Loss of Dioxin Response Element-Mediated Induction of PKM2 Reprograms Hepatic Metabolism in Response to TCDD
Abstract
1. Introduction
2. Results
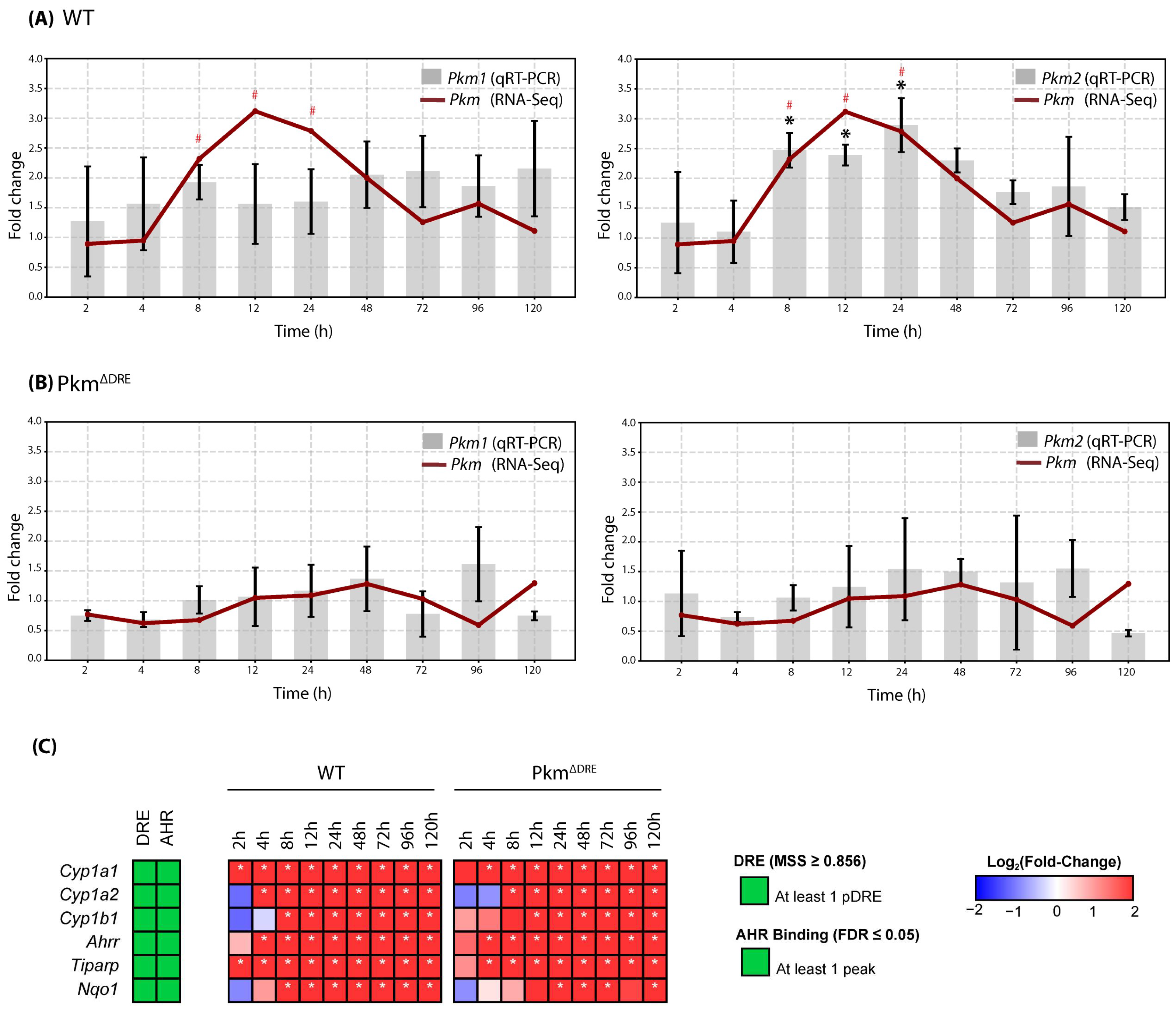
2.1. PkmΔDRE Model Evaluation
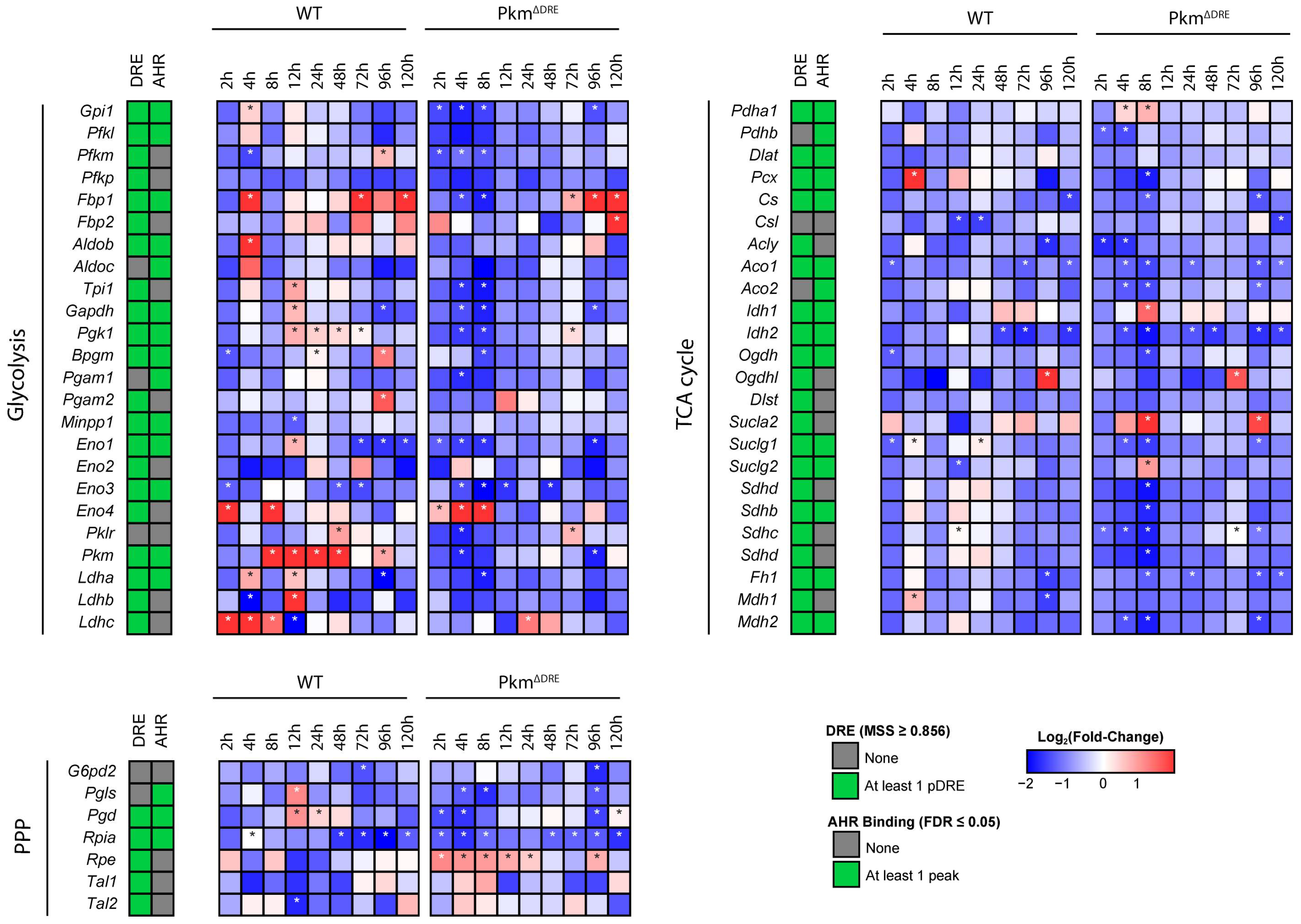
2.2. Effects of TCDD on Gene Expression in Cultured Mouse Primary Hepatocytes (In Vitro)
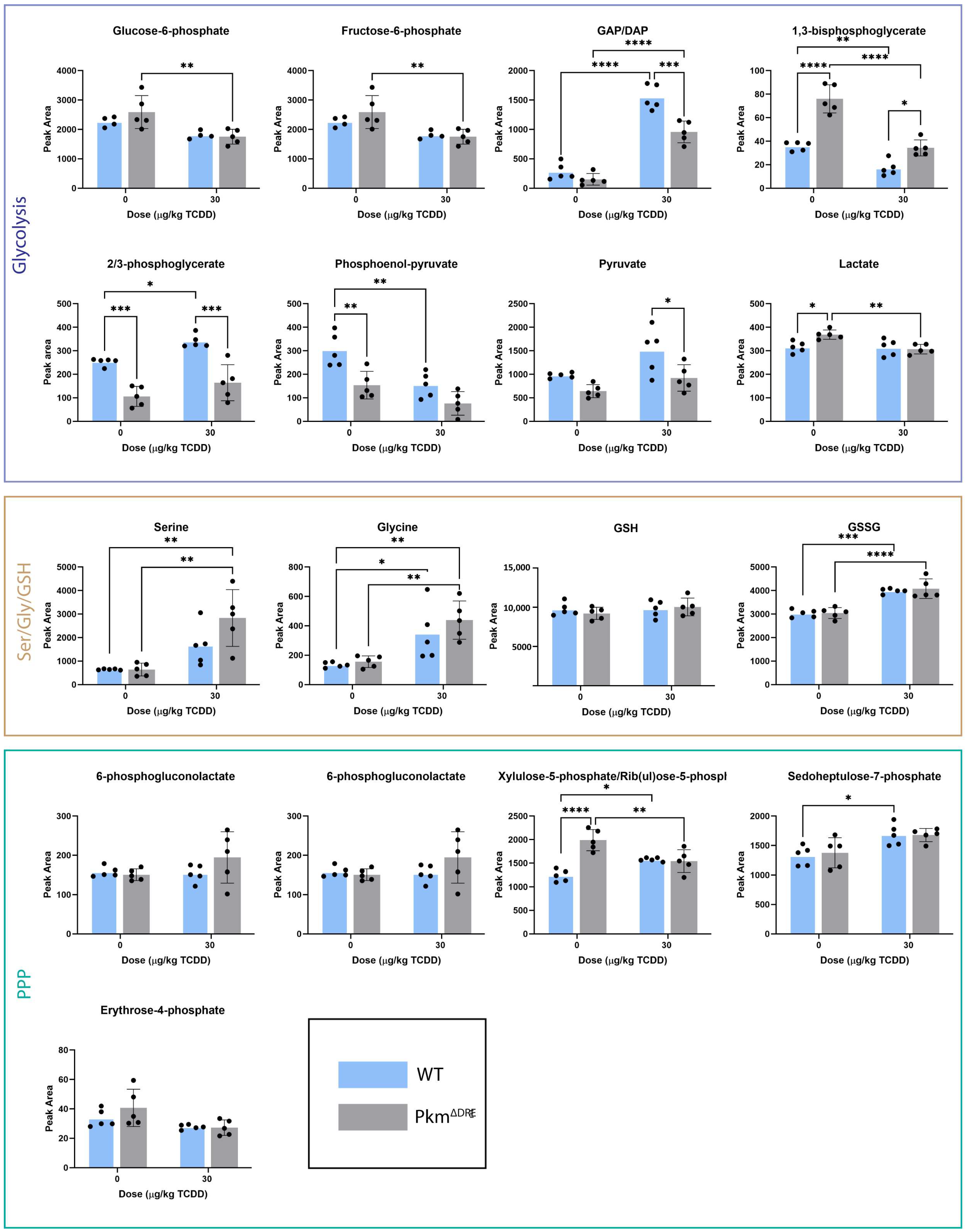
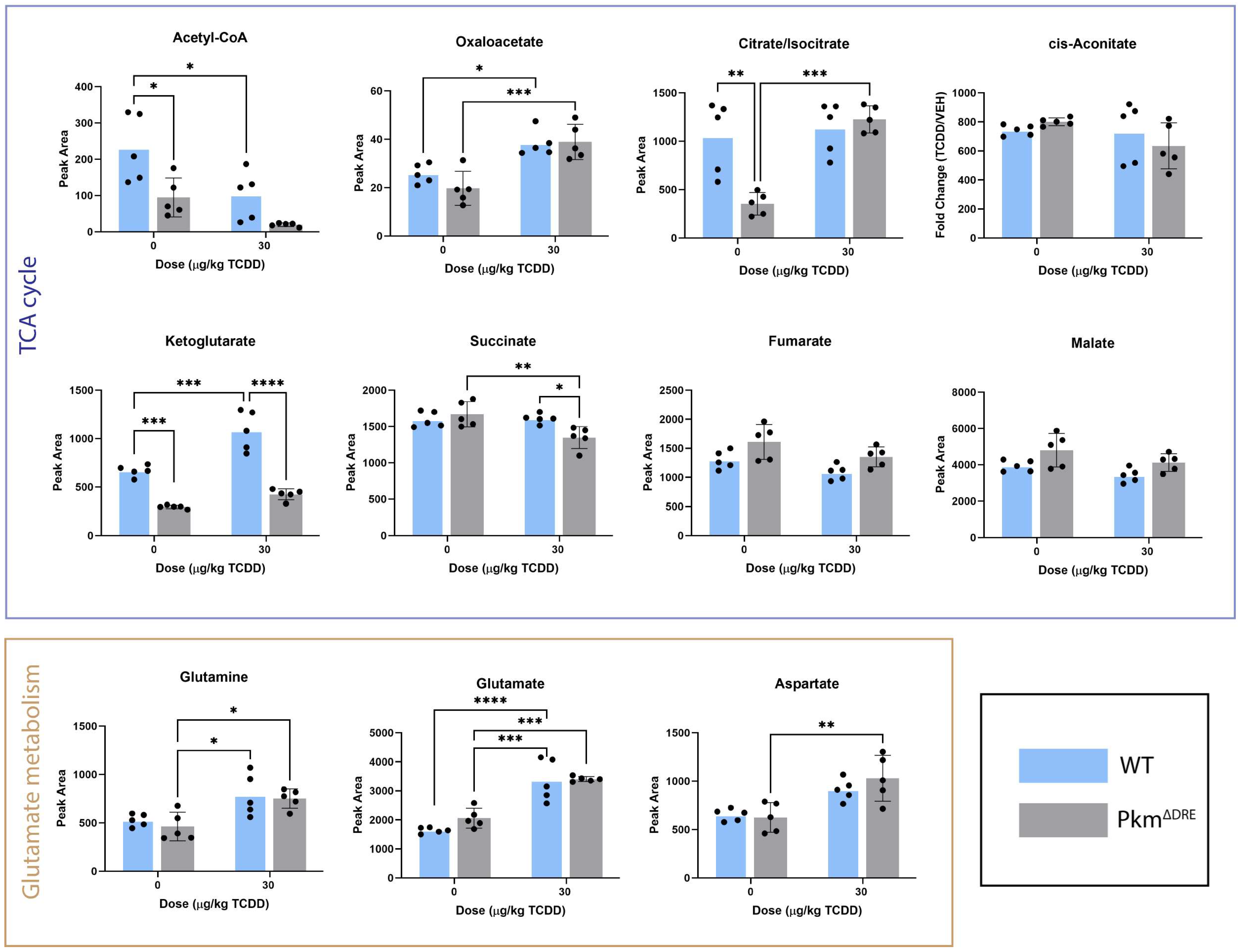
2.3. Effects of TCDD on Hepatic Metabolite Levels (In Vivo)
3. Discussion
4. Materials and Methods
4.1. PkmΔDRE Model Generation
4.2. Primary Hepatocytes Isolation and Culture
4.3. RNA Isolation and Quantitative Real-Time Polymerase Chain Reaction (qRT-PCR)
4.4. RNA-Seq
4.5. Treatment Regimen
4.6. LC-MS Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moffat, I.; Boutros, P.; Chen, H.; Okey, A.; Pohjanvirta, R. Aryl hydrocarbon receptor (AHR)-regulated transcriptomic changes in rats sensitive or resistant to major dioxin toxicities. BMC Genom. 2010, 11, 263. [Google Scholar] [CrossRef]
- Zhao, M.; Ma, J.; Li, M.; Zhang, Y.; Jiang, B.; Zhao, X.; Huai, C.; Shen, L.; Zhang, N.; He, L.; et al. Cytochrome P450 Enzymes and Drug Metabolism in Humans. Int. J. Mol. Sci. 2021, 22, 12808. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; Fang, Y.Z.; Yang, S.; Lupton, J.R.; Turner, N.D. Glutathione metabolism and its implications for health. Recent. Adv. Nutr. Sci. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Leijs, M.M.; Gan, L.; De Boever, P.; Esser, A.; Amann, P.M.; Ziegler, P.; Fietkau, K.; Schettgen, T.; Kraus, T.; Merk, H.F.; et al. Altered Gene Expression in Dioxin-Like and Non-Dioxin-Like PCB Exposed Peripheral Blood Mononuclear Cells. Int. J. Environ. Res. Public Health 2019, 16, 90. [Google Scholar] [CrossRef]
- Denison, M.S.; Soshilov, A.A.; He, G.; DeGroot, D.E.; Zhao, B. Exactly the same but different: Promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicol. Sci. 2011, 124, 1–22. [Google Scholar] [CrossRef]
- Denison, M.S.; Faber, S.C. And Now for Something Completely Different: Diversity in Ligand-Dependent Activation of Ah Receptor Responses. Curr. Opin. Toxicol. 2017, 2, 124–131. [Google Scholar] [CrossRef]
- Wilson, S.R.; Joshi, A.D.; Elferink, C.J. The Tumor Suppressor Kruppel-Like Factor 6 Is a Novel Aryl Hydrocarbon Receptor DNA Binding Partner. J. Pharmacol. Exp. Ther. 2013, 345, 419–429. [Google Scholar] [CrossRef]
- Orlowska, K.; Nault, R.; Ara, J.; LaPres, J.J.; Harkema, J.; Demireva, E.Y.; Xie, H.; Wilson, R.H.; Bradfield, C.A.; Yap, D.; et al. Disruption of canonical AHR-mediated induction of hepatocyte PKM2 expression compromises antioxidant defenses and increases TCDD-induced hepatotoxicity. Redox Biol. 2024, 77, 103405. [Google Scholar] [CrossRef] [PubMed]
- Alquraishi, M.; Puckett, D.L.; Alani, D.S.; Humidat, A.S.; Frankel, V.D.; Donohoe, D.R.; Whelan, J.; Bettaieb, A. Pyruvate kinase M2: A simple molecule with complex functions. Free Radic. Biol. Med. 2019, 143, 176–192. [Google Scholar] [CrossRef]
- Iqbal, M.A.; Gupta, V.; Gopinath, P.; Mazurek, S.; Bamezai, R.N.K. Pyruvate kinase M2 and cancer: An updated assessment. Febs Lett. 2014, 588, 2685–2692. [Google Scholar] [CrossRef]
- Israelsen, W.J.; Dayton, T.L.; Davidson, S.M.; Fiske, B.P.; Hosios, A.M.; Bellinger, G.; Li, J.; Yu, Y.M.; Sasaki, M.; Horner, J.W.; et al. PKM2 Isoform-Specific Deletion Reveals a Differential Requirement for Pyruvate Kinase in Tumor Cells. Cell 2013, 155, 397–409. [Google Scholar] [CrossRef]
- Zhou, X.; Mikaeloff, F.; Curbo, S.; Zhao, Q.; Kuiper, R.; Vegvari, A.; Neogi, U.; Karlsson, A. Coordinated pyruvate kinase activity is crucial for metabolic adaptation and cell survival during mitochondrial dysfunction. Hum. Mol. Genet. 2021, 30, 2012–2026. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.B.; Min, J.K.; Kim, J.G.; Cap, K.C.; Islam, R.; Hossain, A.; Dogsom, O.; Hamza, A.; Mahmud, S.; Choi, D.R.; et al. Multiple functions of pyruvate kinase M2 in various cell types. J. Cell Physiol. 2022, 237, 128–148. [Google Scholar] [CrossRef]
- Diehl, F.F.; Lewis, C.A.; Fiske, B.P.; Vander Heiden, M.G. Cellular redox state constrains serine synthesis and nucleotide production to impact cell proliferation. Nat. Metab. 2019, 1, 861–867. [Google Scholar] [CrossRef]
- Nault, R.; Fader, K.A.; Kirby, M.P.; Ahmed, S.; Matthews, J.; Jones, A.D.; Lunt, S.Y.; Zacharewski, T.R. Pyruvate Kinase Isoform Switching and Hepatic Metabolic Reprogramming by the Environmental Contaminant 2,3,7,8-Tetrachlorodibenzo-p-Dioxin. Toxicol. Sci. 2016, 149, 358–371. [Google Scholar] [CrossRef]
- Kopf, P.G.; Walker, M.K. 2,3,7,8-tetrachlorodibenzo-p-dioxin increases reactive oxygen species production in human endothelial cells via induction of cytochrome P4501A1. Toxicol. Appl. Pharmacol. 2010, 245, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Stohs, S.J. Oxidative stress induced by 2,3,7,8-ttetrachlorodibenzo-p-dioxin (TCDD). Free Radic. Biol. Med. 1990, 9, 79–90. [Google Scholar] [CrossRef]
- Gao, X.; Wang, H.; Yang, J.J.; Liu, X.; Liu, Z.R. Pyruvate kinase M2 regulates gene transcription by acting as a protein kinase. Mol. Cell 2012, 45, 598–609. [Google Scholar] [CrossRef]
- Zheng, D.D.; Jiang, Y.C.; Qu, C.; Yuan, H.; Hu, K.S.; He, L.; Chen, P.; Li, J.Y.; Tu, M.X.; Lin, L.H.; et al. Pyruvate Kinase M2 Tetramerization Protects against Hepatic Stellate Cell Activation and Liver Fibrosis. Am. J. Pathol. 2020, 190, 2267–2281. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. J. Hepatol. 2023, 79, 1542–1556. [Google Scholar] [CrossRef] [PubMed]
- Liang, W.L.; Zhang, Y.T.; Song, L.; Li, Z.Y. 2,3′4,4′, 5-Pentachlorobiphenyl induces hepatocellular carcinoma cell proliferation through pyruvate kinase M2-dependent glycolysis. Toxicol. Lett. 2019, 313, 108–119. [Google Scholar] [CrossRef]
- Matsuda, S.; Adachi, J.; Ihara, M.; Tanuma, N.; Shima, H.; Kakizuka, A.; Ikura, M.; Ikura, T.; Matsuda, T. Nuclear pyruvate kinase M2 complex serves as a transcriptional coactivator of arylhydrocarbon receptor. Nucleic Acids Res. 2016, 44, 636–647. [Google Scholar] [CrossRef]
- Nault, R.; Doskey, C.M.; Fader, K.A.; Rockwell, C.E.; Zacharewski, T. Comparison of Hepatic NRF2 and Aryl Hydrocarbon Receptor Binding in 2,3,7,8-Tetrachlorodibenzo-p-dioxin-Treated Mice Demonstrates NRF2-Independent PKM2 Induction. Mol. Pharmacol. 2018, 94, 876–884. [Google Scholar] [CrossRef]
- Diani-Moore, S.; Marques Pedro, T.; Rifkind, A.B. Organ-specific effects on glycolysis by the dioxin-activated aryl hydrocarbon receptor. PLoS ONE 2020, 15, e0243842. [Google Scholar] [CrossRef]
- Heo, M.J.; Suh, J.H.; Lee, S.H.; Poulsen, K.L.; An, Y.A.; Moorthy, B.; Hartig, S.M.; Moore, D.D.; Kim, K.H. Aryl hydrocarbon receptor maintains hepatic mitochondrial homeostasis in mice. Mol. Metab. 2023, 72, 101717. [Google Scholar] [CrossRef]
- Fan, J.; Ye, J.; Kamphorst, J.J.; Shlomi, T.; Thompson, C.B.; Rabinowitz, J.D. Quantitative flux analysis reveals folate-dependent NADPH production. Nature 2014, 510, 298–302. [Google Scholar] [CrossRef]
- Cholico, G.N.; Orlowska, K.; Fling, R.R.; Sink, W.J.; Zacharewski, N.A.; Fader, K.A.; Nault, R.; Zacharewski, T. Consequences of reprogramming acetyl-CoA metabolism by 2,3,7,8-tetrachlorodibenzo-p-dioxin in the mouse liver. Sci. Rep. 2023, 13, 4138. [Google Scholar] [CrossRef] [PubMed]
- Lunt, S.Y.; Muralidhar, V.; Hosios, A.M.; Israelsen, W.J.; Gui, D.Y.; Newhouse, L.; Ogrodzinski, M.; Hecht, V.; Xu, K.; Acevedo, P.N.; et al. Pyruvate kinase isoform expression alters nucleotide synthesis to impact cell proliferation. Mol. Cell 2015, 57, 95–107. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, B.P.; Kopec, A.K.; Joshi, N.; Cline, H.; Brown, J.A.; Bishop, S.C.; Kassel, K.M.; Rockwell, C.; Mackman, N.; Luyendyk, J.P. Hepatocyte tissue factor activates the coagulation cascade in mice. Blood 2013, 121, 1868–1874. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Eckel, J.E.; Gennings, C.; Chinchilli, V.M.; Burgoon, L.D.; Zacharewski, T.R. Empirical bayes gene screening tool for time-course or dose-response microarray data. J. Biopharm. Stat. 2004, 14, 647–670. [Google Scholar] [CrossRef] [PubMed]
- Wolfe, W.H.; Michalek, J.E.; Miner, J.C.; Pirkle, J.L.; Caudill, S.P.; Patterson, D.G., Jr.; Needham, L.L. Determinants of TCDD half-life in veterans of operation ranch hand. J. Toxicol. Environ. Health 1994, 41, 481–488. [Google Scholar] [CrossRef]
- Gasiewicz, T.A.; Geiger, L.E.; Rucci, G.; Neal, R.A. Distribution, excretion, and metabolism of 2,3,7,8-tetrachlorodibenzo-p-dioxin in C57BL/6J, DBA/2J, and B6D2F1/J mice. Drug Metab. Dispos. 1983, 11, 397–403. [Google Scholar] [CrossRef] [PubMed]

| 2 h | 4 h | 8 h | 12 h | 24 h | 48 h | 72 h | 96 h | 120 h | |
|---|---|---|---|---|---|---|---|---|---|
| PkmΔDRE unique DEGs | 651 | 1380 | 2709 | 176 | 259 | 162 | 96 | 371 | 224 |
| WT unique DEGs | 1743 | 511 | 240 | 214 | 183 | 266 | 368 | 442 | 138 |
| Common DEGs | 105 | 53 | 63 | 25 | 42 | 83 | 78 | 130 | 129 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Orlowska, K.; Nault, R.; Zacharewski, T. Loss of Dioxin Response Element-Mediated Induction of PKM2 Reprograms Hepatic Metabolism in Response to TCDD. Int. J. Mol. Sci. 2025, 26, 10853. https://doi.org/10.3390/ijms262210853
Orlowska K, Nault R, Zacharewski T. Loss of Dioxin Response Element-Mediated Induction of PKM2 Reprograms Hepatic Metabolism in Response to TCDD. International Journal of Molecular Sciences. 2025; 26(22):10853. https://doi.org/10.3390/ijms262210853
Chicago/Turabian StyleOrlowska, Karina, Rance Nault, and Tim Zacharewski. 2025. "Loss of Dioxin Response Element-Mediated Induction of PKM2 Reprograms Hepatic Metabolism in Response to TCDD" International Journal of Molecular Sciences 26, no. 22: 10853. https://doi.org/10.3390/ijms262210853
APA StyleOrlowska, K., Nault, R., & Zacharewski, T. (2025). Loss of Dioxin Response Element-Mediated Induction of PKM2 Reprograms Hepatic Metabolism in Response to TCDD. International Journal of Molecular Sciences, 26(22), 10853. https://doi.org/10.3390/ijms262210853






