Effect of the Gestational Fluoxetine Administration on Behavioral Tests and Hippocampal Structure in Male Offspring of Rats
Abstract
1. Introduction
2. Results
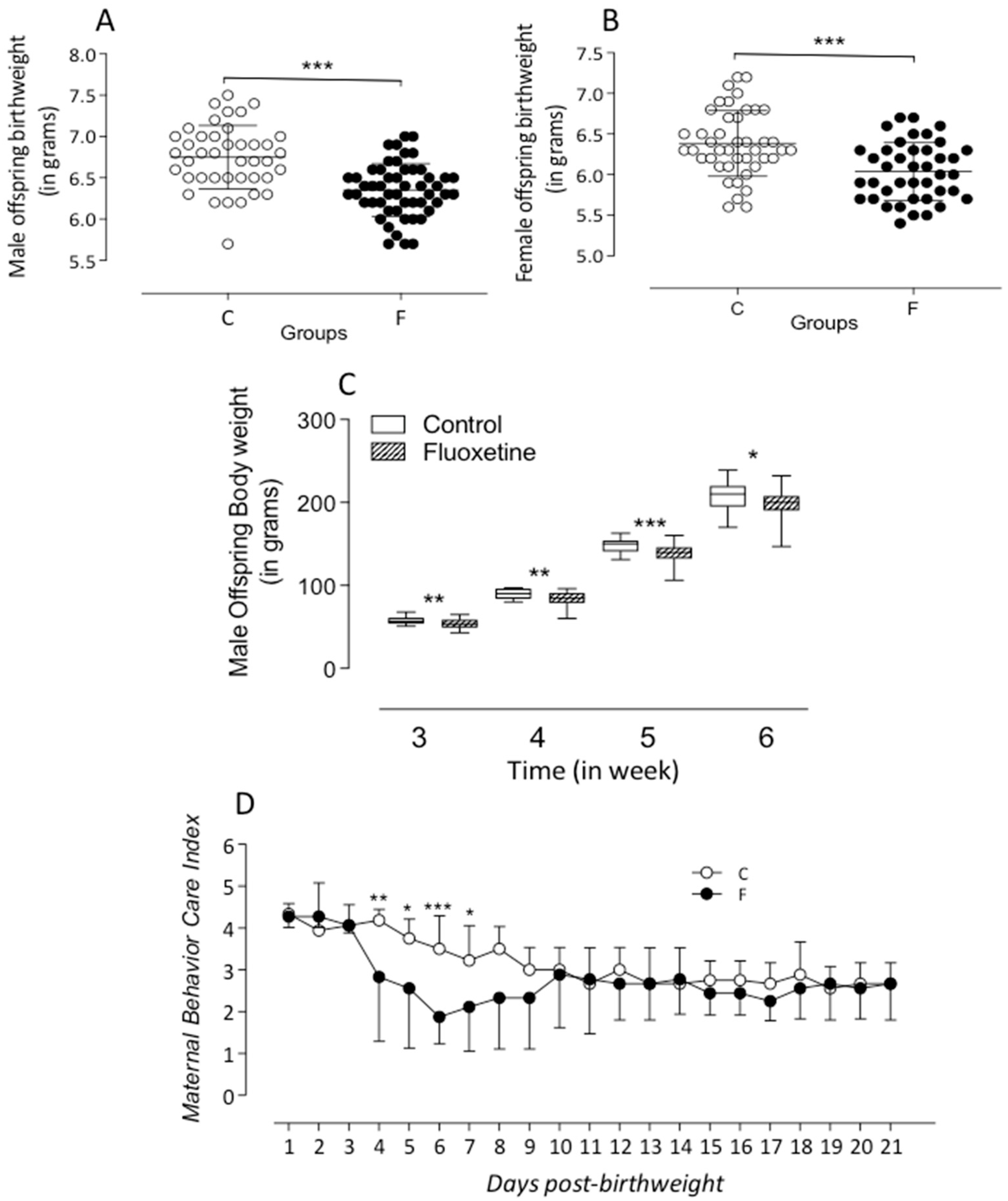
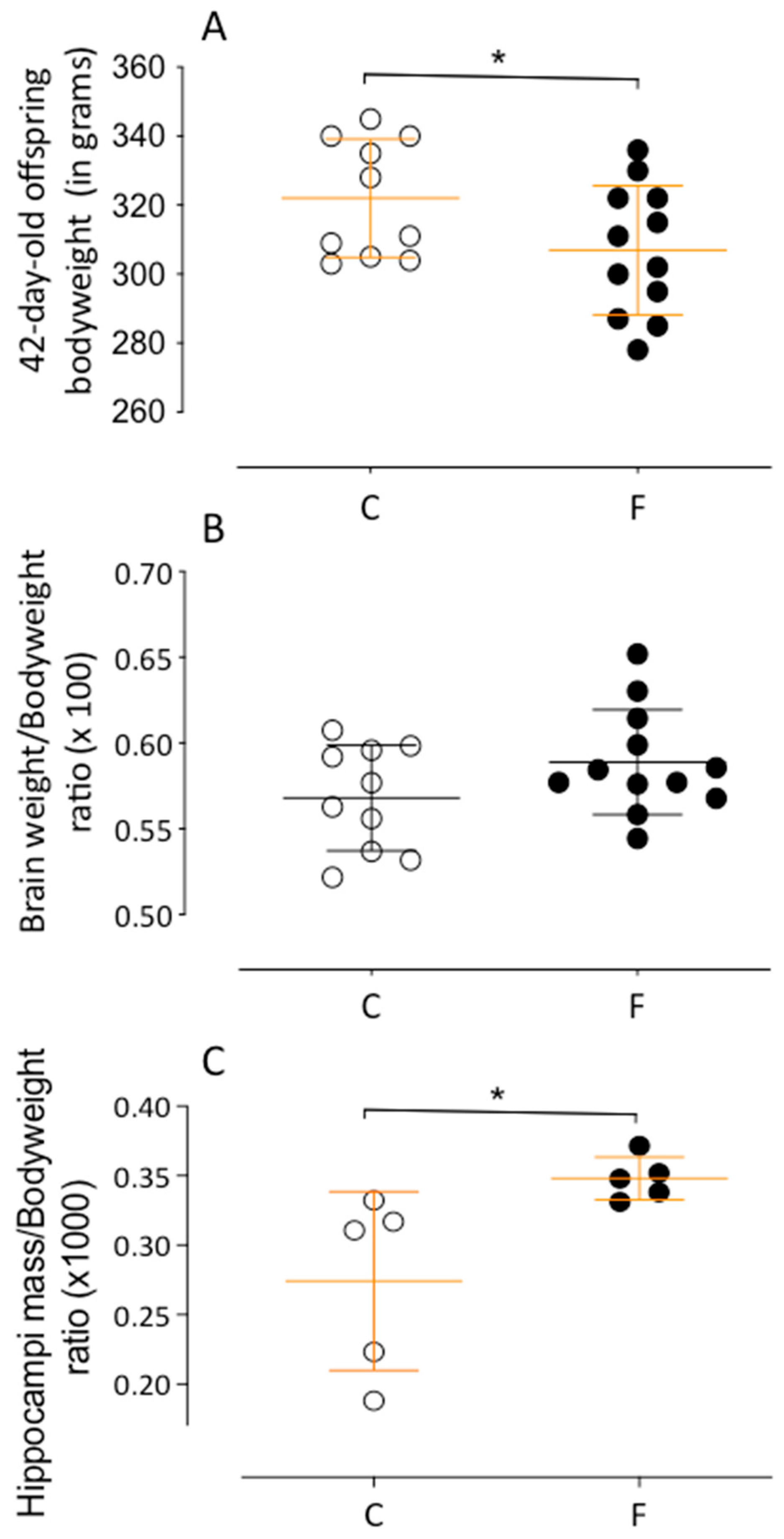
2.1. Animals and Experimental Groups
2.2. Offspring Behavioral Tests
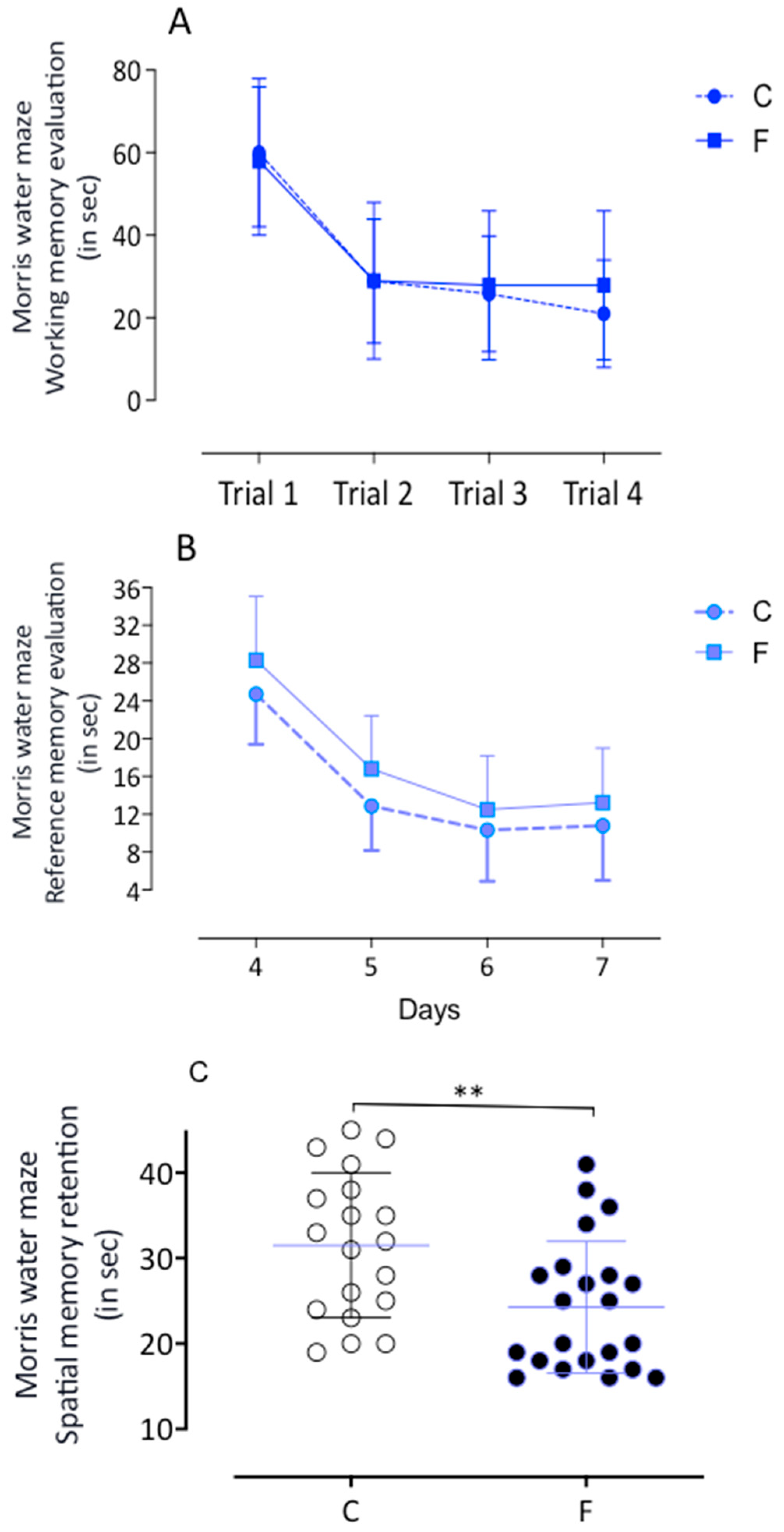
2.2.1. Morris Water Maze
2.2.2. Open Field Activity Monitoring Test
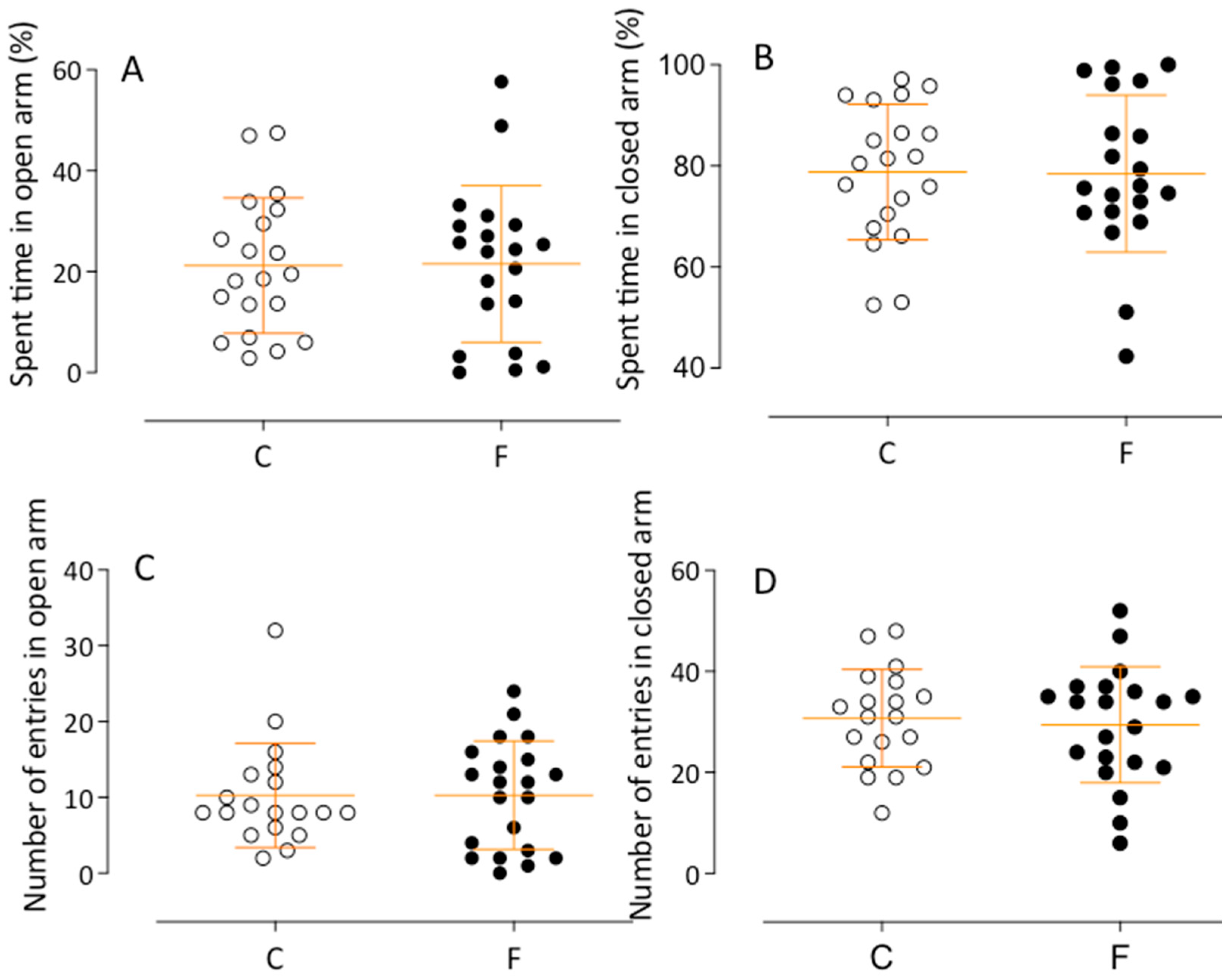
2.2.3. Elevated Plus Maze Test
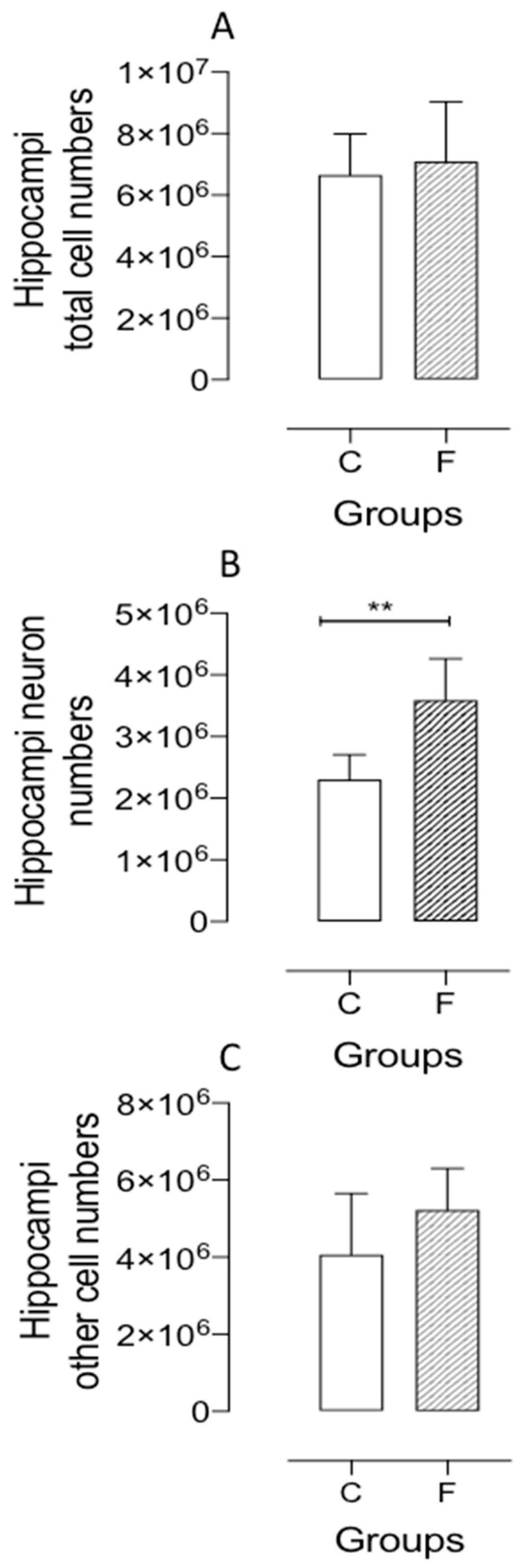
2.3. Isotropic Fractionation
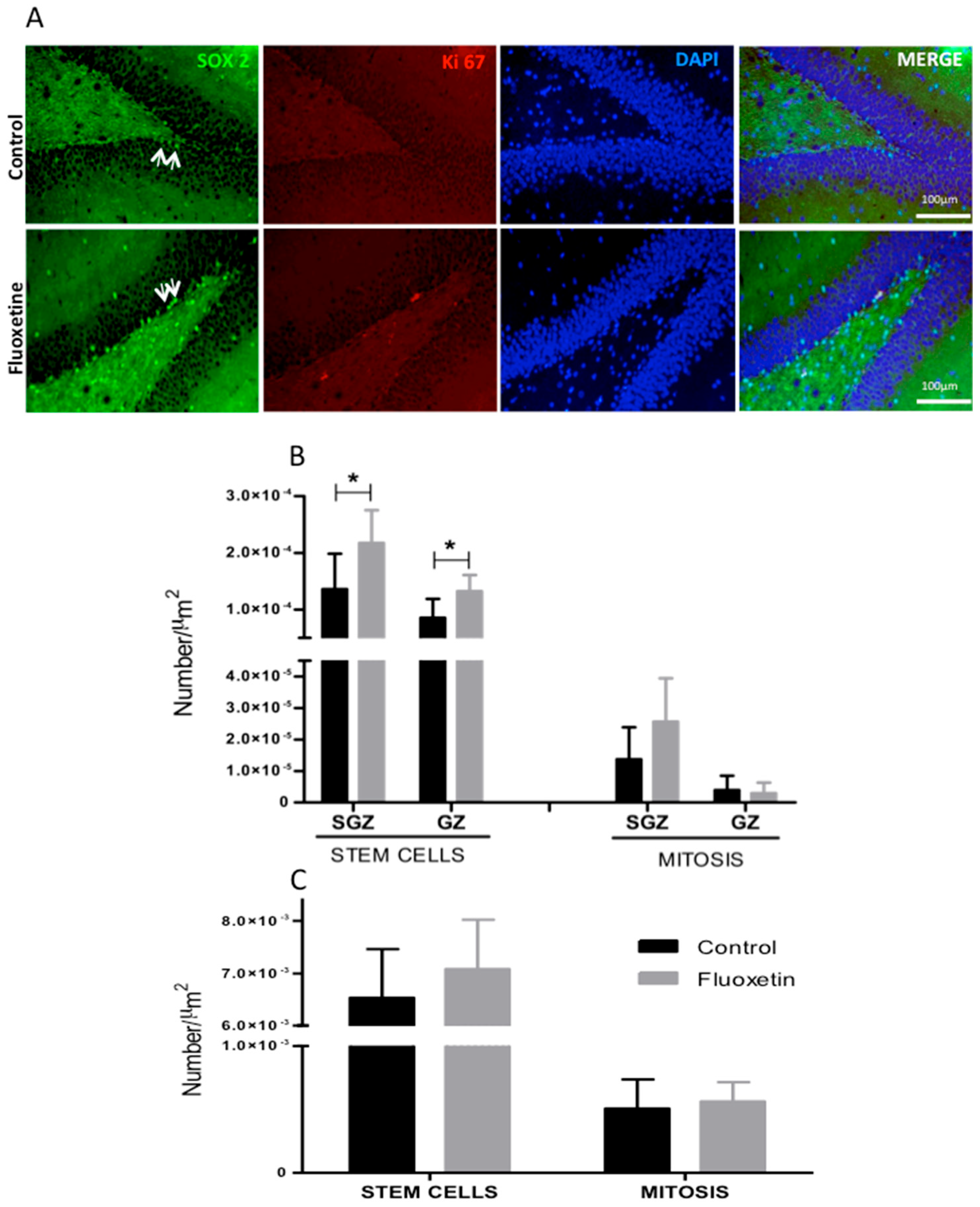
2.4. Immunohistochemistry
3. Discussion
4. Materials and Methods
4.1. Animals and Experimental Groups
4.2. Experimental Procedures—Behavioral Analysis
Maternal Maternal Care Behavior
4.3. Offspring Behavioral Tests
4.3.1. Morris Water Maze (MWM)
4.3.2. Working Memory Task
4.3.3. Reference Memory Task
4.3.4. Activity Monitoring Test
4.3.5. Elevated Plus Maze
4.4. Hippocampal Total Cell and Neuron Quantification by Isotropic Fractionation
4.5. Immunohistochemistry
4.6. Data Presentation and Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
List of Abbreviations
| 5-HT | Serotonin |
| ADHD | Attention deficit hyperactivity disorder |
| ASD | Autism spectrum disorder |
| BDNF | Brain-derived neurotrophic factor |
| C | Control offspring |
| CA | Cornus ammonis |
| CEMIB | Biotherism Center of the State University of Campinas |
| CEUA | Ethics Committee on Animal Experimentation |
| CNS | Central nervous system |
| DAPI | 4′,6-diamidino-2-phenylindole |
| F | Fluoxetine offspring |
| GD | Dentate gyrus |
| GCL | Granular cell layer |
| HPA | Hypothalamus–pituitary–adrenal |
| SGZ | Subgranular zone |
| SRI | Selective serotonin reuptake inhibitors |
| SVZ | Subventricular zone |
References
- Lopes, A.; Torres, D.B.; Rodrigues, A.J.; Cerqueira, J.J.; Pêgo, J.M.; Sousa, N.; Gontijo, J.A.; Boer, P.A. Gestational protein restriction induces CA3 dendritic atrophy in dorsal hippocampal neurons but does not alter learning and memory performance in adult offspring. Int. J. Dev. Neurosci. 2013, 31, 151–156, ISSN 0736-5748. [Google Scholar] [CrossRef] [PubMed]
- Scabora, J.E.; Lopes, A.; Mesquita, F.F.; Torres, D.; Boer, P.A.; Rocha Gontijo, J.A. Early hypothalamic angiotensin II receptor expression changes in gestational protein-restricted offspring: Effect on water intake, blood pressure, and renal sodium handling. J. Renin-Angiotensin-Aldosterone Syst. 2013, 14, 271–282. [Google Scholar] [CrossRef]
- Block, D.B.; Mesquita, F.F.; de Lima, I.P.; Boer, P.A.; Gontijo, J.A. Fetal Kidney Programming by Maternal Smoking Exposure: Effects on Kidney Structure, Blood Pressure, and Urinary Sodium Excretion in Adult Offspring. Nephron 2015, 129, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Torres, D.B.; Lopes, A.; Rodrigues, A.J.; Ventura-Silva, A.P.; Sousa, N.; Gontijo, J.A.R.; Boer, P.A.; Lopes, M.G. Early morphological and neurochemical changes of the stria terminalis (BNST) bed nucleus in gestationally protein-restricted male offspring. Nutr. Neurosci. 2024, 27, 1250–1268. [Google Scholar] [CrossRef]
- Torres, D.B.; Lopes, A.; Rodrigues, A.J.; Lopes, M.G.; Ventura-Silva, A.P.; Sousa, N.; Gontijo, J.A.R.; Boer, P.A. Gestational protein restriction alters early amygdala neurochemistry in male offspring. Nutr. Neurosci. 2022, 26, 1103–1119. [Google Scholar] [CrossRef]
- Torres, D.B.; Lopes, A.; Rodrigues, A.J.; Cerqueira, J.J.; Sousa, N.; Gontijo, J.A.; Boer, P.A. Anxiety-like behavior and structural changes of the bed nucleus of the stria terminalis (BNST) in gestational protein-restricted male offspring. J. Dev. Orig. Health Dis. 2018, 9, 536–543. [Google Scholar] [CrossRef]
- Grigoletti-Lima, G.B.; Lopes, M.G.; Franco, A.T.B.; Damico, A.M.; Boer, P.A.; Rocha Gontijo, J.A. Severe Gestational Low-Protein Intake Impacts Hippocampal Cellularity, Tau, Amyloid-β Levels, and Memory Performance in Male Adult Offspring: An Alzheimer-simile Disease Model? J. Alzheimers Dis. Rep. 2022, 6, 17–30. [Google Scholar] [CrossRef]
- Kessler, R.C.; Berglund, P.; Demler, O.; Jin, R.; Merikangas, K.R.; Walters, E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch. Gen. Psychiatry 2005, 62, 593–602. [Google Scholar] [CrossRef]
- Austin, M.P.; Kildea, S.; Sullivan, E. Maternal mortality and psychiatric morbidity in the perinatal period: Challenges and opportunities for prevention in the Australian setting. Med. J. Aust. 2007, 186, 364–367. [Google Scholar] [CrossRef]
- Gavin, N.I.; Gaynes, B.N.; Lohr, K.N.; Meltzer-Brody, S.; Gartlehner, G.; Swinson, T. Perinatal depression: A systematic review of prevalence and incidence. Obs. Gynecol. 2005, 106 Pt 1, 1071–1083. [Google Scholar] [CrossRef]
- Pawlby, S.; Hay, D.F.; Sharp, D.; Waters, C.S.; O’Keane, V. Antenatal depression predicts depression in adolescent offspring: Prospective longitudinal community-based study. J. Affect. Disord. 2009, 113, 236–243. [Google Scholar] [CrossRef]
- Van Batenburg-Eddes, T.; Brion, M.J.; Henrichs, J.; Jaddoe, V.W.; Hofman, A.; Verhulst, F.C.; Lawlor, D.A.; Davey Smith, G.; Tiemeier, H. Parental depressive and anxiety symptoms during pregnancy and attention problems in children: A cross-cohort consistency study. J. Child Psychol. Psychiatry Allied. Discip. 2013, 54, 591–600. [Google Scholar] [CrossRef] [PubMed]
- Pearson, R.M.; Evans, J.; Kounali, D.; Lewis, G.; Heron, J.; Ramchandani, P.G.; O’Connor, T.G.; Stein, A. Maternal depression during pregnancy and the postnatal period: Risks and possible mechanisms for offspring depression at age 18 years. JAMA Psychiatry 2013, 70, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Alwan, S.; Bandoli, G.; Chambers, C.D. Use of selective serotonin-reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. Clin. Pharmacol. Ther. 2016, 100, 34–41. [Google Scholar] [CrossRef]
- Majeroni, B.A.; Hess, A. The pharmacologic treatment of depression. J. Am. Board Fam. Pract. 1988, 11, 127–139. [Google Scholar] [CrossRef]
- Gentile, S.; Galbally, M. Prenatal exposure to antidepressant medications and neurodevelopmental outcomes: A systematic review. J. Affect. Disord. 2011, 128, 1–9. [Google Scholar] [CrossRef]
- Buznikov, G.A.; Shmukler, Y.B. From oocyte to neuron: Do neurotransmitters function in the same way throughout development? Cell Mol. Neurobiol. 1996, 16, 537–559. [Google Scholar] [CrossRef]
- Lauder, J.M. Hormonal and humoral influences on brain development. Psychoneuroendocrinology 1983, 8, 121–155. [Google Scholar] [CrossRef]
- Bonnin, A.; Peng, W.; Hewlett, W.; Levitt, P. Expression mapping of 5-HT1 serotonin receptor subtypes during fetal and early postnatal mouse forebrain development. Neuroscience 2006, 141, 781–794. [Google Scholar] [CrossRef]
- Lambe, E.K.; Krimer, L.S.; Goldman-Rakic, P.S. Differential postnatal development of catecholamine and serotonin inputs to identified neurons in the prefrontal cortex of the rhesus monkey. J. Neurosci. 2000, 20, 8780–8787. [Google Scholar] [CrossRef]
- Brezun, J.M.; Daszuta, A. Serotonin may stimulate granule cell proliferation in the adult hippocampus, as observed in rats grafted with foetal raphe neurons. Eur. J. Neurosci. 2000, 12, 391–396. [Google Scholar] [CrossRef]
- Banasr, M.; Hery, M.; Printemps, R.; Daszuta, A. Serotonin-induced increases in adult cell proliferation and neurogenesis are mediated through different and common 5-HT receptor subtypes in the dentate gyrus and the subventricular zone. Neuropsychopharmacology 2004, 29, 450–460. [Google Scholar] [CrossRef]
- Velasquez, J.C.; Goeden, N.; Bonnin, A. Placental serotonin: Implications for the developmental effects of SSRIs and maternal depression. Front. Cell. Neurosci. 2013, 7, 47. [Google Scholar] [CrossRef]
- Sit, D.; Perel, J.M.; Wisniewski, S.R.; Helsel, J.C.; Luther, J.F.; Wisner, K.L. Mother-Infant Antidepressant Levels, Maternal Depression and Perinatal Events. J. Clin. Psychiatry 2011, 72, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Hendrick, V.; Stowe, Z.N.; Altshuler, L.L.; Hwang, S.; Lee, E.; Haynes, D. Placental passage of antidepressant medications. Am. J. Psychiatry 2003, 160, 993–996. [Google Scholar] [CrossRef] [PubMed]
- Pohland, R.C.; Byrd, T.K.; Hamilton, M.; Koons, J.R. Placental transfer and fetal distribution of fluoxetine in the rat. Toxicol. Appl. Pharmacol. 1989, 98, 198–205. [Google Scholar] [CrossRef]
- Hendrick, V.; Stowe, Z.N.; Altshuler, L.L.; Mintz, J.; Hwang, S.; Hostetter, A.; Suri, R.; Leight, K.; Fukuchi, A. Fluoxetine and norfluoxetine concentrations in nursing infants and breast milk. Biol. Psychiatry 2001, 50, 775–782. [Google Scholar] [CrossRef] [PubMed]
- Vorhees, C.V.; Acuff-Smith, K.D.; Schilling, M.A.; Fisher, J.; Moran, M.S.; Buelke-Sam, J. A developmental neurotoxicity evaluation of the effects of prenatal exposure to fluoxetine in rats. Fundam. Appl. Toxicol. 1994, 23, 194–205. [Google Scholar] [CrossRef]
- Oberlander, T.F.; Warburton, W.; Misri, S.; Aghajanian, J.; Hertzman, C. Neonatal outcomes after prenatal exposure to selective serotonin reuptake inhibitor antidepressants and maternal depression using population-based linked health data. Arch Gen. Psychiatry 2006, 63, 898–906. [Google Scholar] [CrossRef]
- Casper, R.C.; Fleisher, B.E.; Lee-Ancajas, J.C.; Gilles, A.; Gaylor, E.; DeBattista, A.; Hoyme, H.E. Follow-up of children of depressed mothers exposed or not exposed to antidepressant drugs during pregnancy. J. Pediatr. 2003, 142, 402–408. [Google Scholar] [CrossRef]
- Oberlander, T.F.; Grunau, R.E.; Fitzgerald, C.; Papsdorf, M.; Rurak, D.; Riggs, W. Pain Reactivity in 2-Month-Old Infants After Prenatal and Postnatal Serotonin Reuptake Inhibitor Medication Exposure. Pediatrics 2005, 115, 411–425. [Google Scholar] [CrossRef] [PubMed]
- Croen, L.A.; Grether, J.K.; Yoshida, C.K.; Odouli, R.; Hendrick, V. Antidepressants during pregnancy and childhood autism spectrum disorders. Arch. Gen. Psychiatry 2011, 68, 1104–1112. [Google Scholar] [CrossRef] [PubMed]
- Nulman, I.; Rovet, J.; Stewart, D.E.; Wolpin, J.; Pace-Asciak, P.; Shuhaiber, S.; Koren, G. Child development following exposure to tricyclic antidepressants or fluoxetine throughout fetal life: A prospective, controlled study. Am. J. Psychiatry 2002, 159, 1889–1895. [Google Scholar] [CrossRef] [PubMed]
- Paulson, J.F.; Keefe, H.A.; Leiferman, J.A. Early parental depression and child language development. J. Child Psychol. Psychiatry Allied Discip. 2009, 50, 254–262. [Google Scholar] [CrossRef]
- Bonnin, A.; Levitt, P. Fetal, maternal, and placental sources of serotonin and new implications for developmental programming of the brain. Neuroscience 2011, 197, 1–7. [Google Scholar] [CrossRef]
- Ganapathy, V.; Ramamoorthy, S.; Frederick, H. Leibach FH. Transport and metabolism of monoamines in the human placenta—A review. Placenta 1993, 14, 35–51. [Google Scholar] [CrossRef]
- Nissinen, J.; Halonen, T.; Koivisto, E.; Pitkänen, A. A new model of chronic temporal lobe epilepsy induced by electrical stimulation of the amygdala in rats. Epilepsy Res. 2000, 38, 177–205. [Google Scholar] [CrossRef]
- Verhaagh, S.; Barlow, D.P.; Zwart, R. The extraneuronal monoamine transporter Slc22a3/Orct3 co-localizes with the Maoa metabolizing enzyme in mouse placenta. Mech. Dev. 2001, 100, 127–130. [Google Scholar] [CrossRef]
- Pawluski, J.L.; Charlier, T.D.; Fillet, M.; Houbart, V.; Crispin, H.T.; Steinbusch, H.W.; van den Hove, D.L. Chronic fluoxetine treatment and maternal adversity differentially alter neurobehavioral outcomes in the rat dam. Behav. Brain Res. 2012, 228, 159–168. [Google Scholar] [CrossRef]
- Homberg, J.R.; Lesch, K.P. Looking on the bright side of serotonin transporter gene variation. Biol. Psychiatry 2011, 69, 513–519. [Google Scholar] [CrossRef]
- Ververs, T.; Kaasenbrood, H.; Visser, G.; Schobben, F.; de Jong-van den Berg, L.; Egberts, T. Prevalence and patterns of antidepressant drug use during pregnancy. Eur. J. Clin. Pharmacol. 2006, 62, 863–870. [Google Scholar] [CrossRef] [PubMed]
- Heikkinen, T.; Ekblad, U.; Palo, P.; Laine, K. Pharmacokinetics of fluoxetine and norfluoxetine in pregnancy and lactation. Clin. Pharmacol. Ther. 2003, 73, 330–337. [Google Scholar] [CrossRef] [PubMed]
- Gillette, R.; Reilly, M.P.; Topper, V.Y. Anxiety-like behaviors in adulthood are altered in male but not female rats exposed to low dosages of polychlorinated biphenyls in utero. Horm. Behav. 2017, 87, 8–15. [Google Scholar] [CrossRef]
- Kwong, W.Y.; Wild, A.E.; Roberts, P.; Willis, A.C.; Fleming, T.P. Maternal undernutrition during the preimplantation period of rat development causes blastocyst abnormalities and programming of postnatal hypertension. Development 2000, 127, 4195–4202. [Google Scholar] [CrossRef]
- Bairy, K.L.; Madhyastha, S.; Ashok, K.P.; Bairy, I.; Malini, S. Developmental and behavioral consequences of prenatal fluoxetine. Pharmacology 2007, 79, 1–11. [Google Scholar] [CrossRef]
- Ansorge, M.S.; Zhou, M.; Lira, A.; Hen, R.; Gingrich, J.A. Early-life blockade of the 5-HT transporter alters emotional behavior in adult mice. Science 2004, 306, 879–881. [Google Scholar] [CrossRef]
- McAdam, T.D.; Brien, J.F.; Reynolds, J.N.; Dringenberg, H.C. Altered water-maze search behavior in adult guinea pigs following chronic prenatal ethanol exposure: Lack of mitigation by postnatal fluoxetine treatment. Behav. Brain Res. 2008, 191, 202–209. [Google Scholar] [CrossRef]
- Tuomisto, J.M.P. Neurotransmitter regulation of anterior pituitary hormones. Pharmacol. Rev. 1985, 37, 249–332. [Google Scholar]
- Leibowitz, S.F.; Alexander, J.T. Hypothalamic serotonin controls eating behavior, meal size, and body weight. Biol. Psychiatry 1998, 44, 851–864. [Google Scholar] [CrossRef]
- Adlard, B.P.F.; Smart, J.L. Adrenocortical function in rats subjected to the plenitude of high energy TER, similar to normal rats, and nutritional deprivation in early life. J. Endocrinol. 1972, 54, 99–105. [Google Scholar] [CrossRef]
- Noorlander, C.W.; Ververs, F.F.; Nikkels, P.G.; van Echteld, C.J.; Visser, G.H.; Smidt, M.P. Modulation of serotonin transporter function during fetal development causes dilated cardiomyopathy and lifelong behavioral abnormalities. PLoS ONE 2008, 3, e2782, Correction in PLoS 2009, 4, 9. [Google Scholar] [CrossRef] [PubMed]
- Bourke, C.H.; Capello, C.F.; Rogers, S.M. Prenatal Stress Model of Maternal Depression and Its Treatment. Psychopharmacology 2013, 228, 231–241, Erratum in Psychopharmacology 2014, 228 (2), 231–241. [Google Scholar] [CrossRef] [PubMed]
- Glover, M.E.; Pugh, P.C.; Jackson, N.L.; Cohen, J.L.; Fant, A.D.; Akil, H.; Clinton, S.M. Early-life exposure to the SSRI paroxetine exacerbates depression-like behavior in anxiety/depression-prone rats. Neuroscience 2015, 284, 775–797. [Google Scholar] [CrossRef] [PubMed]
- McAllister, B.B.; Kiryanova, V.; Dyck, R.H. Behavioral outcomes of perinatal maternal fluoxetine treatment. Neuroscience 2012, 226, 356–366. [Google Scholar] [CrossRef]
- Kiryanova, V.; Meunier, S.J.; Vecchiarelli, H.A.; Hill, M.N.; Dyck, R.H. Effects of maternal stress and perinatal fluoxetine exposure on behavioral outcomes of adult male offspring. Neuroscience 2016, 320, 281–296. [Google Scholar] [CrossRef]
- Kiryanova, V.; McAllister, B.B.; Dyck, R.H. Long-Term Outcomes of Developmental Exposure to Fluoxetine: A Review of the Animal Literature. Dev. Neurosci. 2013, 35, 437–447. [Google Scholar] [CrossRef]
- Arlington, V. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Figueroa, R. Use of antidepressants during pregnancy and risk of attention-deficit/ hyperactivity disorder in the offspring. J. Dev. Behav. Pediatr. 2010, 31, 641–648. [Google Scholar] [CrossRef]
- Laugesen, K.; Olsen, M.S.; Andersen, A.B.T.; Frøslev, T.; Sørensen, H.T. In utero exposure to antidepressant drugs and risk of attention deficit hyperactivity disorder: A nationwide Danish cohort study. BMJ Open 2013, 3, e003507. [Google Scholar] [CrossRef]
- Clements, C.C.; Castro, V.M.; Blumenthal, S.R.; Rosenfield, H.R.; Murphy, S.N.; Fava, M.; Erb, J.L.; Churchill, S.E.; Kaimal, A.J.; Doyle, A.E.; et al. Prenatal antidepressant exposure is associated with risk for attention-deficit hyperactivity disorder but not autism spectrum disorder in a large health system. Mol. Psychiatry 2015, 20, 727–734. [Google Scholar] [CrossRef]
- Gemmel, M.; Rayen, I.; Lotus, T.; van Donkelaar, E.; Steinbusch, H.W.; De Lacalle, S.; Kokras, N.; Dalla, C.; Pawluski, J.L. Developmental fluoxetine and prenatal stress effects on serotonin, dopamine, and synaptophysin density in the PFC and hippocampus of offspring at weaning. Dev. Psychobiol. 2016, 58, 315–327. [Google Scholar] [CrossRef]
- Umemori, J.; Winkel, F.; Castrén, E.; Karpova, N.N. Distinct effects of perinatal exposure to fluoxetine or methylmercury on parvalbumin and perineuronal nets, the markers of critical periods in brain development. Int. J. Dev. Neurosci. 2015, 44, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Laine, K.; Heikkinen, T.; Ekblad, U.; Kero, P. Effects of exposure to selective serotonin reuptake inhibitors during pregnancy on serotonergic symptoms in newborns and cord blood monoamine and prolactin concentrations. Arch Gen. Psychiatry 2003, 60, 720–726. [Google Scholar] [CrossRef] [PubMed]
- Weaver, K.J.; Paul, I.A.; Lin, R.C.; Simpson, K.L. Neonatal Exposure to Citalopram Selectively Alters the Expression of the Serotonin Transporter in the Hippocampus: Dose-Dependent Effects. Anat. Rec. 2010, 293, 1920–1932. [Google Scholar] [CrossRef]
- Karpova, N.N.; Lindholm, J.; Pruunsild, P.; Timmusk, T.; Castrén, E. Long-lasting behavioural and molecular alterations induced by early postnatal fluoxetine exposure are restored by chronic fluoxetine treatment in adult mice. Eur. Neuropsychopharmacol. 2009, 19, 97–108. [Google Scholar] [CrossRef]
- Rayen, I.; van den Hove, D.L.; Prickaerts, J.; Steinbusch, H.W.; Pawluski, J.L. Fluoxetine during development reverses the effects of prenatal stress on depressive-like behavior and hippocampal neurogenesis in adolescence. PLoS ONE 2011, 6, e24003. [Google Scholar] [CrossRef]
- Scabora, J.E.; de Lima, M.C.; Lopes, A.; de Lima, I.P.; Mesquita, F.F.; Torres, D.B.; Boer, P.A.; Gontijo, J.A. Impact of taurine supplementation on blood pressure in gestational protein-restricted offspring: Effect on the medial solitary tract nucleus cell numbers, angiotensin receptors, and renal sodium handling. J. Renin-Angiotensin-Aldosterone Syst. 2015, 16, 47–58. [Google Scholar] [CrossRef]
- Södersten, P.; Eneroth, P. Effects of exposure to pups on maternal behaviour, sexual behaviour, and serum prolactin concentrations in male rats. J. Endocrinol. 1984, 102, 115–119. [Google Scholar] [CrossRef] [PubMed]
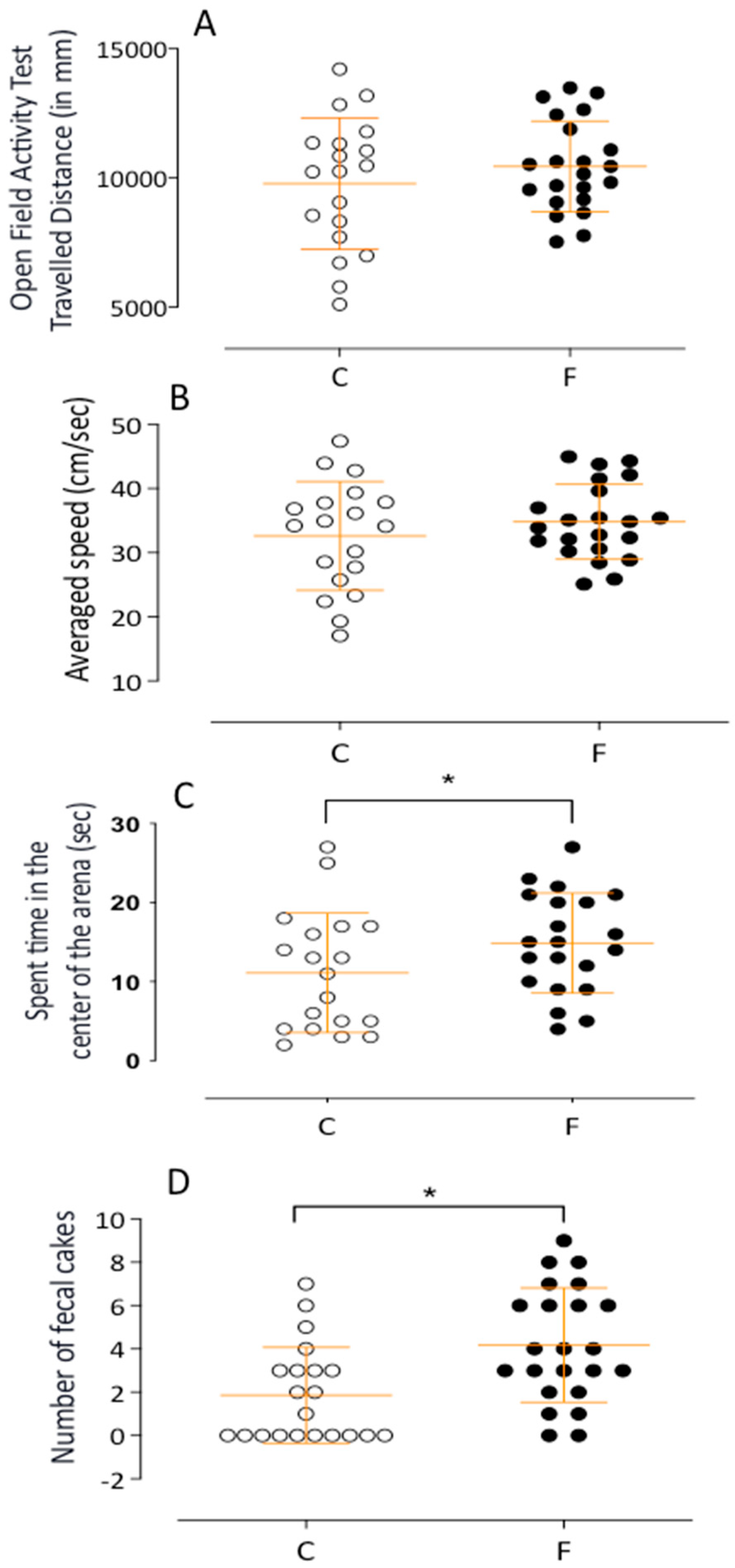
| Parameters | Control (C, n = 20) | Fluoxetine (F, n = 22) | p Values |
|---|---|---|---|
| Orthostatic position (frequency) | 42.65 ± 3.215 | 44.55 ± 1.969 | 0.3053 |
| Activity time (min) | 1.234 ± 0.09 | 1.455 ± 0.056 | 0.0198 * |
| Travelled distance (mm) | 9478 ± 631.2 | 10,450 ± 374 | 0.0926 |
| Speed (cm/sec) | 36.6 ± 8.48 | 34.82 ± 5.84 | 0.82 |
| Time in arena center (sec) | 11.11 ± 1.73 | 14.86 ± 1.38 | 0.0479 * |
| Score | Observed Parameters |
|---|---|
| 0 | Absence of nest |
| 1 | Presence of nest |
| 2 | All the pups in the nest |
| 3 | All the pups in the nest, with their mother |
| 4 | All the pups in the nest, being suckled with arched backs |
| 4.5 | All the pups in the nest, being nursed in a passive position, on their side or supine |
| 5 | All the pups in the nest, being suckled and licked |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopes, M.G.; Grigoletti-Lima, G.B.; Boer, P.A.; Gontijo, J.A.R. Effect of the Gestational Fluoxetine Administration on Behavioral Tests and Hippocampal Structure in Male Offspring of Rats. Int. J. Mol. Sci. 2025, 26, 10758. https://doi.org/10.3390/ijms262110758
Lopes MG, Grigoletti-Lima GB, Boer PA, Gontijo JAR. Effect of the Gestational Fluoxetine Administration on Behavioral Tests and Hippocampal Structure in Male Offspring of Rats. International Journal of Molecular Sciences. 2025; 26(21):10758. https://doi.org/10.3390/ijms262110758
Chicago/Turabian StyleLopes, Marcelo Gustavo, Gabriel Boer Grigoletti-Lima, Patrícia Aline Boer, and José Antonio Rocha Gontijo. 2025. "Effect of the Gestational Fluoxetine Administration on Behavioral Tests and Hippocampal Structure in Male Offspring of Rats" International Journal of Molecular Sciences 26, no. 21: 10758. https://doi.org/10.3390/ijms262110758
APA StyleLopes, M. G., Grigoletti-Lima, G. B., Boer, P. A., & Gontijo, J. A. R. (2025). Effect of the Gestational Fluoxetine Administration on Behavioral Tests and Hippocampal Structure in Male Offspring of Rats. International Journal of Molecular Sciences, 26(21), 10758. https://doi.org/10.3390/ijms262110758







