Cross-Species Validation of Pigeon-Specific CHD1 Primers for Molecular Sexing in Pet Birds
Abstract
1. Introduction
1.1. Molecular Methods for Sexing Birds
1.2. Genes That Are Linked to Bird Sexing
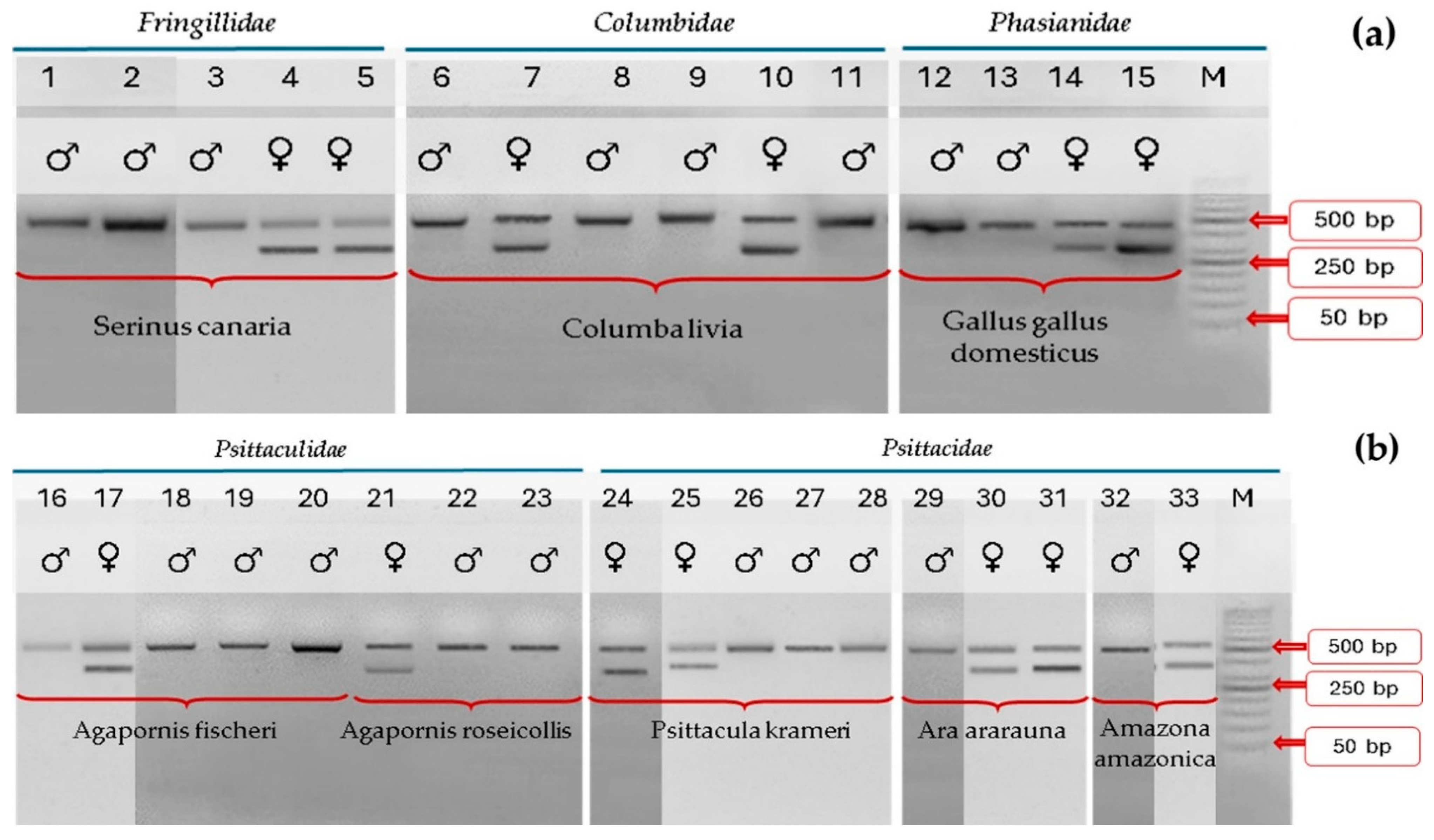
2. Results
3. Discussion
4. Materials and Methods
DNA Isolation, PCR Reaction, and Electrophoresis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Omogiade Idahor, K. Avian Reproduction. In Animal Reproduction; Bozkurt, Y., Bucak, M.N., Eds.; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar]
- Liang, S.-J.; Chen, M.-X.; Gao, C.-Q.; Yan, H.-C.; Zhang, G.-L.; Wang, X.-Q. Sex identification of pigeons using polymerase chain reaction analysis with simple DNA extraction. Avian Biol. Res. 2019, 12, 45–48. [Google Scholar] [CrossRef]
- Rudaya, S.V.; Katerynych, O.O.; Drahulian, M.V.; Chaplygina, A.B.; Pakhomov, O.Y. Sex identification of different species of wild birds using a single universal protocol to the bird sexing method based on gene polymorphism. Regul. Mech. Biosyst. 2020, 11, 399–404. [Google Scholar] [CrossRef]
- Stehlíková Sovadinová, S.; Mekadim, C.; Korpimäki, E.; Mrázek, J.; Kouba, M. Comparison of three primer pairs for molecular sex determination in Eurasian pygmy owls (Glaucidium passerinum). Sci. Rep. 2024, 14, 16397. [Google Scholar] [CrossRef] [PubMed]
- Beaton, L. Pet Bird Segment Enjoys Growth, Faces Pandemic Challenges. Available online: https://www.petfoodindustry.com/pet-food-market/article/15469139/pet-bird-segment-enjoys-growth-faces-pandemic-challenges (accessed on 28 February 2025).
- Shahbandeh, M. Number of Ornamental Birds in the European Union* in 2023, by Country. Available online: https://www.statista.com/statistics/515421/ornamental-bird-population-european-union-eu-by-country/ (accessed on 28 February 2025).
- Cerit, H.; Avanus, K. Sex identification in avian species using DNA typing methods. Poult. Sci. J. 2007, 63, 91–100. [Google Scholar] [CrossRef]
- England, A.D.; Kheravii, S.K.; Musigwa, S.; Kumar, A.; Daneshmand, A.; Sharma, N.K.; Gharib-Naseri, K.; Wu, S.B. Sexing chickens (Gallus gallus domesticus) with high-resolution melting analysis using feather crude DNA. Poult. Sci. 2021, 100, 100924. [Google Scholar] [CrossRef]
- Miąsko, M.; Gruszczynska, J.; Florczuk-Kołomyja, P.; Matuszewski, A. Determining sex in pigeons (Columba livia). World Sci. News 2017, 73, 109–114. [Google Scholar]
- Pollock, C.G.; Orosz, S.E. Avian reproductive anatomy, physiology and endocrinology. Vet. Clin. N. Am. Exot. Anim. Pract. 2002, 5, 441–474. [Google Scholar] [CrossRef]
- Li, W.; Xue, F.; Li, L.; Li, X.; Yue, B.; Li, J. A triple-primer PCR approach for the sex identification of endangered Phasianidae birds. Eur. J. Wildl. Res. 2012, 58, 289–294. [Google Scholar] [CrossRef]
- García-Moreno, J.; Mindell, D.P. Rooting a Phylogeny with Homologous Genes on Opposite Sex Chromosomes (Gametologs): A Case Study Using Avian CHD. Mol. Biol. Evol. 2000, 17, 1826–1832. [Google Scholar] [CrossRef]
- Chang, H.-W.; Cheng, C.-A.; Gu, D.-L.; Chang, C.-C.; Su, S.-H.; Wen, C.-H.; Chou, Y.-C.; Chou, T.-C.; Yao, C.-T.; Tsai, C.-L.; et al. High-throughput avian molecular sexing by SYBR green-based real-time PCR combined with melting curve analysis. BMC Biotechnol. 2008, 8, 12. [Google Scholar] [CrossRef]
- He, P.J.; Yu, J.Q.; Fang, S.G. Sex Identification of the Black Swan (Cygnus atratus) using the Locus-specific PCR and Implications for its Reproduction. Reprod. Domest. Anim. 2005, 40, 196–198. [Google Scholar] [CrossRef]
- Birkhead, T.R.; Hatchwell, B.J.; Lindner, R.; Blomqvist, D.; Pellatt, E.J.; Griffiths, R.; Lifjeld, J.T. Extra-Pair Paternity in the Common Murre. Condor 2001, 103, 158–162. [Google Scholar] [CrossRef]
- Huang, H.-W.; Su, Y.-F.; Yao, C.-T.; Hung, Y.-C.; Chen, C.-C.; Cheng, C.-C.; Li, S.S.-L.; Chang, H.-W. High-throughput gender identification of three Columbidae species using melting curve analysis. Theriogenology 2011, 75, 73–79.e74. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.C.; Liu, Y.S.; Cheng, C.C.; Wang, C.L.; Liao, M.H.; Tseng, C.N.; Chang, H.W. High-throughput sex identification by melting curve analysis in blue-breasted quail and chicken. Theriogenology 2012, 77, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Pastiu, A.I.; Turcu, M.C.; Pusta, D.L. PCR protocols for molecular sexing in monomorphic birds. Sci. Works. Ser. C Vet. Med. 2023, LXIX, 37–40. [Google Scholar]
- Fridolfsson, A.-K.; Ellegren, H. Molecular Evolution of the Avian CHD1 Genes on the Z and W Sex Chromosomes. Genetics 2000, 155, 1903–1912. [Google Scholar] [CrossRef]
- Chou, T.C.; Yao, C.T.; Su, S.H.; Hung, Y.C.; Chen, W.S.; Cheng, C.C.; Tseng, C.N.; Wang, H.M.; Chou, Y.C.; Li, S.S.L.; et al. Validation of Spilornis cheela hoya TaqMan probes for potential gender identification of many Accipitridae species. Theriogenology 2010, 73, 404–411. [Google Scholar] [CrossRef]
- Kroczak, A.; Wołoszyńska, M.; Wierzbicki, H.; Kurkowski, M.; Grabowski, K.A.; Piasecki, T.; Galosi, L.; Urantówka, A.D. New Bird Sexing Strategy Developed in the Order Psittaciformes Involves Multiple Markers to Avoid Sex Misidentification: Debunked Myth of the Universal DNA Marker. Genes 2021, 12, 878. [Google Scholar] [CrossRef]
- Griffiths, R.; Orr, K. The use of amplified fragment length polymorphism (AFLP) in the isolation of sex-specific markers. Mol. Ecol. 1999, 8, 671–674. [Google Scholar] [CrossRef]
- Ito, H.; Sudo-Yamaji, A.; Abe, M.; Murase, T.; Tsubota, T. Sex Identification by Alternative Polymerase Chain Reaction Methods in Falconiformes. Zool. Sci. 2003, 20, 339–344. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Hong, Y.-J.; Park, S.-K.; Kim, Y.; Choi, T.; Lee, H.; Min, M.-S. Application of Two Complementary Molecular Sexing Methods for East Asian Bird Species. Genes Genom 2008, 30, 365–372. [Google Scholar]
- da Silva, E.M.; Wong, M.S.L.; Martins, C.; Wasko, A.P. Screening and characterization of sex-specific DNA fragments in the freshwater fish matrinchã, Brycon amazonicus (Teleostei: Characiformes: Characidae). Fish Physiol. Biochem. 2012, 38, 1487–1496. [Google Scholar] [CrossRef]
- Volodin, I.; Kaiser, M.; Matrosova, V.; Volodina, E.; Klenova, A.; Filatova, O.; Kholodova, M. The technique of noninvasive distant sexing for four monomorphic dendrocygna whistling duck species by their loud whistles. Bioacoustics Int. J. Anim. Sound Its Rec. 2009, 18, 277–290. [Google Scholar] [CrossRef]
- Rosenthal, N.F.; Ellis, H.; Shioda, K.; Mahoney, C.; Coser, K.R.; Shioda, T. High-throughput applicable genomic sex typing of chicken by TaqMan real-time quantitative polymerase chain reaction. Poult. Sci. 2010, 89, 1451–1456. [Google Scholar] [CrossRef]
- Brubaker, J.L.; Karouna-Renier, N.K.; Chen, Y.U.; Jenko, K.; Sprague, D.T.; Henry, P.F.P. A noninvasive, direct real-time PCR method for sex determination in multiple avian species. Mol. Ecol. Resour. 2011, 11, 415–417. [Google Scholar] [CrossRef] [PubMed]
- Suh, A.; Kriegs, J.O.; Brosius, J.; Schmitz, J. Retroposon Insertions and the Chronology of Avian Sex Chromosome Evolution. Mol. Biol. Evol. 2011, 28, 2993–2997. [Google Scholar] [CrossRef] [PubMed]
- Morinha, F.; Cabral, J.; Bastos, E. Molecular sexing of birds: A comparative review of polymerase chain reaction (PCR)-based methods. Theriogenology 2012, 78, 703–714. [Google Scholar] [CrossRef] [PubMed]
- Ramos, P.S.; Bastos, E.; Mannan, R.W.; Guedes-Pinto, H. Polymerase chain reaction-single strand conformation polymorphism applied to sex identification of Accipiter cooperii. Mol. Cell. Probes 2009, 23, 115–118. [Google Scholar] [CrossRef] [PubMed]
- Cortés, O.; Barroso, A.; Dunner, S. Avian sexing: An optimized protocol using polymerase chain reaction-single-strand conformation polymorphism. J. Vet. Diagn. Investig. 1999, 11, 297–299. [Google Scholar] [CrossRef]
- Huang, M.C.; Lin, W.C.; Horng, Y.M.; Rouvier, R.; Huang, C.W. Female-specific DNA sequences in geese. Br. Poult. Sci. 2003, 44, 359–364. [Google Scholar] [CrossRef]
- Lee, J.C.-I.; Tsai, L.-C.; Hwa, P.-Y.; Chan, C.-L.; Huang, A.; Chin, S.-C.; Wang, L.-C.; Lin, J.-T.; Linacre, A.; Hsieh, H.-M. A novel strategy for avian species and gender identification using the CHD gene. Mol. Cell. Probes 2010, 24, 27–31. [Google Scholar] [CrossRef]
- Chang, H.W.; Gu, D.L.; Su, S.H.; Chang, C.C.; Cheng, C.A.; Huang, H.W.; Yao, C.T.; Chou, T.C.; Chuang, L.Y.; Cheng, C.C. High-throughput gender identification of Accipitridae eagles with real-time PCR using TaqMan probes. Theriogenology 2008, 70, 83–90. [Google Scholar] [CrossRef]
- Turcu, M.-C.; Paștiu, A.I.; Bel, L.-V.; Doboși, A.-A.; Pusta, D.L. Application of Minimally Invasive Oral Swab Samples for qPCR-Based Sexing in Neognathae Birds. Vet. Sci. 2025, 12, 73. [Google Scholar] [CrossRef]
- Çakmak, E.; Akın Pekşen, Ç.; Bilgin, C.C. Comparison of three different primer sets for sexing birds. J. Vet. Diagn. Investig. 2016, 29, 59–63. [Google Scholar] [CrossRef]
- Mazzoleni, S.; Němec, P.; Albrecht, T.; Lymberakis, P.; Kratochvíl, L.; Rovatsos, M. Long-term stability of sex chromosome gene content allows accurate qPCR-based molecular sexing across birds. Mol. Ecol. Resour. 2021, 21, 2013–2021. [Google Scholar] [CrossRef]
- Petrou, E.L.; Scott, L.C.; McKeeman, C.M.; Ramey, A.M. Molecular sexing of birds using quantitative PCR (qPCR) of sex-linked genes and logistic regression models. Mol. Ecol. Resour. 2024, 24, e13946. [Google Scholar] [CrossRef] [PubMed]
- Kuroiwa, A. Sex-Determining Mechanism in Avians. In Avian Reproduction: From Behavior to Molecules; Sasanami, T., Ed.; Springer: Singapore, 2017; pp. 19–31. [Google Scholar]
- Lv, X.; Wei, Q.; Liu, X.; Gong, W.; Li, F.; Niu, Y.; Jin, K.; Li, B.; Zuo, Q. Construction and mechanism analysis of the sex reversal model of chicken gonadal somatic cells. Poult. Sci. 2025, 104, 105435. [Google Scholar] [CrossRef] [PubMed]
- Ioannidis, J.; Taylor, G.; Zhao, D.; Liu, L.; Idoko-Akoh, A.; Gong, D.; Lovell-Badge, R.; Guioli, S.; McGrew, M.J.; Clinton, M. Primary sex determination in birds depends on DMRT1 dosage, but gonadal sex does not determine adult secondary sex characteristics. Proc. Natl. Acad. Sci. USA 2021, 118, e2020909118. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, R.; Daan, S.; Dijkstra, C. Sex identification in birds using two CHD genes. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1997, 263, 1251–1256. [Google Scholar] [CrossRef]
- Ellegren, H. First gene on the avian W chromosome (CHD) provides a tag for universal sexing of non-ratite birds. Proc. R. Soc. Lond. Ser. B Biol. Sci. 1997, 263, 1635–1641. [Google Scholar] [CrossRef]
- Vucicevic, M.; Stevanov-Pavlovic, M.; Stevanovic, J.; Bosnjak, J.; Gajic, B.; Aleksic, N.; Stanimirovic, Z. Sex Determination in 58 Bird Species and Evaluation of CHD Gene as a Universal Molecular Marker in Bird Sexing. Zoo Biol. 2013, 32, 269–276. [Google Scholar] [CrossRef]
- Turcu, M.-C.; Paștiu, A.I.; Bel, L.V.; Pusta, D.L. A Comparison of Feathers and Oral Swab Samples as DNA Sources for Molecular Sexing in Companion Birds. Animals 2023, 13, 525. [Google Scholar] [CrossRef]
- Mataragka, A.; Balaskas, C.; Sotirakoglou, K.; Ikonomopoulos, J. Comparative evaluation of the performance of the PCR assays commonly used for the determination of sex in avian species. J. King Saud Univ. –Sci. 2020, 32, 228–234. [Google Scholar] [CrossRef]
- Dyah Argarini, A.; Ari Nugroho, H.; Purwaningrum, M.; Haryanto, A. Molecular Bird Sexing on Fischeri Lovebird (Agapornis fischeri) by Using Polymerase Chain Reaction. BIO Web Conf. 2020, 20, 04003. [Google Scholar] [CrossRef]
- El Islami, S.I.; Purwaningrum, M.; Haryanto, A. Molecular Sex Determination of Masked Lovebird (Agapornis personata) by Polymerase Chain Reaction Method. In Proceedings of the KOBI 2nd International Conference on Management of Tropical Biodiversity for Human Welfare: From Ecosystem to Molecular, Pontianak, Indonesia, 6–8 September 2019; pp. 48–53. [Google Scholar]
- Albino Miranda, S.; Antonio, G.N.M.; Montserrat, S.P.D.; Nanci, V.B.; Fernando, G.-G.; Serio-Silva, J.C. Polymerase Chain Reaction (PCR) is a Useful and Low-Cost Tool for Molecular Sexing Psittaciformes under Human Care: An Example of a Collaborative Approach in Mexico. J. Appl. Anim. Welf. Sci. 2024, 27, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Stothard, P. The Sequence Manipulation Suite: JavaScript Programs for Analyzing and Formatting Protein and DNA Sequences. BioTechniques 2000, 28, 1102–1104. [Google Scholar] [CrossRef]
- Istvan Lazar, P., Jr.; Istvan Lazar, P., Sr. CSc GelAnalyzer 23.1.1. Available online: http://www.gelanalyzer.com/?i=1 (accessed on 6 March 2025).


| Methods | Species in Which It Was Applied | Advantages | Disadvantages | References |
|---|---|---|---|---|
| SSCP | goshawk (Accipiter gentilis), cooper’s hawks (Accipiter cooperii) |
|
| [30,31,32] |
| RFLP | brown skua (Catharacta lonnbergi), Sfinx’s makaw (Cyanopsitta spixii) |
|
| [1,7,22,30] |
| RAPD | Chinese geese (Anser cygnoides), white Roman geese (Coscoroba coscoroba), and Landaise geese |
|
| [30,33] |
| AFLP | ostrich (Struthio camelus), cormorant (Phalacrocorax aristotelis), shag (Phalacrocorax aristotelis) |
|
| [22,30] |
| Microsatellites/STRs/SSRs | American cranes (Grus americana), falcons |
|
| [30] |
| AS-PCR/ARMS | black swans (Cygnus atratus); black kite (Milvus migrans), Northern goshawk (Accipiter gentilis), Eastern marsh harrier (Circus spilonotus), peregrine falcon (Falco peregrinus) etc |
| - | [23,24,30] |
| Capillary electrophoresis | fairy pitta (Pitta nympha), Jungle fowl (Gallus gallus), mute swan (Cygnus olor) etc. |
|
| [30,34] |
| qPCR | domestic pigeon (Columba livia domestica), budgerigar (Melopsittacus undulatus), lovebird (Agapornis roseicollis), rose-ringed parakeet (Psittacula krameri), African grey parrot (Psittacus erithacus), red-rumped parrot (Psephotus haematonotus), etc. |
|
| [20,30,35,36] |
| Real-time PCR combined with melting curve analysis | Eurasian pygmy owls (Glaucidium passerinum), Japanese quail (Coturnix japonica), eastern screech-owls (Megascops asio), etc. |
|
| [4,13,28,30] |
| HRM | common quail (Coturnix coturnix), Japanese quail (Coturnix japonica) |
|
| [28,29,30] |
| Family | Species | Sex | CHD1F/CHD1R (bp) | pCHD1F/p.CHD1R (bp) | ||
|---|---|---|---|---|---|---|
| Z | W | Z | W | |||
| Fringillidae | Serinus canaria | M | 508–529 | - | 452–533 | - |
| F | 502–520 | 311 | 465–471 | 312–316 | ||
| Columbidae | Columba livia | M | 477–515 | - | 443–469 | |
| F | 496 | 297–309 | 460–469 | 311–323 | ||
| Phasianidae | Gallus gallus | M | 440–458 | - | 469–486 | - |
| F | 458 | 308 | 486 | 324 | ||
| Psittaculidae | Agapornis fischeri | M | 506–536 | - | 477–504 | - |
| F | 515 | 331 | 476 | 315 | ||
| Agapornis roseicollis | M | 515–525 | - | 452–469 | - | |
| F | 515 | 324 | 469 | 329 | ||
| Psittacula krameri | M | 514–519 | - | 470 | - | |
| F | 515 | 328–334 | 469 | 306–327 | ||
| Psittacidae | Araararauna | M | 496 | - | 460 | - |
| F | 508–510 | 338 | 458 | 324–329 | ||
| Amazonaamazonica | M | 514 | - | 418 | - | |
| F | 528 | 339 | 504 | 330 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marc, S.; Boldura, O.M.; Paul, C.; Tripon, M.R.; Otavă, G.; Savici, J. Cross-Species Validation of Pigeon-Specific CHD1 Primers for Molecular Sexing in Pet Birds. Int. J. Mol. Sci. 2025, 26, 11142. https://doi.org/10.3390/ijms262211142
Marc S, Boldura OM, Paul C, Tripon MR, Otavă G, Savici J. Cross-Species Validation of Pigeon-Specific CHD1 Primers for Molecular Sexing in Pet Birds. International Journal of Molecular Sciences. 2025; 26(22):11142. https://doi.org/10.3390/ijms262211142
Chicago/Turabian StyleMarc, Simona, Oana Maria Boldura, Cristina Paul, Maria Roberta Tripon, Gabriel Otavă, and Jelena Savici. 2025. "Cross-Species Validation of Pigeon-Specific CHD1 Primers for Molecular Sexing in Pet Birds" International Journal of Molecular Sciences 26, no. 22: 11142. https://doi.org/10.3390/ijms262211142
APA StyleMarc, S., Boldura, O. M., Paul, C., Tripon, M. R., Otavă, G., & Savici, J. (2025). Cross-Species Validation of Pigeon-Specific CHD1 Primers for Molecular Sexing in Pet Birds. International Journal of Molecular Sciences, 26(22), 11142. https://doi.org/10.3390/ijms262211142








