Plant-Derived Modifiers for Antimicrobial Soft Denture Liners: A Review
Abstract
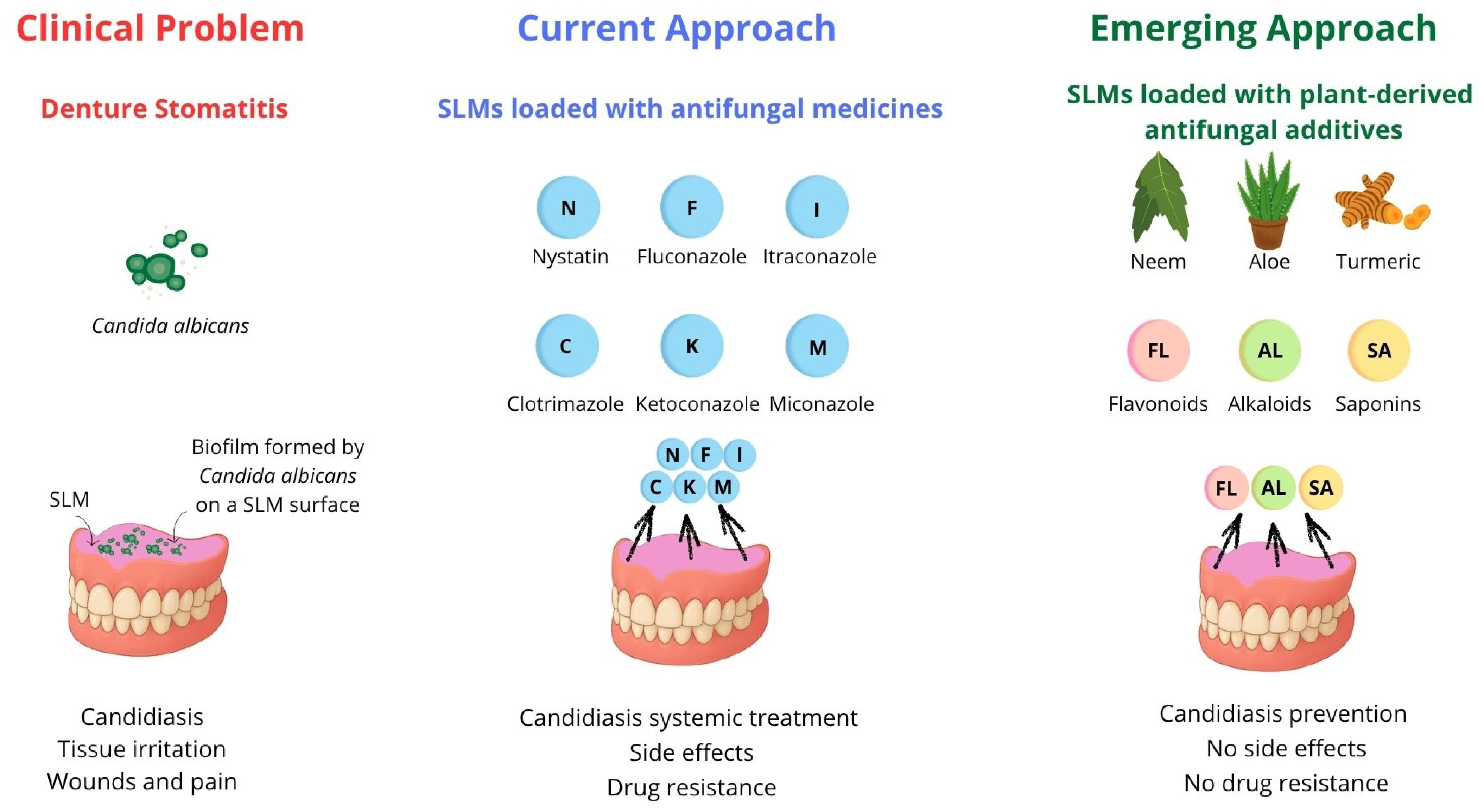
1. Introduction
2. Classification of SLMs
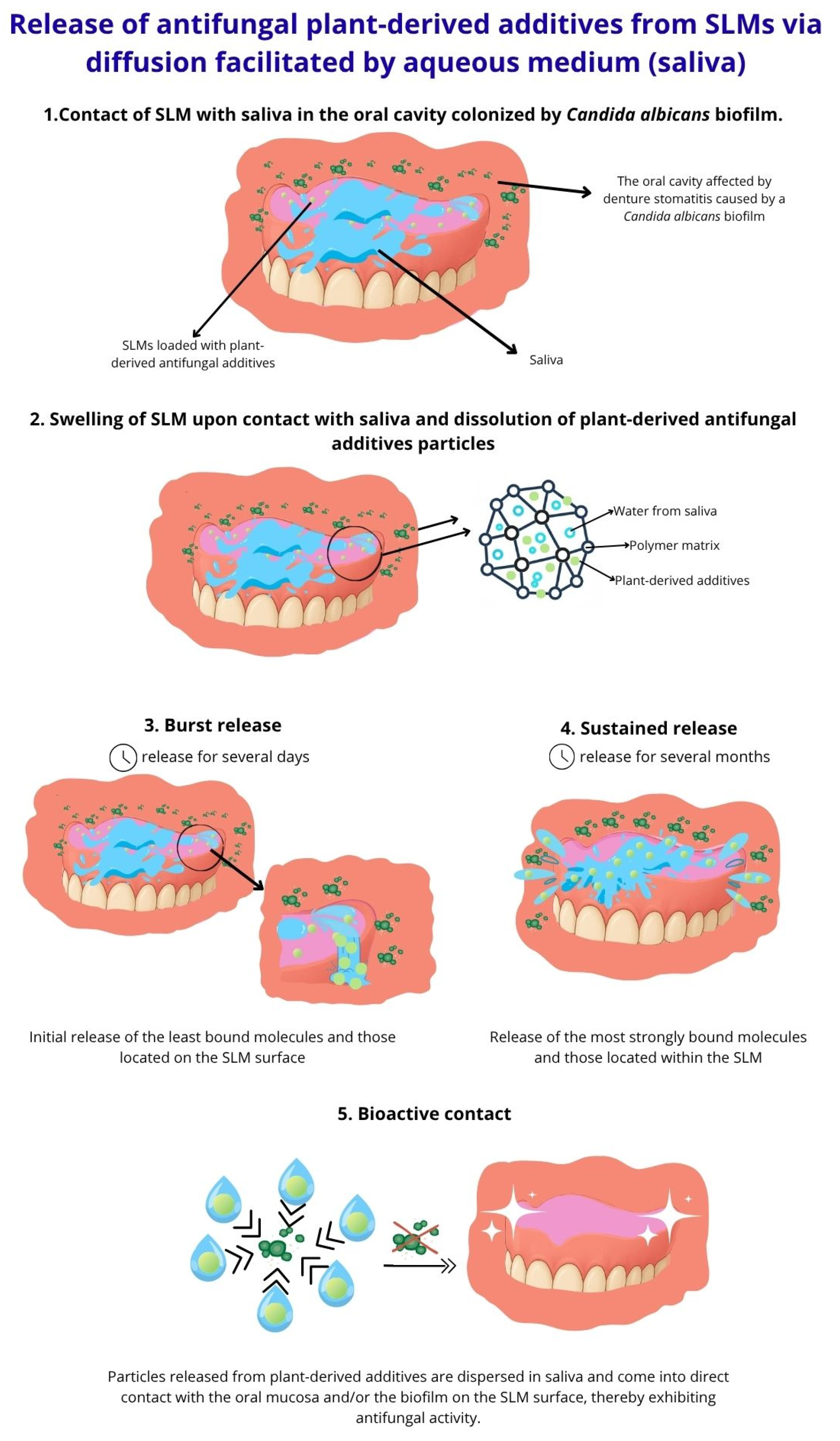
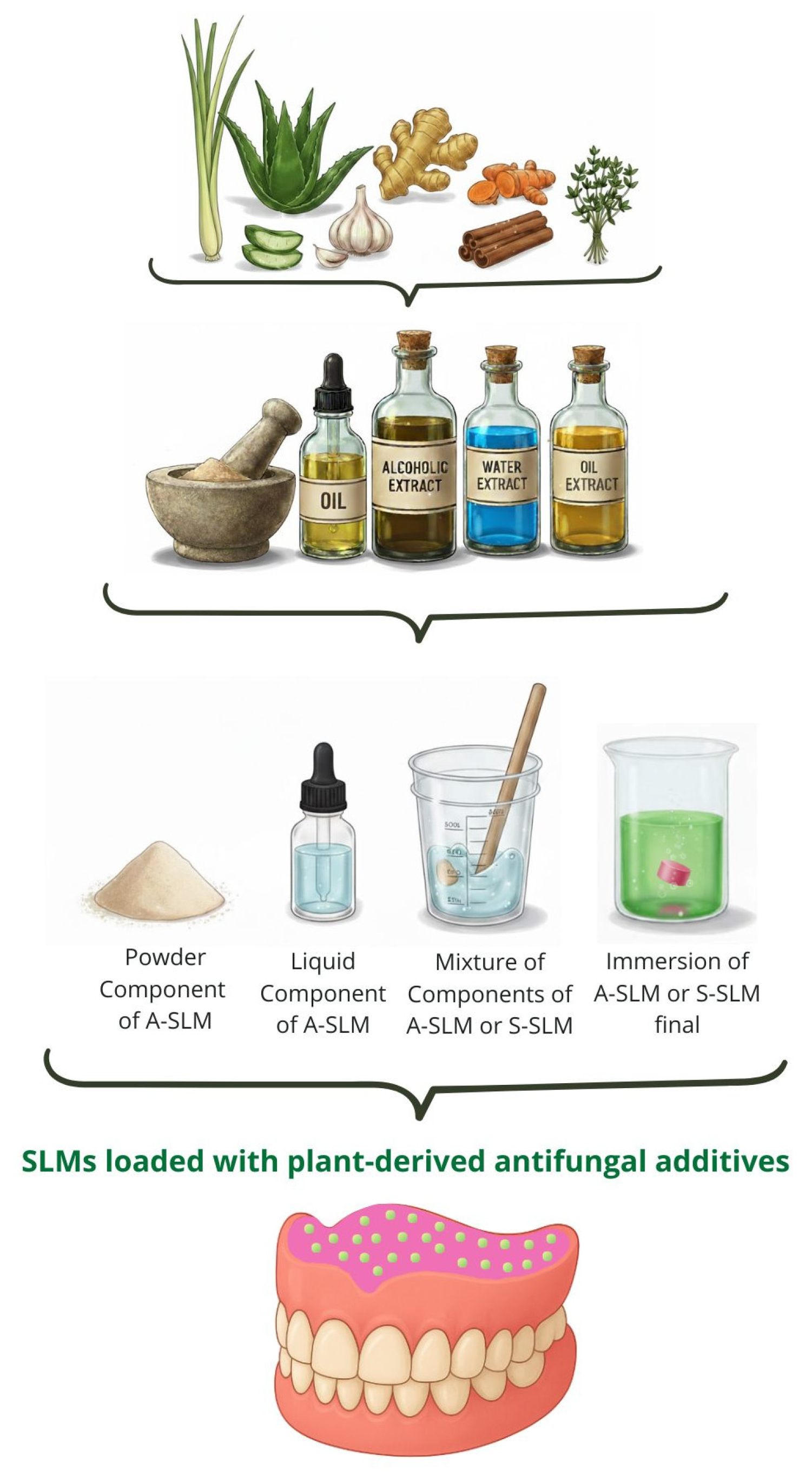
3. The Concept of Antimicrobial SLMs with Plant-Derived Additives
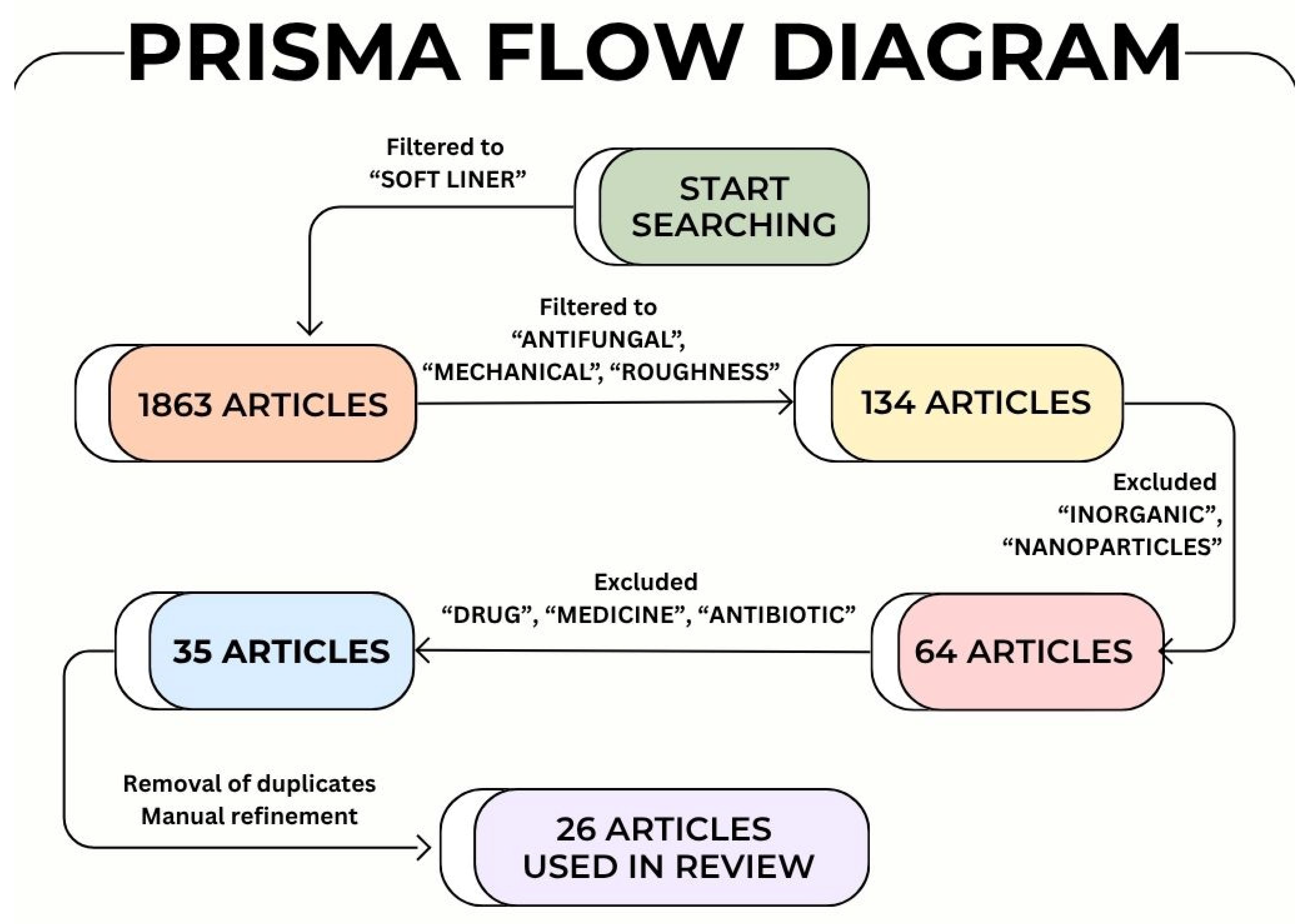
4. Methodology
5. Discussion
- SLM type (TC, ST-SLM, LT-SLM).
- SLM composition, including polymer type (A-SLM, S-SLM), plasticizer, and ethanol content.
- Differences in extraction methods for bioactive compounds.
- Variations in the form of bioactive compounds (essential oil, powder, or dried plant parts).
- Additive incorporation method, such as direct admixing, immersion, or nanoparticle dispersion.
- Additive-enriched component in direct admixing (added to liquid, powder, or pre-formulated mixture).
- Inconsistent reporting of additive concentration, rarely expressed as weight or volume fractions relative to total sample mass. Often, fixed amounts were added to liquid or powder according to manufacturer-recommended ratios, preventing standardized comparison.
- Additive concentration range tested.
- Immersion medium, e.g., pure additive oil or solvent.
5.1. In Vitro Antifungal Efficacy
5.2. In Vivo Antifungal Efficacy
5.3. In Vitro Antibacterial Efficacy
5.4. Biocompatibility
5.5. Intrinsic Mechanical Properties
5.6. Interface Mechanical Properties
5.7. Roughness
5.8. Prospects and Future Directions
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| A-SLM | Acrylic-based Soft Lining Material |
| BPO | Benzoyl Peroxide |
| BuMA | n-Buthyl Methacrylate |
| CFU | Colony Forming Unit |
| CFU/mL | Colony Forming Unit per milliliter |
| DD | Degree of Deacetylation |
| DMSO | Dimethyl Sulfoxide |
| DMPT | N,N-Dimethyl-p-toluidine |
| DNA | Deoxyribonucleic Acid |
| EGDMA | Ethylene Glycol Dimethacrylate |
| EMA | Poly(ethyl methacrylate) |
| HWP1 | Hyphal Wall Protein 1 |
| ISO | International Organization for Standardization |
| IZD | Inhibition Zone Diameter |
| LD50 | Lethal Dose 50% |
| LT-SLM | Long-term Soft Lining Material |
| LT-S-SLM | Long-term Silicone Soft Lining Material |
| MAP | Mitogen-Activated Protein |
| MFC | Minimum Fungicidal Concentration |
| MIC | Minimum Inhibitory Concentration |
| MMA | Methyl Methacrylate |
| mRNA | Messenger Ribonucleic Acid |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| MW | Molecular Weight |
| PEMA | Poly(methyl methacrylate) |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| SLM | Soft Lining Material |
| S-SLM | Silicone-based Soft Lining Material |
| ST-SLM | Short-term Soft Lining Material |
| T-SLM | Temporary Soft Lining Material |
| TC | Tissue Conditioner |
| TEGDMA | Triethylene Glycol Dimethacrylate |
| WCA | Water Contact Angle |
| WHO | World Health Organization |
References
- Hashem, M.I. Advances in soft denture liners: An update. J. Contemp. Dent. Pract. 2015, 16, 314–318. [Google Scholar]
- Barszczewska-Rybarek, I.; Kula, P.; Chladek, G. Review of the Anti-Candida albicans Activity and Physical Properties of Soft Lining Materials Modified with Polyene Antibiotics, Azole Drugs, and Chlorohexidine Salts. Materials 2024, 17, 5383. [Google Scholar] [CrossRef]
- Martins, N.; Ferreira, I.C.F.R.; Barros, L.; Silva, S.; Henriques, M. Candidiasis: Predisposing factors, prevention, diagnosis and alternative treatment. Mycopathologia 2014, 177, 223–240. [Google Scholar] [CrossRef]
- Nittayananta, W.; DeRouen, T.A.; Arirachakaran, P.; Laothumthut, T.; Pangsomboon, K.; Petsantad, S.; Vuddhakul, V.; Sriplung, H.; Jaruratanasirikul, S.; Martin, M.D. A randomized clinical trial of chlorhexidine in the maintenance of oral candidiasis-free period in HIV infection. Oral Dis. 2008, 14, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Abuhajar, E.; Ali, K.; Zulfiqar, G.; Al Ansari, K.; Raja, H.Z.; Bishti, S.; Anweigi, L. Management of chronic atrophic candidiasis (denture stomatitis)—A narrative review. Int. J. Environ. Res. Public Health 2023, 20, 3029. [Google Scholar] [CrossRef]
- Pfaller, M.; Neofytos, D.; Diekema, D.; Azie, N.; Meier-Kriesche, H.; Quan, S.; Horn, D. Epidemiology and outcomes of candidemia in 3648 patients: Data from the prospective antifungal therapy (PATH Alliance) registry, 2004–2008. Diagn. Microbiol. Infect. Dis. 2012, 74, 323–331. [Google Scholar] [CrossRef] [PubMed]
- Millsop, J.W.; Fazel, N. Oral candidiasis. Clin. Dermatol. 2016, 34, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Su, C.; Liu, H.P. Candida albicans hyphal initiation and elongation. Trends Microbiol. 2014, 22, 707–714. [Google Scholar] [CrossRef]
- Martins, K.V.; De Lacerda Gontijo, S.M. Treatment of denture stomatitis: Literature review. Rev. Sur. 2017, 74, 215–220. [Google Scholar]
- Sajjad, A.; Subhani Sajjad, S. Aloe vera: An ancient herb for modern dentistry—A literature review. J. Dent. Surg. 2014, 10463, 210463. [Google Scholar] [CrossRef]
- Pareek, S.; Nagaraj, A.; Sharma, P.; Naidu, S.; Yousuf, A. Aloe-vera: A herb with medicinal properties. Int. J. Oral Care Res. 2013, 1, 47–50. [Google Scholar]
- Deswal, H.; Singh, Y.; Grover, H.S.; Bhardwaj, A.; Verma, S. Neem: A Boon in Medical and Dental Therapies: A review. Innovare J. Agric. Sci. 2016, 4, 1–3. [Google Scholar]
- Deshmukh, N.B.; Aru, P.B.; Lokade, S.K.; Nagre, V.S.; Taware, T.J. A review: The remarkable neem tree: A comprehensive review of its botanical pharmacological and therapeutic properties. Int. J. Creat. Res. Thoughts 2023, 11, 738–744. [Google Scholar]
- Upadhyay, A.; Agrahari, P.; Singh, D.K. A review on the pharmacological aspects of Terminalia chebula. Int. J. Pharmacol. 2014, 10, 289–298. [Google Scholar] [CrossRef]
- Moreira, L.E.A.; de Farias Cabral, V.P.; Rodrigues, D.S.; Barbosa, A.D.; Silveira, M.J.C.B.; Coutinho, T.D.N.P.; Barbosa, S.A.; Sá, L.G.D.A.V.; Neto, J.B.d.A.; da Rocha, S.N.C.; et al. Antifungal activity of tannic acid against Candida spp. And its mechanism of action. Braz. J. Microbiol. 2024, 55, 3679–3690. [Google Scholar] [CrossRef] [PubMed]
- Farhat, M.; Tóth, J.; Héthelyi, B.É.; Szarka, S.; Czigle, S. Analysis of the essential oil compounds of Origanum syriacum L. Acta Fac. Pharm. Univ. Comen. 2012, 59, 6–14. [Google Scholar]
- Veldhuizen, E.J.; Tjeerdsma-van Bokhoven, J.L.; Zweijtzer, C.; Burt, S.A.; Haagsman, H.P. Structural requirements for the antimicrobial activity of carvacrol. J. Agric. Food Chem. 2006, 54, 1874–1879. [Google Scholar] [CrossRef]
- Salehi, B.; Sharifi-Rad, J.; Seca, A.M.L.; Pinto, D.C.G.A.; Michalak, M.; Trincone, F. Thymus spp. Essential oils: Chemical composition, biological activities and new practical applications. Int. J. Mol. Sci. 2018, 19, 3042. [Google Scholar]
- Khan, A.; Ahmad, A.; Akhtar, F.; Yousuf, S.; Xess, I.; Khan, L.A.; Manzoor, N. Ocimum sanctum Essential Oil and Its Active Principles Exert Their Antifungal Activity by Disrupting Ergosterol Biosynthesis and Membrane Integrity. Res. Microbiol. 2010, 161, 816–823. [Google Scholar] [CrossRef]
- Hussain, A.I.; Anwar, F.; Hussain Sherazi, S.T.; Przybylski, R. Chemical composition, antioxidant and antimicrobial activities of basil (Ocimum basilicum) essential oils depends on seasonal variations. Food Chem. 2008, 108, 986–995. [Google Scholar] [CrossRef]
- Rajagopal, P.L.; Rajeev, V.R. Virgin Coconut oil—An updated pharmacological review. World J. Multidiscip. Res. Dev. 2017, 3, 87–92. [Google Scholar]
- Udensi, J.; Umeh, S.; Mgbemena, I.; Emeka-Nwabunnia, I.; Ebe, T.; Aroh, K. Antifungal activities of virgin coconut oil on Candida albicans, Aspergillus niger and mould species. Afr. J. Health Sci. 2021, 7, 889–893. [Google Scholar]
- Wen-Ru, L.; Shi, Q.-S.; Dai, H.-Q.; Liang, Q.; Xie, X.-B.; Huang, X.-M.; Zhao, G.-Z.; Zhang, L.-X. Antifungal activity, kinetics and molecular mechanism of action of garlic oil against Candida albicans. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Borlinghaus, J.; Albrecht, F.; Gruhlke, M.C.H.; Nwachukwu, I.D.; Slusarenko, A.J. Allicin: Chemistry and biological properties. Molecules 2014, 19, 12591–12618. [Google Scholar] [CrossRef]
- Cox, S.D.; Mann, C.M.; Markham, J.L.; Bell, H.C.; Gustafson, J.E.; Warmington, J.R.; Wyllie, S.G. The mode of antimicrobial action of the essential oil of Melaleuca alternifolia (tea tree oil). J. Appl. Microbiol. 2000, 88, 170–175. [Google Scholar] [CrossRef]
- Amornvit, P.; Choonharuangdej, S.; Srithavaj, T. Lemongrass-Incorporated Tissue Conditioner Against Candida albicans Culture. J. Clin. Diagn. Res. 2014, 8, 50–52. [Google Scholar]
- Khan, N.; Shreaz, S.; Bhatia, R.; Ahmad, S.I.; Muralidhar, S.; Manzoor, N.; Khan, L.A. Anticandidal activity of curcumin and methyl cinnamaldehyde. Fitoterapia 2012, 83, 434–440. [Google Scholar] [CrossRef]
- Park, M.; Bae, J.; Lee, D.S. Antibacterial and antifungal effects of ginger essential oil and its components. Food Sci. Biotechnol. 2008, 17, 1368–1372. [Google Scholar]
- El-Baz, A.M.; Mosbah, R.A.; Goda, R.M.; Mansour, B.; Sultana, T.; Dahms, T.E.S.; El-Ganiny, A.M. Back to nature: Combating Candida albicans biofilm, phospholipase and hemolysin using plant essential oils. Antibiotics 2021, 10, 81. [Google Scholar] [CrossRef]
- Abdelillah, A.; Houcine, B.; Halima, D.; Meriem, C.; Imane, Z.; Eddine, S.D.; Abdallah, M.; Daoudi, C.S. Evaluation of antifungal activity of free fatty acids methyl esters fraction isolated from Algerian Linum usitatissimum L. seeds against toxigenic Aspergillus. Asian Pac. J. Trop. Biomed. 2013, 3, 443–448. [Google Scholar] [CrossRef]
- Khan, M.A.; Chen, H.C.; Tania, M. Antifungal activity of thymoquinone against Candida albicans: Role of oxidative stress and apoptosis. Microb. Pathog. 2019, 132, 100–108. [Google Scholar]
- Kumar, S.; Mishra, A.; Singh, S.P.; Singh, A. Anti-Filarial Efficacy of Centratherum anthelminticum: Unravelling the Underlying Mechanisms through Biochemical, HRAMS Proteomics and MD Simulation Approaches. RSC Adv. 2024, 14, 25198–25220. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Y. Chemical composition and antibacterial activity of essential oils from different parts of Litsea cubeba. Chem. Biodivers. 2010, 7, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Poolkerd, P.; Nagaviroj, N.; Eiampongpaiboon, T. Anti-microbial effect of Piper betle crude extract and essential oil incorporated into short-term soft lining material. J. Stomatol. 2023, 76, 226–234. [Google Scholar] [CrossRef]
- Natsir, S.; Khaerawati, N.; Kamril, R.A.; Febriani, N. The effects of fresh leaf-to-water ratio and heating time on the antifungal and antioxidant activities of betel leaf (Piper betle L.) extract. ResearchGate 2020, 10, 117–124. [Google Scholar]
- Rana, J.N.; Gul, K.; Mumtaz, S. Isorhamnetin: Reviewing Recent Developments in Anticancer Mechanisms and Nanoformulation-Driven Delivery. Int. J. Mol. Sci. 2025, 26, 7381. [Google Scholar] [CrossRef] [PubMed]
- Rana, J.N.; Mumtaz, S. Prunin: An Emerging Anticancer Flavonoid. Int. J. Mol. Sci. 2025, 26, 2678. [Google Scholar] [CrossRef]
- Chae, S.Y.; Jang, M.K.; Nah, J.W. Influence of molecular weight on oral absorption of water soluble chitosans. J. Control. Release 2005, 102, 383–394. [Google Scholar] [CrossRef]
- Liu, H.; Du, Y.; Wang, X.; Sun, L. Chitosan kills bacteria through cell membrane damage. Int. J. Food Microbiol. 2004, 95, 147–155. [Google Scholar] [CrossRef]
- Memon, M.R.; Memon, H.; Shoro, M.; Bhurgri, H.; Issrani, R.; Iqbal, A.; Khattak, O.; Altassan, M.; Almabadi, A.A.; Sultan, S.E.S.; et al. Effectiveness of Chitosan versus Natural Aloe Vera on Candida Adherence in Denture Soft Lining Material. Scientifica 2024, 8, 9918914. [Google Scholar] [CrossRef]
- Abdulwahhab, A.; Jassim, R. The Effect of Aloe vera Extract on Adherence of Candida albicans and Other Properties of Heat Cure Denture Soft Lining Material. Int. J. Med. Res. Health Sci. 2018, 7, 94–103. [Google Scholar]
- Noori, A.A.; Jaber, M.A.R. Effect Of Incorporation Of Either Neem Or Aloe Vera Powders On Tear Strength And Hardness Of Acrylic Soft Denture Liner Material. Mustansiria Dent. J. 2021, 18, 65–74. [Google Scholar] [CrossRef]
- Noori, A.A.; Jaber, M.A. Evaluation The Effect of Incorporation of Different Herbal Extract Powders (Either Neem or Aloe Vera) On Thermal Conductivity and Shear Bond Strength of Acrylic Soft Denture Liner Material. Tikrit J. Dent. Sci. 2022, 10, 35–46. [Google Scholar] [CrossRef]
- Madhan Kumar, V.S.; Anand Kumar, V.; Natarajan, P.; Sreenivasan, G. Antifungal Efficacy and the Mechanical Properties of Soft Liners against Candida albicans after the Incorporation of Garlic and Neem: An In Vitro Study. J. Int. Soc. Prev. Community Dent. 2018, 8, 263–268. [Google Scholar] [CrossRef]
- Kumar, P.S. The influence of Azadirachta indica, Melaleuca alternifolia, and Cocos nucifera on Candida albicans strain in tissue conditioner at varying time intervals. J. Indian Prosthodont. Soc. 2020, 20, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Muttagi, S.; Subramanya, J.K. Effect of incorporating seed oils on the antifungal property, surface roughness, wettability, weight change, and glucose sorption of a soft liner. J. Prosthet. Dent. 2017, 117, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, G.A. Studying the Anti Candidal-Activity of Different Herbal Oils Incorporated into Tissue Conditioner: A Comparative Study. Jordan J. Pharm. Sci. 2023, 16, 871–879. [Google Scholar] [CrossRef]
- Hatim, N.A. Effect of Plant Fixed Oil Extracts Incorporation to Heat Cured Denture Soft Lining Material on its Mechanical Properties. Iraqi Dent. J. 2012, 34, 58–70. [Google Scholar]
- Rajali, A.; Zain, N.M.; Amran, N.A.; Azmi, N.H. Antifungal efficacy of Ocimum basilicum essential oil in tissue conditioner against Candida albicans: An in vitro study. Contemp. Clin. Dent. 2023, 14, 115–122. [Google Scholar] [CrossRef]
- Vankadara, S.K.; Hallikerimath, R.B.; Patil, V.; Bhat, K.; Doddamani, M.H. Effect of Melaleuca alternifolia mixed with tissue conditioners in varying doses on colonization and inhibition of Candida albicans: An in vitro study. Contemp. Clin. Dent. 2017, 8, 446–450. [Google Scholar] [CrossRef]
- Naser, H.J.; Abdul-Ameer, F.M. Evaluating the effect of lemongrass essential oil addition on some properties of heat cure acrylic soft-lining material. Med. J. Babylon 2022, 19, 646–652. [Google Scholar] [CrossRef]
- Alamen, B.M.; Naji, G.A.-H.; Alsmael, M.A. The Effect of Virgin Coconut Oil Addition on the Hardness and Wettability of Acrylic Based Denture Soft Lining Material. J. Res. Med. Dent. Sci. 2020, 8, 96–106. [Google Scholar]
- Songsang, N.; Anunmana, C.; Pudla, M.; Eiampongpaiboon, T. Effects of Litsea cubeba Essential Oil Incorporated into Denture Soft Lining Materials. Polymers 2022, 14, 3261. [Google Scholar] [CrossRef]
- Ahmed, A.S.; Ahmed, R.S.; Arab, L.N. The antifungal potential of cinnamon oil incorporated into a heat-polymerized soft liner. J. Dent. Mater. Tech. 2024, 13, 116–120. [Google Scholar]
- Abdallah, R.M.; Aref, N.S. Curcumin Containing Soft Liner as an Alternative Treatment Modality for Oral Candidiasis. World J. Dent. 2021, 12, 435–440. [Google Scholar] [CrossRef]
- Kumpanich, J.; Eiampongpaiboon, T.; Kanchanavasita, W.; Chitmongkolsuk, S.; Puripattanavong, J. Effect of Piper betle extract on anti-candidal activity, gelation time, and surface hardness of a short-term soft lining material. Dent. Mater. J. 2020, 39, 1016–1021. [Google Scholar] [CrossRef] [PubMed]
- Mohammed, H.; Fatalla, A. The effectiveness of chitosan nano-particles addition into soft denture lining material on Candida albicans adherence. Pak. J. Med. Health Sci. 2020, 14, 1734–1739. [Google Scholar]
- Saeed, A.; Haider, A.; Zahid, S.; Khan, S.A.; Faryal, R.; Kaleem, M. In-vitro antifungal efficacy of tissue conditioner-chitosan composites as potential treatment therapy for denture stomatitis. Int. J. Biol. Macromol. 2019, 125, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Baygara, T.; Ugur, A.; Sarac, N.; Balci, U.; Ergun, G. Functional denture soft liner with antimicrobial and antibiofilm properties. J. Dent. Sci. 2018, 13, 213–219. [Google Scholar] [CrossRef]
- Pachava, K.R.; Nadendla, L.K.; Alluri, L.S.; Tahseen, H.; Sajja, N.P. In vitro Antifungal Evaluation of Denture Soft Liner Incorporated with Tea Tree Oil: A New Therapeutic Approach Towards Denture Stomatitis. J. Clin. Diagn. Res. 2015, 9, 62–64. [Google Scholar]
- Singhania, A.; Sathe, S.; Ranka, R.; Godbole, S.; Ranka, R.K.; Dubey, S.A. Individual and Synergistic Effects of Tea Tree Oil and Neem Extract on Candida albicans Adhesion to Denture Soft Liner. Cureus 2022, 14, 27869. [Google Scholar] [CrossRef]
- AlHamdan, E.M. Soft Denture liner and microbial disinfection with contemporary and conventional agents. Photodiagnosis Photodyn. Ther. 2022, 38, 102768. [Google Scholar] [CrossRef]
- Patil, A.R.; Ravi, M.B.; Raghavendra, S.K.N.; Archer, A.C.; Sowmya, S.; Soans, S.H.; Achar, R.R. Lemongrass oil disrupts the biofilm of Candida albicans MTCC 1637T on soft denture reliners at lower concentrations compared to thyme and tea tree oils. J. Appl. Biol. Biotechnol. 2022, 10, 108–115. [Google Scholar]
- Nikawa, H.; Yamamoto, T.; Hamada, T. Effect of components of resilient denture-lining materials on the growth, acid production and colonization of Candida albicans. Oral Microbiol. Immunol. 1995, 10, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Trublar, M.; Truhlar, Z.; Pekař, M.; Štemberek, J. Evaluation of the antifungal activity of nystatin incorporated into two soft lining materials. Biomaterials 2007, 28, 4843–4848. [Google Scholar]
- Guranda, D.F.; Pikhtar, A.V. Use of Benzyl Benzoate for New Purpose as Antimycotic Agent, Producing and Using Antimycotic Pharmaceutical Compositions. RU2573976C2, 18 December 2015. [Google Scholar]
- Yu, L.; Wang, X.; Wei, Y.; Jiang, S.; Ye, J.; Chen, Y.; Xu, F.; Wang, H.; Shao, X. A cyclodextrin metal-organic framework loaded with erpinene-4-ol and its application to control gray mold in strawberry. Food Control 2024, 155, 110053. [Google Scholar] [CrossRef]
- Yadav, E.; Rao, R. A promising bioactive component erpinene-4-ol: A review. Int. J. Pharmacogn. 2016, 3, 336–345. [Google Scholar]
- Fujisawa, H.; Suma, K.; Origuchi, K.; Kumagai, H.; Seki, T.; Ariga, T. Biological and chemical stability of garlic-derived allicin. J. Agric. Food Chem. 2008, 56, 4229–4235. [Google Scholar] [CrossRef] [PubMed]
- ChemicalBook. CAS DataBase List. Allicin. Available online: https://www.chemicalbook.com/ChemicalProductProperty_EN_CB8466037.htm (accessed on 24 September 2025).
- ChemicalBook. Brief Introduction of Thymol. 2022. Available online: https://www.chemicalbook.com/article/brief-introduction-of-thymol.htm (accessed on 24 September 2025).
- Jaapar, S.Z.S.; Morad, N.A.; Iwai, Y. Prediction of Solubilities for Ginger Bioactive Compounds in Hot Water by the COSMO-RS Method. J. Phys. Conf. Ser. 2013, 423, 012066. [Google Scholar] [CrossRef]
- Swetha, T.K.; Vikraman, A.; Nithya, C.; Hari Prasath, N.; Pandian, S.K. Synergistic antimicrobial combination of carvacrol and thymol impairs single and mixed-species biofilms of Candida albicans and Staphylococcus epidermidis. Biofouling 2020, 36, 1256–1271. [Google Scholar] [CrossRef]
- Dantas, T.D.S.; Machado, J.C.B.; Ferreira, M.R.A.; Soares, L.A.L. Bioactive Plant Compounds as Alternatives Against Antifungal Resistance in the Candida Strains. Pharmaceutics 2025, 17, 687. [Google Scholar] [CrossRef]
- Perchyonok, T. Bio-Active Denture Soft Liner Materials from Design to Application: In vitro approach. J. Dent. Health Oral Disord. Ther. 2017, 6, 101–105. [Google Scholar] [CrossRef][Green Version]
- Sánchez-Aliaga, A.; Farago, P.V.; Michél, M.D.; Sugio, C.Y.C.; Neppelenbroek, K.H. Surface morphology and in vitro leachability of soft liners modified by the incorporation of antifungals for denture stomatitis treatment. J. Appl. Oral Sci. 2020, 28, 20200639. [Google Scholar] [CrossRef]
- Ojah, P.; Luniyal, C.; Nair, C.; Astekar, M.; Pal, A.; Chopra, M. Anti candidal efficacy of commercially available triphala, neem, denture cleanser and natural aloevera leaf on heat polymerized acrylic resin. J. Indian Prosthodont. Soc. 2021, 21, 167–172. [Google Scholar] [CrossRef]
- Guo, X.; Mei, N. Aloe vera: A review of toxicity and adverse clinical effects. J. Environ. Sci. Health C Environ. Carcinog. Ecotoxicol. Rev. 2016, 34, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Nalimu, F.; Oloro, J.; Peter, E.L.; Ogwang, P.E. Acute and sub-acute oral toxicity of aqueous whole leaf and green rind extracts of Aloe vera in Wistar rats. BMC Complement. Med. Ther. 2022, 22, 16. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.; Lal, R.; Sankaranarayanan, A.; Banerjee, C.K.; Sharma, P.L. Acute toxicity study of the oil from Azadirachta indica seed (neem oil). J. Ethnopharmacol. 1988, 23, 39–51. [Google Scholar] [CrossRef]
- Dorababu, M.; Joshi, M.C.; Bhawani, G.; Kumar, M.M.; Chaturvedi, A.; Goel, R.K. Effect of aqueous extract of neem (Azadirachta indica) leaves on offensive and diffensive gastric mucosal factors in rats. Indian J. Physiol. Pharmacol. 2006, 50, 241–249. [Google Scholar]
- Kingsley, O.-A.; Oseni, L.A.; Quasie, O.; Antwi, S.; Mavis, T. A comparative evaluation of in vivo antiplasmodial activity of aqueous leaf extracts of Carica papaya, Azadirachta indica, Mangifera indica and the combination thereof using Plasmodium infected BALB/c mice. Int. J. Appl. Biol. Pharm. Technol. 2012, 3, 372–378. [Google Scholar]
- Sani, I.; Umar, R.A.; Hassan, S.W.; Faruq, U.Z.; Bello, F. Median lethal dose and sub-chronic toxicity profile of Azadirachta indica A. Juss. Leaf hexane and ethyl acetate fractionated extracts on albino rats. World J. Biol. Pharm. Health Sci. 2020, 3, 7–22. [Google Scholar] [CrossRef]
- Kupradinun, P.; Tepsuwan, A.; Tanthasri, N.; Meesiripan, N.; Tunsakul, S.; Tompat, W.; Jarratwisarutporn, Y.; Kusamran, W.R. Toxicity testing of flowers of neem tree (Azadirachta indica A. Juss). Thai J. Vet. Med. 2013, 40, 47–55. [Google Scholar] [CrossRef]
- Achi, N.K.; Onyeabo, C.; Nnate, D.A.; Ekeleme-Egedigwe, C.A.; Kalu, I.K.; Chibundu, I.C.; Wokoma, G.C. Therapeutic effects of Azadirachta indica A.Juss. leaves in malaria-induced male Wistar rats. J. Pharm. Pharmacogn. Res. 2018, 6, 191–204. [Google Scholar] [CrossRef]
- Mbaya, A.W.; Ibrahim, U.I.; God, O.T.; Ladi, S. Toxicity and potential anti-trypanosomal activity of ethanolic extract of Azadirachta indica (Maliacea) stem bark: An in vivo and in vitro approach using Trypanosoma brucei. J. Ethnopharmacol. 2010, 128, 495–500. [Google Scholar] [CrossRef]
- Akin-Osanaiye, B.C.; Nok, A.J.; Ibrahim, S.; Inuwa, H.M.; Onyike, E.; Amlabu, E.; Haruna, E. Antimalarial effect of neem leaf and neem stem bark extracts on Plasmodium berghei infected in the pathology and treatment of malaria. Int. J. Res. Biochem. Biophys. 2013, 3, 7–14. [Google Scholar]
- Arpornchayanon, W.; Subhawa, S.; Jaijoy, K.; Lertprasertsuk, N.; Soonthornchareonnon, N.; Sireeratawong, S. Safety of the oral Triphala recipe from acute and chronic toxicity tests in Sprague-Dawley rats. Toxics 2022, 10, 514. [Google Scholar] [CrossRef] [PubMed]
- Jagetia, G.C.; Baliga, M.S.; Malagi, K.J.; Sethukumar Kamath, M. The evaluation of the radioprotective effect of Triphala (an ayurvedic rejuvenating drug) in the mice exposed to gamma-radiation. Phytomedicine 2002, 9, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Gorriti, A.; Arroyo, J.; Quispe, F.; Cisneros, B.; Condorhuamán, M.; Almora, Y.; Chumpitaz, V. Toxicidad oral a 60 días del aceite de sacha inchi (Plukenetia volubilis L.) y linaza (Linum usitatissimum L.) y determinación de la dosis letal 50 en roedores [Oral toxicity at 60-days of sacha inchi oil (Plukenetia volubilis L.) and linseed (Linum usitatissimum L.), and determination of lethal dose 50 in rodents]. Rev. Peru. Med. Exp. Salud Publica 2010, 27, 352–360. [Google Scholar] [PubMed][Green Version]
- Baig, N.; Qureshi, S.A.; Anwar, M.; Rafique, A.; Aziz, S.; Mansoor, A. Centratherum anthelminticum (L.) Kuntze seed oil restoring hepatic balance in type 2 diabetic rats through Nrf2/Keap1/HO-1 and NF-κB pathway modulation. Res. Sq. 2025. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Maisanaba, S.; Puerto, M.; Pichardo, S.; Jos, A.; Moyano, R.; Cameán, A.M. A subchronic 90-day oral toxicity study of Origanum vulgare essential oil in rats. Food Chem. Toxicol. 2017, 101, 36–47. [Google Scholar] [CrossRef]
- Abdelghffar, E.A.R.; El-Nashar, H.A.S.; Fayez, S.; Obaid, W.A.; Eldahshan, O.A. Ameliorative effect of oregano (Origanum vulgare) versus silymarin in experimentally induced hepatic encephalopathy. Sci. Rep. 2022, 12, 17854. [Google Scholar] [CrossRef]
- Kumar, A.; Shukla, R.; Singh, P.; Dubey, N.K. Chemical composition, antifungal and antiaflatoxigenic activities of Ocimum sanctum L. essential oil and its safety assessment as plant-based antimicrobial. Food Chem. Toxicol. 2010, 48, 539–543. [Google Scholar] [CrossRef]
- Chil, I.; Escalona Arranz, J.; Berenguer Rivas, C.; Mendonca, P.; Pérez, K.M.; Dutok Sánchez, C.; Cortinhas, L.; Da Silva, C.; Carvalho, G.; Queiroz, M. Chemical composition and toxicity of Ocimum sanctum L. Var. Cubensis essential oil up-growing in the eastern of Cuba. Int. J. Pharmacogn. Phytochem. Res. 2018, 9, 11175. [Google Scholar] [CrossRef]
- Abrori, C.; Nurfadhila, K.; Sakinah, E.N. Acute toxicity tests of basil leaves (Ocimum sanctum) ethanolic extract determined by LD50 and renal histopathology. J. Agromed. Med. Sci. 2019, 5, 13–19. [Google Scholar]
- Venâncio, A.M.; Onofre, A.S.C.; Lira, A.F.; Alves, P.B.; Blank, A.F.; Antoniolli, Â.R.; Marchioro, M.; Estevam, C.d.S.; de Araujo, B.S. Chemical composition, acute toxicity, and antinociceptive activity of the essential oil of a plant breeding cultivar of basil (Ocimum basilicum L.). Planta Med. 2011, 77, 825–829. [Google Scholar] [CrossRef] [PubMed]
- Fandohan, P. Toxicity and gastric tolerance of essential oils from Cymbopogon citratus, Ocimum gratissimum and Ocimum basilicum in Wistar rats. Food Chem. Toxicol. 2008, 46, 2493–2497. [Google Scholar] [CrossRef]
- Safety Data Sheet: Tea Tree Oil—ATE (Oral). Available online: https://prod.adv-bio.com/sds-us/3037sdsUS.pdf (accessed on 28 September 2025).
- Biochemica®. Safety Data Sheet—COCONUT OIL EV ORG; Hallstar: Chicago, IL, USA, 2024; Available online: https://hallstar-sds.thewercs.com/private/document.aspx?PRD=EVCOPDFMTRAGHHEN2024-05-14+14%3A03%3A44BIOCHEMICA%C2%AE+COCONUT+OIL+EV+ORG+~~ (accessed on 28 September 2025).
- Abere, T. Toxicological evaluation of virgin coconut oil extracted from Cocos nucifera L. (Arecaceae). J. Sci. Pract. Pharm. 2021, 8, 417–424. [Google Scholar] [CrossRef]
- Madaki, F.; Waheed, S.; Musa, B.; Ibrahim, Y.; Kabiru, A.; Ogbadoyi, E.; Mann, A. Antioxidant and anti-trypanosomal activities of the Allium sativum (garlic) bulb aqueous extract on Trypanosoma congolense infected mice. Iran. J. Parasitol. 2022, 16, 153–162. [Google Scholar] [CrossRef]
- Ekong, M.B.; Muonagolu, N.J.; Peter, A.I.; Ekandem, G.J.; Ekanem, T.B. Morphogenesis of the thymus in rabbit during prenatal and early postnatal periods. Histol. Cytol. Embryol. 2017, 1, 1–6. [Google Scholar]
- Nakagawa, S.; Masamoto, K.; Sumiyoshi, H.; Harada, H. Acute toxicity test of garlic extract. J. Toxicol. Sci. 1984, 9, 57–60. [Google Scholar] [CrossRef]
- Ihekwereme, P.; Asomugha, R.; Mbagwu, S.; Oraekei, D.I.; Ajaghaku, D. Phytochemicals, acute toxicities and actual median lethal doses (actual LD50) of Zingiber officinale and Allium sativum given singly and in combination via mice models. GSC Biol. Pharm. Sci. 2023, 25, 8–18. [Google Scholar] [CrossRef]
- Costa, C.A.; Bidinotto, L.T.; Takahira, R.K.; Salvadori, D.M.; Barbisan, L.F.; Costa, M. Cholesterol reduction and lack of genotoxic or toxic effects in mice after repeated 21-day oral intake of lemongrass (Cymbopogon citratus) essential oil. Food Chem. Toxicol. 2011, 49, 2268–2272. [Google Scholar] [CrossRef]
- Offor, V.; Ufele, A.; Ononye, B.; Aghalu, U.; Afoemezie, P.; Azaka, E.; Chude, C. Effects of Aloe barbadensis and Cymbopogon citratus on blood glucose levels of alloxan-induced diabetic albino rats. J. Complement. Altern. Med. Res. 2023, 21, 44–51. [Google Scholar] [CrossRef]
- Rojas-Armas, J.; Arroyo-Acevedo, J.; Ortiz-Sánchez, M.; Palomino-Pacheco, M.; Castro-Luna, A.; Ramos-Cevallos, N.; Justil-Guerrero, H.; Hilario-Vargas, J.; Herrera-Caldern, O. Acute and repeated 28-day oral dose toxicity studies of Thymus vulgaris L. essential oil in rats. Toxicol. Res. 2019, 35, 225–232. [Google Scholar] [CrossRef]
- Olayemi, F.O.; Adebayo, E.A.; Adelowo, F.E. The antimicrobial activity and the median lethal dose of thyme (Thymus vulgaris) ethanolic extract on mice. Int. J. Basic Appl. Sci. 2017, 6, 1–8. [Google Scholar]
- Biolandes. Safety Data Sheet—Thyme (Thymus vulgaris L.); Biolandes: Le Sen, France, 2023; Available online: https://www.biolandes.com/wp-content/uploads/fiche-securite-B915.pdf (accessed on 28 September 2025).
- Chourasiya, P.; Gour, R.; Patel, A.K. Qualitative phytochemical investigation and acute oral toxicity study on ethanolic extract of Curcuma longa leaves. J. Drug Deliv. Ther. 2024, 14, 73–78. [Google Scholar] [CrossRef]
- Mohammed, A.; Wudil, A.M.; Alhassan, A.J.; Muhammad, I.U.; Idi, A.; Abdulmumin, Y. Acute and subchronic toxicity studies of aqueous, methanolic and n-hexane root extracts of Curcuma longa L. on albino rats. J. Pharm. Res. Int. 2016, 14, 1–8. [Google Scholar] [CrossRef]
- Oyemitan, I.A.; Elusiyan, C.A.; Onifade, A.O.; Akanmu, M.A.; Oyedeji, A.O.; McDonald, A.G. Neuropharmacological profile and chemical analysis of essential oil of Curcuma longa. Toxicol. Rep. 2017, 4, 391–398. [Google Scholar] [CrossRef]
- Wakil, A.W.; Stephen, J.; Mohammed, A.; Ekwem, P.C.; Ngulde, S.I.; Sandabe, U.K.; Mbaya, A.W.; Ojo, N.A.; Sodipo, O.A. Acute toxicity and effects of ethanolic extract of turmeric (Curcuma longa) on some serum biochemical parameters in rats. Sahel J. Life Sci. 2024, 2, 149–155. [Google Scholar] [CrossRef]
- Winarsih, W.; Wientarsih, I.; Sulistyawati, N.P.; Wahyudina, I. Uji toksisitas akut ekstrak rimpang kunyit pada mencit: Kajian histopatologis lambung, hati dan ginjal (Acute oral toxicity of turmeric extract in mice: Histopathological studies of stomach, liver and kidney). J. Veteriner 2013, 13, 402–409. [Google Scholar]
- Luo, M.; Jiang, L.K.; Zou, G.L. Acute and genetic toxicity of essential oil extracted from Litsea cubeba (Lour.) Pers. J. Food Prot. 2005, 68, 581–588. [Google Scholar] [CrossRef]
- Golgemma. Fiche de Données de Sécurité—Huile de Litsea Cubeba Chine; Golgemma: Esperaza, France, 2024; Available online: https://golgemma.com/wp-content/uploads/fiche-securite-570.pdf (accessed on 28 September 2025).
- Zaoui, A.; Cherrah, Y.; Alaoui, K.; Mahassine, N.; Amarouch, H.; Hassar, M. Acute and chronic toxicity of Nigella sativa fixed oil. Phytomedicine 2002, 9, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Al-Ali, A.; Alkhawajah, A.A.; Randhawa, M.A.; Shaikh, N.A. Oral and intraperitoneal LD50 of thymoquinone, an active principle of Nigella sativa, in mice and rats. J. Ayub Med. Coll. Abbottabad 2008, 20, 25–27. [Google Scholar]
- Hamman, L.L.; Garba, S.H.; Jacks, T.W.; Zirahei, J.V.; Dibal, N.I.; Attah, M.O.O. Acute toxicity and effect of prolonged oral administration of Zingiber officinale ethanol extract on liver and kidney histology in rats. Arid. J. Basic Appl. Res. 2022, 5, 149–155. [Google Scholar]
- Li, Y.; Zhang, L.; Wang, X.; Zhang, Y.; Zhang, J.; Zhang, Z. Acute toxicity studies of methanolic extracts of Zingiber officinale in mice. J. Ethnopharmacol. 2016, 189, 123–130. [Google Scholar]
- Iu, M.; Jazuli, F.U.; Faruq, F.W.; Imam, A.A.; Alhassan, A.J.; Yaradua, A.I. Phytochemical screening, acute (LD50) and sub-chronic toxicity studies of aqueous stem bark extract of Cinnamomum verum. Saudi J. Med. Pharm. Sci. 2022, 11, 1253–1258. [Google Scholar]
- Safety Data Sheet—Organic Cinnamon Essential Oil—Bark; Spectrum Chemical: New Brunswick, NJ, USA.
- Ranasinghe, P.; Galappaththy, P.; Constantine, G.R.; Jayawardena, R.; Weeratunga, H.D.; Premakumara, S.; Katulanda, P. Cinnamomum zeylanicum (Ceylon cinnamon) as a potential source of bioactive compounds: A review. Phytochem. Rev. 2017, 16, 613–625. [Google Scholar]
- Nayaka, N.M.D.M.W.; Sasadara, M.M.V.; Sanjaya, D.A.; Yuda, P.E.S.K.; Dewi, N.L.K.A.A.; Cahyaningsih, E.; Hartati, R. Piper betle (L): Recent review of antibacterial and antifungal activities. Pharmacogn. J. 2021, 13, 1511–1516. [Google Scholar]
- Punarvasu, T.P.; Harish Prashanth, K.V. Acute and subacute in vivo safety assessment of developed chitosan derivatives for food applications. Food Hydrocoll. Health 2023, 4, 100145. [Google Scholar] [CrossRef]
- O’Neil, M.J. The Merck Index—An Encyclopedia of Chemicals, Drugs, and Biologicals, 13th ed.; Merck and Co., Inc.: Whitehouse Station, NJ, USA, 2001; p. 1208. [Google Scholar]
- Fisher Scientific. Nystatin—Safety Data Sheet. Available online: https://www.fishersci.com/shop/products/nystatin-5g/bp29495 (accessed on 18 September 2025).
- Pfizer Inc. Fluconazole—Material Safety Data Sheet. Available online: https://cdn.pfizer.com/pfizercom/products/material_safety_data/501.pdf (accessed on 18 September 2025).
- McKesson Medical-Surgical Inc. Safety Data Sheet—Fluconazole Injection. Available online: https://imgcdn.mckesson.com/CumulusWeb/Click_and_learn/SDS_9SAGPH_25021011382_FLUCONAZOLE_INJECTION.pdf (accessed on 18 September 2025).
- McKesson Medical-Surgical Inc. Safety Data Sheet—Itraconazole Solution 10 mg/mL, 150 mL. Available online: https://imgcdn.mckesson.com/CumulusWeb/Click_and_learn/SDS_9AMNEL_ITRACONAZOLE_SOL_10MG_ML_150ML.pdf (accessed on 18 September 2025).
- Covetrus North America. Ketoconazole—Material Safety Data Sheet. Available online: https://northamerica.covetrus.com/Content/SDS/071342.pdf (accessed on 18 September 2025).
- InChem. Miconazole—Safety Data Sheet. Available online: https://www.inchem.org/documents/pims/pharm/miconazo.htm (accessed on 18 September 2025).
- Cayman Chemical Company. Clotrimazole—Safety Data Sheet. Available online: https://s3-us-west-2.amazonaws.com/drugbank/cite_this/attachments/files/000/003/124/original/Safety_data_sheet__clotrimazole.pdf?1548367831 (accessed on 18 September 2025).
- Spectrum Chemical Manufacturing Corporation. Safety Data Sheet—Chlorhexidine Acetate, USP (Product Code: C4066); Spectrum Chemical Mfg. Corp.: Gardena, CA, USA, 2016; Available online: https://www.spectrumchemical.com/MSDS/C4066_AGHS.pdf (accessed on 18 September 2025).
- Vista Apex. Safety Data Sheet—CHX-Plus (Chlorhexidine Digluconate); Vista Apex Dental Products: Racine, WI, USA, 2019; Available online: https://vistaapex.com/wp-content/uploads/2020/12/FINAL-CHX-Plus-US-SDS-EN-190201.pdf (accessed on 18 September 2025).
- World Health Organization (WHO). WHO Classification of Toxicity and LD50 Values. Available online: https://www.who.int/ (accessed on 24 September 2025).
- Song, Y.-H.; Song, H.-J.; Han, M.-K.; Yang, H.-S.; Park, Y.-J. Cytotoxicity of soft denture lining materials depending on their component types. Int. J. Prosthodont. 2014, 27, 229–235. [Google Scholar] [CrossRef]
- Naarala, J.; Korpi, A. Cell death and production of reactive oxygen species by murine macrophages after short term exposure to phthalates. Toxicol. Lett. 2009, 188, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Babich, H.; Zuckerbraun, H.L.; Wurzburger, B.J.; Rubin, Y.L.; Borenfreund, E.; Blau, L. Benzoyl peroxide cytotoxicity evaluated in vitro with the human keratinocyte cell line, RHEK-1. Toxicology 1996, 106, 187–196. [Google Scholar] [CrossRef]
- EN ISO 10139-2:2016; Dentistry—Soft Lining Materials for Removable Dentures—Part 2: Materials for Long-Term Use. ISO International Organization for Standardization: London, UK, 2016.
- Sakaguchi, R.L.; Powers, J.M. Craig’s Restorative Dental Materials, 13th ed.; Elsevier/Mosby: St. Louis, MO, USA, 2012. [Google Scholar]
- Mancuso, D.N.; Goiato, M.C.; Zuccolotti, B.C.R.; Moreno, A.; dos Santos, D.M. Evaluation of hardness and colour change of soft liners after accelerated ageing. Prim. Dent. Care 2009, 16, 127–130. [Google Scholar] [CrossRef]
- Aloul, R.K.; Shen, C. The influence of plasticizer loss on the viscoelasticity of temporary soft liners. J. Prosthodont. 2002, 11, 254–262. [Google Scholar] [CrossRef]
- Munksgaard, E.C. Plasticizers in denture soft-lining materials: Leaching and biodegradation. Eur. J. Oral Sci. 2005, 113, 166–169. [Google Scholar] [CrossRef]
- Manurung, M.M.; Leksonowati, N.F.P.; Pamungkas, N.; Stefani, W.; Batubara, N.H.; Purba, A.S. The Impact of Plasticizer Levels in Hardening PVC Plastic. J. Integrasi 2024, 16, 35–46. [Google Scholar] [CrossRef]
- Oguz, S.; Mutluay, M.M.; Dogan, O.M.; Bek, B. Effect of Incorporation of Antifungal Agents on the Ultimate Tensile Strength of Temporary Soft Denture Liners. J. Prosthodont. 2017, 27, 215–220. [Google Scholar] [CrossRef]
- Hasan, W.Y.; Ali, M.M. Evaluation of Thermal Conductivity and Some Other Properties of Heat Cured Denture Soft Liner Reinforced By Halloysite Nanotubes. Biomed. Pharmacology J. 2018, 11, 1491–1500. [Google Scholar] [CrossRef]
- Muddugangadhar, B.C.; Ramaraju, C.; Narayana, A.; Ranganathan, V. Effect of Incorporation of Silver Nanoparticles on the Tensile Bond Strength of a Long term Soft Denture Liner. J. Contemp. Dent. Pract. 2020, 21, 473–477. [Google Scholar]
- Sinobad, D.; Murphy, W.M.; Huggett, R.; Brooks, S. Bond strength and rupture properties of some soft denture liners. J. Oral Rehabil. 1992, 19, 151–160. [Google Scholar] [CrossRef]
- Bueno, M.; Urban, V.M.; Barbério, G.S.; de Lima, A.R.R.; Cislaghi, H.A.; da Silva, W.J.; de Almeida, A.L.F. Effects of Antifungals Incorporated into Soft Denture Liners on the Mechanical Properties and Adherence of Candida albicans. J. Prosthet. Dent. 2017, 117, 178–185. [Google Scholar]
- Machado, A.L.; Giampaolo, E.T.; Vergani, C.E.; Salles, M.M.; Jorge, J.H. Changes in roughness of denture base and reline materials by chemical disinfection or microwave irradiation. J. Prosthet. Dent. 2011, 106, 375–381. [Google Scholar]
- Vasisht, K.; Sharma, N.; Karan, M. Current Perspective in the International Trade of Medicinal Plants Material: An Update. Curr. Pharm. Des. 2016, 22, 4288–4336. [Google Scholar] [CrossRef] [PubMed]
- Global Market Insights. Medicinal and Aromatic Plant Market Size—By Plant Type, Application, Product Form, Cultivation Method, Growth Forecast, 2025–2034. Available online: https://www.gminsights.com/industry-analysis/medicinal-and-aromatic-plant-market (accessed on 28 September 2025).
- Zamani, S.; Fathi, M.; Ebadi, M.-T.; Máthé, Á. Global trade of medicinal and aromatic plants: A review. J. Agric. Food Res. 2025, 21, 101910, Corrigendum in J. Agric. Food Res. 2025, 22, 102040. [Google Scholar] [CrossRef]
- Camaioni, L.; Ustyanowski, B.; Buisine, M.; Lambert, D.; Sendid, B.; Billamboz, M.; Jawhara, S. Natural Compounds with Antifungal Properties against Candida albicans and Identification of Hinokitiol as a Promising Antifungal Drug. Antibiotics 2023, 12, 1603. [Google Scholar] [CrossRef] [PubMed]
| Plant Latin Name (Common Name) (Family) | Additive Content (Form) | SLM Type/Name (Manufacturer, Country) Powder/Liquid Ratio | Antimicrobial Properties | Reference |
|---|---|---|---|---|
| Enriched SLM Component | ||||
| Mechanical Properties | ||||
| Aloe vera (aloe) (Liliaceae) | 2 wt. % (powder) | TC/GC Soft Liner (GC Corp., Tokyo, Japan) 1.2 g/1 mL | C. albicans colony count | [40] |
| powder | - | |||
| 3 and 10 wt.% (powder) | ST-A-SLM/Vertex Soft (Vertex Dental, Soesterberg, The Netherlands) 1.2 g/1 mL | - | [41] | |
| powder | Tear strength, shear bond strength | |||
| 10 wt.% (powder) | ST-A-SLM/Vertex Soft (Vertex Dental, Soesterberg, The Netherlands) 1.2 g/1 mL | - | [42,43] | |
| formulation | Tear strength, shear bond strength | |||
| Azachirachta indica (Neem) (Meliaceae) | 50, 100, 200, 400, 500 µg (powder) | TC/GC Soft Liner (GC Corp., Tokyo, Japan) 1.2 g/1 mL | C. albicans colony count | [44] |
| powder | Shore A hardness | |||
| 5, 10, 15, 20, 25, 30, 35, 40 vol./vol. % (oil extract) | TC/Visco-gel (Dentsply DeTrey, Konstanz, Germany) 3 g/2.2 mL | C. albicans inhibition zone | [45] | |
| liquid | - | |||
| 10 wt.% (powder) | ST-A-SLM/Vertex Soft (Vertex Dental, Soesterberg, The Netherlands) 1.2 g/1 mL | - | [42,43] | |
| formulation | Tear strength, shear bond strength | |||
| Linum usitatissimum (flaxseed) (Linaceae) | 800, 900 and 1000 µL (seed oil) | TC/Visco-gel (Dentsply DeTrey, Konstanz, Germany) 3 g/2.2 mL | C. albicans inhibition zone | [46] |
| liquid | Roughness | |||
| Centratherum anthelminticum (bitter cumin) (Asteraceae) | 600, 700 and 800 µL (seed oil) | TC/Visco-gel (Dentsply DeTrey, Konstanz, Germany) 3 g/2.2 mL | C. albicans inhibition zone | [46] |
| liquid | Roughness | |||
| Origanum vulgare (oregano) (Lamiaceae) | 50 µg/mL (oil) | TC/Acrosoft (Marlik Medical Industries Co., Tehran, Iran) 3.2 g/2.5 mL | C. albicans inhibition zone | [47] |
| formulation | - | |||
| Thymus vulgaris (thyme) (Lamiaceae) | 5 vol.% (oil) | ST-A-SLM/Vertex Soft (Vertex Dental, Soesterberg, The Netherlands) 1.2 g/1 mL | - | [48] |
| liquid | Tensile strength | |||
| Ocimum sanctum (Holy Basil and Tulsi) (Lamiaceae) | 600, 700 and 800 µL (oil) | TC/Visco-gel (Dentsply DeTrey, Konstanz, Germany) 3 g/2.2 mL | C. albicans inhibition zone | [46] |
| liquid | Roughness | |||
| Ocimum basilicum (basil) (Lamiaceae) | 1.25 and 5 vol./vol. % (oil) | TC/Visco-gel (Dentsply DeTrey, Konstanz, Germany) 3 g/2 mL | C. albicans biofilm formation | [49] |
| liquid | Roughness | |||
| 5, 10, 15, 20, 25, 30, 35, 40 vol./vol. % (oil) | TC/Visco-gel (Dentsply DeTrey, Konstanz, Germany) 3 g/2 mL | C. albicans inhibition zone | [45] | |
| liquid | - | |||
| Melaleuca alternifolia (tea tree) (Myrtaceae) | 0.5, 1, 1.5, 2 mL (10, 20, 30, 40 vol./vol. % oil extract) | TC/Visco-gel (Dentsply DeTrey, Konstanz, Germany) 3 g/2.2 mL TC/GC Soft Liner (GC Corp., Tokyo, Japan) 1.2 g/1 mL | C. albicans colony count | [50] |
| liquid | - | |||
| Cymbopogon citratus (lemongrass) (Poaceae) | 2.5 and 5 vol.% (oil) | TC/Moonstar Soft ((Moonstar, Turkey) 10 g/7.8 mL | - | [51] |
| liquid | Peel bond strength, roughness | |||
| Cocos nucifera (coconut) (Arecaceae) | 5, 10, 15, 20, 25, 30, 35, 40 vol./vol. % (oil extract) | TC/Visco-gel (Dentsply DeTrey, Konstanz, Germany) 3 g/2 mL | C. albicans inhibition zone | [45] |
| liquid | - | |||
| 1.5, 2.5 vol/vol. % oil | ST-A-SLM/Vertex Soft (Vertex Dental, Soesterberg, The Netherlands) 1.2 g/1 mL | - | [52] | |
| liquid | Shore A hardness | |||
| Allium sativum (garlic) (Amaryllidaceae) | 50, 100, 200, 400, 500 µg (powder) | TC/GC Soft Liner (GC Corp., Tokyo, Japan) 1.2 g/1 mL | C. albicans colony count | [44] |
| powder | Shore A hardness | |||
| 50 µg/mL (oil) | TC/Acrosoft (Marlik Medical Industries Co., Tehran, Iran) 3.2 g/2.5 mL | C. albicans inhibition zone | [47] | |
| formulation | - | |||
| Litsea cubeba (Lauraceae) | 5, 10, 20, 30 vol./vol. % (oil extract) | TC/GC Soft Liner (GC Corp., Tokyo, Japan) 1.2 g/1 mL TC/Visco-gel (Dentsply DeTrey, Konstanz, Germany) 3 g/2 mL TC/Coe-Comfort (COE, GC America Inc., Alsip, IL, USA) 6 g/5 mL | C. albicans and S. mutans inhibition zone | [53] |
| liquid | Shore A hardness | |||
| Cinnamomum (cinnamon) (Lauraceae) | 1 and 2 wt. % (oil) | ST-A-SLM/Vertex Soft (Vertex Dental, Soesterberg, The Netherlands) 1.2 g/1 mL | C. albicans inhibition zone | [54] |
| liquid | Shore A hardness | |||
| Curcuma longa (curcumin) (Zingiberaceae) | 10 and 20 vol./vol. % (10 vol. % ethanolic extract) | TC/Trusoft (Boswoth Company, Skokie, IL, USA) 9 g/6.8 mL | C. albicans inhibition zone | [55] |
| liquid | Tensile bond strength | |||
| Zingiber officinale (ginger) (Zingiberaceae) | 50 µg/mL (oil) | TC/Acrosoft (Marlik Medical Industries Co., Tehran, Iran) 3.2 g/2.5 mL | C. albicans inhibition zone | [47] |
| formulation | - | |||
| Nigella sativa (black seed) (Ranunculaceae) | 50 µg/mL (oil) | TC/Acrosoft (Marlik Medical Industries Co., Tehran, Iran) 3.2 g/2.5 mL | C. albicans inhibition zone | [47] |
| formulation | - | |||
| Piper bettle (Piperaceae) | 2.5, 5, 10, 20, 30, 40 wt./wt. % (crude extract) and 2.5, 5, 10, 20, 30, 40 vol./vol. % (oil extract) | TC/GC Soft Liner (GC Corp., Tokyo, Japan) 1.2 g/1 mL | C. albicans and S. mutans inhibition zone | [34] |
| formulation | - | |||
| 0.25, 0.5, 1, 2.5, 5, 10, 20 wt./wt. % (oil extract) | TC/GC Soft Liner (GC Corp., Tokyo, Japan) 1.2 g/1 mL | C. albicans inhibition zone | [56] | |
| liquid | Shore A hardness | |||
| Sesamum indicum (sesame) (Pedaliaceae) | 5 vol.% (oil) | ST-A-SLM/Vertex Soft (Vertex Dental, Soesterberg, The Netherlands) 1.2 g/1 mL | - | [48] |
| liquid | Tensile strength | |||
| Chitosan | 2 wt. % (powder) | TC/GC Soft Liner (GC Corp., Tokyo, Japan) 1.2 g/1 mL | C. albicans colony count | [40] |
| powder | - | |||
| 1.5 and 2 wt. % (powder/nanoparticles) | ST-A-SLM/Vertex Soft (Vertex Dental, Soesterberg, The Netherlands) 1.2 g/1 mL | C. albicans colony count | [57] | |
| liquid | - | |||
| 0.625, 1.25 and 2.5 mg/mL (powder) | TC/GC Soft Liner (GC Corp., Tokyo, Japan) 1.2 g/1 mL | C. albicans colony count | [58] | |
| powder | - |
| Plant Latin Name (Common Name) (Family) | Additive Content (Form) | SLM Type/Name (Manufacturer, Country) Base/Catalyst Volume Ratio | Antimicrobial Properties | Reference |
|---|---|---|---|---|
| Enriched SLM Component | Mechanical Properties | |||
| Origanum vulgare (Carvacrol) (Lamiaceae) | 10 µL/5 mm disc (essential oil) | LT-S-SLM/Ufi Gel SC (UG, VOCO GmbH, Cuxhaven, Germany) 1:1 | C. albicans, S. aureus, S. sanguis, S. mutans, B. subtilis, P. aeruginosa, and E. coli inhibition zone | [59] |
| formulation | - | |||
| Melaleuca alternifolia (tea tree) (Myrtaceae) | 15 wt. % (oil) | LT-S-SLM/GC Reline Extra Soft (GC Dental Industrial Corp., Tokyo, Japan) 1:1 | C. albicans colony count | [60] |
| formulation | - |
| Plant Latin Name (Common Name) (Family) | Immersion Liquid (Volume) | SLM Type/Name (Manufacturer, Country) Powder/Liquid Ratio | Antimicrobial Properties | Reference |
|---|---|---|---|---|
| Mechanical Properties | ||||
| Azadirachta indica (Neem) (Meliaceae) | alcoholic extract (10 mL) | TC/GC Soft Liner (GC Corp., Tokyo, Japan) 1.2 g/1 mL | C. albicans colony count | [61] |
| - | ||||
| 10 wt.% alcoholic extract (10 mL) | TC/GC Soft Liner (GC Corp., Tokyo, Japan) 1.2 g/1 mL | C. albicans, S. mutans and E. coli colony count | [62] | |
| - | ||||
| oil (10 mL) | TC/GC Soft Liner (GC Corp., Tokyo, Japan) 1.2 g/1 mL | C. albicans colony count | [61] | |
| - | ||||
| Melaleuca alternifolia (tea tree) (Myrtaceae) | 10 vol. % extract (10 mL) | TC/GC Soft Liner (GC Corp., Tokyo, Japan) 1.2 g/1 mL | C. albicans, S. mutans and E. coli colony count | [62] |
| - | ||||
| 50 vol. % DMSO extract (not specified volume) | TC/GC Soft Liner (GC Corp., Tokyo, Japan) 1.2 g/1 mL | C. albicans biofilm formation | [63] | |
| Shore A hardness | ||||
| Cymbopogon citratus (lemongrass) (Poaceae) | 50 vol. % DMSO extract (aqueous extract) | TC/GC Soft Liner (GC Corp., Tokyo, Japan) 1.2 g/1 mL | C. albicans biofilm formation | [63] |
| Shore A hardness | ||||
| Thymus vulgaris (thyme) (Lamiaceae) | 50 vol. % DMSO extract (not specified volume) | TC/GC Soft Liner (GC Corp., Tokyo, Japan) 1.2 g/1 mL | C. albicans biofilm formation | [63] |
| Shore A hardness |
| Plant Latin Name (Common Name) Family | SLM Type Name (Polymer/Plasticizer) | Results | Reference |
|---|---|---|---|
| Litsea cubeba (Lauraceae) | TCs: Visco-gel (PEMA/triethyl citrate) Coe-Comfort (PEMA)/benzyl benzoate) GC Soft Liner (PMMA/butyl phthalate butyl glycolate) | IZD for 10, 20, and 30 vol./vol.%: Visco-gel: 12.09, 16.56, 24.68 mm Coe-Comfort: 10.22, 14.07, 22.61 mm GC Soft Liner: 7.61, 13.19, 22.42 mm Antifungal efficacy: Visco-gel > Coe-Comfort > GC Soft Liner | [53] |
| Melaleuca alternifolia (tea tree) (Myrtaceae) | TCs: Visco-gel (PEMA/triethyl citrate) GC Soft Liner (PMMA/butyl phthalate butyl glycolate) | IZD for 10 vol./vol.%: Visco-gel: 19.0–20.8 mm GC Soft Liner: 0–17.4 mm IZD for 40 vol./vol.%: Visco-gel: 21.6–26.4 mm GC Soft Liner: 15.2–23.2 mm Fungal reduction (for 2 mL, 40 vol./vol.%): Visco-gel 88% GC Soft Liner 86% Antifungal efficacy: Visco-gel > GC Soft Liner | [50] |
| Melaleuca alternifolia (tea tree) (Myrtaceae) | LT-S-SLM GC Reline Extra Soft (Vinyl dimethyl polysiloxane/hydrogen polysiloxane) | Log CFU/mm2 for 1, 30, 60 days (15 wt.%): 2.1, 2.8, 3.1 Fungal reduction: 70.4%, 56.9%, 54.4% Sustained antifungal activity up to 60 days | [60] |
| Cymbopogon citratus (lemongrass) (Poaceae) | TC GC Soft Liner (PMMA/butyl phthalate butyl glycolate) | MICs/Fungal reduction: C. citratus: 0.03125%/50% T. vulgare: 0.0625%/30% M. aternifolia: 0.25%/20% Antifungal activity: C. citratus > T. vulgare > M. alternifolia | [63] |
| Thymus vulgaris (thyme) (Lamiaceae) | |||
| Melaleuca alternifolia (tea tree) (Myrtaceae) | |||
| Azachirachta indica (Neem) (Meliaceae) | TC GC Soft Liner (PMMA/butyl phthalate butyl glycolate) | Log CFU/mL: M. alternifolia: 2.31 A. indica: 2.15 Antifungal activity: A. indica > M.alternifolia | [62] |
| Melaleuca alternifolia (tea tree) (Myrtaceae) | |||
| Melaleuca alternifolia (tea tree) (Myrtaceae) | TC GC Soft Liner (PMMA/butyl phthalate butyl glycolate) | Log CFU/mL: M. alternifolia: 2.30 M. alternifolia + A. indica: 0.40 Antifungal activity: M. alternifolia + A. indica > A. indica | [61] |
| Azachirachta indica (Neem) (Meliaceae) | |||
| Azachirachta indica (Neem) (Meliaceae) | TC Visco-gel (PEMA/triethyl citrate) | IZDs for 5–40 vol./vol.% at 48 h: A. indica: 6.35–20.10 mm C. nucifera: 5.0–16.55 mm M. alternifolia: 5.0–15.35 mm IZDs for 40 vol./vol.% at 7 days: A. indica: 19.15 mm C. nucifera: 16.20 mm M. alternifolia: 14.75 mm Optimal concentrations: A. indica: 15 vol./vol.% C. nucifera: 20 vol./vol.% M. alternifolia: 25 vol./vol.% Antifungal activity: A. indica > C. nucifera > M. alternifolia | [45] |
| Cocos nucifera (coconut) (Arecaceae) | |||
| Melaleuca alternifolia (tea tree) (Myrtaceae) | |||
| Azachirachta indica (Neem) (Meliaceae) | TC GC Soft Liner (PMMA/butyl phthalate butyl glycolate) | 100% fungal reduction: A. indica: 7 days A. sativum: 2 days Antifungal activity: A. indica > A. sativum | [44] |
| Allium sativum (garlic) (Amaryllidaceae) | |||
| Allium sativum (garlic) (Amaryllidaceae) | TC Acrosoft (PEMA/phthalate ester) | IZD: A. sativum: 8.00 mm O. vulgare: 7.75 mm Z. officinale and N. sativa: negligible Antifungal activity: A. sativum > O. vulgare > Z. officinale ≈ N. sativa | [47] |
| Origanum vulgare (oregano) (Lamiaceae) | |||
| Zingiber officinale (ginger) (Zingiberaceae) | |||
| Nigella sativa (black seed) (Ranunculaceae) | |||
| Origanum vulgare (oregano) (Lamiaceae) (carvacrol) | LT-S-SLM Ufi Gel SC (Mixture of different polyalkylsiloxanes) | IZD: 38.33 mm Fungal reduction: 98% | [59] |
| Centratherum anthelminticum (bitter cumin) (Asteraceae) | TC Visco-gel (PEMA/triethyl citrate) | IZD: C. anthelminticum (600–800 µL): 31.66–18.33 mm (7 days) O. sanctum (800 µL): 21.00–29.66 mm (7 days) L. usitatissimum (800–1000 µL): 20.33–21.00 mm (24 h) Antifungal activity: C. anthelminticum > O. sanctum > L. usitatissimum | [46] |
| Ocimum sanctum (Holy Basil and Tulsi) (Lamiaceae) | |||
| Linum usitatissimum (flaxseed) (Linaceae) | |||
| Piper bettle (Piperaceae) | TC GC Soft Liner (PMMA/butyl phthalate butyl glycolate) | IZD: 40 wt./wt.% extract: 26.63 mm 40 vol./vol.% oil: 13.69 mm Antifungal activity: extract > essential oil | [34] |
| Ocimum basilicum (basil) (Lamiaceae) | TC Visco-gel (PEMA/triethyl citrate) | MICs/Fungal reduction after 24 h and 14 days: 0.0625 mL/60.03% and 65.94% 0.25 mL/55.06% and 52.68 | [49] |
| Curcuma longa (curcumin) (Zingiberaceae) | TC Trusoft (PEMA/benzyl butyl phthalate, dibutyl phthalate) | IZD: 10 vol./vol.%: 6 mm 20 vol./vol.%: 8.2 mm | [55] |
| Cinnamomum (cinnamon) (Lauraceae) | ST-A-SLM Vertex Soft (PEMA/acetyl tributyl citrate) | IZD: 1 wt.%: 12.56 mm 2 wt.% 16.72 mm | [54] |
| Aloe vera (aloe) (Liliaceae) | TC GC Soft Liner (PMMA/butyl phthalate butyl glycolate) | Fungal reduction: 80% | [40] |
| Chitosan | ST-A-SLM Vertex Soft (PEMA/acetyl tributyl citrate) | Fungal reduction: 1.5 wt.%: 50% 2 wt.%: 70% | [57] |
| Chitosan | TC GC Soft Liner (PMMA/butyl phthalate butyl glycolate) | Fungal reduction: 2 wt.%: 50% | [40] |
| Chitosan (commercial) | TC GC Soft Liner (PMMA/butyl phthalate butyl glycolate) | MIC: Chitosan commercial: 0.625 mg/mL Chitosan synthesized: 0.3125 mg/mL Antifungal activity: chitosan synthesized > chitosan commercial | [58] |
| Chitosan (synthesized) |
| Plant Latine Name (Common Name) Family | Methodology | Results | Reference |
|---|---|---|---|
| Azadirachta indica (Neem) (Meliaceae) | 5 mg/mL (equivalent to 2 tablets) aqueous solution (overnight immersion) | Fungal reduction: A. Indica: 71% Triphala: 51% A. vera: 41% Antifungal activity: A. Indica > Triphala > A. vera | [77] |
| Triphala (Combretaceae) | 10 g/100 mL aqueous solution (overnight immersion) | ||
| Aloe vera (aloe) (Liliaceae) | Leaves (rubbing the denture) |
| Plant Latine Name (Common Name) Family | SLM Type Name (Polymer/Plasticizer) | Results/Findings | Reference |
|---|---|---|---|
| Azadirachta indica (Neem) (Meliaceae) | TC GC Soft Liner (PMMA/butyl phthalate butyl glycolate) | Log CFU/mL: S. mutans: 2.14 S. aureus: 2.44 E. coli: 6.22 Antibacterial activity: S. mutans > S. aureus > E. coli | [61] |
| Piper betle (Piperaceae) | TC GC Soft Liner (PMMA/butyl phthalate butyl glycolate) | IZD (S. mutans): 10–70 wt./wt.% extract: 8.38–26.76 mm 60–70 vol./vol.% oil: 8.31–9.30 mm Antibacterial activity: extract (70 wt./wt.%) > oil (70 vol./vol.%) | [34] |
| Melaleuca alternifolia (tea tree) (Myrtaceae) | TC GC Soft Liner (PMMA/butyl phthalate butyl glycolate) | Log CFU/mL: S. aureus: 2.21 S. mutans: 2.33 E. coli: 2.45 Antibacterial activity: S. aureus > S. mutans > E. coli | [62] |
| Litsea cubeba (Lauraceae) | TCs: Coe-Comfort (PEMA)/benzyl benzoate) Visco-gel (PEMA/triethyl citrate) GC Soft Liner (PMMA/butyl phthalate butyl glycolate) | IZD (S. mutans): Coe-Comfort: 8.15 mm Visco-gel: 7.96 mm GC Soft Liner: 7.89 mm Antibacterial activity: Coe-Comfort > Visco-gel > GC Soft Liner | [53] |
| Origanum vulgare (Carvacrol) (Lamiaceae) | LT-S-SLM Ufi Gel SC (Mixture of different polyalkylsiloxanes) | IZD/Bacteria reduction: B. subtilis: 43.67 mm/90% S. mutans: 40.33 mm/99% S. sanguis: 36.67 mm/96% S. aureus: 34.00 mm/97% E. coli: 29.33 mm/70% P. aeruginosa: 15.33 mm/68% Antibacterial activity: B. subtilis > S. mutans > S. sanguis > S. aureus > E. coli > P. aeruginosa | [59] |
| Plant | Preparation Way | Test Species | LD50 (mg/kg) | Reference |
|---|---|---|---|---|
| Aloe vera (aloe) | dried leaf extract | Swiss albino mice | 120.65 | [78] |
| aqueous extract of leaves | female Wistar rats | >5000 | [79] | |
| Azachirachta indica (Neem) | seed oil | rats | >14,000 | [80] |
| mice | 2500 | [81] | ||
| >5000 | [82] | |||
| methanolic extract of leaves | albino rats | >5000 | [83] | |
| methanolic extract of flowers | Wistar rats | >12,000 | [84] | |
| ethanolic extract of leaves | mice | >5000 | [85] | |
| ethanolic extracts of the stem bark | rats | 870 | [86] | |
| mice | 489.90 | [87] | ||
| Triphala | aqueous extract | Sprague-Dawley rats | >5000 | [88] |
| mice | 280 | [89] | ||
| Linum usitatissimum (flaxseed) | crude oil | mice (Balb C57) | 37 240 | [90] |
| Centratherum anthelminticum (black cumin) | oil | Wistar albino rats | >2000 | [91] |
| Origanum vulgare (oregano) | essential oil | rats | >2000 | [92] |
| aqueous extract | 2000 | [93] | ||
| Ocimum sanctum (Holy Basil and Tulsi) | essential oil | mice | 4.571 | [94] |
| aqueous extract | 6200 | [95] | ||
| ethanolic extract | >2000 | [96] | ||
| Ocimum basilicum (basil) | essential oil | Swiss mice | 532 | [97] |
| Wistar rats | 3250 | [98] | ||
| Melaleuca alternifolia (tea tree) | essential oil | rats | 1900 | [99] |
| Cocos nucifera (coconut) | oil | rats | >5000 | [100] |
| mice | >5000 | [101] | ||
| Allium sativum (garlic) | aqueous extract | mice | >5000 | [102] |
| 650 | [103] | |||
| rats | >30 | [104] | ||
| ethanol extract | mice | 4.472 | [105] | |
| Cymbopogon citratus (lemongrass) | essential oil | mice | 3500 | [106] |
| albino rats | >5000 | [107] | ||
| Thymus vulgaris (thyme) | essential oil | rats | 1220 | [108] |
| 4700 | [109] | |||
| ethanolic extract | mice | 4220 | [110] | |
| Curcuma longa (curcumin) | ethanol extract of leaves | Swiss albino mice | 2154.06 | [111] |
| aqueous, methanolic and n-hexane extracts of roots | albino rats | >5000 | [112] | |
| essential oil of rhizomes | mice | 2154 | [113] | |
| ethanolic extract of rhizomes | rats | 3807 | [114] | |
| mice | 27,980 | [115] | ||
| Litsea cubeba | essential oil | mice | 4000 | [116] |
| rats | >5000 | [117] | ||
| Nigella sativa (black seed) | oil | mice | >28,000 | [118] |
| (2-Isopropyl-5-methyl-1, 4-benzoquinone) (a bioactive compound in N. sativa) | rats | 794.3 | [119] | |
| mice | 870.9 | |||
| Zingiber officinale (ginger) | ethanolic extract of the root | Wistar rats | 3800 | [120] |
| methanolic extract of the root | mice | 10,250 | [121] | |
| Cinnamomum (cinnamon) | aqueous extract of bark | Wistar rats | 5000 | [122] |
| essential oil | rats | 2323 | [123] | |
| mice | 1850 | [124] | ||
| Piper betle | methanolic extract | mice | >5000 | [125] |
| Chitosan | powder | rats | >5000 | [126] |
| Medicine | Test Species | LD50 (mg/kg) | Reference |
|---|---|---|---|
| nystatin | mice | 200 | [127] |
| rats | 10,000 | [128] | |
| fluconazole | rats | 1575 | [129] |
| mice | 1408 | [130] | |
| itraconazole | rats | >320 | [131] |
| mice | >320 | ||
| ketoconazole | rats | 166 | [132] |
| mice | 618 | ||
| miconazole | mice | 578.1 | [133] |
| rats | >640 | ||
| clotrimazole | rats | 708 | [134] |
| mice | 761 | ||
| chlorhexidine diacetate | rats | 1180 | [135] |
| mice | 2000 | ||
| chlorhexidine digluconate | rats | 2000 | [136] |
| Plant Latine Name (Common Name) Family | SLM Type Name (Polymer/Plasticizer) | Results/Findings | Reference |
|---|---|---|---|
| Melaleuca alternifolia (tea tree) (Myrtaceae) | TC GC Soft Liner (PMMA/butyl phthalate butyl glycolate) | Shore A hardness after 60 days: M. alternifolia: 52.6 °Sh C. citratus: 57.1 °Sh T. vulgaris: 58.9 °Sh Shore A hardness: M. alternifolia < C. citratus < T. vulgaris | [63] |
| Cymbopogon citratus (lemongrass) (Poaceae) | |||
| Thymus vulgaris (thyme) (Lamiaceae) | |||
| Allium sativum (garlic) (Amaryllidaceae) | TC GC Soft Liner (PMMA/butyl phthalate butyl glycolate) | Shore A hardness: A. sativum: 13.0 °Sh A. indica: 14.1 °Sh Shore A hardness: A. sativum < A. indica | [44] |
| Azachirachta indica (Neem) (Meliaceae) | |||
| Piper bettle (Piperaceae) | TC GC Soft Liner (PMMA/butyl phthalate butyl glycolate) | Shore A hardness after 2 h/7 days: 5 vol.vol.%: 19 °Sh/27 °Sh 10 vol.vol.%: 13 °Sh/23 °Sh 20 vol.vol%: 7 °Sh/15 °Sh Shore A hardness: 20 vol./vol.% < 10 vol./vol.% < 5 vol./vol.% | [56] |
| Litsea cubeba (Lauraceae) | TCs: Coe-Comfort (PEMA)/benzyl benzoate) GC Soft Liner (PMMA/butyl phthalate butyl glycolate) Visco-gel (PEMA/triethyl citrate) | Shore A hardness at 5, 10, 20, and 30 vol./vol.% after 2 h: Coe-Comfort: 13.96, 10.00, 5.20, 2.12 °Sh GC Soft Liner: 14.72, 11.24, 6.88, 4.08 °Sh Visco-gel: 34.67, 32.73, 29.07, 22.27 °Sh Shore A hardness at 5, 10, 20, and 30 vol./vol.% after 7d: Coe-Comfort: 13.96, 10.00, 5.20, 2.12 °Sh GC Soft Liner: 22.36, 18.52, 13.64, 8.84 °Sh Visco-gel: 37.93, 35.87, 32.53, 27.93 °Sh Shore A hardness: Coe-Comfort < GC Soft Liner < Visco-gel | [53] |
| Cocos nucifera (coconut) (Arecaceae) | ST-A-SLM Vertex Soft (PEMA/acetyl tributyl citrate) | Shore A hardness after 2 h/14 days/4 weeks: 1.5%: 39.63 °Sh/45.41 °Sh/42.99 °Sh 2.5%: 50.13 °Sh/55.11 °Sh/52.74 °Sh Shore A hardness: 1.5% < 2.5% | [52] |
| Cinnamomum (cinnamon) (Lauraceae) | ST-A-SLM Vertex Soft (PEMA/acetyl tributyl citrate) | Shore A hardness: 1 wt.%: 45.23 °Sh 2 wt.%: 47.13 °Sh Shore A hardness: 1 wt.% < 2 wt.% | [54] |
| Sesamum indicum (sesame) (Pedaliaceae) | ST-A-SLM Vertex Soft (PEMA/acetyl tributyl citrate) | Tensile Strength after 2 days/30 days: S. indicum: 3 MPa/3 MPa T.vulgaris: 3.75 MPa/3 MPa S. indicum + T.vulgaris: 3.60 MPa/3.20 MPa Tensile strength: S. indicum < T.vulgaris < S. indicum + T.vulgaris | [48] |
| Thymus vulgaris (thyme) (Lamiaceae) | |||
| Aloe vera (aloe) (Liliaceae) | ST-A-SLM Vertex Soft (PEMA/acetyl tributyl citrate) | Tear strength after 24 h/2 weeks/4 weeks: 3 wt.%: 7.093 N/mm/7.92 N/mm/9.2 N/mm 10 wt.%: 5.2 N/mm/5.7 N/mm/6.45 N/mm Tear strength: 10 wt.% < 3 wt.% | [41] |
| Aloe vera (aloe) (Liliaceae) | ST-A-SLM Vertex Soft (PEMA/acetyl tributyl citrate) | Tear strength: A. indica: 6.7 N/mm A. vera: 7.4 N/mm Tear strength: A. indica < A. vera | [42] |
| Azachirachta indica (Neem) (Meliaceae) |
| Plant Latine Name (Common Name) Family | SLM Type Name (Polymer/Plasticizer) | Results/Findings | Reference |
|---|---|---|---|
| Cymbopogon citratus (lemongrass) (Poaceae) | TC Moonstar Soft (PEMA/dibutyl phthalate) | Peel bond strength: 2.5 vol./vol.%: 2.04 MPa 5 vol./vol.%: 1.83 MPa Peel bond strength: 5 vol./vol.% < 2.5 vol./vol.% | [51] |
| Aloe vera (aloe) (Liliaceae) | TC GC Soft Liner (PMMA/butyl phthalate butyl glycolate) | Shear bond strength: A. vera: 0.48 MPa A. indica: 0.56 MPa Shear bond strength: A. vera < A. indica | [43] |
| Azachirachta indica (Neem) (Meliaceae) | |||
| Aloe vera (aloe) (Liliaceae) | ST-A-SLM Vertex Soft (PEMA/acetyl tributyl citrate) | Shear bond strength after 24 h/2 weeks/4 weeks: 3 wt.%: 0.302 MPa/0.345 MPa/0.404 MPa 10 wt.%: 0.288 MPa/0.334 MPa/0.391 MPa Shear bond strength: 10 wt.% < 3 wt.% | [41] |
| Cinnamomum (cinnamon) (Lauraceae) | TC Trusoft (PEMA/benzyl butyl phthalate, dibutyl phthalate) | Tensile bond strength: 10 vol./vol.%: 0.52 MPa 20 vol./vol.%: 0.55 MPa Tensile bond strength: 10 vol./vol.% < 20 vol./vol.% | [55] |
| Plant Latine Name (Common Name) Family | SLM Type Name (Polymer/Plasticizer) | Results/Findings | Reference |
|---|---|---|---|
| Centratherum anthelminticum (bitter cumin) (Asteraceae) | TC Visco-gel (PEMA/triethyl citrate) | Surface roughness (SEM observations): O. sanctum < C. anthelminticum < L. usitatissimum | [46] |
| Ocimum sanctum (Holy Basil and Tulsi) (Lamiaceae) | |||
| Ocimum basilicum (basil) (Lamiaceae) | TC Visco-gel (PEMA/triethyl citrate) | Surface roughness: 1.25 vol.%: 3.2564 µm 5 vol.%: 3.3448 µm Surface roughness: 1.25 vol.% < 5 vol.% | [49] |
| Cymbopogon citratus (lemongrass) (Poaceae) | TC Moonstar Soft (PEMA/dibutyl phthalate) | Surface roughness: 2.5 vol./vol.%: 2.503 µm 5 vol./vol.%: 2.352 µm Surface roughness: 5 vol./vol.% < 2.5 vol.vol.% | [51] |
| Cinnamomum (cinnamon) (Lauraceae) | TC Trusoft (PEMA/benzyl butyl phthalate, dibutyl phthalate) | Surface roughness: 10 vol./vol.%: 3.3 µm 20 vol./vol.%: 2.53 µm Surface roughness: 20 vol./vol.% < 10 vol.vol.% | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kula, P.; Chladek, G.; Barszczewska-Rybarek, I. Plant-Derived Modifiers for Antimicrobial Soft Denture Liners: A Review. Int. J. Mol. Sci. 2025, 26, 10848. https://doi.org/10.3390/ijms262210848
Kula P, Chladek G, Barszczewska-Rybarek I. Plant-Derived Modifiers for Antimicrobial Soft Denture Liners: A Review. International Journal of Molecular Sciences. 2025; 26(22):10848. https://doi.org/10.3390/ijms262210848
Chicago/Turabian StyleKula, Patrycja, Grzegorz Chladek, and Izabela Barszczewska-Rybarek. 2025. "Plant-Derived Modifiers for Antimicrobial Soft Denture Liners: A Review" International Journal of Molecular Sciences 26, no. 22: 10848. https://doi.org/10.3390/ijms262210848
APA StyleKula, P., Chladek, G., & Barszczewska-Rybarek, I. (2025). Plant-Derived Modifiers for Antimicrobial Soft Denture Liners: A Review. International Journal of Molecular Sciences, 26(22), 10848. https://doi.org/10.3390/ijms262210848











