β-Antithrombin Levels in Patients with Venous Thromboembolism
Abstract
1. Introduction
2. Results
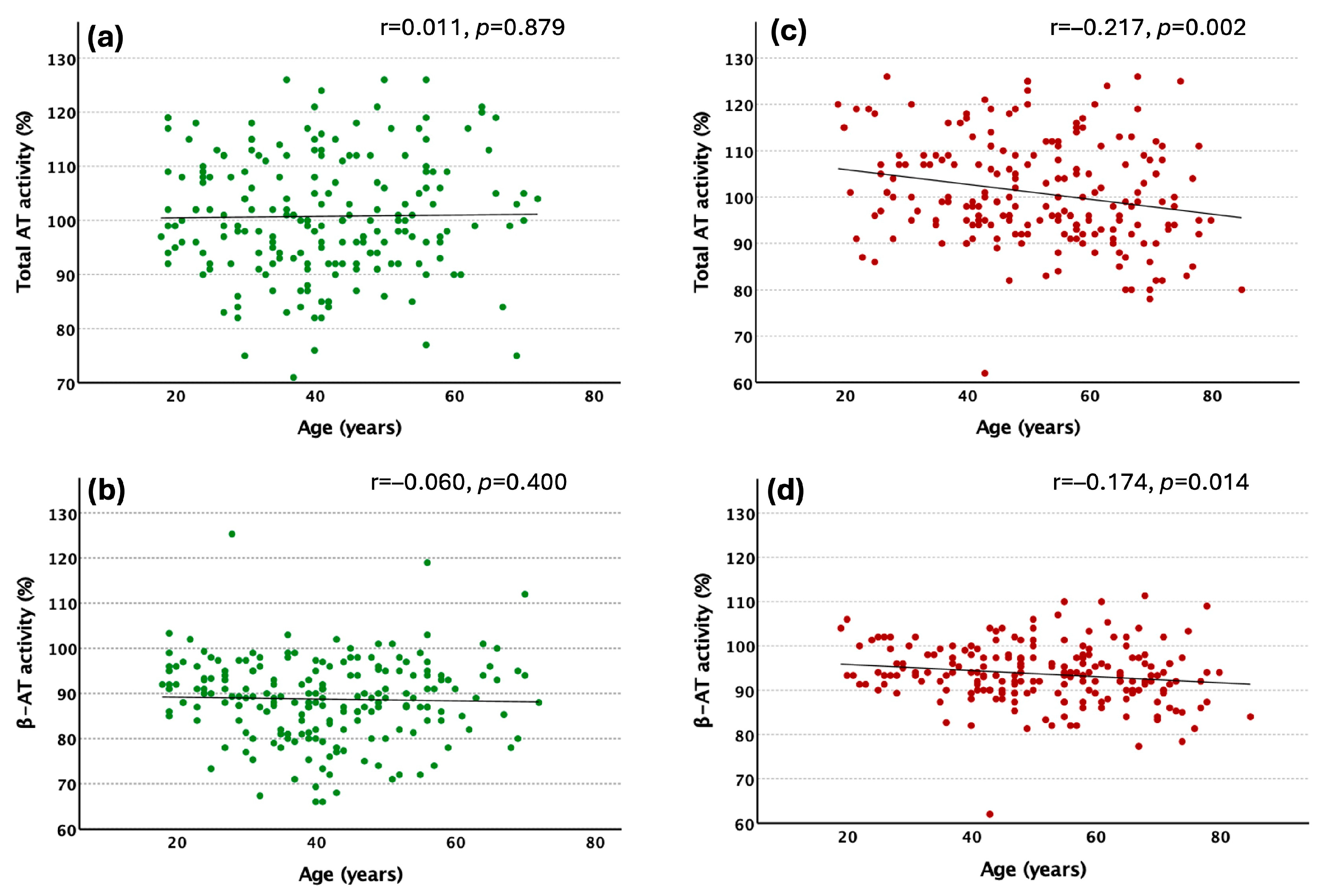
2.1. Characterization of the Study Population
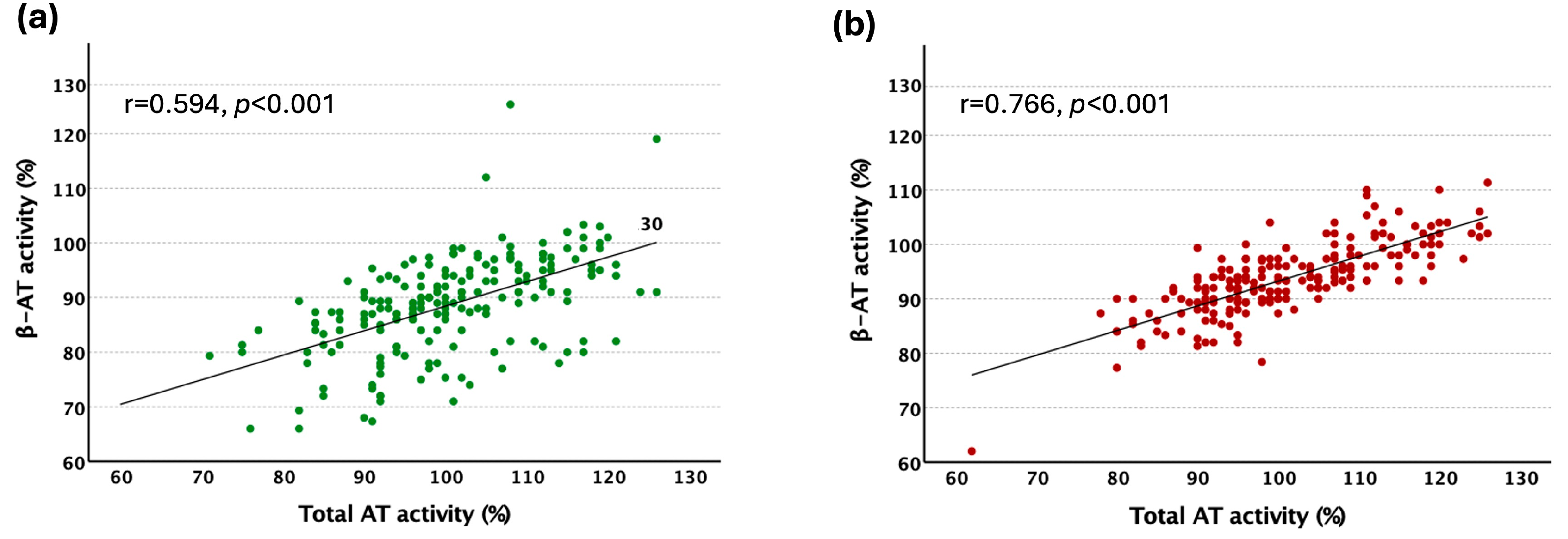
2.2. The Evaluation of β-AT Activity Assay
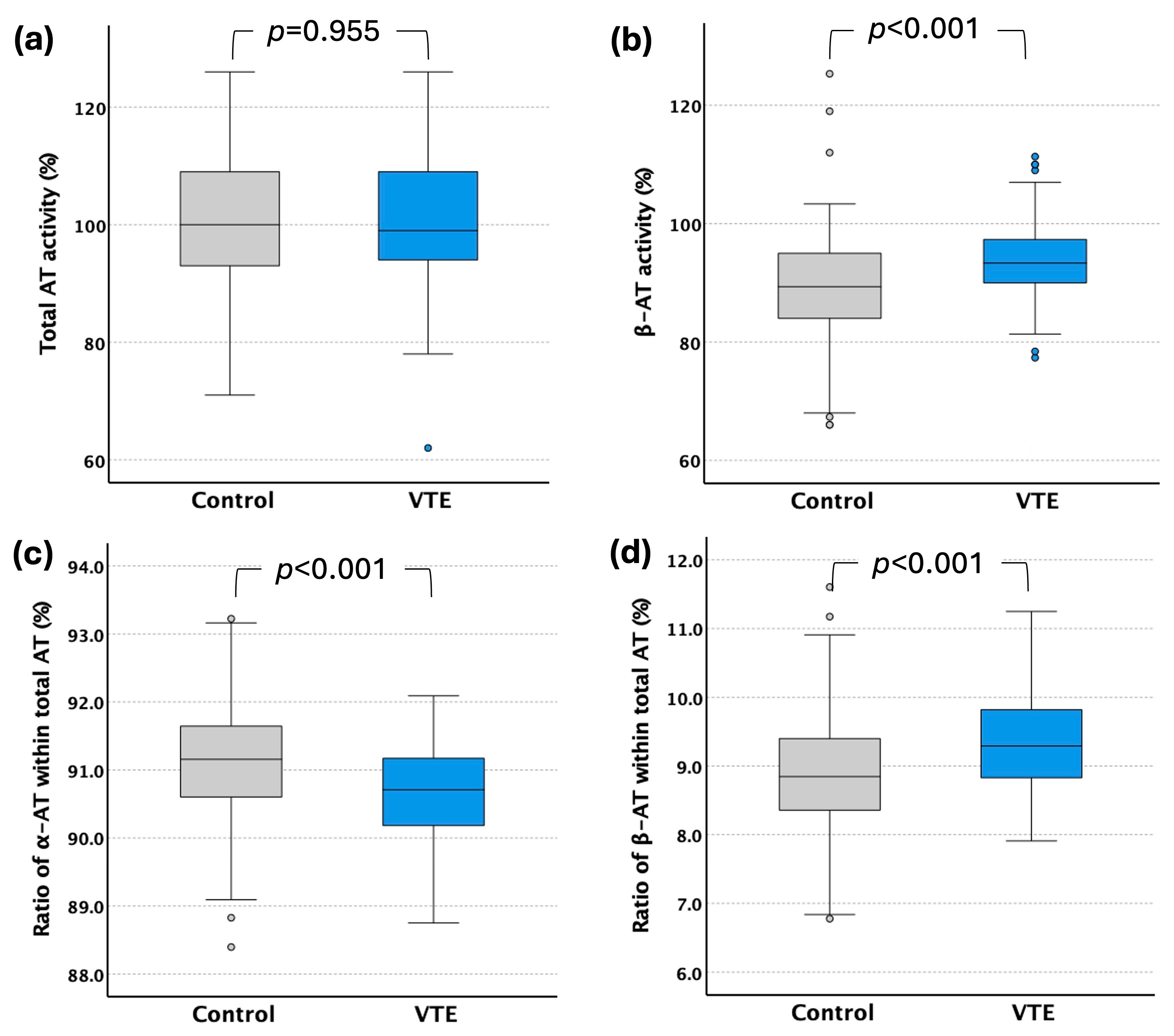
2.3. Total-AT and β-AT Levels in VTE Patients
2.4. β-AT Levels and Thrombotic Risk
3. Discussion
4. Materials and Methods
4.1. Subjects
4.2. Heparin Cofactor Antithrombin Activity Assay
4.3. Method Evaluation
4.4. Other Laboratory Methods
4.5. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AT | Antithrombin |
| BMI | Body mass index |
| CI | Confidence interval |
| CRP | C-reactive protein |
| CV | Coefficient of variation |
| DOAC | Direct oral anticoagulant |
| DVT | Deep vein thrombosis |
| EDTA | Ethylenediaminetetraacetic acid |
| FIIa | Activated factor II |
| FVIIa | Activated factor VII |
| FIXa | Activated factor IX |
| FXa | Activated factor X |
| GAG | Glycosaminoglycan |
| HDL | High density lipoprotein |
| OR | Odds ratio |
| IQR | Interquartile range |
| LDL | Low density lipoprotein |
| NPB-α2 PI | Non-plasminogen-binding α2-plasmin-inhibitor |
| PE | Pulmonary embolism |
| SD | Standard deviation |
| SERPIN | Serin protease inhibitor |
| VTE | Venous thromboembolism |
References
- Olson, S.T.; Bjork, I.; Shore, J.D. Kinetic characterization of heparin-catalyzed and uncatalyzed inhibition of blood coagulation proteinases by antithrombin. Methods Enzymol. 1993, 222, 525–559. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.A.; Caso, R. Antithrombin: Structure, genomic organization, function and inherited deficiency. Baillieres Clin. Haematol. 1989, 2, 961–998. [Google Scholar] [CrossRef] [PubMed]
- Quinsey, N.S.; Greedy, A.L.; Bottomley, S.P.; Whisstock, J.C.; Pike, R.N. Antithrombin: In control of coagulation. Int. J. Biochem. Cell Biol. 2004, 36, 386–389. [Google Scholar] [CrossRef] [PubMed]
- Rezaie, A.R.; Giri, H. Anticoagulant and signaling functions of antithrombin. J. Thromb. Haemost. 2020, 18, 3142–3153. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rosenberg, J.S.; McKenna, P.W.; Rosenberg, R.D. Inhibition of human factor IXa by human antithrombin. J. Biol. Chem. 1975, 250, 8883–8888. [Google Scholar] [CrossRef] [PubMed]
- Scott, C.F.; Schapira, M.; Colman, R.W. Effect of heparin on the inactivation rate of human factor XIa by antithrombin-III. Blood 1982, 60, 940–947. [Google Scholar] [CrossRef] [PubMed]
- Pixley, R.A.; Schapira, M.; Colman, R.W. The regulation of human factor XIIa by plasma proteinase inhibitors. J. Biol. Chem. 1985, 260, 1723–1729. [Google Scholar] [CrossRef] [PubMed]
- Rao, L.V.; Nordfang, O.; Hoang, A.D.; Pendurthi, U.R. Mechanism of antithrombin III inhibition of factor VIIa/tissue factor activity on cell surfaces. Comparison with tissue factor pathway inhibitor/factor Xa-induced inhibition of factor VIIa/tissue factor activity. Blood 1995, 85, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Olson, S.T.; Bjork, I.; Sheffer, R.; Craig, P.A.; Shore, J.D.; Choay, J. Role of the antithrombin-binding pentasaccharide in heparin acceleration of antithrombin-proteinase reactions. Resolution of the antithrombin conformational change contribution to heparin rate enhancement. J. Biol. Chem. 1992, 267, 12528–12538. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Abrahams, J.P.; Skinner, R.; Petitou, M.; Pike, R.N.; Carrell, R.W. The anticoagulant activation of antithrombin by heparin. Proc. Natl. Acad. Sci. USA 1997, 94, 14683–14688. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ishiguro, K.; Kojima, T.; Kadomatsu, K.; Nakayama, Y.; Takagi, A.; Suzuki, M.; Takeda, N.; Ito, M.; Yamamoto, K.; Matsushita, T.; et al. Complete antithrombin deficiency in mice results in embryonic lethality. J. Clin. Investig. 2000, 106, 873–878. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, Y.; Kretz, C.A.; Maeder, M.L.; Richter, C.E.; Tsao, P.; Vo, A.H.; Huarng, M.C.; Rode, T.; Hu, Z.; Mehra, R.; et al. Targeted mutagenesis of zebrafish antithrombin III triggers disseminated intravascular coagulation and thrombosis, revealing insight into function. Blood 2014, 124, 142–150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brennan, S.O.; George, P.M.; Jordan, R.E. Physiological variant of antithrombin-III lacks carbohydrate sidechain at Asn 135. FEBS Lett. 1987, 219, 431–436. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.J.; Pei, X.Y.; Skinner, R.; Abrahams, J.P.; Carrell, R.W. Structure of beta-antithrombin and the effect of glycosylation on antithrombin’s heparin affinity and activity. J. Mol. Biol. 2003, 326, 823–833. [Google Scholar] [CrossRef] [PubMed]
- Witmer, M.R.; Hatton, M.W. Antithrombin III-beta associates more readily than antithrombin III-alpha with uninjured and de-endothelialized aortic wall in vitro and in vivo. Arterioscler. Thromb. 1991, 11, 530–539. [Google Scholar] [CrossRef] [PubMed]
- Frebelius, S.; Isaksson, S.; Swedenborg, J. Thrombin inhibition by antithrombin III on the subendothelium is explained by the isoform AT beta. Arterioscler. Thromb. Vasc. Biol. 1996, 16, 1292–1297. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Sasaki, T.; Ohshima, K.; Sato, K.; Nagaoka, I.; Thachil, J. The Comparison of the Protective Effects of alpha- and beta-Antithrombin against Vascular Endothelial Cell Damage Induced by Histone in Vitro. TH Open 2017, 1, e3–e10. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khor, B.; Van Cott, E.M. Laboratory tests for antithrombin deficiency. Am. J. Hematol. 2010, 85, 947–950. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, B.; Bereczky, Z.; Selmeczi, A.; Gindele, R.; Olah, Z.; Kerenyi, A.; Boda, Z.; Muszbek, L. Progressive chromogenic anti-factor Xa assay and its use in the classification of antithrombin deficiencies. Clin. Chem. Lab. Med. 2014, 52, 1797–1806. [Google Scholar] [CrossRef] [PubMed]
- Van Cott, E.M.; Orlando, C.; Moore, G.W.; Cooper, P.C.; Meijer, P.; Marlar, R.; for the Subcommittee on Plasma Coagulation Inhibitors. Recommendations for clinical laboratory testing for antithrombin deficiency; Communication from the SSC of the ISTH. J. Thromb. Haemost. 2020, 18, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Karlaftis, V.; Sritharan, G.; Attard, C.; Corral, J.; Monagle, P.; Ignjatovic, V. Beta (beta)-antithrombin activity in children and adults: Implications for heparin therapy in infants and children. J. Thromb. Haemost. 2014, 12, 1141–1144. [Google Scholar] [CrossRef] [PubMed]
- de la Morena-Barrio, M.E.; Garcia, A.; Martinez-Martinez, I.; Minano, A.; Padilla, J.; Navarro-Fernandez, J.; Roldán, V.; Águila, S.; Iniesta, J.; Corral, J.; et al. A new method to quantify beta-antithrombin glycoform in plasma reveals increased levels during the acute stroke event. Thromb. Res. 2015, 136, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Croles, F.N.; Mulder, R.; Mulder, A.B.; Lukens, M.V.; Meijer, K. beta-Antithrombin, subtype of antithrombin deficiency and the risk of venous thromboembolism in hereditary antithrombin deficiency: A family cohort study. Thromb. Res. 2018, 168, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Wendelboe, A.; Weitz, J.I. Global Health Burden of Venous Thromboembolism. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1007–1011. [Google Scholar] [CrossRef] [PubMed]
- Maclean, P.S.; Tait, R.C. Hereditary and acquired antithrombin deficiency: Epidemiology, pathogenesis and treatment options. Drugs 2007, 67, 1429–1440. [Google Scholar] [CrossRef] [PubMed]
- Bucciarelli, P.; Passamonti, S.M.; Biguzzi, E.; Gianniello, F.; Franchi, F.; Mannucci, P.M.; Martinelli, I. Low borderline plasma levels of antithrombin, protein C and protein S are risk factors for venous thromboembolism. J. Thromb. Haemost. 2012, 10, 1783–1791. [Google Scholar] [CrossRef] [PubMed]
- Di Minno, M.N.; Dentali, F.; Lupoli, R.; Ageno, W. Mild antithrombin deficiency and risk of recurrent venous thromboembolism: A prospective cohort study. Circulation 2014, 129, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Sokol, J.; Timp, J.F.; le Cessie, S.; van Hylckama-Vlieg, A.; Rosendaal, F.R.; Kubisz, P.; Cannegieter, S.C.; Lijfering, W.M. Mild antithrombin deficiency and risk of recurrent venous thromboembolism: Results from the MEGA follow-up study. J. Thromb. Haemost. 2018, 16, 680–688. [Google Scholar] [CrossRef] [PubMed]
- Di Minno, M.N.; Dentali, F.; Veglia, F.; Russolillo, A.; Tremoli, E.; Ageno, W. Antithrombin levels and the risk of a first episode of venous thromboembolism: A case-control study. Thromb. Haemost. 2013, 109, 167–169. [Google Scholar] [CrossRef]
- Kattula, S.; Byrnes, J.R.; Wolberg, A.S. Fibrinogen and Fibrin in Hemostasis and Thrombosis. Arterioscler. Thromb. Vasc. Biol. 2017, 37, e13–e21. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rahmani, J.; Haghighian Roudsari, A.; Bawadi, H.; Thompson, J.; Khalooei Fard, R.; Clark, C.; Ryan, P.M.; Ajami, M.; Rahimi Sakak, F.; Salehisahlabadi, A.; et al. Relationship between body mass index, risk of venous thromboembolism and pulmonary embolism: A systematic review and dose-response meta-analysis of cohort studies among four million participants. Thromb. Res. 2020, 192, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Dix, C.; Zeller, J.; Stevens, H.; Eisenhardt, S.U.; Shing, K.; Nero, T.L.; Morton, C.J.; Parker, M.W.; Peter, K.; McFadyen, J.D. C-reactive protein, immunothrombosis and venous thromboembolism. Front. Immunol. 2022, 13, 1002652. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kawasaki, T.; Kambayashi, J.; Ariyoshi, H.; Sakon, M.; Suehisa, E.; Monden, M. Hypercholesterolemia as a risk factor for deep-vein thrombosis. Thromb. Res. 1997, 88, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Rafaqat, S.; Khalid, A.; Riaz, S.; Rafaqat, S. Irregularities of Coagulation in Hypertension. Curr. Hypertens. Rep. 2023, 25, 271–286. [Google Scholar] [CrossRef] [PubMed]
- Barath, B.; Bogati, R.; Miklos, T.; Kallai, J.; Mezei, Z.A.; Bereczky, Z.; Muszbek, L.; Katona, É. Effect of alpha2-plasmin inhibitor heterogeneity on the risk of venous thromboembolism. Thromb. Res. 2021, 203, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Balogh, I.; Poka, R.; Pfliegler, G.; Dekany, M.; Boda, Z.; Muszbek, L. High prevalence of factor V Leiden mutation and 20210A prothrombin variant in Hungary. Thromb. Haemost. 1999, 81, 660–661. [Google Scholar] [CrossRef] [PubMed]
- Kolarova, H.; Ambruzova, B.; Svihalkova Sindlerova, L.; Klinke, A.; Kubala, L. Modulation of endothelial glycocalyx structure under inflammatory conditions. Mediat. Inflamm. 2014, 2014, 694312. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, N.; Hao, R.; Ren, P.; Wang, J.; Dong, J.; Ye, T.; Zhao, D.; Qiao, X.; Meng, Z.; Gan, H.; et al. Glycosaminoglycans: Participants in Microvascular Coagulation of Sepsis. Thromb. Haemost. 2024, 124, 599–612. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Konstantinides, S.V.; Torbicki, A.; Agnelli, G.; Danchin, N.; Fitzmaurice, D.; Galie, N.; Gibbs, J.S.R.; Huisman, M.V.; Humbert, M.; Kucher, N.; et al. 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur. Heart J. 2014, 35, 3033–3080, Correction in Eur. Heart J. 2015, 36, 2642. Correction in Eur. Heart J. 2015, 36, 2666. [Google Scholar] [CrossRef] [PubMed]
- Mezei, Z.A.; Katona, E.; Kallai, J.; Bereczky, Z.; Somodi, L.; Molnar, E.; Kovács, B.; Miklós, T.; Ajzner, É.; Muszbek, L. Factor XIII levels and factor XIII B subunit polymorphisms in patients with venous thromboembolism. Thromb. Res. 2017, 158, 93–97. [Google Scholar] [CrossRef] [PubMed]
| Control Group (n = 200) | VTE Group (n = 200) | Significance (p) | |
|---|---|---|---|
| Age (mean ± SD) | 41.1 ± 13.1 | 41.5 ± 13.4 | 0.540 |
| Gender (male/female ratio); n | 89/111 | 101/99 | 0.271 |
| Positive family history; n (%) | 60 (36.1) | 61 (34.3) | 0.736 |
| Smoking; n (%) | 40 (20.3) | 48 (24.0) | 0.399 |
| High blood pressure; n (%) | 40 (20.1) | 97 (48.5) | <0.001 |
| Diabetes mellitus; n (%) | 0 | 24 (12.0) | - |
| Factor V Leiden heterozygotes; n (%) | 17 (12.5) | 62 (31.2) | <0.001 |
| Factor V Leiden homozygotes; n (%) | 0 | 11 (5.5) | <0.001 |
| FII 20210G>A carriers; n (%) | 5 (3.7) | 12 (6.0) | 0.449 |
| Type of VTE | |||
| DVT; n (%) | 0 | 152 (76.0) | - |
| DVT + PE; n (%) | 0 | 42 (21.0) | - |
| Distal; n (%) | 0 | 88 (55.0) | - |
| Proximal; n (%) | 0 | 66 (41.0) | - |
| Upper extremity; n (%) | 0 | 6 (4.0) | - |
| PE; n (%) | 0 | 6 (3.0) | - |
| Anticoagulant therapy; n (%) | |||
| Vitamin K antagonist | 0 | 158 (79.0) | - |
| Heparin | 0 | 12 (6.0) | - |
| DOAC | 0 | 18 (9.0) | - |
| No therapy | 12 (6.0) | ||
| BMI (median (IQR)) | 25.2 (21.9–28.3) | 29.0 (25.7–32.9) | <0.001 |
| Fibrinogen; g/L (mean ± SD) | 3.34 ± 0.60 | 3.65 ± 0.66 | <0.001 |
| CRP; mg/L (median (IQR)) | 1.75 (1.00–3.85) | 3.70 (2.33–6.40) | <0.001 |
| Triglyceride; mmol/L (median (IQR)) | 1.98 (1.44–2.59) | 1.56 (1.10–2.19) | <0.001 |
| Total cholesterol; mmol/L (median (IQR)) | 4.96 (4.41–5.44) | 5.19 (4.51–6.01) | 0.055 |
| HDL cholesterol; mmol/L (median (IQR)) | 1.21 (1.03–1.42) | 1.19 (0.99–1.45) | 0.951 |
| LDL cholesterol; mmol/L (median (IQR)) | 3.19 (2.66–3.67) | 3.30 (2.69–4.01) | 0.382 |
| Total α2-PI antigen; mg/L (mean ± SD) | 65.5 ± 7.9 | 72.3 ± 9.1 | <0.001 |
| NPB-α2-PI antigen; mg/L (mean ± SD) | 23.1 ± 6.8 | 30.6 ± 7.1 | <0.001 |
| Parameter | Group | Total | Men | Women | Significance (p) |
|---|---|---|---|---|---|
| Total AT activity (%) (median, IQR) | Control | 100 (93–109) | 100 (94–112) | 99 (92–106) | 0.223 |
| VTE | 99 (94–109) | 98 (92–107) | 101 (94–111) | 0.098 | |
| β-AT activity (%) (median, IQR) | Control | 89.3 (84.0–95.0) | 89.0 (81.7–94.0) | 90.0 (86.0–95.3) | 0.219 |
| VTE | 93.3 (90.0–97.3) | 93.0 (89–96) | 94.0 (90–99) | 0.031 | |
| Ratio of β-AT within total AT (%) (mean ± SD) | Control | 8.86 ± 0.88 | 8.74 ± 0.99 | 8.96 ± 0.78 | 0.077 |
| VTE | 9.34 ± 0.68 | 9.36 ± 0.68 | 9.31 ± 0.67 | 0.582 |
| Parameter (n, Upper Third/Lowest Third in Controls vs. Patients) | OR | 95% CI | Significance (p) |
|---|---|---|---|
| Total AT activity % (52/52 vs. 52/49) | 0.942 | 0.599–1.482 | 0.797 |
| adjusted | 1.509 | 0.764–2.98 | 0.236 |
| β-AT activity % (52/56 vs. 80/16) | 5.783 | 3.077–10.867 | <0.001 |
| adjusted | 12.207 | 5.248–28.313 | <0.001 |
| Ratio of β-AT within total AT (%) (50/50 vs. 89/12) | 6.147 | 3.362–11.240 | <0.001 |
| adjusted | 6.400 | 2.971–13.788 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uj, E.A.; Molnár, É.; Miklós, T.; Gindele, R.; Shemirani, A.H.; Bereczky, Z.; Katona, É. β-Antithrombin Levels in Patients with Venous Thromboembolism. Int. J. Mol. Sci. 2025, 26, 11151. https://doi.org/10.3390/ijms262211151
Uj EA, Molnár É, Miklós T, Gindele R, Shemirani AH, Bereczky Z, Katona É. β-Antithrombin Levels in Patients with Venous Thromboembolism. International Journal of Molecular Sciences. 2025; 26(22):11151. https://doi.org/10.3390/ijms262211151
Chicago/Turabian StyleUj, Edith Alexandra, Éva Molnár, Tünde Miklós, Réka Gindele, Amir Houshang Shemirani, Zsuzsanna Bereczky, and Éva Katona. 2025. "β-Antithrombin Levels in Patients with Venous Thromboembolism" International Journal of Molecular Sciences 26, no. 22: 11151. https://doi.org/10.3390/ijms262211151
APA StyleUj, E. A., Molnár, É., Miklós, T., Gindele, R., Shemirani, A. H., Bereczky, Z., & Katona, É. (2025). β-Antithrombin Levels in Patients with Venous Thromboembolism. International Journal of Molecular Sciences, 26(22), 11151. https://doi.org/10.3390/ijms262211151







