Plasma Biomarker Profiling of 2-Hydroxypropyl-β-Cyclodextrin (HPβCD) Treatment in an Aged Mouse Model of Ischemic Stroke
Abstract
1. Introduction
2. Results
2.1. HPβCD Treatment Does Not Confer Acute Neuroprotection
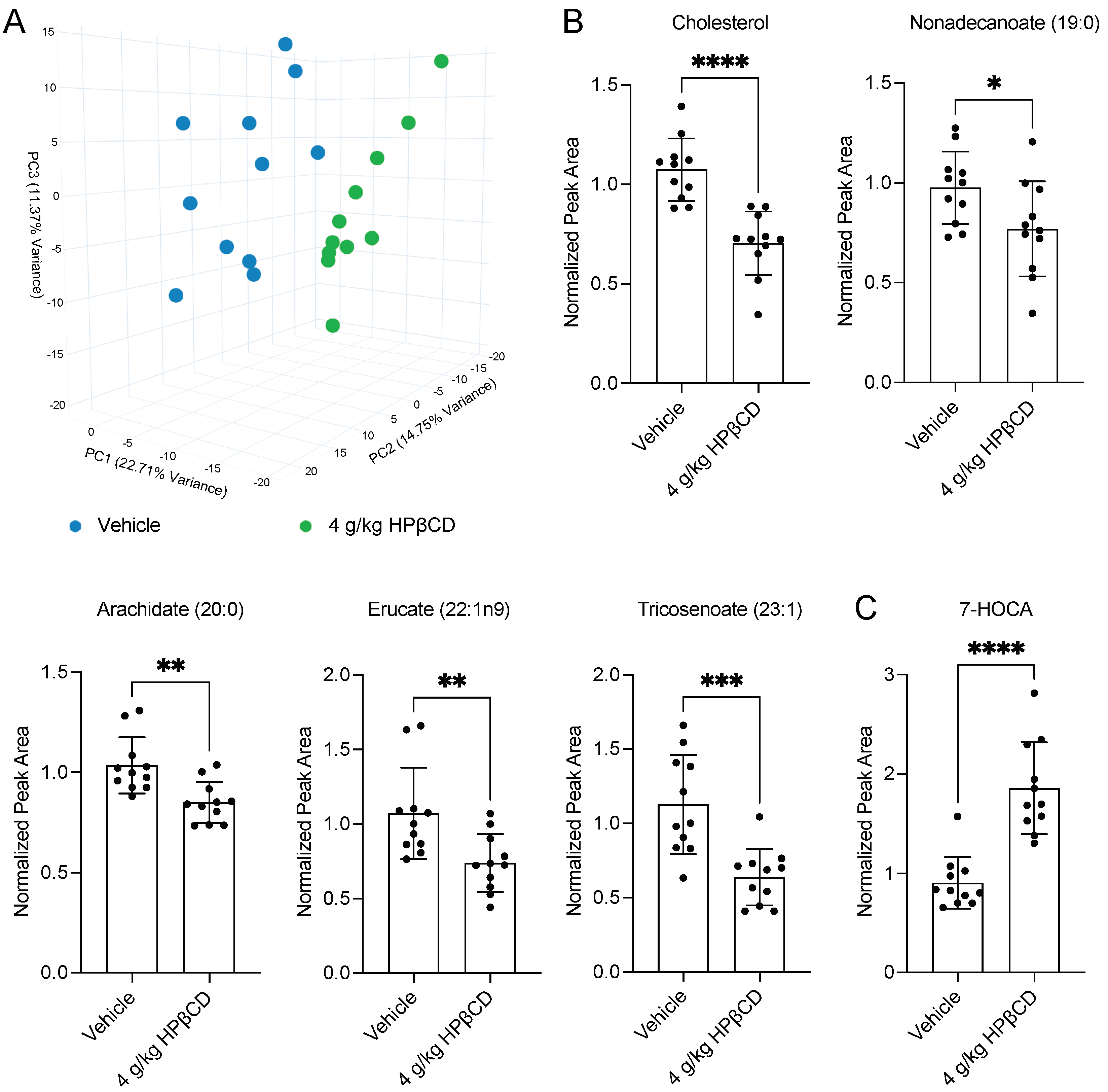
2.2. HPβCD Treatment Induces a Distinct Plasma Metabolomic Signature 4 d After Stroke
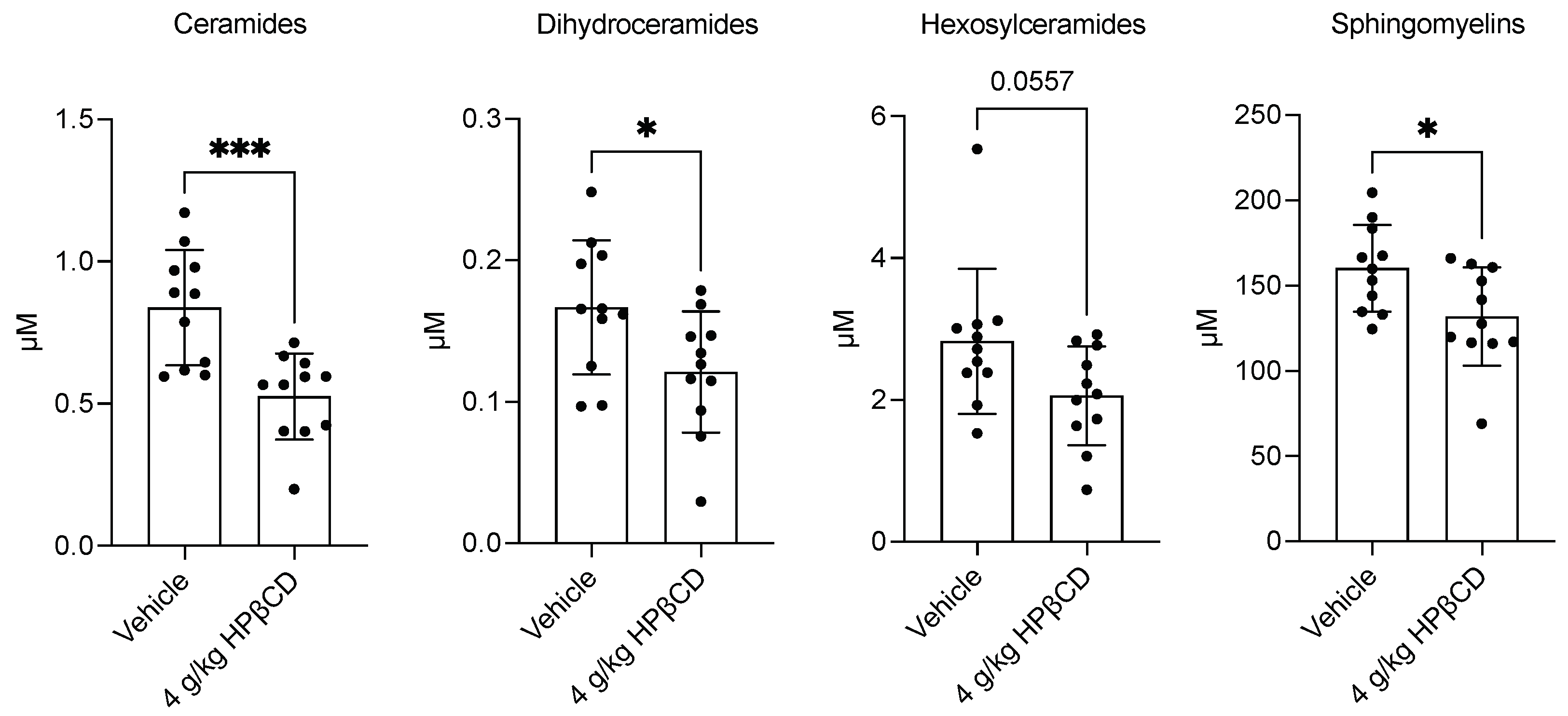
2.3. HPβCD Treatment Decreases Plasma Sphingolipid Levels 4 d After Stroke
2.4. HPβCD Treatment Alters Cholesterol Metabolism Through Oxysterol Pathways 4 d After Stroke
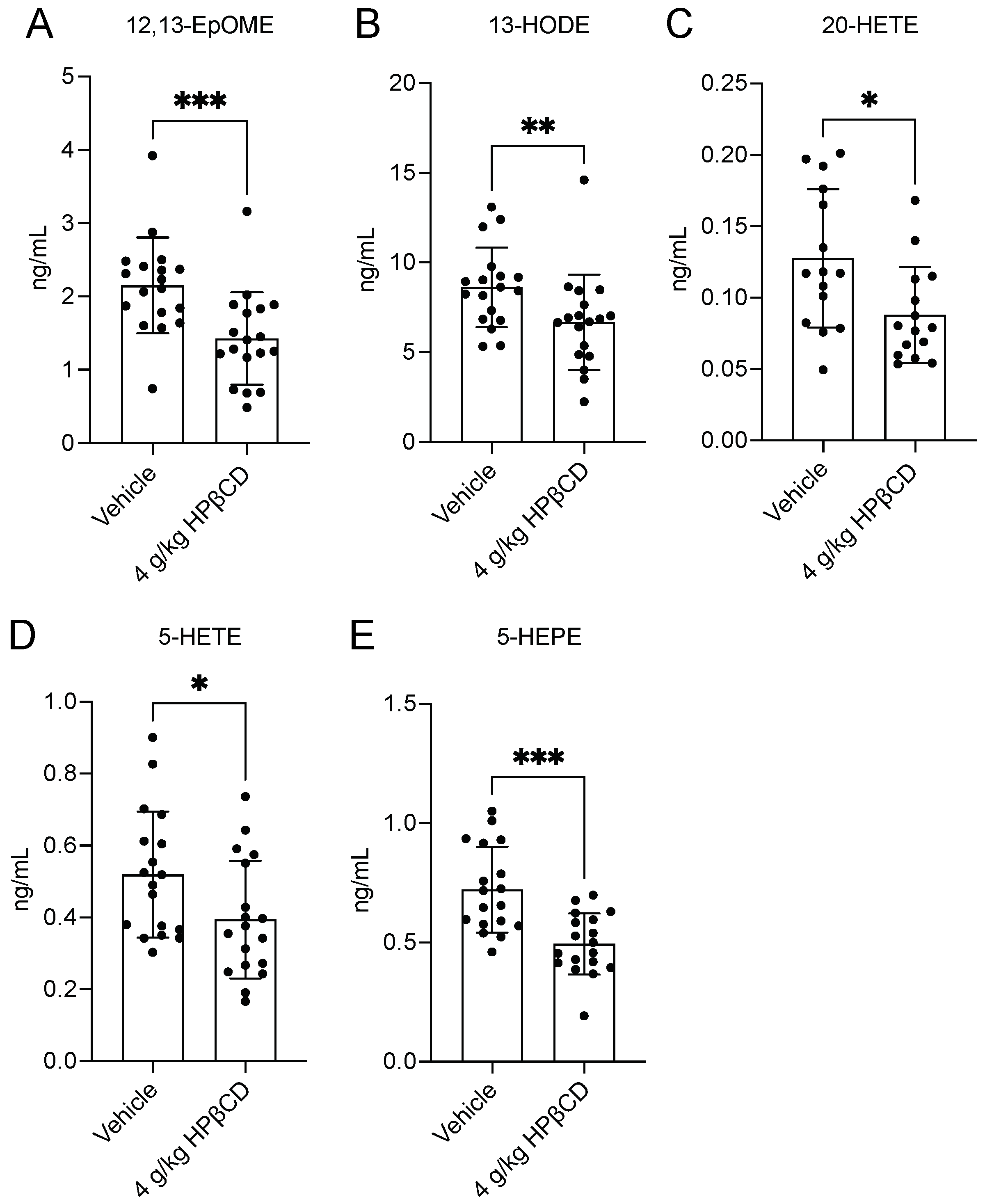
2.5. HPβCD Treatment Attenuates Pro-Inflammatory Plasma Oxylipin Production 4 d After Stroke
3. Discussion
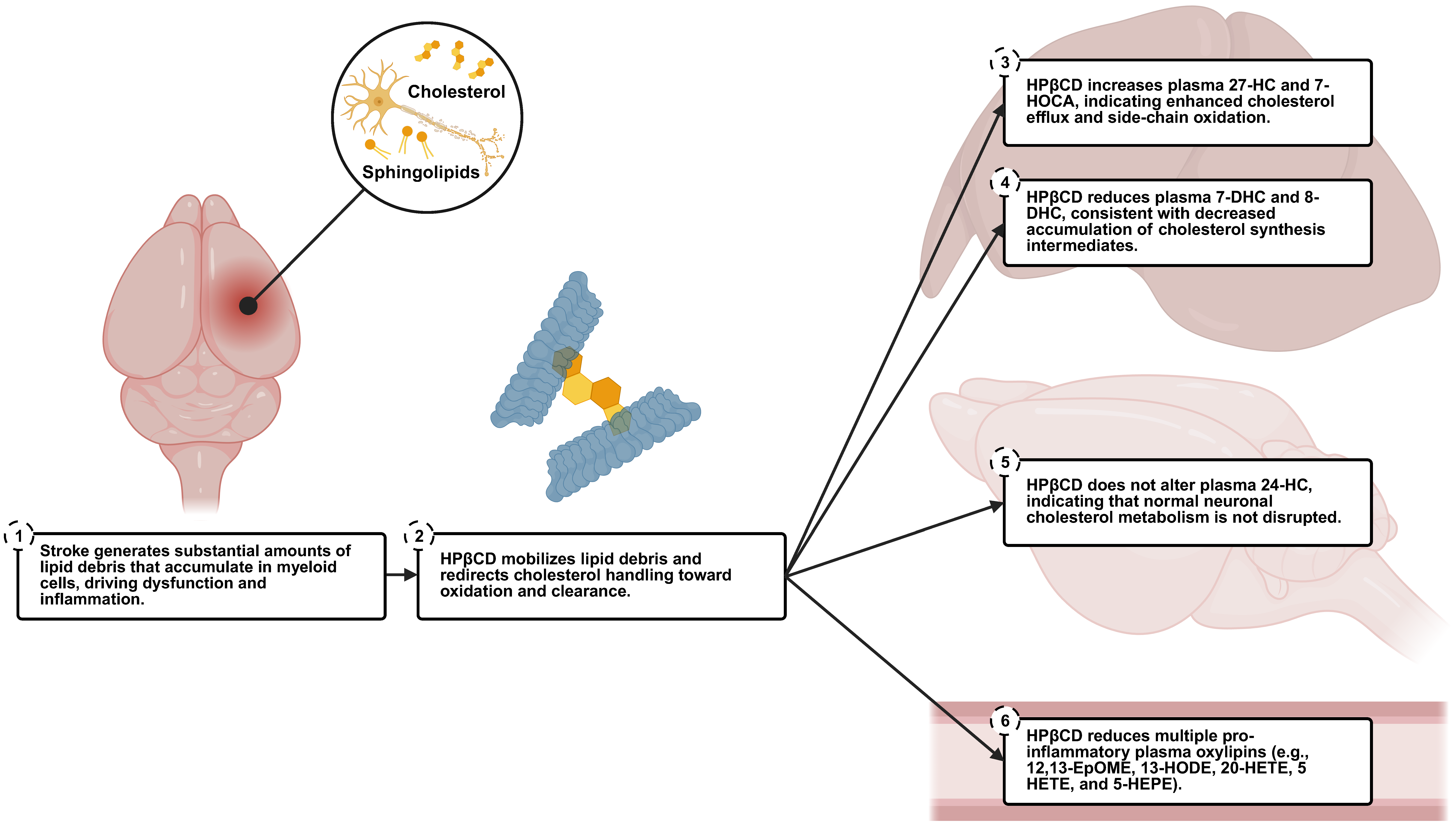
3.1. Summary
3.2. Plasma Biomarkers of HPβCD Activity
3.3. Insights into HPβCD Mechanisms of Action
3.4. Lack of Acute Neuroprotective Effects of HPβCD
3.5. Translational Significance
3.6. Limitations and Future Directions
4. Materials and Methods
4.1. Animals
4.2. Stroke Surgeries
4.3. Drug Treatments
4.4. MRI
4.5. Plasma Neurofilament Light Analysis (Quanterix®)
4.6. Plasma Global Untargeted Metabolomic Analysis—Global Discovery Panel (Metabolon, Inc.)
4.7. Plasma Targeted Lipidomic Analysis—Complex Lipid Panel (Metabolon, Inc.)
4.8. Plasma Targeted Lipidomic Analysis—Oxysterols Targeted Panel (Metabolon, Inc.)
4.9. Plasma Targeted Lipidomic Analysis—Lipid Mediators of Inflammation Targeted Panel (Metabolon, Inc.)
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HEPE | 5-hydroxyeicosapentaenoic acid |
| 5-HETE | 5-hydroxyeicosatetraenoic acid |
| 7-DHC | 7-dehydrocholesterol |
| 7-HOCA | 7α-hydroxy-3-oxo-4-cholestenoic acid |
| 8-DHC | 8-dehydrocholesterol |
| 12,13-EpOME | 12,13-epoxyoctadecenoic acid |
| 13-HODE | 13-hydroxyoctadecadienoic acid |
| 20-HETE | 20-hydroxyeicosatetraenoic acid |
| 24-HC | 24-hydroxycholesterol |
| 27-HC | 27-hydroxycholesterol |
| ANOVA | Analysis of variance |
| BBB | Blood–brain barrier |
| CNS | Central nervous system |
| CYP | Cytochrome P450 |
| DH | Distal middle cerebral artery occlusion + hypoxia |
| EDTA | Ethylenediaminetetraacetic acid |
| ESI | Electrospray ionization |
| FA | Formic acid |
| FDA | Food and Drug Administration |
| HILIC | Hydrophilic interaction liquid chromatography |
| HPβCD | 2-hydroxypropyl-β-cyclodextrin |
| LOX | Lipoxygenase |
| LXR | Liver X receptor |
| MCA | Middle cerebral artery |
| MRI | Magnetic resonance imaging |
| MS | Mass spectrometry |
| NfL | Neurofilament light |
| ns | Not significant |
| NPC | Niemann-Pick disease type C |
| PCA | Principal component analysis |
| PET | Positron emission tomography |
| PFPA | Perfluoropentanoic acid |
| RGP | Resorufin ß-D-galactopyranoside |
| RI | Retention time/index |
| RP | Reverse phase |
| SBG | Streptavidin-ß-galactosidase |
| s.c. | Subcutaneous |
| SD | Standard deviation |
| SREBP2 | Sterol regulatory element-binding protein 2 |
| TSPO | Translocator protein |
| UPLC-MS/MS | Ultra-performance liquid chromatography-tandem mass spectrometry |
References
- Lakhan, S.E.; Kirchgessner, A.; Hofer, M. Inflammatory mechanisms in ischemic stroke: Therapeutic approaches. J. Transl. Med. 2009, 7, 97. [Google Scholar] [CrossRef]
- Vidale, S.; Consoli, A.; Arnaboldi, M.; Consoli, D. Postischemic Inflammation in Acute Stroke. J. Clin. Neurol. 2017, 13, 1–9. [Google Scholar] [CrossRef]
- Wei, W.; Lattau, S.S.J.; Xin, W.; Pan, Y.; Tatenhorst, L.; Zhang, L.; Graf, I.; Kuang, Y.; Zheng, X.; Hao, Z.; et al. Dynamic Brain Lipid Profiles Modulate Microglial Lipid Droplet Accumulation and Inflammation Under Ischemic Conditions in Mice. Adv. Sci. 2024, 11, 2306863. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Huang, Y.; Shi, Z.; Li, J.; Zhang, Y.; Wang, K.; Smith, A.D.; Gong, Y.; Gao, Y. Demyelinating processes in aging and stroke in the central nervous system and the prospect of treatment strategy. CNS Neurosci. Ther. 2020, 26, 1219–1229. [Google Scholar] [CrossRef] [PubMed]
- Hammel, G.; Zivkovic, S.; Ayazi, M.; Ren, Y. Consequences and mechanisms of myelin debris uptake and processing by cells in the central nervous system. Cell. Immunol. 2022, 380, 104591. [Google Scholar] [CrossRef]
- Chung, A.G.; Frye, J.B.; Zbesko, J.C.; Constantopoulos, E.; Hayes, M.; Figueroa, A.G.; Becktel, D.A.; Day, W.A.; Konhilas, J.P.; McKay, B.S.; et al. Liquefaction of the brain following stroke shares a similar molecular and morphological profile with atherosclerosis and mediates secondary neurodegeneration in an osteopontin-dependent mechanism. eNeuro 2018, 5. [Google Scholar] [CrossRef] [PubMed]
- Becktel, D.A.; Zbesko, J.C.; Frye, J.B.; Chung, A.G.; Hayes, M.; Calderon, K.; Grover, J.W.; Li, A.; Garcia, F.G.; Tavera-Garcia, M.A.; et al. Repeated Administration of 2-Hydroxypropyl-β-Cyclodextrin (HPβCD) Attenuates the Chronic Inflammatory Response to Experimental Stroke. J. Neurosci. 2022, 42, 325–348. [Google Scholar] [CrossRef]
- Zbesko, J.C.; Stokes, J.; Becktel, D.A.; Doyle, K.P. Targeting foam cell formation to improve recovery from ischemic stroke. Neurobiol. Dis. 2023, 181, 106130. [Google Scholar] [CrossRef]
- Wang, X.; Cao, K.; Sun, X.; Chen, Y.; Duan, Z.; Sun, L.; Guo, L.; Bai, P.; Sun, D.; Fan, J.; et al. Macrophages in spinal cord injury: Phenotypic and functional change from exposure to myelin debris. Glia 2015, 63, 635–651. [Google Scholar] [CrossRef]
- Kopper, T.J.; Gensel, J.C. Myelin as an inflammatory mediator: Myelin interactions with complement, macrophages, and microglia in spinal cord injury. J. Neurosci. Res. 2017, 96, 969–977. [Google Scholar] [CrossRef]
- Becktel, D.A.; Frye, J.B.; Le, E.H.; Whitman, S.A.; Schnellmann, R.G.; Morrison, H.W.; Doyle, K.P. Discovering novel plasma biomarkers for ischemic stroke: Lipidomic and metabolomic analyses in an aged mouse model. J. Lipid Res. 2024, 65, 100614. [Google Scholar] [CrossRef]
- Bernoud-Hubac, N.; Van, A.L.; Lazar, A.-N.; Lagarde, M. Ischemic Brain Injury: Involvement of Lipids in the Pathophysiology of Stroke and Therapeutic Strategies. Antioxidants 2024, 13, 634. [Google Scholar] [CrossRef]
- Coisne, C.; Tilloy, S.; Monflier, E.; Wils, D.; Fenart, L.; Gosselet, F. Cyclodextrins as Emerging Therapeutic Tools in the Treatment of Cholesterol-Associated Vascular and Neurodegenerative Diseases. Molecules 2016, 21, 1748. [Google Scholar] [CrossRef]
- Davidson, C.D.; Ali, N.F.; Micsenyi, M.C.; Stephney, G.; Renault, S.; Dobrenis, K.; Ory, D.S.; Vanier, M.T.; Walkley, S.U. Chronic Cyclodextrin Treatment of Murine Niemann-Pick C Disease Ameliorates Neuronal Cholesterol and Glycosphingolipid Storage and Disease Progression. PLoS ONE 2009, 4, e6951. [Google Scholar] [CrossRef]
- Davidson, C.D.; Fishman, Y.I.; Puskás, I.; Szemán, J.; Sohajda, T.; McCauliff, L.A.; Sikora, J.; Storch, J.; Vanier, M.T.; Szente, L.; et al. Efficacy and ototoxicity of different cyclodextrins in Niemann–Pick C disease. Ann. Clin. Transl. Neurol. 2016, 3, 366–380. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, S.; Grebe, A.; Bakke, S.S.; Bode, N.; Halvorsen, B.; Ulas, T.; Skjelland, M.; De Nardo, D.; Labzin, L.I.; Kerksiek, A.; et al. Cyclodextrin promotes atherosclerosis regression via macrophage reprogramming. Sci. Transl. Med. 2016, 8, 333ra50. [Google Scholar] [CrossRef] [PubMed]
- Meaney, S.; Heverin, M.; Panzenboeck, U.; Ekström, L.; Axelsson, M.; Andersson, U.; Diczfalusy, U.; Pikuleva, I.; Wahren, J.; Sattler, W.; et al. Novel route for elimination of brain oxysterols across the blood-brain barrier: Conversion into 7α-hydroxy-3-oxo-4-cholestenoic acid. J. Lipid Res. 2007, 48, 944–951. [Google Scholar] [CrossRef] [PubMed]
- Sandebring-Matton, A.; Goikolea, J.; Björkhem, I.; Paternain, L.; Kemppainen, N.; Laatikainen, T.; Ngandu, T.; Rinne, J.; Soininen, H.; Cedazo-Minguez, A.; et al. 27-Hydroxycholesterol, cognition, and brain imaging markers in the FINGER randomized controlled trial. Alzheimer’s Res. Ther. 2021, 13, 56. [Google Scholar] [CrossRef]
- Nitta, S.-I.; Hashimoto, M.; Kazuki, Y.; Takehara, S.; Suzuki, H.; Oshimura, M.; Akita, H.; Chiba, K.; Kobayashi, K. Evaluation of 4β-Hydroxycholesterol and 25-Hydroxycholesterol as Endogenous Biomarkers of CYP3A4: Study with CYP3A-Humanized Mice. AAPS J. 2018, 20, 1–9. [Google Scholar] [CrossRef]
- Björkhem, I.; Meaney, S. Brain Cholesterol: Long Secret Life Behind a Barrier. Arter. Thromb. Vasc. Biol. 2004, 24, 806–815. [Google Scholar] [CrossRef]
- Batta, A.K.; Tint, G.S.; Shefer, S.; Abuelo, D.; Salen, G. Identification of 8-dehydrocholesterol (cholesta-5,8-dien-3 beta-ol) in patients with Smith-Lemli-Opitz syndrome. J. Lipid Res. 1995, 36, 705–713. [Google Scholar] [CrossRef] [PubMed]
- Kölsch, H.; Heun, R.; Kerksiek, A.; Bergmann, K.; Maier, W.; Lütjohann, D. Altered levels of plasma 24S- and 27-hydroxycholesterol in demented patients. Neurosci. Lett. 2004, 368, 303–308. [Google Scholar] [CrossRef] [PubMed]
- Lund, E.G.; Xie, C.; Kotti, T.; Turley, S.D.; Dietschy, J.M.; Russell, D.W. Knockout of the cholesterol 24-hydroxylase gene in mice reveals a brain-specific mechanism of cholesterol turnover. J. Biol. Chem. 2003, 278, 22980–22988. [Google Scholar] [CrossRef]
- Sodero, A.O. 24S-hydroxycholesterol: Cellular effects and variations in brain diseases. J. Neurochem. 2021, 157, 899–918. [Google Scholar] [CrossRef]
- Berrodin, T.J.; Shen, Q.; Quinet, E.M.; Yudt, M.R.; Freedman, L.P.; Nagpal, S. Identification of 5α,6α-Epoxycholesterol as a Novel Modulator of Liver X Receptor Activity. Mol. Pharmacol. 2010, 78, 1046–1058. [Google Scholar] [CrossRef] [PubMed]
- Wen, H.; Östman, J.; Bubb, K.J.; Panayiotou, C.; Priestley, J.V.; Baker, M.D.; Ahluwalia, A. 20-Hydroxyeicosatetraenoic Acid (20-HETE) Is a Novel Activator of Transient Receptor Potential Vanilloid 1 (TRPV1) Channel. J. Biol. Chem. 2012, 287, 13868. [Google Scholar] [CrossRef]
- Saeed, A.; Floris, F.; Andersson, U.; Pikuleva, I.; Lövgren-Sandblom, A.; Bjerke, M.; Paucar, M.; Wallin, A.; Svenningsson, P.; Björkhem, I. 7α-hydroxy-3-oxo-4-cholestenoic acid in cerebrospinal fluid reflects the integrity of the blood-brain barrier. J. Lipid Res. 2014, 55, 313–318. [Google Scholar] [CrossRef]
- Nayeem, M.A. Role of oxylipins in cardiovascular diseases review-article. Acta Pharmacol. Sin. 2018, 39, 1142–1154. [Google Scholar] [CrossRef]
- Tourdot, B.E.; Ahmed, I.; Holinstat, M. The emerging role of oxylipins in thrombosis and diabetes. Front. Pharmacol. 2014, 4, 176. [Google Scholar] [CrossRef]
- Gabbs, M.; Leng, S.; Devassy, J.G.; Monirujjaman, M.; Aukema, H.M. Advances in Our Understanding of Oxylipins Derived from Dietary PUFAs. Adv. Nutr. 2015, 6, 513. [Google Scholar] [CrossRef]
- Noverr, M.C.; Erb-Downward, J.R.; Huffnagle, G.B. Huffnagle. Production of Eicosanoids and Other Oxylipins by Pathogenic Eukaryotic Microbes. Clin. Microbiol. Rev. 2003, 16, 517. [Google Scholar] [CrossRef]
- Nagy, L.; Tontonoz, P.; Alvarez, J.G.; Chen, H.; Evans, R.M. Oxidized LDL Regulates Macrophage Gene Expression through Ligand Activation of PPARγ. Cell 1998, 93, 229–240. [Google Scholar] [CrossRef] [PubMed]
- Vangaveti, V.; Baune, B.T.; Kennedy, R.L. Hydroxyoctadecadienoic acids: Novel regulators of macrophage differentiation and atherogenesis. Ther. Adv. Endocrinol. Metab. 2010, 1, 51. [Google Scholar] [CrossRef] [PubMed]
- Kloska, A.; Malinowska, M.; Gabig-Cimińska, M.; Jakóbkiewicz-Banecka, J. Lipids and Lipid Mediators Associated with the Risk and Pathology of Ischemic Stroke. Int. J. Mol. Sci. 2020, 21, 3618. [Google Scholar] [CrossRef]
- Kim, B.-Y.; Son, Y.; Cho, H.-R.; Lee, D.; Eo, S.-K.; Kim, K. 27-Hydroxycholesterol induces macrophage gene expression via LXR-dependent and -independent mechanisms. Korean J. Physiol. Pharmacol. 2021, 25, 111–118. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Menke, J.G.; Chen, Y.; Zhou, G.; MacNaul, K.L.; Wright, S.D.; Sparrow, C.P.; Lund, E.G. 27-Hydroxycholesterol Is an Endogenous Ligand for Liver X Receptor in Cholesterol-loaded Cells. J. Biol. Chem. 2001, 276, 38378–38387. [Google Scholar] [CrossRef]
- Saito, H.; Tachiura, W.; Nishimura, M.; Shimizu, M.; Sato, R.; Yamauchi, Y. Hydroxylation site–specific and production-dependent effects of endogenous oxysterols on cholesterol homeostasis: Implications for SREBP-2 and LXR. J. Biol. Chem. 2022, 299, 102733. [Google Scholar] [CrossRef]
- Sun, H.; Yang, T.; Simon, R.P.; Xiong, Z.-G.; Leng, T. Role of Cholesterol Metabolic Enzyme CYP46A1 and Its Metabolite 24S-Hydroxycholesterol in Ischemic Stroke. Stroke 2024, 55, 2492–2501. [Google Scholar] [CrossRef]
- Braga, S.S. Cyclodextrins: Emerging Medicines of the New Millennium. Biomolecules 2019, 9, 801. [Google Scholar] [CrossRef]
- Sugiyama, M.G.; Agellon, L.B. Agellon. Sex differences in lipid metabolism and metabolic disease risk. Biochem. Cell Biol. 2012, 90, 124–141. [Google Scholar] [CrossRef]
- Lapchak, P.A.; Zhang, J.H.; Noble-Haeusslein, L.J. RIGOR Guidelines: Escalating STAIR and STEPS for Effective Translational Research. Transl. Stroke Res. 2012, 4, 279–285. [Google Scholar] [CrossRef]
- Landis, S.C.; Amara, S.G.; Asadullah, K.; Austin, C.P.; Blumenstein, R.; Bradley, E.W.; Crystal, R.G.; Darnell, R.B.; Ferrante, R.J.; Fillit, H.; et al. A call for transparent reporting to optimize the predictive value of preclinical research. Nature 2012, 490, 187–191. [Google Scholar] [CrossRef] [PubMed]
- du Sert, N.P.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. PLoS Biol. 2020, 18, e3000410. [Google Scholar] [CrossRef]
- Doyle, K.P.; Fathali, N.; Siddiqui, M.R.; Buckwalter, M.S. Distal hypoxic stroke: A new mouse model of stroke with high throughput, low variability and a quantifiable functional deficit. J. Neurosci. Methods 2012, 207, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-V.V.; Frye, J.B.; Zbesko, J.C.; Stepanovic, K.; Hayes, M.; Urzua, A.; Serrano, G.; Beach, T.G.; Doyle, K.P. Multiplex immunoassay characterization and species comparison of inflammation in acute and non-acute ischemic infarcts in human and mouse brain tissue. Acta Neuropathol. Commun. 2016, 4, 100, Erratum in Acta Neuropathol. Commun. 2016, 4, 104. [Google Scholar] [CrossRef] [PubMed]
- Ford, L.; Kennedy, A.D.; Goodman, K.D.; Pappan, K.L.; Evans, A.M.; Miller, L.A.D.; E Wulff, J.; Wiggs, B.R.; Lennon, J.J.; Elsea, S.; et al. Precision of a Clinical Metabolomics Profiling Platform for Use in the Identification of Inborn Errors of Metabolism. J. Appl. Lab. Med. 2020, 5, 342–356. [Google Scholar] [CrossRef]
- Matyash, V.; Liebisch, G.; Kurzchalia, T.V.; Shevchenko, A.; Schwudke, D. Lipid extraction by methyl-tert-butyl ether for high-throughput lipidomics. J. Lipid Res. 2008, 49, 1137–1146. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becktel, D.A.; Frye, J.B.; Le, E.H.; Schnellmann, R.G.; Doyle, K.P. Plasma Biomarker Profiling of 2-Hydroxypropyl-β-Cyclodextrin (HPβCD) Treatment in an Aged Mouse Model of Ischemic Stroke. Int. J. Mol. Sci. 2025, 26, 10814. https://doi.org/10.3390/ijms262210814
Becktel DA, Frye JB, Le EH, Schnellmann RG, Doyle KP. Plasma Biomarker Profiling of 2-Hydroxypropyl-β-Cyclodextrin (HPβCD) Treatment in an Aged Mouse Model of Ischemic Stroke. International Journal of Molecular Sciences. 2025; 26(22):10814. https://doi.org/10.3390/ijms262210814
Chicago/Turabian StyleBecktel, Danielle A., Jennifer B. Frye, Elizabeth H. Le, Rick G. Schnellmann, and Kristian P. Doyle. 2025. "Plasma Biomarker Profiling of 2-Hydroxypropyl-β-Cyclodextrin (HPβCD) Treatment in an Aged Mouse Model of Ischemic Stroke" International Journal of Molecular Sciences 26, no. 22: 10814. https://doi.org/10.3390/ijms262210814
APA StyleBecktel, D. A., Frye, J. B., Le, E. H., Schnellmann, R. G., & Doyle, K. P. (2025). Plasma Biomarker Profiling of 2-Hydroxypropyl-β-Cyclodextrin (HPβCD) Treatment in an Aged Mouse Model of Ischemic Stroke. International Journal of Molecular Sciences, 26(22), 10814. https://doi.org/10.3390/ijms262210814





