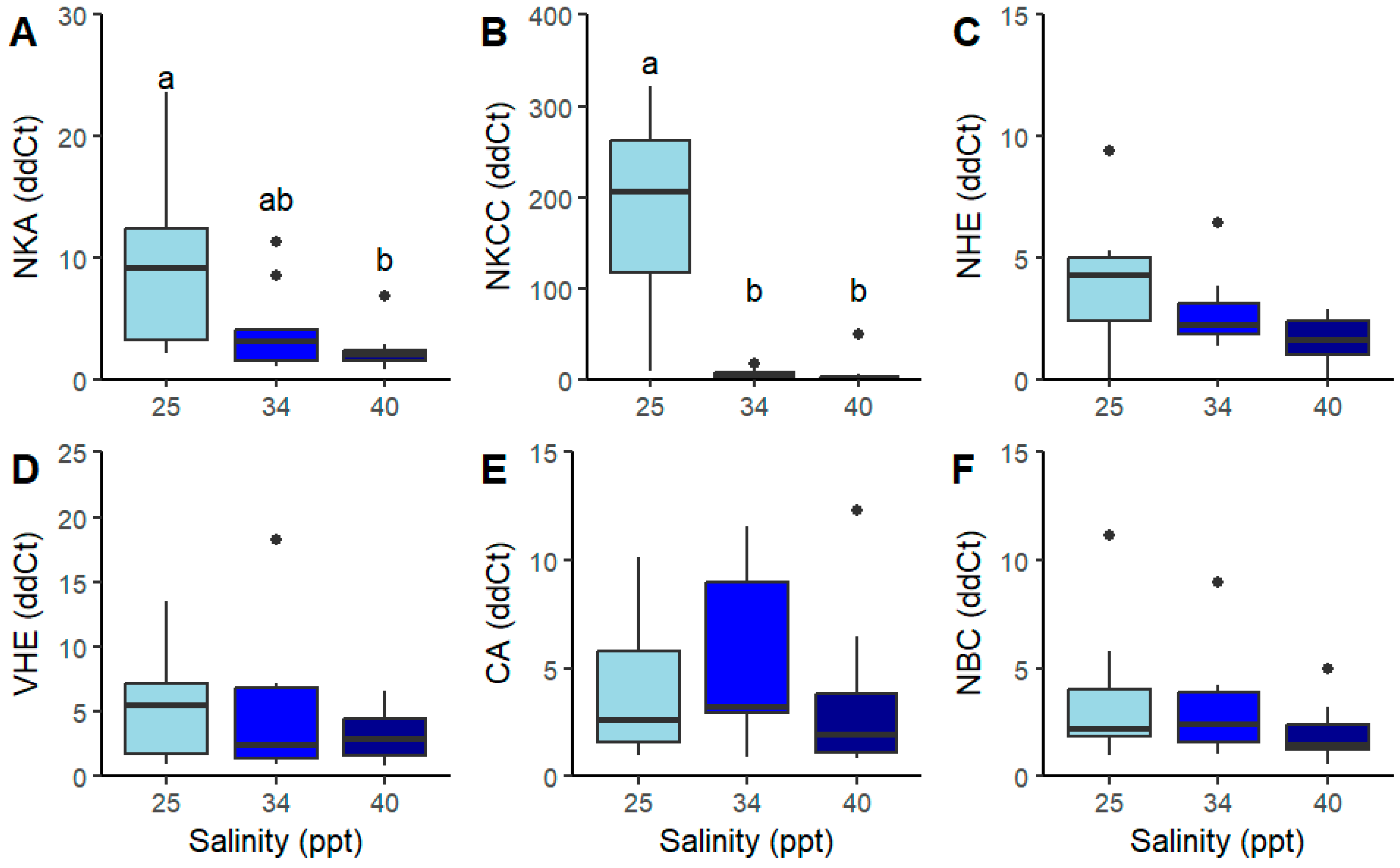
| NBC (ddCt) | 3.56 ± 3.22 | 3.12 ± 2.35 | 1.95 ± 1.31 | 2 | 2.61 | 0.32 | Kruskal–Wallis |
| CA (ddCt) | 11.83 ± 16.40 | 7.96 ± 8.71 | 3.29 ± 3.34 | 2 | 3.40 | 0.27 | Kruskal–Wallis |
| NKA (ddCt) | 9.03 a ± 7.30 | 3.98 ab ± 3.37 | 2.27 b ± 1.55 | 2 | 10.35 | >0.01 * | Kruskal–Wallis |
| VHE (ddCt) | 5.27 ± 4.06 | 17.63 ± 40.93 | 3.28 ± 1.97 | 2 | 0.88 | 0.65 | Kruskal–Wallis |
| NHE (ddCt) | 3.93 ± 2.62 | 2.73 ± 1.52 | 1.62 ± 0.99 | 2 | 6.64 | 0.07 | Kruskal–Wallis |
| NKCC (ddCt) | 283.65 a ± 224.43 | 6.34 b ± 4.71 | 5.94 b ± 14.48 | 2 | 21.50 | >0.01 * | Kruskal–Wallis |
| Gene Name | Primer Sequence | Sequence | Amplicon Size |
|---|---|---|---|
| V-type H+-ATPase | Forward | TGTTTGCGGTCATGTTTGGT | 241 |
| Reverse | CGTATGTGCCAAGATGAGCC | ||
| carbonic anhydrase | Forward | TGGCACTCCTCGTTCAAGAT | 208 |
| Reverse | CGCATGATAGAGGGAGGTGT | ||
| Na+/K+-ATPase | Forward | TAACTCTCACAGCCAAGCGA | 235 |
| Reverse | GAAAGTGCCTTCCAGCCTTC | ||
| Na+/H+ antiporter | Forward | TGCCAACAACAACCACAACT | 242 |
| Reverse | TGGCAGGTCAACACTCGTAT | ||
| Na+/K+/Cl− cotransporter | Forward | GGAGGGTATGCTGATCGTGA | 210 |
| Reverse | ACAGAAGCCCACGATGTACA | ||
| 18s | Forward | AACGGACTTGACGGTTGGTT | 21 |
| Reverse | CTGTTCGGAGCCTGACAGAA | ||
| Na+/HCO3− co-transporter | Forward | ACTCAGGGTCAGGCTTCTTC | 116 |
| Reverse | GCGTGTAATGGAGCCAGATG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Spencer, E.L.; Fitzgibbon, Q.P.; Glendinning, S.; Lewis, C.L.; Banks, T.M.; Trotter, A.J.; Ventura, T.; Smith, G.G. Assessing Molecular Mechanisms of Stress Induced Salinity Adaptation in the Juvenile Ornate Spiny Lobster, Panulirus ornatus. Int. J. Mol. Sci. 2025, 26, 11150. https://doi.org/10.3390/ijms262211150
Spencer EL, Fitzgibbon QP, Glendinning S, Lewis CL, Banks TM, Trotter AJ, Ventura T, Smith GG. Assessing Molecular Mechanisms of Stress Induced Salinity Adaptation in the Juvenile Ornate Spiny Lobster, Panulirus ornatus. International Journal of Molecular Sciences. 2025; 26(22):11150. https://doi.org/10.3390/ijms262211150
Chicago/Turabian StyleSpencer, Eleanor L., Quinn P. Fitzgibbon, Susan Glendinning, Courtney L. Lewis, Thomas M. Banks, Andrew J. Trotter, Tomer Ventura, and Gregory G. Smith. 2025. "Assessing Molecular Mechanisms of Stress Induced Salinity Adaptation in the Juvenile Ornate Spiny Lobster, Panulirus ornatus" International Journal of Molecular Sciences 26, no. 22: 11150. https://doi.org/10.3390/ijms262211150
APA StyleSpencer, E. L., Fitzgibbon, Q. P., Glendinning, S., Lewis, C. L., Banks, T. M., Trotter, A. J., Ventura, T., & Smith, G. G. (2025). Assessing Molecular Mechanisms of Stress Induced Salinity Adaptation in the Juvenile Ornate Spiny Lobster, Panulirus ornatus. International Journal of Molecular Sciences, 26(22), 11150. https://doi.org/10.3390/ijms262211150








