Elevated Levels of Active GSK3β in the Blood of Patients with Myotonic Dystrophy Type 1 Correlate with Muscle Weakness
Abstract
1. Introduction
2. Results
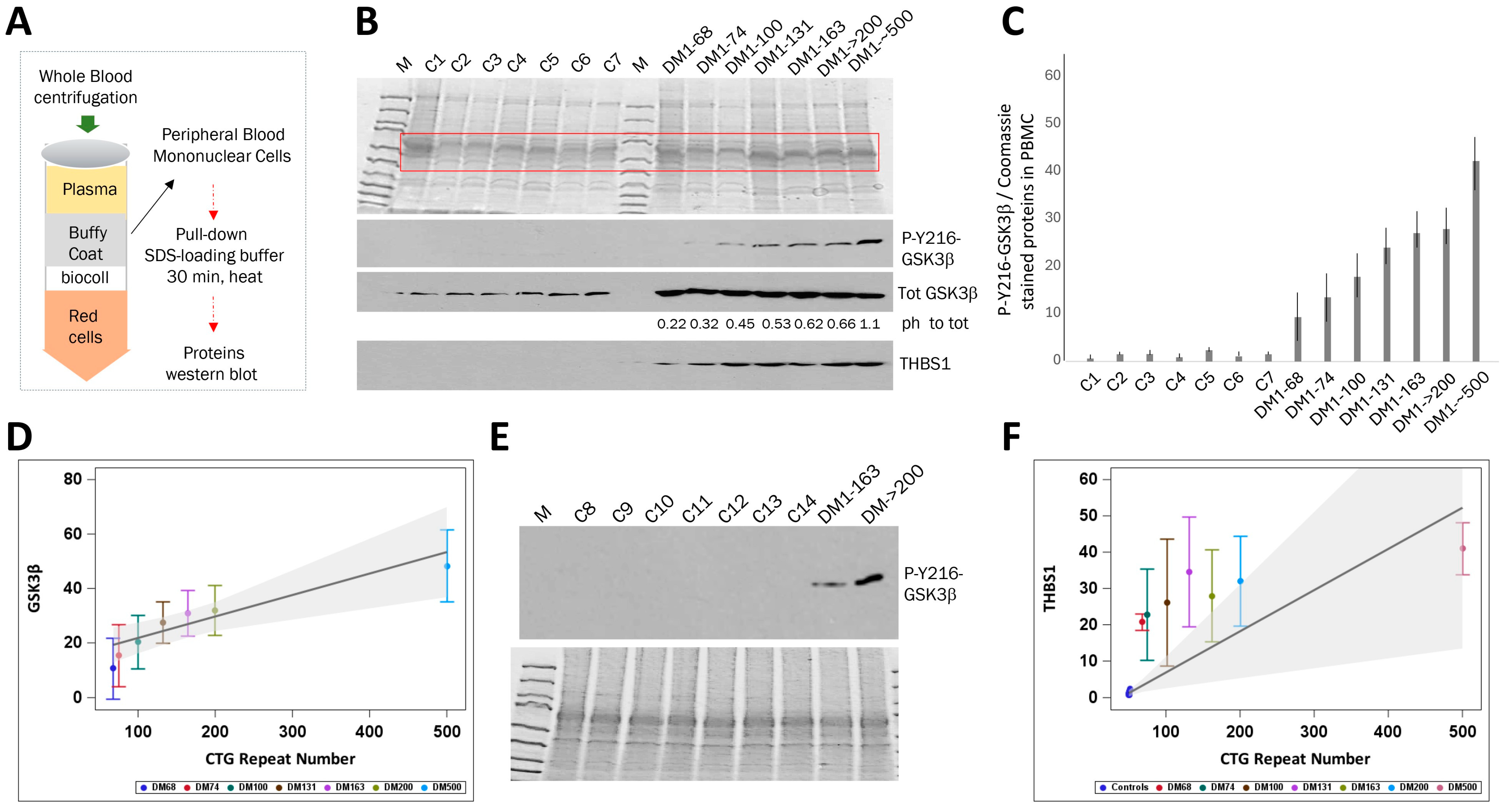
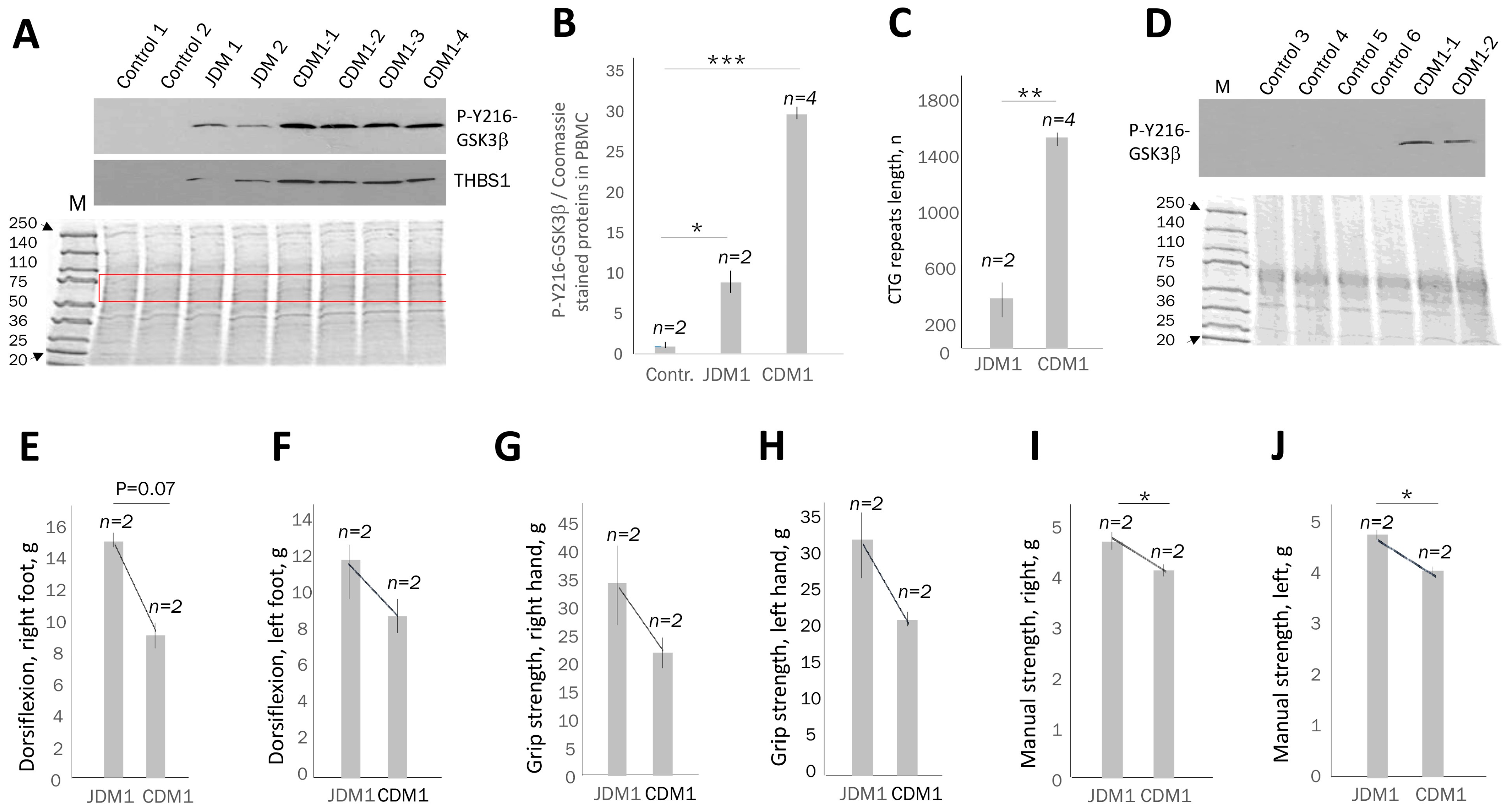
2.1. The Levels of Active GSK3β Are Increased in the Blood of Patients with Adult-Onset DM1 and Correlate with CTG Repeat Number
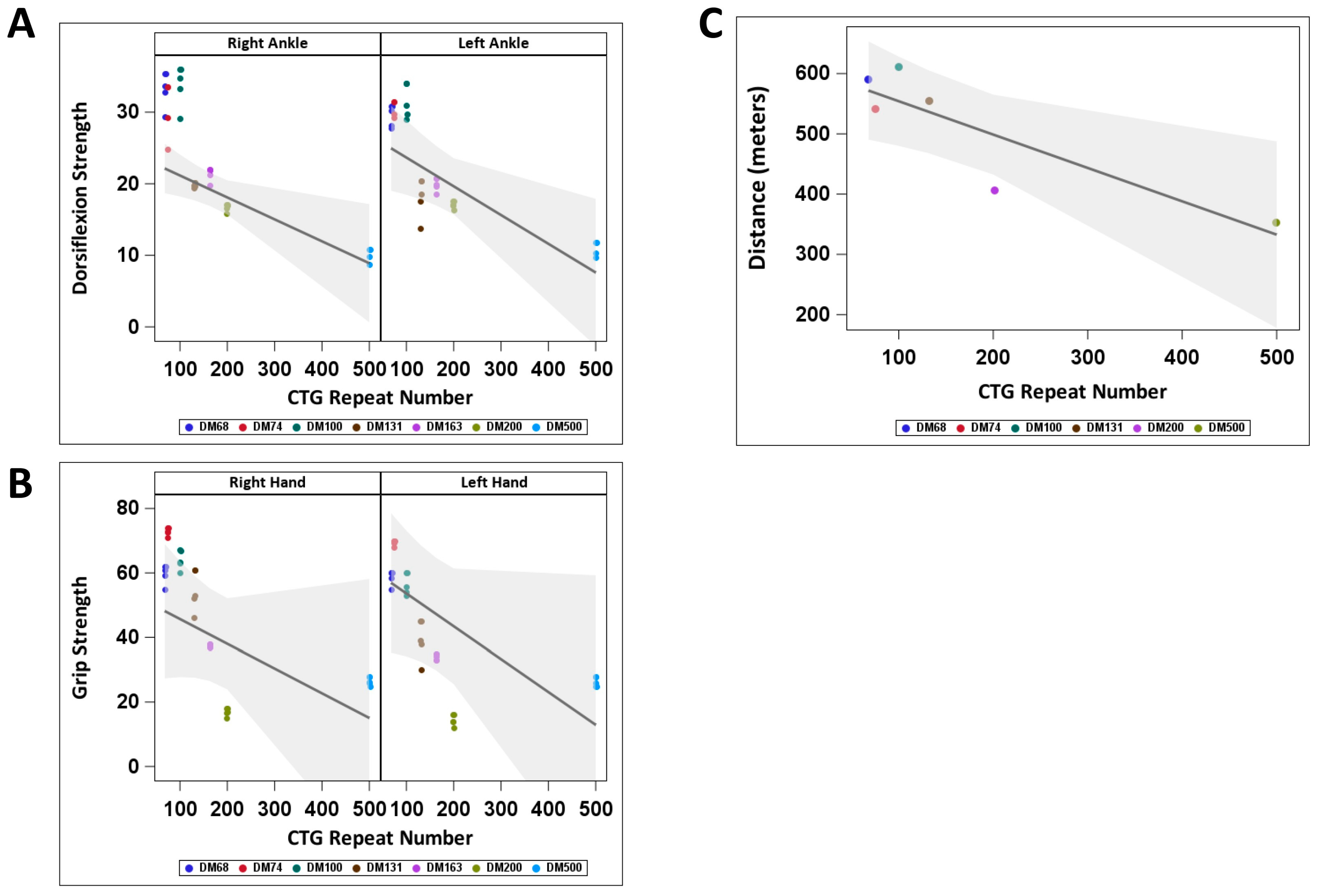
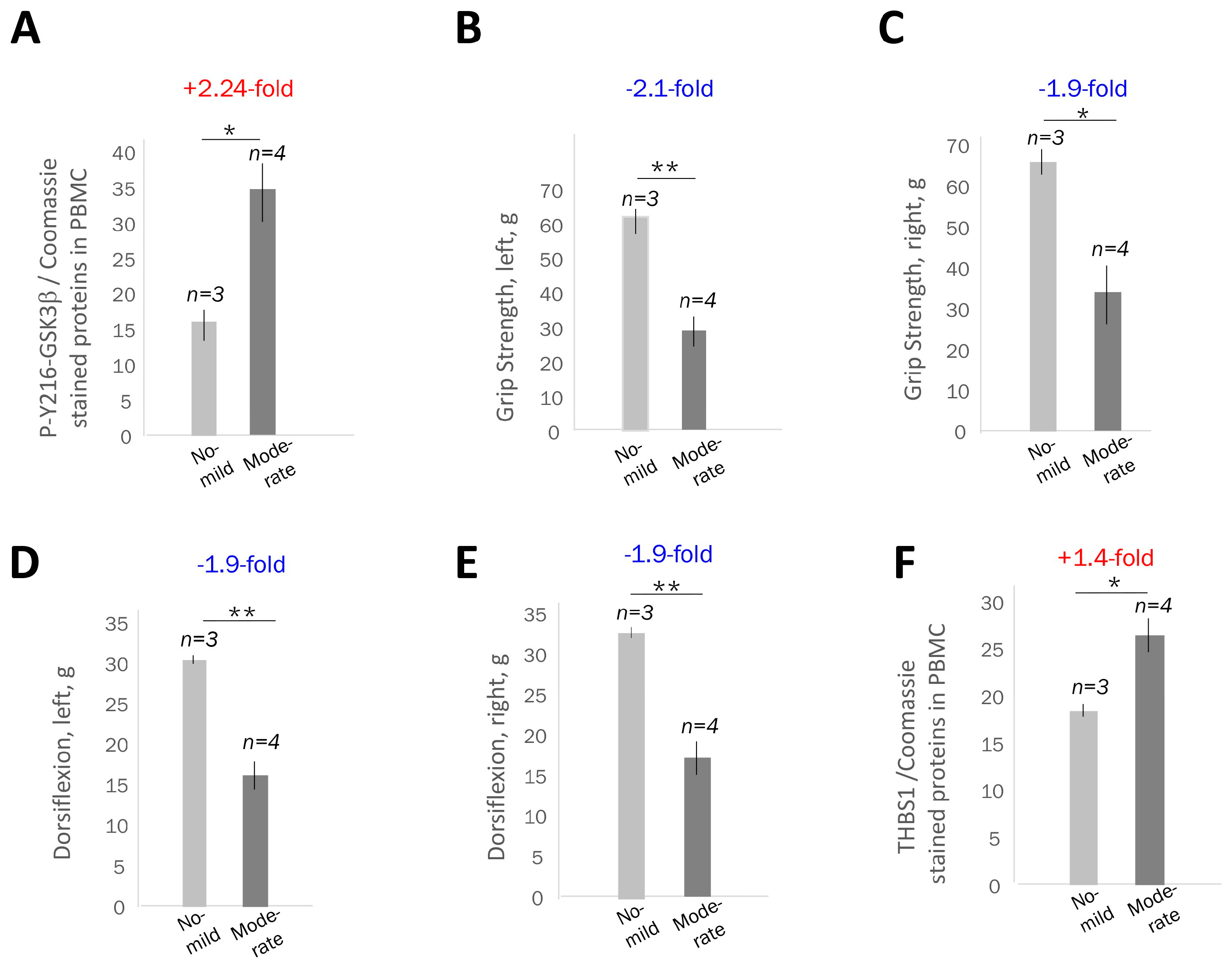
2.2. The Increase in Active GSK3β in PBMCs from Adult-Onset DM1 Patients Correlates with Muscle Weakness
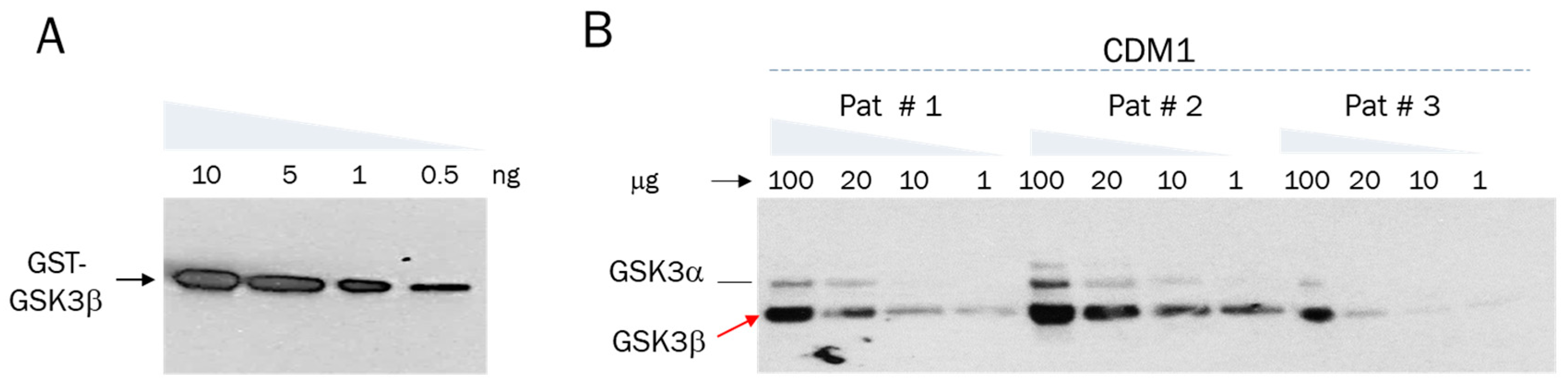
2.3. Active GSK3β Is Increased in PBMCs of Patients with Juvenile and Congenital DM1 Relatively Controls, Correlating with Muscle Weakness
2.4. PBMC Samples from Patients with DM1 Might Be a Source for Evaluation of Proteins Connected to the GSK3β-CUGBP1 Pathway in DM1
3. Discussion
4. Materials and Methods
4.1. Human Subjects
4.2. Muscle Performance Assessments
4.3. Skin Biopsy and Generation of Fibroblasts
4.4. Blood Collection and Preparation of Protein Extracts from PBMCs
4.5. Myoblast and Fibroblast Cell Culture
4.6. Western Blot Analysis of Proteins
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 6MWT | Six-minute walk test |
| CNS | Central Nervous System |
| CDM1 | Congenital Myotonic Dystrophy 1 |
| CUGBP1 | CUG triplet repeat binding protein or CUGBP1 Elav-like family member 1), implicated in DM1 |
| DM1 | Myotonic Dystrophy type 1 |
| DMEM | Dulbecco’s Modified Eagle Medium |
| DMPK | Myotonin Protein Kinase |
| FBS | Fetal Bovine Serum |
| GSK3β | Glycogen Synthase Kinase 3β |
| hnRNP-A3 | Heterogeneous nuclear ribonucleoprotein A3 |
| JDM1 | Juvenile Myotonic Dystrophy 1 |
| MBNL1 | Muscleblind like splicing regulator 1, binding to CUG repeats, implicated in DM1 |
| PBMCs | Peripheral blood mononuclear cells |
| TG | Tideglusib |
| THBS1 | Thrombospondin 1 |
| TGFβ | Transforming Growth Factor β |
Appendix A
References
- Harper, P.S. Myotonic Dystrophy; WB Saunders: London, UK, 2001. [Google Scholar]
- Fu, Y.H.; Pizzuti, A.; Fenwick, R.G., Jr.; King, J.; Rajnarayan, S.; Dunne, P.W.; Dubel, J.; Nasser, G.A.; Ashizawa, T.; de Jong, P.; et al. An unstable triplet repeat in a gene related to myotonic muscular dystrophy. Science 1992, 255, 1256–1258. [Google Scholar] [CrossRef]
- Timchenko, L.T.; Timchenko, N.A.; Caskey, C.T.; Roberts, R. Novel proteins with binding specificity for DNA CTG repeats and RNA CUG repeats: Implications for myotonic dystrophy. Hum. Mol. Genet. 1996, 5, 115–121. [Google Scholar] [CrossRef][Green Version]
- Timchenko, L.T.; Miller, J.W.; Timchenko, N.A.; DeVore, D.R.; Datar, K.V.; Lin, L.; Roberts, R.; Caskey, C.T.; Swanson, M.S. Identification of a (CUG)n triplet repeat RNA-binding protein and its expression in myotonic dystrophy. Nucl. Acids Res. 1996, 24, 4407–4414. [Google Scholar] [CrossRef]
- Miller, J.W.; Urbinati, C.R.; Teng-Umnuay, P.; Stenberg, M.G.; Byrne, B.J.; Thornton, C.A.; Swanson, M.S. Recruitment of human muscleblind proteins to (CUG)n expansions associated with myotonic dystrophy. EMBO J. 2000, 19, 4439–4448. [Google Scholar] [CrossRef]
- Michalowski, S.; Miller, J.W.; Urbinati, C.R.; Paliouras, M.; Swanson, M.S.; Griffith, J. Visualization of double-stranded RNAs from the myotonic dystrophy protein kinase gene and interactions with CUG-binding protein. Nucl. Acids Res. 1999, 27, 3534–3542. [Google Scholar] [CrossRef]
- Timchenko, N.A.; Cai, Z.-J.; Welm, A.L.; Reddy, S.; Ashizawa, T.; Timchenko, L.T. RNA CUG repeats sequester CUGBP1 and alter protein levels and activity of CUGBP1. J. Biol. Chem. 2001, 276, 7820–7826. [Google Scholar] [CrossRef]
- Kuyumcu-Martinez, N.M.; Wang, G.-S.; Cooper, T.A. Increased steady-state levels of CUGBP1 in myotonic dystrophy are due to PKC-mediated hyperphosphorylation. Mol. Cell 2007, 28, 68–78. [Google Scholar] [CrossRef]
- Jones, K.; Wei, C.; Iakova, P.; Bugiardini, E.; Schneider-Gold, C.; Meola, G.; Woodgett, J.; Killian, J.; Timchenko, N.A.; Timchenko, L.T. GSK3β mediates muscle pathology in myotonic dystrophy. J. Clin. Investig. 2012, 122, 4461–4472. [Google Scholar] [CrossRef] [PubMed]
- Timchenko, L.T. Myotonic dystrophy: The role of RNA CUG triplet repeats. Am. J. Hum. Genet. 1999, 64, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Mulders, S.A.; van den Broek, W.J.; Wheeler, T.M.; Croes, H.J.; van Kuik-Romeijn, P.; de Kimpe, S.J.; Furling, D.; Platenburg, G.J.; Gourdon, G.; Thornton, C.A.; et al. Triplet-repeat oligonucleotide-mediated reversal of RNA toxicity in myotonic dystrophy. Proc. Natl. Acad. Sci. USA 2009, 106, 13915–13920. [Google Scholar] [CrossRef] [PubMed]
- Wheeler, T.M.; Leger, A.J.; Pandey, S.K.; MacLeod, A.R.; Nakamori, M.; Cheng, S.H.; Wentworth, B.M.; Bennett, C.F.; Thornton, C.A. Targeting nuclear RNA for in vivo correction of myotonic dystrophy. Nature 2012, 488, 111–115. [Google Scholar] [CrossRef] [PubMed]
- Ait Benichou, S.; Jauvin, D.; De-Serres-Berard, T.; Bennett, F.; Rigo, F.; Gourdon, G.; Boutjdir, M.; Chahine, M.; Puymirat, J. Enhanced Delivery of Ligand-Conjugated Antisense Oligonucleotides (C16-HA-ASO) Targeting DMPK Transcripts for the Treatment of Myotonic Dystrophy Type 1. Hum. Gene Ther. 2022, 33, 810–820. [Google Scholar] [CrossRef]
- Ait Benichou, S.; Jauvin, D.; De Serres-Bérard, T.; Pierre, M.; Ling, K.K.; Bennett, C.F.; Rigo, F.; Gourdon, G.; Chahine, M.; Puymirat, J. Antisense oligonucleotides as a potential treatment for brain deficits observed in myotonic dystrophy type 1. Gene Ther. 2022, 29, 698–709. [Google Scholar] [CrossRef]
- López-Morató, M.; Brook, J.D.; Wojciechowska, M. Small Molecules Which Improve Pathogenesis of Myotonic Dystrophy Type 1. Front. Neurol. 2018, 9, 349. [Google Scholar] [CrossRef]
- Lopez Castel, A.; Overby, S.J.; Artero, R. MicroRNA-Based Therapeutic Perspectives in Myotonic Dystrophy. Int. J. Mol. Sci. 2019, 20, 5600. [Google Scholar] [CrossRef] [PubMed]
- Timchenko, L. Development of Therapeutic Approaches for Myotonic Dystrophies Type 1 and Type 2. Int. J. Mol. Sci. 2022, 23, 10491. [Google Scholar] [CrossRef]
- Wang, M.; Weng, W.C.; Stock, L.; Lindquist, D.; Martinez, A.; Gourdon, G.; Timchenko, N.; Snape, M.; Timchenko, L. Correction of Glycogen Synthase Kinase 3β in Myotonic Dystrophy 1 Reduces the Mutant RNA and Improves Postnatal Survival of DMSXL Mice. Mol. Cell. Biol. 2019, 39, e00155-19. [Google Scholar] [CrossRef] [PubMed]
- Horrigan, J.; Gomes, T.B.; Snape, M.; Nikolenko, N.; McMorn, A.; Evans, S.; Yaroshinsky, A.; Della Pasqua, O.; Oosterholt, S.; Lochmüller, H. A Phase 2 Study of AMO-02 (Tideglusib) in Congenital and Childhood-Onset Myotonic Dystrophy Type 1 (DM1). Pediatr. Neurol. 2020, 112, 84–93. [Google Scholar] [CrossRef]
- AMO Pharma Announces Affirming Data from REACH-CDM Clinical Trial for AMO-02 in Treatment of Myotonic Dystrophy. Available online: https://www.prnewswire.com/news-releases/amo-pharma-announces-affirming-data-from-reach-cdm-clinical-trial-for-amo-02-in-treatment-of-myotonic-dystrophy-301918241.html (accessed on 23 May 2025).
- Phase 3 Trial for Myotonic Dystrophy Agent AMO-02 to Begin Following FDA Meeting. Available online: https://www.neurologylive.com/view/phase-3-trial-dm1-agent-amo-02-begin-following-fda-meeting (accessed on 23 May 2025).
- Nakamori, M.; Sobczak, K.; Puwanant, A.; Welle, S.; Eichinger, K.; Pandya, S.; Dekdebrun, J.; Heatwole, C.R.; McDermott, M.P.; Chen, T.; et al. Splicing biomarkers of disease severity in myotonic dystrophy. Ann. Neurol. 2013, 74, 862–872. [Google Scholar] [CrossRef] [PubMed]
- Provenzano, M.; Ikegami, K.; Bates, K.; Gaynor, A.; Hartman, J.M.; Jones, A.; Butler, A.; Berggren, K.N.; Dekdebrun, J.; Hung, M.; et al. The Splice Index as a prognostic biomarker of strength and function in myotonic dystrophy type 1. J. Clin. Investig. 2025, 135, e185426. [Google Scholar] [CrossRef]
- Antoury, L.; Hu, N.; Balaj, L.; Das, S.; Georghiou, S.; Darras, B.; Clark, T.; Breakefield, X.O.; Wheeler, T.M. Analysis of extracellular mRNA in human urine reveals splice variant biomarkers of muscular dystrophies. Nat. Commun. 2018, 9, 3906. [Google Scholar] [CrossRef] [PubMed]
- Gibreel, W.O.; Selcen, D.; Zeidan, M.M.; Ishitani, M.B.; Moir, C.R.; Zarroug, A.E. Safety and yield of muscle biopsy in pediatric patients in the modern era. J. Pediatr. Surg. 2014, 49, 1429–1432. [Google Scholar] [CrossRef]
- Sethuraman, C. Muscle biopsies in children—A broad overview and recent updates: Where does the future lie? Diagn. Histopathol. 2023, 29, 511–520. [Google Scholar] [CrossRef]
- Lutz, M.; Levanti, M.; Karns, R.; Gourdon, G.; Lindquist, D.; Timchenko, N.A.; Timchenko, L. Therapeutic Targeting of the GSK3β-CUGBP1 Pathway in Myotonic Dystrophy. Int. J. Mol. Sci. 2023, 24, 10650. [Google Scholar] [CrossRef]
- Charlet-B, N.; Savkur, R.S.; Singh, G.; Philips, A.V.; Grice, E.A.; Cooper, T.A. Loss of the muscle-specific chloride channel in type 1 myotonic dystrophy due to misregulated alternative splicing. Mol. Cell 2002, 10, 45–53. [Google Scholar] [CrossRef]
- Mankodi, A.; Takahashi, M.P.; Jiang, H.; Beck, C.L.; Bowers, W.J.; Moxley, R.T.; Cannon, S.C.; Thornton, C.A. Expanded CUG repeats trigger aberrant splicing of ClC-1 chloride channel pre-mRNA and hyperexcitability of skeletal muscle in myotonic dystrophy. Mol. Cell 2002, 10, 35–44. [Google Scholar] [CrossRef]
- Adams, J.C.; Lawler, J. The thrombospondins. Cold Spring Harb. Perspect. Biol. 2011, 3, a009712. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Jiang, Y.; Barnes, R.H., 2nd; Tokunaga, M.; Martinez-Santibañez, G.; Geletka, L.; Lumeng, C.N.; Buchner, D.A.; Chun, T.H. Thrombospondin 1 mediates high-fat diet-induced muscle fibrosis and insulin resistance in male mice. Endocrinology 2013, 154, 4548–4559. [Google Scholar] [CrossRef]
- Ismaeel, A.; Kim, J.S.; Kirk, J.S.; Smith, R.S.; Bohannon, W.T.; Koutakis, P. Role of Transforming Growth Factor-β in Skeletal Muscle Fibrosis: A Review. Int. J. Mol. Sci. 2019, 20, 2446. [Google Scholar] [CrossRef] [PubMed]
- Ceco, E.; McNally, E.M. Modifying muscular dystrophy through transforming growth factor-β. FEBS J. 2013, 280, 4198–4209. [Google Scholar] [CrossRef]
- Mázala, D.A.; Novak, J.S.; Hogarth, M.W.; Nearing, M.; Adusumalli, P.; Tully, C.B.; Habib, N.F.; Gordish-Dressman, H.; Chen, Y.W.; Jaiswal, J.K.; et al. TGF-β-driven muscle degeneration and failed regeneration underlie disease onset in a DMD mouse model. JCI Insight 2020, 5, e135703. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Nihei, Y.; Arzberger, T.; Zhou, Q.; Mackenzie, I.R.; Hermann, A.; Hanisch, F.; German Consortium for Frontotemporal Lobar Degeneration; Bavarian Brain Banking Alliance; Kamp, F.; et al. Reduced hnRNPA3 increases C9orf72 repeat RNA levels and dipeptide-repeat protein deposition. EMBO Rep. 2016, 17, 1314–1325. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Gatt, A.; Lashley, T. HnRNP Pathologies in Frontotemporal Lobar Degeneration. Cells 2023, 12, 1633. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jennings, K.; Tian, C.; Brown, R.L.; Horn, P.S.; Schoser, B.; Kushlaf, H.; Timchenko, N.A.; Timchenko, L. Elevated Levels of Active GSK3β in the Blood of Patients with Myotonic Dystrophy Type 1 Correlate with Muscle Weakness. Int. J. Mol. Sci. 2025, 26, 10760. https://doi.org/10.3390/ijms262110760
Jennings K, Tian C, Brown RL, Horn PS, Schoser B, Kushlaf H, Timchenko NA, Timchenko L. Elevated Levels of Active GSK3β in the Blood of Patients with Myotonic Dystrophy Type 1 Correlate with Muscle Weakness. International Journal of Molecular Sciences. 2025; 26(21):10760. https://doi.org/10.3390/ijms262110760
Chicago/Turabian StyleJennings, Katherine, Cuixia Tian, Rebeccah L. Brown, Paul S. Horn, Benedikt Schoser, Hani Kushlaf, Nikolai A. Timchenko, and Lubov Timchenko. 2025. "Elevated Levels of Active GSK3β in the Blood of Patients with Myotonic Dystrophy Type 1 Correlate with Muscle Weakness" International Journal of Molecular Sciences 26, no. 21: 10760. https://doi.org/10.3390/ijms262110760
APA StyleJennings, K., Tian, C., Brown, R. L., Horn, P. S., Schoser, B., Kushlaf, H., Timchenko, N. A., & Timchenko, L. (2025). Elevated Levels of Active GSK3β in the Blood of Patients with Myotonic Dystrophy Type 1 Correlate with Muscle Weakness. International Journal of Molecular Sciences, 26(21), 10760. https://doi.org/10.3390/ijms262110760








