GLP-1 and the Degenerating Brain: Exploring Mechanistic Insights and Therapeutic Potential
Abstract
1. Introduction
2. Expression and Distribution of Glp-1 and Glp-1r in Nervous System
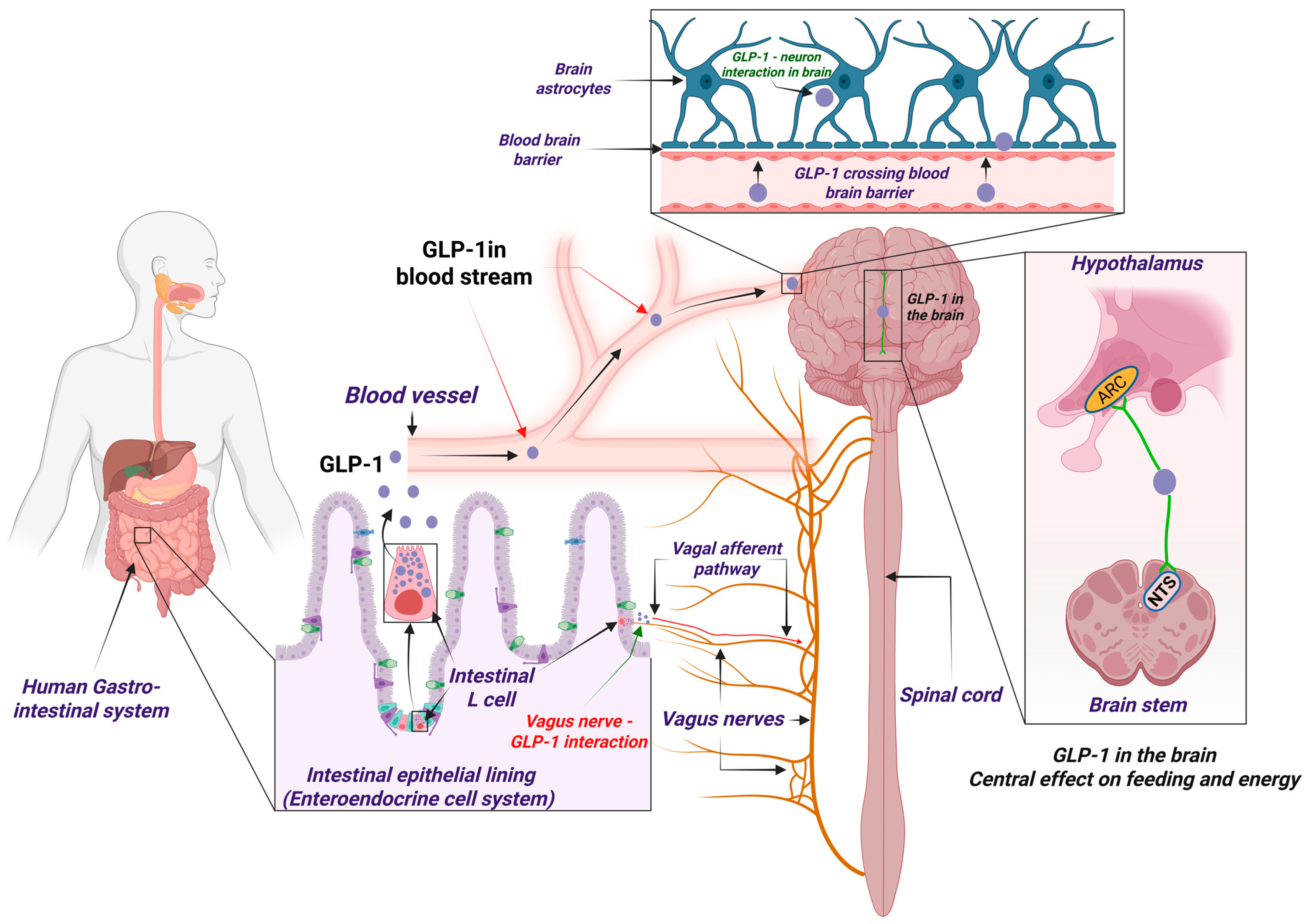
2.1. The Peripheral Nervous Pathway of Glp-1: Vagus-Driven Central Effects
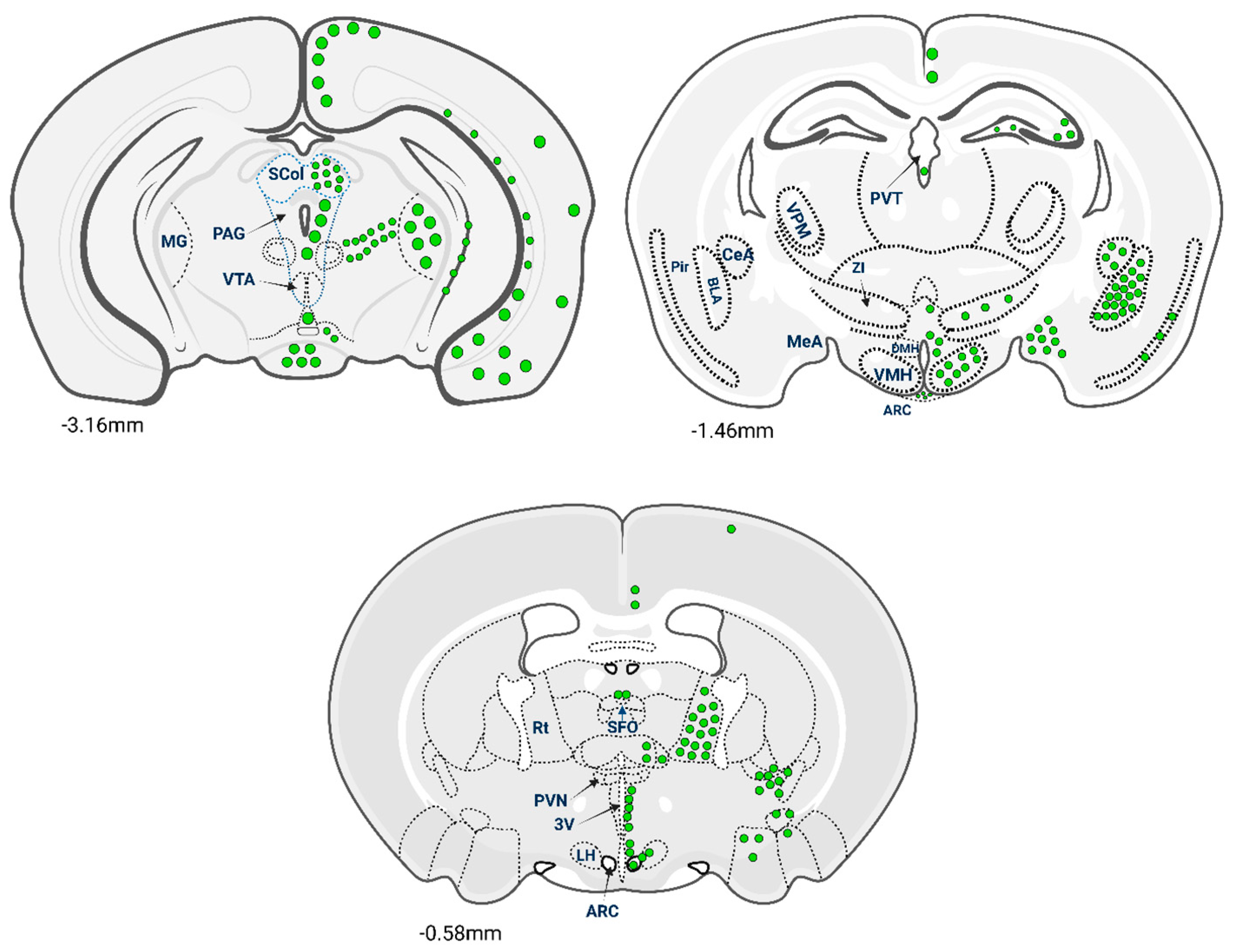
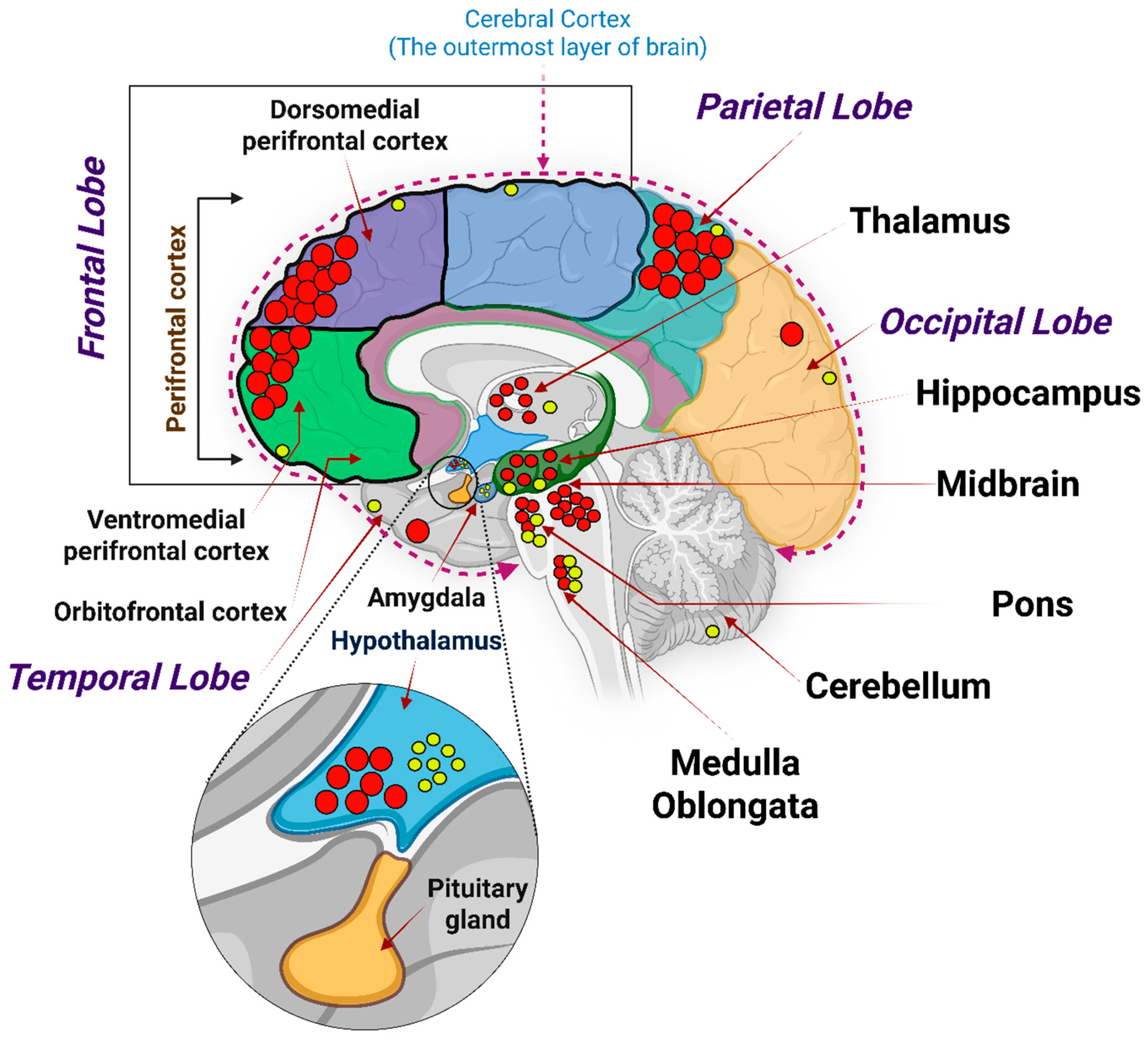
2.2. Glp-1 Receptors in the Central Nervous System (CNS): Regional Density and Physiological Roles
2.3. Species Differences of Glp-1r Expression in Brain: Implications for Translation
3. Effect of Glp-1r Expression in Different Regions of Brain Functionality
3.1. Glp-1r in the Hypothalamus: Appetite and Energy Balance
3.2. Glp-1r in the Hippocampus and Cerebral Cortex: Learning, Memory, and Executive Control
3.3. Glp-1r in Other Brain Regions: Mood, Reward, and Pain/Stress Modulation
3.4. Spatial Mapping of Glp-1r and Its Translational Value for Neurodegenerative Disorders
4. Molecular Mechanisms of Glp-1 Action in Neurons
4.1. Intracellular Signaling Mechanisms of Glp-1 in Neurons
4.2. Glp-1 Enhances Myelination
4.3. Glp-1 Prevents Demyelination by Inhibiting Neuronal Inflammation
4.4. Glp-1 Potentiates Axonal Regeneration Through Modulating Autophagy and Neuronal Apoptosis
4.5. Glp-1 Action in Microglia
4.6. Glp-1 Enhances Neuronal Survival
5. Neuroprotective Actions of Glp-1 (Summarized in Table 2)
5.1. Glp-1 in Alzheimer’s Disease (AD)
5.1.1. Efforts in Elucidating the Role of GLP-1RAs in Dementia Management
5.1.2. Diabetes and Dementia: Connecting Brain Insulin Signaling, GLP-1 Pathways
5.2. Glp-1 in Parkinson’s Disease (PD)
5.3. Glp-1 in Huntington’s Disease
| Neurodegenerative Diseases | Therapeutic Benefits of Using GLP-1Rs | Mechanism of Action | References |
|---|---|---|---|
| Alzheimer’s diseases |
|
| [53,55,56,57,59,60,61,62,67] |
| Dementia |
|
| [58,64,65,66] |
| Parkinson’s disease |
|
| [67,73,74,75,76,77,78] |
| Huntington’s disease |
|
| [79,80,81,82] |
6. Therapeutic Strategies for Neurodegenerative Disorders
6.1. Glp-1 in Neuroprotection: Evidence from Animal Models
6.1.1. Studies in Mice
6.1.2. Studies in Rats
6.2. Safeguarding the Aging Brain: Clinical Evidence Supporting Glp-1ra Therapy
6.2.1. Observational and Pooled Randomized Controlled Trial (RCT) Evidence
6.2.2. REWIND Trial—Dulaglutide
6.2.3. Semaglutide Biomarker/Immune Modulation Study
6.2.4. EVOKE & EVOKE Plus—Semaglutide Phase III Trials
6.2.5. ISAP Trial—Oral Semaglutide in Preclinical/Prodromal AD
6.2.6. Exenatide—Proof-of-Concept in Mild Cognitive Impairment (MCI)
6.2.7. Exenatide—18-Month Phase II Trial in Early AD
6.2.8. Liraglutide—Evaluating the Effects of the Novel GLP-1 Analogue Liraglutide in Alzheimer’s Disease (ELAD) Trial
6.2.9. Liraglutide—Phase IIb (AAIC 2024 and Related Imaging Studies)
7. Limitations and Challenges of GLP-1RAs in Neurodegeneration
7.1. Translational Barriers & CNS Target Engagement
7.2. Tolerability, Adherence, and Safety (And Their Regulatory Implications)
7.3. Methodological Limitations: Power, Endpoints, and Analyses
7.4. Population Heterogeneity and Metabolic/Genetic Confounding
7.5. Publication Bias and Reporting Transparency in the Evidence Base
8. Future Directions for Glp-1r–Based Therapy in Neurodegenerative Diseases
8.1. Precision Neuroincretin Medicine
8.2. Omics-Driven Patient Stratification
8.3. Artificial Intelligence (AI), Predictive Modeling, and Digital Twins
8.4. Next-Generation CNS Delivery Platforms
8.5. Intranasal Route and Other Innovative Delivery Approaches
8.6. Disease-Specific Precision Applications
8.6.1. Alzheimer’s Disease (AD)
8.6.2. Parkinson’s Disease (PD)
8.6.3. Huntington’s Disease (HD)
9. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Reich, N.; Hölscher, C. The neuroprotective effects of glucagon-like peptide 1 in Alzheimer’s and Parkinson’s disease: An in-depth review. Front. Neurosci. 2022, 16, 970925. [Google Scholar] [CrossRef]
- Grieco, M.; Giorgi, A.; Gentile, M.C.; d’Erme, M.; Morano, S.; Maras, B.; Filardi, T. Glucagon-Like Peptide-1: A Focus on Neurodegenerative Diseases. Front. Neurosci. 2019, 13, 1112. [Google Scholar] [CrossRef] [PubMed]
- Heppner, K.M.; Kirigiti, M.; Secher, A.; Paulsen, S.J.; Buckingham, R.; Pyke, C.; Knudsen, L.B.; Vrang, N.; Grove, K.L. Expression and distribution of glucagon-like peptide-1 receptor mRNA, protein and binding in the male nonhuman primate (Macaca mulatta) brain. Endocrinology 2015, 156, 255–267. [Google Scholar] [CrossRef] [PubMed]
- Vrang, N.; Larsen, P.J. Preproglucagon derived peptides GLP-1, GLP-2 and oxyntomodulin in the CNS: Role of peripherally secreted and centrally produced peptides. Prog. Neurobiol. 2010, 92, 442–462. [Google Scholar] [CrossRef]
- Kanoski, S.E.; Fortin, S.M.; Arnold, M.; Grill, H.J.; Hayes, M.R. Peripheral and central GLP-1 receptor populations mediate the anorectic effects of peripherally administered GLP-1 receptor agonists, liraglutide and exendin-4. Endocrinology 2011, 152, 3103–3112. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.; Sparre-Ulrich, A.H.; Hartmann, B.; Grevstad, U.; Rosenkilde, M.M.; Holst, J.J.; Vilsbøll, T.; Knop, F.K. Transfer of liraglutide from blood to cerebrospinal fluid is minimal in patients with type 2 diabetes. Int. J. Obes. 2015, 39, 1651–1654. [Google Scholar] [CrossRef]
- Hunter, K.; Hölscher, C. Drugs developed to treat diabetes, liraglutide and lixisenatide, cross the blood brain barrier and enhance neurogenesis. BMC Neurosci. 2012, 13, 33. [Google Scholar] [CrossRef]
- Trapp, S.; Brierley, D.I. Brain GLP-1 and the regulation of food intake: GLP-1 action in the brain and its implications for GLP-1 receptor agonists in obesity treatment. Br. J. Pharmacol. 2022, 179, 557–570. [Google Scholar] [CrossRef]
- Sisson, E.M. Liraglutide: Clinical pharmacology and considerations for therapy. Pharmacotherapy 2011, 31, 896–911. [Google Scholar] [CrossRef]
- Chen, B.; Yu, X.; Horvath-Diano, C.; Ortuño, M.J.; Tschöp, M.H.; Jastreboff, A.M.; Schneeberger, M. GLP-1 programs the neurovascular landscape. Cell Metab. 2024, 36, 2173–2189. [Google Scholar] [CrossRef]
- Salameh, T.S.; Rhea, E.M.; Talbot, K.; Banks, W.A. Brain uptake pharmacokinetics of incretin receptor agonists showing promise as Alzheimer’s and Parkinson’s disease therapeutics. Biochem. Pharmacol. 2020, 180, 114187, Erratum in Biochem. Pharmacol. 2023, 210, 115474. [Google Scholar] [CrossRef]
- Hall, S.; Isaacs, D.; Clements, J.N. Pharmacokinetics and Clinical Implications of Semaglutide: A New Glucagon-Like Peptide (GLP)-1 Receptor Agonist. Clin. Pharmacokinet. 2018, 57, 1529–1538. [Google Scholar] [CrossRef]
- Yang, X.D.; Yang, Y.Y. Clinical Pharmacokinetics of Semaglutide: A Systematic Review. Drug Des. Dev. Ther. 2024, 18, 2555–2570. [Google Scholar] [CrossRef]
- Chen, S.; Yu, S.J.; Li, Y.; Lecca, D.; Glotfelty, E.; Kim, H.K.; Choi, H.I.; Hoffer, B.J.; Greig, N.H.; Kim, D.S.; et al. Post-treatment with PT302, a long-acting Exendin-4 sustained release formulation, reduces dopaminergic neurodegeneration in a 6-Hydroxydopamine rat model of Parkinson’s disease. Sci. Rep. 2018, 8, 10722. [Google Scholar] [CrossRef]
- Cork, S.C.; Richards, J.E.; Holt, M.K.; Gribble, F.M.; Reimann, F.; Trapp, S. Distribution and characterisation of Glucagon-like peptide-1 receptor expressing cells in the mouse brain. Mol. Metab. 2015, 4, 718–731. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.B.; Pyke, C.; Rasch, M.G.; Dahl, A.B.; Knudsen, L.B.; Secher, A. Characterization of the Glucagonlike Peptide-1 Receptor in Male Mouse Brain Using a Novel Antibody and In Situ Hybridization. Endocrinology 2018, 159, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Brain Expression Cluster (RNA): Glucagon like Peptide 1 Receptor (GLP1R). Available online: https://www.proteinatlas.org/ENSG00000112164-GLP1R/brain (accessed on 5 October 2025).
- Gupta, T.; Kaur, M.; Shekhawat, D.; Aggarwal, R.; Nanda, N.; Sahni, D. Investigating the Glucagon-like Peptide-1 and Its Receptor in Human Brain: Distribution of Expression, Functional Implications, Age-related Changes and Species Specific Characteristics. Basic. Clin. Neurosci. 2023, 14, 341–353. [Google Scholar] [CrossRef]
- Bjerre Knudsen, L.; Madsen, L.W.; Andersen, S.; Almholt, K.; de Boer, A.S.; Drucker, D.J.; Gotfredsen, C.; Egerod, F.L.; Hegelund, A.C.; Jacobsen, H.; et al. Glucagon-like Peptide-1 receptor agonists activate rodent thyroid C-cells causing calcitonin release and C-cell proliferation. Endocrinology 2010, 151, 1473–1486. [Google Scholar] [CrossRef]
- Rosol, T.J. On-target effects of GLP-1 receptor agonists on thyroid C-cells in rats and mice. Toxicol. Pathol. 2013, 41, 303–309. [Google Scholar] [CrossRef]
- Feng, J.; Jin, T. Current understanding and controversy on brain access of GLP-1 and GLP-1 receptor agonists. J. Transl. Intern. Med. 2025, 13, 201–210. [Google Scholar] [CrossRef]
- Fu, Z.; Gong, L.; Liu, J.; Wu, J.; Barrett, E.J.; Aylor, K.W.; Liu, Z. Brain Endothelial Cells Regulate Glucagon-Like Peptide 1 Entry Into the Brain via a Receptor-Mediated Process. Front. Physiol. 2020, 11, 555. [Google Scholar] [CrossRef]
- Jensen, M.N.; Israelsen, I.M.E.; Wardman, J.H.; Jensen, D.B.; Andersen, D.B.; Toft-Bertelsen, T.L.; Rath, M.F.; Holst, J.J.; Rosenkilde, M.M.; MacAulay, N. Glucagon-like peptide-1 receptor modulates cerebrospinal fluid secretion and intracranial pressure in rats. Fluids Barriers CNS 2025, 22, 41. [Google Scholar] [CrossRef]
- Deden, L.N.; Booij, J.; Grandjean, J.; Homberg, J.R.; Hazebroek, E.J.; Gotthardt, M.; Boss, M. Brain Imaging of the GLP-1 Receptor in Obesity Using (68)Ga-NODAGA-Exendin-4 PET. Brain Sci. 2021, 11, 1647. [Google Scholar] [CrossRef] [PubMed]
- Tokgöz, S.; Boss, M.; Prasad, S.; Shah, P.; Laverman, P.; van Riel, M.; Gotthardt, M. Protocol for Clinical GLP-1 Receptor PET/CT Imaging with [(68)Ga]Ga-NODAGA-Exendin-4. Methods Mol. Biol. 2023, 2592, 143–153. [Google Scholar] [CrossRef]
- Lockie, S.H.; Heppner, K.M.; Chaudhary, N.; Chabenne, J.R.; Morgan, D.A.; Veyrat-Durebex, C.; Ananthakrishnan, G.; Rohner-Jeanrenaud, F.; Drucker, D.J.; DiMarchi, R.; et al. Direct control of brown adipose tissue thermogenesis by central nervous system glucagon-like peptide-1 receptor signaling. Diabetes 2012, 61, 2753–2762. [Google Scholar] [CrossRef]
- van Bloemendaal, L.; IJzerman, R.G.; Ten Kulve, J.S.; Barkhof, F.; Konrad, R.J.; Drent, M.L.; Veltman, D.J.; Diamant, M. GLP-1 receptor activation modulates appetite- and reward-related brain areas in humans. Diabetes 2014, 63, 4186–4196. [Google Scholar] [CrossRef]
- Hansen, H.H.; Fabricius, K.; Barkholt, P.; Niehoff, M.L.; Morley, J.E.; Jelsing, J.; Pyke, C.; Knudsen, L.B.; Farr, S.A.; Vrang, N. The GLP-1 Receptor Agonist Liraglutide Improves Memory Function and Increases Hippocampal CA1 Neuronal Numbers in a Senescence-Accelerated Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 46, 877–888. [Google Scholar] [CrossRef]
- Diz-Chaves, Y.; Herrera-Pérez, S.; González-Matías, L.C.; Mallo, F. Effects of Glucagon-like peptide 1 (GLP-1) analogs in the hippocampus. Vitam. Horm. 2022, 118, 457–478. [Google Scholar] [CrossRef]
- Terrill, S.J.; Jackson, C.M.; Greene, H.E.; Lilly, N.; Maske, C.B.; Vallejo, S.; Williams, D.L. Role of lateral septum glucagon-like peptide 1 receptors in food intake. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2016, 311, R124–R132. [Google Scholar] [CrossRef] [PubMed]
- Rebosio, C.; Balbi, M.; Passalacqua, M.; Ricciarelli, R.; Fedele, E. Presynaptic GLP-1 receptors enhance the depolarization-evoked release of glutamate and GABA in the mouse cortex and hippocampus. Biofactors 2018, 44, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Hendershot, C.S.; Bremmer, M.P.; Paladino, M.B.; Kostantinis, G.; Gilmore, T.A.; Sullivan, N.R.; Tow, A.C.; Dermody, S.S.; Prince, M.A.; Jordan, R.; et al. Once-Weekly Semaglutide in Adults With Alcohol Use Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2025, 82, 395–405. [Google Scholar] [CrossRef]
- Fortin, S.M.; Roitman, M.F. Central GLP-1 receptor activation modulates cocaine-evoked phasic dopamine signaling in the nucleus accumbens core. Physiol. Behav. 2017, 176, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Abbas, T.; Faivre, E.; Hölscher, C. Impairment of synaptic plasticity and memory formation in GLP-1 receptor KO mice: Interaction between type 2 diabetes and Alzheimer’s disease. Behav. Brain Res. 2009, 205, 265–271. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liang, F.; Wang, Y.; Wei, Y.; Ma, T. Liraglutide-associated depression in a patient with type 2 diabetes: A case report and discussion. Medicine 2024, 103, e37928. [Google Scholar] [CrossRef]
- Gu, G.; Roland, B.; Tomaselli, K.; Dolman, C.S.; Lowe, C.; Heilig, J.S. Glucagon-like peptide-1 in the rat brain: Distribution of expression and functional implication. J. Comp. Neurol. 2013, 521, 2235–2261. [Google Scholar] [CrossRef]
- Xie, Y.; Zheng, J.; Li, S.; Li, H.; Zhou, Y.; Zheng, W.; Zhang, M.; Liu, L.; Chen, Z. GLP-1 improves the neuronal supportive ability of astrocytes in Alzheimer’s disease by regulating mitochondrial dysfunction via the cAMP/PKA pathway. Biochem. Pharmacol. 2021, 188, 114578. [Google Scholar] [CrossRef]
- Yang, J.L.; Chen, W.Y.; Chen, Y.P.; Kuo, C.Y.; Chen, S.D. Activation of GLP-1 Receptor Enhances Neuronal Base Excision Repair via PI3K-AKT-Induced Expression of Apurinic/Apyrimidinic Endonuclease 1. Theranostics 2016, 6, 2015–2027. [Google Scholar] [CrossRef]
- Matsuzaki, H.; Tamatani, M.; Mitsuda, N.; Namikawa, K.; Kiyama, H.; Miyake, S.; Tohyama, M. Activation of Akt kinase inhibits apoptosis and changes in Bcl-2 and Bax expression induced by nitric oxide in primary hippocampal neurons. J. Neurochem. 1999, 73, 2037–2046. [Google Scholar] [PubMed]
- Torii, T.; Miyamoto, Y.; Yamauchi, J. Cellular Signal-Regulated Schwann Cell Myelination and Remyelination. Adv. Exp. Med. Biol. 2019, 1190, 3–22. [Google Scholar] [CrossRef]
- Takaku, S.; Tsukamoto, M.; Niimi, N.; Yako, H.; Sango, K. Exendin-4 Promotes Schwann Cell Survival/Migration and Myelination In Vitro. Int. J. Mol. Sci. 2021, 22, 2971. [Google Scholar] [CrossRef]
- Lee, C.H.; Jeon, S.J.; Cho, K.S.; Moon, E.; Sapkota, A.; Jun, H.S.; Ryu, J.H.; Choi, J.W. Activation of Glucagon-Like Peptide-1 Receptor Promotes Neuroprotection in Experimental Autoimmune Encephalomyelitis by Reducing Neuroinflammatory Responses. Mol. Neurobiol. 2018, 55, 3007–3020. [Google Scholar] [CrossRef]
- Chiou, H.C.; Lin, M.W.; Hsiao, P.J.; Chen, C.L.; Chiao, S.; Lin, T.Y.; Chen, Y.C.; Wu, D.C.; Lin, M.H. Dulaglutide Modulates the Development of Tissue-Infiltrating Th1/Th17 Cells and the Pathogenicity of Encephalitogenic Th1 Cells in the Central Nervous System. Int. J. Mol. Sci. 2019, 20, 1584. [Google Scholar] [CrossRef] [PubMed]
- Li, H.T.; Zhao, X.Z.; Zhang, X.R.; Li, G.; Jia, Z.Q.; Sun, P.; Wang, J.Q.; Fan, Z.K.; Lv, G. Exendin-4 Enhances Motor Function Recovery via Promotion of Autophagy and Inhibition of Neuronal Apoptosis After Spinal Cord Injury in Rats. Mol. Neurobiol. 2016, 53, 4073–4082. [Google Scholar] [CrossRef]
- Spencer, B.; Potkar, R.; Trejo, M.; Rockenstein, E.; Patrick, C.; Gindi, R.; Adame, A.; Wyss-Coray, T.; Masliah, E. Beclin 1 gene transfer activates autophagy and ameliorates the neurodegenerative pathology in alpha-synuclein models of Parkinson’s and Lewy body diseases. J. Neurosci. 2009, 29, 13578–13588. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Yang, B.; Mo, X.; Xiao, H. Mechanism and Regulation of Autophagy and Its Role in Neuronal Diseases. Mol. Neurobiol. 2015, 52, 1190–1209. [Google Scholar] [CrossRef]
- Turcano, P.; Savica, R.; Benarroch, E. What Is the Role of Glucagon-Like Peptide 1 Signaling in the Nervous System and Its Potential Neuroprotective Effects? Neurology 2024, 103, e209781. [Google Scholar] [CrossRef]
- Kopp, K.O.; Li, Y.; Glotfelty, E.J.; Tweedie, D.; Greig, N.H. Incretin-Based Multi-Agonist Peptides Are Neuroprotective and Anti-Inflammatory in Cellular Models of Neurodegeneration. Biomolecules 2024, 14, 872. [Google Scholar] [CrossRef]
- Wu, H.Y.; Tang, X.Q.; Liu, H.; Mao, X.F.; Wang, Y.X. Both classic Gs-cAMP/PKA/CREB and alternative Gs-cAMP/PKA/p38β/CREB signal pathways mediate exenatide-stimulated expression of M2 microglial markers. J. Neuroimmunol. 2018, 316, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.T.; Tsai, Y.H.; Fülöp, F.; Chang, F.R.; Lo, Y.C. 2-Iodo-4’-Methoxychalcone Attenuates Methylglyoxal-Induced Neurotoxicity by Activation of GLP-1 Receptor and Enhancement of Neurotrophic Signal, Antioxidant Defense and Glyoxalase Pathway. Molecules 2019, 24, 2249. [Google Scholar] [CrossRef]
- Erbil, D.; Eren, C.Y.; Demirel, C.; Küçüker, M.U.; Solaroğlu, I.; Eser, H.Y. GLP-1’s role in neuroprotection: A systematic review. Brain Inj. 2019, 33, 734–819. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Mao, Y.; Zheng, Z.; Chen, Y.; Khor, S.; Shi, K.; He, Z.; Li, J.; Gong, F.; et al. Liraglutide activates autophagy. Oncotarget 2017, 8, 85949–85968. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ma, N.; Yang, X.; Sun, M.; Li, X.; Liu, Y.; Chang, Q.; Hei, C. The Association of Circulating Glucagon-Like Peptide-1 with Cognitive Functions and Biomarkers in Alzheimer’s Disease. J. Alzheimer’s Dis. 2024, 99, 525–533. [Google Scholar] [CrossRef]
- Pelle, M.C.; Zaffina, I.; Giofrè, F.; Pujia, R.; Arturi, F. Potential Role of Glucagon-like Peptide-1 Receptor Agonists in the Treatment of Cognitive Decline and Dementia in Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 11301. [Google Scholar] [CrossRef]
- Qian, Z.; Chen, H.; Xia, M.; Chang, J.; Li, X.; Ye, S.; Wu, S.; Jiang, S.; Bao, J.; Wang, B.; et al. Activation of glucagon-like peptide-1 receptor in microglia attenuates neuroinflammation-induced glial scarring via rescuing Arf and Rho GAP adapter protein 3 expressions after nerve injury. Int. J. Biol. Sci. 2022, 18, 1328–1346. [Google Scholar] [CrossRef]
- Song, S.; Guo, R.; Mehmood, A.; Zhang, L.; Yin, B.; Yuan, C.; Zhang, H.; Guo, L.; Li, B. Liraglutide attenuate central nervous inflammation and demyelination through AMPK and pyroptosis-related NLRP3 pathway. CNS Neurosci. Ther. 2022, 28, 422–434. [Google Scholar] [CrossRef]
- Li, Y.; Glotfelty, E.J.; Karlsson, T.; Fortuno, L.V.; Harvey, B.K.; Greig, N.H. The metabolite GLP-1 (9-36) is neuroprotective and anti-inflammatory in cellular models of neurodegeneration. J. Neurochem. 2021, 159, 867–886. [Google Scholar] [CrossRef]
- Lv, D.; Feng, P.; Guan, X.; Liu, Z.; Li, D.; Xue, C.; Bai, B.; Hölscher, C. Neuroprotective effects of GLP-1 class drugs in Parkinson’s disease. Front. Neurol. 2024, 15, 1462240. [Google Scholar] [CrossRef]
- McClean, P.L.; Parthsarathy, V.; Faivre, E.; Hölscher, C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J. Neurosci. 2011, 31, 6587–6594. [Google Scholar] [CrossRef]
- Duarte, A.I.; Candeias, E.; Alves, I.N.; Mena, D.; Silva, D.F.; Machado, N.J.; Campos, E.J.; Santos, M.S.; Oliveira, C.R.; Moreira, P.I. Liraglutide Protects Against Brain Amyloid-β(1-42) Accumulation in Female Mice with Early Alzheimer’s Disease-Like Pathology by Partially Rescuing Oxidative/Nitrosative Stress and Inflammation. Int. J. Mol. Sci. 2020, 21, 1746. [Google Scholar] [CrossRef] [PubMed]
- Bomba, M.; Ciavardelli, D.; Silvestri, E.; Canzoniero, L.M.; Lattanzio, R.; Chiappini, P.; Piantelli, M.; Di Ilio, C.; Consoli, A.; Sensi, S.L. Exenatide promotes cognitive enhancement and positive brain metabolic changes in PS1-KI mice but has no effects in 3xTg-AD animals. Cell Death Dis. 2013, 4, e612. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Zhou, Y.; Zhang, M.; Xie, Y.; Ke, S.; Liu, L.; Pan, X.; Chen, Z. Exenatide alleviates mitochondrial dysfunction and cognitive impairment in the 5×FAD mouse model of Alzheimer’s disease. Behav. Brain Res. 2019, 370, 111932. [Google Scholar] [CrossRef] [PubMed]
- Athauda, D.; Maclagan, K.; Budnik, N.; Zampedri, L.; Hibbert, S.; Skene, S.S.; Chowdhury, K.; Aviles-Olmos, I.; Limousin, P.; Foltynie, T. What Effects Might Exenatide have on Non-Motor Symptoms in Parkinson’s Disease: A Post Hoc Analysis. J. Park. Dis. 2018, 8, 247–258. [Google Scholar] [CrossRef] [PubMed]
- Siddeeque, N.; Hussein, M.H.; Abdelmaksoud, A.; Bishop, J.; Attia, A.S.; Elshazli, R.M.; Fawzy, M.S.; Toraih, E.A. Neuroprotective effects of GLP-1 receptor agonists in neurodegenerative Disorders: A Large-Scale Propensity-Matched cohort study. Int. Immunopharmacol. 2024, 143, 113537. [Google Scholar] [CrossRef]
- Inoue, K.; Saliba, D.; Gotanda, H.; Moin, T.; Mangione, C.M.; Klomhaus, A.M.; Tsugawa, Y. Glucagon-Like Peptide-1 Receptor Agonists and Incidence of Dementia Among Older Adults With Type 2 Diabetes: A Target Trial Emulation. Ann. Intern. Med. 2025, 178, 1258–1267. [Google Scholar] [CrossRef]
- Liang, Y.; Doré, V.; Rowe, C.C.; Krishnadas, N. Clinical Evidence for GLP-1 Receptor Agonists in Alzheimer’s Disease: A Systematic Review. J. Alzheimer’s Dis. Rep. 2024, 8, 777–789. [Google Scholar] [CrossRef] [PubMed]
- Hölscher, C. Glucagon-like peptide-1 class drugs show clear protective effects in Parkinson’s and Alzheimer’s disease clinical trials: A revolution in the making? Neuropharmacology 2024, 253, 109952. [Google Scholar] [CrossRef]
- Xu, W.; Caracciolo, B.; Wang, H.X.; Winblad, B.; Bäckman, L.; Qiu, C.; Fratiglioni, L. Accelerated progression from mild cognitive impairment to dementia in people with diabetes. Diabetes 2010, 59, 2928–2935. [Google Scholar] [CrossRef]
- Peng, Y.; Yao, S.Y.; Chen, Q.; Jin, H.; Du, M.Q.; Xue, Y.H.; Liu, S. True or false? Alzheimer’s disease is type 3 diabetes: Evidences from bench to bedside. Ageing Res. Rev. 2024, 99, 102383. [Google Scholar] [CrossRef]
- Bhalla, S.; Mehan, S.; Khan, A.; Rehman, M.U. Protective role of IGF-1 and GLP-1 signaling activation in neurological dysfunctions. Neurosci. Biobehav. Rev. 2022, 142, 104896. [Google Scholar] [CrossRef]
- Zheng, M.; Wang, C.; Hu, M.; Li, Q.; Li, J.; Quan, S.; Zhang, X.; Gu, L. Research progress on the association of insulin resistance with type 2 diabetes mellitus and Alzheimer’s disease. Metab. Brain Dis. 2024, 40, 35. [Google Scholar] [CrossRef]
- Husain, K.H.; Sarhan, S.F.; AlKhalifa, H.K.A.A.; Buhasan, A.; Moin, A.S.M.; Butler, A.E. Dementia in Diabetes: The Role of Hypoglycemia. Int. J. Mol. Sci. 2023, 24, 9846. [Google Scholar] [CrossRef] [PubMed]
- Dahiya, S.; Tisch, S.; Greenfield, J. The effect of GLP-1 receptor agonists in pre-clinical rodent models of Parkinson’s disease: A systematic review and meta-analysis. Clin. Park. Relat. Disord. 2022, 6, 100133, Erratum in Clin. Park. Relat. Disord. 2023, 23, 100193. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Dong, Y.; Chen, J.; Guan, T.; Cao, B.; Zhang, Y.; Qi, Y.; Guan, Z.; Wang, Y. Liraglutide Regulates Mitochondrial Quality Control System Through PGC-1α in a Mouse Model of Parkinson’s Disease. Neurotox. Res. 2022, 40, 286–297. [Google Scholar] [CrossRef]
- Dilan, A.; James, E.; Laura, M.-S.; Gurvir, V.; Patricia, L.-G.; Anna, W.; Aaron, W.; Karishma, D.S.; Joanne, L.; Stephanie, S.; et al. GLP1 receptor agonism ameliorates Parkinson’s disease through modulation of neuronal insulin signalling and glial suppression. bioRxiv 2024. [Google Scholar] [CrossRef]
- Liu, C.; Liu, W.H.; Yang, W.; Chen, L.; Xue, Y.; Chen, X.Y. GLP-1 modulated the firing activity of nigral dopaminergic neurons in both normal and parkinsonian mice. Neuropharmacology 2024, 252, 109946. [Google Scholar] [CrossRef]
- Hogg, E.; Wu, T.; Gottuso, A.; Gold, D.; Tagliati, M. A Randomized, Double-blinded, Placebo-controlled Trial of Liraglutide in Patients with Parkinson’s Disease: Neuropsychological Outcomes. J. Int. Neuropsychol. Soc. 2023, 29, 109. [Google Scholar] [CrossRef]
- Manfready, R.A.; Forsyth, C.B.; Voigt, R.M.; Hall, D.A.; Goetz, C.G.; Keshavarzian, A. Gut-Brain Communication in Parkinson’s Disease: Enteroendocrine Regulation by GLP-1. Curr. Neurol. Neurosci. Rep. 2022, 22, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Saudou, F.; Humbert, S. The Biology of Huntingtin. Neuron 2016, 89, 910–926. [Google Scholar] [CrossRef]
- Chang, C.C.; Lin, T.C.; Ho, H.L.; Kuo, C.Y.; Li, H.H.; Korolenko, T.A.; Chen, W.J.; Lai, T.J.; Ho, Y.J.; Lin, C.L. GLP-1 Analogue Liraglutide Attenuates Mutant Huntingtin-Induced Neurotoxicity by Restoration of Neuronal Insulin Signaling. Int. J. Mol. Sci. 2018, 19, 2505. [Google Scholar] [CrossRef]
- Shawki, S.M.; Saad, M.A.; Rahmo, R.M.; Wadie, W.; El-Abhar, H.S. Liraglutide Improves Cognitive and Neuronal Function in 3-NP Rat Model of Huntington’s Disease. Front. Pharmacol. 2021, 12, 731483. [Google Scholar] [CrossRef] [PubMed]
- Martin, B.; Golden, E.; Carlson, O.D.; Pistell, P.; Zhou, J.; Kim, W.; Frank, B.P.; Thomas, S.; Chadwick, W.A.; Greig, N.H.; et al. Exendin-4 improves glycemic control, ameliorates brain and pancreatic pathologies, and extends survival in a mouse model of Huntington’s disease. Diabetes 2009, 58, 318–328. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.Y.; Wang, L.X.; Chen, Z.; Liu, L.B. Liraglutide prevents beta-amyloid-induced neurotoxicity in SH-SY5Y cells via a PI3K-dependent signaling pathway. Neurol. Res. 2016, 38, 313–319. [Google Scholar] [CrossRef]
- Panagaki, T.; Michael, M.; Hölscher, C. Liraglutide restores chronic ER stress, autophagy impairments and apoptotic signalling in SH-SY5Y cells. Sci. Rep. 2017, 7, 16158. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zhou, M.; Sun, J.; Xiong, H.; Peng, P.; Gu, Z.; Deng, Y. The protective effects of liraglutide on AD-like neurodegeneration induced by oxidative stress in human neuroblastoma SH-SY5Y cells. Chem. Biol. Interact. 2019, 310, 108688. [Google Scholar] [CrossRef]
- Carranza-Naval, M.J.; Del Marco, A.; Hierro-Bujalance, C.; Alves-Martinez, P.; Infante-Garcia, C.; Vargas-Soria, M.; Herrera, M.; Barba-Cordoba, B.; Atienza-Navarro, I.; Lubian-Lopez, S.; et al. Liraglutide Reduces Vascular Damage, Neuronal Loss, and Cognitive Impairment in a Mixed Murine Model of Alzheimer’s Disease and Type 2 Diabetes. Front. Aging Neurosci. 2021, 13, 741923. [Google Scholar] [CrossRef]
- Paladugu, L.; Gharaibeh, A.; Kolli, N.; Learman, C.; Hall, T.C.; Li, L.; Rossignol, J.; Maiti, P.; Dunbar, G.L. Liraglutide Has Anti-Inflammatory and Anti-Amyloid Properties in Streptozotocin-Induced and 5xFAD Mouse Models of Alzheimer’s Disease. Int. J. Mol. Sci. 2021, 22, 860. [Google Scholar] [CrossRef]
- McClean, P.L.; Jalewa, J.; Hölscher, C. Prophylactic liraglutide treatment prevents amyloid plaque deposition, chronic inflammation and memory impairment in APP/PS1 mice. Behav. Brain Res. 2015, 293, 96–106. [Google Scholar] [CrossRef]
- Chen, S.; Sun, J.; Zhao, G.; Guo, A.; Chen, Y.; Fu, R.; Deng, Y. Liraglutide Improves Water Maze Learning and Memory Performance While Reduces Hyperphosphorylation of Tau and Neurofilaments in APP/PS1/Tau Triple Transgenic Mice. Neurochem. Res. 2017, 42, 2326–2335. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Chen, S.; Peng, P.; Gu, Z.; Yu, J.; Zhao, G.; Deng, Y. Dulaglutide ameliorates STZ induced AD-like impairment of learning and memory ability by modulating hyperphosphorylation of tau and NFs through GSK3β. Biochem. Biophys. Res. Commun. 2019, 511, 154–160. [Google Scholar] [CrossRef]
- Li, Y.; Duffy, K.B.; Ottinger, M.A.; Ray, B.; Bailey, J.A.; Holloway, H.W.; Tweedie, D.; Perry, T.; Mattson, M.P.; Kapogiannis, D.; et al. GLP-1 receptor stimulation reduces amyloid-beta peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer’s disease. J. Alzheimer’s Dis. 2010, 19, 1205–1219. [Google Scholar] [CrossRef]
- Zhang, M.; Wu, Y.; Gao, R.; Chen, X.; Chen, R.; Chen, Z. Glucagon-like peptide-1 analogs mitigate neuroinflammation in Alzheimer’s disease by suppressing NLRP2 activation in astrocytes. Mol. Cell. Endocrinol. 2022, 542, 111529. [Google Scholar] [CrossRef]
- Wang, V.; Kuo, T.T.; Huang, E.Y.; Ma, K.H.; Chou, Y.C.; Fu, Z.Y.; Lai, L.W.; Jung, J.; Choi, H.I.; Choi, D.S.; et al. Sustained Release GLP-1 Agonist PT320 Delays Disease Progression in a Mouse Model of Parkinson’s Disease. ACS Pharmacol. Transl. Sci. 2021, 4, 858–869. [Google Scholar] [CrossRef] [PubMed]
- Robinson, A.; Lubitz, I.; Atrakchi-Baranes, D.; Licht-Murava, A.; Katsel, P.; Leroith, D.; Liraz-Zaltsman, S.; Haroutunian, V.; Beeri, M.S. Combination of Insulin with a GLP1 Agonist Is Associated with Better Memory and Normal Expression of Insulin Receptor Pathway Genes in a Mouse Model of Alzheimer’s Disease. J. Mol. Neurosci. 2019, 67, 504–510. [Google Scholar] [CrossRef]
- Ohtake, N.; Saito, M.; Eto, M.; Seki, K. Exendin-4 promotes the membrane trafficking of the AMPA receptor GluR1 subunit and ADAM10 in the mouse neocortex. Regul. Pept. 2014, 190–191, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.Y.; Yang, J.T.; Wang, Z.J.; Zhang, J.; Yang, W.; Wu, M.N.; Qi, J.S. Lixisenatide reduces amyloid plaques, neurofibrillary tangles and neuroinflammation in an APP/PS1/tau mouse model of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2018, 495, 1034–1040. [Google Scholar] [CrossRef]
- Liu, W.; Jalewa, J.; Sharma, M.; Li, G.; Li, L.; Hölscher, C. Neuroprotective effects of lixisenatide and liraglutide in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model of Parkinson’s disease. Neuroscience 2015, 303, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Jiang, R.; Yuan, Y.; Yu, Q.; Li, Y. Exendin-4 antagonizes Aβ1-42-induced suppression of long-term potentiation by regulating intracellular calcium homeostasis in rat hippocampal neurons. Brain Res. 2015, 1627, 101–108. [Google Scholar] [CrossRef]
- Zago, A.M.; Carvalho, F.B.; Rahmeier, F.L.; Santin, M.; Guimarães, G.R.; Gutierres, J.M.; da Cruz Fernandes, M. Exendin-4 Prevents Memory Loss and Neuronal Death in Rats with Sporadic Alzheimer-Like Disease. Mol. Neurobiol. 2024, 61, 2631–2652. [Google Scholar] [CrossRef]
- Garabadu, D.; Verma, J. Exendin-4 attenuates brain mitochondrial toxicity through PI3K/Akt-dependent pathway in amyloid beta (1-42)-induced cognitive deficit rats. Neurochem. Int. 2019, 128, 39–49. [Google Scholar] [CrossRef]
- Solmaz, V.; Çınar, B.P.; Yiğittürk, G.; Çavuşoğlu, T.; Taşkıran, D.; Erbaş, O. Exenatide reduces TNF-α expression and improves hippocampal neuron numbers and memory in streptozotocin treated rats. Eur. J. Pharmacol. 2015, 765, 482–487. [Google Scholar] [CrossRef]
- Chen, S.; Liu, A.R.; An, F.M.; Yao, W.B.; Gao, X.D. Amelioration of neurodegenerative changes in cellular and rat models of diabetes-related Alzheimer’s disease by exendin-4. Age 2012, 34, 1211–1224. [Google Scholar] [CrossRef]
- Li, Y.; Perry, T.; Kindy, M.S.; Harvey, B.K.; Tweedie, D.; Holloway, H.W.; Powers, K.; Shen, H.; Egan, J.M.; Sambamurti, K.; et al. GLP-1 receptor stimulation preserves primary cortical and dopaminergic neurons in cellular and rodent models of stroke and Parkinsonism. Proc. Natl. Acad. Sci. USA 2009, 106, 1285–1290. [Google Scholar] [CrossRef]
- Yu, S.J.; Chen, S.; Yang, Y.Y.; Glotfelty, E.J.; Jung, J.; Kim, H.K.; Choi, H.I.; Choi, D.S.; Hoffer, B.J.; Greig, N.H.; et al. PT320, Sustained-Release Exendin-4, Mitigates L-DOPA-Induced Dyskinesia in a Rat 6-Hydroxydopamine Model of Parkinson’s Disease. Front. Neurosci. 2020, 14, 785. [Google Scholar] [CrossRef]
- Keerie, A.; Brown-Wright, H.; Kirkland, I.; Grierson, A.; Alix, J.J.P.; Holscher, C.; Mead, R.J. The GLP-1 receptor agonist, liraglutide, fails to slow disease progression in SOD1(G93A) and TDP-43(Q331K) transgenic mouse models of ALS. Sci. Rep. 2021, 11, 17027. [Google Scholar] [CrossRef]
- Akimoto, H.; Negishi, A.; Oshima, S.; Wakiyama, H.; Okita, M.; Horii, N.; Inoue, N.; Ohshima, S.; Kobayashi, D. Antidiabetic Drugs for the Risk of Alzheimer Disease in Patients With Type 2 DM Using FAERS. Am. J. Alzheimer’s Dis. Other Dement. 2020, 35, 1533317519899546. [Google Scholar] [CrossRef] [PubMed]
- Nørgaard, C.H.; Friedrich, S.; Hansen, C.T.; Gerds, T.; Ballard, C.; Møller, D.V.; Knudsen, L.B.; Kvist, K.; Zinman, B.; Holm, E.; et al. Treatment with glucagon-like peptide-1 receptor agonists and incidence of dementia: Data from pooled double-blind randomized controlled trials and nationwide disease and prescription registers. Alzheimer’s Dement. 2022, 8, e12268. [Google Scholar] [CrossRef] [PubMed]
- Cukierman-Yaffe, T.; Gerstein, H.C.; Colhoun, H.M.; Diaz, R.; García-Pérez, L.E.; Lakshmanan, M.; Bethel, A.; Xavier, D.; Probstfield, J.; Riddle, M.C.; et al. Effect of dulaglutide on cognitive impairment in type 2 diabetes: An exploratory analysis of the REWIND trial. Lancet Neurol. 2020, 19, 582–590. [Google Scholar] [CrossRef] [PubMed]
- A Randomised Double-Blind Placebo-Controlled Clinical Study Investigating the Effects of Semaglutide s.c. Once-Weekly Versus Placebo on Central and Peripheral Inflammation in Participants with Alzheimer’s Disease. Phase 3, Interventional Clinical Trial. Available online: https://www.medifind.com/articles/clinical-trial/448266600 (accessed on 25 March 2025).
- Cummings, J.L.; Atri, A.; Feldman, H.H.; Hansson, O.; Sano, M.; Knop, F.K.; Johannsen, P.; León, T.; Scheltens, P. evoke and evoke+: Design of two large-scale, double-blind, placebo-controlled, phase 3 studies evaluating efficacy, safety, and tolerability of semaglutide in early-stage symptomatic Alzheimer’s disease. Alzheimer’s Res. Ther. 2025, 17, 14. [Google Scholar] [CrossRef]
- Koychev, I.; Adler, A.I.; Edison, P.; Tom, B.; Milton, J.E.; Butchart, J.; Hampshire, A.; Marshall, C.; Coulthard, E.; Zetterberg, H.; et al. Protocol for a double-blind placebo-controlled randomised controlled trial assessing the impact of oral semaglutide in amyloid positivity (ISAP) in community dwelling UK adults. BMJ Open 2024, 14, e081401. [Google Scholar] [CrossRef]
- Dei Cas, A.; Micheli, M.M.; Aldigeri, R.; Gardini, S.; Ferrari-Pellegrini, F.; Perini, M.; Messa, G.; Antonini, M.; Spigoni, V.; Cinquegrani, G.; et al. Long-acting exenatide does not prevent cognitive decline in mild cognitive impairment: A proof-of-concept clinical trial. J. Endocrinol. Investig. 2024, 47, 2339–2349. [Google Scholar] [CrossRef]
- Mullins, R.J.; Mustapic, M.; Chia, C.W.; Carlson, O.; Gulyani, S.; Tran, J.; Li, Y.; Mattson, M.P.; Resnick, S.; Egan, J.M.; et al. A Pilot Study of Exenatide Actions in Alzheimer’s Disease. Curr. Alzheimer Res. 2019, 16, 741–752. [Google Scholar] [CrossRef]
- Femminella, G.D.; Frangou, E.; Love, S.B.; Busza, G.; Holmes, C.; Ritchie, C.; Lawrence, R.; McFarlane, B.; Tadros, G.; Ridha, B.H.; et al. Evaluating the effects of the novel GLP-1 analogue liraglutide in Alzheimer’s disease: Study protocol for a randomised controlled trial (ELAD study). Trials 2019, 20, 191. [Google Scholar] [CrossRef]
- Vargas-Soria, M.; Carranza-Naval, M.J.; Del Marco, A.; Garcia-Alloza, M. Role of liraglutide in Alzheimer’s disease pathology. Alzheimer’s Res. Ther. 2021, 13, 112. [Google Scholar] [CrossRef]
- Edison, P. Evaluation of Novel GLP-1 analogue in the treatment of Alzheimer’s disease. Alzheimer’s Dement. 2025, 20, e089799. [Google Scholar] [CrossRef]
- Gejl, M.; Gjedde, A.; Egefjord, L.; Møller, A.; Hansen, S.B.; Vang, K.; Rodell, A.; Brændgaard, H.; Gottrup, H.; Schacht, A.; et al. In Alzheimer’s Disease, 6-Month Treatment with GLP-1 Analog Prevents Decline of Brain Glucose Metabolism: Randomized, Placebo-Controlled, Double-Blind Clinical Trial. Front. Aging Neurosci. 2016, 8, 108. [Google Scholar] [CrossRef]
- Gejl, M.; Brock, B.; Egefjord, L.; Vang, K.; Rungby, J.; Gjedde, A. Blood-Brain Glucose Transfer in Alzheimer’s disease: Effect of GLP-1 Analog Treatment. Sci. Rep. 2017, 7, 17490. [Google Scholar] [CrossRef] [PubMed]
- Javonillo, D.I.; Tran, K.M.; Phan, J.; Hingco, E.; Kramár, E.A.; da Cunha, C.; Forner, S.; Kawauchi, S.; Milinkeviciute, G.; Gomez-Arboledas, A.; et al. Systematic Phenotyping and Characterization of the 3xTg-AD Mouse Model of Alzheimer’s Disease. Front. Neurosci. 2021, 15, 785276. [Google Scholar] [CrossRef]
- Watson, K.T.; Wroolie, T.E.; Tong, G.; Foland-Ross, L.C.; Frangou, S.; Singh, M.; McIntyre, R.S.; Roat-Shumway, S.; Myoraku, A.; Reiss, A.L.; et al. Neural correlates of liraglutide effects in persons at risk for Alzheimer’s disease. Behav. Brain Res. 2019, 356, 271–278. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.H.; Fabricius, K.; Barkholt, P.; Kongsbak-Wismann, P.; Schlumberger, C.; Jelsing, J.; Terwel, D.; Termont, A.; Pyke, C.; Knudsen, L.B.; et al. Long-Term Treatment with Liraglutide, a Glucagon-Like Peptide-1 (GLP-1) Receptor Agonist, Has No Effect on β-Amyloid Plaque Load in Two Transgenic APP/PS1 Mouse Models of Alzheimer’s Disease. PLoS ONE 2016, 11, e0158205. [Google Scholar] [CrossRef]
- Koshatwar, M.; Acharya, S.; Prasad, R.; Lohakare, T.; Wanjari, M.; Taksande, A.B. Exploring the Potential of Antidiabetic Agents as Therapeutic Approaches for Alzheimer’s and Parkinson’s Diseases: A Comprehensive Review. Cureus 2023, 15, e44763. [Google Scholar] [CrossRef]
- Dong, M.; Wen, S.; Zhou, L. The Relationship Between the Blood-Brain-Barrier and the Central Effects of Glucagon-Like Peptide-1 Receptor Agonists and Sodium-Glucose Cotransporter-2 Inhibitors. Diabetes Metab. Syndr. Obes. 2022, 15, 2583–2597. [Google Scholar] [CrossRef]
- Achar, A.; Myers, R.; Ghosh, C. Drug Delivery Challenges in Brain Disorders across the Blood-Brain Barrier: Novel Methods and Future Considerations for Improved Therapy. Biomedicines 2021, 9, 1834. [Google Scholar] [CrossRef]
- Gabery, S.; Salinas, C.G.; Paulsen, S.J.; Ahnfelt-Rønne, J.; Alanentalo, T.; Baquero, A.F.; Buckley, S.T.; Farkas, E.; Fekete, C.; Frederiksen, K.S.; et al. Semaglutide lowers body weight in rodents via distributed neural pathways. JCI Insight 2020, 5, 1834. [Google Scholar] [CrossRef]
- Aroda, V.R.; Ahmann, A.; Cariou, B.; Chow, F.; Davies, M.J.; Jódar, E.; Mehta, R.; Woo, V.; Lingvay, I. Comparative efficacy, safety, and cardiovascular outcomes with once-weekly subcutaneous semaglutide in the treatment of type 2 diabetes: Insights from the SUSTAIN 1-7 trials. Diabetes Metab. 2019, 45, 409–418. [Google Scholar] [CrossRef]
- Kushner, R.F.; Calanna, S.; Davies, M.; Dicker, D.; Garvey, W.T.; Goldman, B.; Lingvay, I.; Thomsen, M.; Wadden, T.A.; Wharton, S.; et al. Semaglutide 2.4 mg for the Treatment of Obesity: Key Elements of the STEP Trials 1 to 5. Obesity 2020, 28, 1050–1061. [Google Scholar] [CrossRef]
- Aroda, V.R.; Bauer, R.; Christiansen, E.; Haluzík, M.; Kallenbach, K.; Montanya, E.; Rosenstock, J.; Meier, J.J. Efficacy and safety of oral semaglutide by subgroups of patient characteristics in the PIONEER phase 3 programme. Diabetes Obes. Metab. 2022, 24, 1338–1350. [Google Scholar] [CrossRef]
- Blonde, L.; Russell-Jones, D. The safety and efficacy of liraglutide with or without oral antidiabetic drug therapy in type 2 diabetes: An overview of the LEAD 1–5 studies. Diabetes Obes. Metab. 2009, 11 (Suppl. S3), 26–34. [Google Scholar] [CrossRef] [PubMed]
- Ard, J.; Cannon, A.; Lewis, C.E.; Lofton, H.; Vang Skjøth, T.; Stevenin, B.; Pi-Sunyer, X. Efficacy and safety of liraglutide 3.0 mg for weight management are similar across races: Subgroup analysis across the SCALE and phase II randomized trials. Diabetes Obes. Metab. 2016, 18, 430–435. [Google Scholar] [CrossRef]
- Jendle, J.; Grunberger, G.; Blevins, T.; Giorgino, F.; Hietpas, R.T.; Botros, F.T. Efficacy and safety of dulaglutide in the treatment of type 2 diabetes: A comprehensive review of the dulaglutide clinical data focusing on the AWARD phase 3 clinical trial program. Diabetes Metab. Res. Rev. 2016, 32, 776–790. [Google Scholar] [CrossRef] [PubMed]
- Grimm, M.; Han, J.; Weaver, C.; Griffin, P.; Schulteis, C.T.; Dong, H.; Malloy, J. Efficacy, safety, and tolerability of exenatide once weekly in patients with type 2 diabetes mellitus: An integrated analysis of the DURATION trials. Postgrad. Med. 2013, 125, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Klonoff, D.C.; Buse, J.B.; Nielsen, L.L.; Guan, X.; Bowlus, C.L.; Holcombe, J.H.; Wintle, M.E.; Maggs, D.G. Exenatide effects on diabetes, obesity, cardiovascular risk factors and hepatic biomarkers in patients with type 2 diabetes treated for at least 3 years. Curr. Med. Res. Opin. 2008, 24, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Feng, W.; Wang, W.; Meng, R.; Wu, G.; Zhang, M.; Zhang, X.; Yin, H.; Zhu, D. Lixisenatide is effective and safe as add-on treatment to basal insulin in Asian individuals with type 2 diabetes and different body mass indices: A pooled analysis of data from the GetGoal Studies. BMJ Open Diabetes Res. Care 2021, 9, e002290. [Google Scholar] [CrossRef]
- Smits, M.M.; Van Raalte, D.H. Safety of Semaglutide. Front. Endocrinol. 2021, 12, 645563. [Google Scholar] [CrossRef]
- Bettge, K.; Kahle, M.; Abd El Aziz, M.S.; Meier, J.J.; Nauck, M.A. Occurrence of nausea, vomiting and diarrhoea reported as adverse events in clinical trials studying glucagon-like peptide-1 receptor agonists: A systematic analysis of published clinical trials. Diabetes Obes. Metab. 2017, 19, 336–347. [Google Scholar] [CrossRef] [PubMed]
- Shetty, R.; Basheer, F.T.; Poojari, P.G.; Thunga, G.; Chandran, V.P.; Acharya, L.D. Adverse drug reactions of GLP-1 agonists: A systematic review of case reports. Diabetes Metab. Syndr. 2022, 16, 102427. [Google Scholar] [CrossRef]
- Consoli, A.; Formoso, G. Potential side effects to GLP-1 agonists: Understanding their safety and tolerability. Expert Opin. Drug Saf. 2015, 14, 207–218. [Google Scholar] [CrossRef]
- Jespersen, M.J.; Knop, F.K.; Christensen, M. GLP-1 agonists for type 2 diabetes: Pharmacokinetic and toxicological considerations. Expert Opin. Drug Metab. Toxicol. 2013, 9, 17–29. [Google Scholar] [CrossRef]
- Aviles-Olmos, I.; Dickson, J.; Kefalopoulou, Z.; Djamshidian, A.; Ell, P.; Soderlund, T.; Whitton, P.; Wyse, R.; Isaacs, T.; Lees, A.; et al. Exenatide and the treatment of patients with Parkinson’s disease. J. Clin. Investig. 2013, 123, 2730–2736. [Google Scholar] [CrossRef] [PubMed]
- Athauda, D.; Maclagan, K.; Skene, S.S.; Bajwa-Joseph, M.; Letchford, D.; Chowdhury, K.; Hibbert, S.; Budnik, N.; Zampedri, L.; Dickson, J.; et al. Exenatide once weekly versus placebo in Parkinson’s disease: A randomised, double-blind, placebo-controlled trial. Lancet 2017, 390, 1664–1675. [Google Scholar] [CrossRef]
- Vijiaratnam, N.; Girges, C.; Auld, G.; McComish, R.; King, A.; Skene, S.S.; Hibbert, S.; Wong, A.; Melander, S.; Gibson, R.; et al. Exenatide once a week versus placebo as a potential disease-modifying treatment for people with Parkinson’s disease in the UK: A phase 3, multicentre, double-blind, parallel-group, randomised, placebo-controlled trial. Lancet 2025, 405, 627–636. [Google Scholar] [CrossRef]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood-brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Masoudi Asil, S.; Ahlawat, J.; Guillama Barroso, G.; Narayan, M. Nanomaterial based drug delivery systems for the treatment of neurodegenerative diseases. Biomater. Sci. 2020, 8, 4109–4128. [Google Scholar] [CrossRef]
- Stokes, J.M.; Yang, K.; Swanson, K.; Jin, W.; Cubillos-Ruiz, A.; Donghia, N.M.; MacNair, C.R.; French, S.; Carfrae, L.A.; Bloom-Ackermann, Z.; et al. A Deep Learning Approach to Antibiotic Discovery. Cell 2020, 181, 475–483. [Google Scholar] [CrossRef]
- Taléns-Visconti, R.; de Julián-Ortiz, J.V.; Vila-Busó, O.; Diez-Sales, O.; Nácher, A. Intranasal Drug Administration in Alzheimer-Type Dementia: Towards Clinical Applications. Pharmaceutics 2023, 15, 1399. [Google Scholar] [CrossRef]
- Deng, S.; Chen, Z.; Shi, Y. Roles of glucagon-like peptide 1 receptor agonists in immune cell biology and autoimmune/autoinflammatory diseases. Cell Biosci. 2025, 15, 137. [Google Scholar] [CrossRef]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290.e1217. [Google Scholar] [CrossRef]
- Dangol, M.; Kim, S.; Li, C.G.; Fakhraei Lahiji, S.; Jang, M.; Ma, Y.; Huh, I.; Jung, H. Anti-obesity effect of a novel caffeine-loaded dissolving microneedle patch in high-fat diet-induced obese C57BL/6J mice. J. Control. Release 2017, 265, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Olah, M.; Menon, V.; Habib, N.; Taga, M.F.; Ma, Y.; Yung, C.J.; Cimpean, M.; Khairallah, A.; Coronas-Samano, G.; Sankowski, R.; et al. Single cell RNA sequencing of human microglia uncovers a subset associated with Alzheimer’s disease. Nat. Commun. 2020, 11, 6129. [Google Scholar] [CrossRef]
- Rayon, T.; Maizels, R.J.; Barrington, C.; Briscoe, J. Single-cell transcriptome profiling of the human developing spinal cord reveals a conserved genetic programme with human-specific features. Development 2021, 148, dev199711. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Tang, X.; Qin, D.; Yu, L.; Zhou, X.; Feng, C.; Mi, J.; Pan, H.; Wu, J.; Huang, B.; et al. Engineered GLP-1R-targeting nanoplatforms: Multimodal therapeutics in human diseases. J. Nanobiotechnol. 2025, 23, 682. [Google Scholar] [CrossRef]
- Xin, H.; Jiang, X.; Gu, J.; Sha, X.; Chen, L.; Law, K.; Chen, Y.; Wang, X.; Jiang, Y.; Fang, X. Angiopep-conjugated poly(ethylene glycol)-co-poly(ε-caprolactone) nanoparticles as dual-targeting drug delivery system for brain glioma. Biomaterials 2011, 32, 4293–4305. [Google Scholar] [CrossRef]
- Xin, H.; Sha, X.; Jiang, X.; Zhang, W.; Chen, L.; Fang, X. Anti-glioblastoma efficacy and safety of paclitaxel-loading Angiopep-conjugated dual targeting PEG-PCL nanoparticles. Biomaterials 2012, 33, 8167–8176. [Google Scholar] [CrossRef]
- Hoyos-Ceballos, G.P.; Ruozi, B.; Ottonelli, I.; Da Ros, F.; Vandelli, M.A.; Forni, F.; Daini, E.; Vilella, A.; Zoli, M.; Tosi, G.; et al. PLGA-PEG-ANG-2 Nanoparticles for Blood-Brain Barrier Crossing: Proof-of-Concept Study. Pharmaceutics 2020, 12, 72. [Google Scholar] [CrossRef]
- Habib, S.; Singh, M. Angiopep-2-Modified Nanoparticles for Brain-Directed Delivery of Therapeutics: A Review. Polymers 2022, 14, 712. [Google Scholar] [CrossRef]
- Abdulhameed, N.; Babin, A.; Hansen, K.; Weaver, R.; Banks, W.A.; Talbot, K.; Rhea, E.M. Comparing regional brain uptake of incretin receptor agonists after intranasal delivery in CD-1 mice and the APP/PS1 mouse model of Alzheimer’s disease. Alzheimer’s Res. Ther. 2024, 16, 173. [Google Scholar] [CrossRef] [PubMed]
- Hanson, L.R.; Frey, W.H., II. Intranasal delivery bypasses the blood-brain barrier to target therapeutic agents to the central nervous system and treat neurodegenerative disease. BMC Neurosci. 2008, 9 (Suppl. S3), S5. [Google Scholar] [CrossRef]
- Yun, S.P.; Kam, T.I.; Panicker, N.; Kim, S.; Oh, Y.; Park, J.S.; Kwon, S.H.; Park, Y.J.; Karuppagounder, S.S.; Park, H.; et al. Block of A1 astrocyte conversion by microglia is neuroprotective in models of Parkinson’s disease. Nat. Med. 2018, 24, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Aviles-Olmos, I.; Dickson, J.; Kefalopoulou, Z.; Djamshidian, A.; Kahan, J.; Ell, P.; Whitton, P.; Wyse, R.; Isaacs, T.; Lees, A.; et al. Motor and cognitive advantages persist 12 months after exenatide exposure in Parkinson’s disease. J. Park. Dis. 2014, 4, 337–344. [Google Scholar] [CrossRef] [PubMed]

| Brain Area | Human [Relevant Reference [18]] | Non-Human Primate [Relevant Reference [3]] | Rodents [Relevant Reference [36]] |
|---|---|---|---|
| Cerebral Cortex | Present in all areas except in orbitofrontal cortex | Absent | Absent, except in mouse prefrontal cortex |
| Hippocampus | Present | Present | Present |
| Cerebellum | Absent | Present | Present |
| Hypothalamus | Present | Most abundant expression | Most abundant expression |
| Amygdala, Thalamus and Brain Stem | Present | Present | Present |
| Disease Model | Species/Strain | Compound/Formulation | Dose & Route | Treatment Duration | Main Outcomes | Reference |
|---|---|---|---|---|---|---|
| Alzheimer’s disease (APP/PS1) | Mouse | Liraglutide (Victoza®, i.p.) | 25 nmol/kg daily, i.p. | 8–12 weeks | ↓ Aβ plaque load; ↑ synaptic density; improved Morris water maze performance | [59] |
| Alzheimer’s disease (3xTg-AD) | Mouse | Exenatide (Byetta®, i.p.) | 0.1 μg/g (100 μg/kg) daily | 9 months | No effects on memory performance, Aβ or tau pathology in 3xTg-AD mice | [61] |
| Parkinson’s disease (MPTP-induced) | Mouse | Exenatide (synthetic, i.p.) | 10 µg/kg daily, i.p. | 7 days | Preserved TH+ neurons; improved rotarod and open-field scores | [103] |
| Parkinson’s disease (6-OHDA-induced) | Rat | PT320 (sustained-release exenatide, s.c.) | 100 mg/kg weekly (containing 2 mg/kg exendin-4) | 3 weeks | ↓ L-DOPA-induced dyskinesia; normalized DA turnover | [104] |
| Amyotrophic lateral sclerosis (SOD1^G93A) | Mouse | Liraglutide, i.p. | 25 nmol/kg daily i.p. | From 50 days of age until endpoint | No effect on disease progression, motor neuron counts, glial activation, or survival | [105] |
| Huntington’s disease (R6/2) | Mouse | Exendin-4, i.p. | 10 µg/kg daily | 8–12 weeks | Improved motor coordination; ↓ neuronal inclusion bodies; extended survival | [82] |
| Knowledge Gap | Scientific/Clinical Challenge | Proposed Future Direction |
|---|---|---|
| Blood–brain barrier penetration and receptor occupancy | Limited in vivo evidence confirming central target engagement in humans | Apply radiolabeled ligand PET and CSF biomarker studies in early-phase trials |
| Species-specific receptor distribution | Rodent–human divergence in GLP-1R localization and signaling bias | Employ humanized or non-human primate models; correlate expression with pharmacodynamics |
| Heterogeneity in clinical outcomes | Variation in disease stage, comorbid diabetes, and APOE genotype | Stratified trial designs and AI-driven patient selection |
| Biomarker inconsistency | Weak linkage between imaging, CSF biomarkers, and cognition | Harmonize biomarker endpoints and integrate multi-modal readouts |
| Long-term safety in non-diabetic elderly populations | Possible GI, cardiovascular, or weight-loss adverse effects | Longitudinal registries and real-world pharmacovigilance |
| Translational modeling | Preclinical success not reliably predictive of human efficacy | Systems-level modeling of neuro-metabolic networks; combination therapy trials |
| Diversity and generalizability | Underrepresentation of non-European populations in trials | Expand recruitment across global cohorts to ensure external validity |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moaket, O.S.; Obaid, S.E.; Obaid, F.E.; Shakeeb, Y.A.; Elsharief, S.M.; Tania, A.; Darwish, R.; Butler, A.E.; Moin, A.S.M. GLP-1 and the Degenerating Brain: Exploring Mechanistic Insights and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 10743. https://doi.org/10.3390/ijms262110743
Moaket OS, Obaid SE, Obaid FE, Shakeeb YA, Elsharief SM, Tania A, Darwish R, Butler AE, Moin ASM. GLP-1 and the Degenerating Brain: Exploring Mechanistic Insights and Therapeutic Potential. International Journal of Molecular Sciences. 2025; 26(21):10743. https://doi.org/10.3390/ijms262110743
Chicago/Turabian StyleMoaket, Osama Sobhi, Sarah Eyad Obaid, Fawaz Eyad Obaid, Yusuf Abdulkarim Shakeeb, Samir Mohammed Elsharief, Afrin Tania, Radwan Darwish, Alexandra E. Butler, and Abu Saleh Md Moin. 2025. "GLP-1 and the Degenerating Brain: Exploring Mechanistic Insights and Therapeutic Potential" International Journal of Molecular Sciences 26, no. 21: 10743. https://doi.org/10.3390/ijms262110743
APA StyleMoaket, O. S., Obaid, S. E., Obaid, F. E., Shakeeb, Y. A., Elsharief, S. M., Tania, A., Darwish, R., Butler, A. E., & Moin, A. S. M. (2025). GLP-1 and the Degenerating Brain: Exploring Mechanistic Insights and Therapeutic Potential. International Journal of Molecular Sciences, 26(21), 10743. https://doi.org/10.3390/ijms262110743






