Molecular Network Analysis of Circulating microRNAs Highlights miR-17-5p and miR-29a-3p as Potential Biomarkers of Aortic Valve Calcification
Abstract
1. Introduction
2. Results
2.1. MiRNA Expression Profiling in Calcified Aortic Valves and Patients Sera
2.2. Shared miRNAs Between Valve Tissue and Serum
2.3. Pathway Enrichment Analysis of Shared Differentially Expressed miRNAs
| Term Name | Term Genes | miRNAs (n) | miRNA Names | Merged p-Value | Merged FDR |
|---|---|---|---|---|---|
| Signaling by TGFB family members | 104 | 11 | hsa-miR-17-5p hsa-miR-20a-5p hsa-miR-22-3p hsa-miR-24-3p hsa-miR-25-3p hsa-miR-26a-5p hsa-miR-26b-5p hsa-miR-27a-3p hsa-miR-92a-3p hsa-miR-125b-5p hsa-miR-126b-5p | 7.5 × 10−61 | 4.89 × 10−59 |
| Signaling by WNT | 366 | 7 | hsa-miR-17-5p hsa-miR-20a-5p hsa-miR-22-3p hsa-miR-24-3p hsa-miR-29a-3p hsa-miR-92a-3p hsa-miR-125b-5p | 1.68 × 10−20 | 1.68 × 10−18 |
| PI3K-Akt signaling pathway | 372 | 10 | hsa-miR-17-5p hsa-miR-20a-5p hsa-miR-22-3p hsa-miR-24-3p hsa-miR-25-3p hsa-miR-26a-5p hsa-miR-26b-5p hsa-miR-27a-3p hsa-miR-29a-3p hsa-miR-145-5p | 1.59 × 10−29 | 1.06 × 10−28 |
| Signaling pathway regulating pluripotency of Stem Cells | 156 | 10 | hsa-miR-17-5p hsa-miR-22-3p hsa-miR-24-3p hsa-miR-26a-5p hsa-miR-26b-5p hsa-miR-27a-3p hsa-miR-29a-3p hsa-miR-92a-3p hsa-miR-125b-5p hsa-miR-106b-5p | 7.16 × 10−27 | 4.08 × 10−26 |
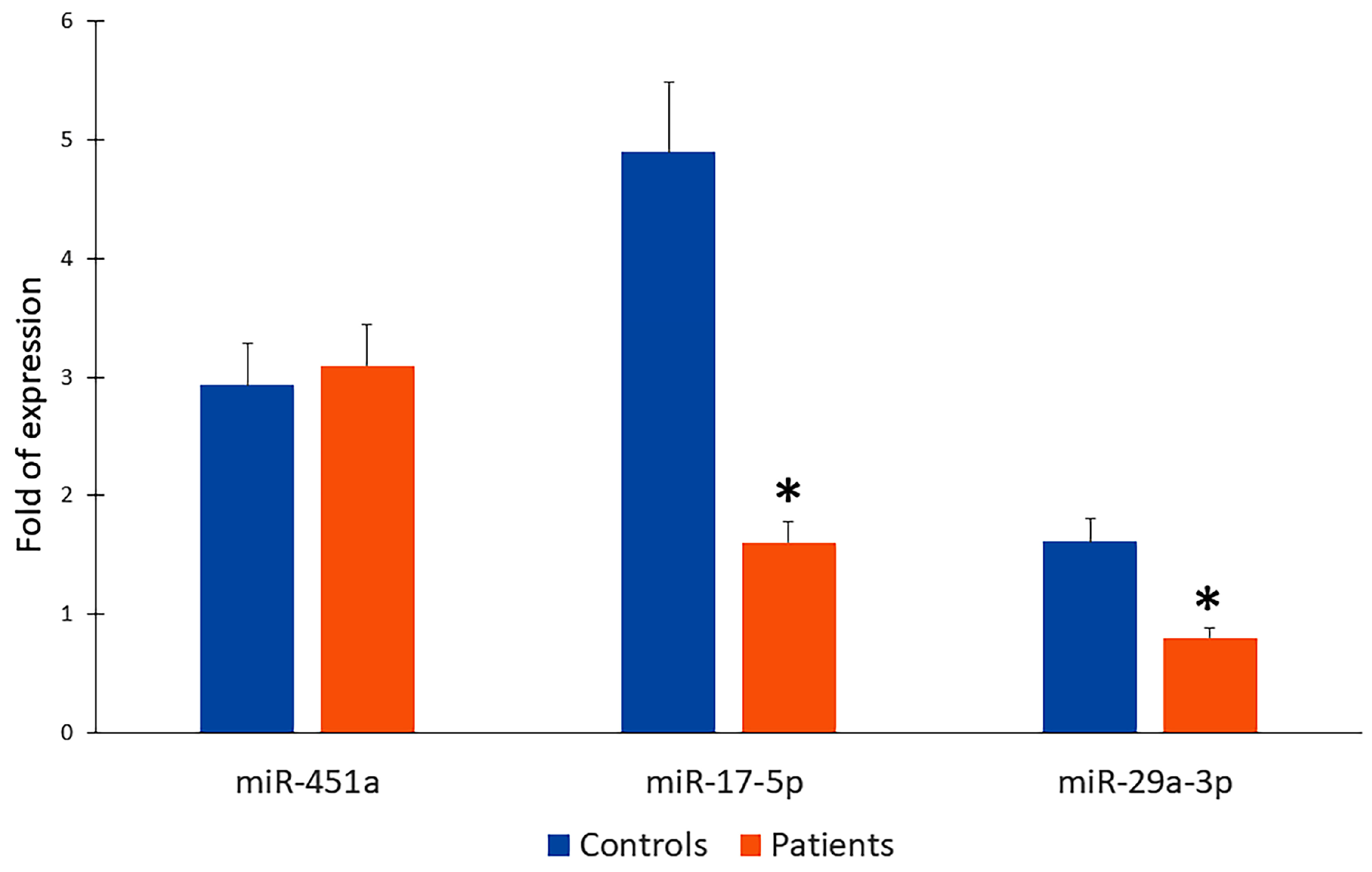
2.4. Downregulation of miR-17-5p and miT-29a-3p in Patient Serum as Potential Circulating Biomarkers for Detection of Valve Calcification
3. Discussion
4. Materials and Methods
4.1. Aortic Valves and Sera
4.2. Tissue Processing and Homogenization
4.3. miRNA Extraction and Quantification
4.4. TaqMan Gene Expression Array
4.5. Gene Expression Analysis/ Real Time-PCR
4.6. Pathway Enrichment Analysis
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BAV | Bicuspid Aortic Valve |
| CAVD | Calcific Aortic Valve Disease |
| DOACs | Direct Oral Anticoagulants |
| e-GRF | Estimated Glomerular Filtration Rate |
| GO | Gene Ontology |
| PCR | Polymerase Chain Reaction |
| miRNA | Micro RNA |
| qRT-PCR | Quantitative Real-Time Polymerase Chain Reaction |
| SAVR | Surgical Aortic Valve Replacement |
| VEGFA | Vascular Endothelial Growth Factor A |
| VSMC | Vascular Smooth Muscular Cell |
References
- Moncla, L.M.; Briend, M.; Bossé, Y.; Mathieu, P. Calcific aortic valve disease: Mechanisms, prevention and treatment. Nat. Rev. Cardiol. 2023, 20, 546–559. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Malashicheva, A.; Sullivan, G.; Bogdanova, M.; Kostareva, A.; Stensløkken, K.O.; Fiane, A.; Vaage, J. Valve Interstitial Cells: The Key to Understanding the Pathophysiology of Heart Valve Calcification. J. Am. Heart Assoc. 2017, 6, e006339. [Google Scholar] [CrossRef] [PubMed]
- Galeone, A.; Paparella, D.; Colucci, S.; Grano, M.; Brunetti, G. The role of TNF-α and TNF superfamily members in the pathogenesis of calcific aortic valvular disease. Sci. World J. 2013, 2013, 875363. [Google Scholar] [CrossRef] [PubMed]
- Di Vito, A.; Donato, A.; Presta, I.; Mancuso, T.; Brunetti, F.S.; Mastroroberto, P.; Amorosi, A.; Malara, N.; Donato, G. Extracellular Matrix in Calcific Aortic Valve Disease: Architecture, Dynamic and Perspectives. Int. J. Mol. Sci. 2021, 22, 913. [Google Scholar] [CrossRef] [PubMed]
- Galeone, A.; Brunetti, G.; Oranger, A.; Greco, G.; Di Benedetto, A.; Mori, G.; Colucci, S.; Zallone, A.; Paparella, D.; Grano, M. Aortic valvular interstitial cells apoptosis and calcification are mediated by TNF-related apoptosis-inducing ligand. Int. J. Cardiol. 2013, 169, 296–304. [Google Scholar] [CrossRef]
- Abbas, M.; Gaye, A. Emerging roles of noncoding RNAs in cardiovascular pathophysiology. Am. J. Physiol. Heart Circ. Physiol. 2025, 328, H603–H621. [Google Scholar] [CrossRef]
- Goettsch, C.; Hutcheson, J.D.; Aikawa, E. MicroRNA in cardiovascular calcification: Focus on targets and extracellular vesicle delivery mechanisms. Circ. Res. 2013, 112, 1073–1084, Erratum in Circ. Res. 2013, 113, e106. [Google Scholar] [CrossRef]
- Sivan, S.; Vijayakumar, G.; Pillai, I.C. Non-coding RNAs mediating the regulation of genes and signaling pathways in aortic valve calcification. Gene 2025, 936, 149117. [Google Scholar] [CrossRef]
- Tijsen, A.J.; Pinto, Y.M.; Creemers, E.E. Circulating microRNAs as diagnostic biomarkers for cardiovascular diseases. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H1085–H1095. [Google Scholar] [CrossRef]
- Oury, C.; Servais, L.; Bouznad, N.; Hego, A.; Nchimi, A.; Lancellotti, P. MicroRNAs in Valvular Heart Diseases: Potential Role as Markers and Actors of Valvular and Cardiac Remodeling. Int. J. Mol. Sci. 2016, 17, 1120. [Google Scholar] [CrossRef]
- Chen, J.; Simmons, C.A. Cell-matrix interactions in the pathobiology of calcific aortic valve disease: Critical roles for matricellular, matricrine, and matrix mechanics cues. Circ. Res. 2011, 108, 1510–1524. [Google Scholar] [CrossRef] [PubMed]
- Clark-Greuel, J.N.; Connolly, J.M.; Sorichillo, E.; Narula, N.R.; Rapoport, H.S.; Mohler, E.R., 3rd; Gorman, J.H., 3rd; Gorman, R.C.; Levy, R.J. Transforming growth factor-beta1 mechanisms in aortic valve calcification: Increased alkaline phosphatase and related events. Ann. Thorac. Surg. 2007, 83, 946–953. [Google Scholar] [CrossRef]
- Albanese, I.; Yu, B.; Al-Kindi, H.; Barratt, B.; Ott, L.; Al-Refai, M.; de Varennes, B.; Shum-Tim, D.; Cerruti, M.; Gourgas, O.; et al. Role of Noncanonical Wnt Signaling Pathway in Human Aortic Valve Calcification. Arterioscler. Thromb. Vasc. Biol. 2017, 37, 543–552. [Google Scholar] [CrossRef]
- Ghafouri-Fard, S.; Khanbabapour Sasi, A.; Hussen, B.M.; Shoorei, H.; Siddiq, A.; Taheri, M.; Ayatollahi, S.A. Interplay between PI3K/AKT pathway and heart disorders. Mol. Biol. Rep. 2022, 49, 9767–9781. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xiao, X.; Zhou, T.; Han, D.; Dong, N. Novel mechanisms for osteogenic differentiation of human aortic valve interstitial cells. J. Thorac. Cardiovasc. Surg. 2020, 159, 1742–1753.e7. [Google Scholar] [CrossRef]
- Huang, Y.; Xu, K.; Zhou, T.; Zhu, P.; Dong, N.; Shi, J. Comparison of Rapidly Proliferating, Multipotent Aortic Valve-Derived Stromal Cells and Valve Interstitial Cells in the Human Aortic Valve. Stem Cells Int. 2019, 2019, 7671638. [Google Scholar] [CrossRef]
- New, S.E.; Aikawa, E. Role of extracellular vesicles in de novo mineralization: An additional novel mechanism of cardiovascular calcification. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 1753–1758. [Google Scholar] [CrossRef]
- Bartel, D.P. MicroRNAs: Target recognition and regulatory functions. Cell 2009, 136, 215–233. [Google Scholar] [CrossRef]
- Wang, H.; Shi, J.; Li, B.; Zhou, Q.; Kong, X.; Bei, Y. MicroRNA Expression Signature in Human Calcific Aortic Valve Disease. BioMed Res. Int. 2017, 2017, 4820275. [Google Scholar] [CrossRef] [PubMed]
- Ni, W.J.; Wu, Y.Z.; Ma, D.H.; Leng, X.M. Noncoding RNAs in Calcific Aortic Valve Disease: A Review of Recent Studies. J. Cardiovasc. Pharmacol. 2018, 71, 317–323. [Google Scholar] [CrossRef]
- Nigam, V.; Srivastava, D. Notch1 represses osteogenic pathways in aortic valve cells. J. Mol. Cell Cardiol. 2009, 47, 828–834. [Google Scholar] [CrossRef] [PubMed]
- Baba, I.; Matoba, T.; Katsuki, S.; Koga, J.I.; Kawahara, T.; Kimura, M.; Akita, H.; Tsutsui, H. EVs-miR-17-5p attenuates the osteogenic differentiation of vascular smooth muscle cells potentially via inhibition of TGF-β signaling under high glucose conditions. Sci. Rep. 2024, 14, 16323. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Garcia, A.d.J.; Soule-Egea, M.; Fuentevilla-Alvarez, G.; Vargas-Alarcon, G.; Hernández-Mejia, B.I.; Martínez-Hernández, H.; Mora-Canela, S.L.; Santibanez-Escobar, F.; Ávila-Martinez, V.; Castrejón-Tellez, V.; et al. Role of miRNAs in Regulating Ascending Aortic Dilation in Bicuspid Aortic Valve Patients Operated for Aortic Stenosis. Int. J. Mol. Sci. 2025, 26, 779. [Google Scholar] [CrossRef]
- Zhang, Y.; Jing, X.; Li, Z.; Tian, Q.; Wang, Q.; Chen, X. Investigation of the role of the miR17-92 cluster in BMP9-induced osteoblast lineage commitment. J. Orthop. Surg. Res. 2021, 16, 652. [Google Scholar] [CrossRef]
- Bai, X.; Hua, S.; Zhang, J.; Xu, S. The MicroRNA Family Both in Normal Development and in Different Diseases: The miR-17-92 Cluster. BioMed Res. Int. 2019, 2019, 9450240. [Google Scholar] [CrossRef] [PubMed]
- Girdauskas, E.; Petersen, J.; Neumann, N.; Ungelenk, M.; Kurth, I.; Reichenspurner, H.; Zeller, T. MiR-145 expression and rare NOTCH1 variants in bicuspid aortic valve-associated aortopathy. PLoS ONE 2018, 13, e0200205. [Google Scholar] [CrossRef]
- Sabatino, J.; Wicik, Z.; De Rosa, S.; Eyileten, C.; Jakubik, D.; Spaccarotella, C.; Mongiardo, A.; Postula, M.; Indolfi, C. MicroRNAs fingerprint of bicuspid aortic valve. J. Mol. Cell. Cardiol. 2019, 134, 98–106. [Google Scholar] [CrossRef]
- Koide, T.; Mandai, S.; Kitaoka, R.; Matsuki, H.; Chiga, M.; Yamamoto, K.; Yoshioka, K.; Yagi, Y.; Suzuki, S.; Fujiki, T.; et al. Circulating Extracellular Vesicle-Propagated microRNA Signature as a Vascular Calcification Factor in Chronic Kidney Disease. Circ. Res. 2023, 132, 415–431. [Google Scholar] [CrossRef]
- Luo, B.; Liang, Z.; Lin, W.; Li, Y.; Zhong, W.; Bai, D.; Hu, X.; Xie, J.; Li, Y.; Wang, P.; et al. Aqueous extract of Rehmanniae Radix Praeparata improves bone health in ovariectomized rats by modulating the miR-29a-3p/NFIA/Wnt signaling pathway axis. J. Ethnopharmacol. 2025, 344, 119549. [Google Scholar] [CrossRef]
- Fu, C.; Liang, Q.; Ma, L.; Liu, W.; Guo, W.; Wang, G. miR-29a-3p/Vegfa axis modulates high phosphate-induced vascular smooth muscle cell calcification. Ren. Fail. 2025, 47, 2489712. [Google Scholar] [CrossRef]
- Shin, B.; Hrdlicka, H.C.; Karki, S.; Fraser, B.; Lee, S.K.; Delany, A.M. The miR-29-3p family suppresses inflammatory osteolysis. J. Cell. Physiol. 2024, 239, e31299. [Google Scholar] [CrossRef] [PubMed]
- Ejaz, M.; Usman, S.M.; Amir, S.; Khan, M.J. Holistic expression of miR-17-92 cluster in obesity, kidney diseases, cardiovascular diseases, and diabetes. Mol. Biol. Rep. 2023, 50, 6913–6925. [Google Scholar] [CrossRef]
- Cao, Y.; Zheng, M.; Sewani, M.A.; Wang, J. The miR-17-92 cluster in cardiac health and disease. Birth Defects Res. 2024, 116, e2273. [Google Scholar] [CrossRef] [PubMed]
- Roncarati, R.; Viviani Anselmi, C.; Losi, M.A.; Papa, L.; Cavarretta, E.; Da Costa Martins, P.; Contaldi, C.; Saccani Jotti, G.; Franzone, A.; Galastri, L.; et al. Circulating miR-29a, among other up-regulated microRNAs, is the only biomarker for both hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy. J. Am. Coll. Cardiol. 2014, 63, 920–927. [Google Scholar] [CrossRef]
- Torres-Do Rego, A.; Barrientos, M.; Ortega-Hernández, A.; Modrego, J.; Gómez-Gordo, R.; Álvarez-Sala, L.A.; Cachofeiro, V.; Gómez-Garre, D. Identification of a Plasma Microrna Signature as Biomarker of Subaneurysmal Aortic Dilation in Patients with High Cardiovascular Risk. J. Clin. Med. 2020, 9, 2783. [Google Scholar] [CrossRef] [PubMed]
- Costé, É.; Rouleux-Bonnin, F. The crucial choice of reference genes: Identification of miR-191-5p for normalization of miRNAs expression in bone marrow mesenchymal stromal cell and HS27a/HS5 cell lines. Sci. Rep. 2020, 10, 17728. [Google Scholar] [CrossRef]
- Zheng, G.; Wang, H.; Zhang, X.; Yang, Y.; Wang, L.; Du, L.; Li, W.; Li, J.; Qu, A.; Liu, Y.; et al. Identification and validation of reference genes for qPCR detection of serum microRNAs in colorectal adenocarcinoma patients. PLoS ONE 2013, 8, e83025. [Google Scholar] [CrossRef]
- Tastsoglou, S.; Skoufos, G.; Miliotis, M.; Karagkouni, D.; Koutsoukos, I.; Karavangeli, A.; Kardaras, F.S.; Hatzigeorgiou, A.G. DIANA-miRPath v4.0: Expanding target-based miRNA functional analysis. Nucleic Acids Res. 2023, 51, W154–W159. [Google Scholar] [CrossRef]

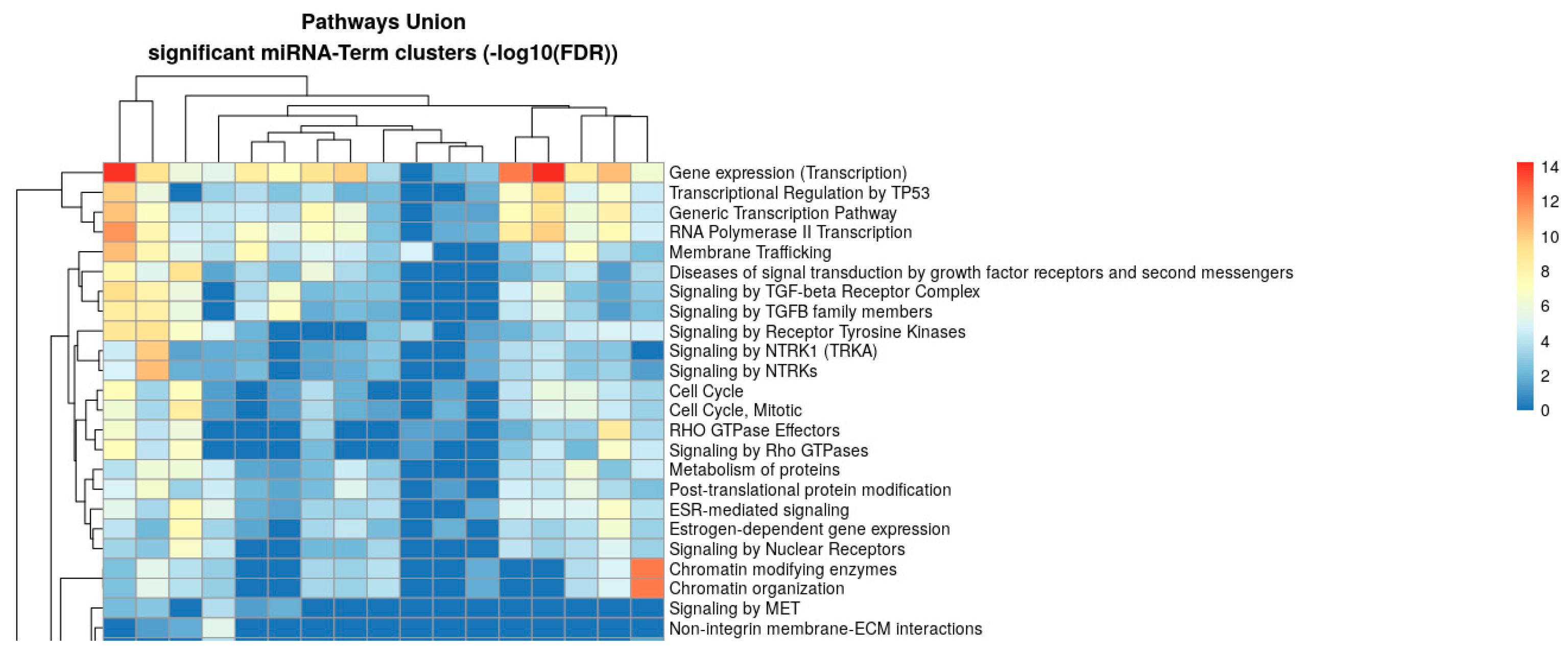
| GO Term | No. of Genes | No. of miRNAs | Representative miRNAs | p-Value/FDR |
|---|---|---|---|---|
| Heart development | 251 | 7 | hsa-miR-17-5p, hsa-miR-22-3p, hsa-miR-24-3p, hsa-miR-27a-3p, hsa-miR-29a-3p, hsa-miR-125b-5p, hsa-miR-106b-5p | p = 2.53 × 10−30; FDR = 2.40 × 10−29 |
| Step | Temperature | Time | Cycles |
|---|---|---|---|
| Enzyme activation | 95 °C | 20 sec | 1 |
| Denature | 95 °C | 3 sec | 40 |
| Anneal/Extend | 60 °C | 30 sec | 40 |
| Gene | Origin | Assay ID |
|---|---|---|
| hsa-miR-191-5p | Thermo Fisher Scientific | 477952_mir |
| hsa-miR-17-5p | Thermo Fisher Scientific | 478447_mir |
| hsa-miR-451a | Thermo Fisher Scientific | 478107_mir |
| hsa- miR-29a-3p | Thermo Fisher Scientific | 478587_mir |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galeone, A.; Minoia, A.; Braggio, M.; Cominacini, M.; Romanelli, M.G.; Dalle Carbonare, L.; Faggian, G.; Luciani, G.B.; Valenti, M.T. Molecular Network Analysis of Circulating microRNAs Highlights miR-17-5p and miR-29a-3p as Potential Biomarkers of Aortic Valve Calcification. Int. J. Mol. Sci. 2025, 26, 10813. https://doi.org/10.3390/ijms262210813
Galeone A, Minoia A, Braggio M, Cominacini M, Romanelli MG, Dalle Carbonare L, Faggian G, Luciani GB, Valenti MT. Molecular Network Analysis of Circulating microRNAs Highlights miR-17-5p and miR-29a-3p as Potential Biomarkers of Aortic Valve Calcification. International Journal of Molecular Sciences. 2025; 26(22):10813. https://doi.org/10.3390/ijms262210813
Chicago/Turabian StyleGaleone, Antonella, Arianna Minoia, Michele Braggio, Mattia Cominacini, Maria Grazia Romanelli, Luca Dalle Carbonare, Giuseppe Faggian, Giovanni Battista Luciani, and Maria Teresa Valenti. 2025. "Molecular Network Analysis of Circulating microRNAs Highlights miR-17-5p and miR-29a-3p as Potential Biomarkers of Aortic Valve Calcification" International Journal of Molecular Sciences 26, no. 22: 10813. https://doi.org/10.3390/ijms262210813
APA StyleGaleone, A., Minoia, A., Braggio, M., Cominacini, M., Romanelli, M. G., Dalle Carbonare, L., Faggian, G., Luciani, G. B., & Valenti, M. T. (2025). Molecular Network Analysis of Circulating microRNAs Highlights miR-17-5p and miR-29a-3p as Potential Biomarkers of Aortic Valve Calcification. International Journal of Molecular Sciences, 26(22), 10813. https://doi.org/10.3390/ijms262210813











