From Electron Imbalance to Network Collapse: Decoding the Redox Code of Ischemic Stroke for Biomarker-Guided Precision Neuroprotection
Abstract
1. Prologue: Redox Reprogramming as the Missing Dimension in Acute Ischemic Stroke
2. The Redox Code of the Ischemic Brain
2.1. NADH/NAD+: Surplus Electrons and the Shifting Architecture of Complex I
2.2. Network Reverberations and Translational Choreography
- Hyperacute (0–6 h)—RET- and ferroptosis inhibitors, DNase I, iron chelators [62].
- Acute (6–24 h)—fasudil, sulodexide, sepiapterin, arginase inhibitors [63].
- Subacute (1–7 days)—NRF2 activators, HIF modulators, mitochondrial biogenesis inducers [64].
- Chronic (>7 days)—rehabilitation under the auspice of redox biomarker measurement [65].
3. Mitochondrial Reprogramming and Redox Plasticity
3.1. Complex I Conformers and the RET Paradox
3.2. The Role of Organelle Consortia and the Dynamics–Mitophagy–mPTP Axis
3.3. Heterogeneity, Messengers and Systemic Interfaces
3.4. Sex Differences, Immunometabolic Crosstalk and Intercellular Transfer
3.5. DHODH–CoQ Ferroptotic Control, Network Coupling and Therapeutic Timing
4. Cytosolic Redox Signaling and Pathway Crosstalk
4.1. Reactive Oxygen and Nitrogen Species as Coded Messages
4.2. The Collapse and Inversion of Antioxidant Defenses
4.3. Convergence with Regulated Cell Death Programs
4.4. Redox Condensates and the Architecture of Stress
4.5. Immune Infiltration as Redox Amplifier and Buffer
4.6. The Ionic Symphony: Calcium, Zinc, and Copper
4.7. Translational Horizons: Coding Therapy in Time and Space
- Acute timing (6–24 h) increase FSP1, decreases ACSL4, or decreases NLRP3 inflammasome together, managing the lipid metabolism and curbing the low-magnitude inflammation [126].
- Subacute timing (2–7 days), stimulus-activated Nrf2, or HIF-PHD wisely suppressed, or integrated stress unified; boosts repairs and suppresses edema [127].
- Finally, chronic timing, redox-boosted E-exercise, dietary electrophiles, and rehabilitation will provide mechanistic functions of resilience [128].
5. The Neurovascular Unit and the No-Reflow Problem: Microvascular Redox, Barrier Failure, and Edema
5.1. Capillaries as the Decisive Battlefield
5.2. Pericyte Constriction as a Redox Chokehold
5.3. Endothelial Dysfunction and Barrier Failure
5.4. Astrocytic End-Feet and Polarity Collapse
5.5. Thrombo-Inflammation as a Redox Amplifier
5.6. Erythrocytes as Redox Propagators
5.7. Edema as a Redox-Governed Choreography
5.8. Translational Horizons for NVU Redox Biology
5.9. From Microvascular Collapse to Network Disintegration
5.10. Holistic Analytical Integration
6. Redox-Mediated Immunological and Glial Reprogramming in Acute Ischemic Stroke
6.1. Microglial Plasticity Under Redox Pressure
6.2. Infiltrating Leukocytes and Oxidative Traps
6.3. Astrocytes as Immune–Neurovascular Hubs
6.4. Pathways to Clinical Translation
- Microglia and astrocytes: Nrf2 activators such as dimethyl fumarate and synthetic triterpenoids shift phenotypes to antioxidant states [207].
- Oligodendrocytes: Ferroptosis inhibitors (liproxstatin-1, ferrostatin-1) and iron chelators (next-generation deferoxamine derivatives) protect the integrity of myelin [210].
- Adaptive immunity: Modulating thioredoxin-dependent Treg survival gives new points of leverage [211].
- Biomarkers: Astrocytic and oligodendrocytic vesicles with oxidized proteins are being developed as plasma biomarkers for patient stratification [212].
- Nanotechnology: Redox-sensitive nanoparticles developed to release antioxidants only in ischemic microenvironments are now entering preclinical work [213].
- Gene editing: CRISPR directed toward Keap1-Nrf2 and Mfsd2a pathways will provide futuristic trials for tuning immune-glial redox signaling [214].
7. Redox Control of Network-Level Dysfunction and Plasticity After Ischemic Stroke
7.1. Synaptic Integrity and Redox-Dependent Pruning
7.2. Oscillatory Dynamics and Network Coherence
7.3. Connectome Disintegration Under Oxidative Stress
7.4. Redox-Dependent Plasticity and Recovery Potential
7.5. Advanced Network Metrics and Computational Frameworks
7.6. Translational Perspectives
7.7. Integrative Synthesis
8. Clinical Translation and Therapeutic Frontiers in Redox-Guided Stroke Care
8.1. Biomarkers for Patient Stratification
8.2. Imaging Windows into Redox–Network Interactions
8.3. Pharmacological Pathways to Redox Modulation
8.4. Neuromodulation and Closed-Loop Strategies
8.5. Rehabilitation and Lifestyle Interventions
8.6. Clinical Trials and Precision Frameworks
9. Future Directions and Integrative Perspectives
9.1. Temporal Choreography: Building a Redox Clock of Recovery
9.2. Spatial Atlases: Mapping Redox Heterogeneity Across Networks
9.3. Systematic and Epigenetic Convergence: Stroke as Systemic Redox Phenotype
9.4. The Gut–Brain–Redox Axis
9.5. Single-Cell and Spatial Omics: Redox Landscapes at Cellular Resolution
9.6. The Neurovascular–Glymphatic–Immune Continuum
9.7. Computational and Bioengineering Perspectives
9.8. Equity, Consortia, and Neuroethics
10. Conclusions and Outlook
- RET, occurring at complex I, is the major mechanism generating ROS during the initial phase of reperfusion;
- Ferroptosis, characterized by the inhibition of GPX4 via the lipid peroxidation of lipids dependent on iron, and subsequent loss of glutathione stores, is the major type of death for both neurons and oligodendrocytes;
- Failure of microcirculation due to constriction of pericytes, destruction of the glycocalyx, and decoupling of eNOS unite redox injury with vascular dysfunction;
- The amplification of inflammation through the activation of the NLRP3 inflammasome, subsequent release of pro-inflammatory cytokines via NF-kappaB, and formation of NETs link oxidative stress with the innate immune response;
- Both neuroimmune and metabolic adaptations, such as antioxidant defenses mediated by Nrf2 and sirtuins, transfer of mitochondria from glia to neurons, and redox modulation by the microbiota affecting recovery trajectories.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Feigin, V.L.; Brainin, M.; Norrving, B.; Martins, S.O.; Pandian, J.; Lindsay, P.; Grupper, M.F.; Rautalin, I. World Stroke Organization: Global Stroke Fact Sheet 2025. Int. J. Stroke 2025, 20, 132–144. [Google Scholar] [CrossRef]
- Donkor, E.S. Stroke in the 21st Century: A Snapshot of the Burden, Epidemiology, and Quality of Life. Stroke Res. Treat. 2018, 2018, 3238165. [Google Scholar] [CrossRef]
- Boehme, A.K.; Esenwa, C.; Elkind, M.S.V. Stroke Risk Factors, Genetics, and Prevention. Circ. Res. 2017, 120, 472–495. [Google Scholar] [CrossRef]
- Kuriakose, D.; Xiao, Z. Pathophysiology and Treatment of Stroke: Present Status and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 7609. [Google Scholar] [CrossRef]
- Salaudeen, M.A.; Bello, N.; Danraka, R.N.; Ammani, M.L. Understanding the Pathophysiology of Ischemic Stroke: The Basis of Current Therapies and Opportunity for New Ones. Biomolecules 2024, 14, 305. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, S.; Kostev, K.; Tanislav, C.; Hammed, A. Acute Ischemic Stroke Treatment in Germany (2015–2023): Nationwide Trends in Thrombolysis and Thrombectomy by Age and Sex. Brain Sci. 2025, 15, 832. [Google Scholar] [CrossRef]
- Alfei, S.; Schito, G.C.; Schito, A.M.; Zuccari, G. Reactive Oxygen Species (ROS)-Mediated Antibacterial Oxidative Therapies: Available Methods to Generate ROS and a Novel Option Proposal. Int. J. Mol. Sci. 2024, 25, 7182. [Google Scholar] [CrossRef]
- Chavda, V.; Lu, B. Reverse Electron Transport at Mitochondrial Complex I in Ischemic Stroke, Aging, and Age-Related Diseases. Antioxidants 2023, 12, 895. [Google Scholar] [CrossRef] [PubMed]
- Asatiani, N.; Sapojnikova, N.; Kartvelishvili, T.; Asanishvili, L.; Sichinava, N.; Chikovani, Z. Blood Catalase, Superoxide Dismutase, and Glutathione Peroxidase Activities in Alcohol- and Opioid-Addicted Patients. Medicina 2025, 61, 204. [Google Scholar] [CrossRef] [PubMed]
- Jomova, K.; Raptova, R.; Alomar, S.Y.; Alwasel, S.H.; Nepovimova, E.; Kuca, K.; Valko, M. Reactive oxygen species, toxicity, oxidative stress, and antioxidants: Chronic diseases and aging. Arch. Toxicol. 2023, 97, 2499–2574. [Google Scholar] [CrossRef]
- Sahni, P.V.; Zhang, J.; Sosunov, S.; Galkin, A.; Niatsetskaya, Z.; Starkov, A.; Brookes, P.S.; Ten, V.S. Krebs cycle metabolites and preferential succinate oxidation following neonatal hypoxic-ischemic brain injury in mice. Pediatr. Res. 2018, 83, 491–497. [Google Scholar] [CrossRef]
- Tabata Fukushima, C.; Dancil, I.-S.; Clary, H.; Shah, N.; Nadtochiy, S.M.; Brookes, P.S. Reactive oxygen species generation by reverse electron transfer at mitochondrial complex I under simulated early reperfusion conditions. Redox. Biol. 2024, 70, 103047. [Google Scholar] [CrossRef]
- Bao, Y.; Hu, C.; Wang, B.; Liu, X.; Wu, Q.; Xu, D.; Shi, Z.; Sun, C. Mitochondrial Reverse Electron Transport: Mechanisms, Pathophysiological Roles, and Therapeutic Potential. Biology 2025, 14, 1140. [Google Scholar] [CrossRef] [PubMed]
- Atallah, R.; Gindlhuber, J.; Platzer, W.; Rajesh, R.; Heinemann, A. Succinate Regulates Endothelial Mitochondrial Function and Barrier Integrity. Antioxidants 2024, 13, 1579. [Google Scholar] [CrossRef]
- Ao, Q.; Hu, H.; Huang, Y. Ferroptosis and endoplasmic reticulum stress in rheumatoid arthritis. Front. Immunol. 2024, 15, 1438803. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Jin, X.; Ye, F.; Liu, X.; Yu, B.; Li, Z.; Zhao, T.; Chen, W.; Liu, X.; Di, C.; et al. Ferroptosis: A novel regulated cell death participating in cellular stress response, radiotherapy, and immunotherapy. Exp. Hematol. Oncol. 2023, 12, 65. [Google Scholar] [CrossRef]
- Xie, C.; Wu, N.; Guo, J.; Ma, L.; Zhang, C. The key role of the ferroptosis mechanism in neurological diseases and prospects for targeted therapy. Front. Neurosci. 2025, 19, 1591417. [Google Scholar] [CrossRef]
- Shi, Z.; Chen, K.; Wang, Y.; Du, H. The Crosstalk Between Ferritinophagy and Ferroptosis in Ischemic Stroke: Regulatory Mechanisms and Therapeutic Implications. Cell. Mol. Neurobiol. 2025, 45, 73. [Google Scholar] [CrossRef] [PubMed]
- Varughese, J.T.; Buchanan, S.K.; Pitt, A.S. The Role of Voltage-Dependent Anion Channel in Mitochondrial Dysfunction and Human Disease. Cells 2021, 10, 1737. [Google Scholar] [CrossRef]
- Zhao, B.-H.; Ruze, A.; Zhao, L.; Li, Q.-L.; Tang, J.; Xiefukaiti, N.; Gai, M.-T.; Deng, A.-X.; Shan, X.-F.; Gao, X.-M. The role and mechanisms of microvascular damage in the ischemic myocardium. Cell. Mol. Life Sci. 2023, 80, 341. [Google Scholar] [CrossRef]
- Beard, D.J.; Brown, L.S.; Morris, G.P.; Couch, Y.; Adriaanse, B.A.; Karali, C.S.; Schneider, A.M.; Howells, D.W.; Redzic, Z.B.; Sutherland, B.A.; et al. Rapamycin Treatment Reduces Brain Pericyte Constriction in Ischemic Stroke. Transl. Stroke Res. 2025, 16, 1185–1197. [Google Scholar] [CrossRef]
- Iova, O.-M.; Marin, G.-E.; Lazar, I.; Stanescu, I.; Dogaru, G.; Nicula, C.A.; Bulboacă, A.E. Nitric Oxide/Nitric Oxide Synthase System in the Pathogenesis of Neurodegenerative Disorders—An Overview. Antioxidants 2023, 12, 753. [Google Scholar] [CrossRef] [PubMed]
- Jabrah, D.; Rossi, R.; Molina, S.; Douglas, A.; Pandit, A.; McCarthy, R.; Gilvarry, M.; Ceder, E.; Fitzgerald, S.; Dunker, D.; et al. White blood cell subtypes and neutrophil extracellular traps content as biomarkers for stroke etiology in acute ischemic stroke clots retrieved by mechanical thrombectomy. Thromb. Res. 2024, 234, 1–8. [Google Scholar] [CrossRef]
- Neves, V.H.; Palazzi, C.; Bonjour, K.; Ueki, S.; Weller, P.F.; Melo, R.C.N. In Vivo ETosis of Human Eosinophils: The Ultrastructural Signature Captured by TEM in Eosinophilic Diseases. Front. Immunol. 2022, 13, 938691. [Google Scholar] [CrossRef]
- Bakleh, M.Z.; Al Haj Zen, A. The Distinct Role of HIF-1α and HIF-2α in Hypoxia and Angiogenesis. Cells 2025, 14, 673. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, Z.; Hu, M.; Zhou, S.; Xu, S.; Zhou, G.; Zhou, J.; Li, Y.; Chen, B.; Yao, D.; et al. EEG brain network variability is correlated with other pathophysiological indicators of critical patients in neurology intensive care unit. Brain Res. Bull. 2024, 207, 110881. [Google Scholar] [CrossRef] [PubMed]
- Roth, M.; Carlsson, R.; Buizza, C.; Enström, A.; Paul, G. Pericyte response to ischemic stroke precedes endothelial cell death and blood-brain barrier breakdown. J. Cereb. Blood Flow Metab. 2024, 45, 617–629. [Google Scholar] [CrossRef]
- Alonso-Alonso, M.L.; Sampedro-Viana, A.; Fernández-Rodicio, S.; Bazarra-Barreiros, M.; Ouro, A.; Sobrino, T.; Campos, F.; Castillo, J.; Hervella, P.; Iglesias-Rey, R. Need for a Paradigm Shift in the Treatment of Ischemic Stroke: The Blood-Brain Barrier. Int. J. Mol. Sci. 2022, 23, 9486. [Google Scholar] [CrossRef]
- Liu, H.; Wang, S.; Wang, J.; Guo, X.; Song, Y.; Fu, K.; Gao, Z.; Liu, D.; He, W.; Yang, L.-L. Energy metabolism in health and diseases. Signal Transduct. Target. Ther. 2025, 10, 69. [Google Scholar] [CrossRef]
- Milliken, A.S.; Kulkarni, C.A.; Brookes, P.S. Acid enhancement of ROS generation by complex-I reverse electron transport is balanced by acid inhibition of complex-II: Relevance for tissue reperfusion injury. Redox Biol. 2020, 37, 101733. [Google Scholar] [CrossRef]
- Guaita, M.; Watters, S.C.; Loerch, S. Recent advances and current trends in cryo-electron microscopy. Curr. Opin. Struct. Biol. 2022, 77, 102484. [Google Scholar] [CrossRef]
- Murata, M.M.; Kong, X.; Moncada, E.; Chen, Y.; Imamura, H.; Wang, P.; Berns, M.W.; Yokomori, K.; Digman, M.A. NAD+ consumption by PARP1 in response to DNA damage triggers metabolic shift critical for damaged cell survival. Mol. Biol. Cell 2019, 30, 2584–2597. [Google Scholar] [CrossRef] [PubMed]
- Hurtado-Bagès, S.; Knobloch, G.; Ladurner, A.G.; Buschbeck, M. The taming of PARP1 and its impact on NAD+ metabolism. Mol. Metab. 2020, 38, 100950. [Google Scholar] [CrossRef]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD+ metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 2021, 22, 119–141. [Google Scholar] [CrossRef]
- Kushkevych, I.; Dordević, D.; Alberfkani, M.I.; Gajdács, M.; Ostorházi, E.; Vítězová, M.; Rittmann, S.K.-M.R. NADH and NADPH peroxidases as antioxidant defense mechanisms in intestinal sulfate-reducing bacteria. Sci. Rep. 2023, 13, 13922. [Google Scholar] [CrossRef] [PubMed]
- Xiao, W.; Wang, R.-S.; Handy, D.E.; Loscalzo, J. NAD(H) and NADP(H) Redox Couples and Cellular Energy Metabolism. Antioxid. Redox Signal. 2018, 28, 251–272. [Google Scholar] [CrossRef] [PubMed]
- Park, M.W.; Cha, H.W.; Kim, J.; Kim, J.H.; Yang, H.; Yoon, S.; Boonpraman, N.; Yi, S.S.; Yoo, I.D.; Moon, J.-S. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol. 2021, 41, 101947. [Google Scholar] [CrossRef]
- Zhang, S.; Gou, S.; Zhang, Q.; Yong, X.; Gan, B.; Jia, D. FSP1 oxidizes NADPH to suppress ferroptosis. Cell Res. 2023, 33, 967–970. [Google Scholar] [CrossRef]
- Yang, J.; Lee, Y.; Hwang, C.-S. The ubiquitin-proteasome system links NADPH metabolism to ferroptosis. Trends Cell Biol. 2023, 33, 1088–1103. [Google Scholar] [CrossRef]
- Endale, H.T.; Tesfaye, W.; Mengstie, T.A. ROS induced lipid peroxidation and their role in ferroptosis. Front. Cell Dev. Biol. 2023, 11, 1226044. [Google Scholar] [CrossRef]
- Georgiou-Siafis, S.K.; Tsiftsoglou, A.S. The Key Role of GSH in Keeping the Redox Balance in Mammalian Cells: Mechanisms and Significance of GSH in Detoxification via Formation of Conjugates. Antioxidants 2023, 12, 1953. [Google Scholar] [CrossRef]
- Chu, J.; Li, J.; Sun, L.; Wei, J. The Role of Cellular Defense Systems of Ferroptosis in Parkinson’s Disease and Alzheimer’s Disease. Int. J. Mol. Sci. 2023, 24, 14108. [Google Scholar] [CrossRef]
- Sun, D.; Wang, L.; Wu, Y.; Yu, Y.; Yao, Y.; Yang, H.; Hao, C. Lipid metabolism in ferroptosis: Mechanistic insights and therapeutic potential. Front. Immunol. 2025, 16, 1545339. [Google Scholar] [CrossRef]
- Wardlaw, K.S.; Hardingham, G.E. Long-term plasticity of astrocytic phenotypes and their control by neurons in health and disease. Essays Biochem. 2023, 67, 39–47. [Google Scholar] [CrossRef]
- Feng, Y.; Feng, Y.; Gu, L.; Liu, P.; Cao, J.; Zhang, S. The Critical Role of Tetrahydrobiopterin (BH4) Metabolism in Modulating Radiosensitivity: BH4/NOS Axis as an Angel or a Devil. Front. Oncol. 2021, 11, 720632. [Google Scholar] [CrossRef]
- Ismaeel, A.; Papoutsi, E.; Miserlis, D.; Lavado, R.; Haynatzki, G.; Casale, G.P.; Bohannon, W.T.; Smith, R.S.; Eidson, J.L.; Brumberg, R.; et al. The Nitric Oxide System in Peripheral Artery Disease: Connection with Oxidative Stress and Biopterins. Antioxidants 2020, 9, 590. [Google Scholar] [CrossRef]
- Janaszak-Jasiecka, A.; Płoska, A.; Wierońska, J.M.; Dobrucki, L.W.; Kalinowski, L. Endothelial dysfunction due to eNOS uncoupling: Molecular mechanisms as potential therapeutic targets. Cell. Mol. Biol. Lett. 2023, 28, 21. [Google Scholar] [CrossRef]
- Mao, C.; Zhao, J.; Cheng, N.; Xu, Z.; Ma, H.; Song, Y.; Sun, X. S-Nitrosylation in Cardiovascular Disorders: The State of the Art. Biomolecules 2025, 15, 1073. [Google Scholar] [CrossRef] [PubMed]
- Wen, F.; Gao, J.; Zhang, G.; Guo, S.; Zhang, X.; Han, S.; Feng, X.; Chen, X.; Hu, J. ROS-DRP1-mediated excessive mitochondrial fission and autophagic flux inhibition contribute to heat stress-induced apoptosis in goat Sertoli cells. J. Anim. Sci. Biotechnol. 2025, 16, 58. [Google Scholar] [CrossRef] [PubMed]
- Stancill, J.S.; Corbett, J.A. The Role of Thioredoxin/Peroxiredoxin in the β-Cell Defense Against Oxidative Damage. Front. Endocrinol. 2021, 12, 718235. [Google Scholar] [CrossRef] [PubMed]
- Divecha, Y.A.; Rampes, S.; Tromp, S.; Boyanova, S.T.; Fleckney, A.; Fidanboylu, M.; Thomas, S.A. The microcirculation, the blood-brain barrier, and the neurovascular unit in health and Alzheimer disease: The aberrant pericyte is a central player. Pharmacol. Rev. 2025, 77, 100052. [Google Scholar] [CrossRef] [PubMed]
- Schick, V.C.; Neumann, T.; Illerhaus, A.; Timmer, M.; Fuchs, A.; Grau, S.; Annecke, T. Release of Hyaluronan in Aneurysmal Subarachnoid Hemorrhage and Cerebral Vasospasm: A Pilot Study Indicating a Shedding of the Endothelial Glycocalyx. J. Neurosurg. Anesthesiol. 2023, 35, 232–237. [Google Scholar] [CrossRef]
- Burn, G.L.; Raisch, T.; Tacke, S.; Winkler, M.; Prumbaum, D.; Thee, S.; Gimber, N.; Raunser, S.; Zychlinsky, A. Myeloperoxidase transforms chromatin into neutrophil extracellular traps. Nature 2025. [Google Scholar] [CrossRef]
- Li, Z.; He, X.; Fang, Q.; Yin, X. Gut Microbe-Generated Metabolite Trimethylamine-N-Oxide and Ischemic Stroke. Biomolecules 2024, 14, 1463. [Google Scholar] [CrossRef]
- Guo, Z.; Mo, Z. Keap1-Nrf2 signaling pathway in angiogenesis and vascular diseases. J. Tissue Eng. Regen. Med. 2020, 14, 869–883. [Google Scholar] [CrossRef]
- Ting, K.K.Y. Revisiting the role of hypoxia-inducible factors and nuclear factor erythroid 2-related factor 2 in regulating macrophage inflammation and metabolism. Front. Cell. Infect. Microbiol. 2024, 14, 1403915. [Google Scholar] [CrossRef] [PubMed]
- Buttari, B.; Tramutola, A.; Rojo, A.I.; Chondrogianni, N.; Saha, S.; Berry, A.; Giona, L.; Miranda, J.P.; Profumo, E.; Davinelli, S.; et al. Proteostasis Decline and Redox Imbalance in Age-Related Diseases: The Therapeutic Potential of NRF2. Biomolecules 2025, 15, 113. [Google Scholar] [CrossRef]
- Gajewska, J.; Chełchowska, M.; Rychłowska-Pruszyńska, M.; Klepacka, T.; Ambroszkiewicz, J. Oxidative and Antioxidative Status Expressed as OSI Index and GSH/GSSG Ratio in Children with Bone Tumors after Anticancer Therapy Completion. J. Clin. Med. 2022, 11, 1663. [Google Scholar] [CrossRef]
- Guan, A.; Wang, S.; Huang, A.; Qiu, C.; Li, Y.; Li, X.; Wang, J.; Wang, Q.; Deng, B. The role of gamma oscillations in central nervous system diseases: Mechanism and treatment. Front. Cell. Neurosci. 2022, 16, 962957. [Google Scholar] [CrossRef]
- Ghatak, S.; Nakamura, T.; Lipton, S.A. Aberrant protein S-nitrosylation contributes to hyperexcitability-induced synaptic damage in Alzheimer’s disease: Mechanistic insights and potential therapies. Front. Neural. Circuits 2023, 17, 1099467. [Google Scholar] [CrossRef] [PubMed]
- Kolenc, O.I.; Quinn, K.P. Evaluating Cell Metabolism Through Autofluorescence Imaging of NAD(P)H and FAD. Antioxid. Redox Signal. 2019, 30, 875–889. [Google Scholar] [CrossRef]
- Zhang, L.; Luo, Y.L.; Xiang, Y.; Bai, X.Y.; Qiang, R.R.; Zhang, X.; Yang, Y.L.; Liu, X.L. Ferroptosis inhibitors: Past, present and future. Front. Pharmacol. 2024, 15, 1407335. [Google Scholar] [CrossRef]
- Şahin, N.; Bora, E.S.; Çınaroğlu, O.S.; Erbaş, O. Rho-Associated Kinase Inhibitor Fasudil Protects from Sepsis-Induced Acute Kidney Injury in Rat via Suppressing STAT-3 and NLRP-3 Pathway. Curr. Issues Mol. Biol. 2025, 47, 340. [Google Scholar] [CrossRef]
- Baiskhanova, D.; Schäfer, H. The Role of Nrf2 in the Regulation of Mitochondrial Function and Ferroptosis in Pancreatic Cancer. Antioxidants 2024, 13, 696. [Google Scholar] [CrossRef]
- Soler-López, A.; Moreno-Villanueva, A.; Gómez-Carmona, C.D.; Pino-Ortega, J. The Role of Biomarkers in Monitoring Chronic Fatigue Among Male Professional Team Athletes: A Systematic Review. Sensors 2024, 24, 6862. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Zanini, J.; Pietzner, M.; Koprulu, M.; Wheeler, E.; Kerrison, N.D.; Wareham, N.J.; Langenberg, C. Proteomic prediction of diverse incident diseases: A machine learning-guided biomarker discovery study using data from a prospective cohort study. Lancet Digit. Health 2024, 6, e470–e479. [Google Scholar] [CrossRef] [PubMed]
- Fang, S.; Huang, W.; Qu, X.; Chai, W. The mitochondria as a potential therapeutic target in cerebral I/R injury. Front. Neurosci. 2025, 18, 1500647. [Google Scholar] [CrossRef] [PubMed]
- Zhu, D.; Cao, W.; Li, J.; Wu, C.; Cao, D.; Zhang, X. Correction of preferred orientation–induced distortion in cryo–electron microscopy maps. Sci. Adv. 2024, 10, eadn0092. [Google Scholar] [CrossRef]
- Brzezinski, P.; Moe, A.; Ädelroth, P. Structure and Mechanism of Respiratory III–IV Supercomplexes in Bioenergetic Membranes. Chem. Rev. 2021, 121, 9644–9673. [Google Scholar] [CrossRef]
- García-Sánchez, A.; Miranda-Díaz, A.G.; Cardona-Muñoz, E.G. The Role of Oxidative Stress in Physiopathology and Pharmacological Treatment with Pro- and Antioxidant Properties in Chronic Diseases. Oxidative Med. Cell. Longev. 2020, 2020, 2082145. [Google Scholar] [CrossRef]
- Wong, H.-S.; Monternier, P.-A.; Brand, M.D. S1QELs suppress mitochondrial superoxide/hydrogen peroxide production from site IQ without inhibiting reverse electron flow through Complex I. Free Radic. Biol. Med. 2019, 143, 545–559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lang, R. Succinate metabolism: A promising therapeutic target for inflammation, ischemia/reperfusion injury and cancer. Front. Cell Dev. Biol. 2023, 11, 1266973. [Google Scholar] [CrossRef]
- Huang, H.; Li, G.; He, Y.; Chen, J.; Yan, J.; Zhang, Q.; Li, L.; Cai, X. Cellular succinate metabolism and signaling in inflammation: Implications for therapeutic intervention. Front. Immunol. 2024, 15, 1404441. [Google Scholar] [CrossRef]
- Fremder, M.; Kim, S.W.; Khamaysi, A.; Shimshilashvili, L.; Eini-Rider, H.; Park, I.S.; Hadad, U.; Cheon, J.H.; Ohana, E. A transepithelial pathway delivers succinate to macrophages, thus perpetuating their pro-inflammatory metabolic state. Cell Rep. 2021, 36, 109521. [Google Scholar] [CrossRef]
- Oh, T.S.; Hutchins, D.C.; Mainali, R.; Goslen, K.H.; Quinn, M.A. Itaconate and Its Derivatives Repress Early Myogenesis In Vitro and In Vivo. Front. Immunol. 2022, 13, 748375. [Google Scholar] [CrossRef]
- Chen, C.; Dai, G.; Fan, M.; Wang, X.; Niu, K.; Gao, W. Mitochondria-associated endoplasmic reticulum membranes and myocardial ischemia: From molecular mechanisms to therapeutic strategies. J. Transl. Med. 2025, 23, 277. [Google Scholar] [CrossRef]
- Lindsay, R.T.; Rhodes, C.J. Reactive Oxygen Species (ROS) in Metabolic Disease—Don’t Shoot the Metabolic Messenger. Int. J. Mol. Sci. 2025, 26, 2622. [Google Scholar] [CrossRef] [PubMed]
- Guan, F.; Wu, X.; Zhou, J.; Lin, Y.; He, Y.; Fan, C.; Zeng, Z.; Xiong, W. Mitochondrial transfer in tunneling nanotubes—A new target for cancer therapy. J. Exp. Clin. Cancer Res. 2024, 43, 147. [Google Scholar] [CrossRef]
- Zerihun, M.; Sukumaran, S.; Qvit, N. The Drp1-Mediated Mitochondrial Fission Protein Interactome as an Emerging Core Player in Mitochondrial Dynamics and Cardiovascular Disease Therapy. Int. J. Mol. Sci. 2023, 24, 5785. [Google Scholar] [CrossRef] [PubMed]
- Sever, T.; Erbaş, O. The Role of the PINK1-Parkin Pathway in Mitophagy and Its Implications in Neurodegenerative Disorders. J. Exp. Basic Med. Sci. 2024, 5, 264–270. [Google Scholar]
- Xu, S.; Wang, Z.; Guo, F.; Zhang, Y.; Peng, H.; Zhang, H.; Liu, Z.; Cao, C.; Xin, G.; Chen, Y.Y.; et al. Mitophagy in ischemic heart disease: Molecular mechanisms and clinical management. Cell Death Dis. 2024, 15, 934. [Google Scholar] [CrossRef]
- Endlicher, R.; Drahota, Z.; Štefková, K.; Červinková, Z.; Kučera, O. The Mitochondrial Permeability Transition Pore—Current Knowledge of Its Structure, Function, and Regulation, and Optimized Methods for Evaluating Its Functional State. Cells 2023, 12, 1273. [Google Scholar] [CrossRef]
- Berndt, C.; Alborzinia, H.; Amen, V.S.; Ayton, S.; Barayeu, U.; Bartelt, A.; Bayir, H.; Bebber, C.M.; Birsoy, K.; Böttcher, J.P.; et al. Ferroptosis in health and disease. Redox Biol. 2024, 75, 103211. [Google Scholar] [CrossRef]
- Pekkurnaz, G.; Wang, X. Mitochondrial heterogeneity and homeostasis through the lens of a neuron. Nat. Metab. 2022, 4, 802–812. [Google Scholar] [CrossRef]
- Carinci, M.; Vezzani, B.; Patergnani, S.; Ludewig, P.; Lessmann, K.; Magnus, T.; Casetta, I.; Pugliatti, M.; Pinton, P.; Giorgi, C. Different Roles of Mitochondria in Cell Death and Inflammation: Focusing on Mitochondrial Quality Control in Ischemic Stroke and Reperfusion. Biomedicines 2021, 9, 169. [Google Scholar] [CrossRef]
- Méndez-García, A.; García-Mendoza, M.A.; Zárate-Peralta, C.P.; Flores-Perez, F.V.; Carmona-Ramirez, L.F.; Pathak, S.; Banerjee, A.; Duttaroy, A.K.; Paul, S. Mitochondrial microRNAs (mitomiRs) as emerging biomarkers and therapeutic targets for chronic human diseases. Front. Genet. 2025, 16, 1555563. [Google Scholar] [CrossRef]
- Xian, H.; Karin, M. Oxidized mitochondrial DNA: A protective signal gone awry. Trends Immunol. 2023, 44, 188–200. [Google Scholar] [CrossRef]
- Almansa-Ordonez, A.; Bellido, R.; Vassena, R.; Barragan, M.; Zambelli, F. Oxidative Stress in Reproduction: A Mitochondrial Perspective. Biology 2020, 9, 269. [Google Scholar] [CrossRef] [PubMed]
- Lejri, I.; Grimm, A.; Eckert, A. Mitochondria, Estrogen and Female Brain Aging. Front. Aging Neurosci. 2018, 10, 124. [Google Scholar] [CrossRef] [PubMed]
- Torrens-Mas, M.; Pons, D.-G.; Sastre-Serra, J.; Oliver, J.; Roca, P. Sexual hormones regulate the redox status and mitochondrial function in the brain. Pathological implications. Redox Biol. 2020, 31, 101505. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Wang, Y.; Chen, S.; Liang, F. Glycometabolic Reprogramming of Microglia in Neurodegenerative Diseases: Insights from Neuroinflammation. Aging Dis. 2024, 15, 1155–1175. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xia, X.; Wang, Y.; Zheng, J.C. Mitochondrial dysfunction in microglia: A novel perspective for pathogenesis of Alzheimer’s disease. J. Neuroinflammation 2022, 19, 248. [Google Scholar] [CrossRef]
- Velmurugan, G.V.; Vekaria, H.J.; Patel, S.P.; Sullivan, P.G.; Hubbard, W.B. Astrocytic mitochondrial transfer to brain endothelial cells and pericytes in vivo increases with aging. J. Cereb. Blood Flow Metab. 2024, 271678X241306054. [Google Scholar] [CrossRef] [PubMed]
- Geng, Z.; Guan, S.; Wang, S.; Yu, Z.; Liu, T.; Du, S.; Zhu, C. Intercellular mitochondrial transfer in the brain, a new perspective for targeted treatment of central nervous system diseases. CNS Neurosci. Ther. 2023, 29, 3121–3135. [Google Scholar] [CrossRef] [PubMed]
- Lyamzaev, K.G.; Panteleeva, A.A.; Simonyan, R.A.; Avetisyan, A.V.; Chernyak, B.V. Mitochondrial Lipid Peroxidation Is Responsible for Ferroptosis. Cells 2023, 12, 611. [Google Scholar] [CrossRef]
- Cao, J.; Chen, X.; Chen, L.; Lu, Y.; Wu, Y.; Deng, A.; Pan, F.; Huang, H.; Liu, Y.; Li, Y.; et al. DHODH-mediated mitochondrial redox homeostasis: A novel ferroptosis regulator and promising therapeutic target. Redox Biol. 2025, 85, 103788. [Google Scholar] [CrossRef]
- Odnokoz, O.; Nakatsuka, K.; Wright, C.; Castellanos, J.; Klichko, V.I.; Kretzschmar, D.; Orr, W.C.; Radyuk, S.N. Mitochondrial Redox Signaling Is Critical to the Normal Functioning of the Neuronal System. Front. Cell Dev. Biol. 2021, 9, 613036. [Google Scholar] [CrossRef]
- Catalani, E.; Brunetti, K.; Del Quondam, S.; Cervia, D. Targeting Mitochondrial Dysfunction and Oxidative Stress to Prevent the Neurodegeneration of Retinal Ganglion Cells. Antioxidants 2023, 12, 2011. [Google Scholar] [CrossRef]
- Yu, T.; Wang, L.; Zhang, L.; Deuster, P.A. Mitochondrial Fission as a Therapeutic Target for Metabolic Diseases: Insights into Antioxidant Strategies. Antioxidants 2023, 12, 1163. [Google Scholar] [CrossRef]
- Kalinichenko, S.G.; Pushchin, I.I.; Matveeva, N.Y. Neurotoxic and cytoprotective mechanisms in the ischemic neocortex. J. Chem. Neuroanat. 2023, 128, 102230. [Google Scholar] [CrossRef]
- Liu, X.R.; Zhang, M.M.; Gross, M.L. Mass Spectrometry-Based Protein Footprinting for Higher-Order Structure Analysis: Fundamentals and Applications. Chem. Rev. 2020, 120, 4355–4454. [Google Scholar] [CrossRef]
- Topf, U.; Suppanz, I.; Samluk, L.; Wrobel, L.; Böser, A.; Sakowska, P.; Knapp, B.; Pietrzyk, M.K.; Chacinska, A.; Warscheid, B. Quantitative proteomics identifies redox switches for global translation modulation by mitochondrially produced reactive oxygen species. Nat. Commun. 2018, 9, 324. [Google Scholar] [CrossRef]
- Cyr, A.R.; Huckaby, L.V.; Shiva, S.S.; Zuckerbraun, B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020, 36, 307–321. [Google Scholar] [CrossRef]
- Chai, Y.-C.; Mieyal, J.J. Glutathione and Glutaredoxin—Key Players in Cellular Redox Homeostasis and Signaling. Antioxidants 2023, 12, 1553. [Google Scholar] [CrossRef]
- Wang, W.; Mu, S.; Yan, D.; Qin, H.; Zheng, Z. Comprehending toll-like receptors: Pivotal element in the pathogenesis of sepsis and its complications. Front. Immunol. 2025, 16, 1591011. [Google Scholar] [CrossRef]
- Möller, M.N.; Orrico, F.; Villar, S.F.; López, A.C.; Silva, N.; Donzé, M.; Thomson, L.; Denicola, A. Oxidants and Antioxidants in the Redox Biochemistry of Human Red Blood Cells. ACS Omega 2023, 8, 147–168. [Google Scholar] [CrossRef]
- Bayir, H.; Kagan, V.E. Bench-to-bedside review: Mitochondrial injury, oxidative stress and apoptosis—There is nothing more practical than a good theory. Crit. Care 2008, 12, 206. [Google Scholar] [CrossRef] [PubMed]
- Feng, S.; Tang, D.; Wang, Y.; Li, X.; Bao, H.; Tang, C.; Dong, X.; Li, X.; Yang, Q.; Yan, Y.; et al. The mechanism of ferroptosis and its related diseases. Mol. Biomed. 2023, 4, 33. [Google Scholar] [CrossRef] [PubMed]
- Tortajada-Pérez, J.; Carranza, A.d.V.; Río, C.T.-D.; Collado-Pérez, M.; Millán, J.M.; García-García, G.; Vázquez-Manrique, R.P. Lipid Oxidation at the Crossroads: Oxidative Stress and Neurodegeneration Explored in Caenorhabditis elegans. Antioxidants 2025, 14, 78. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.; Shen, J.; Jiang, J.; Wang, F.; Min, J. Targeting ferroptosis opens new avenues for the development of novel therapeutics. Signal Transduct. Target. Ther. 2023, 8, 372. [Google Scholar] [CrossRef]
- Wang, X.; Wang, Z.; Cao, J.; Dong, Y.; Chen, Y. Ferroptosis Mechanisms Involved in Hippocampal-Related Diseases. Int. J. Mol. Sci. 2021, 22, 9902. [Google Scholar] [CrossRef]
- Rochette, L.; Dogon, G.; Rigal, E.; Zeller, M.; Cottin, Y.; Vergely, C. Lipid Peroxidation and Iron Metabolism: Two Corner Stones in the Homeostasis Control of Ferroptosis. Int. J. Mol. Sci. 2023, 24, 449. [Google Scholar] [CrossRef]
- Al-Mubarak, B.R.; Bell, K.F.S.; Chowdhry, S.; Meakin, P.J.; Baxter, P.S.; McKay, S.; Dando, O.; Ashford, M.L.J.; Gazaryan, I.; Hayes, J.D.; et al. Non-canonical Keap1-independent activation of Nrf2 in astrocytes by mild oxidative stress. Redox Biol. 2021, 47, 102158. [Google Scholar] [CrossRef]
- Song, J.; Yang, P.; Chen, C.; Ding, W.; Tillement, O.; Bai, H.; Zhang, S. Targeting epigenetic regulators as a promising avenue to overcome cancer therapy resistance. Signal Transduct. Target. Ther. 2025, 10, 219. [Google Scholar] [CrossRef]
- Zheng, X.; Sawalha, A.H. The Role of Oxidative Stress in Epigenetic Changes Underlying Autoimmunity. Antioxid. Redox Signal. 2022, 36, 423–440. [Google Scholar] [CrossRef]
- Wang, F.; Li, J.; Fan, S.; Jin, Z.; Huang, C. Targeting stress granules: A novel therapeutic strategy for human diseases. Pharmacol. Res. 2020, 161, 105143. [Google Scholar] [CrossRef] [PubMed]
- Lu, P.; Ruan, D.; Huang, M.; Tian, M.; Zhu, K.; Gan, Z.; Xiao, Z. Harnessing the potential of hydrogels for advanced therapeutic applications: Current achievements and future directions. Signal Transduct. Target. Ther. 2024, 9, 166. [Google Scholar] [CrossRef]
- Arnhold, J. The Dual Role of Myeloperoxidase in Immune Response. Int. J. Mol. Sci. 2020, 21, 8057. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lyu, J.; Li, R.; Jain, V.; Shen, Y.; del Águila, Á.; Hoffmann, U.; Sheng, H.; Yang, W. Single-cell transcriptomic analysis of the immune cell landscape in the aged mouse brain after ischemic stroke. J. Neuroinflammation 2022, 19, 83. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ping, Y.; Sun, Q.; Yu, M.; Yang, C.; Liu, X.; Wang, L.; Huang, J. Single-cell transcriptomics reveals cellular dynamics and chemokine CXCL2-mediated smooth muscle cell proliferation in arterial repair. Front. Immunol. 2025, 16, 1591557. [Google Scholar] [CrossRef]
- De Nicolo, B.; Cataldi-Stagetti, E.; Diquigiovanni, C.; Bonora, E. Calcium and Reactive Oxygen Species Signaling Interplays in Cardiac Physiology and Pathologies. Antioxidants 2023, 12, 353. [Google Scholar] [CrossRef] [PubMed]
- Koh, J.-Y.; Lee, S.-J. Metallothionein-3 as a multifunctional player in the control of cellular processes and diseases. Mol. Brain 2020, 13, 116. [Google Scholar] [CrossRef]
- Marreiro, D.D.N.; Cruz, K.J.C.; Morais, J.B.S.; Beserra, J.B.; Severo, J.S.; De Oliveira, A.R.S. Zinc and Oxidative Stress: Current Mechanisms. Antioxidants 2017, 6, 24. [Google Scholar] [CrossRef] [PubMed]
- Șerban, M.; Toader, C.; Covache-Busuioc, R.-A. The Redox Revolution in Brain Medicine: Targeting Oxidative Stress with AI, Multi-Omics and Mitochondrial Therapies for the Precision Eradication of Neurodegeneration. Int. J. Mol. Sci. 2025, 26, 7498. [Google Scholar] [CrossRef] [PubMed]
- Ishii, T.; Yasuda, K.; Miyazawa, M.; Onouchi, H.; Yanase, S.; Ishii, N. Mitochondrial oxidative stress, cellular damages and stem cell aging in premature aging models with complex II electron transport defect. J. Clin. Biochem. Nutr. 2025, 77, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; De Leon-Oliva, D.; García-Montero, C.; Fraile-Martinez, O.; Boaru, D.L.; de Castro, A.V.; Saez, M.A.; Lopez-Gonzalez, L.; Bujan, J.; Alvarez-Mon, M.A.; et al. Reframing the link between metabolism and NLRP3 inflammasome: Therapeutic opportunities. Front. Immunol. 2023, 14, 1232629. [Google Scholar] [CrossRef]
- Bondi, C.D.; Rush, B.M.; Hartman, H.L.; Wang, J.; Al-Bataineh, M.M.; Hughey, R.P.; Tan, R.J. Suppression of NRF2 Activity by HIF-1α Promotes Fibrosis after Ischemic Acute Kidney Injury. Antioxidants 2022, 11, 1810. [Google Scholar] [CrossRef]
- Tkaczenko, H.; Kurhaluk, N. Antioxidant-Rich Functional Foods and Exercise: Unlocking Metabolic Health Through Nrf2 and Related Pathways. Int. J. Mol. Sci. 2025, 26, 1098. [Google Scholar] [CrossRef]
- Geissel, F.; Lang, L.; Husemann, B.; Morgan, B.; Deponte, M. Deciphering the mechanism of glutaredoxin-catalyzed roGFP2 redox sensing reveals a ternary complex with glutathione for protein disulfide reduction. Nat. Commun. 2024, 15, 1733. [Google Scholar] [CrossRef]
- Ono, M.; Toyomoto, M.; Yamauchi, M.; Hagiwara, M. Platelets accelerate lipid peroxidation and induce pathogenic neutrophil extracellular trap release. Cell Chem. Biol. 2024, 31, 2085–2095.e4. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, Z.; Li, L.; Zhang, Z.; Zhang, K.; Chu, M.; Liu, Y.; Mao, X.; Wu, D.; Xu, D.; et al. Anti-ferroptosis exosomes engineered for targeting M2 microglia to improve neurological function in ischemic stroke. J. Nanobiotechnol. 2024, 22, 291. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, W.; Zhang, Q.; He, D.; Zhao, Y.; Liu, Z.; Xia, P.; Li, Q.; Ye, Z. OSGEP, A Negative Ferroptotic Regulator, Alleviates Cerebral Ischemia-Reperfusion Injury Through Modulating m6A Methylation of GPX4 mRNA. Neurochem. Res. 2025, 50, 122. [Google Scholar] [CrossRef]
- Shi, W.; Yuan, S.; Cheng, G.; Zhang, H.; Liu, K.J.; Ji, X.; Du, L.; Qi, Z. Blood brain barrier-targeted lipid nanoparticles improved the neuroprotection of Ferrostatin-1 against cerebral ischemic damage in an experimental stroke model. Exp. Neurol. 2024, 379, 114849. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Mo, J.; Liu, K.; Chen, Q.; Li, Z.; He, Y.; Chang, Y.; Lin, C.; Yu, M.; Xu, Y.; et al. Glymphatic System Impairment Contributes to the Formation of Brain Edema After Ischemic Stroke. Stroke 2024, 55, 1393–1404. [Google Scholar] [CrossRef]
- Veciana de las Heras, M.; Sala-Padro, J.; Pedro-Perez, J.; García-Parra, B.; Hernández-Pérez, G.; Falip, M. Utility of Quantitative EEG in Neurological Emergencies and ICU Clinical Practice. Brain Sci. 2024, 14, 939. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, Y.; Zhang, Y.; Liu, X.; Qing, K. EEG biomarkers analysis in different cognitive impairment after stroke: An exploration study. Front. Neurol. 2024, 15, 1358167. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.-X.; Wang, J.-Y.; Yang, H.; Zhang, R.; Cao, R.; Hong, W.; Jiang, S. Serum Glutathione and Malondialdehyde Levels as Predictors of Early Neurological Deficits and Short-Term Outcomes in Acute Cerebral Infarction. Physiol. Res. 2025, 74, 327–336. [Google Scholar] [CrossRef]
- Jiang, W.; Zhao, Y.; Liu, R.; Zhang, B.; Xie, Y.; Gao, B.; Shi, K.; Zou, M.; Jia, D.; Ding, J.; et al. Histidine-rich glycoprotein modulates neutrophils and thrombolysis-associated hemorrhagic transformation. EMBO Mol. Med. 2024, 16, 2146–2169. [Google Scholar] [CrossRef]
- Su, X.; Zhang, D.; Zhang, N. Chrysophanol accelerates astrocytic mitochondria transfer to neurons and attenuates the cerebral ischemia-reperfusion injury in rats. Biochem. Biophys. Res. Commun. 2024, 704, 149712. [Google Scholar] [CrossRef]
- Sun, F.; Zhou, J.; Chen, X.; Yang, T.; Wang, G.; Ge, J.; Zhang, Z.; Mei, Z. No-reflow after recanalization in ischemic stroke: From pathomechanisms to therapeutic strategies. J. Cereb. Blood Flow Metab. 2024, 44, 857–880. [Google Scholar] [CrossRef]
- Stergiou, Y.G.; Keramydas, A.T.; Anastasiou, A.D.; Mouza, A.A.; Paras, S.V. Experimental and Numerical Study of Blood Flow in μ-vessels: Influence of the Fahraeus–Lindqvist Effect. Fluids 2019, 4, 143. [Google Scholar] [CrossRef]
- Poster Viewing Sessions PB01-B01 to PB03-V09. J. Cereb. Blood Flow Metab. 2019, 39 (Suppl. 1), 167–523, Erratum in J. Cereb. Blood Flow Metab. 2020, 40, NP11–NP21. https://doi.org/10.1177/0271678X19864534. [CrossRef]
- Xu, J.; Zhao, W. Bidirectional Role of Pericytes in Ischemic Stroke. Brain Sci. 2025, 15, 605. [Google Scholar] [CrossRef] [PubMed]
- Gürler, G.; Soylu, K.O.; Yemisci, M. Importance of Pericytes in the Pathophysiology of Cerebral Ischemia. Arch. Neuropsychiatry 2022, 59 (Suppl. 1), S29–S35. [Google Scholar] [CrossRef]
- ul Islam, B.; Habib, S.; Ahmad, P.; Allarakha, S.; Moinuddin; Ali, A. Pathophysiological Role of Peroxynitrite Induced DNA Damage in Human Diseases: A Special Focus on Poly(ADP-ribose) Polymerase (PARP). Indian J. Clin. Biochem. 2015, 30, 368–385. [Google Scholar] [CrossRef] [PubMed]
- Ghenciu, L.A.; Andrei, D.; Borza, C.; Iacob, R.; Stoicescu, E.R.; Bolintineanu, S.L.; Iacob, D.; Haţegan, O.A. Therapeutic Potential of Rho Kinase Inhibitors in Corneal Disease: A Systematic Review of Preclinical and Clinical Studies. Biomedicines 2025, 13, 1602. [Google Scholar] [CrossRef]
- Daiber, A.; Xia, N.; Steven, S.; Oelze, M.; Hanf, A.; Kröller-Schön, S.; Münzel, T.; Li, H. New Therapeutic Implications of Endothelial Nitric Oxide Synthase (eNOS) Function/Dysfunction in Cardiovascular Disease. Int. J. Mol. Sci. 2019, 20, 187. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, N.; Millican, K.; Ebong, E.E. Unraveling neurovascular mysteries: The role of endothelial glycocalyx dysfunction in Alzheimer’s disease pathogenesis. Front. Physiol. 2024, 15, 1394725. [Google Scholar] [CrossRef]
- Kuo, W.-T.; Odenwald, M.A.; Turner, J.R.; Zuo, L. Tight junction proteins occludin and ZO-1 as regulators of epithelial proliferation and survival. Ann. N. Y. Acad. Sci. 2022, 1514, 21–33. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhan, J.; Cai, Q.; Xu, F.; Chai, R.; Lam, K.; Luan, Z.; Zhou, G.; Tsang, S.; Kipp, M.; et al. The Water Transport System in Astrocytes–Aquaporins. Cells 2022, 11, 2564. [Google Scholar] [CrossRef]
- Stewart, R.; Hope Hutson, K.; Nestorova, G.G. Therapeutic potential of astrocyte-derived extracellular vesicles in mitigating cytotoxicity and transcriptome changes in human brain endothelial cells. Neuroscience 2024, 560, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Park, R.; Peng, Y.; Yslas, A.R.; Lee, E. Astrocyte-driven vasoconstriction impairs glymphatic clearance in a human tauopathy-on-chip model. APL Bioeng. 2025, 9, 026126. [Google Scholar] [CrossRef]
- Lee, H.-T.; Lin, C.-S.; Liu, C.-Y.; Chen, P.; Tsai, C.-Y.; Wei, Y.-H. Mitochondrial Plasticity and Glucose Metabolic Alterations in Human Cancer under Oxidative Stress—From Viewpoints of Chronic Inflammation and Neutrophil Extracellular Traps (NETs). Int. J. Mol. Sci. 2024, 25, 9458. [Google Scholar] [CrossRef]
- Kindberg, K.M.; Nordeng, J.; Langseth, M.S.; Schandiz, H.; Roald, B.; Solheim, S.; Seljeflot, I.; Stokke, M.K.; Helseth, R. IL-6R Signaling Is Associated with PAD4 and Neutrophil Extracellular Trap Formation in Patients with STEMI. Int. J. Mol. Sci. 2025, 26, 5348. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, D.; Li, C.; Liu, R.; Wang, X. Mechanism of hypoxia-induced damage to the mechanical property in human erythrocytes—Band 3 phosphorylation and sulfhydryl oxidation of membrane proteins. Front. Physiol. 2024, 15, 1399154. [Google Scholar] [CrossRef] [PubMed]
- Cyboran-Mikołajczyk, S.; Męczarska, K.; Solarska-Ściuk, K.; Ratajczak-Wielgomas, K.; Oszmiański, J.; Jencova, V.; Bonarska-Kujawa, D. Protection of Erythrocytes and Microvascular Endothelial Cells against Oxidative Damage by Fragaria vesca L. and Rubus idaeus L. Leaves Extracts—The Mechanism of Action. Molecules 2022, 27, 5865. [Google Scholar] [CrossRef]
- Grigorev, G.V.; Lebedev, A.V.; Wang, X.; Qian, X.; Maksimov, G.V.; Lin, L. Advances in Microfluidics for Single Red Blood Cell Analysis. Biosensors 2023, 13, 117. [Google Scholar] [CrossRef]
- Cotinat, M.; Messaoudi, N.; Robinet, E.; Suissa, L.; Doche, E.; Guye, M.; Audoin, B.; Bensoussan, L.; Ranjeva, J.; Zaaraoui, W. Dynamics of Ionic and Cytotoxic Edema During Acute and Subacute Stages of Patients with Ischemic Stroke: Complementarity of 23Na MRI and Diffusion MRI. NMR Biomed. 2025, 38, e70028. [Google Scholar] [CrossRef]
- Muer, J.D.; Didier, K.D.; Wannebo, B.M.; Sanchez, S.; Khademi Motlagh, H.; Haley, T.L.; Carter, K.J.; Banks, N.F.; Eldridge, M.W.; Serlin, R.C.; et al. Sex differences in gray matter, white matter, and regional brain perfusion in young, healthy adults. Am. J. Physiol. Heart Circ. Physiol. 2024, 327, H847–H858. [Google Scholar] [CrossRef] [PubMed]
- Yamada, S.; Sakai, K.; Kubo, M.; Okumura, H.; Asakura, H.; Miyamoto, T.; Matsumoto, M. Excessive cleavage of von Willebrand factor multimers by ADAMTS13 may predict the progression of transplant-associated thrombotic microangiopathy. Res. Pract. Thromb. Haemost. 2024, 8, 102517. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Buonfiglio, F.; Li, J.; Pfeiffer, N.; Gericke, A. Mechanisms Underlying Vascular Inflammaging: Current Insights and Potential Treatment Approaches. Aging Dis. 2025, 16, 1889–1917. [Google Scholar] [CrossRef] [PubMed]
- Trombka, D.; Meiron, O. Common Genomic and Proteomic Alterations Related to Disturbed Neural Oscillatory Activity in Schizophrenia. Int. J. Mol. Sci. 2025, 26, 7514. [Google Scholar] [CrossRef]
- Monsour, M.; Borlongan, C.V. Neurovascular unit permeability in neuroinflammatory diseases: A common pathologic and therapeutic target? Neural Regen. Res. 2022, 18, 1715–1716. [Google Scholar] [CrossRef]
- Girolamo, F.; Errede, M.; Bizzoca, A.; Virgintino, D.; Ribatti, D. Central Nervous System Pericytes Contribute to Health and Disease. Cells 2022, 11, 1707. [Google Scholar] [CrossRef]
- Kan, Y.; Li, S.; Zhang, B.; Ding, Y.; Zhao, W.; Ji, X. No-reflow phenomenon following stroke recanalization therapy: Clinical assessment advances: A narrative review. Brain Circ. 2023, 9, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Dong, J.; Wu, Y.; Zhao, L.; Wang, H.; Zhang, J.; Yao, B.; Xu, X.; Zou, Y.; Zhao, H.; et al. Lipid Peroxides Mediated Ferroptosis in Electromagnetic Pulse-Induced Hippocampal Neuronal Damage via Inhibition of GSH/GPX4 Axis. Int. J. Mol. Sci. 2022, 23, 9277. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, L.; Peng, J.; Zhang, X.; Zhang, F.; Wu, Y.; Huang, A.; Du, F.; Liao, Y.; He, Y.; et al. Astrocytic LRP1 enables mitochondria transfer to neurons and mitigates brain ischemic stroke by suppressing ARF1 lactylation. Cell Metab. 2024, 36, 2054–2068.e14. [Google Scholar] [CrossRef] [PubMed]
- Perumal, V.; Ravula, A.R.; Agas, A.; Kannan, M.; Liu, X.; I, S.S.; Vijayaraghavalu, S.; Haorah, J.; Zhang, Y.; Chandra, N. Transferrin-Grafted Albumin Nanoparticles for the Targeted Delivery of Apocynin and Neuroprotection in an In Vitro Model of the BBB. Micro 2023, 3, 84–106. [Google Scholar] [CrossRef]
- Lim, S.H.; Yee, G.T.; Khang, D. Nanoparticle-Based Combinational Strategies for Overcoming the Blood-Brain Barrier and Blood-Tumor Barrier. Int. J. Nanomed. 2024, 19, 2529–2552. [Google Scholar] [CrossRef]
- Xie, K.; Mo, Y.; Yue, E.; Shi, N.; Liu, K. Exosomes derived from M2-type microglia ameliorate oxygen-glucose deprivation/reoxygenation-induced HT22 cell injury by regulating miR-124-3p/NCOA4-mediated ferroptosis. Heliyon 2023, 9, e17592. [Google Scholar] [CrossRef]
- Li, Y.; Jia, X.; Tang, J.; Qiao, H.; Zhou, J.; Ma, Y. Heparin-Binding Hemagglutinin-Induced Trained Immunity in Macrophages: Implications for Antimycobacterial Defense. Biomolecules 2025, 15, 959. [Google Scholar] [CrossRef] [PubMed]
- Beignon, A.-S.; Galeotti, C.; Menager, M.M.; Schvartz, A. Trained immunity as a possible newcomer in autoinflammatory and autoimmune diseases pathophysiology. Front. Med. 2023, 9, 1085339. [Google Scholar] [CrossRef]
- Flores-Gomez, D.; Hobo, W.; van Ens, D.; Kessler, E.L.; Novakovic, B.; Schaap, N.P.M.; Rijnen, W.H.C.; Joosten, L.A.B.; Netea, M.G.; Riksen, N.P.; et al. Interleukin-1β induces trained innate immunity in human hematopoietic progenitor cells in vitro. Stem Cell Rep. 2024, 19, 1651–1664. [Google Scholar] [CrossRef]
- Erdem, A.; Kaye, S.; Caligiore, F.; Johanns, M.; Leguay, F.; Schuringa, J.J.; Ito, K.; Bommer, G.; van Gastel, N. Lactate dehydrogenase A-coupled NAD+ regeneration is critical for acute myeloid leukemia cell survival. Cancer Metab. 2025, 13, 22. [Google Scholar] [CrossRef]
- Chen, Z.; Liu, K. Mechanism and Applications of Vagus Nerve Stimulation. Curr. Issues Mol. Biol. 2025, 47, 122. [Google Scholar] [CrossRef] [PubMed]
- Keute, M.; Gharabaghi, A. Brain plasticity and vagus nerve stimulation. Auton. Neurosci. 2021, 236, 102876. [Google Scholar] [CrossRef]
- Peng, Z.; Hou, T.; Yang, K.; Zhang, J.; Mao, Y.-H.; Hou, X. Microecologics and Exercise: Targeting the Microbiota–Gut–Brain Axis for Central Nervous System Disease Intervention. Nutrients 2025, 17, 1769. [Google Scholar] [CrossRef]
- Cintado, E.; Muela, P.; Martín-Rodríguez, L.; Alcaide, I.; Tezanos, P.; Vlckova, K.; Valderrama, B.; Bastiaanssen, T.F.S.; Rodríguez-Muñoz, M.; de Ceballos, M.L.; et al. Gut microbiota regulates exercise-induced hormetic modulation of cognitive function. EBioMedicine 2025, 119, 105876. [Google Scholar] [CrossRef]
- Pluta, R.; Januszewski, S.; Czuczwar, S.J. Neuroinflammation in Post-Ischemic Neurodegeneration of the Brain: Friend, Foe, or Both? Int. J. Mol. Sci. 2021, 22, 4405. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Jiao, Y.; Zhao, X.; Kong, X.; Chen, Y.; Li, L.; Chen, X.; Tao, X.; Dong, D. Examining the Impact of Microglia on Ischemic Stroke with an Emphasis on the Metabolism of Immune Cells. CNS Neurosci. Ther. 2025, 31, e70229. [Google Scholar] [CrossRef]
- Wang, W.; Chang, R.; Wang, Y.; Hou, L.; Wang, Q. Mitophagy-dependent mitochondrial ROS mediates 2,5-hexanedione-induced NLRP3 inflammasome activation in BV2 microglia. Neurotoxicology 2023, 99, 50–58. [Google Scholar] [CrossRef]
- Tang, Y.; Wu, X.; Li, J.; Li, Y.; Xu, X.; Li, G.; Zhang, P.; Qin, C.; Wu, L.-J.; Tang, Z.; et al. The Emerging Role of Microglial Hv1 as a Target for Immunomodulation in Myelin Repair. Aging Dis. 2024, 15, 1176–1203. [Google Scholar] [CrossRef]
- Ma, H.; Li, H.; Zhang, Y.; Zhou, Y.; Liu, H.; Xu, H.; Zhu, L.; Zhang, G.; Wang, J.; Li, Z.; et al. Microglia Exhibit Distinct Heterogeneity Rather than M1/M2 Polarization within the Early Stage of Acute Ischemic Stroke. Aging Dis. 2023, 14, 2284–2302. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Malovic, E.; Ealy, A.; Jin, H.; Anantharam, V.; Kanthasamy, A.; Kanthasamy, A.G. Microglial immune regulation by epigenetic reprogramming through histone H3K27 acetylation in neuroinflammation. Front. Immunol. 2023, 14, 1052925. [Google Scholar] [CrossRef]
- Pereira-Santos, A.R.; Candeias, E.; Magalhães, J.D.; Empadinhas, N.; Esteves, A.R.; Cardoso, S.M. Neuronal control of microglia through the mitochondria. Biochim. Biophys. Acta Mol. Basis Dis. 2024, 1870, 167167. [Google Scholar] [CrossRef] [PubMed]
- Fairley, L.H.; Grimm, A.; Eckert, A. Mitochondria Transfer in Brain Injury and Disease. Cells 2022, 11, 3603. [Google Scholar] [CrossRef]
- Ren, J.; Xiang, B.; Xueling, L.; Han, X.; Yang, Z.; Zhang, M.; Zhang, Y. Molecular mechanisms of mitochondrial homeostasis regulation in neurons and possible therapeutic approaches for Alzheimer’s disease. Heliyon 2024, 10, e36470. [Google Scholar] [CrossRef]
- Zambrano, F.; Uribe, P.; Schulz, M.; Hermosilla, C.; Taubert, A.; Sánchez, R. Antioxidants as Modulators of NETosis: Mechanisms, Evidence, and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 5272. [Google Scholar] [CrossRef] [PubMed]
- Gysemans, C.; Beya, M.; Pedace, E.; Mathieu, C. Exploring Neutrophil Heterogeneity and Plasticity in Health and Disease. Biomedicines 2025, 13, 597. [Google Scholar] [CrossRef]
- Sae-Khow, K.; Charoensappakit, A.; Leelahavanichkul, A. Neutrophil Diversity (Immature, Aged, and Low-Density Neutrophils) and Functional Plasticity: Possible Impacts of Iron Overload in β-Thalassemia. Int. J. Mol. Sci. 2024, 25, 10651. [Google Scholar] [CrossRef]
- Xu, J.; Zheng, Y.; Zhao, Y.; Zhang, Y.; Li, H.; Zhang, A.; Wang, X.; Wang, W.; Hou, Y.; Wang, J. Succinate/IL-1β Signaling Axis Promotes the Inflammatory Progression of Endothelial and Exacerbates Atherosclerosis. Front. Immunol. 2022, 13, 817572. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.A.; Akbar, N.; Baidžajevas, K.; Choudhury, R.P. Trained immunity in diabetes and hyperlipidemia: Emerging opportunities to target cardiovascular complications and design new therapies. FASEB J 2023, 37, e23231. [Google Scholar] [CrossRef]
- Chen, X.; Lin, P.; Lu, Y.; Zheng, J.; Lin, Y.; Zhou, Z.; Cui, L.; Zhao, X. Mitochondrial Regulation of CD8+ T Cells: Mechanisms and Therapeutic Modulation. Adv. Sci. 2025, 12, e03095. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Pang, Y.; Fan, X. Mitochondria in oxidative stress, inflammation and aging: From mechanisms to therapeutic advances. Signal Transduct. Target. Ther. 2025, 10, 190. [Google Scholar] [CrossRef] [PubMed]
- Bellanti, F.; Coda, A.R.D.; Trecca, M.I.; Lo Buglio, A.; Serviddio, G.; Vendemiale, G. Redox Imbalance in Inflammation: The Interplay of Oxidative and Reductive Stress. Antioxidants 2025, 14, 656. [Google Scholar] [CrossRef]
- Alqrad, M.A.I.; El-Agamy, D.S.; Ibrahim, S.R.M.; Sirwi, A.; Abdallah, H.M.; Abdel-Sattar, E.; El-Halawany, A.M.; Elsaed, W.M.; Mohamed, G.A. SIRT1/Nrf2/NF-κB Signaling Mediates Anti-Inflammatory and Anti-Apoptotic Activities of Oleanolic Acid in a Mouse Model of Acute Hepatorenal Damage. Medicina 2023, 59, 1351. [Google Scholar] [CrossRef]
- Chen, Y.; Qin, C.; Huang, J.; Tang, X.; Liu, C.; Huang, K.; Xu, J.; Guo, G.; Tong, A.; Zhou, L. The role of astrocytes in oxidative stress of central nervous system: A mixed blessing. Cell Prolif. 2020, 53, e12781. [Google Scholar] [CrossRef]
- Liu, X.; Lv, X.; Liu, Z.; Zhang, M.; Leng, Y. MircoRNA-29a in Astrocyte-derived Extracellular Vesicles Suppresses Brain Ischemia Reperfusion Injury via TP53INP1 and the NF-κB/NLRP3 Axis. Cell. Mol. Neurobiol. 2021, 42, 1487–1500. [Google Scholar] [CrossRef]
- Forró, T.; Manu, D.R.; Băjenaru, O.-L.; Bălașa, R. GFAP as Astrocyte-Derived Extracellular Vesicle Cargo in Acute Ischemic Stroke Patients—A Pilot Study. Int. J. Mol. Sci. 2024, 25, 5726. [Google Scholar] [CrossRef]
- Flender, D.; Vilenne, F.; Adams, C.; Boonen, K.; Valkenborg, D.; Baggerman, G. Exploring the dynamic landscape of immunopeptidomics: Unravelling posttranslational modifications and navigating bioinformatics terrain. Mass Spectrom. Rev. 2025, 44, 599–629. [Google Scholar] [CrossRef]
- Li, Y.; Zhao, Q.; Wang, Y.; Du, W.; Yang, R.; Wu, J.; Li, Y. Lipid droplet accumulation in microglia and their potential roles. Lipids Health Dis. 2025, 24, 215, Erratum in Lipids Health Dis. 2025, 24, 247. https://doi.org/10.1186/s12944-025-02665-9. [Google Scholar] [CrossRef]
- Wang, J.; Shen, Y.; Liao, P.; Yang, B.; Jiang, R. Roles of Ion Channels in Oligodendrocyte Precursor Cells: From Physiology to Pathology. Int. J. Mol. Sci. 2025, 26, 7336. [Google Scholar] [CrossRef]
- Cheli, V.T.; Sekhar, M.; Santiago González, D.A.; Angeliu, C.G.; Denaroso, G.E.; Smith, Z.; Wang, C.; Paez, P.M. The expression of ceruloplasmin in astrocytes is essential for postnatal myelination and myelin maintenance in the adult brain. Glia 2023, 71, 2323–2342. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Liu, W.; Geng, P.; Du, W.; Guo, C.; Wang, Q.; Zheng, G.; Jin, X. Role of Crosstalk between Glial Cells and Immune Cells in Blood-Brain Barrier Damage and Protection after Acute Ischemic Stroke. Aging Dis. 2023, 15, 2507–2525. [Google Scholar] [CrossRef] [PubMed]
- Spaas, J.; van Veggel, L.; Schepers, M.; Tiane, A.; van Horssen, J.; Wilson, D.M.; Moya, P.R.; Piccart, E.; Hellings, N.; Eijnde, B.O.; et al. Oxidative stress and impaired oligodendrocyte precursor cell differentiation in neurological disorders. Cell. Mol. Life Sci. 2021, 78, 4615–4637. [Google Scholar] [CrossRef]
- Voicu, V.; Toader, C.; Șerban, M.; Covache-Busuioc, R.-A.; Ciurea, A.V. Systemic Neurodegeneration and Brain Aging: Multi-Omics Disintegration, Proteostatic Collapse, and Network Failure Across the CNS. Biomedicines 2025, 13, 2025. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-T.; Uruno, A.; Katsuoka, F.; Yamamoto, M. Role of NRF2 in Pathogenesis of Alzheimer’s Disease. Antioxidants 2024, 13, 1529. [Google Scholar] [CrossRef]
- Dabravolski, S.A.; Bukrinsky, M.I.; Utkina, A.S.; Ravani, A.L.; Sukhorukov, V.N.; Orekhov, A.N. Exploring Neutrophil Extracellular Traps in Cardiovascular Pathologies: The Impact of Lipid Profiles, PAD4, and Radiation. Biocell 2025, 49, 931–959. [Google Scholar] [CrossRef]
- Dinc, R.; Ardic, N. Inhibition of Neutrophil Extracellular Traps: A Potential Therapeutic Strategy for Hemorrhagic Stroke. J. Integr. Neurosci. 2025, 24, 26357. [Google Scholar] [CrossRef]
- Li, Y.; Sun, M.; Cao, F.; Chen, Y.; Zhang, L.; Li, H.; Cao, J.; Song, J.; Ma, Y.; Mi, W.; et al. The Ferroptosis Inhibitor Liproxstatin-1 Ameliorates LPS-Induced Cognitive Impairment in Mice. Nutrients 2022, 14, 4599. [Google Scholar] [CrossRef]
- Okeke, E.B.; Uzonna, J.E. The Pivotal Role of Regulatory T Cells in the Regulation of Innate Immune Cells. Front. Immunol. 2019, 10, 680. [Google Scholar] [CrossRef]
- Bi, Y.; Wang, L.; Li, C.; Shan, Z.; Bi, L. Unveiling exosomal biomarkers in neurodegenerative diseases: LC-MS-based profiling. Extracell. Vesicle 2025, 5, 100071. [Google Scholar] [CrossRef]
- Islam, S.; Ahmed, M.M.S.; Islam, M.A.; Hossain, N.; Chowdhury, M.A. Advances in nanoparticles in targeted drug delivery–A review. Results Surf. Interfaces 2025, 19, 100529. [Google Scholar] [CrossRef]
- Kurzhagen, J.T.; Noel, S.; Lee, K.; Sadasivam, M.; Gharaie, S.; Ankireddy, A.; Lee, S.A.; Newman-Rivera, A.; Gong, J.; Arend, L.J.; et al. T Cell Nrf2/Keap1 Gene Editing Using CRISPR/Cas9 and Experimental Kidney Ischemia-Reperfusion Injury. Antioxid. Redox Signal. 2023, 38, 959–973. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Huang, X.; Fang, M.; Feng, X.; Zhang, X.-Y.; Xie, X.; Zheng, Z.; Dong, D. Artificial intelligence in medical imaging empowers precision neoadjuvant immunochemotherapy in esophageal squamous cell carcinoma. J. Immunother. Cancer 2025, 13, e012468. [Google Scholar] [CrossRef]
- Tobin, M.K.; Bonds, J.A.; Minshall, R.D.; Pelligrino, D.A.; Testai, F.D.; Lazarov, O. Neurogenesis and inflammation after ischemic stroke: What is known and where we go from here. J. Cereb. Blood Flow Metab. 2014, 34, 1573–1584. [Google Scholar] [CrossRef]
- Ionescu, C.; Ghidersa, M.; Ciobica, A.; Mavroudis, I.; Kazis, D.; Petridis, F.E.; Gorgan, D.L.; Balmus, I.-M. Potential Correlation Between Molecular Biomarkers and Oxidative Stress in Traumatic Brain Injury. Int. J. Mol. Sci. 2025, 26, 3858. [Google Scholar] [CrossRef] [PubMed]
- Coley, A.A.; Gao, W.-J. PSD-95 deficiency disrupts PFC-associated function and behavior during neurodevelopment. Sci. Rep. 2019, 9, 9486. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 1–37. [Google Scholar] [CrossRef]
- Perluigi, M.; Di Domenico, F.; Butterfield, D.A. Oxidative damage in neurodegeneration: Roles in the pathogenesis and progression of Alzheimer disease. Physiol. Rev. 2024, 104, 103–197. [Google Scholar] [CrossRef]
- Negro-Demontel, L.; Maleki, A.F.; Reich, D.S.; Kemper, C. The complement system in neurodegenerative and inflammatory diseases of the central nervous system. Front. Neurol. 2024, 15, 1396520. [Google Scholar] [CrossRef] [PubMed]
- Navlakha, S.; Barth, A.L.; Bar-Joseph, Z. Decreasing-Rate Pruning Optimizes the Construction of Efficient and Robust Distributed Networks. PLoS Comput. Biol. 2015, 11, e1004347. [Google Scholar] [CrossRef]
- Kanayama, H.; Tominaga, T.; Tominaga, Y.; Kato, N.; Yoshimura, H. Action of GABAB receptor on local network oscillation in somatosensory cortex of oral part: Focusing on NMDA receptor. J. Physiol. Sci. 2024, 74, 16. [Google Scholar] [CrossRef] [PubMed]
- Rochart, R.; Liu, Q.; Fonteh, A.N.; Harrington, M.G.; Arakaki, X. Compromised Behavior and Gamma Power During Working Memory in Cognitively Healthy Individuals with Abnormal CSF Amyloid/Tau. Front. Aging Neurosci. 2020, 12, 574214. [Google Scholar] [CrossRef]
- Akbari-Gharalari, N.; Khodakarimi, S.; Nezhadshahmohammad, F.; Karimipour, M.; Ebrahimi-Kalan, A.; Wu, J. Exosomes in neuron-glia communication: A review on neurodegeneration. Bioimpacts 2024, 14, 30153. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, X.; Li, J.; Liu, C.; Li, W.; Zhao, J.; Li, Z.; Wang, N.; Wang, F.; Dong, J.; et al. Neutrophil extracellular traps mediated by platelet microvesicles promote thrombosis and brain injury in acute ischemic stroke. Cell Commun. Signal. 2024, 22, 50. [Google Scholar] [CrossRef]
- Soni, N.; Vegh, V.; To, X.V.; Mohamed, A.Z.; Borges, K.; Nasrallah, F.A. Combined Diffusion Tensor Imaging and Quantitative Susceptibility Mapping Discern Discrete Facets of White Matter Pathology Post-injury in the Rodent Brain. Front. Neurol. 2020, 11, 153. [Google Scholar] [CrossRef]
- Beckhauser, T.F.; Francis-Oliveira, J.; De Pasquale, R. Reactive Oxygen Species: Physiological and Physiopathological Effects on Synaptic Plasticity. J. Exp. Neurosci. 2016, 10 (Suppl. 1), 23–48. [Google Scholar] [CrossRef]
- Bacanoiu, M.V.; Danoiu, M.; Rusu, L.; Marin, M.I. New Directions to Approach Oxidative Stress Related to Physical Activity and Nutraceuticals in Normal Aging and Neurodegenerative Aging. Antioxidants 2023, 12, 1008. [Google Scholar] [CrossRef] [PubMed]
- Jelinek, M.; Jurajda, M.; Duris, K. Oxidative Stress in the Brain: Basic Concepts and Treatment Strategies in Stroke. Antioxidants 2021, 10, 1886. [Google Scholar] [CrossRef]
- Naze, S.; Proix, T.; Atasoy, S.; Kozloski, J.R. Robustness of connectome harmonics to local gray matter and long-range white matter connectivity changes. Neuroimage 2021, 224, 117364. [Google Scholar] [CrossRef] [PubMed]
- Salatin, S.; Farhoudi, M.; Farjami, A.; Maleki Dizaj, S.; Sharifi, S.; Shahi, S. Nanoparticle Formulations of Antioxidants for the Management of Oxidative Stress in Stroke: A Review. Biomedicines 2023, 11, 3010. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Toschi, N.; Babiloni, C.; Baldacci, F.; Black, K.L.; Bokde, A.L.W.; Bun, R.S.; Cacciola, F.; Cavedo, E.; Chiesa, P.A.; et al. Revolution of Alzheimer Precision Neurology: Passageway of Systems Biology and Neurophysiology. J. Alzheimer’s Dis. 2018, 64 (Suppl. 1), S47–S105. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, N.; Simo, L.; Ma, Q.; Li, C. A narrative review of reperfusion therapy in acute ischemic stroke: Emerging advances, current challenges, and future directions. Brain Circ. 2025, 11, 187–199. [Google Scholar] [CrossRef]
- Li, B.; Ming, H.; Qin, S.; Nice, E.C.; Dong, J.; Du, Z.; Huang, C. Redox regulation: Mechanisms, biology and therapeutic targets in diseases. Signal Transduct. Target Ther. 2025, 10, 72. [Google Scholar] [CrossRef] [PubMed]
- Dhama, K.; Latheef, S.K.; Dadar, M.; Samad, H.A.; Munjal, A.; Khandia, R.; Karthik, K.; Tiwari, R.; Yatoo, M.I.; Bhatt, P.; et al. Biomarkers in Stress Related Diseases/Disorders: Diagnostic, Prognostic, and Therapeutic Values. Front. Mol. Biosci. 2019, 6, 91. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Yang, Z.; Wang, B.; Gong, H.; Zhang, K.; Lin, Y.; Sun, M. Extracellular vesicles: Biological mechanisms and emerging therapeutic opportunities in neurodegenerative diseases. Transl. Neurodegener. 2024, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Zanirati, G.; dos Santos, P.G.; Alcará, A.M.; Bruzzo, F.; Ghilardi, I.M.; Wietholter, V.; Xavier, F.A.C.; Gonçalves, J.I.B.; Marinowic, D.; Shetty, A.K.; et al. Extracellular Vesicles: The Next Generation of Biomarkers and Treatment for Central Nervous System Diseases. Int. J. Mol. Sci. 2024, 25, 7371. [Google Scholar] [CrossRef]
- Hajjar, I.; Hayek, S.S.; Goldstein, F.C.; Martin, G.; Jones, D.P.; Quyyumi, A. Oxidative stress predicts cognitive decline with aging in healthy adults: An observational study. J. Neuroinflammation 2018, 15, 17. [Google Scholar] [CrossRef]
- Hasan, A.; Scuderi, S.A.; Capra, A.P.; Giosa, D.; Bonomo, A.; Ardizzone, A.; Esposito, E. An Updated and Comprehensive Review Exploring the Gut–Brain Axis in Neurodegenerative Disorders and Neurotraumas: Implications for Therapeutic Strategies. Brain Sci. 2025, 15, 654. [Google Scholar] [CrossRef]
- Matei, B.; Winters-Stone, K.M.; Raber, J. Examining the Mechanisms behind Exercise’s Multifaceted Impacts on Body Composition, Cognition, and the Gut Microbiome in Cancer Survivors: Exploring the Links to Oxidative Stress and Inflammation. Antioxidants 2023, 12, 1423. [Google Scholar] [CrossRef] [PubMed]
- Gong, J.; Li, J.; Li, J.; He, A.; Ren, B.; Zhao, M.; Li, K.; Zhang, Y.; He, M.; Liu, Y.; et al. Impact of Microglia-Derived Extracellular Vesicles on Resident Central Nervous System Cell Populations After Acute Brain Injury Under Various External Stimuli Conditions. Mol. Neurobiol. 2025, 62, 9586–9603. [Google Scholar] [CrossRef]
- Eissa, T.; Leonardo, C.; Kepesidis, K.V.; Fleischmann, F.; Linkohr, B.; Meyer, D.; Zoka, V.; Huber, M.; Voronina, L.; Richter, L.; et al. Plasma infrared fingerprinting with machine learning enables single-measurement multi-phenotype health screening. Cell Rep. Med. 2024, 5, 101625. [Google Scholar] [CrossRef]
- Mitolo, M.; Lombardi, G.; Manca, R.; Nacmias, B.; Venneri, A. Association between blood-based protein biomarkers and brain MRI in the Alzheimer’s disease continuum: A systematic review. J. Neurol. 2024, 271, 7120–7140. [Google Scholar] [CrossRef]
- Blair, D.S.; Miller, R.L.; Calhoun, V.D. A Dynamic Entropy Approach Reveals Reduced Functional Network Connectivity Trajectory Complexity in Schizophrenia. Entropy 2024, 26, 545. [Google Scholar] [CrossRef]
- Prabhu, P.; Morise, H.; Kudo, K.; Beagle, A.; Mizuiri, D.; Syed, F.; Kotegar, K.A.; Findlay, A.; Miller, B.L.; Kramer, J.H.; et al. Abnormal gamma phase-amplitude coupling in the parahippocampal cortex is associated with network hyperexcitability in Alzheimer’s disease. Brain Commun. 2024, 6, fcae121. [Google Scholar] [CrossRef]
- Chu, Q.; Guo, X.; Zhang, T.; Huo, C.; Zhang, X.; Xu, G.; Lun, Z.; Cheng, S.; Xie, P. Stroke-Related Alterations in the Brain’s Functional Connectivity Response Associated with Upper Limb Multi-Joint Linkage Movement. Brain Sci. 2023, 13, 338. [Google Scholar] [CrossRef]
- Saha, S.; Saso, L. Pharmacological Modulation of Oxidative Stress. Int. J. Mol. Sci. 2023, 24, 14455. [Google Scholar] [CrossRef]
- Zan, G.; He, H.; Wang, X.; Zhou, J.; Wang, X.; Yan, H. Morin Reactivates Nrf2 by Targeting Inhibition of Keap1 to Alleviate Deoxynivalenol-Induced Intestinal Oxidative Damage. Int. J. Mol. Sci. 2025, 26, 1086. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.-X.; Ma, H.-Y.; Yin, W.-J.; Li, Y.-K.; Lei, S.-Y.; Liu, J.-C.; Yang, Y.; Guo, Z.-N. Emerging Targeted Delivery Strategies of Nanosystems for Ischemic Stroke Treatment. Int. J. Nanomed. 2025, 20, 8143–8171. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Yi, Y.G.; Chang, M.C. The effect of transcranial alternating current stimulation on functional recovery in patients with stroke: A narrative review. Front. Neurol. 2024, 14, 1327383. [Google Scholar] [CrossRef]
- Zrenner, C.; Ziemann, U. Closed-Loop Brain Stimulation. Biol. Psychiatry 2024, 95, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Lonergan, B.; Seemungal, B.M.; Ciocca, M.; Tai, Y.F. The Effects of Deep Brain Stimulation on Balance in Parkinson’s Disease as Measured Using Posturography—A Narrative Review. Brain Sci. 2025, 15, 535. [Google Scholar] [CrossRef]
- Bonanno, M.; De Pasquale, P.; Lombardo Facciale, A.; Dauccio, B.; De Luca, R.; Quartarone, A.; Calabrò, R.S. May Patients with Chronic Stroke Benefit from Robotic Gait Training with an End-Effector? A Case-Control Study. J. Funct. Morphol. Kinesiol. 2025, 10, 161. [Google Scholar] [CrossRef]
- Gut, P.; Lizzo, G.; Migliavacca, E.; Karagounis, L.; Heise, T.; Eynatten, M. Effects of glycine and n-acetylcysteine on glutathione levels and mitochondrial energy metabolism in healthy aging. Innov. Aging 2021, 5 (Suppl. 1), 685. [Google Scholar] [CrossRef]
- Meng, Q.; Su, C.-H. The Impact of Physical Exercise on Oxidative and Nitrosative Stress: Balancing the Benefits and Risks. Antioxidants 2024, 13, 573. [Google Scholar] [CrossRef]
- Bastian, L.; Samanta, A.; Ribeiro de Paula, D.; Weber, F.D.; Schoenfeld, R.; Dresler, M.; Genzel, L. Spindle–slow oscillation coupling correlates with memory performance and connectivity changes in a hippocampal network after sleep. Hum. Brain Mapp. 2022, 43, 3923–3943. [Google Scholar] [CrossRef] [PubMed]
- Goyal, M.; McDonough, R.; Fisher, M.; Ospel, J. The Challenge of Designing Stroke Trials That Change Practice: MCID vs. Sample Size and Pragmatism. J. Stroke 2022, 24, 49–56. [Google Scholar] [CrossRef]
- Kowal, J.; Arras, G.; Colombo, M.; Jouve, M.; Morath, J.P.; Primdal-Bengtson, B.; Dingli, F.; Loew, D.; Tkach, M.; Théry, C. Proteomic comparison defines novel markers to characterize heterogeneous populations of extracellular vesicle subtypes. Proc. Natl. Acad. Sci. USA 2016, 113, E968–E977. [Google Scholar] [CrossRef]
- Zang, Y.; Lee, J.J. Adaptive clinical trial designs in oncology. Chin. Clin. Oncol. 2014, 3, 49. [Google Scholar] [CrossRef] [PubMed]
- Golenia, A.; Olejnik, P. The Role of Oxidative Stress in Ischaemic Stroke and the Influence of Gut Microbiota. Antioxidants 2025, 14, 542. [Google Scholar] [CrossRef]
- Lee, J.; Kim, H.; Kim, J.; Chang, W.H.; Kim, Y.-H. Multimodal Imaging Biomarker-Based Model Using Stratification Strategies for Predicting Upper Extremity Motor Recovery in Severe Stroke Patients. Neurorehabil. Neural Repair 2022, 36, 217–226. [Google Scholar] [CrossRef]
- Kamal, F.Z.; Lefter, R.; Jaber, H.; Balmus, I.-M.; Ciobica, A.; Iordache, A.-C. The Role of Potential Oxidative Biomarkers in the Prognosis of Acute Ischemic Stroke and the Exploration of Antioxidants as Possible Preventive and Treatment Options. Int. J. Mol. Sci. 2023, 24, 6389. [Google Scholar] [CrossRef] [PubMed]
- Afzal, S.; Abdul Manap, A.S.; Attiq, A.; Albokhadaim, I.; Kandeel, M.; Alhojaily, S.M. From imbalance to impairment: The central role of reactive oxygen species in oxidative stress-induced disorders and therapeutic exploration. Front. Pharmacol. 2023, 14, 1269581. [Google Scholar] [CrossRef] [PubMed]
- Menezes Junior, A.d.S.; França-e-Silva, A.L.G.d.; Oliveira, H.L.d.; Lima, K.B.A.d.; Porto, I.d.O.P.; Pedroso, T.M.A.; Silva, D.d.M.e.; Freitas, A.F., Jr. Genetic Mutations and Mitochondrial Redox Signaling as Modulating Factors in Hypertrophic Cardiomyopathy: A Scoping Review. Int. J. Mol. Sci. 2024, 25, 5855. [Google Scholar] [CrossRef]
- Stewart, N.J.; Sato, T.; Takeda, N.; Hirata, H.; Matsumoto, S. Hyperpolarized 13C Magnetic Resonance Imaging as a Tool for Imaging Tissue Redox State, Oxidative Stress, Inflammation, and Cellular Metabolism. Antioxid. Redox Signal. 2022, 36, 81–94. [Google Scholar] [CrossRef]
- Korac, B.; Kalezic, A.; Pekovic-Vaughan, V.; Korac, A.; Jankovic, A. Redox changes in obesity, metabolic syndrome, and diabetes. Redox Biol. 2021, 42, 101887. [Google Scholar] [CrossRef] [PubMed]
- Moradi Kashkooli, F.; Bhandari, A.; Gu, B.; Kolios, M.C.; Kohandel, M.; Zhan, W. Multiphysics modelling enhanced by imaging and artificial intelligence for personalised cancer nanomedicine: Foundations for clinical digital twins. J. Control. Release 2025, 386, 114138. [Google Scholar] [CrossRef]
- Lee, S.-S.; Yoo, Y.-C. NOX-NOS crosstalk in the liver-brain axis: Novel insights for redox regulation and neurodegenerative diseases. Redox Biol. 2025, 86, 103807. [Google Scholar] [CrossRef]
- Cocco, C.; Siotto, M.; Guerrini, A.; Germanotta, M.; Galluccio, C.; Cipollini, V.; Cortellini, L.; Pavan, A.; Lattanzi, S.; Insalaco, S.; et al. Systemic Oxidative Stress in Subacute Stroke Patients Undergoing Rehabilitation Treatment. Antioxidants 2024, 13, 354. [Google Scholar] [CrossRef]
- Cognetti, J.S.; Miller, B.L. Real-Time, Continuous Monitoring of Tissue Chips as an Emerging Opportunity for Biosensing. Sensors 2025, 25, 5153. [Google Scholar] [CrossRef]
- Malik, A.; Kundur, S.P.; Sivalokanathan, S. Hyperpolarized-MRI in Hypertrophic Cardiomyopathy: A Narrative Review. Clin. Med. Insights Cardiol. 2025, 19, 11795468251369234. [Google Scholar] [CrossRef]
- Wang, B.; Hu, S.; Teng, Y.; Chen, J.; Wang, H.; Xu, Y.; Wang, K.; Xu, J.; Cheng, Y.; Gao, X. Current advance of nanotechnology in diagnosis and treatment for malignant tumors. Signal Transduct. Target. Ther. 2024, 9, 200. [Google Scholar] [CrossRef]
- Rao, N.L.; Kotian, G.B.; Shetty, J.K.; Shelley, B.P.; Dmello, M.K.; Lobo, E.C.; Shankar, S.P.; Almeida, S.D.; Shah, S.R. Receptor for Advanced Glycation End Product, Organ Crosstalk, and Pathomechanism Targets for Comprehensive Molecular Therapeutics in Diabetic Ischemic Stroke. Biomolecules 2022, 12, 1712. [Google Scholar] [CrossRef]
- Dobrovinskaya, O.; Alamilla, J.; Olivas-Aguirre, M. Impact of Modern Lifestyle on Circadian Health and Its Contribution to Adipogenesis and Cancer Risk. Cancers 2024, 16, 3706. [Google Scholar] [CrossRef]
- Lu, X.; Xie, Q.; Pan, X.; Zhang, R.; Zhang, X.; Peng, G.; Zhang, Y.; Shen, S.; Tong, N. Type 2 diabetes mellitus in adults: Pathogenesis, prevention and therapy. Signal Transduct. Target. Ther. 2024, 9, 262. [Google Scholar] [CrossRef]
- Singh, C.K.; Chhabra, G.; Ndiaye, M.A.; Garcia-Peterson, L.M.; Mack, N.J.; Ahmad, N. The Role of Sirtuins in Antioxidant and Redox Signaling. Antioxid. Redox Signal. 2018, 28, 643–661. [Google Scholar] [CrossRef] [PubMed]
- Carollo, C.; Sorce, A.; Cirafici, E.; Mulè, G.; Caimi, G. Sirtuins and Resveratrol in Cardiorenal Diseases: A Narrative Review of Mechanisms and Therapeutic Potential. Nutrients 2025, 17, 1212. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Wu, Q.; Wang, H.; Gao, X.; Xu, R.; Cui, Z.; Zhu, J.; Zeng, X.; Zhou, H.; He, Y.; et al. Dysbiosis of Gut Microbiota and Short-Chain Fatty Acids in Acute Ischemic Stroke and the Subsequent Risk for Poor Functional Outcomes. J. Parenter. Enter. Nutr. 2021, 45, 518–529. [Google Scholar] [CrossRef] [PubMed]
- Rode, J.; Yang, L.; König, J.; Hutchinson, A.N.; Wall, R.; Venizelos, N.; Brummer, R.-J.; Rangel, I.; Vumma, R. Butyrate Rescues Oxidative Stress-Induced Transport Deficits of Tryptophan: Potential Implication in Affective or Gut-Brain Axis Disorders. Neuropsychobiology 2021, 80, 253–263. [Google Scholar] [CrossRef]
- Mousavinasab, F.; Karimi, R.; Taheri, S.; Ahmadvand, F.; Sanaaee, S.; Najafi, S.; Halvaii, M.S.; Haghgoo, A.; Zamany, M.; Majidpoor, J.; et al. Microbiome modulation in inflammatory diseases: Progress to microbiome genetic engineering. Cancer Cell Int. 2023, 23, 271. [Google Scholar] [CrossRef]
- Averina, O.V.; Poluektova, E.U.; Marsova, M.V.; Danilenko, V.N. Biomarkers and Utility of the Antioxidant Potential of Probiotic Lactobacilli and Bifidobacteria as Representatives of the Human Gut Microbiota. Biomedicines 2021, 9, 1340. [Google Scholar] [CrossRef] [PubMed]
- Mou, D.; Ding, D.; Pu, J.; Zhou, P.; Cao, E.; Zhang, X.; Lan, J.; Ye, L.; Wen, W. Effects of Dietary Pretreatment with All-trans Lycopene on Lipopolysaccharide-Induced Jejunal Inflammation: A Multi-Pathway Phenomenon. Foods 2025, 14, 794. [Google Scholar] [CrossRef]
- Abdol Samat, H.N.; Razali, N.N.; Mahadzir, H.; Tengku Muhammad, T.S.; Ling, K.-H.; Mansor, N.I.; Abidin, S.Z. The Interplay of Inflammation and Gut-Microbiota Dysbiosis in Alzheimer’s Disease: Mechanisms and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 8905. [Google Scholar] [CrossRef] [PubMed]
- Conrad, T.; Altmüller, J. Single cell- and spatial ’Omics revolutionize physiology. Acta Physiol. 2022, 235, e13848. [Google Scholar] [CrossRef]
- Schumann, U.; Liu, L.; Aggio-Bruce, R.; Cioanca, A.V.; Shariev, A.; Madigan, M.C.; Valter, K.; Wen, J.; Natoli, R. Spatial transcriptomics reveals regionally altered gene expression that drives retinal degeneration. Commun. Biol. 2025, 8, 629, Erratum in Commun. Biol. 2025, 8, 1013. https://doi.org/10.1038/s42003-025-08451-8. [Google Scholar] [CrossRef] [PubMed]
- Martinotti, S.; Ranzato, E. Targeting the Unfolded Protein Response with Natural Products: Therapeutic Potential in ER Stress-Related Diseases. Int. J. Mol. Sci. 2025, 26, 8814. [Google Scholar] [CrossRef]
- Brezic, N.; Gligorevic, S.; Sic, A.; Knezevic, N.N. Protein Misfolding and Aggregation as a Mechanistic Link Between Chronic Pain and Neurodegenerative Diseases. Curr. Issues Mol. Biol. 2025, 47, 259. [Google Scholar] [CrossRef]
- Jurcau, M.C.; Jurcau, A.; Cristian, A.; Hogea, V.O.; Diaconu, R.G.; Nunkoo, V.S. Inflammaging and Brain Aging. Int. J. Mol. Sci. 2024, 25, 10535. [Google Scholar] [CrossRef]
- Chini, C.C.S.; Cordeiro, H.S.; Tran, N.L.K.; Chini, E.N. NAD metabolism: Role in senescence regulation and aging. Aging Cell 2023, 23, e13920. [Google Scholar] [CrossRef]
- Krings, T.; Takemoto, Y.; Mori, K.; Kee, T.P. The Glymphatic System and Its Role in Neurovascular Diseases. J. Neuroendovascular Ther. 2025, 19, ra.2025-0020. [Google Scholar] [CrossRef]
- Yang, H.-M. Vascular Dementia: From Pathophysiology to Therapeutic Frontiers. J. Clin. Med. 2025, 14, 6611. [Google Scholar] [CrossRef]
- Kelley, N.; Jeltema, D.; Duan, Y.; He, Y. The NLRP3 Inflammasome: An Overview of Mechanisms of Activation and Regulation. Int. J. Mol. Sci. 2019, 20, 3328. [Google Scholar] [CrossRef] [PubMed]
- Emmert-Streib, F.; Cherifi, H.; Kaski, K.; Kauffman, S.; Yli-Harja, O. Complexity data science: A spin-off from digital twins. PNAS Nexus 2024, 3, pgae456. [Google Scholar] [CrossRef]
- Liu, D.; Chen, H.; Wang, J.; Wang, Y. MultiGNN: A Graph Neural Network Framework for Inferring Gene Regulatory Networks from Single-Cell Multi-Omics Data. Computation 2025, 13, 124. [Google Scholar] [CrossRef]
- Nuñez-Selles, A.J.; Nuñez-Musa, R.A.; Guillen-Marmolejos, R.A. Linking oxidative stress biomarkers to disease progression and antioxidant therapy in hypertension and diabetes mellitus. Front. Mol. Biosci. 2025, 12, 1611842. [Google Scholar] [CrossRef] [PubMed]
- Revete, A.; Aparicio, A.; Cisterna, B.A.; Revete, J.; Luis, L.; Ibarra, E.; Segura González, E.A.; Molino, J.; Reginensi, D. Advancements in the Use of Hydrogels for Regenerative Medicine: Properties and Biomedical Applications. Int. J. Biomater. 2022, 2022, 3606765. [Google Scholar] [CrossRef]
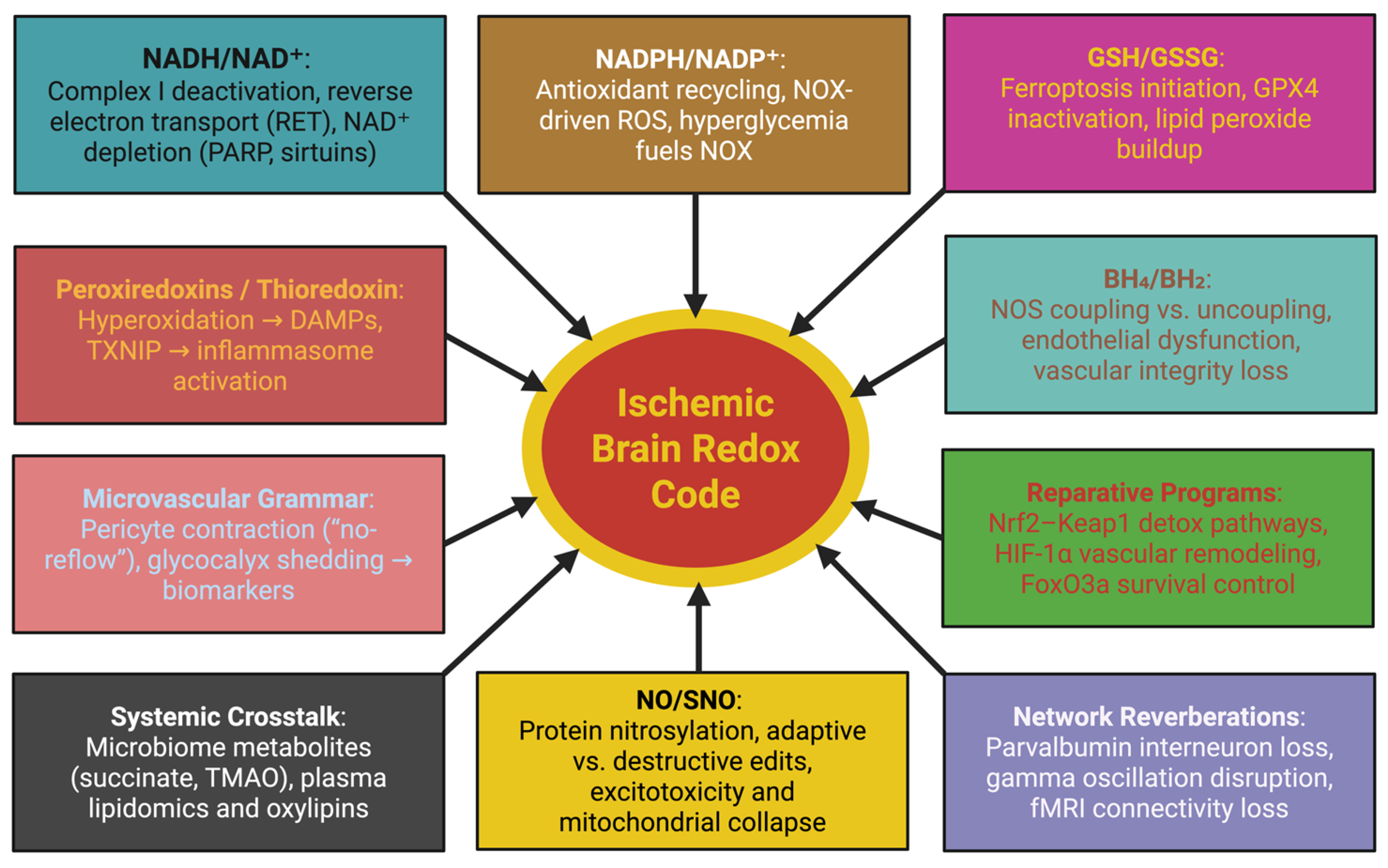
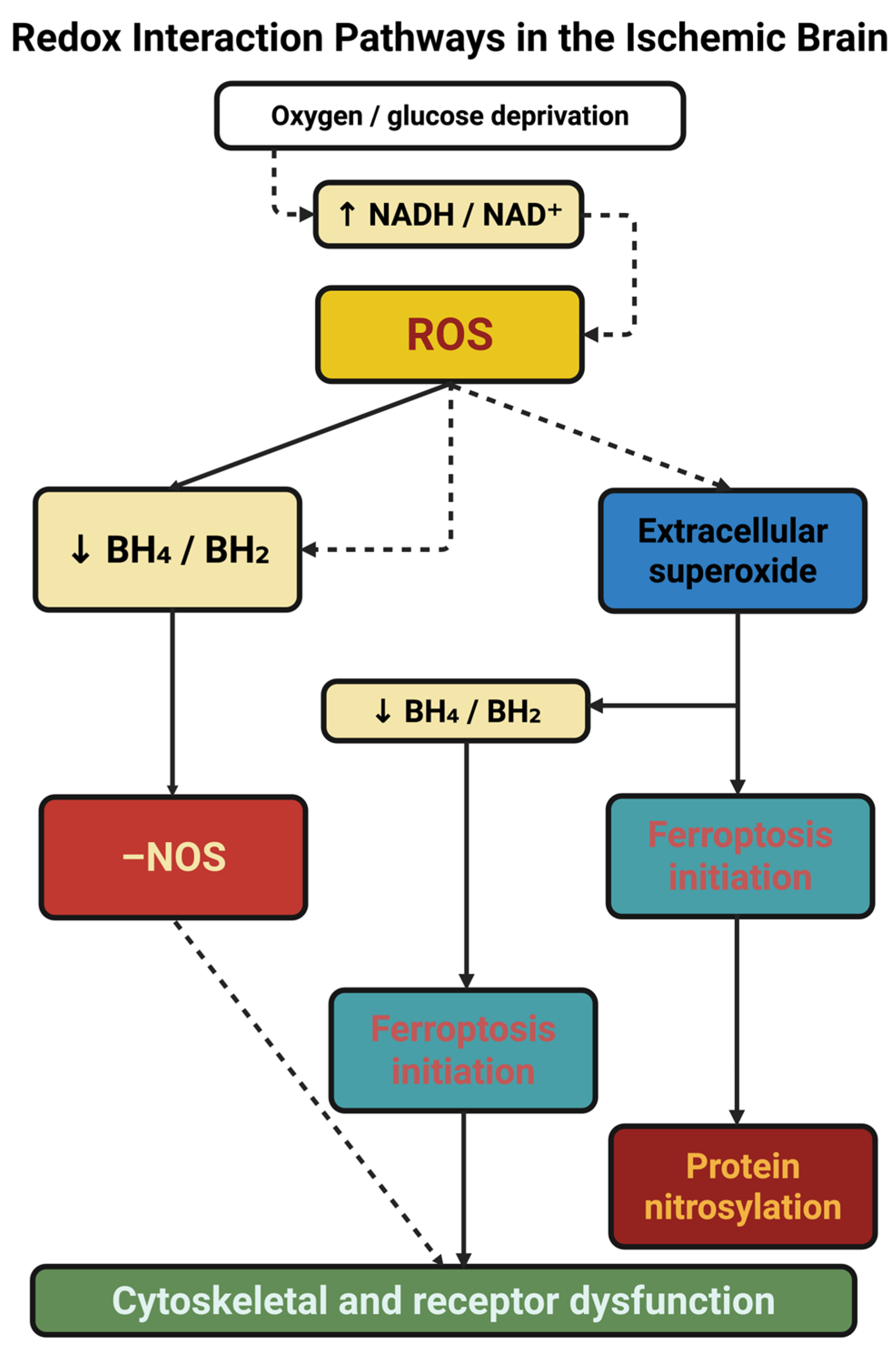
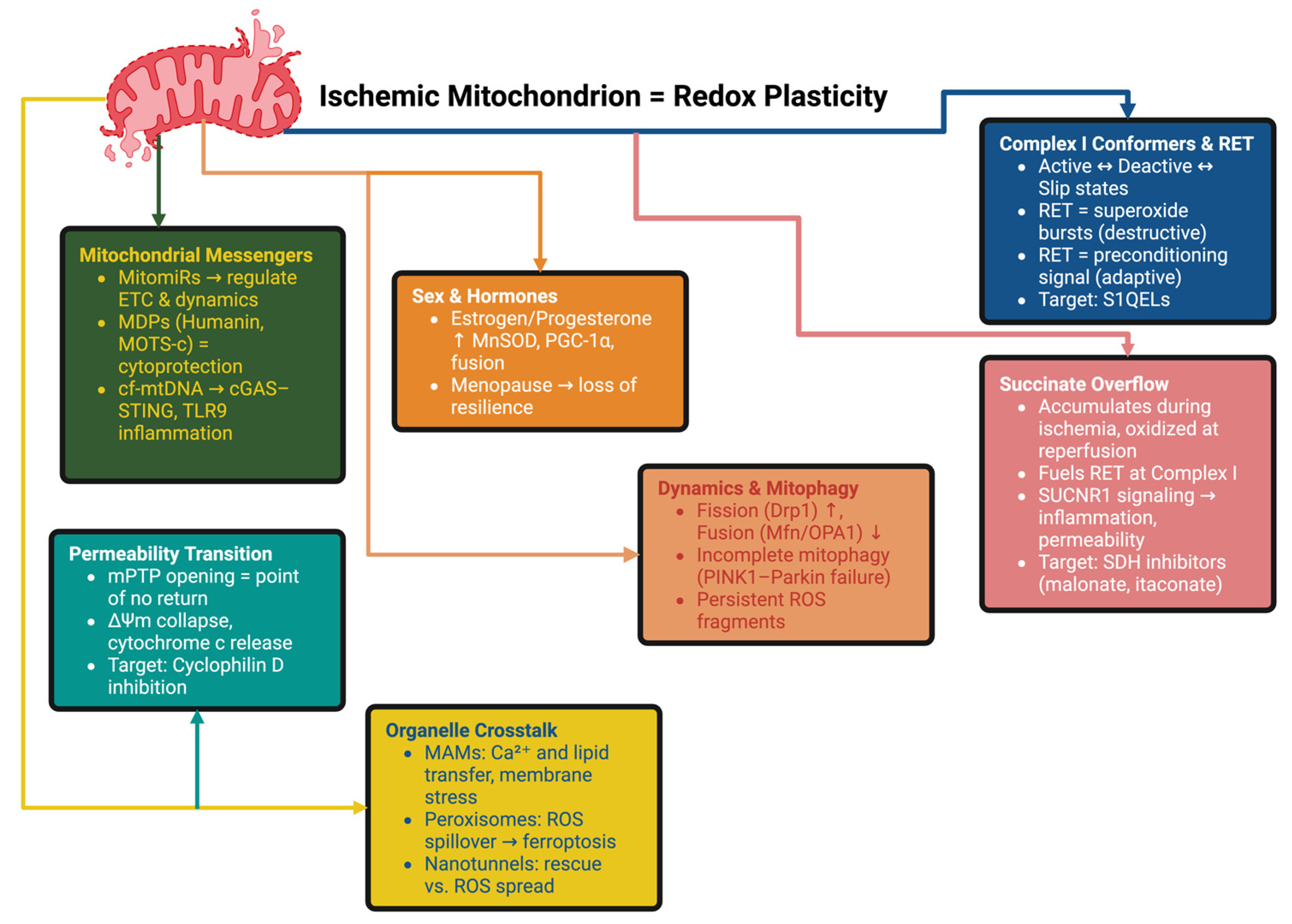
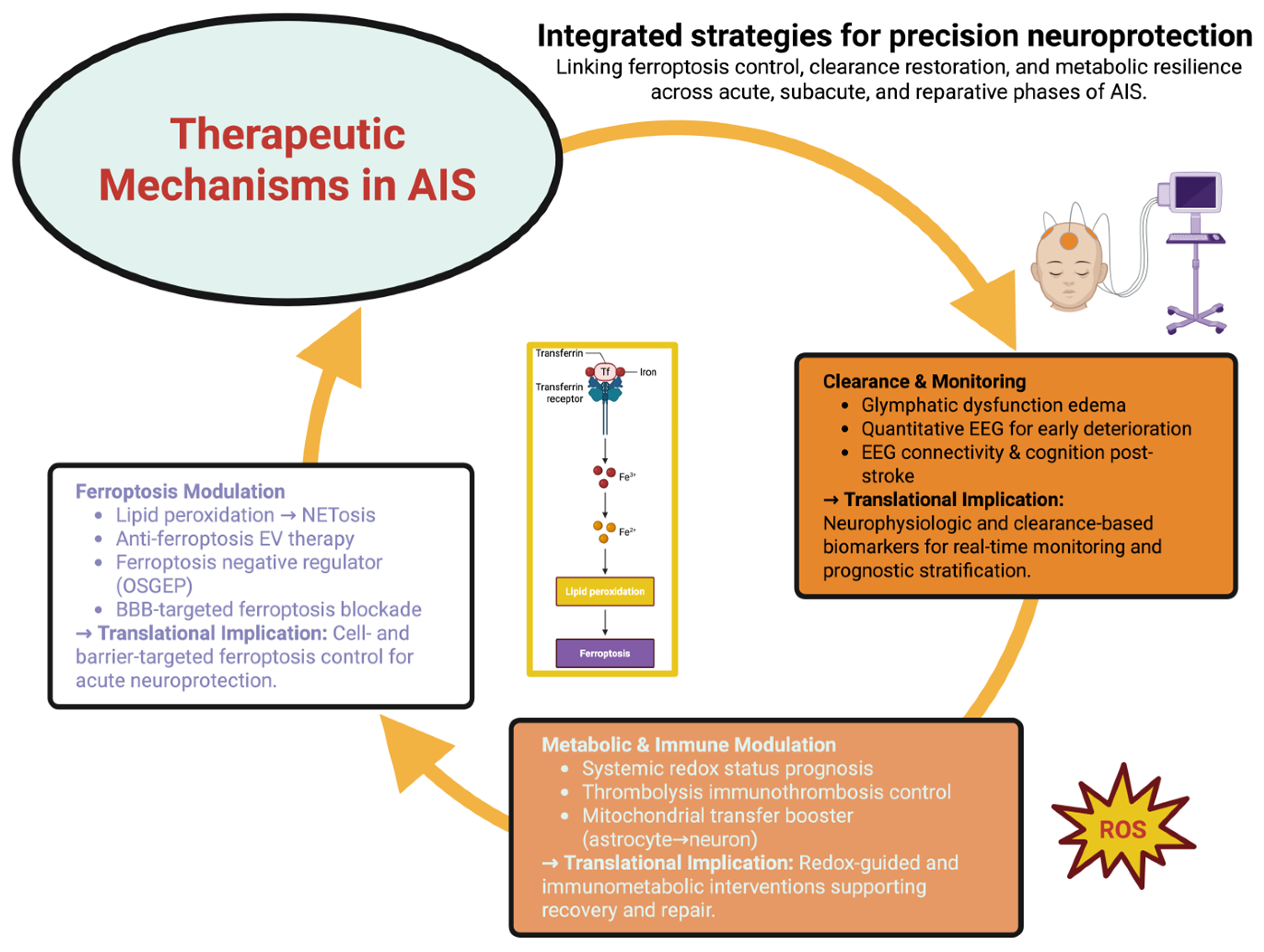
| Mechanism/Strategy | References | Models/Methods | Key Findings | Translational Implications |
|---|---|---|---|---|
| Lipid peroxidation → NETosis | [130] | Human neutrophils + platelets; in vivo validation | Iron accumulation and lipid peroxides trigger NETosis; small-molecule LP inhibitors suppress NETs | Platelets accelerate LP in neutrophils to induce pathogenic NETs; LP inhibition may serve as an anti-NET adjunct in AIS (acute) |
| Anti-ferroptosis EV therapy | [131] | MCAO mice | Engineered exosomes targeting M2 microglia prevent ferroptosis (↑GPX4, ↓lipid-ROS), reducing infarct volume | Cell-type-specific nanotherapies to preserve reparative microglia (acute–subacute) |
| Ferroptosis negative regulator (OSGEP) | [132] | tMCAO mice; OSGEP gain/loss; intranasal Fer-1/deferoxamine | OSGEP restrains ferroptosis; intranasal Fer-1 is immediately protective | OSGEP as targetable ferroptosis node; intranasal ferroptosis blockade in hyperacute AIS |
| BBB-targeted ferroptosis blockade | [133] | MCAO rodents; BBB-targeted lipid nanoparticles | Targeted LNPs deliver Fer-1 efficiently, enhancing neuroprotection vs. free drug | BBB-targeting makes ferroptosis inhibitors feasible for AIS therapy |
| Glymphatic dysfunction edema | [134] | MCAO mice + AIS patients (EVT); tracer assays; DTI-ALPS | Impaired glymphatic clearance contributes to brain edema; partial recovery post-recanalization | DTI-ALPS as biomarker of edema/clearance in AIS trials (hyperacute–subacute) |
| Quantitative EEG for early deterioration | [135] | LVO-AIS patient cohort; qEEG indices | qEEG detects early neurological deterioration beyond standard monitoring | Bedside electrophysiology to triage neuroprotectives/ICU resources (first 24–48 h) |
| EEG connectivity & cognition post-stroke | [136] | 32 post-stroke participants (graded CI); gamma-band PSD; connectivity maps | EEG biomarkers classify cognitive impairment; gamma-band changes are discriminative | Noninvasive biomarker to pair with redox-directed rehab (subacute) |
| Systemic redox status prognosis | [137] | AIS patient cohort; serum GSH & MDA | GSH/MDA ratio predicts early post-stroke outcomes | Peripheral redox markers to enrich and stratify neuroprotection trials |
| Thrombolysis immunothrombosis control | [138] | AIS patients (proteomics, validation cohort); photothrombosis mice | tPA raises HRG; HRG dampens NETosis and reduces hemorrhagic transformation risk | HRG as biomarker/adjunct to extend safer thrombolysis windows |
| Small-molecule booster of mitochondrial transfer | [139] | OGD/R models; MCAO mice; chrysophanol treatment | Chrysophanol accelerates astrocyte→neuron mitochondrial transfer, improving neuronal survival | Tool compound suggesting druggable mito-donation pathways (hours–days) |
| Therapeutic Strategy | Mechanistic Target | Mode of Action | Preclinical/Clinical Evidence | Translational Potential | Limitations/Open Questions | References |
|---|---|---|---|---|---|---|
| Ferroptosis inhibitors (Fer-1, Liproxstatin-1, Deferoxamine, OSGEP upregulation) | Iron-driven lipid peroxidation, GPX4/GSH depletion | Block lipid ROS accumulation; restore GPX4 axis | Intranasal Fer-1 protects MCAO mice; OSGEP overexpression suppresses ferroptosis; BBB-targeted LNP-Fer-1 improves outcomes | First-in-class neuroprotective adjunct to reperfusion | BBB delivery challenges, optimal timing, systemic iron interactions | [166] |
| Mitochondrial transfer enhancers (Chrysophanol, LRP1–ARF1 lactylation axis modulators) | Neuronal bioenergetic collapse | Promote astrocyte-to-neuron mitochondrial donation | Chrysophanol accelerates mitochondrial transfer in MCAO; LRP1 knockdown ↑ transfer, ↓ injury | Metabolic rescue in penumbra; druggable axis for precision therapy | Integration with existing therapies, long-term safety, dosing optimization | [139,167] |
| Nanoparticle-based delivery (BBB-targeted LNPs, ROS-responsive nanocarriers) | ROS surge, antioxidant instability | Encapsulate antioxidants (Fer-1, edaravone, melatonin) for controlled lesion-site release | BBB-targeted Fer-1 LNPs outperform free Fer-1 in rodents; ROS-cleavable carriers validated in I/R | Overcome pharmacokinetic failure of antioxidants | Manufacturing complexity, immune clearance, scalability | [168,169] |
| Exosome therapies (engineered EVs targeting M2 microglia) | Microglial ferroptosis, immune-redox imbalance | Deliver miRNAs/GPX4 stabilizers to preserve M2 phenotype | Engineered exosomes prevent M2 ferroptosis, ↓ lipid ROS, ↓ infarct size | Cell-specific nanotherapy with natural biocompatibility | Standardization, off-target effects, GMP manufacturing hurdles | [131,170] |
| Immune-redox modulators (IL-1β blockade, CCR2/5 inhibitors, HRG augmentation) | Maladaptive immune memory, NETosis, BBB injury | Block IL-1β to prevent trained immunity; HRG suppresses tPA-induced NETosis | IL-1 blockade prevents cardiac fibrosis in post-stroke mice; HRG reduces hemorrhagic transformation in AIS patients treated with tPA | Extend thrombolysis safety, systemic protection | Risk of immunosuppression, patient stratification, timing window | [171,172,173] |
| Antioxidant cocktails with metabolic coupling (Lactate + NAD+ boosters, CoQ10 analogs) | Redox–metabolic axis disruption | Buffer ROS while supporting ATP/lactate metabolism | NAD+ precursors synergize with lactate shuttle in MCAO models | Personalized metabolic–redox therapy | Human dosing strategies, feasibility of combination regimens | [174] |
| Neurotechnology-based redox control (Vagus nerve stimulation, tDCS) | Excitotoxic–oxidative interplay | Modulate neuronal excitability to rebalance ROS | VNS reduces oxidative markers, improves plasticity in rodents; pilot tDCS data emerging | Noninvasive, scalable adjunct | Need for biomarker guidance, mechanistic precision | [175,176] |
| Systemic interventions (Microbiome modulation, exercise-induced ROS hormesis, caloric restriction mimetics) | Systemic oxidative background, gut–brain–redox axis | Modify microbiota; induce adaptive ROS hormesis | Exercise and microbiome-targeting shift redox tone in stroke-prone rodents; early human pilot data (2024) | Accessible, low-cost adjunct for prevention and recovery | Interindividual variability, standardization across populations | [177,178] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Diaconescu, I.B.; Dumitru, A.V.; Tataru, C.P.; Toader, C.; Șerban, M.; Covache-Busuioc, R.-A.; Eva, L. From Electron Imbalance to Network Collapse: Decoding the Redox Code of Ischemic Stroke for Biomarker-Guided Precision Neuroprotection. Int. J. Mol. Sci. 2025, 26, 10835. https://doi.org/10.3390/ijms262210835
Diaconescu IB, Dumitru AV, Tataru CP, Toader C, Șerban M, Covache-Busuioc R-A, Eva L. From Electron Imbalance to Network Collapse: Decoding the Redox Code of Ischemic Stroke for Biomarker-Guided Precision Neuroprotection. International Journal of Molecular Sciences. 2025; 26(22):10835. https://doi.org/10.3390/ijms262210835
Chicago/Turabian StyleDiaconescu, Ionut Bogdan, Adrian Vasile Dumitru, Calin Petru Tataru, Corneliu Toader, Matei Șerban, Răzvan-Adrian Covache-Busuioc, and Lucian Eva. 2025. "From Electron Imbalance to Network Collapse: Decoding the Redox Code of Ischemic Stroke for Biomarker-Guided Precision Neuroprotection" International Journal of Molecular Sciences 26, no. 22: 10835. https://doi.org/10.3390/ijms262210835
APA StyleDiaconescu, I. B., Dumitru, A. V., Tataru, C. P., Toader, C., Șerban, M., Covache-Busuioc, R.-A., & Eva, L. (2025). From Electron Imbalance to Network Collapse: Decoding the Redox Code of Ischemic Stroke for Biomarker-Guided Precision Neuroprotection. International Journal of Molecular Sciences, 26(22), 10835. https://doi.org/10.3390/ijms262210835





