Divergent Effects of Calcium Channel Modulators on H-Reflex Excitability in Fatigued Rat Muscle
Abstract
1. Introduction
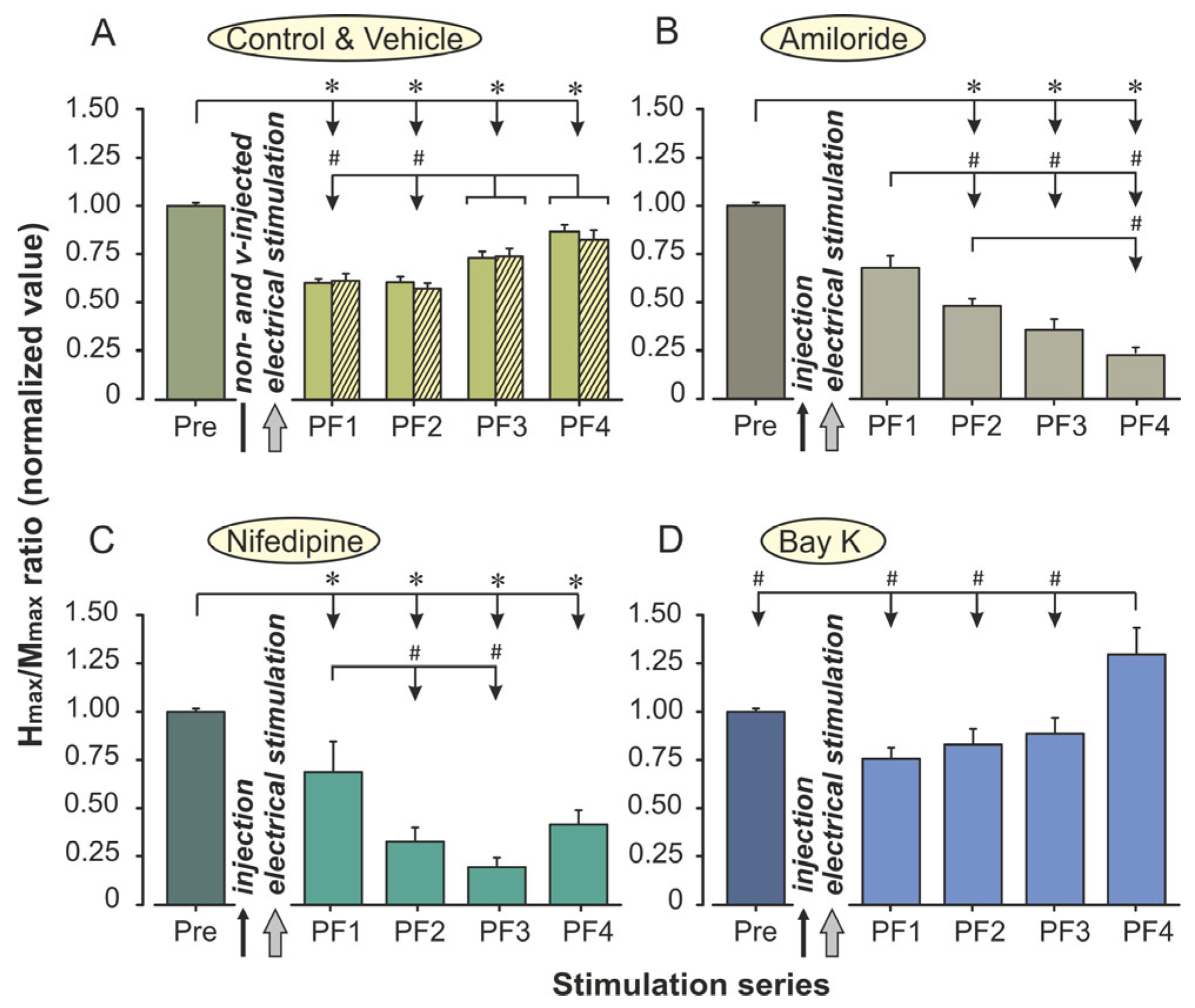
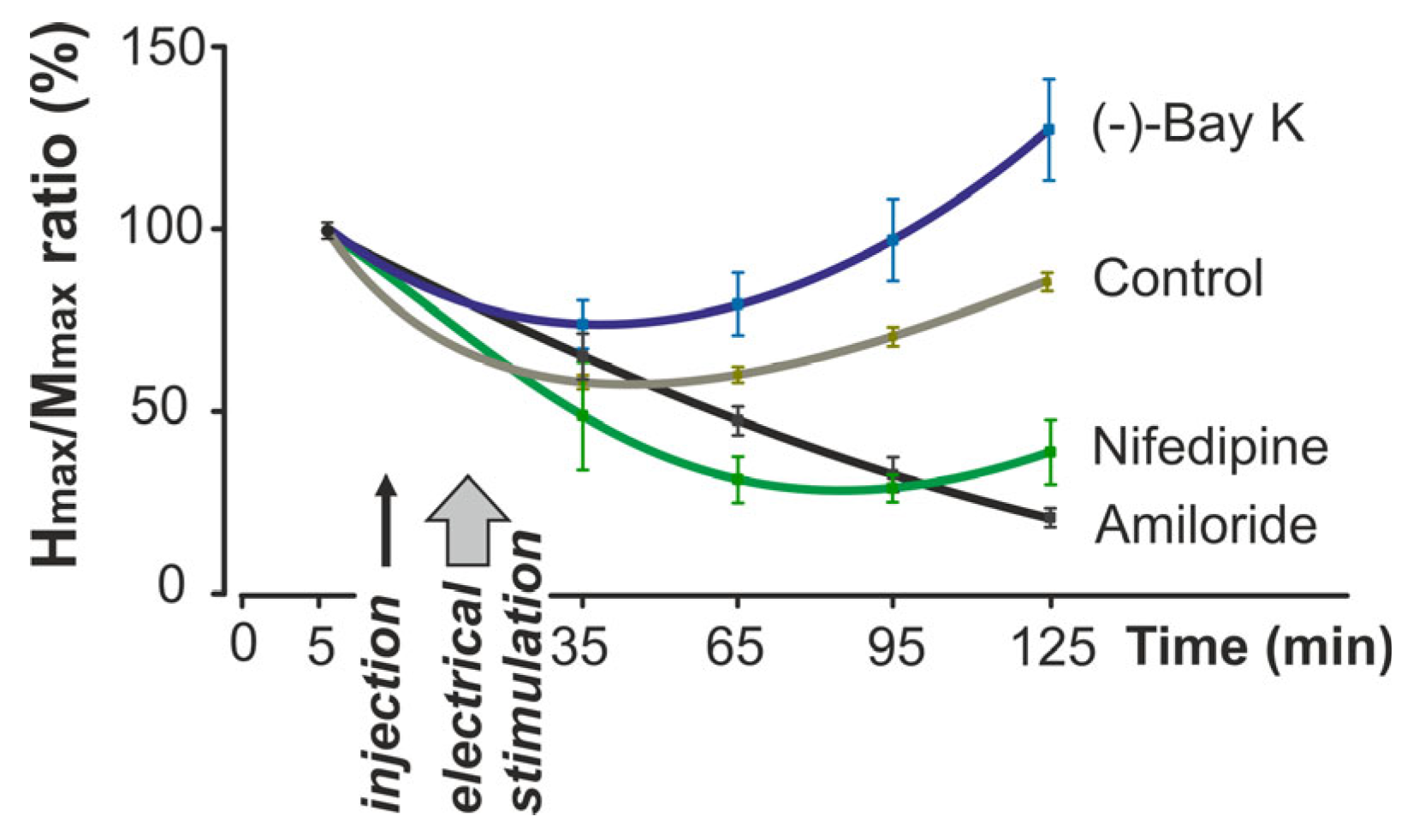
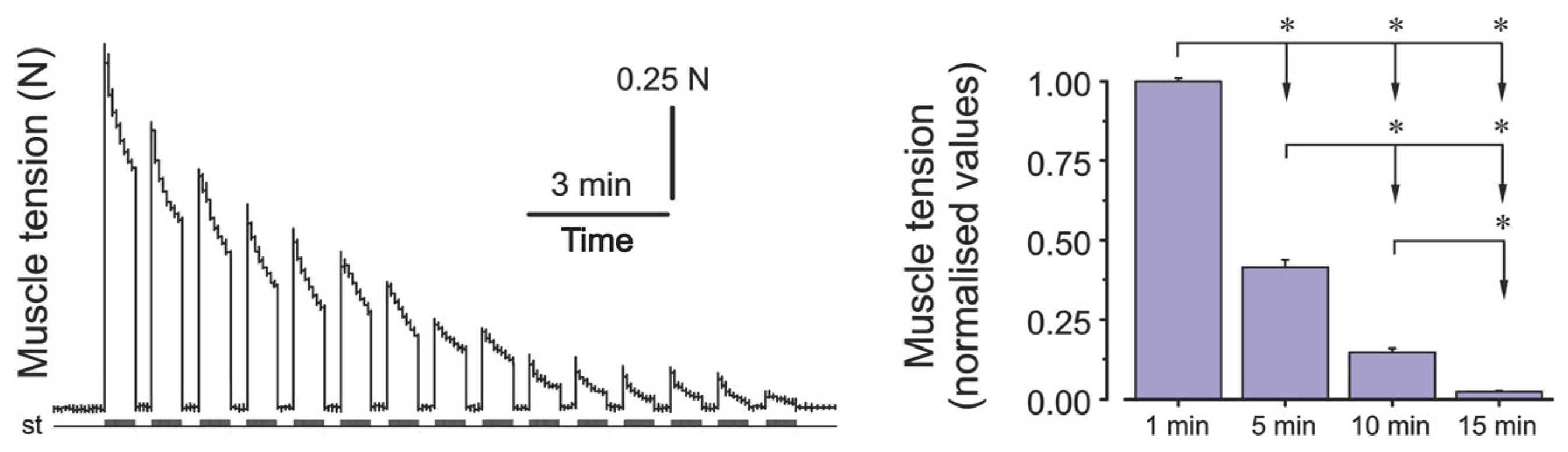
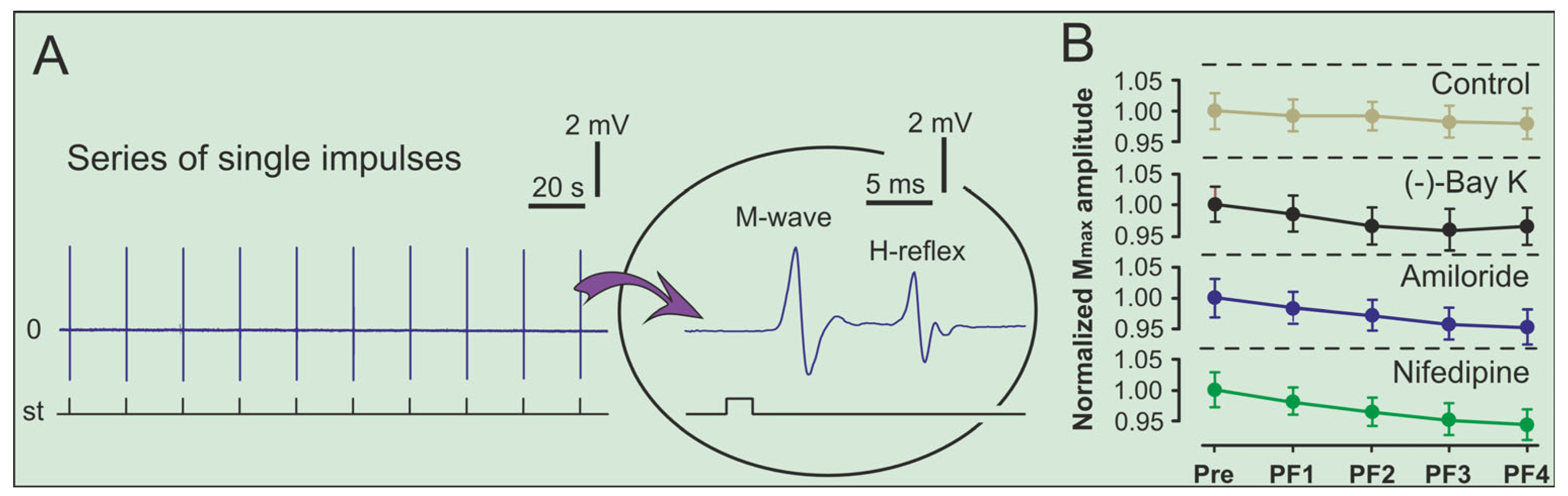
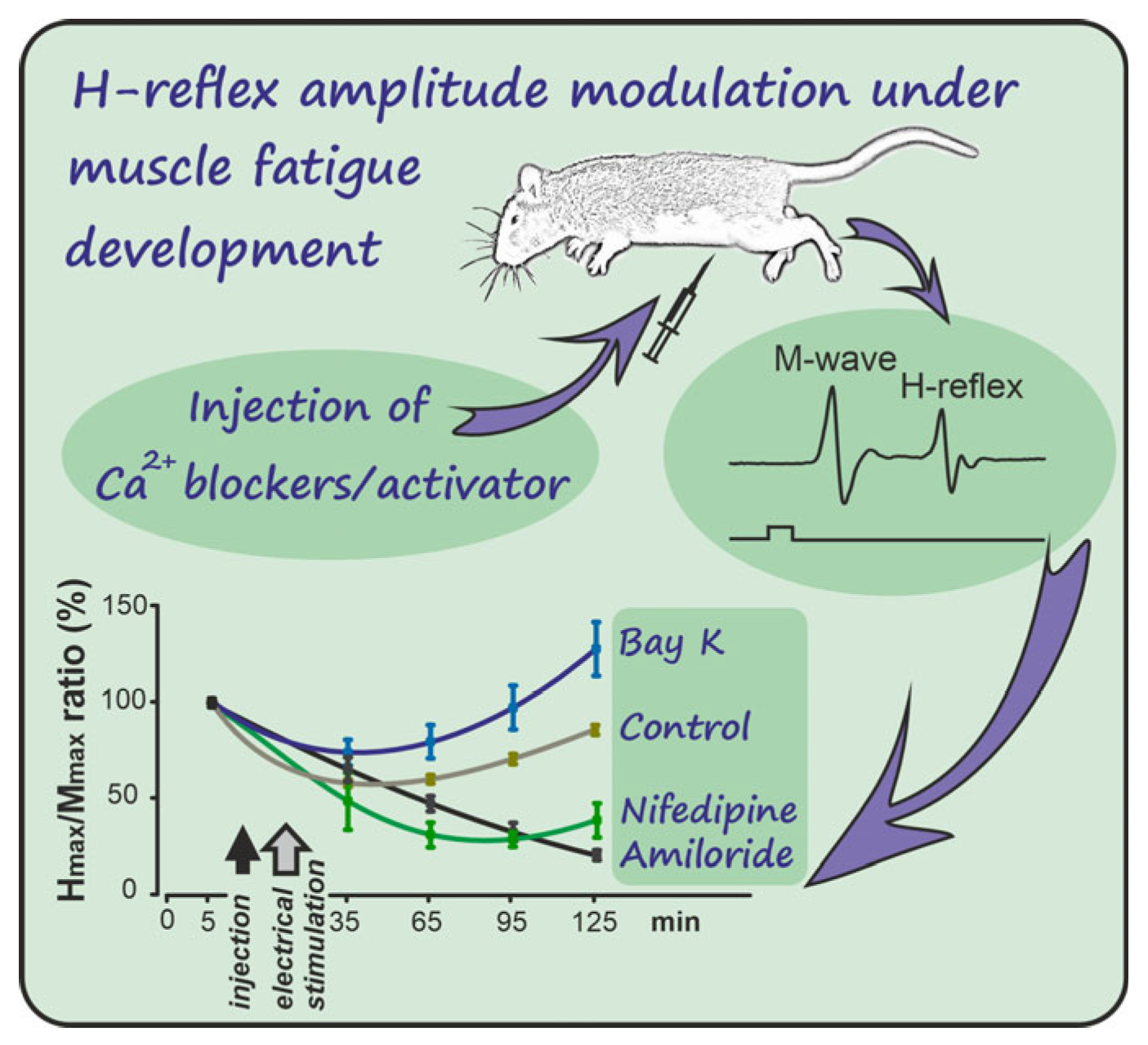
2. Results
3. Discussion
4. Materials and Methods
4.1. Procedure and Experimental Animals
4.2. Surgical Preparation and Electrical Stimulation Protocols
4.3. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| BBB | Blood–brain barrier |
| DAC-ADC | Digital-to-Analog Converter–Analog-to-Digital Converter |
| DHPR | Dihydropyridine receptor Ca2+ channel |
| Pre | Prefatigue recordings of H-reflex amplitudes |
| PF1–PF4 | Postfatigue recordings 1–4 of H-reflex amplitudes in different periods of time |
| RyR | Ryanodine receptor |
| SEM | Standard error of mean |
| SR | Sarcoplasmic reticulum |
References
- Gandevia, S.C. Spinal and supraspinal factors in human muscle fatigue. Physiol. Rev. 2001, 81, 1725–1789. [Google Scholar] [CrossRef] [PubMed]
- Enoka, R.M.; Duchateau, J. Muscle fatigue: What, why and how it influences muscle function. J. Physiol. 2008, 586, 11–23. [Google Scholar] [CrossRef]
- Schiaffino, S.; Reggiani, C. Fiber types in mammalian skeletal muscles. Physiol. Rev. 2011, 91, 1447–1531. [Google Scholar] [CrossRef]
- Allen, D.G.; Lamb, G.D.; Westerblad, H. Skeletal muscle fatigue: Cellular mechanisms. Physiol. Rev. 2008, 88, 287–332. [Google Scholar] [CrossRef]
- Place, N.; Yamada, T.; Bruton, J.D.; Westerblad, H. Muscle fatigue: From observations in humans to underlying mechanisms studied in intact single muscle fibres. Eur. J. Appl. Physiol. 2010, 110, 1–15. [Google Scholar] [CrossRef]
- Taylor, J.L.; Todd, G.; Gandevia, S.C. Evidence for a supraspinal contribution to human muscle fatigue. Clin. Exp. Pharmacol. Physiol. 2006, 33, 400–405. [Google Scholar] [CrossRef]
- Hostrup, M.; Bangsbo, J. Limitations in intense exercise performance of athletes—Effect of speed endurance training on ion handling and fatigue development. J. Physiol. 2017, 595, 2897–2913. [Google Scholar] [CrossRef]
- Shishmarev, D. Excitation-contraction coupling in skeletal muscle: Recent progress and unanswered questions. Biophys. Rev. 2020, 12, 143–153. [Google Scholar] [CrossRef]
- Proske, U.; Gandevia, S.C. The proprioceptive senses: Their roles in signaling body shape, body position and movement, and muscle force. Physiol. Rev. 2012, 92, 1651–1697. [Google Scholar] [CrossRef]
- Taylor, J.L.; Gandevia, S.C. A comparison of central aspects of fatigue in submaximal and maximal voluntary contractions. J. Appl. Physiol. 2008, 104, 542–550. [Google Scholar] [CrossRef]
- Birznieks, I.; Boonstra, T.W.; Macefield, V.G. Modulation of human muscle spindle discharge by arterial pulsations-functional effects and consequences. PLoS ONE 2012, 7, e35091. [Google Scholar] [CrossRef] [PubMed]
- Amann, M.; Sidhu, S.K.; Weavil, J.C.; Mangum, T.S.; Venturelli, M. Autonomic responses to exercise: Group III/IV muscle afferents and fatigue. Auton. Neurosci. 2015, 188, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Therkildsen, E.R.; Nielsen, J.B.; Lorentzen, J. The calcium channel blocker nimodipine inhibits spinal reflex pathways in humans. J. Neurophysiol. 2025, 133, 428–439. [Google Scholar] [CrossRef]
- Triggle, D.J. Calcium channel antagonists: Clinical uses—Past, present and future. Biochem. Pharmacol. 2007, 74, 1–9. [Google Scholar] [CrossRef]
- Kleyman, T.R.; Cragoe Jr, E.J. Amiloride and its analogs as tools in the study of ion transport. J. Membr. Biol. 1988, 105, 1–21. [Google Scholar] [CrossRef]
- Schramm, M.; Thomas, G.; Towart, R.; Franckowiak, G. Novel dihydropyridines with positive inotropic action through activation of Ca2+ channels. Nature 1983, 303, 535–537. [Google Scholar] [CrossRef]
- Zehr, P.E. Considerations for use of the Hoffmann reflex in exercise studies. Eur. J. Appl. Physiol. 2002, 86, 455–468. [Google Scholar] [CrossRef]
- Maznychenko, A.V.; Yang, X.; Dornowski, M.; Gorkovenko, A.V.; Kolosova, O.V.; Zasada, M.; Kostyukov, A.I.; Tomiak, T.; Sokolowska, I.V. Running-induced changes in H-reflex amplitudes in non-trained men. Acta Kinesiol. 2021, 15, 19–22. [Google Scholar] [CrossRef]
- Gajos, A.; Kujawski, S.; Gajos, M.; Chatys, Ż.; Bogacki, P. Applications of the H-reflex in kinesiology: A systematic review. Biomed. Hum. Kinet. 2014, 6, 99–108. [Google Scholar] [CrossRef]
- Palmieri, R.M.; Ingersoll, C.D.; Hoffman, M.A. The Hoffmann reflex: Methodologic considerations and applications for use in sports medicine and athletic training research. J. Athl. Train. 2004, 39, 268–277. [Google Scholar] [PubMed]
- Holtermann, A.; Roeleveld, K.; Engstrøm, M.; Sand, T. Enhanced H-reflex with resistance training is related to increased rate of force development. Eur. J. Appl. Physiol. 2007, 101, 301–312. [Google Scholar] [CrossRef]
- Tang, C.M.; Presser, F.; Morad, M. Amiloride selectively blocks the low threshold (T) calcium channel. Science 1988, 240, 213–215. [Google Scholar] [CrossRef]
- Bannister, R.A.; Pessah, I.N.; Beam, K.G. The skeletal L-type Ca2+ current is a major contributor to excitation-coupled Ca2+ entry. J. Gen. Physiol. 2009, 133, 79–91. [Google Scholar] [CrossRef] [PubMed]
- Godfraind, T.; Miller, R.; Wibo, M. Calcium antagonists and calcium entry blockade. Pharmacol. Rev. 1986, 38, 321–416. [Google Scholar] [CrossRef] [PubMed]
- Bourson, A.; Moser, P.C.; Gower, A.J.; Mir, A.K. Central and peripheral effects of the dihydropyridine calcium channel activator BAY K 8644 in the rat. Eur. J. Pharmacol. 1989, 160, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Hunter, S.K.; Duchateau, J.; Enoka, R.M. Muscle fatigue and the mechanisms of task failure. Exerc. Sport. Sci. Rev. 2004, 32, 44–49. [Google Scholar] [CrossRef]
- Calderón, J.C.; Bolaños, P.; Caputo, C. The excitation-contraction coupling mechanism in skeletal muscle. Biophys. Rev. 2014, 6, 133–160. [Google Scholar] [CrossRef]
- Garland, S.J.; McComas, A.J. Reflex inhibition of human soleus muscle during fatigue. J. Physiol. 1990, 429, 17–27. [Google Scholar] [CrossRef]
- Schillings, M.L.; Hoefsloot, W.; Stegeman, D.F.; Zwarts, M.J. Relative contributions of central and peripheral factors to fatigue during a maximal sustained effort. Eur. J. Appl. Physiol. 2003, 90, 562–568. [Google Scholar] [CrossRef]
- Gruet, M.; Temesi, J.; Rupp, T.; Levy, P.; Verges, S.; Millet, G.Y. Dynamics of corticospinal changes during and after high-intensity quadriceps exercise. Exp. Physiol. 2014, 99, 1053–1064. [Google Scholar] [CrossRef]
- Zavecz, J.H.; Anderson, W.M.; Adams, B. Effect of amiloride on diaphragmatic contractility: Evidence of a role for Na(+)-Ca2+ exchange. J. Appl. Physiol. 1991, 70, 1309–1314. [Google Scholar] [CrossRef] [PubMed]
- Durham-Lee, J.C.; Mokkapati, V.U.; Johnson, K.M.; Nesic, O. Amiloride improves locomotor recovery after spinal cord injury. J. Neurotrauma 2011, 28, 1319–1326. [Google Scholar] [CrossRef] [PubMed]
- Tanabe, T.; Beam, K.G.; Powell, J.A.; Numa, S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature 1988, 336, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Melzer, W.; Herrmann-Frank, A.; Lüttgau, H.C. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim. Biophys. Acta 1995, 1241, 59–116. [Google Scholar] [CrossRef]
- Dayal, A.; Schrötter, K.; Pan, Y.; Föhr, K.; Melzer, W.; Grabner, M. The Ca2+ influx through the mammalian skeletal muscle dihydropyridine receptor is irrelevant for muscle performance. Nat. Commun. 2017, 8, 475. [Google Scholar] [CrossRef]
- Janicki, P.K.; Siembab, D.; Paulo, E.A.; Krzaścik, P. Single-dose kinetics of nifedipine in rat plasma and brain. Pharmacology 1988, 36, 183–187. [Google Scholar] [CrossRef]
- Manita, S.; Ross, W.N. Synaptic activation and membrane potential changes modulate the frequency of spontaneous elementary Ca2+ release events in the dendrites of pyramidal neurons. J. Neurosci. 2009, 29, 7833–7845. [Google Scholar] [CrossRef]
- Chick, T.W.; Halperin, A.K.; Jackson, J.E.; Van As, A. The effect of nifedipine on cardiopulmonary responses during exercise in normal subjects. Chest 1986, 89, 641–646. [Google Scholar] [CrossRef]
- Oz, M.; Frank, G.B.; Dunn, S.M.J. Voltage-dependent calcium fluxes in skeletal muscle transverse tubule membranes in the range of late after potentials. Can. J. Physiol. Pharmacol. 1993, 71, 518–521. [Google Scholar] [CrossRef]
- Frank, G.B. Dihydropyridine calcium channel antagonists block and agonists potentiate high potassium contractures but not twitches in frog skeletal muscle. Jpn. J. Physiol. 1990, 40, 205–224. [Google Scholar] [CrossRef]
- Frank, G.B.; Oz, M. The functional role of T-tubular Ca2+ channels in skeletal muscle contractions. Adv. Exp. Med. Biol. 1992, 311, 123–136. [Google Scholar] [CrossRef]
- Garcia, J.; Avila-Sakar, A.J.; Stefani, E. Repetitive stimulation increases the activation rate of skeletal muscle Ca2+ current. Pfluegers Arch. 1990, 486, 210–212. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.B. Effects of low calcium and calcium antagonists on skeletal muscle staircase and fatigue. Muscle Nerve 1990, 13, 1118–1124. [Google Scholar] [CrossRef] [PubMed]
- Oz, M.; Frank, G.B. Effect of the calcium channel agonist Bay K8644 on mechanical and electrical responses of frog skeletal muscle. Can. J. Physiol. Pharmacol. 1994, 72, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.H.; Ward, C.W. Effects of the calcium channel agonist, BAY K 8644, on the mechanical output of skeletal muscle fibers. Gen. Pharmacol. 1991, 22, 735–740. [Google Scholar] [CrossRef]
- Haida, M.; Shinohara, Y.; Yamamoto, M.; Nagayama, T.; Kurita, D. The Effect of BAY K-8644 on Cytotoxic Edema Induced by Total Ischemia of Rat Brain. In Brain Edema IX; Ito, U., Baethmann, A., Hossmann, K.-A., Kuroiwa, T., Marmarou, A., Reulen, H.-J., Takakura, K., Eds.; Acta Neurochirurgica; Springer: Vienna, Austria, 1994; Volume 60, pp. 293–295. [Google Scholar] [CrossRef]
- Gerin, C.; Becquet, D.; Privat, A. Direct evidence for the link between monoaminergic descending pathways and motor activity. I. A study with microdialysis probes implanted in the ventral funiculus of the spinal cord. Brain Res. 1995, 704, 191–201. [Google Scholar] [CrossRef]
- Jordan, L.M.; Liu, J.; Hedlund, P.B.; Akay, T. Descending command systems for the initiation of locomotion in mammals. Brain Res. Rev. 2008, 57, 183–191. [Google Scholar] [CrossRef]
- Maznychenko, A.V. Changes in the gene c-fos expression in the rat spinal cord after suppression of activity of the cerebral monoaminergic systems. Neurophysiology 2014, 46, 461–470. [Google Scholar] [CrossRef]
- Miller, R.L.; Denny, G.O.; Knuepfer, M.M.; Kleyman, T.R.; Jackson, E.K.; Salkoff, L.B.; Loewy, A.D. Blockade of ENaCs by amiloride induces c-Fos activation of the area postrema. Brain Res. 2015, 1601, 40–51. [Google Scholar] [CrossRef]
- Wagatsuma, A.; Fujimoto, K.; Yamada, S. Effect of treatment with nifedipine, an L-type calcium channel blocker, on muscular atrophy induced by hindlimb immobilization. Scand. J. Med. Sci. Sports 2002, 12, 26–30. [Google Scholar] [CrossRef]
- de Beun, R.; Schneider, R.; Klein, A.; Lohmann, A.; Schreiber, R.; De Vry, J. The calcium channel agonist BAY k 8644 reduces ethanol intake and preference in alcohol-preferring AA rats. Psychopharmacology 1996, 127, 302–310. [Google Scholar] [CrossRef]
- Kleinbloesem, C.H.; Van Brummelen, P.; Breimer, D.D. Nifedipine: Relationship between pharmacokinetics and pharmacodynamics. Clin. Pharmacokinet. 1987, 12, 12–29. [Google Scholar] [CrossRef]
- Segre, G.; Cerretani, D.; Bruni, G.; Urso, R.; Giorgi, G. Amiloride pharmacokinetics in rat. Eur. J. Drug Metab. Pharmacokinet. 1998, 23, 218–222. [Google Scholar] [CrossRef]
- Maznychenko, A.V.; Pilyavskii, A.I.; Kostyukov, A.I.; Lyskov, E.; Vlasenko, O.V.; Maisky, V.A. Coupling of c-fos expression in the spinal cord and amygdala induced by dorsal neck muscles fatigue. Histochem. Cell Biol. 2007, 128, 85–90. [Google Scholar] [CrossRef]
- Maznychenko, A.V.; Bulgakova, N.V.; Sokolowska, I.V.; Butowska, K.; Borowik, A.; Mankivska, O.P.; Piosik, J.; Tomiak, T.; Gonchar, O.O.; Maisky, V.O.; et al. Fatigue-induced Fos immunoreactivity within the lumbar cord and amygdala decreases after C60 fullerene pretreatment. Sci. Rep. 2020, 10, 9826. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maznychenko, A.; Abramovych, T.I.; Bulgakova, N.V.; Melenko, V.; Levchuk, Y.A.; Shevchuk, T.; Sokolowska, I.; Kostyukov, A.I. Divergent Effects of Calcium Channel Modulators on H-Reflex Excitability in Fatigued Rat Muscle. Int. J. Mol. Sci. 2025, 26, 10749. https://doi.org/10.3390/ijms262110749
Maznychenko A, Abramovych TI, Bulgakova NV, Melenko V, Levchuk YA, Shevchuk T, Sokolowska I, Kostyukov AI. Divergent Effects of Calcium Channel Modulators on H-Reflex Excitability in Fatigued Rat Muscle. International Journal of Molecular Sciences. 2025; 26(21):10749. https://doi.org/10.3390/ijms262110749
Chicago/Turabian StyleMaznychenko, Andriy, Tetiana I. Abramovych, Nataliya V. Bulgakova, Vasyl Melenko, Yuliia A. Levchuk, Tatyana Shevchuk, Inna Sokolowska, and Alexander I. Kostyukov. 2025. "Divergent Effects of Calcium Channel Modulators on H-Reflex Excitability in Fatigued Rat Muscle" International Journal of Molecular Sciences 26, no. 21: 10749. https://doi.org/10.3390/ijms262110749
APA StyleMaznychenko, A., Abramovych, T. I., Bulgakova, N. V., Melenko, V., Levchuk, Y. A., Shevchuk, T., Sokolowska, I., & Kostyukov, A. I. (2025). Divergent Effects of Calcium Channel Modulators on H-Reflex Excitability in Fatigued Rat Muscle. International Journal of Molecular Sciences, 26(21), 10749. https://doi.org/10.3390/ijms262110749





