Microgravity Impacts the Expression of Aging-Associated Candidate Gene Targets in the p53 Regulatory Network
Abstract
1. Introduction
Objective
2. Results and Discussion
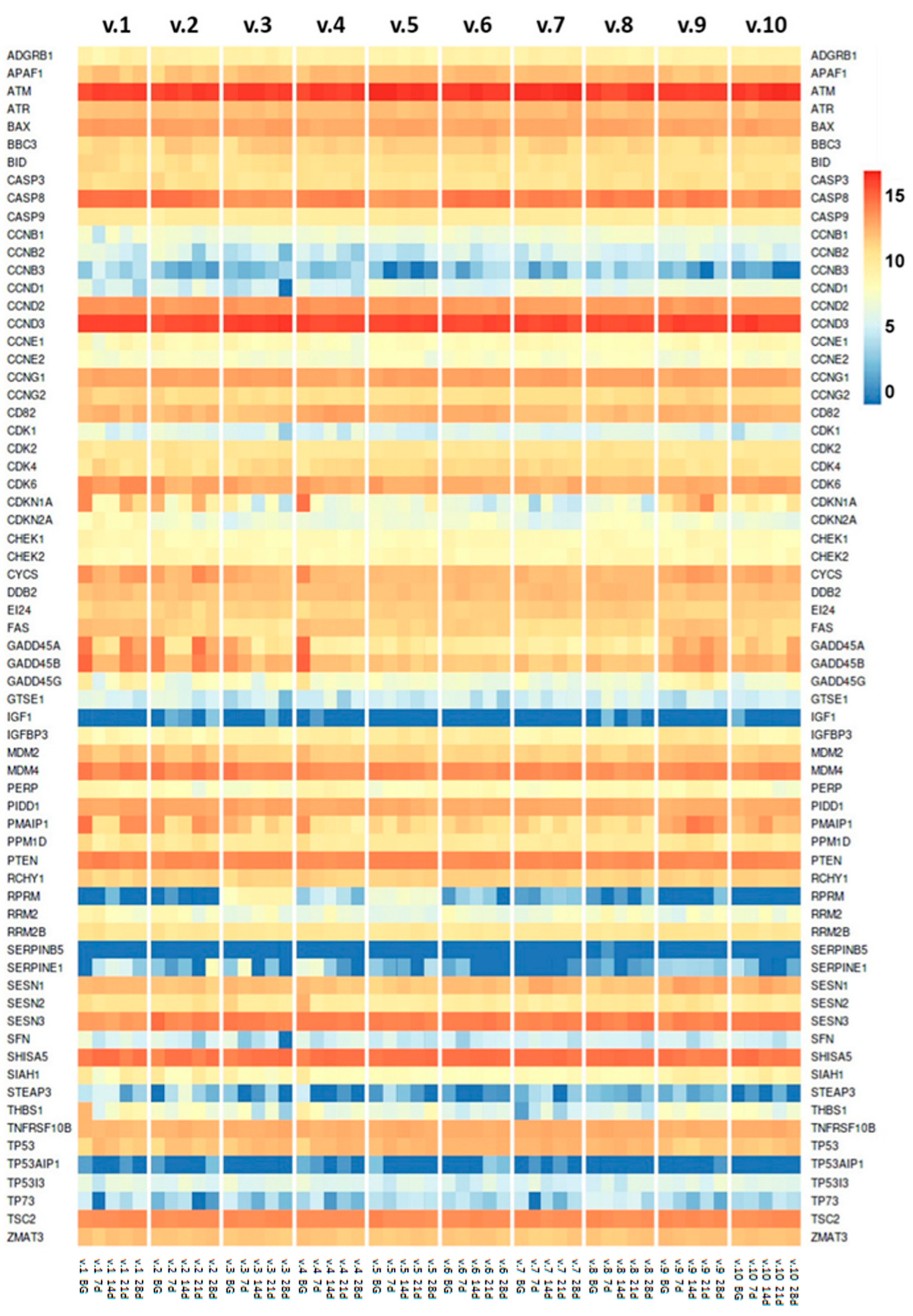
2.1. RNA-Seq Data Output Summary
2.2. TP53 Tumor Suppressor Gene Expression
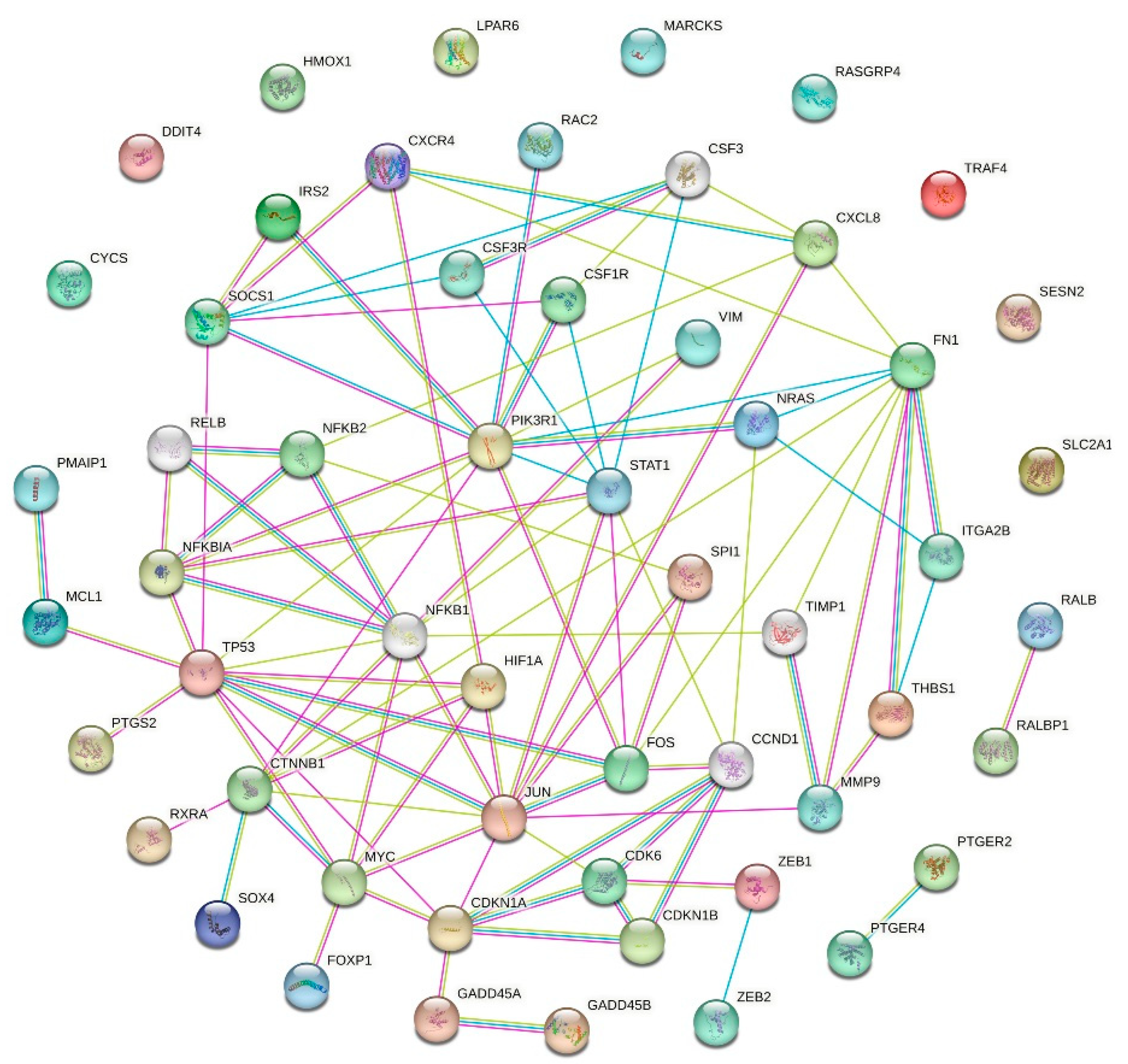
2.3. p53 Gene Network
2.4. Aging-Associated Genes in p53 Gene Network and Interacting Pathways
| # | NCBI Gene ID | Gene Symbol | Timepoint/ log2FC/ q-Value | Gene Name | Cite |
|---|---|---|---|---|---|
| 1 | 317 | APAF1 | 14 day/0.9944/0.0115 | apoptotic peptidase activating factor 1 | [29] |
| 2 | 581 | BAX | 7 day/0.6738/0.0464 | BCL2-associated X, apoptosis regulator | [30] |
| 3 | 27113 | BBC3 | 7 day/1.0363/0.0245 | BCL2 binding component 3 | [31] |
| 4 | 891 | CCNB1 | 21 day/−1.1572/0.0027 | cyclin B1 | [32] |
| 5 | 9133 | CCNB2 | 21 day/−2.1904/0.0038 | cyclin B2 | [33] |
| 6 | 85417 | CCNB3 | 7 day/−2.3464/0.0014 | cyclin B3 | [34] |
| 7 | 3732 | CD82 | 14 day/0.6182/0.0440 | CD82 molecule | [35] |
| 8 | 983 | CDK1 | 14 day/−1.6466/0.0474 | cyclin-dependent kinase 1 | [36] |
| 9 | 1019 | CDK4 | 7 day/1.1546/0.0060 | cyclin-dependent kinase 4 | [37] |
| 10 | 1021 | CDK6 | 14 day/−1.355/3.34 × 10−6 | cyclin-dependent kinase 6 | [38] |
| 11 | 1026 | CDKN1A | 7 day/−5.2107/2.27 × 10−32 | cyclin-dependent kinase inhibitor 1A | [39] |
| 12 | 1026 | CDKN2A | 21 day/1.8168/0.0341 | cyclin-dependent kinase inhibitor 2A | [40] |
| 13 | 54205 | CYCS | 7 day/−1.4602/3.51 × 10−8 | cytochrome c, somatic | [41] |
| 14 | 1647 | GADD45A | 7 day/−3.9401/2.21 × 10−37 | growth arrest and DNA damage-inducible alpha | [42] |
| 15 | 4616 | GADD45B | 7 day/−2.3869/3.54 × 10−32 | growth arrest and DNA damage-inducible beta | [43] |
| 16 | 10912 | GADD45G | 28 day/−3.1241/4.80 × 10−3 | growth arrest and DNA damage-inducible gamma | [44] |
| 17 | 51512 | GTSE1 | 28 day/−1.9802/1 × 10−5 | G2 and S-phase expressed 1 | [45] |
| 18 | 3479 | IGF1 | 28 day/1.0213/0.0041 | insulin-like growth factor 1 | [46,47] |
| 19 | 3486 | IGFBP3 | 7 day/0.8320/0.0199 | insulin-like growth factor binding protein 3 | [48] |
| 20 | 4194 | MDM4 | 7 day/−0.7708/0.0022 | MDM4 regulator of p53 | [49] |
| 21 | 5366 | PMAIP1 | 7 day/−2.4366/2.24 × 10−11 | phorbol-12-myristate-13-acetate-induced protein 1 | [50] |
| 22 | 27244 | SESN1 | 14 day/0.9173/0.0069 | sestrin 1 | [51] |
| 23 | 2810 | SFN | 7 day/−2.5663/4.25 × 10−5 | stratifin | [52] |
| 24 | 6477 | SIAH1 | 7 day/−2.134/0.0121 | siah E3 ubiquitin protein ligase 1 | [53] |
| 25 | 55240 | STEAP3 | 21 day/−3.4598/0.0452 | STEAP3 metalloreductase | [54] |
| 26 | 7057 | THBS1 | 7 day/−5.8423/4.97 × 10−13 | thrombospondin 1 | [55] |
| 27 | 7157 | TP53 | 21 day/1.1392/0.00134 | tumor protein p53 | [56,57] |
| 28 | 63970 | TP53AIP1 | 28 day/0.89438/0.0434 | tp53-regulated apoptosis-inducing protein 1 | [58] |
| 29 | 9540 | TP53I3 | 14 day/1.2081/0.0103 | tumor protein p53-inducible protein 3 | [59] |
| 30 | 7161 | TP73 | 14 day/−2.3214/0.0387 | tumor protein p73 | [60] |
2.5. Limitations of the Study and Future Perspectives
3. Materials and Methods
3.1. Dry Immersion Study Ethical Approval
3.2. Dry Immersion Experiment Setup
3.3. Peripheral Blood Sample Collection
3.4. CD3+ T Cell Isolation
3.5. RNA Extraction
3.6. RNA-Seq and Bioinformatic Analysis
3.7. Statistical Analysis
3.8. Protein–Protein Interaction (PPI) Network
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
| BG | background |
| DI | dry immersion |
| FPKM | fragments per kilobase of transcript per million mapped fragments |
| MG | microgravity |
| PBMC | peripheral blood mononuclear cells |
| PPI | protein–protein interactions |
| QC | quality control |
| SMG | simulated microgravity |
References
- Goswami, N.; van Loon, J.J.; Roessler, A.; Blaber, A.P.; White, O. Gravitational physiology, aging and medicine. Front. Physiol. 2019, 10, 1338. [Google Scholar] [CrossRef] [PubMed]
- Kuznetsov, N.V.; Statsenko, Y.; Ljubisavljevic, M. An Update on Neuroaging on Earth and in Spaceflight. Int. J. Mol. Sci. 2025, 26, 1738. [Google Scholar] [CrossRef] [PubMed]
- 5 Hazards of Human Spaceflight. Available online: https://www.nasa.gov/hrp/hazards/ (accessed on 30 December 2024).
- Tomilovskaya, E.; Shigueva, T.; Sayenko, D.; Rukavishnikov, I.; Kozlovskaya, I. Dry immersion as a ground-based model of microgravity physiological effects. Front. Physiol. 2019, 10, 284. [Google Scholar] [CrossRef] [PubMed]
- Petitjean, A.; Mathe, E.; Kato, S.; Ishioka, C.; Tavtigian, S.V.; Hainaut, P.; Olivier, M. Impact of mutant p53 functional properties on TP53 mutation patterns and tumor phenotype: Lessons from recent developments in the IARC TP53 database. Hum. Mutat. 2007, 28, 622–629. [Google Scholar] [CrossRef]
- Lane, D.P. p53, guardian of the genome. Nature 1992, 358, 6381. [Google Scholar] [CrossRef]
- Zaĭchuk, T.A.; Kuznetsov, N.V.; Osovskaia, V.S.; Kopnin, B.P.; Chumakov, P.M. Specific DNA-binding properties of oncoprotein p53 in human tumor cells. Dokl. Akad. Nauk. 1993, 330, 379–382. [Google Scholar]
- Zaichuk, T.A.; Kuznetsov, N.V.; Ossovskaya, V.S.; Kopnin, B.J.; Chumakov, P.M. Specific DNA-binding properties of p53 oncoprotein from human tumor cells. Proc. Russ. Acad. Sci. 1993, 330, 386–389. [Google Scholar]
- Kuznetsov, N.V. Analysis of DNA-Binding Properties of Protein P53. 1995. Available online: www.dslib.net/molekula/analiz-dnk-svjazyvajuwih-svojstv-belka-r53.html (accessed on 30 December 2024).
- Kondratov, R.V.; Kuznetsov, N.V.; Pugacheva, E.N.; Almazov, V.P.; Prasolov, V.S.; Kopnin, B.P.; Chumakov, P.M. Functional heterogeneity of p53-responsive elements. Mol. Biol. 1996, 30, 613–620. [Google Scholar]
- Kondratov, R.V.; Pugacheva, E.N.; Kuznetsov, N.V.; Prasolov, V.S.; Kopnin, B.P.; Chumakov, P.M. The human adenosine deaminase gene contains a p53-responsive element. Dokl. Akad. Nauk. 1996, 346, 260–262. [Google Scholar]
- Chumakov, P.M.; Pugacheva, E.N.; Ossovskaya, V.S.; Kondratov, R.V.; Kuznetsov, N.V.; Ivanov, A.V.; Prassolov, V.S.; Mazo, I.A.; Gudkov, A.V.; Kopnin, B.P. Functions of the p53 involved in control of malignant transformation. In Cancer and the Cell Cycle; ISREC Press: Lausanne, Switzerland, 1996. [Google Scholar]
- Kondratov, R.V.; Kuznetsov, N.V.; Pugacheva, E.N.; Almazov, V.P.; Prasolov, V.S.; Chumakov, P.M.; Kopnin, B.P. Functional. Mol. Biol. 1996, 30 (Suppl. 2), 363–367. [Google Scholar]
- Chumakov, P.M. Versatile functions of p53 protein in multicellular organisms. Biochemistry 2007, 72, 1399–1421. [Google Scholar] [CrossRef]
- Tyner, S.D.; Venkatachalam, S.; Choi, J.; Jones, S.; Ghebranious, N.; Igelmann, H.; Lu, X.; Soron, G.; Cooper, B.; Brayton, C.; et al. p53 mutant mice that display early ageing-associated phenotypes. Nature 2002, 415, 45–53. [Google Scholar] [CrossRef]
- Maier, B.; Gluba, W.; Bernier, B.; Turner, T.; Mohammad, K.; Guise, T.; Sutherland, A.; Thorner, M.; Scrable, H. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 2004, 18, 306–319. [Google Scholar] [CrossRef] [PubMed]
- Reinhardt, H.C.; Schumacher, B. The p53 network: Cellular and systemic DNA damage responses in aging and cancer. Trends Genet. 2012, 28, 128–136. [Google Scholar] [CrossRef] [PubMed]
- Ohnishi, T.; Takahashi, A.; Wang, X.; Ohnishi, K.; Ohira, Y.; Nagaoka, S. Accumulation of a tumor suppressor p53 protein in rat muscle during a space flight. Mutat. Res. Fundam. Mol. Mech. Mutagen. 1999, 430, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Zhang, H.; Miao, G.Y.; Yi, X.I.; Chao, S.U.; Di, C.X.; Yang, L.I.; Liu, Y.Y.; Zhang, X.; Fei, X.; et al. Simulated microgravity conditions and carbon ion irradiation induce spermatogenic cell apoptosis and sperm DNA damage. Biomed. Environ. Sci. 2013, 26, 726–734. [Google Scholar]
- Takahashi, A.; Suzuki, H.; Omori, K.; Seki, M.; Hashizume, T.; Shimazu, T.; Ishioka, N.; Ohnishi, T. The expression of p53-regulated genes in human cultured lymphoblastoid TSCE5 and WTK1 cell lines during spaceflight. Int. J. Radiat. Biol. 2010, 86, 669–681. [Google Scholar] [CrossRef]
- Takahashi, A.; Suzuki, H.; Omori, K.; Seki, M.; Hashizume, T.; Shimazu, T.; Ishioka, N.; Ohnishi, T. Expression of p53-regulated proteins in human cultured lymphoblastoid TSCE5 and WTK1 cell lines during spaceflight. J. Radiat. Res. 2012, 53, 168–175. [Google Scholar] [CrossRef]
- Shi, L.; Tian, H.; Wang, P.; Li, L.; Zhang, Z.; Zhang, J.; Zhao, Y. Spaceflight and simulated microgravity suppresses macrophage development via altered RAS/ERK/NFκB and metabolic pathways. Cell. Mol. Immunol. 2021, 18, 1489–1502. [Google Scholar] [CrossRef]
- Popov, D.V.; Makhnovskii, P.A.; Zgoda, V.G.; Gazizova, G.R.; Vepkhvadze, T.F.; Lednev, E.M.; Motanova, E.S.; Lysenko, E.A.; Orlov, O.I.; Tomilovskaya, E.S. Rapid changes in transcriptomic profile and mitochondrial function in human soleus muscle after 3-day dry immersion. J. Appl. Physiol. 2023, 134, 1256–1264. [Google Scholar] [CrossRef]
- Battista, N.; Meloni, M.A.; Bari, M.; Mastrangelo, N.; Galleri, G.; Rapino, C.; Dainese, E.; Agro, A.F.; Pippia, P.; Maccarrone, M. 5-Lipoxygenase-dependent apoptosis of human lymphocytes in the International Space Station: Data from the ROALD experiment. FASEB J. 2012, 26, 1791–1798. [Google Scholar] [CrossRef]
- Rai, N.; Dey, S. Protective response of Sestrin under stressful conditions in aging. Ageing Res. Rev. 2020, 64, 101186. [Google Scholar] [CrossRef]
- Mathew, R.; Pal Bhadra, M.; Bhadra, U. Insulin/insulin-like growth factor-1 signalling (IIS) based regulation of lifespan across species. Biogerontology 2017, 18, 35–53. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.; Kim, M.M. IGFBP-3 plays an important role in senescence as an aging marker. Environ. Toxicol. Pharmacol. 2018, 59, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Eom, S.Y.; Kim, M.M. The effect of IGFBP3 gene knockout by the CRISPR/Cas9 system on the IGF-1 pathway in murine cells. Arch. Gerontol. Geriatr. 2024, 125, 105484. [Google Scholar] [CrossRef]
- Robles, A.I.; Bemmels, N.A.; Foraker, A.B.; Harris, C.C. APAF-1 is a transcriptional target of p53 in DNA damage-induced apoptosis. Cancer Res. 2001, 61, 6660–6664. [Google Scholar]
- Qian, S.; Wei, Z.; Yang, W.; Huang, J.; Yang, Y.; Wang, J. The role of BCL-2 family proteins in regulating apoptosis and cancer therapy. Front. Oncol. 2022, 12, 985363. [Google Scholar] [CrossRef]
- Hikisz, P.; Kiliańska, Z. PUMA, a critical mediator of cell death—One decade on from its discovery. Cell. Mol. Biol. Lett. 2012, 17, 646–669. [Google Scholar] [CrossRef]
- Moore, J.D.; Kirk, J.A.; Hunt, T. Unmasking the S-phase-promoting potential of cyclin B1. Science 2003, 300, 987–990. [Google Scholar] [CrossRef]
- Jackman, M.; Firth, M.; Pines, J. Human cyclins B1 and B2 are localized to strikingly different structures: B1 to microtubules, B2 primarily to the Golgi apparatus. EMBO J. 1995, 14, 1646–1654. [Google Scholar] [CrossRef] [PubMed]
- Lozano, J.C.; Perret, E.; Schatt, P.; Arnould, C.; Peaucellier, G.; Picard, A. Molecular cloning, gene localization, and structure of human cyclin B3. Biochem. Biophys. Res. Commun. 2002, 291, 406–413. [Google Scholar] [CrossRef] [PubMed]
- Christgen, M.; Bruchhardt, H.; Ballmaier, M.; Krech, T.; Länger, F.; Kreipe, H.; Lehmann, U. KAI1/CD82 is a novel target of estrogen receptor-mediated gene repression and downregulated in primary human breast cancer. Int. J. Cancer 2008, 123, 2239–2246. [Google Scholar] [CrossRef] [PubMed]
- Kipreos, E.T.; Wang, J.Y. Differential phosphorylation of c-Abl in cell cycle determined by cdc 2 kinase and phosphatase activity. Science 1990, 248, 217–220. [Google Scholar] [CrossRef]
- Ziegler, D.V.; Parashar, K.; Fajas, L. Beyond cell cycle regulation: The pleiotropic function of cdk4 in cancer. In Seminars in Cancer Biology; Academic Press: Cambridge, MA, USA, 2024; Volume 98, pp. 51–63. [Google Scholar]
- Nagasawa, M.; Melamed, I.; Kupfer, A.; Gelfand, E.W.; Lucas, J.J. Rapid nuclear translocation and increased activity of cyclin-dependent kinase 6 after T cell activation. J. Immunol. 1997, 158, 5146–5154. [Google Scholar] [CrossRef]
- Harper, J.W.; Adami, G.R.; Wei, N.; Keyomarsi, K.; Elledge, S.J. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell 1993, 75, 805–816. [Google Scholar] [CrossRef]
- Wu, Y.; Jiang, D.; Liu, Q.; Yan, S.; Liu, X.; Wu, T.; Sun, W.; Li, G. Cathepsin L induces cellular senescence by upregulating CUX1 and p16INK4a. Aging 2024, 16, 10749. [Google Scholar] [CrossRef]
- Chapman, J.; Fielder, E.; Passos, J.F. Mitochondrial dysfunction and cell senescence: Deciphering a complex relationship. FEBS Lett. 2019, 593, 1566–1579. [Google Scholar] [CrossRef]
- Smith, M.L.; Chen, I.T.; Zhan, Q.; Bae, I.; Chen, C.Y.; Gilmer, T.M.; Kastan, M.B.; O’Connor, P.M.; Fornace, A.J. Interaction of the p53-regulated protein Gadd45 with proliferating cell nuclear antigen. Science 1994, 266, 1376–1380. [Google Scholar] [CrossRef]
- Verzella, D.; Bennett, J.; Fischietti, M.; Thotakura, A.K.; Recordati, C.; Pasqualini, F.; Capece, D.; Vecchiotti, D.; d’Andrea, D.; Di Francesco, B.; et al. GADD45β loss ablates innate immunosuppression in cancer. Cancer Res. 2018, 78, 1275–1292. [Google Scholar] [CrossRef]
- Zhang, P.; You, N.; Ding, Y.; Zhu, W.; Wang, N.; Xie, Y.; Huang, W.; Ren, Q.; Qin, T.; Fu, R.; et al. Gadd45g insufficiency drives the pathogenesis of myeloproliferative neoplasms. Nat. Commun. 2024, 15, 2989. [Google Scholar] [CrossRef]
- Lin, F.; Xie, Y.J.; Zhang, X.K.; Huang, T.J.; Xu, H.F.; Mei, Y.; Liang, H.; Hu, H.; Lin, S.T.; Luo, F.F.; et al. GTSE1 is involved in breast cancer progression in p53 mutation-dependent manner. J. Exp. Clin. Cancer Res. 2019, 38, 1–6. [Google Scholar] [CrossRef]
- Khan, M.Z.; Zugaza, J.L.; Aleman, I.T. The signaling landscape of insulin-like growth factor 1. J. Biol. Chem. 2024, 3, 108047. [Google Scholar] [CrossRef] [PubMed]
- Conover, C.A.; Oxvig, C. The Insulin-like Growth Factor System and Aging. Endocr. Rev. 2024, 17, bnae029. [Google Scholar]
- Almeida, O.P.; Hankey, G.J.; Yeap, B.B.; Paul Chubb, S.A.; Gollege, J.; Flicker, L. Risk of prevalent and incident dementia associated with insulin-like growth factor and insulin-like growth factor-binding protein 3. Mol. Psychiatry 2018, 23, 1825–1829. [Google Scholar] [CrossRef] [PubMed]
- Francoz, S.; Froment, P.; Bogaerts, S.; De Clercq, S.; Maetens, M.; Doumont, G.; Bellefroid, E.; Marine, J.C. Mdm4 and Mdm2 cooperate to inhibit p53 activity in proliferating and quiescent cells in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 3232–3237. [Google Scholar] [CrossRef]
- Morsi, R.Z.; Hage-Sleiman, R.; Kobeissy, H.; Dbaibo, G. Noxa: Role in cancer pathogenesis and treatment. Curr. Cancer Drug Targets 2018, 18, 914–928. [Google Scholar] [CrossRef]
- Budanov, A.V.; Sablina, A.A.; Feinstein, E.; Koonin, E.V.; Chumakov, P.M. Regeneration of peroxiredoxins by p53-regulated sestrins, homologs of bacterial AhpD. Science 2004, 304, 596–600. [Google Scholar] [CrossRef]
- Kim, Y.; Shiba-Ishii, A.; Nakagawa, T.; Iemura, S.I.; Natsume, T.; Nakano, N.; Matsuoka, R.; Sakashita, S.; Lee, S.; Kawaguchi, A.; et al. Stratifin regulates stabilization of receptor tyrosine kinases via interaction with ubiquitin-specific protease 8 in lung adenocarcinoma. Oncogene 2018, 37, 5387–5402. [Google Scholar] [CrossRef]
- Fujita, K.; Horikawa, I.; Mondal, A.M.; Jenkins, L.M.; Appella, E.; Vojtesek, B.; Bourdon, J.C.; Lane, D.P.; Harris, C.C. Positive feedback between p53 and TRF2 during telomere-damage signalling and cellular senescence. Nat. Cell Biol. 2010, 12, 1205–1212. [Google Scholar] [CrossRef]
- Wang, B.; Han, J.; Elisseeff, J.H.; Demaria, M. The senescence-associated secretory phenotype and its physiological and pathological implications. Nat. Rev. Mol. Cell Biol. 2024, 25, 958–978. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, A.J.; Kelm, N.Q. Thrombospondin-1, free radicals, and the coronary microcirculation: The aging conundrum. Antioxid. Redox Signal. 2017, 27, 785–801. [Google Scholar] [CrossRef] [PubMed]
- Lane, D.; Levine, A. p53 Research: The past thirty years and the next thirty years. Cold Spring Harb. Perspect. Biol. 2010, 2, a000893. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M. Gene regulation by the tumor suppressor p53–The omics era. Biochim. Biophys. Acta (BBA) Rev. Cancer 2024, 11, 189111. [Google Scholar] [CrossRef]
- Liu, S.; Xu, T.; Chen, X.; Tang, L.; Li, L.; Zhang, L.; Yang, Y.; Huang, J. TP53AIP1 induce autophagy via the AKT/mTOR signaling pathway in the breast cancer cells. Cancer Biol. Ther. 2024, 25, 2398297. [Google Scholar] [CrossRef]
- Chaudhry, S.R.; Lopes, J.; Levin, N.K.; Kalpage, H.; Tainsky, M.A. Germline mutations in apoptosis pathway genes in ovarian cancer; the functional role of a TP53I3 (PIG3) variant in ROS production and DNA repair. Cell Death Discov. 2021, 7, 62. [Google Scholar] [CrossRef]
- Lopriore, P.; Capitanio, N.; Panatta, E.; Di Daniele, N.; Gambacurta, A.; Melino, G.; Amelio, I. TAp73 regulates ATP7A: Possible implications for ageing-related diseases. Aging 2018, 10, 3745. [Google Scholar] [CrossRef]
- Prochownik, E.V.; Wang, H. Lessons in aging from Myc knockout mouse models. Front. Cell Dev. Biol. 2023, 11, 1244321. [Google Scholar] [CrossRef]
- Yu, X.; Wang, Y.; Song, Y.; Gao, X.; Deng, H. AP-1 is a regulatory transcription factor of inflammaging in the murine kidney and liver. Aging Cell 2023, 22, e13858. [Google Scholar] [CrossRef]
- Cai, D.; Han, J.D. Aging-associated lncRNAs are evolutionarily conserved and participate in NFκB signaling. Nat. Aging 2021, 1, 438–453. [Google Scholar] [CrossRef]
- Haga, M.; Okada, M. Systems approaches to investigate the role of NF-κB signaling in aging. Biochem. J. 2022, 479, 161–183. [Google Scholar] [CrossRef] [PubMed]
- Alique, M.; Sánchez-López, E.; Bodega, G.; Giannarelli, C.; Carracedo, J.; Ramírez, R. Hypoxia-inducible factor-1α: The master regulator of endothelial cell senescence in vascular aging. Cells 2020, 9, 195. [Google Scholar] [CrossRef] [PubMed]
- Cancemi, P.; Aiello, A.; Accardi, G.; Caldarella, R.; Candore, G.; Caruso, C.; Ciaccio, M.; Cristaldi, L.; Di Gaudio, F.; Siino, V.; et al. The role of matrix metalloproteinases (MMP-2 and MMP-9) in ageing and longevity: Focus on sicilian long-living individuals (LLIs). Mediat. Inflamm. 2020, 2020, 8635158. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Tang, W.; Chen, Y.; Xue, L.; Dai, J.; Li, Y.; Zhu, X.; Wu, C.; Xiong, J.; Zhang, J.; et al. Spatiotemporal transcriptomic changes of human ovarian aging and the regulatory role of FOXP1. Nat. Aging 2024, 4, 527–545. [Google Scholar] [CrossRef]
- Kutko, O.V.; Rykova, M.P.; Antropova, E.N.; Kalinin, S.A.; Shulgina, S.M.; Sadova, A.A.; Orlova, K.D.; Kiseleva, D.D.; Smarov, V.A.; Vassilieva, G.Y.; et al. Effect of 21-day dry immersion on the production of cytokines involved in the regulation of bone metabolism by T cells. Hum. Physiol. 2020, 46, 787–791. [Google Scholar] [CrossRef]
- Kuznetsov, N.V.; Almuzzaini, B.; Kritikou, J.S.; Baptista, M.A.P.; Oliveira, M.M.S.; Keszei, M.; Snapper, S.B.; Percipalle, P.; Westerberg, L.S. Nuclear Wiskott-Aldrich syndrome protein co-regulates T cell factor 1-mediated transcription in T cells. Genome Med. 2017, 9, 91. [Google Scholar] [CrossRef]
- Kouznetsov, N.V. Cell responses to simulated microgravity and hydrodynamic stress can be distinguished by using comparative transcriptomic analysis. bioRxiv 2021. [Google Scholar] [CrossRef]
- Kuznetsov, N.V. Expression of the p53 Network Aging-associated Genes “shaken, not stirred” in a 3-Week Microgravity Simulation Human Study. bioRxiv 2025. [Google Scholar] [CrossRef]
- Kouznetsov, N.V. Cell Responses to Simulated Microgravity and Hydrodynamic Stress Can Be Distinguished by Comparative Transcriptomics. Int. J. Transl. Med. 2022, 2, 364–386. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsov, N.V.; Vlasova, D.D.; Kotikova, A.A.; Tomilovskaya, E.; Ljubisavljevic, M. Microgravity Impacts the Expression of Aging-Associated Candidate Gene Targets in the p53 Regulatory Network. Int. J. Mol. Sci. 2025, 26, 11140. https://doi.org/10.3390/ijms262211140
Kuznetsov NV, Vlasova DD, Kotikova AA, Tomilovskaya E, Ljubisavljevic M. Microgravity Impacts the Expression of Aging-Associated Candidate Gene Targets in the p53 Regulatory Network. International Journal of Molecular Sciences. 2025; 26(22):11140. https://doi.org/10.3390/ijms262211140
Chicago/Turabian StyleKuznetsov, Nik V., Daria D. Vlasova, Anastasia A. Kotikova, Elena Tomilovskaya, and Milos Ljubisavljevic. 2025. "Microgravity Impacts the Expression of Aging-Associated Candidate Gene Targets in the p53 Regulatory Network" International Journal of Molecular Sciences 26, no. 22: 11140. https://doi.org/10.3390/ijms262211140
APA StyleKuznetsov, N. V., Vlasova, D. D., Kotikova, A. A., Tomilovskaya, E., & Ljubisavljevic, M. (2025). Microgravity Impacts the Expression of Aging-Associated Candidate Gene Targets in the p53 Regulatory Network. International Journal of Molecular Sciences, 26(22), 11140. https://doi.org/10.3390/ijms262211140








