Abstract
Cholangiocarcinoma (CCA) is a biliary tract cancer that accounts for approximately 3% of all gastrointestinal cancers. CCA is a “silent” disease that remains undetected for a long period of time, often presenting at an advanced stage with minimal treatment options and a poor prognosis. Advanced CCA remains largely inoperable, and combination gemcitabine plus cisplatin (GemCis) chemotherapy remains the standard treatment for patients affected by this disease. There is a desperate need for new therapeutic alternatives, and extensive research is ongoing to address this gap. Targeted therapies represent a rapidly expanding area of cancer treatment and are currently under active investigation in CCA. The FDA has approved the targeted therapies ivosidenib, pemigatinib, infigratinib, and futibatinib, as well as the immunotherapy durvalumab, for patients with CCA in recent years. Several other therapeutic strategies are still under investigation, targeting molecular pathways including p53/MDM2, JAK/STAT, KRAS, HER2, VEGFR, PDGFR, MET, ALK, MAPK, PI3K/AKT, BRAF, and DNA damage repair signaling. While several promising advancements have been made, further research is required to improve outcomes for patients with CCA. This review provides an up-to-date, comprehensive overview of currently approved targeted therapies in CCA, as well as those under investigation.
1. Introduction
Cholangiocarcinoma (CCA) is a biliary tract cancer characterized by the formation of malignant tumors within the biliary tree [1,2]. It is the second most common primary liver cancer [3,4,5] and accounts for approximately 3% of all gastrointestinal cancers [6]. The global incidence of CCA is relatively low in Western countries, at less than 2 per 100,000 people, but is markedly higher in Thailand and other Eastern countries, where rates exceed 6 per 100,000 people [4,6]. Although several risk factors are known, its predominance in the East is largely attributed to liver fluke infections caused by Opisthorchis viverrini and Clonorchis sinensis, associated with poor sanitation and the consumption of raw fish in traditional dishes [7,8,9].
CCA is classified as intrahepatic (iCCA, 10–20%), perihilar (pCCA, 50–60%), or distal (dCCA, 10–20%) based on tumor location within the biliary tree [1,2,3,10,11,12]. iCCA arises above the second-order bile ducts, pCCA occurs between the second-order bile ducts and the junction of the cystic duct and common bile duct, and dCCA arises below this junction (Figure 1) [3,10]. Perihilar and distal CCA are collectively termed extrahepatic CCA (eCCA). CCA is often referred to as a “silent” disease due to its asymptomatic nature in the early stages, leading to late diagnosis and limited treatment options [6,13]. The disease exhibits high therapeutic resistance due to factors such as a complex tumor microenvironment (TME) and diverse genetic manifestations, resulting in poor prognosis and low five-year overall survival rate of 15–30% [2]. For patients with unresectable CCA, median overall survival (mOS) is approximately one year, with a five-year survival rate not exceeding 10% [14].
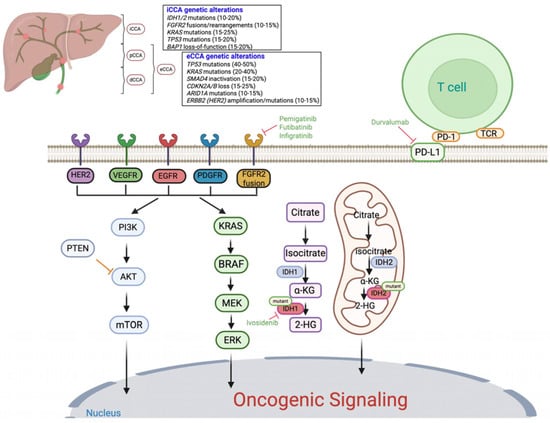
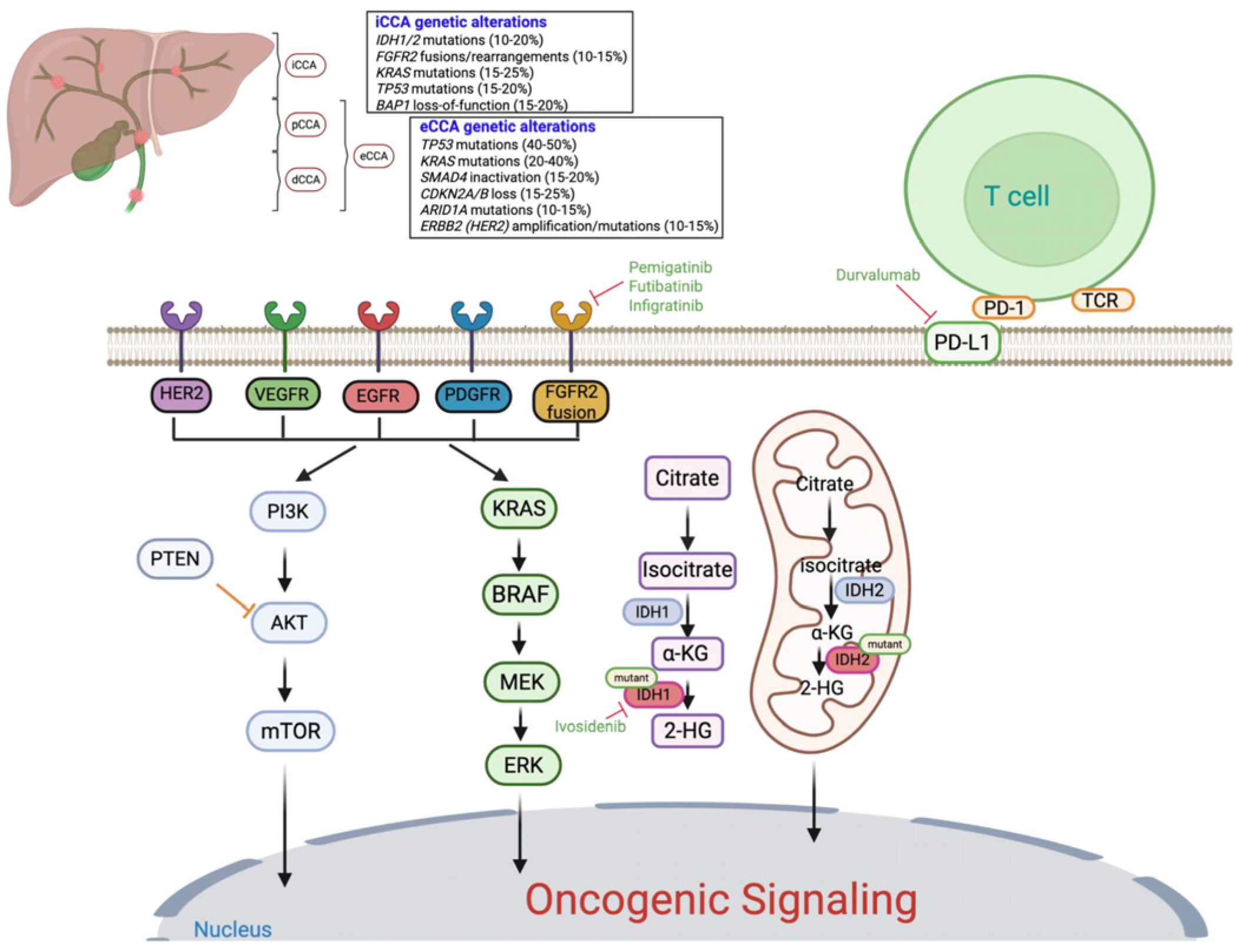
Figure 1.
Anatomical and molecular landscape of cholangiocarcinoma and key oncogenic signaling pathways: The figure illustrates the anatomical classification of cholangiocarcinoma (CCA) into intrahepatic (iCCA), perihilar (pCCA), and distal (dCCA) subtypes, along with representative genetic alterations commonly observed in each. Major oncogenic signaling cascades implicated in CCA pathogenesis are shown, including the RAS/MAPK and PI3K/AKT/mTOR pathways, which regulate cell proliferation, apoptosis, and survival. Tyrosine kinase receptor alterations (e.g., FGFR2, HER2, EGFR, VEGFR, PDGFR) and downstream mutations (KRAS, BRAF, PTEN) contribute to aberrant signaling. Mutations in IDH1 and IDH2 lead to accumulation of the oncometabolite 2-hydroxyglutarate (2HG), promoting epigenetic dysregulation. Approved targeted therapies are indicated in green text, including FGFR inhibitors (pemigatinib, futibatinib, infigratinib), the IDH1 inhibitor (ivosidenib), and immune checkpoint blockade (durvalumab, targeting PD-L1). Additional potential therapeutic targets with investigational agents currently under evaluation are also shown.
Surgery remains the only curative treatment option but is often not feasible at diagnosis. Even when resection is possible, recurrence rates approach 50% within the first year [13,15,16]. A combination of gemcitabine and cisplatin (GemCis) chemotherapy is the standard of care for advanced, inoperable CCA and has been shown to extend mOS compared to best supportive care (12.8 vs. 6.1 months) [2,17,18,19,20]. The FOLFOX regimen (5-fluorouracil plus oxaliplatin) has been approved as a second-line therapy for patients who progress on GemCis, based on the ABC-06 trial, which demonstrated clinically meaningful improvements in 6-month and 12-month survival rates [21]. More recently, the TOPAZ-1 trial led to the approval of durvalumab (an anti-PD-L1 antibody) combined with GemCis as the preferred first-line treatment for advanced or metastatic CCA, improving overall survival compared to GemCis alone (mOS: 12.9 vs. 11.3 months) [22]. In parallel, the KEYNOTE-966 trial evaluated pembrolizumab (a humanized anti-PD-1 antibody) in combination with GemCis in a similar population and demonstrated a comparable survival benefit (mOS: 12.7 vs. 10.9 months) without introducing new safety concerns (NCT04003636). Together, these two pivotal phase III studies establish chemoimmunotherapy as the current standard first-line approach for advanced or metastatic CCA [23].
Given the poor prognosis associated with CCA and limited treatment options, there remains a dire need to develop new therapies for patients affected by this deadly disease. This review provides a comprehensive overview of the current state of CCA research and highlights emerging targeted therapies that may offer potential new treatment avenues.
2. Recent Success of Targeted Therapies in CCA: Tyrosine Kinase Inhibitors
Although chemotherapy has long been the standard treatment for unresectable or metastatic CCA, its clinical benefit is limited by toxicity and modest survival gains [24,25].
Advances in genomic profiling have revealed a spectrum of actionable molecular alterations, paving the way for precision medicine approaches. TP53 and KRAS mutations are the most common [26,27,28]. In iCCA, frequent alterations include IDH1/2 mutations (10–20%), FGFR2 fusions/rearrangements (10–15%), KRAS mutations (15–25%), TP53 mutations (15–20%), BAP1 loss-of-function (15–20%), ARID1A mutations (10–15%), PIK3CA mutations (5–10%), SMAD4 mutations (5–10%), ATM mutations (5–10%), and BRCA1/2 alterations (5–7%) (Figure 1) [29,30]. In eCCA, frequent alterations include TP53 mutations (40–50%), KRAS mutations (20–40%), SMAD4 inactivation (15–20%), CDKN2A/B loss (15–25%), ARID1A mutations (10–15%), ERBB2 (HER2) amplification/mutations (10–15%), PIK3CA mutations (5–10%), BAP1 alterations (5–10%), ATM mutations (5–8%), and BRCA1/2 alterations (3–5%) (Figure 1) [31,32,33]. Between the two classifications of eCCA, KRAS and TP53 mutations predominate in pCCA, whereas SMAD4 and ERBB2 amplification/mutations (~10–15%) are more common in dCCA. These molecular distinctions underscore potential differences in tumor biology and therapeutic vulnerabilities between pCCA and dCCA.
Identification of actionable mutations, particularly IDH1 and FGFR2 alterations, has enabled targeted therapies to improve patient outcomes, as discussed in the following sections. Currently approved agents include the IDH1 inhibitor ivosidenib and the FGFR2 inhibitors pemigatinib, infigratinib, and futibatinib [34]. These advances provide hope for more effective, personalized treatment strategies for CCA patients.
2.1. FGFR Inhibitors
FGFR2 gene fusions occur in ~10–15% of iCCA cases, making FGFR2 a compelling therapeutic target [35,36]. Several FGFR inhibitors have demonstrated clinical efficacy in iCCA. Pemigatinib, a selective FGFR1–3 inhibitor, demonstrated efficacy in the phase I/II FIGHT-101 trial (NCT02393248) in patients with previously treated solid tumors with or without FGFR aberrations [37]. Subsequently, the phase II FIGHT-202 trial (NCT02924376) enrolled previously treated CCA patients harboring FGFR2 gene fusions or rearrangements, other FGFR aberrations, or no FGFR aberrations. This trial showed notable efficacy in patients with FGFR2 fusions or rearrangements, with a median progression-free survival (mPFS) of 6.9 months and mOS of 21.1 months. Based on these findings, the FDA approved pemigatinib in 2020 for previously treated, unresectable, advanced, or metastatic CCA patients harboring FGFR2 fusions or other alterations. Final results from the FIGHT-202 trial, published in June 2024, showed an mPFS of 7.0 months (95% CI: 6.1–10.5) and an mOS of 17.5 months (95% CI: 14.4–22.9) (Table 1) [38]. A Phase III study (NCT03656536) comparing pemigatinib vs. GemCis in treatment-naïve patients with unresectable or metastatic CCA harboring FGFR2 fusions or rearrangements is currently ongoing.
Similarly, infigratinib, another FGFR1–3 inhibitor, demonstrated an mPFS of 7.3 months and mOS 12.2 months in a phase II study, leading to FDA approval in 2021 for patients with FGFR2 fusions or alterations [39]. On 16 May 2024, FDA approval for infigratinib was withdrawn at the sponsor’s request due to the inability to complete confirmatory studies (https://www.fda.gov/drugs/resources-information-approved-drugs/withdrawn-fda-grants-accelerated-approval-infigratinib-metastatic-cholangiocarcinoma (accessed on 23 October 2025)) (Table 1). More recently, futibatinib, an irreversible FGFR1–4 inhibitor, demonstrated an mPFS of 8.9 months and mOS of 20.0 months in a phase II study and received FDA approval in 2022 for iCCA patients with FGFR2 fusions or other alterations (Table 1) [40].
Beyond direct oncogenic signaling, activated FGFR pathways contribute to an immunosuppressive TME by upregulating immune checkpoint molecules such as PD-L1, suppressing T-cell function, and promoting tumor-associated macrophage polarization. These findings provide a strong rationale for evaluating FGFR inhibitors in combination with immune checkpoint inhibitors (ICIs) in FGFR-altered cancers, including CCA [41]. A phase I/II trial investigating the combination of pemigatinib plus pembrolizumab demonstrated tolerability and preliminary antitumor activity in patients with advanced malignancies, including those with FGF/FGFR alterations [42].

Table 1.
List of FDA-approved small-molecule targeted therapies for cholangiocarcinoma (CCA), organized by molecular target and clinical indication.
Table 1.
List of FDA-approved small-molecule targeted therapies for cholangiocarcinoma (CCA), organized by molecular target and clinical indication.
| Drug | Approval Year | Target | Patients | Efficacy Results | Most Common Grade ≥ 3 AEs | Trial No./Reference |
|---|---|---|---|---|---|---|
| Pemigatinib | 2020 | FGFR1, FGFR2, FGFR3 Little to no activity against FGFR4 | Previously treated, advanced or metastatic CCA harboring FGFR2 fusions or rearrangements | mPFS: 7.0 mo mOS: 17.5 mo | Hyperphosphatemia 14.3% Fatigue 5% Stomatitis 7% Arthralgia 6% | NCT02924376 [38] |
| Infigratinib | 2021 Withdrawn 2024 | Selective for FGFR1–3 | Previously treated, unresectable locally advanced or metastatic CCA with FGFR2 fusion/rearrangement | mPFS: 7.3 mo mOS: 12.2 mo | Hypophosphatemia 14.1% Hyperphosphatemia 12.7% Hyponatremia 11.3% Stomatitis 9.9% | NCT02150967 [39] |
| Futibatinib | 2022 | Selective for FGFR1–4 | Previously treated, unresectable locally advanced or metastatic iCCA with FGFR2 fusion/rearrangement | mPFS: 9.0 mo mOS: 21.7 mo | Hyperphosphatemia 30% Elevated AST level 7% Stomatitis 6% Fatigue 6% | NCT04093362 [40] |
| Ivosidenib | 2021 | Mutant IDH1 enzyme | Previously treated, locally advanced/metastatic CCA with an IDH1 mutation | mPFS: 2.7 months mOS: 10.3 months | Anemia 7% Increased blood bilirubin 6% Hyponatremia 6% Ascites 9% | NCT02989857 [43] |
2.2. IDH1 Inhibitors
Mutations in IDH1/2 are present in 10–30% of iCCA cases and are less common in eCCA (~7%). IDH1 mutations are predominant, representing 90% of IDH mutations, with the remainder being IDH2 mutations [44,45,46]. Ivosidenib, an inhibitor specific to mutant IDH1, suppresses production of the oncometabolite 2-hydroxyglutarate, exhibiting antitumor effects. In a phase III randomized trial, ivosidenib significantly improved PFS (2.7 vs. 1.4 months) and OS (10.3 vs. 7.5 months) compared with placebo in patients with previously treated, locally advanced, or metastatic IDH1-mutant CCA [43]. Common treatment-related adverse events (AEs) included nausea, ascites, anemia, hyperbilirubinemia, and hyponatremia. Based on these findings, the FDA approved ivosidenib in 2021 for adult patients with IDH1-mutant CCA (Table 1) [43]. Although data on resistance mechanisms are limited [47], in vitro studies suggest potential resistance through isoform switching between mutant IDH1 and IDH2 [48].
Considering that IDH1 mutations can promote an immunosuppressive TME, there is potential for synergy between IDH1 inhibition and ICIs [49]. A phase II trial (NCT04056910) of ivosidenib plus nivolumab (a fully human anti-PD-1 antibody) in patients with an advanced or refractory IDH1-mutant solid tumor, including CCA, demonstrated that all four patients with iCCA had stable disease (SD) [50]. Additionally, a phase I/II study (NCT05921760) is ongoing to assess the safety, tolerability, and preliminary efficacy of ivosidenib in combination with nivolumab and ipilimumab in previously treated patients with unresectable or metastatic IDH1-mutant CCA [51].
Overall, the cumulative evidence from phase II and III trials supports FGFR2 and IDH1 inhibitors as the most validated molecularly targeted approaches in advanced CCA. These therapies offer meaningful clinical benefit in biomarker-selected patients, marking a paradigm shift toward precision medicine. However, expanding these benefits beyond select genomic subgroups will require continued investigation into resistance mechanisms and combination strategies.
3. Immunotherapy in CCA
Immunotherapy has emerged as a promising approach in CCA, though its success has been limited by the unique features of the TME. In CCA, the TME comprises various immune cells such as B lymphocytes, cytotoxic CD8+ T cells, regulatory T cells (Tregs), natural killer (NK) cells, and tumor-associated macrophages [52]. Recent advances in ICIs, particularly those targeting the PD-1/PD-L1 axis, have shown potential in improving clinical outcomes for CCA patients, although their effectiveness as monotherapies is limited by a suppressive immune microenvironment [53].
Several studies have explored ICIs as second-line therapy for advanced CCAs (Table 2). The phase II KEYNOTE-158 trial evaluated pembrolizumab monotherapy in patients with advanced or metastatic solid tumors, including CCA, who had microsatellite instability-high (MSI-H) or mismatch repair-deficient (dMMR) tumors and had failed prior therapy. The study reported an objective response rate (ORR) of 5.8%, mPFS of 2.0 months, and mOS of 7.4 months [54]. Similarly, nivolumab monotherapy in a phase II trial showed an ORR of 10.9%, mPFS of 3.7 months, and mOS of 14.2 months, with PD-L1 positivity correlating with improved outcomes [55]. In the CA209-538 phase II trial, nivolumab plus ipilimumab (a fully human anti-CTLA-4 antibody) achieved an ORR of 23.1% and disease control rate (DCR) of 44%, with mPFS of 2.9 months and OS of 5.7 months [56]. A phase II trial (NCT03704480) assessing durvalumab (a human anti-PD-L1 antibody) plus tremelimumab (a human anti-CTLA-4 antibody) in patients who had progressed after platinum-based chemotherapy reported an ORR of 9.7%, DCR of 40.8%, mPFS of 2.5 months, and mOS of 8.0 months [57].

Table 2.
Summary of Phase II and III clinical trials evaluating immunotherapy, either as monotherapy or in combination with chemotherapy, for the treatment of cholangiocarcinoma (CCA).
The most significant success in utilizing immunotherapy for CCAs has come from combining ICIs with first-line chemotherapy (Table 2). Preclinical studies suggest that the GemCis regimen enhances immunogenicity within the TME, providing a rationale for adding ICIs to this treatment [60]. A phase II BilT-01 trial evaluated GemCis with nivolumab and a separate regimen of nivolumab plus ipilimumab in advanced/metastatic CCA. The mPFS and mOS were 6.6 months and 10.6 months, respectively, in the chemoimmunotherapy arm, compared to 3.9 months and 8.2 months, respectively, in the dual ICI arm. Neither regimen significantly exceeded the historical control PFS threshold, indicating that adding nivolumab to standard chemotherapy did not provide a clear benefit, and the nivolumab-ipilimumab combination was less effective [58]. Another phase II trial (NCT03046862) investigated GemCis followed by GemCis plus durvalumab and tremelimumab; GemCis plus durvalumab; and GemCis plus durvalumab and tremelimumab. The ORRs were 50.0, 72.3, and 70.2%; mPFS were 6.6, 11.8 and 12.3 months; and mOS were 15.0, 20.2 and 18.7 months, respectively. These findings suggested that initiating immunotherapy early in treatment was beneficial, while the addition of dual ICI therapy provided minimal additional benefit compared to monotherapy [59].
The most notable success in integrating ICIs into first-line treatment was demonstrated in the phase III TOPAZ-1 trial, where GemCis plus durvalumab significantly improved outcomes over GemCis (ORR: 26.7 vs. 18.7%; mPFS: 7.2 vs. 5.7 months; mOS: 12.9 vs. 11.3 months), leading to FDA approval of the GemCis plus durvalumab regimen in September 2022. The incidence of grade 3 or 4 adverse events was similar between the durvalumab (75.7%) and placebo (77.8%) groups (Table 2) [22]. Similarly, the KEYNOTE-966 trial showed improved outcomes with pembrolizumab combined with GemCis compared with chemotherapy alone (mPFS: 6.5 vs. 5.6 months; mOS: 12.7 vs. 10.9 months) (Table 2) [23].
Ongoing first-line trials continue to explore various ICIs combined with chemotherapy. A phase III trial (NCT03478488) is investigating the efficacy of the subcutaneously administered anti-PD-L1 antibody envafolimab in combination with gemcitabine/oxaliplatin (GEMOX) [61]. Several phase II trials are also underway, including NCT04191343 (toripalimab plus GEMOX), NCT03796429 (toripalimab plus gemcitabine and S-1), and NCT04172402 (nivolumab plus gemcitabine and S-1).
Although immune checkpoint blockade—particularly PD-1/PD-L1 inhibitors—has improved outcomes for some patients, its benefits remain limited by an immunosuppressive TME. To expand therapeutic options beyond immune-based approaches and established FGFR2 and IDH1 inhibitors, current research is exploring additional molecular targets that drive CCA progression. The following section highlights these emerging investigational targeted therapies and their potential to reshape treatment paradigms.
4. Investigational Targeted Therapies in CCA
Building on the positive outcomes and approval of small-molecule inhibitors targeting FGFR and IDH oncogenic signaling in CCA, several other therapeutic strategies focusing on different oncogenic pathways are currently being explored for advanced-stage CCA. Some of the most promising signaling pathways with therapeutic potential include the following:
4.1. P53-MDM-2 Signaling
MDM2-p53 antagonists have primarily been investigated in hematologic malignancies. However, agents such as idasanutlin (a selective MDM2 antagonist) and ALRN-6924 (a dual MDM2/MDMX inhibitor) have demonstrated tolerability and promising efficacy in patients with advanced solid tumors [62,63]. These antagonists are also being evaluated in combination with ICIs, as they may promote a pro-immunogenic TME and exhibit synergy with anti-PD-1 antibodies in preclinical models [64].
Milademetan (RAIN-32), an MDM2-p53 antagonist, has been assessed in solid tumors, including CCA, with phase I trials showing manageable safety profiles and modest antitumor activity [65]. In a Phase II study, milademetan was evaluated in patients with advanced or metastatic solid tumors, including CCA, whose tumors harbored MDM2 gene amplification and wild-type p53. In CCA patients, the treatment demonstrated approximately 29% tumor regression. The ORR across all tumor types was 19.4% (Supplementary Table S1) [66]. Currently, brigimadlin (BI 907828), another potent MDM2-p53 antagonist, is under development for CCA patients (Table 3). This compound has demonstrated encouraging antitumor effects in preclinical models of dedifferentiated liposarcoma with MDM2 amplification [67] and showed synergistic efficacy when combined with a PD-1 checkpoint inhibitor in a colon cancer mouse model [68].

Table 3.
Selected Phase II and III clinical trials of targeted therapies demonstrating clinical efficacy or modest activity, administered as monotherapy or in combination with chemotherapy and/or immunotherapy, in cholangiocarcinoma (CCA).
Preliminary data from phase Ia/Ib trials evaluating brigimadlin, either as monotherapy or in combination with the PD-1 inhibitor ezabenlimab, in CCA patients have been encouraging. Among treated patients, six achieved a partial response (PR), and four had SD. Responses were durable, and no unexpected toxicities were reported, though some patients experienced grade 3/4 treatment-related AEs such as neutropenia, thrombocytopenia, and anemia [69]. Based on these promising results, a phase IIa/IIb trial of brigimadlin is currently recruiting patients with advanced CCA, pancreatic ductal adenocarcinoma (PDAC), or other solid tumors (NCT05512377) [87].
4.2. ErbB Inhibitors
The ErbB family consists of receptor tyrosine kinases ErbB1 (EGFR), ErbB2 (HER2), ErbB3, and ErbB4 [88]. Epidermal growth factor receptor (EGFR) and HER2 activate various pathways that stimulate cell proliferation and differentiation [89]. In iCCA, approximately 10–32% of cases exhibit EGFR and/or HER2/neu overexpression [88,90,91].
For EGFR-targeted therapies, a randomized phase III trial investigated the addition of the EGFR inhibitor erlotinib to GEMOX chemotherapy. GEMOX and GEMOX + erlotinib resulted in ORRs of 15.8 vs. 29.6%, mPFS of 4.2 vs. 5.8 months, and mOS of 9.5 months in both groups, indicating no OS benefit with the addition of erlotinib (NCT01149122) (Table 3) [70]. A phase II study of the EGFR monoclonal antibody cetuximab in combination with GEMOX showed promising antitumor activity (ORR: 23%) (NCT01216345) [71]. However, another phase II study assessing GEMOX with and without cetuximab reported an improvement in mPFS but no significant difference in mOS between treatment arms (NCT01267344) (Table 3) [72]. Other ErbB inhibitors, such as the EGFR inhibitor panitumumab and the dual EGFR/HER2 inhibitor lapatinib, failed to show significant clinical benefit in biliary tract cancer patients (Supplementary Table S1) [92,93].
For HER2-targeted therapies, a retrospective study found that the HER2 monoclonal antibody trastuzumab provided clinical benefit in gallbladder cancer with HER2/neu overexpression but not in CCA [94]. In a phase II study, the HER2 inhibitor vandetanib failed to increase PFS in CCA patients (NCT00753675) (Supplementary Table S1) [95]. In the phase II SUMMIT basket trial (NCT01953926), the pan-HER inhibitor neratinib demonstrated modest activity in HER2-mutant biliary tract cancers, with PFS and OS outcomes comparable to standard therapies, suggesting limited but potential therapeutic relevance in selected patients (Table 3) [73].
4.3. VEGFR and PDGFR Inhibitors
Targeting the tyrosine kinase receptors VEGF and PDGF may provide therapeutic benefit for CCA patients, as these RTKs are commonly overexpressed [91,96]. In a phase II study, the VEGFR inhibitor bevacizumab combined with gemcitabine-capecitabine failed to show significant clinical improvement (mPFS: 8.1 months, mOS: 10.2 months) in CCA patients (Supplementary Table S1) [97]. However, an earlier phase II study combining bevacizumab with GEMOX demonstrated antitumor activity in CCA patients (mPFS: 7.0 months; mOS: 12.7 months) (Table 3) [74].
Sorafenib, a multikinase inhibitor targeting VEGFR, PDGFR, and Raf, has been extensively evaluated in clinical trials for CCA but has failed to produce significant benefits, either as monotherapy or in combination. In a phase II trial, sorafenib monotherapy was evaluated in patients with advanced CCA and showed modest efficacy, reporting an mPFS of 2.3 months and an mOS of 4.4 months (Table 3) [75]. The SWOG 0514 phase II trial involving chemotherapy-naïve patients with unresectable or metastatic gallbladder carcinoma or CCA, single-agent sorafenib yielded no confirmed objective responses, an mPFS of ~3.0 months, and an mOS of ~9.0 months (Supplementary Table S1) [98]. The SWOG phase II trial investigated the combination of sorafenib and erlotinib in advanced gallbladder carcinoma or CCA. The regimen demonstrated limited activity, with an mPFS of 2 months and an mOS of 6 months (Table 3) [76]. Ramucirumab, a VEGFR2 antibody, showed promising results in a single-arm phase II trial with PFS comparable to that of current chemotherapy regimens (mPFS: 3.2 months; mOS: 9.5 months) (Table 3) [77]. Another promising VEGF inhibitor regorafenib (an oral multikinase inhibitor of VEGFR, PDGFR, and RAF kinases), in a phase II study of chemotherapy refractory advanced CCA patients, demonstrated mPFS of 3.6 months and mOS of 7.3 months (NCT02053376), highlighting its potential as a future treatment for patients with advanced CCA (Table 3) [78].
4.4. MET Inhibitors
Overexpression of MET, another RTK, is associated with poor prognosis in CCA and is detected in approximately 35–50% of iCCAs, making it a promising therapeutic target [89,99]. However, clinical trials evaluating MET inhibitors have yielded mixed results (Table 3).
Cabozantinib, a dual MET/VEGFR2 inhibitor, was assessed in a phase II clinical trial for CCA patients but exhibited significant toxicity and failed to demonstrate meaningful antitumor activity (mPFS: 1.8 months, mOS: 5.2 months) (Supplementary Table S1) [100]. A phase II trial of ramucirumab plus the MET inhibitor merestinib with GemCis showed tolerability but no improvement in mPFS in CCA patients (Supplementary Table S1) [101]. The MET inhibitor tivantinib in combination with gemcitabine, demonstrated low toxicity and potential antitumor activity in a phase I trial of advanced CCA patients [102]. Tepotinib, a selective MET inhibitor, showed promising clinical activity in the phase II VISION trial involving NSCLC patients with MET exon 14 mutations (NCT02864992) [103]. A long-term follow-up of the VISION trial reaffirmed tepotinib’s efficacy in this patient population [104]. Following its FDA approval, tepotinib may represent a viable treatment option for CCA patients harboring MET mutations [105].
4.5. ALK Inhibitors
The receptor tyrosine kinases ALK and ROS1 are altered in approximately 1.1–8.8% of iCCA cases [89,106]. Due to structural similarities between ALK and ROS1 proteins, ALK inhibitors have been investigated as potential therapies for ROS1-mutant CCA patients [89]. In a phase III trial in NSCLC patients with ALK mutations, the ALK inhibitor ceritinib significantly improved PFS compared to chemotherapy, suggesting potential efficacy for ROS1-mutant CCA patients as well [107]. Ceritinib was investigated in CCA patients with ROS1 or ALK overexpression (NCT02374489), but the trial was terminated due to insufficient patient recruitment. Alectinib and crizotinib, two other ALK inhibitors, have been evaluated in ALK-mutated non-lung solid tumors [108]. Results revealed that alectinib, and possibly crizotinib, may benefit these patients, suggesting potential therapeutic relevance for CCA patients with ROS1 mutations [108]. Entrectinib, an ALK and ROS1 inhibitor, was tested in two phase I clinical trials in patients with solid tumors, including gastrointestinal tract tumors. These trials demonstrated that entrectinib was well tolerated and exhibited promising antitumor activity across multiple tumor types [109]. Entrectinib is currently being investigated in a phase II basket study in solid tumors, including CCA harboring ROS1, ALK, or NTRK1/2/3 mutations (NCT02568267).
4.6. MAPK Signaling Inhibitors
Oncogenic activation of the RAS/RAF/MEK/ERK (MAPK) signaling pathway is driven by specific mutations in the RAS genes, leading to constitutive activation of RAS proteins and subsequent uncontrolled proliferation, differentiation, migration, and metastasis [110]. MAPK pathway components are well-known oncogenic drivers in many cancers, making them attractive targets for therapy [110,111]. BRAF mutations are found in only 3.3% of iCCA cases [112]. Vemurafenib, a BRAF V600-specific inhibitor, was tested in eight CCA patients with BRAF V600 mutation, but only one partial response was observed [113]. However, a case study reported complete response in a CCA patient with BRAF V600 mutation treated with vemurafenib, panitumumab, and irinotecan [114]. A next-generation BRAF inhibitor PLX8394 has also been evaluated in BRAF-mutant solid tumors, including CCA. A phase II trial evaluated selumetinib, a MEK1/2 inhibitor, in patients with metastatic CCA who had received prior chemotherapy, demonstrated limited clinical activity, with a mPFS of 3.7 months and a mOS of 9.8 months, highlighting modest benefit in the second-line setting (Table 3) [79]. A phase 1b trial of selumetinib combination with GemCis in chemotherapy-naïve advanced CCA patients demonstrated promising activity, with an mPFS of 6.4 months [115]. However, a randomized phase II study of selumetinib plus GemCis failed to improve efficacy and increased toxicity in advanced CCA patients compared to GemCis (mPFS: 6.0–7.0 vs. 6.3 months; mOS: 11.7 vs. 12.8 months) (Supplementary Table S1) [116]. Another MEK inhibitor, trametinib, was tested in a phase II trial in Japan in CCA patients, demonstrating SD in 65% of patients and progressive disease (PD) in 35%, with mPFS of 10.6 weeks and ~20% 1-year OS (Table 3) [80]. A phase II trial comparing trametinib to chemotherapy (5-FU or capecitabine) in refractory CCA patients failed to demonstrate any benefit of trametinib over chemotherapy (ORR: 0 vs. 5%; mPFS: 1.5 vs. 3.3 months; mOS: 4.3 vs. 6.6 months) (Supplementary Table S1) [117]. A combination of trametinib and the VEGF receptor inhibitor pazopanib in advanced CCA patients who were refractory to or refused standard-of-care treatment options showed modest activity, with an ORR of 5%, DCR of 75%, mPFS of 3.6 months, and mOS of 6.4 months. These results suggest that while the combination therapy demonstrated some clinical activity, the overall efficacy was limited and it did not result in a statistically significant improvement in PFS (Table 3) [81]. By contrast, in a phase II trial of dabrafenib (a BRAF inhibitor) plus trametinib in BRAF V600-mutant CCA patients showed promising activity with low toxicity (ORR: 47%; mPFS: 7.2 months; mOS: 11.3 months) (Table 3) [82]. A phase II trial of atezolizumab (anti-PD-L1 antibody) with or without the MEK inhibitor cobimetinib in CCA patients demonstrated improved PFS with the combination (3.65 vs. 1.87 months). However, the low response rate in both arms highlighted the immune-resistant nature of CCA (Table 3) [83]. Similarly, a randomized phase II study evaluated atezolizumab plus the CD27 immune agonist varlilumab (CDX-1127), with or without cobimetinib, and was terminated early due to lack of clinical benefit (ORR: 0 vs. 4%; mPFS: 2.2 vs. 1.8 months) (Supplementary Table S1) [118].
4.7. PI3K/AKT/mTOR Signaling Inhibitors
The PI3K/AKT/mTOR pathway is a key regulator of cell growth and survival, frequently dysregulated in CCA. Mutations in PI3K occur in approximately 6% of eCCA and 4% of intrahepatic iCCA tumors, while PTEN loss or mutation is detected in 4–5% of cases [112]. Overactivation of phosphorylated AKT is observed in the majority of CCA samples, underscoring the pathway’s therapeutic relevance [119,120].
The mTOR inhibitor everolimus demonstrated modest activity (mPFS: 3.2 months; mOS: 7.7 months) in patients with previously treated CCA in a phase II trial [84]. Another phase II trial evaluating everolimus monotherapy as first-line treatment in advanced biliary tract cancer reported a mPFS of 5.5 months and mOS of 9.5 months but failed to meet its primary endpoint (Supplementary Table S1) [121]. The PI3K inhibitor copanlisib plus GemCis achieved an ORR of 17.4% [122], while the AKT inhibitor MK2206 showed no clinical activity (ORR: 0%; mPFS: 1.7 months; mOS: 3.5 months) in a small trial of eight patients (Supplementary Table S1) [123].
Overall, while preclinical rationale is strong, clinical benefits from PI3K/AKT/mTOR inhibitors in CCA remain limited, highlighting the need for better patient selection and combination strategies.
4.8. JAK/STAT Pathway Inhibitors
The JAK/STAT pathway, particularly STAT3, regulates cell proliferation, differentiation, and apoptosis and is frequently activated in CCA through cytokine (e.g., IL-6) and RTK signaling [124,125,126]. Persistent STAT3 activation promotes tumor growth, and elevated STAT3 expression has been documented in iCCA tissues [127]. AZD9150, a novel targeted drug, has shown efficacy in reducing STAT3 expression in various preclinical cancer models [128,129], and is currently being evaluated in pancreatic cancer (NCT02983578) [128]. Other STAT3 inhibitors, such as OPB-31121 for hepatocellular carcinoma (NCT01406574) and BBI608 for colorectal cancer (NCT01830621), are under clinical investigation [128]. While mechanistically promising, JAK/STAT inhibitors remain in early stages of evaluation for CCA, warranting further preclinical and translational research.
4.9. PARP Inhibitors
BRCA1-associated protein 1 (BAP1) functions as a tumor suppressor that regulates cell cycle progression, differentiation, cell death, and DNA damage response. Loss-of-function BAP1 mutations sensitize cancer cells to PARP inhibitors (PARPi), similar to BRCA-mutated cancers [130]. In CCA, BAP1 mutations occur exclusively in iCCA patients (~15–20%) [29,30], though some series have reported frequencies as high as 35% [131].
A case report described a refractory metastatic CCA patient with a novel BAP1 mutation who experienced a prolonged response to the PARP inhibitor olaparib (>11 months) [132]. In a retrospective study in iCCA patients with IDH1 mutation who had previously received at least one line of cisplatin-based therapy, PARPi monotherapy showed a PFS ranging from 1.4 to 18.5 months and OS from 2.8 to 42.4 months [133]. Several trials are currently investigating PARPi in combination with ICIs in advanced CCA. The phase II BilT-02 trial evaluating rucaparib (a PARPi) plus nivolumab as maintenance therapy after first-line platinum-based therapy did not meet its primary endpoint (PFS at four months) but demonstrated a high DCR of 77%, with a preliminary mPFS of 4.6 months and OS of 15.9 months (Table 3) [85]. A phase II trial of olaparib (a PARPi) plus pembrolizumab in advanced CCA patients demonstrated acceptable safety but did not significantly improve outcomes (ORR: 15.4%; mPFS: 5.45 months; mOS: 7.2 months) (Table 3) [86]. Similarly, a phase II study of olaparib and durvalumab in IDH-mutated CCA was discontinued after stage I due to lack of efficacy (no complete or partial responses; mPFS: 1.97 months) (Supplementary Table S1) [134]. However, a case report described a complete response to olaparib plus pembrolizumab after platinum-based induction chemotherapy in an iCCA patient with a BRCA2 mutation and PD-L1-positive recurrent iCCA [135].
Although biologically compelling, PARPi use in CCA remains exploratory, and future studies should refine patient selection based on molecular profiles such as BAP1 or BRCA alterations.
5. Potential of Targeting KRAS in CCA
Gain-of-function mutations in the KRAS gene are among the most common genetic alterations in CCA, occurring in approximately 15–25% of iCCA cases and up to ~40% of pCCA and dCCA cases [94,136,137]. These mutations lead to constitutive activation of the MAPK and PI3K/AKT/mTOR signaling pathways, promoting cancer cell proliferation and survival. KRAS mutations are associated with a more aggressive tumor phenotype and reduced survival rates in CCA [138], highlighting the potential of mutant KRAS as a therapeutic target.
Historically, direct targeting of KRAS has been challenging. However, the development of KRASG12C-specific inhibitors, such as sotorasib, has provided a new avenue for therapy [139]. The prevalence of different KRAS missense mutations varies across cancer types. For instance, KRASG12C mutations are rare in pancreatic cancer (2–3% of all KRAS mutations) and CCA (8%) but represent the predominant KRAS mutation subtype in NSCLC (39–42%) [140]. As a result, KRAS inhibitors like sotorasib and adagrasib, which specifically target the KRASG12C mutation, are more effective in subsets of lung adenocarcinoma rather than CCA. In CCA, the most prevalent KRAS mutations are G12D (~48%), G12V (~18%), and G12S (~12%) [141]. Therefore, the development of mutant allele-specific inhibitors for KRASG12D, KRASG12V or KRASG12S, or broad-spectrum pan-KRAS inhibitors, holds significant therapeutic promise for CCA.
Several KRAS inhibitors are currently being evaluated in clinical trials for tumors harboring KRAS mutations. In a phase II study (NCT03785249) of adagrasib in patients with advanced solid tumors carrying the KRASG12C mutation, a subgroup of 8 CCA patients demonstrated an ORR of 50.0%, an mPFS of 11.3 months, and an mOS of 15.1 months (Table 4) [142]. Moreover, an exploratory analysis from the TOPAZ-1 trial, which evaluated chemotherapy plus immunotherapy, found that KRAS-mutated CCAs were more frequently observed among long-term survivors who lived more than 18 months after randomization [143]. An ongoing phase I study (NCT06607185) is assessing the safety and tolerability of LY4066434, a pan-KRAS inhibitor, in patients with KRAS-mutant advanced or metastatic solid tumors, including CCA (Table 4). In addition, a phase I/II study of the KRASG12D inhibitor MRTX1133 was initiated in solid tumors including CCA with KRASG12D mutation, but the trial was terminated early before proceeding to phase II (NCT05737706) (Table 4).

Table 4.
Ongoing clinical trials of KRAS inhibitors as monotherapy and in combination in cholangiocarcinoma (CCA).
Overall, investigational targeted therapies for CCA encompass a diverse range of pathways, including p53/MDM2, ErbB, VEGFR/PDGFR, MET, ALK, MAPK, PI3K/AKT/mTOR, JAK/STAT, PARP, and KRAS signaling. While preclinical and early-phase clinical data show encouraging activity, most of these agents remain exploratory, with limited evidence from randomized trials. Notably, recent advances in the direct targeting of KRAS using pan-KRAS inhibitors and isoform-selective inhibitors represent a significant milestone, expanding therapeutic potential beyond previously validated FGFR2 and IDH1 pathways. These developments, together with emerging combination approaches and biomarker-driven trial designs, highlight a rapidly evolving landscape that may redefine precision treatment paradigms in CCA.
6. Comparative Stratification of Therapeutic Modalities
Given the expanding therapeutic landscape in CCA, a comparative understanding of efficacy, safety, and patient selection is essential. Among approved treatments, FGFR2 inhibitors (pemigatinib, futibatinib, infigratinib) and the IDH1 inhibitor ivosidenib provide the greatest benefit in molecularly defined subgroups with manageable toxicity. Chemoimmunotherapy (GemCis plus durvalumab) remains the preferred first-line option for patients without targetable alterations, offering the best overall survival improvement in unselected populations. Investigational agents, including KRAS, MDM2, and PARP inhibitors, show emerging promise but await phase III validation.
Treatment selection should be guided by comprehensive genomic profiling, prior therapy, and individual patient factors. In practice, FGFR2 or IDH1 alterations should prompt use of targeted therapy, whereas chemoimmunotherapy remains the standard backbone for biomarker-negative disease. Rational combinations integrating targeted agents with immunotherapy or chemotherapy may further enhance efficacy and overcome resistance.
Predictive clinico-genomic parameters are increasingly critical for refining patient selection. FGFR2 fusions and IDH1 mutations predict response to targeted inhibitors; MSI-H/dMMR status, PD-L1 positivity, and high tumor mutational burden correlate with improved immunotherapy outcomes; and BAP1 or BRCA1/2 alterations indicate sensitivity to PARP inhibition. Incorporating these biomarkers into routine diagnostics will be key to achieving truly personalized treatment in CCA.
7. Conclusions and Future Perspective
CCA remains a highly aggressive malignancy with limited therapeutic options and poor overall prognosis. In recent years, the emergence of molecularly targeted therapies has provided new opportunities to improve clinical outcomes. The discovery of actionable genetic alterations has already been translated into approved therapies, including the IDH1 inhibitor ivosidenib and the FGFR2 inhibitors pemigatinib, infigratinib, and futibatinib, which represent the first steps toward precision medicine in CCA. Moreover, the integration of immunotherapy into the treatment paradigm, highlighted by the approval of durvalumab in combination with chemotherapy, underscores the potential of harnessing the immune system against this disease.
Beyond these established approaches, several other molecular targets are being actively investigated, such as the p53/MDM2 axis, VEGFR, MET, ALK, MAPK, PI3K/AKT/mTOR, JAK/STAT, and BAP1-related pathways. KRAS, long considered an “undruggable” oncogene, is now a particularly promising target due to recent advances in the development of selective KRAS inhibitors. Continued exploration of these and other pathways holds the potential to expand the therapeutic armamentarium against CCA.
The future of CCA management lies in the integration of comprehensive genomic profiling, biomarker-driven clinical trials, and rational drug combinations that overcome resistance. Incorporating targeted, immunotherapeutic, and chemotherapeutic strategies, guided by tumor heterogeneity and molecular signatures, will be key to achieving durable responses. Adaptive and platform-based trial designs will further accelerate evaluation of emerging therapies and enable real-time learning.
Translating these advances into clinical practice remains challenging. Barriers such as limited access to next-generation sequencing, inconsistent biomarker testing, high treatment costs, and disparities in healthcare infrastructure hinder global implementation of precision oncology. Addressing these limitations through collaborative research networks and data sharing will be essential to ensure equitable access to emerging therapies.
Moving forward, CCA treatment should prioritize molecular stratification at diagnosis, combination regimens that jointly target oncogenic signaling and immune evasion, and adaptive trial platforms that rapidly incorporate novel biomarkers. Such an integrated approach will optimize treatment selection, accelerate development of durable personalized therapies, and ultimately improve survival and quality of life for patients with this devastating disease.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms262110755/s1.
Author Contributions
Conceptualization, L.D. and N.A.; design of the article, L.D. and N.A.; formal analysis, L.D., Q.K., M.S.H. and U.v.H.; investigation, L.D., Q.K. and N.A.; writing—original draft preparation, L.D. and N.A.; writing—review and editing, L.D., Q.K., U.v.H. and N.A.; funding acquisition, N.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Indiana University School of Medicine funds.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CCA | Cholangiocarcinoma |
| iCCA | Intrahepatic cholangiocarcinoma |
| pCCA | Perihilar cholangiocarcinoma |
| dCCA | Distal cholangiocarcinoma |
| eCCA | Extrahepatic cholangiocarcinoma |
| FGFR | Fibroblast growth factor receptor |
| IDH | Isocitrate dehydrogenase |
| KRAS | Kirsten rat sarcoma viral oncogene homolog |
References
- Ilyas, S.I.; Affo, S.; Goyal, L.; Lamarca, A.; Sapisochin, G.; Yang, J.D.; Gores, G.J. Cholangiocarcinoma—Novel biological insights and therapeutic strategies. Nat. Rev. Clin. Oncol. 2023, 20, 470–486. [Google Scholar] [CrossRef] [PubMed]
- Razumilava, N.; Gores, G.J. Cholangiocarcinoma. Lancet 2014, 383, 2168–2179. [Google Scholar] [CrossRef]
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [Google Scholar] [CrossRef]
- Banales, J.M.; Cardinale, V.; Carpino, G.; Marzioni, M.; Andersen, J.B.; Invernizzi, P.; Lind, G.E.; Folseraas, T.; Forbes, S.J.; Fouassier, L.; et al. Expert consensus document: Cholangiocarcinoma: Current knowledge and future perspectives consensus statement from the European Network for the Study of Cholangiocarcinoma (ENS-CCA). Nat. Rev. Gastroenterol. Hepatol. 2016, 13, 261–280. [Google Scholar] [CrossRef]
- Global Burden of Disease Cancer Collaboration. The Global Burden of Cancer 2013. JAMA Oncol. 2015, 1, 505–527. [Google Scholar] [CrossRef]
- Sarcognato, S.; Sacchi, D.; Fassan, M.; Fabris, L.; Cadamuro, M.; Zanus, G.; Cataldo, I.; Capelli, P.; Baciorri, F.; Cacciatore, M.; et al. Cholangiocarcinoma. Pathologica 2021, 113, 158–169. [Google Scholar] [CrossRef]
- Sripa, B.; Kaewkes, S.; Sithithaworn, P.; Mairiang, E.; Laha, T.; Smout, M.; Pairojkul, C.; Bhudhisawasdi, V.; Tesana, S.; Thinkamrop, B.; et al. Liver Fluke Induces Cholangiocarcinoma. PLoS Med. 2007, 4, e201. [Google Scholar] [CrossRef] [PubMed]
- Sripa, B.; Tangkawattana, S.; Brindley, P.J. Chapter Five—Update on Pathogenesis of Opisthorchiasis and Cholangiocarcinoma. In Advances in Parasitology; Sripa, B., Brindley, P.J., Eds.; Academic Press: Cambridge, MA, USA, 2018; Volume 102, pp. 97–113. [Google Scholar]
- Zheng, S.; Zhu, Y.; Zhao, Z.; Wu, Z.; Okanurak, K.; Lv, Z. Liver fluke infection and cholangiocarcinoma: A review. Parasitol. Res. 2017, 116, 11–19. [Google Scholar] [CrossRef]
- Brindley, P.J.; Bachini, M.; Ilyas, S.I.; Khan, S.A.; Loukas, A.; Sirica, A.E.; Teh, B.T.; Wongkham, S.; Gores, G.J. Cholangiocarcinoma. Nat. Rev. Dis. Primers 2021, 7, 65. [Google Scholar] [CrossRef]
- Anderson, C.D.; Wright Pinson, C.; Berlin, J.; Chari, R.S. Diagnosis and Treatment of Cholangiocarcinoma. Oncologist 2004, 9, 43–57. [Google Scholar] [CrossRef] [PubMed]
- DeOliveira, M.L.; Cunningham, S.C.; Cameron, J.L.; Kamangar, F.; Winter, J.M.; Lillemoe, K.D.; Choti, M.A.; Yeo, C.J.; Schulick, R.D. Cholangiocarcinoma: Thirty-one-year experience with 564 patients at a single institution. Ann. Surg. 2007, 245, 755–762. [Google Scholar] [CrossRef] [PubMed]
- Banales, J.M.; Cardinale, V.; Macias, R.I.R.; Andersen, J.B.; Braconi, C.; Carpino, G.; Alvaro, D.; Calvisi, D.F. Cholangiocarcinoma: State-of-the-art knowledge and challenges. Liver Int. 2019, 39, 5–6. [Google Scholar] [CrossRef]
- Valle, J.W.; Borbath, I.; Khan, S.A.; Huguet, F.; Gruenberger, T.; Arnold, D. Biliary cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2016, 27, v28–v37. [Google Scholar] [CrossRef]
- Jarnagin, W.R.; Fong, Y.; DeMatteo, R.P.; Gonen, M.; Burke, E.C.; Bodniewicz, B.J.; Youssef, B.M.; Klimstra, D.; Blumgart, L.H. Staging, resectability, and outcome in 225 patients with hilar cholangiocarcinoma. Ann. Surg. 2001, 234, 507–517. [Google Scholar] [CrossRef]
- Jung Hyun, J.; Si Young, S. Chemotherapy of Cholangiocarcinoma: Current Management and Future Directions. In Topics in the Surgery of the Biliary Tree; Hesham, A., Ed.; IntechOpen: Rijeka, Croatia, 2018; Chapter 3. [Google Scholar]
- Zhu, A.X. Future directions in the treatment of cholangiocarcinoma. Best Pract. Res. Clin. Gastroenterol. 2015, 29, 355–361. [Google Scholar] [CrossRef]
- Halder, R.; Amaraneni, A.; Shroff, R.T. Cholangiocarcinoma: A review of the literature and future directions in therapy. Hepatobiliary Surg. Nutr. 2022, 11, 555–566. [Google Scholar] [CrossRef] [PubMed]
- Shin, D.W.; Kim, M.J.; Lee, J.-c.; Kim, J.; Woo, S.M.; Lee, W.J.; Lee, K.H.; Hwang, J.-H. Gemcitabine Plus Cisplatin Chemotherapy Prolongs the Survival in Advanced Hilar Cholangiocarcinoma: A Large Multicenter Study. Am. J. Clin. Oncol. 2020, 43, 422–427. [Google Scholar] [CrossRef]
- Valle, J.W.; Furuse, J.; Jitlal, M.; Beare, S.; Mizuno, N.; Wasan, H.; Bridgewater, J.; Okusaka, T. Cisplatin and gemcitabine for advanced biliary tract cancer: A meta-analysis of two randomised trials. Ann. Oncol. 2014, 25, 391–398. [Google Scholar] [CrossRef]
- Lamarca, A.; Palmer, D.H.; Wasan, H.S.; Ross, P.J.; Ma, Y.T.; Arora, A.; Falk, S.; Gillmore, R.; Wadsley, J.; Patel, K.; et al. Second-line FOLFOX chemotherapy versus active symptom control for advanced biliary tract cancer (ABC-06): A phase 3, open-label, randomised, controlled trial. Lancet Oncol. 2021, 22, 690–701. [Google Scholar] [CrossRef]
- Oh, D.Y.; He, A.R.; Bouattour, M.; Okusaka, T.; Qin, S.; Chen, L.T.; Kitano, M.; Lee, C.K.; Kim, J.W.; Chen, M.H.; et al. Durvalumab or placebo plus gemcitabine and cisplatin in participants with advanced biliary tract cancer (TOPAZ-1): Updated overall survival from a randomised phase 3 study. Lancet Gastroenterol. Hepatol. 2024, 9, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Kelley, R.K.; Ueno, M.; Yoo, C.; Finn, R.S.; Furuse, J.; Ren, Z.; Yau, T.; Klümpen, H.J.; Chan, S.L.; Ozaka, M.; et al. Pembrolizumab in combination with gemcitabine and cisplatin compared with gemcitabine and cisplatin alone for patients with advanced biliary tract cancer (KEYNOTE-966): A randomised, double-blind, placebo-controlled, phase 3 trial. Lancet 2023, 401, 1853–1865. [Google Scholar] [CrossRef]
- Ramírez-Merino, N.; Aix, S.P.; Cortés-Funes, H. Chemotherapy for cholangiocarcinoma: An update. World J. Gastrointest. Oncol. 2013, 5, 171–176. [Google Scholar] [CrossRef]
- Park, J.O.; Oh, D.Y.; Hsu, C.; Chen, J.S.; Chen, L.T.; Orlando, M.; Lim, H.Y. Gemcitabine Plus Cisplatin for Advanced Biliary Tract Cancer: A Systematic Review. Cancer Res. Treat. 2015, 47, 343–361. [Google Scholar] [CrossRef] [PubMed]
- Lamarca, A.; Kapacee, Z.; Breeze, M.; Bell, C.; Belcher, D.; Staiger, H.; Taylor, C.; McNamara, M.G.; Hubner, R.A.; Valle, J.W. Molecular Profiling in Daily Clinical Practice: Practicalities in Advanced Cholangiocarcinoma and Other Biliary Tract Cancers. J. Clin. Med. 2020, 9, 2854. [Google Scholar] [CrossRef]
- Berchuck, J.E.; Facchinetti, F.; DiToro, D.F.; Baiev, I.; Majeed, U.; Reyes, S.; Chen, C.; Zhang, K.; Sharman, R.; Uson Junior, P.L.S.; et al. The clinical landscape of cell-free DNA alterations in 1671 patients with advanced biliary tract cancer. Ann. Oncol. 2022, 33, 1269–1283. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Huang, T.; Jiang, T.; Wang, H. Current status and prospects of targeted therapy for cholangiocarcinoma based on molecular characteristics. Cancer Lett. 2025, 614, 217540. [Google Scholar] [CrossRef]
- Lowery, M.A.; Ptashkin, R.; Jordan, E.; Berger, M.F.; Zehir, A.; Capanu, M.; Kemeny, N.E.; O’Reilly, E.M.; El-Dika, I.; Jarnagin, W.R.; et al. Comprehensive Molecular Profiling of Intrahepatic and Extrahepatic Cholangiocarcinomas: Potential Targets for Intervention. Clin. Cancer Res. 2018, 24, 4154–4161. [Google Scholar] [CrossRef]
- Tsilimigras, D.I.; Stecko, H.; Ntanasis-Stathopoulos, I.; Pawlik, T.M. Racial and Sex Differences in Genomic Profiling of Intrahepatic Cholangiocarcinoma. Ann. Surg. Oncol. 2024, 31, 9071–9078. [Google Scholar] [CrossRef]
- Testa, U.; Pelosi, E.; Castelli, G. Cholangiocarcinoma: Molecular Abnormalities and Cells of Origin. Technol. Cancer Res. Treat. 2023, 22, 15330338221128689. [Google Scholar] [CrossRef] [PubMed]
- Tian, W.; Hu, W.; Shi, X.; Liu, P.; Ma, X.; Zhao, W.; Qu, L.; Zhang, S.; Shi, W.; Liu, A.; et al. Comprehensive genomic profile of cholangiocarcinomas in China. Oncol. Lett. 2020, 19, 3101–3110. [Google Scholar] [CrossRef]
- Carotenuto, M.; Sacco, A.; Forgione, L.; Normanno, N. Genomic alterations in cholangiocarcinoma: Clinical significance and relevance to therapy. Explor. Target. Antitumor Ther. 2022, 3, 200–223. [Google Scholar] [CrossRef] [PubMed]
- Lodl, E.; Ramnaraign, B.; Sahin, I.; Wheeler, S. Updates in the use of targeted therapies for the treatment of cholangiocarcinoma. J. Oncol. Pharm. Pract. 2023, 29, 1206–1217. [Google Scholar] [CrossRef] [PubMed]
- Vogel, A.; Segatto, O.; Stenzinger, A.; Saborowski, A. FGFR2 Inhibition in Cholangiocarcinoma. Annu. Rev. Med. 2023, 74, 293–306. [Google Scholar] [CrossRef]
- Salati, M.; Caputo, F.; Baldessari, C.; Carotenuto, P.; Messina, M.; Caramaschi, S.; Dominici, M.; Bonetti, L.R. The Evolving Role of FGFR2 Inhibitors in Intrahepatic Cholangiocarcinoma: From Molecular Biology to Clinical Targeting. Cancer Manag. Res. 2021, 13, 7747–7757. [Google Scholar] [CrossRef] [PubMed]
- Subbiah, V.; Iannotti, N.O.; Gutierrez, M.; Smith, D.C.; Féliz, L.; Lihou, C.F.; Tian, C.; Silverman, I.M.; Ji, T.; Saleh, M. FIGHT-101, a first-in-human study of potent and selective FGFR 1-3 inhibitor pemigatinib in pan-cancer patients with FGF/FGFR alterations and advanced malignancies. Ann. Oncol. 2022, 33, 522–533. [Google Scholar] [CrossRef]
- Vogel, A.; Sahai, V.; Hollebecque, A.; Vaccaro, G.M.; Melisi, D.; Al Rajabi, R.M.; Paulson, A.S.; Borad, M.J.; Gallinson, D.; Murphy, A.G.; et al. An open-label study of pemigatinib in cholangiocarcinoma: Final results from FIGHT-202. ESMO Open 2024, 9, 103488. [Google Scholar] [CrossRef]
- Javle, M.M.; Roychowdhury, S.; Kelley, R.K.; Sadeghi, S.; Macarulla, T.; Waldschmidt, D.T.; Goyal, L.; Borbath, I.; El-Khoueiry, A.B.; Yong, W.-P.; et al. Final results from a phase II study of infigratinib (BGJ398), an FGFR-selective tyrosine kinase inhibitor, in patients with previously treated advanced cholangiocarcinoma harboring an FGFR2 gene fusion or rearrangement. J. Clin. Oncol. 2021, 39, 265. [Google Scholar] [CrossRef]
- Goyal, L.; Meric-Bernstam, F.; Hollebecque, A.; Valle, J.W.; Morizane, C.; Karasic, T.B.; Abrams, T.A.; Furuse, J.; Kelley, R.K.; Cassier, P.A.; et al. Futibatinib for FGFR2-Rearranged Intrahepatic Cholangiocarcinoma. N. Engl. J. Med. 2023, 388, 228–239. [Google Scholar] [CrossRef]
- Ruan, R.; Li, L.; Li, X.; Huang, C.; Zhang, Z.; Zhong, H.; Zeng, S.; Shi, Q.; Xia, Y.; Zeng, Q.; et al. Unleashing the potential of combining FGFR inhibitor and immune checkpoint blockade for FGF/FGFR signaling in tumor microenvironment. Mol. Cancer 2023, 22, 60. [Google Scholar] [CrossRef] [PubMed]
- Gutierrez, M.; Subbiah, V.; Nemunaitis, J.J.; Mettu, N.B.; Papadopoulos, K.P.; Barve, M.A.; Féliz, L.; Lihou, C.F.; Tian, C.; Ji, T.; et al. Safety and efficacy of pemigatinib plus pembrolizumab combination therapy in patients (pts) with advanced malignancies: Results from FIGHT-101, an open-label phase I/II study. J. Clin. Oncol. 2020, 38, 3606. [Google Scholar] [CrossRef]
- Zhu, A.X.; Macarulla, T.; Javle, M.M.; Kelley, R.K.; Lubner, S.J.; Adeva, J.; Cleary, J.M.; Catenacci, D.V.T.; Borad, M.J.; Bridgewater, J.A.; et al. Final Overall Survival Efficacy Results of Ivosidenib for Patients with Advanced Cholangiocarcinoma with IDH1 Mutation: The Phase 3 Randomized Clinical ClarIDHy Trial. JAMA Oncol. 2021, 7, 1669–1677. [Google Scholar] [CrossRef] [PubMed]
- Simbolo, M.; Fassan, M.; Ruzzenente, A.; Mafficini, A.; Wood, L.D.; Corbo, V.; Melisi, D.; Malleo, G.; Vicentini, C.; Malpeli, G.; et al. Multigene mutational profiling of cholangiocarcinomas identifies actionable molecular subgroups. Oncotarget 2014, 5, 2839–2852. [Google Scholar] [CrossRef] [PubMed]
- Kipp, B.R.; Voss, J.S.; Kerr, S.E.; Barr Fritcher, E.G.; Graham, R.P.; Zhang, L.; Highsmith, W.E.; Zhang, J.; Roberts, L.R.; Gores, G.J.; et al. Isocitrate dehydrogenase 1 and 2 mutations in cholangiocarcinoma. Hum. Pathol. 2012, 43, 1552–1558. [Google Scholar] [CrossRef]
- Lamarca, A.; Barriuso, J.; McNamara, M.G.; Valle, J.W. Molecular targeted therapies: Ready for “prime time” in biliary tract cancer. J. Hepatol. 2020, 73, 170–185. [Google Scholar] [CrossRef]
- Marin, J.J.; Macias, R.I. Understanding drug resistance mechanisms in cholangiocarcinoma: Assisting the clinical development of investigational drugs. Expert. Opin. Investig. Drugs 2021, 30, 675–679. [Google Scholar] [CrossRef]
- Harding, J.J.; Lowery, M.A.; Shih, A.H.; Schvartzman, J.M.; Hou, S.; Famulare, C.; Patel, M.; Roshal, M.; Do, R.K.; Zehir, A.; et al. Isoform Switching as a Mechanism of Acquired Resistance to Mutant Isocitrate Dehydrogenase Inhibition. Cancer Discov. 2018, 8, 1540–1547. [Google Scholar] [CrossRef] [PubMed]
- Brandi, G.; Rizzo, A. IDH Inhibitors and Immunotherapy for Biliary Tract Cancer: A Marriage of Convenience? Int. J. Mol. Sci. 2022, 23, 10869. [Google Scholar] [CrossRef]
- Nguyen, M.K.; Jelinek, M.; Singh, A.; Isett, B.; Myers, E.S.; Mullett, S.J.; Eisele, Y.; Beumer, J.; Parise, R.; Urban, J.; et al. Clinical and translational study of ivosidenib plus nivolumab in advanced solid tumors harboring IDH1 mutations. medRxiv 2025. [Google Scholar] [CrossRef]
- Kelley, R.K.; Cleary, J.M.; Sahai, V.; Baretti, M.; Bridgewater, J.A.; Hua, Z.; Gliser, C.; Bian, Y.; Abou-Alfa, G.K. A phase 1/2, safety lead-in and dose expansion, open-label, multicenter trial investigating the safety, tolerability, and preliminary activity of ivosidenib in combination with nivolumab and ipilimumab in previously treated subjects with IDH1-mutated nonresectable or metastatic cholangiocarcinoma. J. Clin. Oncol. 2024, 42, TPS4197. [Google Scholar]
- Fabris, L.; Sato, K.; Alpini, G.; Strazzabosco, M. The Tumor Microenvironment in Cholangiocarcinoma Progression. Hepatology 2021, 73 (Suppl. S1), 75–85. [Google Scholar] [CrossRef]
- Lo, J.H.; Agarwal, R.; Goff, L.W.; Heumann, T.R. Immunotherapy in Biliary Tract Cancers: Current Standard-of-Care and Emerging Strategies. Cancers 2023, 15, 3312. [Google Scholar] [CrossRef] [PubMed]
- Piha-Paul, S.A.; Oh, D.Y.; Ueno, M.; Malka, D.; Chung, H.C.; Nagrial, A.; Kelley, R.K.; Ros, W.; Italiano, A.; Nakagawa, K.; et al. Efficacy and safety of pembrolizumab for the treatment of advanced biliary cancer: Results from the KEYNOTE-158 and KEYNOTE-028 studies. Int. J. Cancer 2020, 147, 2190–2198. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.D.; Chung, V.; Alese, O.B.; El-Rayes, B.F.; Li, D.; Al-Toubah, T.E.; Schell, M.J.; Zhou, J.M.; Mahipal, A.; Kim, B.H.; et al. A Phase 2 Multi-institutional Study of Nivolumab for Patients with Advanced Refractory Biliary Tract Cancer. JAMA Oncol. 2020, 6, 888–894. [Google Scholar] [CrossRef]
- Klein, O.; Kee, D.; Nagrial, A.; Markman, B.; Underhill, C.; Michael, M.; Jackett, L.; Lum, C.; Behren, A.; Palmer, J.; et al. Evaluation of Combination Nivolumab and Ipilimumab Immunotherapy in Patients with Advanced Biliary Tract Cancers: Subgroup Analysis of a Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2020, 6, 1405–1409. [Google Scholar] [CrossRef]
- Delaye, M.; Assenat, E.; Dahan, L.; Blanc, J.-F.; Tougeron, D.; Metges, J.-P.; Lievre, A.; Turpin, A.; Fares, N.; Fouchardiere, C.D.L.; et al. Durvalumab (D) plus tremelimumab (T) immunotherapy in patients (Pts) with advanced biliary tract carcinoma (BTC) after failure of platinum-based chemotherapy (CTx): Interim results of the IMMUNOBIL GERCOR D18-1 PRODIGE-57 study. J. Clin. Oncol. 2022, 40, 4108. [Google Scholar] [CrossRef]
- Sahai, V.; Griffith, K.A.; Beg, M.S.; Shaib, W.L.; Mahalingam, D.; Zhen, D.B.; Deming, D.A.; Zalupski, M.M. A randomized phase 2 trial of nivolumab, gemcitabine, and cisplatin or nivolumab and ipilimumab in previously untreated advanced biliary cancer: BilT-01. Cancer 2022, 128, 3523–3530. [Google Scholar] [CrossRef]
- Oh, D.Y.; Lee, K.H.; Lee, D.W.; Yoon, J.; Kim, T.Y.; Bang, J.H.; Nam, A.R.; Oh, K.S.; Kim, J.M.; Lee, Y.; et al. Gemcitabine and cisplatin plus durvalumab with or without tremelimumab in chemotherapy-naive patients with advanced biliary tract cancer: An open-label, single-centre, phase 2 study. Lancet Gastroenterol. Hepatol. 2022, 7, 522–532. [Google Scholar] [CrossRef]
- Li, X.M.; Zhang, X.M.; Li, J.Y.; Jiang, N.; Chen, L.; Tang, L.L.; Mao, Y.P.; Li, W.F.; Zhou, G.Q.; Li, Y.Q.; et al. The immune modulation effects of gemcitabine plus cisplatin induction chemotherapy in nasopharyngeal carcinoma. Cancer Med. 2022, 11, 3437–3444. [Google Scholar] [CrossRef]
- Papadopoulos, K.P.; Harb, W.; Peer, C.J.; Hua, Q.; Xu, S.; Lu, H.; Lu, N.; He, Y.; Xu, T.; Dong, R.; et al. First-in-Human Phase I Study of Envafolimab, a Novel Subcutaneous Single-Domain Anti-PD-L1 Antibody, in Patients with Advanced Solid Tumors. Oncologist 2021, 26, e1514–e1525. [Google Scholar] [CrossRef]
- Italiano, A.; Miller, W.H.; Blay, J.-Y.; Gietema, J.A.; Bang, Y.-J.; Mileshkin, L.R.; Hirte, H.W.; Higgins, B.; Blotner, S.; Nichols, G.L.; et al. Phase I study of daily and weekly regimens of the orally administered MDM2 antagonist idasanutlin in patients with advanced tumors. Investig. New Drugs 2021, 39, 1587–1597. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.N.; Patel, M.R.; Bauer, T.M.; Goel, S.; Falchook, G.S.; Shapiro, G.I.; Chung, K.Y.; Infante, J.R.; Conry, R.M.; Rabinowits, G.; et al. Phase 1 Trial of ALRN-6924, a Dual Inhibitor of MDMX and MDM2, in Patients with Solid Tumors and Lymphomas Bearing Wild-type TP53. Clin. Cancer Res. 2021, 27, 5236–5247. [Google Scholar] [CrossRef]
- Fang, D.D.; Tang, Q.; Kong, Y.; Wang, Q.; Gu, J.; Fang, X.; Zou, P.; Rong, T.; Wang, J.; Yang, D.; et al. MDM2 inhibitor APG-115 synergizes with PD-1 blockade through enhancing antitumor immunity in the tumor microenvironment. J. Immunother. Cancer 2019, 7, 327. [Google Scholar] [CrossRef]
- Takahashi, S.; Fujiwara, Y.; Nakano, K.; Shimizu, T.; Tomomatsu, J.; Koyama, T.; Ogura, M.; Tachibana, M.; Kakurai, Y.; Yamashita, T.; et al. Safety and pharmacokinetics of milademetan, a MDM2 inhibitor, in Japanese patients with solid tumors: A phase I study. Cancer Sci. 2021, 112, 2361–2370. [Google Scholar] [CrossRef]
- Dumbrava, E.E.; Stinchcombe, T.E.; Gounder, M.; Cote, G.M.; Hanna, G.J.; Sumrall, B.; Wise-Draper, T.M.; Kanaan, M.; Duffy, S.; Sumey, C.; et al. Milademetan in advanced solid tumors with MDM2 amplification and wild-type TP53: Pre-clinical and phase 2 clinical trial results. Clin. Cancer Res. 2025, 31, 4255–4264. [Google Scholar] [CrossRef]
- Cornillie, J.; Wozniak, A.; Li, H.; Gebreyohannes, Y.K.; Wellens, J.; Hompes, D.; Debiec-Rychter, M.; Sciot, R.; Schöffski, P. Anti-tumor activity of the MDM2-TP53 inhibitor BI-907828 in dedifferentiated liposarcoma patient-derived xenograft models harboring MDM2 amplification. Clin. Transl. Oncol. 2020, 22, 546–554. [Google Scholar] [CrossRef]
- Rudolph, D.; Reschke, M.; Blake, S.; Rinnenthal, J.; Wernitznig, A.; Weyer-Czernilofsky, U.; Gollner, A.; Haslinger, C.; Garin-Chesa, P.; Quant, J.; et al. Abstract 4866: BI 907828: A novel, potent MDM2 inhibitor that induces antitumor immunologic memory and acts synergistically with an anti-PD-1 antibody in syngeneic mouse models of cancer. Cancer Res. 2018, 78, 4866. [Google Scholar] [CrossRef]
- Yamamoto, N.; Tolcher, A.; Hafez, N.; Lugowska, I.; Ramlau, R.; Macarulla, T.; Geng, J.; Li, J.; Teufel, M.; Märten, A.; et al. Efficacy and Safety of the MDM2-p53 Antagonist Brigimadlin (BI 907828) in Patients with Advanced Biliary Tract Cancer: A Case Series. Onco Targets Ther. 2024, 17, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Park, S.H.; Chang, H.M.; Kim, J.S.; Choi, H.J.; Lee, M.A.; Jang, J.S.; Jeung, H.C.; Kang, J.H.; Lee, H.W.; et al. Gemcitabine and oxaliplatin with or without erlotinib in advanced biliary-tract cancer: A multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2012, 13, 181–188. [Google Scholar] [CrossRef] [PubMed]
- Gruenberger, B.; Schueller, J.; Heubrandtner, U.; Wrba, F.; Tamandl, D.; Kaczirek, K.; Roka, R.; Freimann-Pircher, S.; Gruenberger, T. Cetuximab, gemcitabine, and oxaliplatin in patients with unresectable advanced or metastatic biliary tract cancer: A phase 2 study. Lancet Oncol. 2010, 11, 1142–1148. [Google Scholar] [CrossRef]
- Chen, J.S.; Hsu, C.; Chiang, N.J.; Tsai, C.S.; Tsou, H.H.; Huang, S.F.; Bai, L.Y.; Chang, I.C.; Shiah, H.S.; Ho, C.L.; et al. A KRAS mutation status-stratified randomized phase II trial of gemcitabine and oxaliplatin alone or in combination with cetuximab in advanced biliary tract cancer. Ann. Oncol. 2015, 26, 943–949. [Google Scholar] [CrossRef]
- Harding, J.J.; Cleary, J.M.; Quinn, D.I.; Braña, I.; Moreno, V.; Borad, M.J.; Loi, S.; Spanggaard, I.; Park, H.; Ford, J.M.; et al. Targeting HER2 (ERBB2) mutation-positive advanced biliary tract cancers with neratinib: Results from the phase II SUMMIT ‘basket’ trial. J. Clin. Oncol. 2021, 39, 320. [Google Scholar] [CrossRef]
- Zhu, A.X.; Meyerhardt, J.A.; Blaszkowsky, L.S.; Kambadakone, A.R.; Muzikansky, A.; Zheng, H.; Clark, J.W.; Abrams, T.A.; Chan, J.A.; Enzinger, P.C.; et al. Efficacy and safety of gemcitabine, oxaliplatin, and bevacizumab in advanced biliary-tract cancers and correlation of changes in 18-fluorodeoxyglucose PET with clinical outcome: A phase 2 study. Lancet Oncol. 2010, 11, 48–54. [Google Scholar] [CrossRef]
- Bengala, C.; Bertolini, F.; Malavasi, N.; Boni, C.; Aitini, E.; Dealis, C.; Zironi, S.; Depenni, R.; Fontana, A.; Del Giovane, C.; et al. Sorafenib in patients with advanced biliary tract carcinoma: A phase II trial. Br. J. Cancer 2010, 102, 68–72. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Rankin, C.; Siegel, A.B.; Iqbal, S.; Gong, I.Y.; Micetich, K.C.; Kayaleh, O.R.; Lenz, H.J.; Blanke, C.D. S0941: A phase 2 SWOG study of sorafenib and erlotinib in patients with advanced gallbladder carcinoma or cholangiocarcinoma. Br. J. Cancer 2014, 110, 882–887. [Google Scholar] [CrossRef]
- Lee, S.; Shroff, R.T.; Makawita, S.; Xiao, L.; Danner De Armas, A.; Bhosale, P.; Reddy, K.; Shalaby, A.; Raghav, K.; Pant, S.; et al. Phase II Study of Ramucirumab in Advanced Biliary Tract Cancer Previously Treated by Gemcitabine-Based Chemotherapy. Clin. Cancer Res. 2022, 28, 2229–2236. [Google Scholar] [CrossRef]
- Sun, W.; Patel, A.; Normolle, D.; Patel, K.; Ohr, J.; Lee, J.J.; Bahary, N.; Chu, E.; Streeter, N.; Drummond, S. A phase 2 trial of regorafenib as a single agent in patients with chemotherapy-refractory, advanced, and metastatic biliary tract adenocarcinoma. Cancer 2019, 125, 902–909. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.; Phelps, M.A.; Li, X.; Saji, M.; Goff, L.; Kauh, J.S.; O’Neil, B.H.; Balsom, S.; Balint, C.; Liersemann, R.; et al. Multi-institutional phase II study of selumetinib in patients with metastatic biliary cancers. J. Clin. Oncol. 2011, 29, 2357–2363. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, M.; Ioka, T.; Fukutomi, A.; Morizane, C.; Kasuga, A.; Takahashi, H.; Todaka, A.; Okusaka, T.; Creasy, C.L.; Gorman, S.; et al. Efficacy and safety of trametinib in Japanese patients with advanced biliary tract cancers refractory to gemcitabine. Cancer Sci. 2018, 109, 215–224. [Google Scholar] [CrossRef] [PubMed]
- Shroff, R.T.; Yarchoan, M.; O’Connor, A.; Gallagher, D.; Zahurak, M.L.; Rosner, G.; Ohaji, C.; Sartorius-Mergenthaler, S.; Subbiah, V.; Zinner, R.; et al. The oral VEGF receptor tyrosine kinase inhibitor pazopanib in combination with the MEK inhibitor trametinib in advanced cholangiocarcinoma. Br. J. Cancer 2017, 116, 1402–1407, Erratum in Br. J. Cancer 2018, 118, e2. [Google Scholar] [CrossRef]
- Subbiah, V.; Lassen, U.; Élez, E.; Italiano, A.; Curigliano, G.; Javle, M.; de Braud, F.; Prager, G.W.; Greil, R.; Stein, A.; et al. Dabrafenib plus trametinib in patients with BRAF(V600E)-mutated biliary tract cancer (ROAR): A phase 2, open-label, single-arm, multicentre basket trial. Lancet Oncol. 2020, 21, 1234–1243. [Google Scholar] [CrossRef] [PubMed]
- Yarchoan, M.; Cope, L.; Ruggieri, A.N.; Anders, R.A.; Noonan, A.M.; Goff, L.W.; Goyal, L.; Lacy, J.; Li, D.; Patel, A.K.; et al. Multicenter randomized phase II trial of atezolizumab with or without cobimetinib in biliary tract cancers. J. Clin. Investig. 2021, 131, e152670. [Google Scholar] [CrossRef]
- Buzzoni, R.; Pusceddu, S.; Bajetta, E.; De Braud, F.; Platania, M.; Iannacone, C.; Cantore, M.; Mambrini, A.; Bertolini, A.; Alabiso, O.; et al. Activity and safety of RAD001 (everolimus) in patients affected by biliary tract cancer progressing after prior chemotherapy: A phase II ITMO study. Ann. Oncol. 2014, 25, 1597–1603. [Google Scholar] [CrossRef]
- Sahai, V.; Griffith, K.A.; Goff, L.W.; Crysler, O.V.; Enzler, T.; Zalupski, M. Phase 2 multicenter trial of rucaparib and nivolumab as maintenance therapy following first-line platinum-based chemotherapy in patients with advanced biliary tract cancer (BTC): BilT-02. J. Clin. Oncol. 2023, 41, 4113. [Google Scholar] [CrossRef]
- Sadagopan, N.; Wang, H.; Yin, C.; Weinberg, B.A.; Noel, M.S.; Mukherji, R.; Geng, X.; Marshall, J.L.; He, A.R. A phase II single-arm study of combination pembrolizumab and olaparib in the treatment of patients with advanced biliary tract cancer. Npj Precis. Oncol. 2025, 9, 229. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.; Lamarca, A.; Choi, H.J.; Vogel, A.; Pishvaian, M.J.; Goyal, L.; Ueno, M.; Märten, A.; Teufel, M.; Geng, L.; et al. Brightline-2: A phase IIa/IIb trial of brigimadlin (BI 907828) in advanced biliary tract cancer, pancreatic ductal adenocarcinoma or other solid tumors. Future Oncol. 2024, 20, 1069–1077. [Google Scholar] [CrossRef]
- Sirica, A.E. Role of ErbB family receptor tyrosine kinases in intrahepatic cholangiocarcinoma. World J. Gastroenterol. 2008, 14, 7033–7058. [Google Scholar] [CrossRef]
- Simile, M.M.; Bagella, P.; Vidili, G.; Spanu, A.; Manetti, R.; Seddaiu, M.A.; Babudieri, S.; Madeddu, G.; Serra, P.A.; Altana, M.; et al. Targeted Therapies in Cholangiocarcinoma: Emerging Evidence from Clinical Trials. Medicina 2019, 55, 42. [Google Scholar] [CrossRef] [PubMed]
- Sia, D.; Tovar, V.; Moeini, A.; Llovet, J.M. Intrahepatic cholangiocarcinoma: Pathogenesis and rationale for molecular therapies. Oncogene 2013, 32, 4861–4870. [Google Scholar] [CrossRef]
- Yoshikawa, D.; Ojima, H.; Iwasaki, M.; Hiraoka, N.; Kosuge, T.; Kasai, S.; Hirohashi, S.; Shibata, T. Clinicopathological and prognostic significance of EGFR, VEGF, and HER2 expression in cholangiocarcinoma. Br. J. Cancer 2008, 98, 418–425. [Google Scholar] [CrossRef]
- Leone, F.; Marino, D.; Cereda, S.; Filippi, R.; Belli, C.; Spadi, R.; Nasti, G.; Montano, M.; Amatu, A.; Aprile, G.; et al. Panitumumab in combination with gemcitabine and oxaliplatin does not prolong survival in wild-type KRAS advanced biliary tract cancer: A randomized phase 2 trial (Vecti-BIL study). Cancer 2016, 122, 574–581. [Google Scholar] [CrossRef]
- Peck, J.; Wei, L.; Zalupski, M.; O’Neil, B.; Villalona Calero, M.; Bekaii-Saab, T. HER2/neu may not be an interesting target in biliary cancers: Results of an early phase II study with lapatinib. Oncology 2012, 82, 175–179. [Google Scholar] [CrossRef]
- Javle, M.; Bekaii-Saab, T.; Jain, A.; Wang, Y.; Kelley, R.K.; Wang, K.; Kang, H.C.; Catenacci, D.; Ali, S.; Krishnan, S.; et al. Biliary cancer: Utility of next-generation sequencing for clinical management. Cancer 2016, 122, 3838–3847. [Google Scholar] [CrossRef]
- Santoro, A.; Gebbia, V.; Pressiani, T.; Testa, A.; Personeni, N.; Arrivas Bajardi, E.; Foa, P.; Buonadonna, A.; Bencardino, K.; Barone, C.; et al. A randomized, multicenter, phase II study of vandetanib monotherapy versus vandetanib in combination with gemcitabine versus gemcitabine plus placebo in subjects with advanced biliary tract cancer: The VanGogh study. Ann. Oncol. 2015, 26, 542–547. [Google Scholar] [CrossRef]
- Boonjaraspinyo, S.; Wu, Z.; Boonmars, T.; Kaewkes, S.; Loilome, W.; Sithithaworn, P.; Nagano, I.; Takahashi, Y.; Yongvanit, P.; Bhudhisawasdi, V. Overexpression of PDGFA and its receptor during carcinogenesis of Opisthorchis viverrini-associated cholangiocarcinoma. Parasitol. Int. 2012, 61, 145–150. [Google Scholar] [CrossRef]
- Iyer, R.V.; Pokuri, V.K.; Groman, A.; Ma, W.W.; Malhotra, U.; Iancu, D.M.; Grande, C.; Saab, T.B. A Multicenter Phase II Study of Gemcitabine, Capecitabine, and Bevacizumab for Locally Advanced or Metastatic Biliary Tract Cancer. Am. J. Clin. Oncol. 2018, 41, 649–655. [Google Scholar] [CrossRef]
- El-Khoueiry, A.B.; Rankin, C.J.; Ben-Josef, E.; Lenz, H.J.; Gold, P.J.; Hamilton, R.D.; Govindarajan, R.; Eng, C.; Blanke, C.D. SWOG 0514: A phase II study of sorafenib in patients with unresectable or metastatic gallbladder carcinoma and cholangiocarcinoma. Investig. New Drugs 2012, 30, 1646–1651. [Google Scholar] [CrossRef] [PubMed]
- Miyamoto, M.; Ojima, H.; Iwasaki, M.; Shimizu, H.; Kokubu, A.; Hiraoka, N.; Kosuge, T.; Yoshikawa, D.; Kono, T.; Furukawa, H.; et al. Prognostic significance of overexpression of c-Met oncoprotein in cholangiocarcinoma. Br. J. Cancer 2011, 105, 131–138. [Google Scholar] [CrossRef]
- Goyal, L.; Zheng, H.; Yurgelun, M.B.; Abrams, T.A.; Allen, J.N.; Cleary, J.M.; Knowles, M.; Regan, E.; Reardon, A.; Khachatryan, A.; et al. A phase 2 and biomarker study of cabozantinib in patients with advanced cholangiocarcinoma. Cancer 2017, 123, 1979–1988. [Google Scholar] [CrossRef] [PubMed]
- Valle, J.W.; Vogel, A.; Denlinger, C.S.; He, A.R.; Bai, L.-Y.; Orlova, R.; Van Cutsem, E.; Adeva, J.; Chen, L.-T.; Obermannova, R.; et al. Addition of ramucirumab or merestinib to standard first-line chemotherapy for locally advanced or metastatic biliary tract cancer: A randomised, double-blind, multicentre, phase 2 study. Lancet Oncol. 2021, 22, 1468–1482, Correction in Lancet Oncol. 2021, 22, e472. [Google Scholar] [CrossRef] [PubMed]
- Pant, S.; Saleh, M.; Bendell, J.; Infante, J.R.; Jones, S.; Kurkjian, C.D.; Moore, K.M.; Kazakin, J.; Abbadessa, G.; Wang, Y.; et al. A phase I dose escalation study of oral c-MET inhibitor tivantinib (ARQ 197) in combination with gemcitabine in patients with solid tumors. Ann. Oncol. 2014, 25, 1416–1421. [Google Scholar] [CrossRef]
- Paik Paul, K.; Felip, E.; Veillon, R.; Sakai, H.; Cortot Alexis, B.; Garassino Marina, C.; Mazieres, J.; Viteri, S.; Senellart, H.; Van Meerbeeck, J.; et al. Tepotinib in Non–Small-Cell Lung Cancer with MET Exon 14 Skipping Mutations. N. Engl. J. Med. 2020, 383, 931–943. [Google Scholar] [CrossRef]
- Mazieres, J.; Paik, P.K.; Garassino, M.C.; Le, X.; Sakai, H.; Veillon, R.; Smit, E.F.; Cortot, A.B.; Raskin, J.; Viteri, S.; et al. Tepotinib Treatment in Patients with MET Exon 14–Skipping Non–Small Cell Lung Cancer: Long-Term Follow-Up of the VISION Phase 2 Nonrandomized Clinical Trial. JAMA Oncol. 2023, 9, 1260–1266, Correction in JAMA Oncol. 2023, 9, 1300. [Google Scholar] [CrossRef] [PubMed]
- Administration USFD. FDA Approves Tepotinib for Metastatic Non-Small Cell Lung Cancer. Food Drug Adm. 2024. Available online: https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-tepotinib-metastatic-non-small-cell-lung-cancer (accessed on 23 October 2025).
- Rahnemai-Azar, A.A.; Weisbrod, A.B.; Dillhoff, M.; Schmidt, C.; Pawlik, T.M. Intrahepatic cholangiocarcinoma: Current management and emerging therapies. Expert Rev. Gastroenterol. Hepatol. 2017, 11, 439–449. [Google Scholar] [CrossRef] [PubMed]
- Soria, J.-C.; Tan, D.S.W.; Chiari, R.; Wu, Y.-L.; Paz-Ares, L.; Wolf, J.; Geater, S.L.; Orlov, S.; Cortinovis, D.; Yu, C.-J.; et al. First-line ceritinib versus platinum-based chemotherapy in advanced ALK-rearranged non-small-cell lung cancer (ASCEND-4): A randomised, open-label, phase 3 study. Lancet 2017, 389, 917–929. [Google Scholar] [CrossRef] [PubMed]
- Takeyasu, Y.; Okuma, H.S.; Kojima, Y.; Nishikawa, T.; Tanioka, M.; Sudo, K.; Shimoi, T.; Noguchi, E.; Arakawa, A.; Mori, T.; et al. Impact of ALK Inhibitors in Patients with ALK-Rearranged Nonlung Solid Tumors. JCO Precis. Oncol. 2021, 5, 756–766. [Google Scholar] [CrossRef]
- Drilon, A.; Siena, S.; Ou, S.I.; Patel, M.; Ahn, M.J.; Lee, J.; Bauer, T.M.; Farago, A.F.; Wheler, J.J.; Liu, S.V.; et al. Safety and Antitumor Activity of the Multitargeted Pan-TRK, ROS1, and ALK Inhibitor Entrectinib: Combined Results from Two Phase I Trials (ALKA-372-001 and STARTRK-1). Cancer Discov. 2017, 7, 400–409. [Google Scholar] [CrossRef]
- Palomba, G.; Doneddu, V.; Cossu, A.; Paliogiannis, P.; Manca, A.; Casula, M.; Colombino, M.; Lanzillo, A.; Defraia, E.; Pazzola, A.; et al. Prognostic impact of KRAS, NRAS, BRAF, and PIK3CA mutations in primary colorectal carcinomas: A population-based study. J. Transl. Med. 2016, 14, 292. [Google Scholar] [CrossRef]
- Bahar, M.E.; Kim, H.J.; Kim, D.R. Targeting the RAS/RAF/MAPK pathway for cancer therapy: From mechanism to clinical studies. Signal Transduct. Target. Ther. 2023, 8, 455. [Google Scholar] [CrossRef]
- Walter, D.; Hartmann, S.; Waidmann, O. Update on cholangiocarcinoma: Potential impact of genomic studies on clinical management. Z. Gastroenterol. 2017, 55, 575–581. [Google Scholar] [CrossRef]
- Subbiah, V.; Puzanov, I.; Blay, J.Y.; Chau, I.; Lockhart, A.C.; Raje, N.S.; Wolf, J.; Baselga, J.; Meric-Bernstam, F.; Roszik, J.; et al. Pan-Cancer Efficacy of Vemurafenib in BRAF (V600)-Mutant Non-Melanoma Cancers. Cancer Discov. 2020, 10, 657–663. [Google Scholar] [CrossRef]
- Silkin, S.V.; Startsev, S.S.; Krasnova, M.E.; Raskin, G.A.; Mitiushkina, N.V.; Iyevleva, A.G.; Sokolenko, A.P.; Imyanitov, E.N. Complete Clinical Response of BRAF-Mutated Cholangiocarcinoma to Vemurafenib, Panitumumab, and Irinotecan. J. Gastrointest. Cancer 2016, 47, 502–505. [Google Scholar] [CrossRef]
- Bridgewater, J.; Lopes, A.; Beare, S.; Duggan, M.; Lee, D.; Ricamara, M.; McEntee, D.; Sukumaran, A.; Wasan, H.; Valle, J.W. A phase 1b study of Selumetinib in combination with Cisplatin and Gemcitabine in advanced or metastatic biliary tract cancer: The ABC-04 study. BMC Cancer 2016, 16, 153, Erratum in BMC Cancer 2016, 16, 369. [Google Scholar]
- Doherty, M.K.; Tam, V.C.; McNamara, M.G.; Jang, R.; Hedley, D.; Chen, E.; Dhani, N.; Tang, P.; Sim, H.W.; O’Kane, G.M.; et al. Randomised, Phase II study of selumetinib, an oral inhibitor of MEK, in combination with cisplatin and gemcitabine chemotherapy for patients with advanced biliary tract cancer. Br. J. Cancer 2022, 127, 1473–1478. [Google Scholar] [CrossRef]
- Kim, R.D.; McDonough, S.; El-Khoueiry, A.B.; Bekaii-Saab, T.S.; Stein, S.M.; Sahai, V.; Keogh, G.P.; Kim, E.J.; Baron, A.D.; Siegel, A.B.; et al. Randomised phase II trial (SWOG S1310) of single agent MEK inhibitor trametinib Versus 5-fluorouracil or capecitabine in refractory advanced biliary cancer. Eur. J. Cancer 2020, 130, 219–227. [Google Scholar] [CrossRef]
- Heumann, T.R.; Yarchoan, M.; Murray, J.; Lu, J.; Li, D.; Kunk, P.R.; Iyer, R.V.; Dayyani, F.; Fonkoua, L.A.K.; Kalyan, A.; et al. A randomized phase 2 study of combination atezolizumab and varlilumab (CDX-1127) with or without addition of cobimetinib in previously treated unresectable biliary tract cancer. J. Clin. Oncol. 2024, 42, 4017. [Google Scholar] [CrossRef]
- Schmitz, K.J.; Lang, H.; Wohlschlaeger, J.; Sotiropoulos, G.C.; Reis, H.; Schmid, K.W.; Baba, H.A. AKT and ERK1/2 signaling in intrahepatic cholangiocarcinoma. World J. Gastroenterol. 2007, 13, 6470–6477. [Google Scholar] [CrossRef]
- Javle, M.M.; Yu, J.; Khoury, T.; Chadha, K.S.; Iyer, R.V.; Foster, J.; Kuvshinoff, B.W.; Gibbs, J.F.; Geradts, J.; Black, J.D.; et al. Akt expression may predict favorable prognosis in cholangiocarcinoma. J. Gastroenterol. Hepatol. 2006, 21, 1744–1751. [Google Scholar] [CrossRef]
- Lau, D.K.; Tay, R.Y.; Yeung, Y.H.; Chionh, F.; Mooi, J.; Murone, C.; Skrinos, E.; Price, T.J.; Mariadason, J.M.; Tebbutt, N.C. Phase II study of everolimus (RAD001) monotherapy as first-line treatment in advanced biliary tract cancer with biomarker exploration: The RADiChol Study. Br. J. Cancer 2018, 118, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.D.; Alberts, S.R.; Peña, C.; Genvresse, I.; Ajavon-Hartmann, A.; Xia, C.; Kelly, A.; Grilley-Olson, J.E. Phase I dose-escalation study of copanlisib in combination with gemcitabine or cisplatin plus gemcitabine in patients with advanced cancer. Br. J. Cancer 2018, 118, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Ahn, D.H.; Li, J.; Wei, L.; Doyle, A.; Marshall, J.L.; Schaaf, L.J.; Phelps, M.A.; Villalona-Calero, M.A.; Bekaii-Saab, T. Results of an abbreviated phase-II study with the Akt. Inhibitor MK-2206 in Patients with Advanced Biliary Cancer. Sci. Rep. 2015, 5, 12122. [Google Scholar] [CrossRef]
- Yang, R.; Song, Y.; Shakoor, K.; Yi, W.; Peng, C.; Liu, S. Insights into the role of STAT3 in intrahepatic cholangiocarcinoma (Review). Mol. Med. Rep. 2022, 25, 171. [Google Scholar] [CrossRef]
- Jarnicki, A.; Putoczki, T.; Ernst, M. Stat3: Linking inflammation to epithelial cancer—More than a “gut” feeling? Cell Div. 2010, 5, 14. [Google Scholar] [CrossRef]
- Isomoto, H.; Mott, J.L.; Kobayashi, S.; Werneburg, N.W.; Bronk, S.F.; Haan, S.; Gores, G.J. Sustained IL-6/STAT-3 signaling in cholangiocarcinoma cells due to SOCS-3 epigenetic silencing. Gastroenterology 2007, 132, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.W.; Li, L.; Hou, G.J.; Yan, X.Z.; Xu, Q.G.; Chen, L.; Zhang, B.H.; Shen, F. STAT3 overexpression promotes metastasis in intrahepatic cholangiocarcinoma and correlates negatively with surgical outcome. Oncotarget 2017, 8, 7710–7721. [Google Scholar] [PubMed]
- Li, X.; Jiang, W.; Dong, S.; Li, W.; Zhu, W.; Zhou, W. STAT3 Inhibitors: A Novel Insight for Anticancer Therapy of Pancreatic Cancer. Biomolecules 2022, 12, 1450. [Google Scholar] [CrossRef]
- Hong, D.; Kurzrock, R.; Kim, Y.; Woessner, R.; Younes, A.; Nemunaitis, J.; Fowler, N.; Zhou, T.; Schmidt, J.; Jo, M.; et al. AZD9150, a next-generation antisense oligonucleotide inhibitor of STAT3 with early evidence of clinical activity in lymphoma and lung cancer. Sci. Transl. Med. 2015, 7, 314ra185. [Google Scholar] [CrossRef]
- Han, A.; Purwin, T.J.; Aplin, A.E. Roles of the BAP1 Tumor Suppressor in Cell Metabolism. Cancer Res. 2021, 81, 2807–2814. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, A.; Carloni, R.; Ricci, A.D.; Di Federico, A.; Guven, D.C.; Yalcin, S.; Brandi, G. Molecular Profile and Prognostic Value of BAP1 Mutations in Intrahepatic Cholangiocarcinoma: A Genomic Database Analysis. J. Pers. Med. 2022, 12, 1247. [Google Scholar] [CrossRef]
- Sabbatino, F.; Liguori, L.; Malapelle, U.; Schiavi, F.; Tortora, V.; Conti, V.; Filippelli, A.; Tortora, G.; Ferrone, C.R.; Pepe, S. Case Report: BAP1 Mutation and RAD21 Amplification as Predictive Biomarkers to PARP Inhibitor in Metastatic Intrahepatic Cholangiocarcinoma. Front. Oncol. 2020, 10, 567289. [Google Scholar] [CrossRef]
- Mohan, A.; Quingalahua, E.; Gunchick, V.; Paul, S.; Kumar-Sinha, C.; Crysler, O.; Zalupski, M.M.; Sahai, V. PARP inhibitor therapy in patients with IDH1 mutated cholangiocarcinoma. Oncologist 2024, 29, 725–730. [Google Scholar] [CrossRef]
- Tsang, E.S.; O’Kane, G.M.; Knox, J.J.; Chen, E.X. A phase II study of olaparib and durvalumab in patients with IDH-mutated cholangiocarcinoma. J. Clin. Oncol. 2023, 41, 4099. [Google Scholar] [CrossRef]
- Zhou, T.; Mahn, R.; Möhring, C.; Sadeghlar, F.; Meyer, C.; Toma, M.; Kreppel, B.; Essler, M.; Glowka, T.; Matthaei, H.; et al. Case Report: Sustained complete remission on combination therapy with olaparib and pembrolizumab in BRCA2-mutated and PD-L1-positive metastatic cholangiocarcinoma after platinum derivate. Front. Oncol. 2022, 12, 933943. [Google Scholar] [CrossRef]
- Zou, S.; Li, J.; Zhou, H.; Frech, C.; Jiang, X.; Chu, J.S.; Zhao, X.; Li, Y.; Li, Q.; Wang, H.; et al. Mutational landscape of intrahepatic cholangiocarcinoma. Nat. Commun. 2014, 5, 5696. [Google Scholar] [CrossRef]
- Riener, M.O.; Bawohl, M.; Clavien, P.A.; Jochum, W. Rare PIK3CA hotspot mutations in carcinomas of the biliary tract. Genes Chromosomes Cancer 2008, 47, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, M.; Ohnishi, H.; Ohtsuka, K.; Matsushima, S.; Ohkura, Y.; Furuse, J.; Watanabe, T.; Mori, T.; Sugiyama, M. KRAS Mutation as a Potential Prognostic Biomarker of Biliary Tract Cancers. Jpn. Clin. Med. 2016, 7, 33–39. [Google Scholar] [CrossRef]
- The Lancet Oncology. Undruggable KRAS-Time Rebrand? Lancet Oncol. 2021, 22, 289. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, C.R.; Garassino, M.C.; Nadal, E.; Öhrling, K.; Scheffler, M.; Mazières, J. On target: Rational approaches to KRAS inhibition for treatment of non-small cell lung carcinoma. Lung Cancer 2021, 160, 152–165. [Google Scholar] [CrossRef]
- Uprety, D.; Adjei, A.A. KRAS: From undruggable to a druggable Cancer Target. Cancer Treat. Rev. 2020, 89, 102070. [Google Scholar] [CrossRef]
- Bekaii-Saab, T.S.; Yaeger, R.; Spira, A.I.; Pelster, M.S.; Sabari, J.K.; Hafez, N.; Barve, M.; Velastegui, K.; Yan, X.; Shetty, A.; et al. Adagrasib in Advanced Solid Tumors Harboring a KRAS(G12C) Mutation. J. Clin. Oncol. 2023, 41, 4097–4106. [Google Scholar] [CrossRef]
- Bouattour, M.; Valle, J.W.; Vogel, A.; Kim, J.W.; Kitano, M.; Chen, J.-S.; Zaucha, R.; Qin, S.; Evesque, L.; Zhen, D.B.; et al. Characterization of long-term survivors in the TOPAZ-1 study of durvalumab or placebo plus gemcitabine and cisplatin in advanced biliary tract cancer. J. Clin. Oncol. 2023, 41, 531. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).