Spatiotemporal Distribution Patterns of Osthole and Expression Correlation of the MOT1 Homologue in Cultivated Angelica biserrata
Abstract
1. Introduction
2. Results
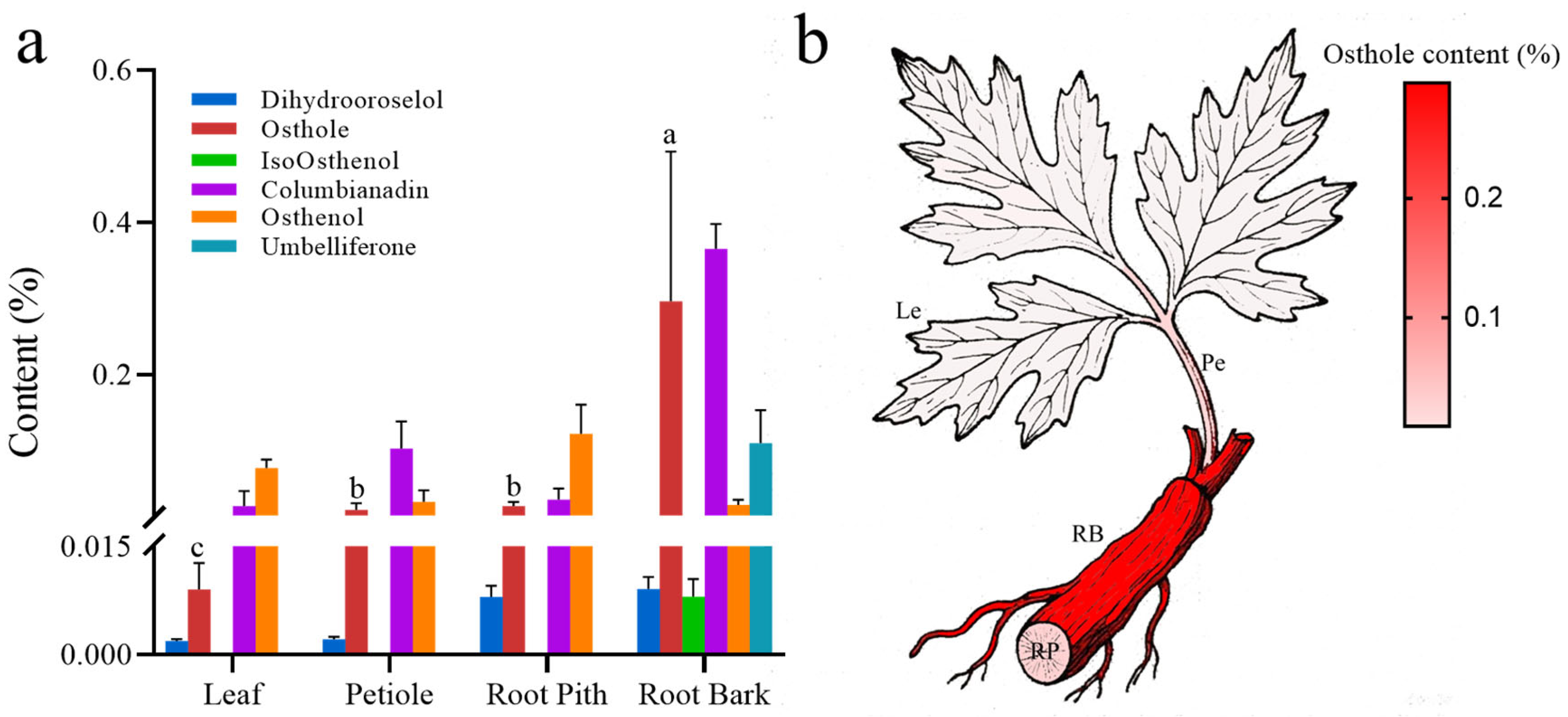
2.1. Hierarchical Accumulation of Osthole as a Functional Metabolite
2.2. Metabolic Variability and Core Germplasm Identification
2.3. Temporal Dynamics and Environmental Induction of Osthole Biosynthesis
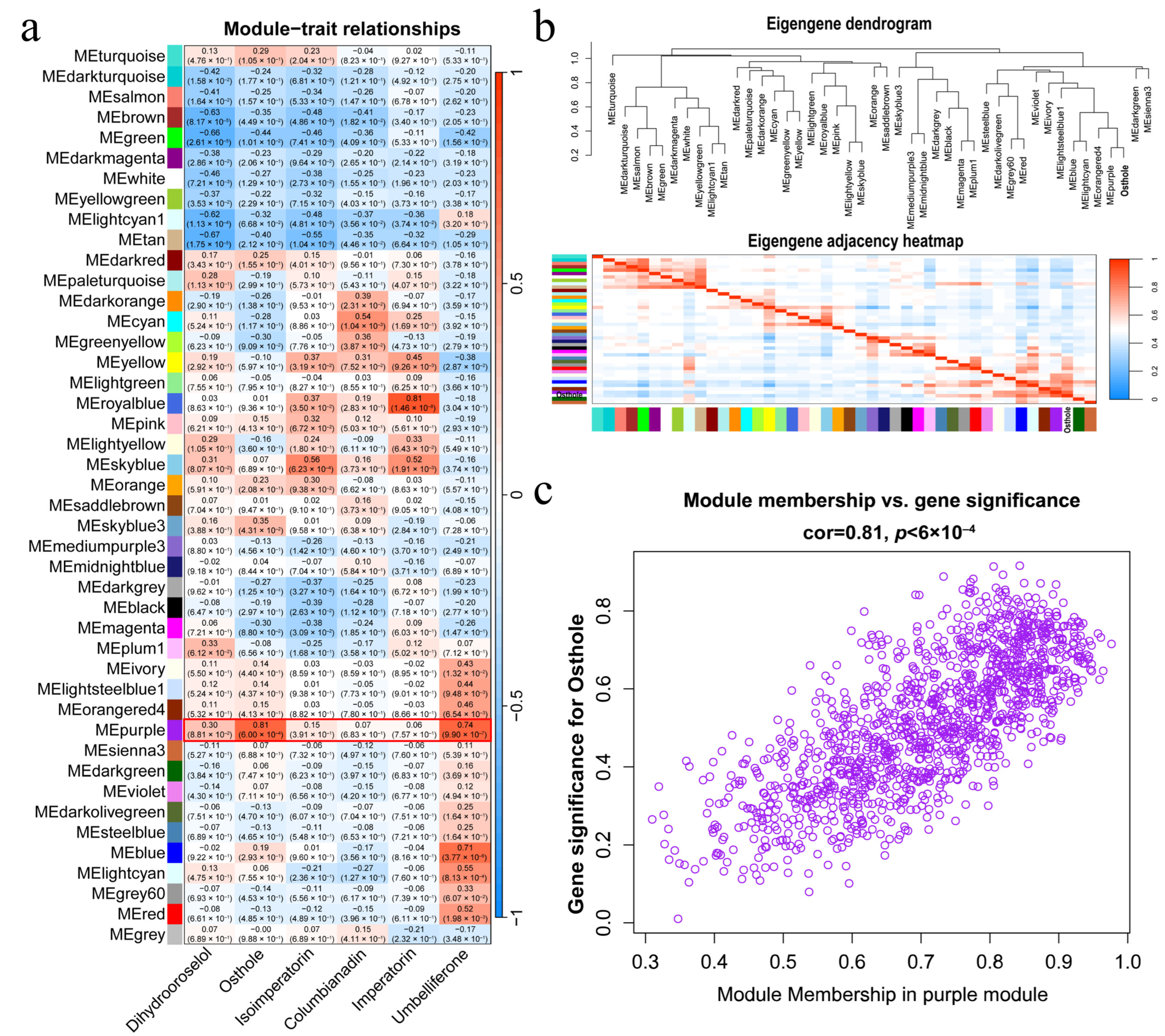
2.4. Transcriptomic Profiling and Co-Expression Network Analysis of A. biserrata
2.5. Identification of AbOMT1 as a Central Hub Gene in the Osthole-Associated Co-Expression Module
3. Discussion
3.1. Spatiotemporal Heterogeneity in Osthole Accumulation
3.2. Expression of the AbOMT1 Correlates with Osthole Accumulation and Chemotype Stability
3.3. Frost Stress Association with Osthole Levels and AbOMT1 Expression
4. Materials and Methods
4.1. Plant Materials
4.2. Sample Collection for RNA Sequencing and Metabolomic Profiling
4.3. Metabolite Extraction and Quantification
4.4. Transcriptome Profiling and Co-Expression Network Analysis
4.5. Statistical Analysis of Metabolite Variation and Sample Classification
4.6. qRT-Pcr Validation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| COX | Coumarin oxygenase |
| CV | Coefficient of variation |
| DEGs | Differentially expressed genes |
| FPKM | Fragments per kilobase transcript per million |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LN | Liquid nitrogen |
| OMT | O-methyltransferase |
| PCA | Principal component analysis |
| qRT-PCR | Quantitative real-time polymerase chain reaction |
| SAM | S-adenosyl methionine |
| WGCNA | Weighted gene co-expression network analysis |
References
- Ji, J.; Han, X.; Zang, L.; Li, Y.; Lin, L.; Hu, D.; Sun, S.; Ren, Y.; Maker, G.; Lu, Z.; et al. Integrative multi-omics data provide insights into the biosynthesis of furanocoumarins and mechanisms regulating their accumulation in Angelica dahurica. Commun. Biol. 2025, 8, 649. [Google Scholar] [CrossRef]
- Tan, Z.; Yuan, Y.; Huang, S.; Ma, Y.; Hong, Z.; Wang, Y.; Wu, X.; Li, Z.; Ye, J.; Zhang, L. Geographical distribution and predict potential distribution of Angelica, L. genus. Environ. Sci. Pollut. Res. Int. 2023, 30, 46562–46573. [Google Scholar] [CrossRef]
- Yu, C.; Xie, T.; Liu, S.; Bai, L. Fabrication of a biochar-doped monolithic adsorbent and its application for the extraction and determination of coumarins from Angelicae Pubescentis Radix. J. Chromatogr. A 2024, 1714, 464564. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Ji, T.; Lv, W.; Zhang, J.; Hao, X.; Ling, X. Economic and facile extraction and analysis of Osthole in Fructus cnidii for large-scale production. Anal. Methods 2025, 17, 3953–3962. [Google Scholar] [CrossRef] [PubMed]
- Ai, C.; Miao, X.; Wang, L.; He, J. Discovery and Activity Evaluation of the Inhibitory Effect of Four Kinds of Traditional Chinese Medicine Extracts on the CYP3A4 Enzyme. Comb. Chem. High Throughput Screen. 2023, 26, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.W.; Yeh, W.L.; Charoensaensuk, V.; Lin, C.; Yang, L.Y.; Chen, M.K.; Yeh, T.; Tsai, C.F.; Lu, D.Y. Oral administration of osthole mitigates maladaptive behaviors through PPARα activation in mice subjected to repeated social defeat stress. Neurochem. Int. 2024, 179, 105811. [Google Scholar] [CrossRef]
- Li, S.H.; Li, M.Y.; Yuan, T.T.; Wang, G.W.; Zeng, J.B.; Shi, Z.; Liu, J.H.; Su, J.C. Osthole Activates the Cholinergic Anti-Inflammatory Pathway via α7nAChR Upregulation to Alleviate Inflammatory Responses. Chem. Biodivers. 2024, 21, e202400290. [Google Scholar] [CrossRef]
- Lv, X.; Yang, H.; Zhong, H.; He, L.; Wang, L. Osthole exhibits an antitumor effect in retinoblastoma through inhibiting the PI3K/AKT/mTOR pathway via regulating the hsa_circ_0007534/miR-214-3p axis. Pharm. Biol. 2022, 60, 417–426. [Google Scholar] [CrossRef]
- Yang, S.; Dai, W.; Wang, J.; Zhang, X.; Zheng, Y.; Bi, S.; Pang, L.; Ren, T.; Yang, Y.; Sun, Y.; et al. Osthole: An up-to-date review of its anticancer potential and mechanisms of action. Front. Pharmacol. 2022, 13, 945627. [Google Scholar] [CrossRef]
- Lin, H.; You, Q.; Wei, X.; Chen, Z.; Wang, X. Osthole, a Coumarin from Cnidium monnieri: A Review on Its Pharmacology, Pharmacokinetics, Safety, and Innovative Drug Delivery Platforms. Am. J. Chin. Med. 2024, 52, 1397–1425. [Google Scholar] [CrossRef]
- Chen, J.; Liao, X.; Gan, J. Review on the protective activity of osthole against the pathogenesis of osteoporosis. Front. Pharmacol. 2023, 14, 1236893. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Chen, X.; Men, X.; Li, Z.; Kong, Y.; Yuan, Y.; Ge, F. Contact Toxicity, Antifeedant Activity, and Oviposition Preference of Osthole against Agricultural Pests. Insects 2023, 14, 725. [Google Scholar] [CrossRef] [PubMed]
- Hao, M.; Jiang, L.; Lv, M.; Ding, H.; Zhou, Y.; Xu, H. Plant natural product-based pesticides in crop protection: Semi-synthesis, mono-crystal structures and agrochemical activities of osthole ester derivatives, and study of their toxicology against Tetranychus cinnabarinus (Boisduval). Pest Manag. Sci. 2024, 80, 6356–6365. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Feng, L.; Sun, C.; Wang, L. Visualizing the Spatial Distribution of Metabolites in Angelica sinensis Roots by Matrix-Assisted Laser Desorption/Ionization Mass Spectrometry Imaging. Phytochem. Anal. 2025, 36, 1245–1251. [Google Scholar] [CrossRef]
- Li, S.; Chiu, T.Y.; Jin, X.; Cao, D.; Xu, M.; Zhu, M.; Zhou, Q.; Liu, C.; Zong, Y.; Wang, S.; et al. Integrating genomic and multiomic data for Angelica sinensis provides insights into the evolution and biosynthesis of pharmaceutically bioactive compounds. Commun. Biol. 2023, 6, 1198. [Google Scholar] [CrossRef]
- Wu, Q.; Jiang, L.; Yan, Y.; Yan, Q.; Zhu, X.; Zhang, J.; Huang, C.; Zhou, T.; Ren, C.; Wen, F.; et al. Geographical distribution-based differentiation of cultivated Angelica dahurica, exploring the relationship between the secretory tract and the quality. Sci. Rep. 2023, 13, 21733. [Google Scholar] [CrossRef]
- Li, X.; Zhang, C.; Wu, E.; Han, L.; Deng, X.; Shi, Z. UPLC-Q-TOF/MS-Based Metabolomics Approach Reveals Osthole Intervention in Breast Cancer 4T1 Cells. Int. J. Mol. Sci. 2023, 24, 1168. [Google Scholar] [CrossRef]
- Gao, H.; Li, Q. Study on the spatial distribution of coumarins in Angelica dahurica root by MALDI-TOF-MSI. Phytochem. Anal. 2023, 34, 139–148. [Google Scholar] [CrossRef]
- Ji, J.; Zang, L.; Lu, T.; Li, C.; Han, X.; Lee, S.R.; Wang, L. Widely targeted metabolomics analysis reveals differences in volatile metabolites among four Angelica species. Nat. Prod. Bioprospecting 2025, 15, 2. [Google Scholar] [CrossRef]
- Wu, Q.; Yan, Q.; Jiang, L.; Chen, C.; Huang, X.; Zhu, X.; Zhou, T.; Chen, J.; Yan, J.; Wen, F.; et al. Metabolomics analysis reveals metabolite changes during freeze-drying and oven-drying of Angelica dahurica. Sci. Rep. 2023, 13, 6022. [Google Scholar] [CrossRef]
- Jiang, J.; Fan, G.; Wen, R.; Liu, T.; He, S.; Yang, S.; Zi, S. Effects of osthole and Bacillus amyloliquefaciens on the physiological growth of Panax quinquefolius in a forest. Front. Microbiol. 2024, 15, 1497987. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Y.; Gao, H.; Li, Z.; Qiu, D.; Hu, G. In situ analysis of volatile oil in Angelica sinensis roots by fluorescence imaging combined with mass spectrometry imaging. Talanta 2023, 255, 124253. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.; Liu, S.; Tang, X.; Jia, G.; Yang, S.; Ren, C.; Pei, J. Integrative analysis of metabolite and transcriptome reveals the biosynthetic pathway and candidate genes for iridoid glycoside biosynthesis in Neopicrorhiza scrophulariiflora (Pennell) D.Y.Hong. Front. Plant Sci. 2025, 16, 1527477. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ge, W.; Miao, Z. Integrative metabolomic and transcriptomic analyses reveals the accumulation patterns of key metabolites associated with flavonoids and terpenoids of Gynostemma pentaphyllum (Thunb.) Makino. Sci. Rep. 2024, 14, 8644. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.C.; Tang, H.; Wei, X.; He, Y.; Hu, S.; Wu, J.Y.; Xu, D.; Qiao, F.; Xue, J.Y.; Zhao, Y. The gradual establishment of complex coumarin biosynthetic pathway in Apiaceae. Nat. Commun. 2024, 15, 6864. [Google Scholar] [CrossRef]
- He, J.; Yao, L.; Pecoraro, L.; Liu, C.; Wang, J.; Huang, L.; Gao, W. Cold stress regulates accumulation of flavonoids and terpenoids in plants by phytohormone, transcription process, functional enzyme, and epigenetics. Crit. Rev. Biotechnol. 2023, 43, 680–697. [Google Scholar] [CrossRef]
- Eom, S.H.; Ahn, M.A.; Kim, E.; Lee, H.J.; Lee, J.H.; Wi, S.H.; Kim, S.K.; Lim, H.B.; Hyun, T.K. Plant Response to Cold Stress: Cold Stress Changes Antioxidant Metabolism in Heading Type Kimchi Cabbage (Brassica rapa L. ssp. Pekinensis). Antioxidants 2022, 11, 700. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Ma, Y.; Gao, R.; Wang, Y.; An, Z.; Tian, Y.; Wan, H.; Wei, D.; Wang, F.; et al. Lonicera caerulea genome reveals molecular mechanisms of freezing tolerance and anthocyanin biosynthesis. J. Adv. Res. 2024, 76, 293–305. [Google Scholar] [CrossRef]
- He, Y.; Zhang, J.; He, Y.; Liu, H.; Wang, C.; Guan, G.; Zhao, Y.; Tian, Y.; Zhong, X.; Lu, X. Two O-methyltransferases are responsible for multiple O-methylation steps in the biosynthesis of furanocoumarins from Angelica decursiva. Plant Physiol. Biochem. 2023, 204, 108142. [Google Scholar] [CrossRef]
- Koeduka, T.; Watanabe, B.; Shirahama, K.; Nakayasu, M.; Suzuki, S.; Furuta, T.; Suzuki, H.; Matsui, K.; Kosaka, T.; Ozaki, S.I. Biosynthesis of dillapiole/apiole in dill (Anethum graveolens): Characterization of regioselective phenylpropene O-methyltransferase. Plant J. 2023, 113, 562–575. [Google Scholar] [CrossRef]
- Zhan, C.; Lei, L.; Guo, H.; Zhou, S.; Xu, C.; Liu, Z.; Wu, Z.; Deng, Y.; Miao, Y.; Han, Y.; et al. Disease resistance conferred by components of essential chrysanthemum oil and the epigenetic regulation of OsTPS1. Sci. China Life Sci. 2023, 66, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Yan, T.; Sun, X.; Wilson, I.; Li, G.; Hong, Z.; Shao, F.; Qiu, D. Identification and characterization of two O-methyltransferases involved in biosynthesis of methylated 2-(2-phenethyl) chromones in agarwood. J. Exp. Bot. 2024, 75, 3452–3466. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Zhao, L.; Ma, W.; Peng, Z.; Shi, J.; Pan, F.; Gao, Y.; Sui, X.; Rengel, Z.; Chen, Q.; et al. Ethylene inhibits ABA-induced stomatal closure via regulating NtMYB184-mediated flavonol biosynthesis in tobacco. J. Exp. Bot. 2023, 74, 6735–6748. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Ding, Y.; Shi, Y.; Ma, L.; Wang, Y.; Song, C.; Wilkins, K.A.; Davies, J.M.; Knight, H.; Knight, M.R.; et al. The calcium transporter ANNEXIN1 mediates cold-induced calcium signaling and freezing tolerance in plants. EMBO J. 2021, 40, e104559. [Google Scholar] [CrossRef]
- Kang, X.; Wei, F.; Chai, S.; Peng, S.; Huang, B.; Han, Q.; Zhao, T.; Zhang, P.; Tian, Y.; Xia, R.; et al. The OST1-HOS1-HAT1 module regulates cold response in Arabidopsis thaliana. New Phytol. 2025, 247, 209–223. [Google Scholar] [CrossRef]
- Huang, S.; Wang, H.; Liu, S.; Lu, S.; Hua, J.; Zou, B. Ethylene antagonizes ABA and inhibits stomatal closure and chilling tolerance in rice. J. Exp. Bot. 2025, eraf052. [Google Scholar] [CrossRef]
- Wang, X.; Lu, W.; Zhao, Z.; Hao, W.; Du, R.; Li, Z.; Wang, Z.; Lv, X.; Wang, J.; Liang, D.; et al. Abscisic acid promotes selenium absorption, metabolism and toxicity via stress-related phytohormones regulation in Cyphomandra betacea Sendt. (Solanum betaceum Cav.). J. Hazard. Mater. 2024, 461, 132642. [Google Scholar] [CrossRef]
- An, S.; Liu, Y.; Sang, K.; Wang, T.; Yu, J.; Zhou, Y.; Xia, X. Brassinosteroid signaling positively regulates abscisic acid biosynthesis in response to chilling stress in tomato. J. Integr. Plant Biol. 2023, 65, 10–24. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, K.; Yang, Y.; Luo, Y.; Jiang, X.; Guo, J.; Guo, X. Spatiotemporal Distribution Patterns of Osthole and Expression Correlation of the MOT1 Homologue in Cultivated Angelica biserrata. Int. J. Mol. Sci. 2025, 26, 10746. https://doi.org/10.3390/ijms262110746
Yu K, Yang Y, Luo Y, Jiang X, Guo J, Guo X. Spatiotemporal Distribution Patterns of Osthole and Expression Correlation of the MOT1 Homologue in Cultivated Angelica biserrata. International Journal of Molecular Sciences. 2025; 26(21):10746. https://doi.org/10.3390/ijms262110746
Chicago/Turabian StyleYu, Kaidi, Yuying Yang, Yuan Luo, Xiaogang Jiang, Jie Guo, and Xiaoliang Guo. 2025. "Spatiotemporal Distribution Patterns of Osthole and Expression Correlation of the MOT1 Homologue in Cultivated Angelica biserrata" International Journal of Molecular Sciences 26, no. 21: 10746. https://doi.org/10.3390/ijms262110746
APA StyleYu, K., Yang, Y., Luo, Y., Jiang, X., Guo, J., & Guo, X. (2025). Spatiotemporal Distribution Patterns of Osthole and Expression Correlation of the MOT1 Homologue in Cultivated Angelica biserrata. International Journal of Molecular Sciences, 26(21), 10746. https://doi.org/10.3390/ijms262110746




