Abstract
The PER2 gene is a crucial component responsible for the proper functioning of the mammalian core circadian clock. The circadian nature of the murine Per2 (mPer2) promoter’s activity has been thoroughly investigated to identify important elements responsible for its oscillatory behavior; however, its human counterpart has not. While there are similarities between murine and human core clocks, there are differences and unconserved elements between their promoter sequences that may influence the nature of rhythms. Further, most studies to date have used murine-based sequences in human cell lines. To fully understand the role(s) of and factors involved in the human PER2 (hPER2) gene, human-derived sequences should be used. To this end, we developed two lentiviral luciferase reporters in well-established, circadian model U2OS cells using different hPER2 promoter regions. Their rhythmic nature was compared to that of the standard mPer2 promoter reporter. We found that hPER2 reporters exhibited stronger oscillations than the mPer2 reporter, and that the frame of the hPER2 promoter affected the period and phase. This work introduces a human sequence-based PER2 promoter in U2OS cells, which should be used for further in vitro tracking of hPER2 activity and to understand PER2 gene dynamics, in lieu of the murine iteration.
1. Introduction
Circadian rhythms are innate biological processes that regulate various functions in an organism. They follow an approximately 24 h cycle that is driven by the master clock, located in the suprachiasmatic nucleus (SCN) [1,2]. The SCN receives external environmental cues, primarily light, to entrain itself, as well as peripheral oscillators distributed throughout the body [2,3]. The widely accepted molecular model of the core clock is composed of a cell-autonomous, auto-regulatory transcriptional-translational feedback loop (TTFL) [4]. The positive arm of the TTFL includes brain and muscle ARNT-like-1 (BMAL1), and circadian locomotor output cycles kaput (CLOCK), which heterodimerize and drive the expression of various clock-controlled genes, including periods (PER1/2/3) and cryptochromes (CRY1/2). Upon reaching threshold concentrations, PER and CRY form an inhibitory complex that interacts with CLOCK:BMAL1, resulting in the negative regulation of the TTFL. The use of various biochemical assays, including protein pulldown assays [5,6], chromatin immunoprecipitation (ChIP) [5,7], and RT-PCR [5,6,7,8], has been important in providing us with information about the key aspects of the negative arm of the TTFL. Reporters have also played a crucial role in monitoring the real-time dynamics of core clock components. The generation of promoter-based reporters, as well as fusion proteins expressing luminescent or fluorescent reporters, has facilitated the monitoring of circadian dynamics at both transcriptional and translational levels [9,10,11,12,13,14].
Seminal work by Yoo et al. introduced PER2::luciferase (Per2::luc) mice, which allowed tracking of PER2 protein oscillations in vivo [9]. This study highlighted the importance of PER2 in producing self-sustained oscillations in peripheral tissues. To date, Per2::luc mice and tissues isolated from this reporter model have been widely used, expanding our knowledge of the circadian clock [12,15,16,17,18]. Some of these studies have shown that the period and phase of the molecular clock are controlled by PER2, thereby making it a stoichiometrically rate-limiting component of the primary TTFL [19,20]. To understand the transcriptional regulation of the Per2 promoter, several iterations of mouse Per2 (mPer2) promoter sequences with varying lengths have been used to develop mPer2:luc promoter reporters [21,22]. These studies have revealed the presence of an E’-box (5′-CACGTT-3′), which is essential for retaining the rhythmicity of the Per2 promoter. Additionally, it was established that using a truncated minimal proximal mPer2 promoter of approximately 200 base pairs (bp) around the transcript start site (TSS) (containing the E2 enhancer) was sufficient to drive robust rhythms for Per2 in peripheral cells. These studies also showed that introducing mutations to the E2-enhancer resulted in the loss of rhythms.
The transcriptional-level circadian nature of Per2 has been extensively studied and monitored for various applications in vitro, using reporters expressing truncated murine Per2 promoters in NIH3T3 (mouse fibroblast) [23,24] and U2OS (human bone osteosarcoma) cells [25,26], among others [10,27,28]. To date, it has been standard practice to use mouse sequence-derived circadian reporters in human cell lines in vitro, particularly U2OS [10,25,26,28,29,30,31], which serves as a standard in vitro model to study circadian rhythms [29]. This practice is supported by the presence of conserved regions, including the E’-box, hypoxia response element (HRE), and the mPer2 transcription start site (+1), which is shared by both the mPer2 and hPER2 promoters. However, it is important to also recognize that there are differences between the murine and human core clock in terms of genomic regulation. A recent transcriptomic study revealed differences in the rhythmicity of various clock-controlled transcripts in human and mouse prefrontal cortex (PFC) samples [32]. This could arise from cross-species variations in the unconserved regions, which may alter the circadian nature of the gene and, in turn, could introduce deviations in comparing the nature of oscillations of Per2 at the promoter level to those of the translated PER2 protein. In addition to the variations in the genomic sequence, the co-evolution of transcription factors across species [33] might also have an effect on the nature of the promoter activity. In fact, previous in vitro studies reported discrepancies between assessments of murine promoter activity and endogenous protein expression levels in human cells [10,27,34,35]. There have been a few studies that have used the hPER2 promoter sequences to evaluate circadian effects due to mutations in E-boxes [35,36] and secondary circadian regulators [37]. However, these studies did not assess the rhythmic activity of the hPER2 promoter. Therefore, our knowledge of the transcriptional regulation of hPER2 is limited.
Here, we sought to bridge this gap in knowledge by using lentiviral luciferase reporters bearing shifted frames of the hPER2 promoter fragment, hPER2.1:luc and hPER2.2:luc. These reporters were incorporated into U2OS cells, and their rhythmic nature and characteristics were evaluated and compared to those derived from mPer2:luc promoter-reporters. We observed that the hPER2 reporters exhibited strong oscillations with amplitudes higher than those of mPer2 reporters. Additionally, our findings revealed that the upstream shifted hPER2 promoter-reporter (hPER2.1:luc) resulted in a higher period and phase delay, while the downstream shifted promoter-reporter (hPER2.2:luc) exhibited a period and phase similar to the previously established mPer2:luc promoter-reporter. Taken together, our study introduces human-derived PER2:luc promoter-reporters and demonstrates that shifting the frame of the hPER2 promoter affects the nature of PER2 oscillations in vitro. These reporter systems should be used to monitor the rhythmic activity of PER2 transcription in human-derived in vitro models in the future, in order to provide a better understanding of its dynamics in health and disease, as well as its responses to perturbation.
2. Results and Discussion
2.1. Generation and Validation of hPER2 Promoter Reporter Constructs
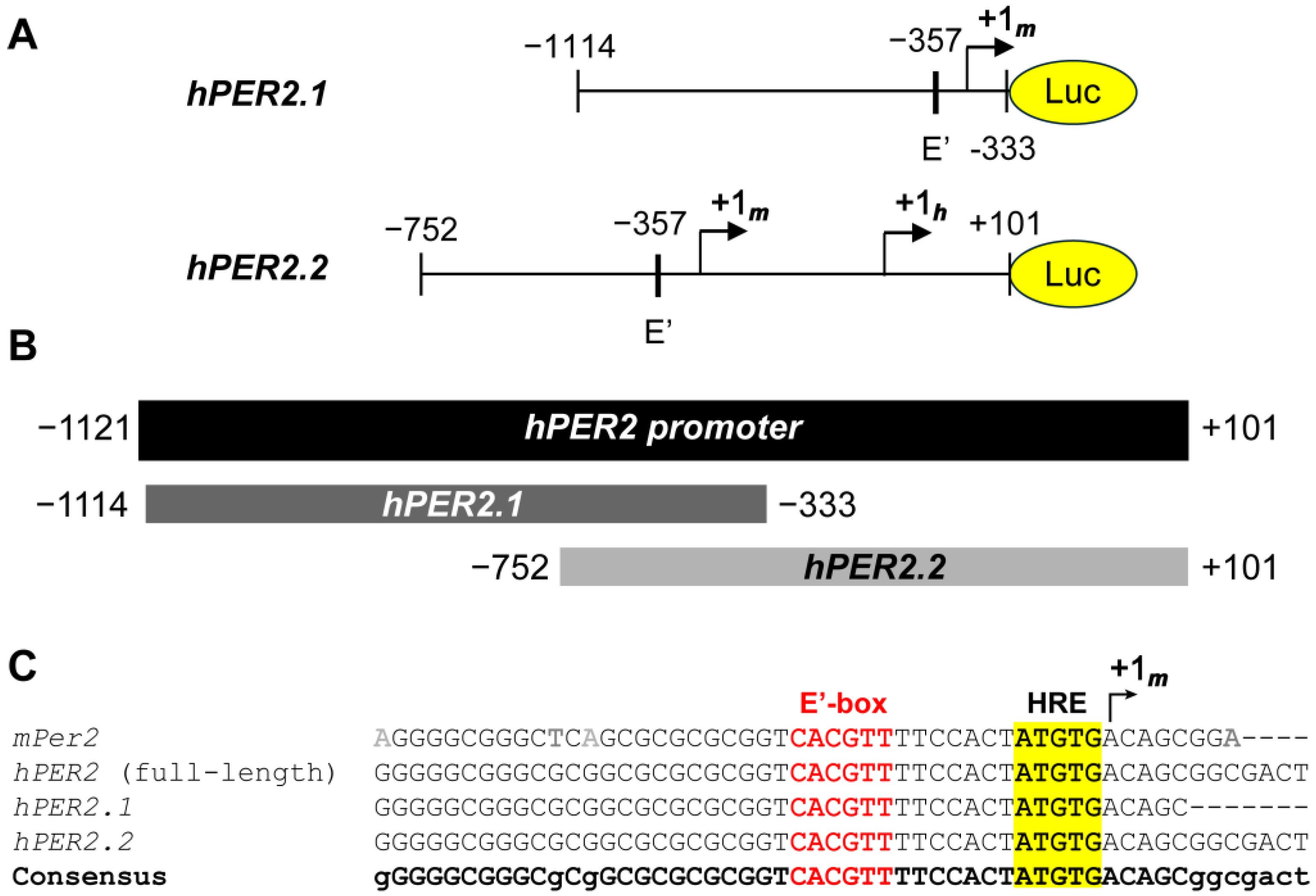
We obtained two truncated sequences of the hPER2 promoter [38,39], and generated lentiviral luciferase promoter reporters using each, resulting in hPER2.1:luc and hPER2.2:luc, respectively (Figure 1A). To compare the frames of the two promoter fragments, we performed sequence alignment of the two promoter sequences against the full-length hPER2 promoter, using the T-Coffee multiple sequence alignment (MSA) program [40]. We identified the full-length hPer2 promoter by comparing the genomic sequence and the mRNA transcript sequence for the hPER2 gene. We found the +1h site by analyzing the functional and regulatory elements in the hPER2 genomic sequence (Figure S1), which aligned with previous literature [21,41]. The hPER2.1 promoter sequence aligned with the upstream region (−1114 to −333, relative to the hPER2 transcription start site, +1h), while the hPER2.2 fragment aligned with the downstream region (−752 to +101, relative to +1h) of the full-length hPER2 promoter (Figure 1B). There was a 419-base pair (bp) region of overlap between hPER2.1 and hPER2.2 sequences, which contained E’-box (E2 enhancer; 5′-CACGTT-3′) and hypoxia response element (HRE) sequences and aligned with the consensus sequence. Further alignment with the truncated mPer2 promoter from a previously established mPer2:luc promoter reporter [10,21] revealed a 34 bp conserved region with it, the E’-box, HRE, and the mPer2 transcription start site (TSS), +1m (Figure 1C). We identified the conserved +1m in both hPER2.1 and hPER2.2 sequences. In addition to the +1m, the hPER2.2 sequence also contained the +1h site [41]. We also found a non-canonical E-box (5′-CAGGTG-3′; five bases upstream of +1h) in hPER2.2, which closely resembled the “minimal proximal promoter” organization described previously for mPer2 [21]. Additionally, there were significantly longer frames of unconserved regions in both of the hPER2 promoter sequences, which could contain regulatory elements unique to hPER2. Both hPER2.1:luc and hPER2.2:luc promoter reporter constructs were validated, by Sanger sequencing and whole plasmid sequencing, respectively.
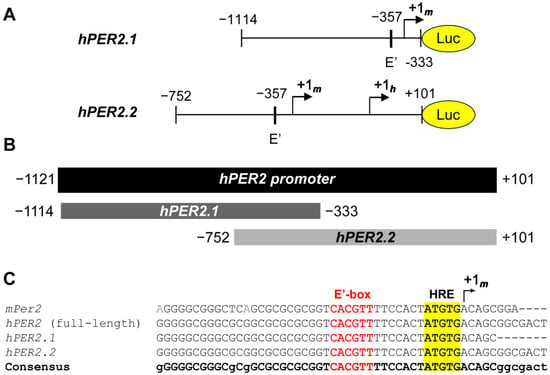
Figure 1.
Analysis of hPER2 promoter fragments. (A) A 781 bp truncated promoter (hPER2.1) and an 853 bp truncated promoter (hPER2.2) were sub-cloned upstream of the luciferase gene. (B) Graphical representation of the alignments of the two truncated hPER2 promoter sequences against the full-length hPER2 promoter sequence. (C) Sequence alignments of the 34 bp conserved regions for hPER2.1 and hPER2.2 promoter sequences against the full-length hPER2 promoter sequence and the mPer2 promoter sequence. The conserved E’-box (also known as the E2 enhancer; text in red) and the hypoxia response element (HRE; highlighted in yellow) are indicated in the sequences.
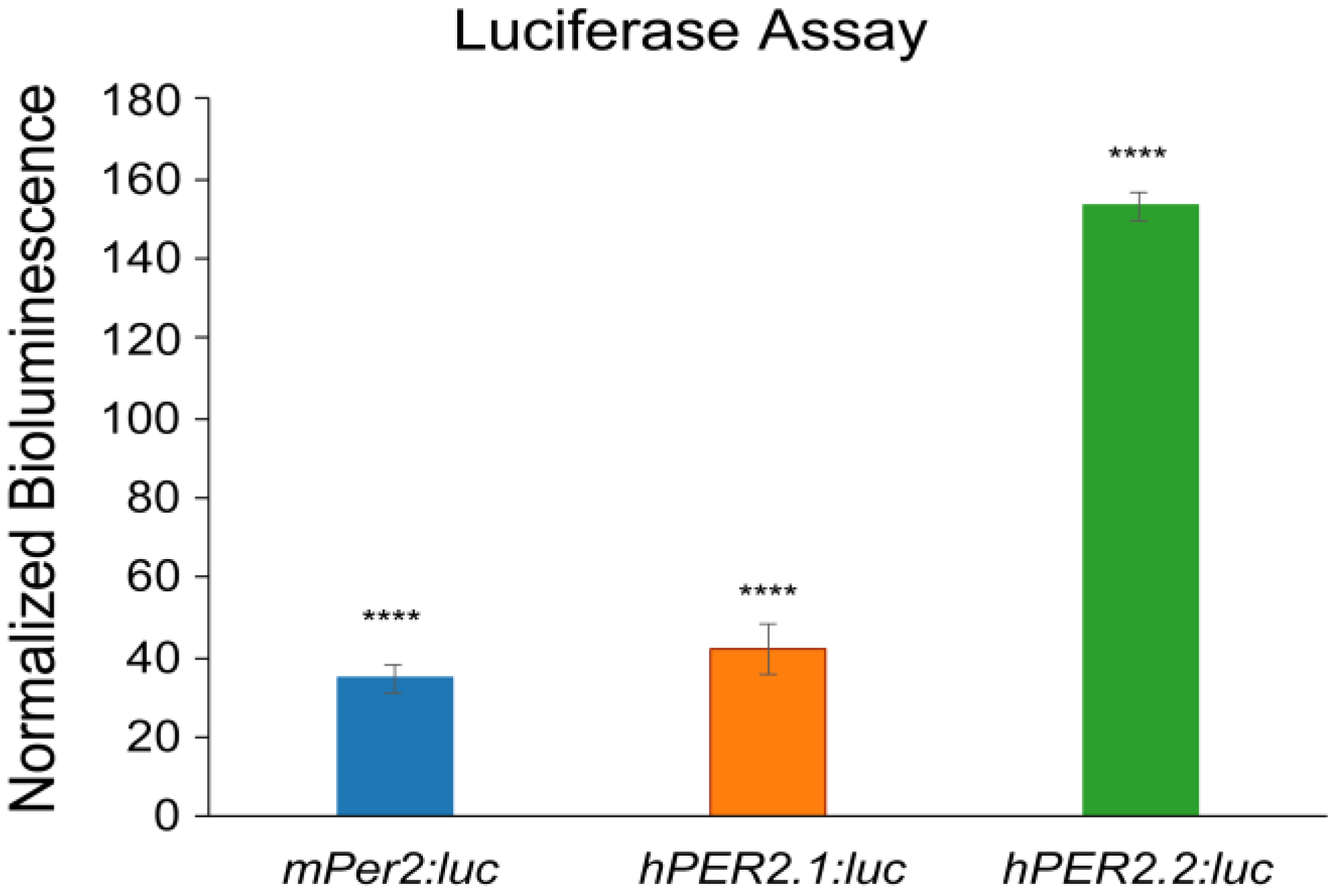
Following validations, we stably transfected the hPER2.1:luc and hPER2.2:luc reporter constructs into U2OS (human bone osteosarcoma) cells using lentiviral transductions. After selection of the positively transfected cells, we performed a luciferase assay to validate the generation of a functional luciferase (Figure 2). We compared the bioluminescence intensities of U2OS-hPER2.1:luc, U2OS-hPER2.2:luc, and a U2OS-mPer2:luc cell line previously generated in our lab [26]. We observed that the normalized bioluminescence intensities of U2OS-mPer2:luc, U2OS-hPER2.1:luc, and U2OS-hPER2.2:luc were approximately 35-, 42-, and 153-fold, respectively, higher than the non-transfected U2OS control. The promoter activities of mPer2:luc and hPER2.1:luc have been established previously [26,38]. To discern the promoter activity of the newly established hPER2.2:luc promoter-reporter from baseline, we compared the luminescence signal from hPER2.2:luc against the same lentiviral vector backbone containing a non-specific (scrambled) insert upstream of the luciferase gene. A luciferase assay in U2OS cells transiently transfected with the two vectors revealed a significantly higher bioluminescence intensity in the U2OS-hPER2.2 reporter cells, indicative of efficient hPER2.2 promoter activity (Figure S2).
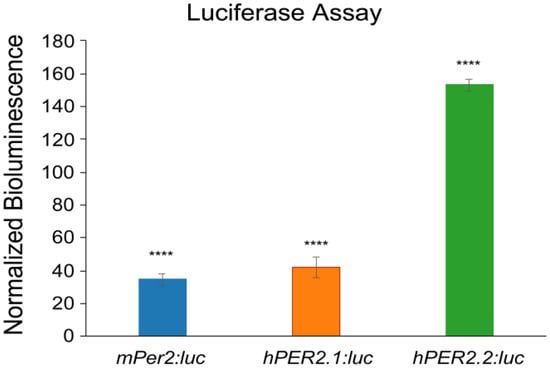
Figure 2.
PER2:luc promoter reporter validation using luciferase assay. U2OS-mPer2:luc, U2OS-hPER2.1:luc, and U2OS-hPER2.2:luc cells were assessed for bioluminescence against the non-transfected (control) U2OS cell line using a luciferase assay. The data shown for each condition is an average of three replicates (N = 3), normalized to the control U2OS cells. Error bars represent standard deviation of the mean. A t-test was performed to compare the average bioluminescence intensities of the biological replicates for each cell line versus the control. (**** p < 0.0001).
2.2. Assessment of hPER2 Promoter Frame Effects on Oscillations and Comparisons to mPer2 Traces
We performed luminometry assays to monitor the circadian behavior of our hPER2 promoter-reporters and compare the nature(s) of their oscillations against the mPer2 promoter reporter. We concurrently synchronized U2OS-mPer2:luc, U2OS-hPER2.1:luc, and U2OS-hPER2.2:luc cells for 2 h using a dexamethasone pulse, a synchronization method widely used for U2OS cells [25,26,31,42]. We recorded the bioluminescence signals for each cell line for seven days. The raw data was pre-processed by removing the first 24 h to eliminate the signal from transient peak expression. The data were detrended by subtracting a 24 h window moving average (Figure 3 and Figures S3–S5). The detrended data was then fit to a damped cosine curve.
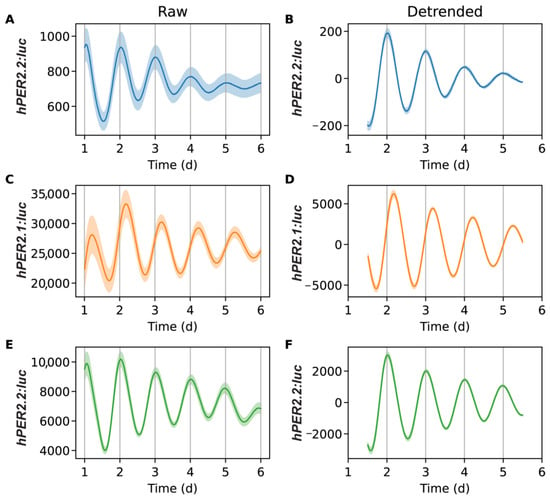
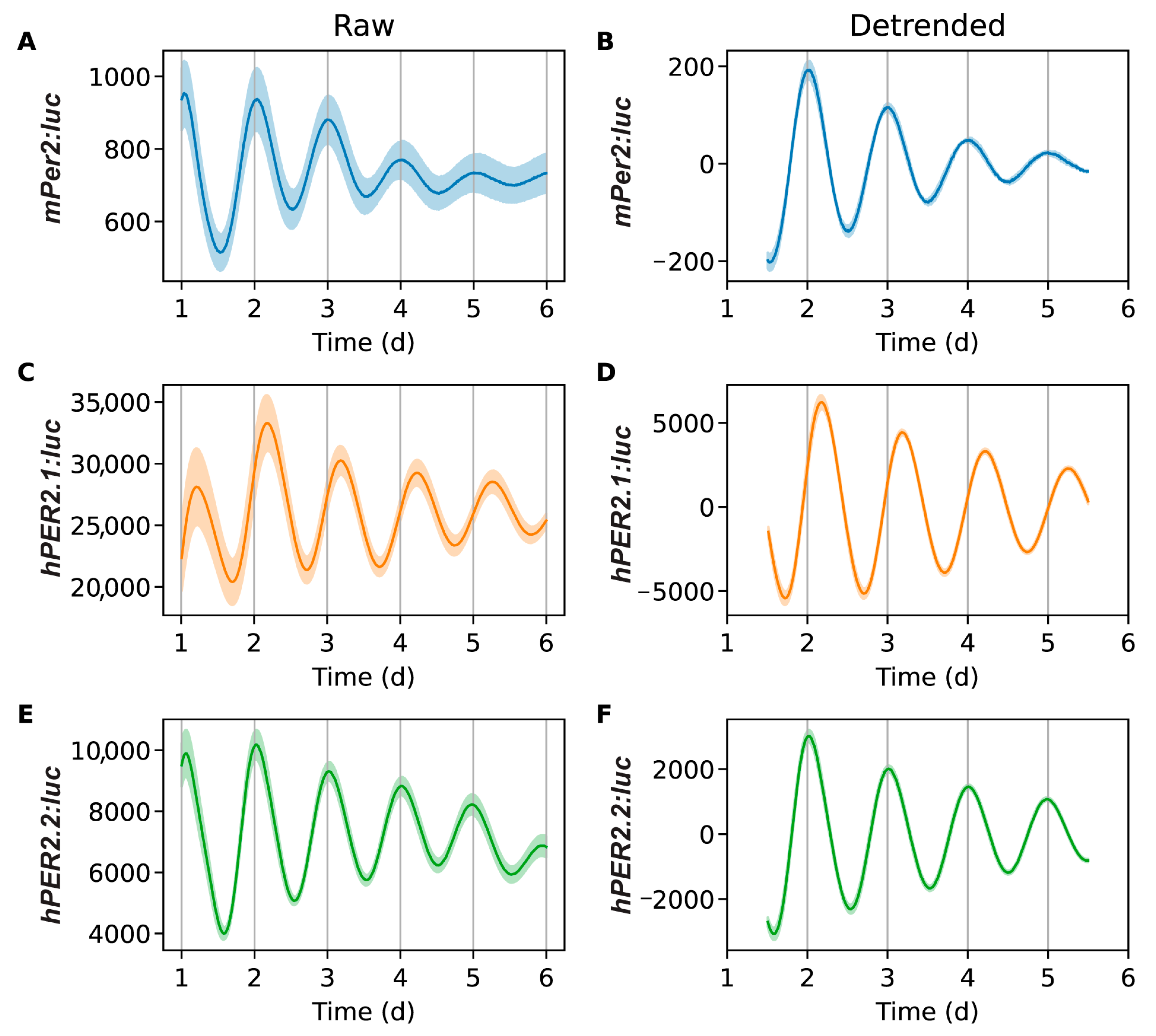
Figure 3.
Bioluminescence time-series for mPer2:luc (A,B), hPER2.1:luc (C,D), and hPER2.2:luc (E,F). Excluding a 24 h transient, shown are raw time-series (A,C,E) and time-series after detrending (B,D,F) by subtracting the average of a 24 h moving window. The mean (raw or detrended) time-series is plotted as a solid line, with the standard error of the mean as a semi-transparent envelope around it. (N = 17 for mPer2:luc from three independent experiments, N = 24 for hPER2.1: from three independent experiments, and N = 16 for hPER2.2:luc from four independent experiments).
The average bioluminescence intensity and oscillations of mPer2 (Figure 3A,B) were consistent with our previously reported results [25,26,31]. Both hPER2.1 and hPER2.2 also exhibited robust oscillations (Figure 3C–F). We noted minor differences in the signals observed across different experiments (see Figures S3–S5); however, they remained largely consistent within each experiment. Our results showed that the amplitudes of the oscillations were stronger in both hPER2.1 and hPER2.2 when compared with the mPer2 promoter reporter. This could be attributed to the use of longer lengths of the hPER2 promoters (781 bp for hPER2.1 and 853 bp for hPER2.2) compared to ~200 bp of the mPer2. While previous reports suggest that the use of the minimal mPer2 promoter with the E’-box is sufficient to elicit rhythmic oscillations [21], there may be additional elements in the promoter that might be responsible for regulating the strengths and amplitudes of oscillations. Another reason for the higher amplitude of the hPER2.1 promoter could be the presence of a miniCMV promoter downstream of the hPER2.1 promoter. Furthermore, we used lentiviral transduction to generate stable reporter cell lines; however, we cannot control the number of promoter-reporter copies transfected per cell, which could be a contributing factor resulting in higher amplitudes. Off-target insertion of the reporter sequence into the genome is another pitfall of lentiviral transduction and could result in disruptions to normal cellular processes. We note that the cells did not have any detrimental changes to their physical (i.e., changes in cell morphology or cell division, vacuole indicative of cell stress, and cell death), or circadian attributes.
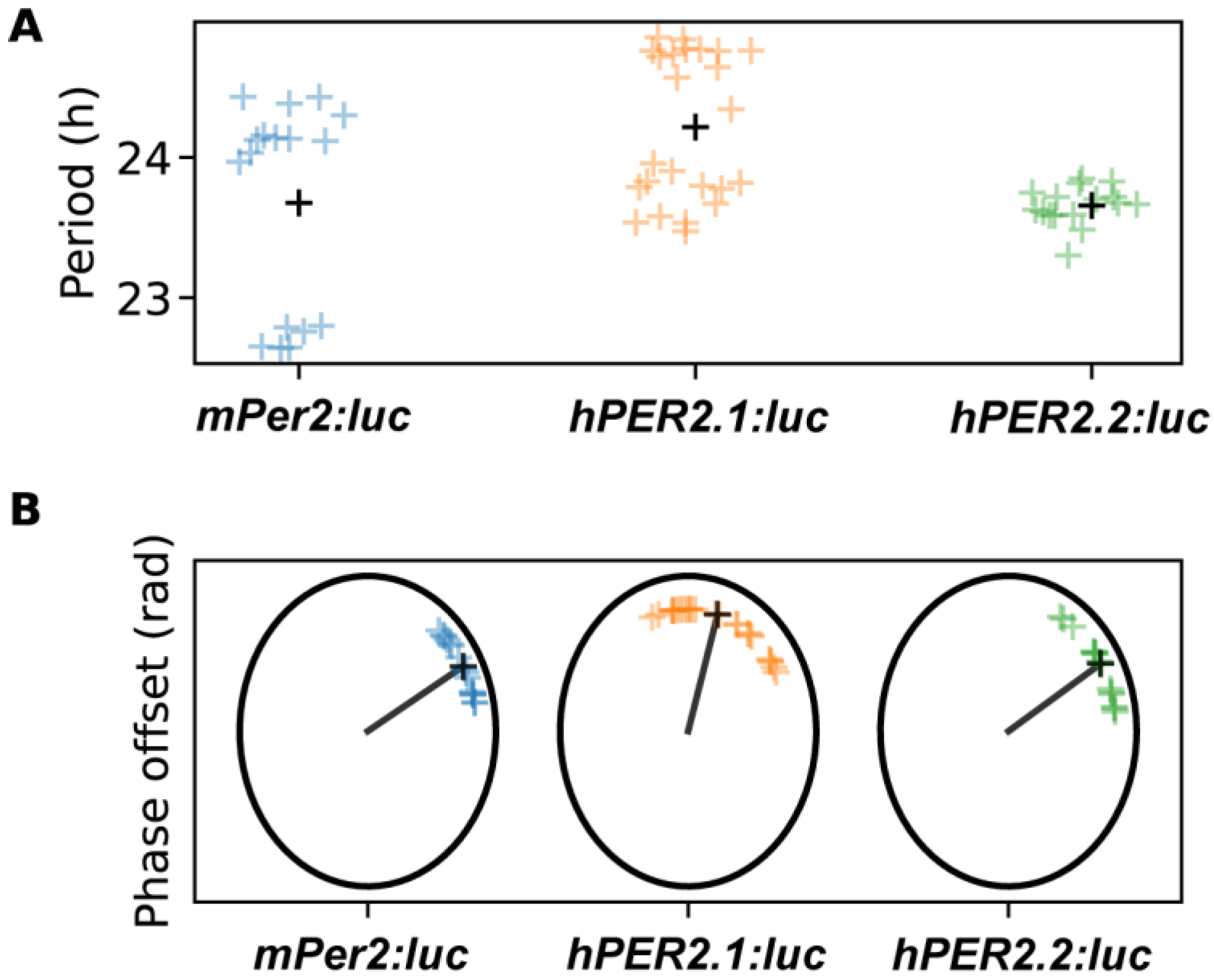
We then evaluated circadian parameters, including period and phase offsets (Figure 4 and Figure S6). Both were estimated by fitting a damped cosine curve to the data (see Section 3). The phase offset, measured in radians, can be interpreted as the portion of a cycle between the time of synchronization (end of dexamethasone pulse) and the time of the first peak. Time-series were labeled as outliers if the period or phase offset values were more than two standard deviations away from the mean calculated across all of the replicates for a given reporter. Excluding outliers (one replicate from mPer2:luc and two replicates from the hPER2.2:luc time-series), we found the period of hPER2.1 to be slightly longer than that of mPer2, while the period of hPER2.2 was similar to mPer2. The average period of mPer2 was determined to be 23.68 ± 0.72 h, which aligned with mPer2 period values previously calculated in U2OS cells [25,26,31,43]. The average periods of hPER2.1 and hPER2.2 were determined to be 24.22 ± 0.51 h and 23.66 ± 0.13 h, respectively (Figure 4 and Figure S6A,B). We also determined periods by estimating the average differences in timing of the first four peaks starting 24 h after the end of the dexamethasone treatment. In these instances, the periods were found to be 23.83 ± 0.89 h, 24.65 ± 0.57 h, and 23.75 ± 0.15 h for mPer2, hPER2.1, and hPER2.2, respectively (Figure S6C).
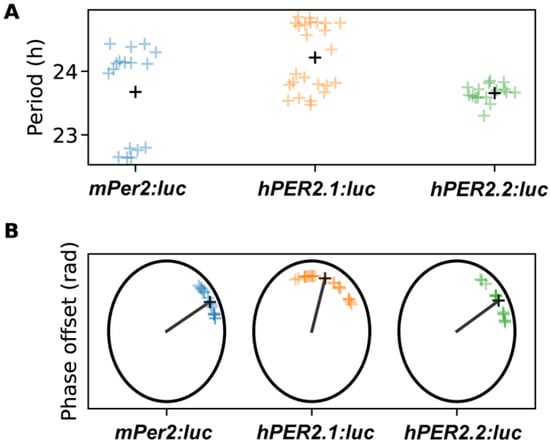
Figure 4.
Shown are the period (A) and phase-offset (B) values estimated by fitting a damped cosine curve to de-trended mPer2:luc, hPER2.1:luc, and hPER2.2:luc time-series. The black plus (+) signs in (A) indicate average period, and the black line and x’s indicate average phase in (B). (N = 17 for mPer2:luc from three independent experiments, N = 24 for hPER2.1:luc from three independent experiments, and N = 16 for hPER2.2:luc from four independent experiments).
The phase offset values for mPer2 and hPER2.2 were found to be similar (0.16π ± 0.07π rad and 0.17π ± 0.09π rad, respectively), while we observed a phase delay for hPER2.1 (Figure 4; phase offset = 0.41π ± 0.15π rad). These results align with observations previously reported using mPer2 promoter sequences [44]. We note that in one experiment presented here, mPer2 periods were shorter but their phase offsets had little effect on the mean of standard deviation of the distribution. Excluding this “short-period” experiment led to an mPer2 period of 24.2 ± 0.15 h and a phase offset of 0.12π ± 0.04π rad. We also determined the phases by calculating the difference between the first peak and its ideal timing, given a peak of bioluminescence at t = 0 h and the period estimated with damped cosine-fitting (Figure S6D). Including all experiments, those phase estimates were 2.00 ± 0.85 h for mPer2, 4.35 ± 1.92 h for hPER2.1, and 1.47 ± 1.07 h for hPER2.2. Excluding the short mPer2-period experiment, the alternate phase estimate for mPer2 was 1.62 ± 0.77 h. Our phase offset estimates capture the phase at the end of the synchronization treatment, so period length alone does not explain the differences. In fact, the short-period mPer2 time-series were not phase-advanced in comparison to the remaining mPer2 time-series. hPER2.1 time-series exhibited both delays and longer periods.
While the period and phase offsets showed variations across experiments, the values within each experiment were tightly grouped. The similarities in the period and phases of mPer2 and hPER2.2 can be attributed to the conserved “rhythm-generating element” located upstream of +1m [21]. We hypothesize that the presence of regulatory elements in the upstream region of hPER2.1, in addition to the rhythm-generating element, could be responsible for the longer periods and phase delays.
3. Materials and Methods
3.1. Plasmid Construction
The mPer2:luc reporter construct (pMA3160 lentiviral backbone) was previously generated by our group [10]. The hPER2.1:luc lentiviral reporter construct containing a 781 bp truncated human PER2 promoter fragment (−1114 to −333; relative to +1h) was obtained from Dr. Benedetto Grimaldi (Istituto Italiano di Tecnologia; Genova, Italy) [38]. For generating the hPER2.2:luc promoter reporter plasmid, a 941 bp truncated human PER2 promoter fragment (−752 to +101, relative to +1h) was PCR-amplified from a pGL4[Luc2P/Neo] backbone obtained from Dr. Helmut Zarbl (Rutgers University; New Brunswick, NJ, USA) [39]. The primers used for PCR were: forward primer (containing EcoRI restriction site, underlined) = 5′-ccg gaa ttc AGG TGG AGG TCT CCC TCG TCC GGC T-3′; reverse primer (containing NotI restriction site, underlined) = 5′-AAA TAT gcg gcc gcG GAG GGT TCC CAA AAG AGA A-3′. The EcoRI/NotI containing hPER2.2 promoter fragment was subcloned into a pMA3160 lentiviral vector (Addgene plasmid #35043, deposited by Dr. Mikhail Alexeyev) [45], thereby generating the hPER2.2:luc promoter reporter construct. The ligated plasmid DNA was transformed into the electrocompetent Stbl3 strain of E. coli (Thermo Fisher, Waltham, MA, USA), plated on LB agar plates containing ampicillin, and incubated for approximately 16–20 h at 37 °C. The recombinant clones were further expanded, and plasmid DNA was isolated using a GeneJET plasmid midiprep kit (Thermo Fisher Scientific, #K0481). The reporter plasmid was validated using Sanger sequencing and whole plasmid sequencing (Azenta Life Sciences, South Plainfield, NJ, USA).
3.2. Cell Culture
HEK293T (ATCC) cells were maintained in Dulbecco’s Modified Eagle Medium/Nutrient Mixture F12 (DMEM/F12; Gibco, Waltham, MA, USA) containing 10% fetal bovine serum (FBS; Corning, Corning, NY, USA) and 100 U/mL penicillin-streptomycin (Gibco). U2OS (ATCC) cells and derived reporter cell lines were cultured in Dulbecco’s Modified Eagle Medium (DMEM; Gibco) supplemented with 10% FBS, 100 U/mL penicillin-streptomycin (Gibco), 2mM glutamine (Gibco), 1mM sodium pyruvate (Gibco), and 1% non-essential amino acids (Cytiva, Marlborough, MA, USA). The cells were grown in an incubator maintained at 37 °C under 5% CO2 atmosphere.
3.3. Lentiviral Transductions
The generation of the U2OS-mPer2:luc cell line has been previously described [26]. For U2OS-hPER2.1:luc and -hPER2.2:luc cells, the procedure was as follows. HEK293T cells were used as the viral packaging cell line and seeded into 60 mm dishes at a density of 2.5 × 106 cells per dish. At 70–90% confluence (approximately 24 h), the cell culture media was replaced with fresh HEK293T culture media. The cells were further treated with a transfection mixture containing 5 μg of the target DNA (hPER2.1:luc, or hPER2.2:luc), 3 μg of lentiviral packaging plasmid (psPAX2; Addgene plasmid #12260, deposited by Dr. Didier Trono), and 2 μg of lentiviral envelope plasmid (pMD2.G; Addgene plasmid #12259, deposited by Dr. Didier Trono), along with Lipofectamine 3000 (Invitrogen, Waltham, MA, USA), following the manufacturer’s protocol. Meanwhile, the target cell line, U2OS, was seeded into T25 flasks at a density of 6 × 105 cells/flask and grown to 60–70% confluence.
After 48 h incubation, the viral supernatant from HEK293T cells was collected and sterile-filtered using a 0.45 μm syringe filter before combining it with U2OS culture media containing 4 μg/mL polybrene (Sigma-Aldrich, St. Louis, MO, USA) in a 1:1 ratio. Culture media from the U2OS cells was replaced with 6 mL of viral media, and the infections were repeated every 12 h over two days. The viral media was replaced with fresh media 24 h following the final infection. U2OS-hPER2.1:luc cells were passaged and screened for copGFP using flow cytometry (BD FACSAria Fusion at the Flow Cytometry Core Facility, Institute for Applied Life Sciences, UMass Amherst) to select for stably transfected cells, which were further cultured for future use. U2OS-hPER2.2:luc cells were treated with U2OS media containing 4 μg/mL of puromycin (Gibco) for 3–5 weeks, before expanding and freezing for future use.
3.4. Cell Synchronization and Bioluminescence Recording
For luminometry experiments, the U2OS-mPer2:luc, -hPER2.1:luc, and -hPER2.2:luc cells were seeded in 35 mm dishes at a density of 4 × 105 cells per dish. After approximately 24 h, the cells were rinsed with PBS and replaced with synchronization media containing 100 nM dexamethasone (Sigma-Aldrich) in U2OS cell culture media for two hours. After synchronization, the cells were rinsed with phosphate-buffered saline (PBS) and treated with recording media. Recording media was prepared by dissolving powdered DMEM (Sigma-Aldrich) in water (11.25 mg/mL). The resulting solution was sterile-filtered using a 0.22 μm syringe filter and further supplemented with 5% FBS, 10 mM HEPES (Cytiva), 4 mM sodium bicarbonate (Fisher Bioreagents, Waltham, MA, USA), 500 U/mL penicillin-streptomycin, and 500 μM D-luciferin (Thermo Scientific) dissolved in water. After the addition of recording media, the dishes were sealed with 40 mm cover glasses using autoclaved silicone vacuum grease, and the bioluminescence recordings were monitored using a Lumicycle 32 system (Actimetrics, Wilmette, IL, USA) for 7 days at 37 °C.
3.5. Data Analysis
The bioluminescence recordings were preprocessed to exclude the first 24 h of transient expression and discard the oscillations after 6 days. The data was then detrended by removing a 24 h sliding window and fit into a damped cosine curve with a linear baseline , where τ is the period in hours, θ is the phase in radians, and t is the time in hours. The bioluminescence time series was considered an outlier if the goodness-of-fit value was less than 0.8, or if the period and phase offset values were greater than two standard deviations away from the mean for all the replicates, for a given reporter.
4. Conclusions
PER2 is an integral component of the mammalian core clock. Prior studies have shown that the negative feedback established by PER2 governs the length of the circadian period [44,46]. The murine Per2 (mPer2) promoter has been studied extensively to recognize important regulatory elements that are responsible for the rhythmic behavior of the mammalian clock. Luciferase-based reporters driven by truncated sections of mPer2 promoters have been used to understand the fundamental circadian behavior of Per2 in homeostasis and disease. However, most of the reporters developed to date have utilized murine sequences in in vitro cell lines of both murine and human origin. While known, important regulatory elements are conserved across species, we should not disregard any possible variations and their effects arising from the unconserved sections of the Per2 gene.
In this work, we aimed to minimize species-specific variability by developing luciferase reporters driven by a human-derived PER2 promoter for use in in vitro cell lines of human origin. We utilized lentiviral luciferase constructs with two different sections of the hPER2 promoter (hPER2.1 and hPER2.2). Both promoters had a region of overlap, with hPER2.1 aligning with the upstream region of the endogenous promoter, while hPER2.2 aligned with the downstream region. Comparing the oscillations of both hPER2:luc reporters with murine Per2:luc in the commonly employed U2OS circadian model, we found that the human-derived promoters exhibited robust oscillations. Interestingly, we also noted that the upstream frame of the hPER2 promoter triggered a phase delay and a slightly longer period. This finding corroborated previous observations in mPer2 promoter sequences, highlighting the presence of phase-delaying elements in addition to the rhythm-generating element.
A possible explanation for the higher amplitudes in hPER2:luc cell lines is the use of longer promoter sequence frames. The mPer2:luc reporter used was designed to include the minimal region of the Per2 promoter that establishes circadian gene expression [21]. For mPer2, it has been shown that promoter lengths can affect amplitudes and that increasing them upstream of the E’-box can result in phase delays [22]. The use of longer frames for the hPER2:luc reporters could allow for synergistic cooperation between neighboring elements, resulting in a more dynamic expression pattern. While more research needs to be conducted to understand the regulatory mechanisms responsible for the variations in amplitudes and phases in human-derived clock components, our work provides a solid foundation and introduces reliable human-derived reporter sequences to track hPER2 transcription. In the future, these hPER2:luc sequences should be used to investigate circadian changes within the human core clock and to better understand the fundamental aspects of the negative feedback regulation in both health and disease.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms262110785/s1.
Author Contributions
Conceptualization, B.K. and M.E.F.; methodology, B.K., S.R.T. and M.E.F.; validation, B.K.; formal analysis, B.K., S.R.T. and M.E.F.; investigation, B.K. and G.V.; resources, M.E.F.; data curation, B.K.; writing—original draft preparation, B.K.; writing—review and editing, B.K., G.V., S.R.T. and M.E.F.; supervision, M.E.F.; project administration, M.E.F.; funding acquisition, M.E.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Institute of General Medical Sciences of the National Institutes of Health, under award number R35 GM143016. G.V. was supported in part by a Fellowship from the University of Massachusetts as part of the Biotechnology Training Program (National Research Service Award T32 GM135096).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original data presented in this study are openly available via the Data Repository at ScholarWorks. The URL is https://hdl.handle.net/20.500.14394/57756 (accessed on 24 September 2025).
Acknowledgments
We wish to thank Benedetto Grimaldi (Istituto Italiano di Tecnologia, Italy) and Helmut Zarbl (Rutgers University) for providing us with hPER2 promoter sequence-containing plasmids. We would also like to thank Amy Burnside and the Flow Cytometry Core Facility of the Institute for Applied Life Sciences (IALS), UMass Amherst.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| SCN | Suprachiasmatic nucleus |
| TTFL | Transcriptional-translational feedback loop |
| BMAL1 | Brain and muscle Arnt-like-1 |
| CLOCK | Circadian locomotor output cycles kaput |
| PER | Period |
| CRY | Cryptochrome |
| ChIP | Chromatin immunoprecipitation |
| RT-PCR | Reverse transcription-polymerase chain reaction |
| T-Coffee | Tree-based consistency objective function for alignment evaluation |
| MSA | Multiple sequence alignment |
| HRE | Hypoxia response element |
| luc | Luciferase |
| TSS | Transcription start site |
| PCR | Polymerase chain reaction |
| LB | Luria broth |
| DMEM | Dulbecco’s modified eagle medium |
| FBS | Fetal bovine serum |
| PBS | Phosphate-buffered saline |
References
- Mohawk, J.A.; Takahashi, J.S. Cell Autonomy and Synchrony of Suprachiasmatic Nucleus Circadian Oscillators. Trends Neurosci. 2011, 34, 349–358. [Google Scholar] [CrossRef]
- Welsh, D.K.; Takahashi, J.S.; Kay, S.A. Suprachiasmatic Nucleus: Cell Autonomy and Network Properties. Annu. Rev. Physiol. 2010, 72, 551–577. [Google Scholar] [CrossRef] [PubMed]
- Honma, S. The Mammalian Circadian System: A Hierarchical Multi-Oscillator Structure for Generating Circadian Rhythm. J. Physiol. Sci. 2018, 68, 207–219. [Google Scholar] [CrossRef]
- Takahashi, J.S. Molecular Components of the Circadian Clock in Mammals. Diab. Obes. Metab. 2015, 17, 6–11. [Google Scholar] [CrossRef]
- Cao, X.; Yang, Y.; Selby, C.P.; Liu, Z.; Sancar, A. Molecular Mechanism of the Repressive Phase of the Mammalian Circadian Clock. Proc. Natl. Acad. Sci. USA 2021, 118, e2021174118. [Google Scholar] [CrossRef] [PubMed]
- Ye, R.; Selby, C.P.; Ozturk, N.; Annayev, Y.; Sancar, A. Biochemical Analysis of the Canonical Model for the Mammalian Circadian Clock. J. Biol. Chem. 2011, 286, 25891–25902. [Google Scholar] [CrossRef] [PubMed]
- Schmutz, I.; Ripperger, J.A.; Baeriswyl-Aebischer, S.; Albrecht, U. The Mammalian Clock Component PERIOD2 Coordinates Circadian Output by Interaction with Nuclear Receptors. Genes Dev. 2010, 24, 345–357. [Google Scholar] [CrossRef]
- Xu, H.; Gustafson, C.L.; Sammons, P.J.; Khan, S.K.; Parsley, N.C.; Ramanathan, C.; Lee, H.W.; Liu, A.C.; Partch, C.L. Cryptochrome 1 Regulates the Circadian Clock through Dynamic Interactions with the BMAL1 C-Terminus. Nat. Struct. Mol. Biol. 2015, 22, 476–484. [Google Scholar] [CrossRef]
- Yoo, S.H.; Yamazaki, S.; Lowrey, P.L.; Shimomura, K.; Ko, C.H.; Buhr, E.D.; Siepka, S.M.; Hong, H.K.; Oh, W.J.; Yoo, O.J.; et al. PERIOD2:: LUCIFERASE Real-Time Reporting of Circadian Dynamics Reveals Persistent Circadian Oscillations in Mouse Peripheral Tissues. Proc. Natl. Acad. Sci. USA 2004, 101, 5339–5346. [Google Scholar] [CrossRef]
- Lin, H.H.; Qraitem, M.; Lian, Y.; Taylor, S.R.; Farkas, M.E. Analyses of BMAL1 and PER2 Oscillations in a Model of Breast Cancer Progression Reveal Changes With Malignancy. Integr. Cancer Ther. 2019, 18, 1534735419836494. [Google Scholar] [CrossRef]
- Ramanathan, C.; Khan, S.K.; Kathale, N.D.; Xu, H.; Liu, A.C. Monitoring Cell-Autonomous Circadian Clock Rhythms of Gene Expression Using Luciferase Bioluminescence Reporters. J. Vis. Exp. 2012, 67, 4234. [Google Scholar]
- Yang, N.; Smyllie, N.J.; Morris, H.; Gonçalves, C.F.; Dudek, M.; Pathiranage, D.; Chesham, J.E.; Adamson, A.; Spiller, D.; Zindy, E.; et al. Quantitative Live Imaging of Venus::BMAL1 in a Mouse Model Reveals Complex Dynamics of the Master Circadian Clock Regulator. PLoS Genet. 2020, 16, e1008729. [Google Scholar] [CrossRef] [PubMed]
- Smyllie, N.J.; Pilorz, V.; Boyd, J.; Meng, Q.J.; Saer, B.; Chesham, J.E.; Maywood, E.S.; Krogager, T.P.; Spiller, D.G.; Boot-Handford, R.; et al. Visualizing and Quantifying Intracellular Behavior and Abundance of the Core Circadian Clock Protein PERIOD2. Curr. Biol. 2016, 26, 1880–1886. [Google Scholar] [CrossRef] [PubMed]
- Koch, A.A.; Bagnall, J.S.; Smyllie, N.J.; Begley, N.; Adamson, A.; Fribourgh, J.L.; Spiller, D.G.; Meng, Q.J.; Partch, C.L.; Strimmer, K.; et al. Quantification of Protein Abundance and Interaction Defines a Mechanism for Operation of the Circadian Clock. eLife 2022, 11, e73976. [Google Scholar] [CrossRef] [PubMed]
- Maywood, E.S.; Drynan, L.; Chesham, J.E.; Edwards, M.D.; Dardente, H.; Fustin, J.M.; Hazlerigg, D.G.; O’Neill, J.S.; Codner, G.F.; Smyllie, N.J.; et al. Analysis of Core Circadian Feedback Loop in Suprachiasmatic Nucleus of MCry1-Luc Transgenic Reporter Mouse. Proc. Natl. Acad. Sci. USA 2013, 110, 9547–9552. [Google Scholar] [CrossRef]
- Ono, D.; Honma, K.I.; Honma, S. Circadian and Ultradian Rhythms of Clock Gene Expression in the Suprachiasmatic Nucleus of Freely Moving Mice. Sci. Rep. 2015, 5, 12310. [Google Scholar] [CrossRef]
- Liu, A.C.; Tran, H.G.; Zhang, E.E.; Priest, A.A.; Welsh, D.K.; Kay, S.A. Redundant Function of REV-ERBα and β and Non-Essential Role for Bmal1 Cycling in Transcriptional Regulation of Intracellular Circadian Rhythms. PLoS Genet. 2008, 4, e1000023. [Google Scholar] [CrossRef]
- Smyllie, N.J.; Bagnall, J.; Koch, A.A.; Niranjan, D.; Polidarova, L.; Chesham, J.E.; Chin, J.W.; Partch, C.L.; Loudon, A.S.I.; Hastings, M.H. Cryptochrome Proteins Regulate the Circadian Intracellular Behavior and Localization of PER2 in Mouse Suprachiasmatic Nucleus Neurons. Proc. Natl. Acad. Sci. USA 2022, 119, e2113845119. [Google Scholar] [CrossRef]
- D’Alessandro, M.; Beesley, S.; Kim, J.K.; Jones, Z.; Chen, R.; Wi, J.; Kyle, K.; Vera, D.; Pagano, M.; Nowakowski, R.; et al. Stability of Wake-Sleep Cycles Requires Robust Degradation of the PERIOD Protein. Curr. Biol. 2017, 27, 3454–3467. [Google Scholar] [CrossRef]
- Lee, Y.; Chen, R.; Lee, H.M.; Lee, C. Stoichiometric Relationship among Clock Proteins Determines Robustness of Circadian Rhythms. J. Biol. Chem. 2011, 286, 7033–7042. [Google Scholar] [CrossRef]
- Yoo, S.H.; Ko, C.H.; Lowrey, P.L.; Buhr, E.D.; Song, E.J.; Chang, S.; Yoo, O.J.; Yamazaki, S.; Lee, C.; Takahashi, J.S. A Noncanonical E-Box Enhancer Drives Mouse Period2 Circadian Oscillations in Vivo. Proc. Natl. Acad. Sci. USA 2005, 102, 2608–2613. [Google Scholar] [CrossRef]
- Yamajuku, D.; Shibata, Y.; Kitazawa, M.; Katakura, T.; Urata, H.; Kojima, T.; Nakata, O.; Hashimoto, S. Identification of Functional Clock-Controlled Elements Involved in Differential Timing of Per1 and Per2 Transcription. Nucleic Acids Res. 2010, 38, 7964–7973. [Google Scholar] [CrossRef]
- Ramanathan, C.; Liu, A.C. Developing Mammalian Cellular Clock Models Using Firefly Luciferase Reporter. Methods Mol. Biol. 2018, 1755, 49–64. [Google Scholar]
- Nakajima, Y.; Yamazaki, T.; Nishii, S.; Noguchi, T.; Hoshino, H.; Niwa, K.; Viviani, V.R.; Ohmiya, Y. Enhanced Beetle Luciferase for High-Resolution Bioluminescence Imaging. PLoS ONE 2010, 5, e10011. [Google Scholar] [CrossRef]
- Chhe, K.; Hegde, M.S.; Taylor, S.R.; Farkas, M.E. Circadian Effects of Melatonin Receptor-Targeting Molecules In Vitro. Int. J. Mol. Sci. 2024, 25, 13508. [Google Scholar] [CrossRef]
- Lin, H.H.; Robertson, K.L.; Bisbee, H.A.; Farkas, M.E. Oncogenic and Circadian Effects of Small Molecules Directly and Indirectly Targeting the Core Circadian Clock. Integr. Cancer Ther. 2020, 19, 1534735420924094. [Google Scholar] [CrossRef] [PubMed]
- Lellupitiyage Don, S.S.; Lin, H.H.; Furtado, J.J.; Qraitem, M.; Taylor, S.R.; Farkas, M.E. Circadian Oscillations Persist in Low Malignancy Breast Cancer Cells. Cell Cycle 2019, 18, 2447–2453. [Google Scholar] [CrossRef] [PubMed]
- Altman, B.J.; Hsieh, A.L.; Sengupta, A.; Krishnanaiah, S.Y.; Stine, Z.E.; Walton, Z.E.; Gouw, A.M.; Venkataraman, A.; Li, B.; Goraksha-Hicks, P.; et al. MYC Disrupts the Circadian Clock and Metabolism in Cancer Cells. Cell Metab. 2015, 22, 1009–1019. [Google Scholar] [CrossRef]
- Zhang, E.E.; Liu, A.C.; Hirota, T.; Miraglia, L.J.; Welch, G.; Pongsawakul, P.Y.; Liu, X.; Atwood, A.; Huss, J.W.; Janes, J.; et al. A Genome-Wide RNAi Screen for Modifiers of the Circadian Clock in Human Cells. Cell 2009, 139, 199–210. [Google Scholar] [CrossRef]
- Hirota, T.; Lee, J.W.; St. John, P.C.; Sawa, M.; Iwaisako, K.; Noguchi, T.; Pongsawakul, P.Y.; Sonntag, T.; Welsh, D.K.; Brenner, D.A.; et al. Identification of Small Molecule Activators of Cryptochrome. Science 2012, 337, 1094–1097. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.H.; Robertson, K.L.; Lellupitiyage Don, S.S.; Taylor, S.R.; Farkas, M.E. Chemical Modulation of Circadian Rhythms and Assessment of Cellular Behavior via Indirubin and Derivatives. Methods Enzymol. 2020, 639, 115–140. [Google Scholar]
- Burns, J.N.; Jenkins, A.K.; Xue, X.; Petersen, K.A.; Ketchesin, K.D.; Perez, M.S.; Vadnie, C.A.; Scott, M.R.; Seney, M.L.; Tseng, G.C.; et al. Comparative Transcriptomic Rhythms in the Mouse and Human Prefrontal Cortex. Front. Neurosci. 2024, 18, 1524615. [Google Scholar] [CrossRef]
- Goity, A.; Dovzhenok, A.; Lim, S.; Honh, C.; Loros, J.; Dunlap, J.C.; Larrondo, L.F. Trancriptional Rewiring of an Evolutionarily Conserved Circadian Clock. EMBO J. 2024, 43, 2015–2034. [Google Scholar] [CrossRef] [PubMed]
- Maier, B.; Wendt, S.; Vanselow, J.T.; Wallaeh, T.; Reischl, S.; Oehmke, S.; Sehlosser, A.; Kramer, A. A Large-Scale Functional RNAi Screen Reveals a Role for CK2 in the Mammalian Circadian Clock. Genes. Dev. 2009, 23, 708–718. [Google Scholar] [CrossRef]
- Crosby, P.; Hamnett, R.; Putker, M.; Hoyle, N.P.; Reed, M.; Karam, C.J.; Maywood, E.S.; Stangherlin, A.; Chesham, J.E.; Hayter, E.A.; et al. Insulin/IGF-1 Drives PERIOD Synthesis to Entrain Circadian Rhythms with Feeding Time. Cell 2019, 177, 896–909.e20. [Google Scholar] [CrossRef]
- Xu, Y.; Toh, K.L.; Jones, C.R.; Shin, J.Y.; Fu, Y.H.; Ptáček, L.J. Modeling of a Human Circadian Mutation Yields Insights into Clock Regulation by PER2. Cell 2007, 128, 59–70. [Google Scholar] [CrossRef] [PubMed]
- Vakili, H.; Jin, Y.; Cattini, P.A. Evidence for a Circadian Effect on the Reduction of Human Growth Hormone Gene Expression in Response to Excess Caloric Intake. J. Biol. Chem. 2016, 291, 13823–13833. [Google Scholar] [CrossRef] [PubMed]
- Blaževitš, O.; Bolshette, N.; Vecchio, D.; Guijarro, A.; Croci, O.; Campaner, S.; Grimaldi, B. MYC-Associated Factor MAX Is a Regulator of the Circadian Clock. Int. J. Mol. Sci. 2020, 21, 2294. [Google Scholar] [CrossRef]
- Fang, M.; Kang, H.G.; Park, Y.; Estrella, B.; Zarbl, H. In Vitro Bioluminescence Assay to Characterize Circadian Rhythm in Mammary Epithelial Cells. J. Vis. Exp. 2017, 127, 55832. [Google Scholar]
- Madeira, F.; Madhusoodanan, N.; Lee, J.; Eusebi, A.; Niewielska, A.; Tivey, A.R.N.; Lopez, R.; Butcher, S. The EMBL-EBI Job Dispatcher Sequence Analysis Tools Framework in 2024. Nucleic. Acids. Res. 2024, 52, W521–W525. [Google Scholar] [CrossRef]
- Nakahata, Y.; Yoshida, M.; Takano, A.; Soma, H.; Yamamoto, T.; Yasuda, A.; Nakatsu, T.; Takumi, T. A Direct Repeat of E-Box-like Elements Is Required for Cell-Autonomous Circadian Rhythm of Clock Genes. BMC Mol. Biol. 2008, 9, 1. [Google Scholar] [CrossRef]
- Zhuang, Y.; Li, Z.; Xiong, S.; Sun, C.; Li, B.; Wu, S.A.; Lyu, J.; Shi, X.; Yang, L.; Chen, Y.; et al. Circadian Clocks Are Modulated by Compartmentalized Oscillating Translation. Cell 2023, 186, 3245–3260.e23. [Google Scholar] [CrossRef]
- Lellupitiyage Don, S.S.; Robertson, K.L.; Lin, H.H.; Labriola, C.; Harrington, M.E.; Taylor, S.R.; Farkas, M.E. Nobiletin Affects Circadian Rhythms and Oncogenic Characteristics in a Cell-Dependent Manner. PLoS ONE 2020, 15, e0236315. [Google Scholar] [CrossRef] [PubMed]
- Akashi, M.; Ichise, T.; Mamine, T.; Takumi, T. Molecular Mechanism of Cell-Autonomous Circadian Gene Expression of Period2, a Crucial Regulator of the Mammalian Circadian Clock. Mol. Biol. Cell 2006, 17, 555–565. [Google Scholar] [CrossRef] [PubMed]
- Alexeyev, M.F.; Fayzulin, R.; Shokolenko, I.N.; Pastukh, V. A Retro-Lentiviral System for Doxycycline-Inducible Gene Expression and Gene Knockdown in Cells with Limited Proliferative Capacity. Mol. Biol. Rep. 2010, 37, 1987–1991. [Google Scholar] [CrossRef]
- Wilkins, A.K.; Barton, P.I.; Tidor, B. The Per2 Negative Feedback Loop Sets the Period in the Mammalian Circadian Clock Mechanism. PLoS Comput. Biol. 2007, 3, e242. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).