Abstract
Chitosan (CS) is a biodegradable and biocompatible polysaccharide, obtained by the deacetylation of chitin. It has gained significant attention as a versatile material for biomedical applications due to its mucoadhesive properties, ease of chemical modification and intrinsic pharmacological activities. This review synthesizes two decades (2005–2025) of literature, focusing on chemical modifications of chitosan for pharmacological purposes and their therapeutic implications in non-communicable diseases (NCDs) and wound healing. Evidence highlights the roles of chitosan-based materials in anticancer, anti-inflammatory, antidiabetic, antihypertensive, and neuroprotective activities, alongside their integration in advanced wound healing strategies. Clinical trials have demonstrated the translational potential of chitosan-based materials. In general, chitosan-based materials exhibit promising dual functions as bioactive agents and drug carriers, necessitating additional investigation in clinical and regulatory frameworks to accelerate therapeutic adoption. In contrast to other studies, this study offers a mechanistic and integrative viewpoint that links chitosan’s chemical modification techniques with their pharmacological effects and clinical translation potential, providing novel perspectives on structure–activity correlations and therapeutic design.
1. Introduction
Non-communicable diseases (NCDs) are classified as a group of chronic illnesses that cannot be transmitted from one person to another. These encompass a range of conditions including cancer, diabetes, neurodegenerative disorders and chronic inflammatory disease, and are among the leading causes of morbidity and mortality worldwide. It is estimated that around 70% of deaths are NCD-related [1]. NCDs have placed a deep burden on health care facilities and systems, notably in low and middle-income countries; for instance, 80% of NCD-related deaths occur in these countries. Key risk factors include unhealthy diets (particularly those high in saturated fats), tobacco use, and physical inactivity [2,3]. Conventional pharmacological treatments for these conditions are often limited by poor bioavailability, rapid metabolism, and inadequate tissue targeting [4,5]. These shortcomings highlight the urgent need for improved drug delivery strategies and safer therapeutic agents.
Natural products are receiving growing attention in drug discovery, and chitosan-based materials are among the most promising candidates. Chitosan is a biocompatible, biodegradable, and low-toxicity polymer obtained through the deacetylation of chitin. Structurally, it is a versatile linear biopolymer composed of β-(1→4)-linked 2-amino-2-deoxy-D-glucose (D-glucosamine) units (see Figure 1). Its physicochemical properties, including a positive surface charge [6], mucoadhesiveness [7], and ease of chemical modification [8], make it highly suitable for diverse pharmacological applications. Beyond its role as a drug carrier, chitosan also exhibits intrinsic pharmacological activities, including anticancer, anti-inflammatory, antimicrobial, antioxidant, and antidiabetic effects [9,10,11,12,13]. However, the mechanistic and pharmacodynamic contributions remain only partially understood.
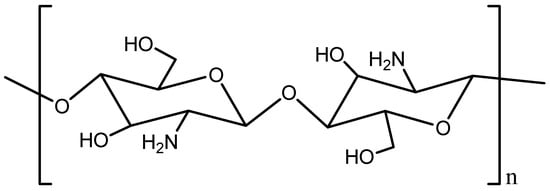
Figure 1.
Chemical structure of chitosan.
Scopus database analysis (2005–2025, English-language articles) indicates growing research interest in chitosan for therapeutic applications, with 3598 publications currently available (Figure 2). Numerous review articles have focused on the general uses of chitosan derivatives in biomedical and pharmaceutical contexts. These include studies on the design of encapsulated polyphenolic compounds, antimicrobial activities of chitosan, the role of biopolymers in drug delivery, and the use of chitosan biopolymer and its modified nanocomposites in pharmaceuticals. Other reviews have also highlighted the therapeutic potential of chitosan and its derivatives in the treatment of osteoarthritis as well as a wide variety of bioactivity supporting its versatile role as a bio-macromolecule in biomedical applications [14,15,16,17,18,19]. Few review articles have provided a comprehensive and mechanistic overview linking chemical modifications of chitosan to their pharmacological activities across the spectrum of NCDs, including wound healing. Therefore, this review provides insights into the novel approach and offers an integrated perspective on the pharmacological applications and mechanistic interactions of chitosan-based materials, including their use in the treatment of NCDs and wounds.
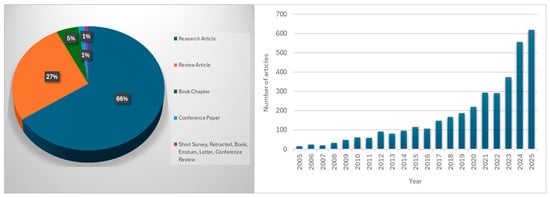
Figure 2.
Number of published articles on “therapeutic AND properties AND of AND chitosan AND chitosan” based on the Scopus database (accessed on 15 September 2025).
2. Methodology
This article was prepared as a traditional narrative review article; nevertheless, data collection in this study was conducted using multiple databases to ensure the information was comprehensive, aligned, and dynamic. Reputable online databases such as Google Scholar, Scopus, PubMed, and Web of Science were utilized to facilitate this process. The search targeted reliable and high-quality studies from 2005 to 2025 (two decades), aiming for the most recent studies. Data collection involved a thorough review of peer-reviewed journals, scientific articles, review articles, and book chapters in pharmacology, toxicology and therapeutics, chemistry, biochemistry, immunology, material sciences, and related fields. The keywords used included “Pharmacological Applications of Chitosan AND Its Derivatives,” “Biological potency of chitosan AND its derivatives,” “Modifications of chitosan for pharmacological applications,” and “Chitosan Derivatives AND Their Biological Efficacy”. Selected articles were categorized based on modifications of chitosan for therapeutic or pharmacological applications and their roles in pharmacological or biological efficacy (e.g., anti-inflammatory, anticancer, neuroprotective, metabolic disorders, wound healing). The synthesized results were integrated into a cohesive discussion, and the overall therapeutic efficacy of chitosan-based materials across various biological models. Only articles written in English were included.
3. Chemical Modifications and Functionalization Strategies
Chitosan is a naturally occurring polysaccharide derived from the deacetylation of chitin and is mostly extracted from the shells of crustaceans, insects, and fungi [20]. It has gained extensive interest in the biomedical field due to its inherent biocompatibility, biodegradability, and bioactivity [21]. However, its pharmacological applications are limited by poor solubility at physiological pH and variability in physicochemical properties [22]. Some of the modifications made to chitosan for biomedical applications are shown in Figure 3, along with the functional groups that are most favored by these modifications.
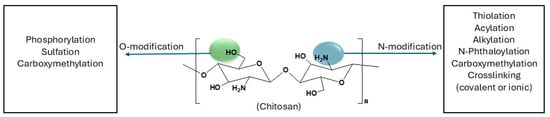
Figure 3.
Chemical modifications of chitosan for biomedical applications [23,24].
3.1. Acylated CS
Acylation of chitosan involves the introduction of acyl groups in the chitosan chain, on either the amino or hydroxyl group, though mostly on the amino group. It is one of the most frequently used modifications of chitosan, which involves reacting chitosan with various organic acids and their derivatives, such as anhydrides and acyl chlorides, to incorporate aliphatic or aromatic acyl groups into the chitosan chain (see Figure 4) [25]. Acylation improves the physicochemical and biomedical properties of chitosan, particularly solubility, stability, and hydrophobic interactions [26,27,28]. For example, Almeida et al. [29] synthesized an amphiphilic O-acylated chitosan derivative that self-assembled into micelles. This derivative self-assembles into micelles capable of encapsulating a hydrophobic anticancer drug (camptothecin) with high efficiency (78%), providing controlled release and stabilization of the active drug form.
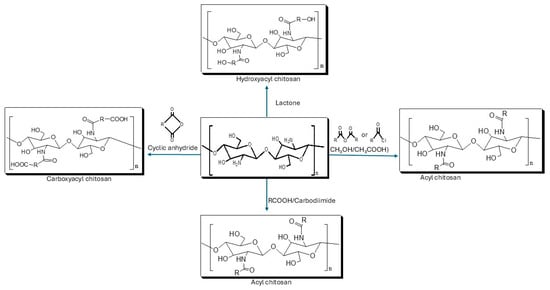
Figure 4.
Acylation of chitosan.
A special subclass of acylation reactions involves the use of thiol-containing carboxylic acids, such as thioglycolic acid, to introduce sulfhydryl (–SH) functional groups through acylation. Thiolation of chitosan was made based on the hypothesis that a disulfide bond will be formed between the polymer-bound free thiol groups and the thiol group present in mucus [30]. See its synthetic protocol in Figure 5. It has been reported that thiolated chitosan improves the mucoadhesive strength and permeability [31,32]. Additionally, the creation of intramolecular and intermolecular disulfide bonds results in a compact, three-dimensional network with increased cohesiveness, enabling regulated drug release [33].

Figure 5.
Synthesis of Thiolated Chitosan [34].
3.2. Alkylated Chitosan
The introduction of an alkyl group on the chitosan chain significantly weakens intermolecular hydrogen bonds, thereby improving solubility for biomedical applications [35]. The hydrophobic nature of alkyl groups means that solubility can be tuned by varying chain length: short chains enhance solubility, while long chains reduce it [36,37]. Alkylated chitosan derivatives can be prepared through the reductive alkylation of chitosan. Reductive alkylation of chitosan proceeds through imine formation between chitosan amine groups and aldehydes, followed by NaBH4 reduction to produce N-alkyl chitosan derivatives (see Figure 6) [38]. Alkylated chitosan has been used extensively in the treatment of wounds and hemostasis therapy [39,40,41,42,43].
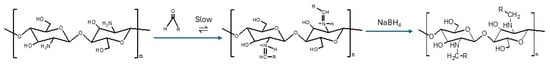
Figure 6.
Reductive alkylation of chitosan [38].
3.3. N-Phthaloylation of Chitosan
N-phthaloylation of chitosan is another method of modifying chitosan that is mostly used for biomedical applications. This modification introduces phthalimide groups into the chitosan chain. These groups act as a protective group which further increases the solubility and stability of the chitosan, enabling selective click chemistry or polymer grafting which results in functional derivatives such as micellar systems, tailored scaffolds, and stimuli-responsive drug carriers [44].
3.4. Other Chitosan Modifications
Other types of modifications and their applications are discussed in Table 1. It is important to note that the optimal modification depends on the intended application; for instance, overall, among the various chitosan-based materials listed in Table 1 below, carboxymethyl chitosan stands out as a broadly applicable material for wound dressing, owing to its high-water solubility, excellent biocompatibility, and intrinsic antimicrobial activity, while phosphorylated chitosan is preferred for bone tissue engineering due to its calcium-binding and osteoconductive properties [45,46]. For drug delivery systems, thiolated chitosan is most advantageous owing to its mucoadhesive and permeation-enhancing effects [31].

Table 1.
Overview of Chemically Modified Chitosan Derivatives and their Biomedical Applications.
4. Chitosan-Based Materials’ Efficacy in Wound Dressing and Healing
Chitosan-based materials have been reported to play a major role in wound dressing since they promote cell growth, minimize scarring, have good clotting capabilities, and absorb exudate (see Figure 7) [69]. For instance, Zhang et al. [70] synthesized a near-infrared (NIR)-responsive double-network hydrogel, composed of chitosan, which was incorporated with hollow copper sulfide nanoparticles (HCuS) and sodium nitroprusside (SNP). The hydrogel showed excellent mechanical stability, strong antibacterial activity (>99% MRSA elimination), anti-inflammatory effects, and accelerated wound healing (93% closure in 9 days). Kumi et al. [71] designed a 3D printed chitosan-based porous flexible hydrogel electrode, creating a material that conforms to wounds for electrical stimulation treatment. The chitosan-based material demonstrated a tensile strength of around 2.43 MPa, stretchability of approximately 48.9%, and a hydrophilic, porous structure that improved biocompatibility and moisture retention. In vivo diabetic wound models demonstrated faster closure (~99% by Day 14) with improved re-epithelialization and collagen deposition, highlighting its potential for customized wound healing applications. It also achieved >85% bacterial suppression against Methicillin-resistant Staphylococcus aureus (MRSA) and Escherichia coli (E. coli).
Figure 7.
Chitosan-based material in wound dressing.
In vivo study conducted by Xiao et al. [72] on mice showed that acid-responsive hydrogels based on carboxymethyl chitosan can efficiently accelerate wound healing. According to bacterial culture data, the chitosan hydrogel had good antibacterial action. Lastly, there was a remarkable increase in the production of M1 macrophages for pathogen clearance and a switch to M2 macrophages to assist tissue regeneration, demonstrating the hydrogel’s capacity to alter the Reactive Oxygen Species (ROS)-macrophage axis. Ren et al. [73] prepared a chitosan/glycerophosphate (CS/GP) hydrogel loaded with hydrothermally prepared Cu3SnS4 nanoparticles to construct a novel wound dressing (CS/GP/Cu3SnS4). The hydrogel showed >95% antibacterial activity against Staphylococcus aureus (S. aureus) and E. coli, with confirmed biocompatibility in vitro. In vivo studies demonstrated superior burn wound healing. A chitosan-based hydrogel (COG-Z@P200) containing polydopamine-coated ZIF-8 nanoparticles was synthesized in the study by Gao et al. [74] to work in tandem with metabolic-immune reprogramming and moderate photothermal treatment. The chitosan-based hydrogel provided an antibiotic-free strategy against resistant infections by achieving >99.5% eradication of MRSA, reprogramming macrophages towards pro-healing phenotypes, enhancing angiogenesis, and accelerating diabetic wound repair by 48%.
Yu et al. [75] synthesized a CS/ZnO-NPs@Exos hydrogel by combining zinc oxide nanoparticles, exosomes, and chitosan, which produced prolonged exosome release in diabetic wounds. The hydrogel showed great therapeutic promise for the healing of diabetic skin injuries by speeding wound closure, lowering inflammation, facilitating re-epithelialization, collagen deposition, and angiogenesis. Methacrylate-grafted quaternary ammonium chitosan, polyvinyl alcohol, ZIF-8, and nicotinamide mononucleotide were used by Zhang et al. [76] to create a multifunctional hydrogel that could be applied to irregular wounds. With the help of quaternary ammonium groups in chitosan and Zn2+ release, the hydrogel exhibited long-lasting antibacterial action, which in turn led to decreased infection, improved epithelium regeneration, and collagen deposition in vivo. Extensive research by Salarizadeh et al. [77] created a two-layer sponge wound dressing by combining polyvinyl alcohol, sodium alginate, and a chitosan active layer that was freeze-dried and contained zinc oxide nanoparticles and Hyssopus officinalis extract. The dressing’s antimicrobial activity, clotting ability, and porosity were all good. After 14 days, in vivo burn models showed superior biocompatibility and quicker healing, with 92.7% wound closure as opposed to 73.2% with Alpha ointment.
A multifunctional hemostatic bandage has also been created by anchoring silica nanoparticles onto polydopamine-coated chitosan non-woven fabric, combined with polyphosphate. The bandage showed strong flexibility, cytocompatibility, adhesion, and antibacterial activity, while promoting coagulation and hemostasis. In rat liver and femoral artery injury models, PSPC achieved hemostatic performance comparable to QuickClot Combat Gauze® and significantly enhanced full-thickness wound healing [78]. Self-healing hydrogel was created by Zhou et al. [79] using guanidinium-functionalized chitosan (GCS) and aldehyde-modified chitosan (ACS) and a Schiff base reaction. The hydrogel exhibited potent antibacterial and anti-inflammatory properties. It outperformed commercial Aquacel™ Ag+ dressings in infected full-thickness rat wounds, achieving 94.5% closure in 14 days after infection. Dai et al. [80] created a cellulose-based composite hydrogel by mixing acrylamide, carboxymethyl chitosan, and cellulose dialdehyde to create a double network using free radical polymerization and the Schiff base reaction. High levels of toughness, stretchability, tensile strength, self-adhesion, and biocompatibility were all demonstrated by the chitosan-based hydrogel. The full-thickness wound model in mice showed a considerable acceleration in healing.
Another research developed a chitosan-based Janus wound dressing with asymmetric wettability, consisting of a methacrylated chitosan (CSMA), incorporating polydopamine-coated silica (PDA-SiO2) hydrophilic sponge for hemostasis and angiogenesis, and a TPU/ZIF-8 nanofiber outer layer for antibacterial protection. The dressing showed strong bacterial resistance, promoted coagulation, and in vivo reduced bleeding time and blood loss while enhancing collagen deposition, angiogenesis, and full-thickness wound healing [81]. Purified chitosan carbonate liquid dressing via an ammonium bicarbonate-mediated acetate replacement strategy was also synthesized. In full-thickness wound models, it promoted rapid repair with hair follicle regeneration within 10 days, outperforming commercial benchmarks like 3M Cavilon [82]. Another injectable multifunctional hydrogel (RM-CHβ) using chitosan hydrochloride, hydroxyethyl cellulose, β-glycerol phosphate, and resveratrol micelles was used to treat S. aureus-infected full-thickness wounds. The hydrogel showed strong adhesion, antioxidant, antibacterial, and hemostatic properties, while in vivo it accelerated healing by enhancing angiogenesis, collagen deposition, and inflammation control [83]. A wide range of chitosan-based materials have shown great promise as wound dressings due to their distinct physicochemical characteristics, which include superior adhesion, swelling capacity, biodegradability, and increased mechanical strength. Furthermore, a lot of these systems include inherent or functionalized hemostatic, antioxidant, and antibacterial properties, which support their function in hastening tissue regeneration and wound healing [84,85,86,87,88]. Table 2 highlights some of the recent studies (strictly 2024–2025) on chitosan-based materials in wound healing. In these studies, rats, mice, and rabbits are used because they provide well-characterized, reproducible in vivo models with healing properties closer to human skin [89,90].

Table 2.
Chitosan-based materials in wound healing.
5. Biological Activities and Clinical Trials
Beyond wound healing, chitosan-based materials exhibit a wide range of biological activities, which further underscore their pharmacological versatility. These biological activities include anticancer, anti-inflammatory, antidiabetic, antihypertensive, and neuroprotective effects, many of which share underlying mechanisms, such as modulation of oxidative stress, control of inflammation, and enhanced tissue regeneration, previously discussed in the context of wound repair.
5.1. Anticancer Activity
Cancer is a chronic illness that is mostly caused by the body’s cells proliferating out of control, producing tumors that may or may not be malignant [104]. Numerous anticancer medications on the market today have unfavorable pharmacological characteristics, such as low aqueous solubility, irritation, instability, fast metabolism, and nonselective drug distribution. These characteristics can lead to negative outcomes, such as suboptimal therapeutic activity, dose-limiting side effects, and poor patient quality of life [105,106,107,108,109,110]. Hence, scientists are looking for less harmful and effective medications for cancer victims. One naturally occurring polysaccharide, considered to have anticancer properties, is a chitosan-based material. For instance, the most recent research by Yu et al. [111] created a gellan gum/chitosan-based bilayer scaffold, loaded with green tea extract and curcumin, and it demonstrated potent antibacterial and antioxidant properties. When tested on MCF-7 breast cancer cells, the scaffold remained biocompatible with normal fibroblasts but decreased cell viability from around 92% (control) to about 23%. Another work related to anticancer properties (breast cancer) of chitosan-based materials was demonstrated by Mirzaie et al. [112] where they developed Docetaxel–chitosan nanoparticles (DTX–CS NPs), assembled with hyaluronic acid, and tested them against MCF-7 breast cancer cells. The nanoparticles showed enhanced cellular uptake, apoptosis induction, and altered gene expression, with a significantly higher BAX/BCL-2 ratio compared to free Docetaxel. Importantly, the material exhibited lower cytotoxicity toward normal fibroblasts, suggesting improved selectivity and reduced off-target toxicity. Mirzaie et al. [112] examined the effects of chitosan nanoparticles loaded with ethanolic and n-hexane extracts from Moringa oleifera seeds on MCF-7 breast cancer cells. The IC50 values for both nano formulations were 1843 µg/mL and 382 µg/mL, respectively, indicating dose-dependent cytotoxicity. They dramatically decreased the expression of genes linked to metastasis (Wnt/β-catenin, cyclin D1, TGF-β, and Snail) and inhibited cell proliferation (Ki-67 marker). Chitosan-coated PLGA nanoparticles loaded with ursolic acid for breast cancer therapy were tested on MCF-7 and MDA-MB-231 breast cancer cells. The chitosan-based nanoparticles showed high encapsulation efficiency of around 80%, controlled release, and significant cytotoxicity compared to free ursolic acid [113].
Poly(ethylene glycol) methacrylate-grafted chitosan (PEGMA-g-Cs) microbeads with gold-coated SPIONs were created by Olaoye et al. [114] for the transport of lactoferrin to the colon. The formulation demonstrated decreased cancer cell survival, pH-responsive release, and good encapsulation efficiency when tested against Caco-2 colorectal cancer cells. In studies on HCT-116 colon cancer cells, the chitosan–graphene oxide–silver (Cs/GO/AgNP) nanocomposite demonstrated dose-dependent cytotoxicity with around 72% reduction in cell viability at 100 µg/mL, showing high anticancer activity [115]. Chang et al. [116] demonstrated that colon cancer cells (HT29, DLD-1, HCT116, SW480) cultured on chitosan membranes acquired stronger stem-like features, enhanced motility, drug resistance, self-renewal, and upregulation of stemness markers by activation of the canonical Wnt/β-catenin-CD44 signaling pathway.
Qu et al. [117] created anisamide-functionalized, pH-responsive chitosan micelles for paclitaxel delivery, which demonstrated improved uptake in sigma-1 receptor–positive prostate cancer cells (PC-3), pH-triggered drug release, stronger tumor inhibition in vivo, and decreased systemic toxicity. Another study that demonstrated that chitosan-based materials have anticancer properties (prostate cancer) was performed by Khan et al. [118]. The study showed that oral chitosan encapsulated with epigallocatechin-3-gallate (EGCG) nanoparticles enhances bioavailability, inhibits prostate tumor growth, reduces PSA, induces apoptosis, and suppresses proliferation and angiogenesis, offering a promising nanochemopreventive strategy for prostate cancer. The effectiveness of chitosan-based materials has also been confirmed in /DU145, LNCaP, and PC-3 cell models, demonstrating strong anticancer potential in prostate cancer by suppressing cell proliferation, significantly reducing tumor size, and persistently inhibiting tumor growth [119,120,121].
5.2. Anti-Inflammatory Activity
Inflammation is the second body defense mechanism. In reaction to viruses, irritants, and damaged cells, among other damaging and alien stimuli, the immune system uses this mechanism to identify, reject, and start the healing process. Chronic inflammation can persist for months or years. The source of the damage and the body’s capacity to heal and reverse it usually dictate the degree and length of chronic inflammation [122,123]. Numerous studies have been carried out to investigate the potential of chitosan-based formulations in inflammation. Xu et al. [124] reviewed that chitosan and its derivatives show anti-inflammatory activity mainly through cytokine modulation and signaling pathway regulation. For instance, Zhong et al. [125] found that the deferoxamine-loaded chitosan–chlorogenic acid/oxidized hyaluronic acid hydrogel (CCOD) dramatically reduces inflammatory responses in diabetic and infected wound models by upregulating the anti-inflammatory cytokine IL-10 and downregulating pro-inflammatory cytokines (IL-1β, IL-6, TNF-α, and CRP). Goyal et al. [126] found that chitosan-based nanocarriers primarily induce anti-inflammatory effects by modulating cytokines, suppressing MCP-1–mediated monocyte infiltration, and downregulating TNF-α, IL-1β, and IL-6, while simultaneously promoting tissue repair. This was confirmed by the ability of the material to reduce joint inflammation, synovial hyperplasia, and intestinal barrier defects in models of rheumatoid arthritis and inflammatory bowel disease.
Lu et al. [127] created a gallic acid–chitosan methacrylate (GA-CS-MA) hydrogel that effectively suppressed pro-inflammatory signals, reduced ROS and Nitric Oxide (NO) production, and modulated macrophage polarization in response to Lipopolysaccharide (LPS) stimulation. Its potent anti-inflammatory properties were validated through in vitro and in vivo studies, revealing decreased cell infiltration, thinner fibrous capsules, and increased M2 polarization. Chitosan nanoparticles have also been proven to inhibit the inflammation response of LPS-stimulated macrophages, by altering common inflammation variables and biomarkers [128]. Guan et al. [129] demonstrated that Ibuprofen-chitosan methacrylate (IBU-CS-MA) hydrogel dramatically decreases the amount of ROS that LPS-stimulated macrophages produce. It is well recognized that ROS contributes to inflammation and oxidative damage. Numerous studies have demonstrated that chitosan-based materials reduce inflammation through various mechanisms by suppressing NF-κB, which downregulates pro-inflammatory mediators (IL-1β, IL-6, IL-12, and TNF-α) and decreases the production of NO, IL-6, and TNF-α in macrophages. Additionally, they stimulate inflammation resolution and tissue repair by promoting angiogenesis and enhancing anti-inflammatory cytokines (TGF-β, IL-10) [130,131,132,133,134].
5.3. Antidiabetic Activity
Diabetes is a general term for several metabolic diseases caused by partial or total insulin insufficiency and marked by hyperglycemia. It is a long-term condition that impacts how your body uses food as fuel. The body transforms most of the food we eat into glucose, or sugar, which is then delivered into the bloodstream [135,136]. Chitosan-based formulations have shown potential as a key remedy to treat diabetes. The potential of chitosan to address the shortcomings of traditional antidiabetic treatments by enhancing medication bioavailability, lowering insulin resistance, and improving glycaemic control makes it practically significant [137,138]. For instance, Wadasinghe et al. [139] demonstrated that chitosan–tripolyphosphate nanoparticles (CS–TPP) encapsulating Gmelina arborea and Spondias pinnata extracts (GAE–CS–TPP, SAE–CS–TPP) exhibited strong antidiabetic potential, with high encapsulation efficiency and enhanced inhibitory activity against α-glucosidase and DPP-IV enzymes compared to crude extracts, thereby supporting their role in controlling hyperglycemia in diabetes. Abdel-Baky et al. [140] conducted another in vitro study which also demonstrated strong anti-diabetic effects. They found that chitosan-quinoline Schiff base increased glucose uptake and achieved high inhibition of key enzymes that break down carbohydrates (92.10% for α-glucosidase and 99.78% for α-amylase). These findings were further supported by molecular-docking analysis and a favorable safety profile.
Many in vivo studies have also been carried out to evaluate the antidiabetic activities of chitosan-based formulations. According to research by Priyanka et al. [138] male Wistar rats with type 1 diabetes (T1DM) show significant anti-hyperglycemic benefits from low-molecular-weight chitosan (LMWC), a chitosan derivative, mostly by modulating the AKT/PI3K/FOXO pathway to enhance insulin sensitivity and glucose metabolism. Yang et al. [141] also performed the in vivo study, which demonstrated that a chitosan-based hydrogel combined with umbilical cord mesenchymal stem cells (UC-MSCs) can significantly enhance glucose regulation and restore pancreatic islet integrity in Type 2 Diabetes Mellitus (T2DM) mice by stimulating β-cell function and promoting macrophage polarization toward the M2 phenotype. Furthermore, chitosan-based embelin nanoparticles (ECNPs) significantly lowered blood glucose, as demonstrated by Maanvizhi et al. [142] in streptozotocin-induced diabetic rats, showing dose-dependent antidiabetic activity comparable to glibenclamide and demonstrating good histological safety up to 25 mg/kg, highlighting their potential for hyperglycemia management. Similarly, chitosan extracted from crayfish shells through acid demineralization and alkali deproteinization showed marked anti-hyperglycemic activity in alloxan-induced diabetic rats, with the chitosan-only group returning blood glucose to near-normal levels within 2 h postprandially, highlighting chitosan’s ability to improve glucose tolerance and enhance the efficacy of oral hypoglycemic agents [143].
5.4. Anti-Hypertensive Activity
Hypertension (high blood pressure) is a chronic disorder in which the blood pressure in the arteries is too high. Hypertension is a significant public health problem in both developing and developed countries [144]. Research on the antihypertensive potential of chitosan-based materials has gained importance, driven by the worldwide prevalence of hypertension and its contribution to cardiovascular diseases, responsible for over 17.5 million deaths each year and expected to rise by >23.6 million by 2030 [145,146]. As an example, Chitosan/carboxymethyl-cellulose (CS/CMC) biomaterials containing captopril were used by Kim et al. [147] as a chitosan-based antihypertensive system by releasing the drug by non-Fickian diffusion through artificial skin and pseudo-Fickian diffusion in buffer. They demonstrated excellent angiotensin-converting enzyme (ACE)-blocking action for blood pressure regulation in an artificial skin model, achieving sustained release for 36 h and inhibiting ACE by up to 88.4%. Batista et al. [148] conducted research that resulted in the development of chitosan microparticles-in-films that were loaded with the antihypertensive peptide KGYGGVSLPEW. These microparticles were able to reduce blood pressure by inhibiting ACE and enhancing buccal absorption. The efficacy of these microparticles was demonstrated in vitro using the human buccal epithelium TR146 cell model, demonstrating rapid film disintegration and controlled peptide release. In a related study, Auwal et al. [149] developed chitosan–antihypertensive biopeptide nanoparticles (Chit-AntBiop-NPs) that lower blood pressure by inhibiting ACE and providing sustained peptide release. Their efficacy was evaluated in vivo using hypertensive rats, achieving a dose-dependent systolic blood pressure reduction of up to 59.8 ± 7.7 mmHg at 6 h post-administration. Du et al. [150] also reported that water-soluble chitosan (WSC) lowers blood pressure by inhibiting vascular remodeling through suppression of Nuclear Factor of Activated T-Cells, cytoplasmic 1 (NFATc1) expression and c-myc–mediated smooth-muscle proliferation, as shown in vitro in primary rat aortic smooth-muscle cells and in vivo in spontaneously hypertensive Wistar-Kyoto rats treated with 150 mg/kg/day WSC.
Another study by Khalid Danish et al. [151] used spontaneous hypertensive rats (SHR) to confirm that chitosan–zein nanoparticles (CZ NP) encapsulating the antihypertensive tripeptides IPP and LKP were effective through sustained release and ACE inhibition, which enhanced peptide stability and bioavailability. Oral administration of the nanoparticles lowered systolic blood pressure for up to 8 h. Extending these findings, the antihypertensive effects of chitosan-based materials, such as chitosan–ferulic acid salts, miR-29b–chitosan nanoparticles, and chitooligosaccharide–polyphenol conjugates, are mediated by distinct mechanisms, including bile acid adsorption and ferulic acid release, enhanced nitric oxide bioavailability with antifibrotic action, and ACE/renin mixed-type inhibition. Their effectiveness was confirmed in angiotensin II- or SHR, showing notable reductions in blood pressure and associated complications [152,153,154].
5.5. Neuroprotective Activity
Neurological illnesses impact the brain, spinal cord, and the peripheral nervous system. Anxiety, depression, stroke, Alzheimer’s, Parkinson’s, and other similar illnesses are among them. The growing incidence of neurodegenerative diseases and neurological injuries, which pose serious global health challenges, has made the investigation of chitosan-based materials for their neuroprotective potential a critical research area [155,156]. Recent research indicates that chitosan is a good candidate in neurotherapeutics since it promotes neural repair, enhances drug bioavailability, and modulates neuroinflammation [157,158]. For instance, according to the research conducted by Dadkhah et al. [159] Fluoxetine-loaded pegylated chitosan nanoparticles significantly enhanced the neuroprotective effects in a rat model of hippocampal demyelination, improving memory function, reducing anxiety-like behaviors, increasing Brain-Derived Neurotrophic Factor (BDNF) levels in the hippocampus, and more effectively reducing demyelination lesions compared to fluoxetine alone. Similarly, in a rat model of intracerebral hemorrhage (ICH) stroke, Lin et al. [160] created a chitosan micellar self-healing hydrogel (CMD hydrogel), which showed in vivo neuroprotective efficacy. Significant brain tissue regeneration and functional improvement were demonstrated by approximately 84% behavioral recovery, balanced brain midline shift, increased neurogenesis (doublecortin/nestin-positive cells), and promoted angiogenesis that resulted from intracerebral injection of CMD hydrogel.
It is worth noting that chitosan is compatible with other materials, as Wang et al. [161] demonstrated that valproic acid–labeled chitosan nanoparticles (VA-CN) exhibited strong in vivo neuroprotective effects in a rat spinal cord injury (SCI) model. VA-CN treatment significantly improved tissue repair and locomotor function, enhanced neural stem cell proliferation and differentiation (Nestin+/Ki67+ and Tuj-1+ cells), increased neurotrophic factors (BDNF, NGF, NTF-3), and reduced microglial activation compared with chitosan or valproic acid alone. Saleem et al. [162] found that chrysin-loaded chitosan nanoparticles were neuroprotective in an Aβ1-42-induced Alzheimer’s zebrafish model, lowering neuronal apoptosis, oxidative stress, and amyloid aggregation while maintaining synaptic integrity. These nanoparticles also boosted learning and memory ability in many behavioral tests. Overall, Chitosan-based materials, such as 3D scaffolds, dopamine-loaded nanoparticles, magnoflorine-collagen nanocapsules, and citric acid cross-linked hydrogels, demonstrated in vitro neuroprotective effects by supporting neural cell survival, enhancing antioxidant enzyme activity, reducing oxidative stress, and inhibiting acetylcholinesterase, highlighting their potential for nerve repair and treatment of neurodegenerative diseases [163,164,165,166].
5.6. Clinical Studies
The translation of chitosan-based research into clinical practice necessitates the implementation of rigorous preclinical and clinical trials to guarantee the safety, efficacy, and regulatory conformance of the drug formulations. In this context, Wang et al. [167] conducted a registered clinical study (ClinicalTrials.gov NCT03907111) that compared chitosan fiber (CF) and chitosan sponge (CP) dressings in patients following abdominal surgery. The study found that CF dressings achieved quicker hemostasis and higher blood absorption than normal gauze. In a clinical experiment (ClinicalTrials.gov NCT03280849), Ramos-Zúñiga et al. [168] used a bilaminar chitosan scaffold to rebuild the sellar floor following pituitary surgery, resulting in watertight closure and high biocompatibility. No CSF fluid leaking, infection, or inflammation was noted throughout nearly three years of follow-up. Similarly, in a randomized trial of 59 cesarean-section patients, Kao et al. [169] (ClinicalTrials.gov NCT04211597) found that a chitosan-microencapsulated recombinant human epidermal growth factor (Me-EGF) spray plus silicone gel significantly improved wound healing and decreased Vancouver Scar Scale scores for vascularity, pigmentation, and pliability compared to silicone gel or no treatment. ElGendy et al. [170] conducted a double-blind RCT (NCT05212311) in 54 patients with mild–moderate cubital tunnel syndrome, a neurological illness, showing that adding chitosan nanoparticle gel phonophoresis to standard hand therapy improved ulnar nerve conduction, reduced pain, and enhanced hand function. The chitosan gel was applied through ultrasound (0.5 W/cm2, 3 MHz) three times per week for five weeks. There are currently 190 clinical studies involving the use of chitosan-based materials. Figure 8 indicates their recruitment statuses, while Table 3 summarizes selected chitosan-based clinical trials that have been completed, as indicated by their status of ‘Completed’ on ClinicalTrials.gov (Search ClinicalTrials.gov for: Other terms: Chitosan | List Results | ClinicalTrials.gov).
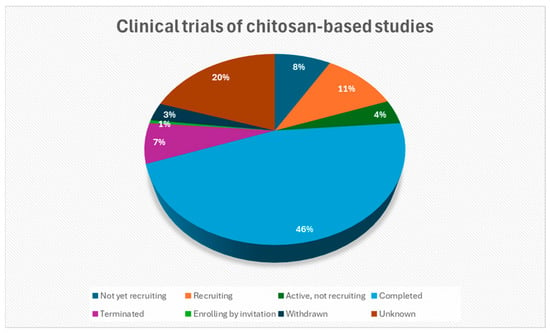
Figure 8.
The recruitment statuses of clinical studies concerning chitosan-based materials (Search ClinicalTrials.gov for: Other terms: Chitosan|List Results|ClinicalTrials.gov, accessed on 14 September 2025) [171].

Table 3.
Some of the clinical trials involving the use of chitosan-based materials (Search ClinicalTrials.gov for: Other terms: Chitosan | List Results | ClinicalTrials.gov, accessed on 14 September 2025) [171].
6. Conclusions, Future Work, and Recommendations
This review highlights two decades (2005–2025) of research on chitosan-based materials; these materials have shown remarkable promise for use in formulations. One of the key advantages of chitosan is its ease of chemical modification, which allows improvement of critical properties, e.g., solubility, which further expands its therapeutic potential. These materials’ flexibility to be used both as drug carriers and pharmacologically active agents drives their unique position in modern therapeutics. This has been evidenced from the in vivo and in vitro models, which support their anticancer, anti-inflammatory, antidiabetic, antihypertensive, and neuroprotective activities, while recent clinical trials highlight their safety, efficacy, and feasibility in diverse biomedical applications.
The significance of these findings lies in the uniqueness and ability of chitosan to bridge material science and pharmacology, offering a natural, modifiable, and safe platform for next-generation biomedical applications. Its dual role, such as therapeutic and structural, makes it a promising cornerstone for the design of multifunctional biomaterials.
Despite these advances, certain limitations remain; for instance, most studies do not adequately evaluate the cytotoxicity of chitosan-based materials, which is essential for ensuring their safety of use. Additionally, clinical translation remains limited, as some studies were withdrawn and others were terminated, as shown in Figure 8. Furthermore, a lot of findings are constrained in laboratory-scale studies.
Future research should focus on large-scale preclinical validation and well-designed clinical trials to confirm therapeutic efficacy and safety. Integration of computational modeling, green synthesis approaches, and advanced biofabrication (such as 3D printing and hybrid scaffolds) could accelerate the clinical translation of chitosan-based therapeutics, and lastly, the cytotoxicity of every chitosan-based material should be evaluated. With continued interdisciplinary collaboration, these materials hold strong potential to transform the treatment of chronic and degenerative diseases and could enter the market in high quantities in the near future.
Author Contributions
Conceptualization, A.D., M.A.S. and P.P.M.; methodology, A.D. and M.A.S.; resources, A.D.; data curation, A.D. and M.A.S.; writing—original draft preparation, A.D.; writing—review and editing, A.D., M.A.S., P.P.M. and M.E.M.; visualization, A.D.; supervision, M.A.S., P.P.M. and M.E.M.; project administration, M.A.S.; funding acquisition, A.D. All authors have read and agreed to the published version of the manuscript.
Funding
This study was funded by the National Research Foundation (Sasol Foundation), grant number PMDS240613227379.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors would like to thank the University of the Free State (Department of Chemistry) and the University of Johannesburg (Department of Metallurgical Engineering) for providing a conducive research environment, as well as the NRF (Sasol Foundation) for financial support.
Conflicts of Interest
The authors declare no conflicts of interest.
Correction Statement
This article has been republished with a minor correction to the Data Availability Statement. This change does not affect the scientific content of the article.
References
- Boopathi, S.; Priya, P.S.; Haridevamuthu, B.; Nayak, S.R.R.; Chandrasekar, M.; Arockiaraj, J.; Jia, A.-Q. Expanding germ-organ theory: Understanding non-communicable diseases through enterobacterial translocation. Pharmacol. Res. 2023, 194, 106856. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.D.; Mehta, A.; Kautsar, H.; Kak, M.; Karem, G.; Misra, M.; Joshi, H.; Herbst, C.H.; Perry, H.B. Improving quality of non-communicable disease services at primary care facilities in middle-income countries: A scoping review. Soc. Sci. Med. 2023, 320, 115679. [Google Scholar] [CrossRef]
- Brenyah, J.K.; Nonvignon, J.; Singh, A.; Owusu-Dabo, E. Tobacco consumption and non-communicable diseases in Ghana; Identifying accentuating factors and further evidence from 2014 Ghana demographic and health survey. Sci. Afr. 2023, 20, e01665. [Google Scholar] [CrossRef]
- Singh, A.P.; Biswas, A.; Shukla, A.; Maiti, P. Targeted therapy in chronic diseases using nanomaterial-based drug delivery vehicles. Signal Transduct. Target. Ther. 2019, 4, 33. [Google Scholar] [CrossRef]
- Gorantla, S.; Singhvi, G.; Rapalli, V.K.; Waghule, T.; Dubey, S.K.; Saha, R.N. Targeted drug-delivery Systems in the Treatment of Rheumatoid Arthritis: Recent Advancement and Clinical Status. Ther. Deliv. 2020, 11, 269–284. [Google Scholar] [CrossRef]
- Padhi, S.; Behera, A.; Hasnain, S.; Nayak, A.K. Chapter 7—Chitosan-based drug delivery systems in cancer therapeutics. In Chitosan in Drug Delivery; Hasnain, M.S., Beg, S., Nayak, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 159–193. [Google Scholar] [CrossRef]
- Ahmad, K.; Zhang, Y.; Chen, P.; Yang, X.; Hou, H. Chitosan interaction with stomach mucin layer to enhances gastric retention and mucoadhesive properties. Carbohydr. Polym. 2024, 333, 121926. [Google Scholar] [CrossRef]
- Shete, A.; Chavan, A.; Potekar, P.; Yadav, G.; Shah, N. Modification of physicochemical properties of chitosan to improve its pharmaceutical and agrochemical potential applications. Int. J. Biol. Macromol. 2024, 267, 131404. [Google Scholar] [CrossRef]
- Sivasuriyan, K.S.; Namasivayam, S.K.R.; Pandian, A. Molecular insights into the anti-cancer activity of chitosan-okra mucilage polymeric nanocomposite doped with nano zero-valent iron against multi-drug-resistant oral carcinoma cells. Int. J. Biol. Macromol. 2025, 286, 138495. [Google Scholar] [CrossRef] [PubMed]
- Ulu, Ö.D.; Birhanlı, E.; Ulu, A.; Ateş, B. Enhanced antioxidant and antimicrobial activities of chitosan/oxidized microcrystalline cellulose blended films with Tribulus terrestris extract for food packaging applications. Int. J. Biol. Macromol. 2025, 291, 139036. [Google Scholar] [CrossRef]
- Jamiri, F.; Fasaei, B.N.; Joghataei, S.M.; Yahyaraeyat, R.; Mazloom-Jalali, A. Synergistic antibacterial activity of chitosan-polyethylene glycol nanocomposites films containing ZIF-8 and doxycycline. BMC Biotechnol. 2025, 25, 19. [Google Scholar] [CrossRef]
- Rajkumar, M.; Presley, S.I.D.; Govindaraj, P.; Kirubakaran, D.; Farahim, F.; Ali, T.; Shkir, M.; Latha, S. Synthesis of chitosan/PVA/copper oxide nanocomposite using Anacardium occidentale extract and evaluating its antioxidant, antibacterial, anti-inflammatory and cytotoxic activities. Sci. Rep. 2025, 15, 3931. [Google Scholar] [CrossRef]
- Alsolami, A.; Bazaid, A.S.; Alshammari, M.A.; Qanash, H.; Amin, B.H.; Bakri, M.M.; Abdelghany, T.M. Ecofriendly fabrication of natural jojoba nanoemulsion and chitosan/jojoba nanoemulsion with studying the antimicrobial, anti-biofilm, and anti-diabetic activities In Vitro. Biomass Convers. Biorefin. 2025, 15, 1283–1294. [Google Scholar] [CrossRef]
- Di Santo, M.C.; Antoni, C.L.D.; Rubio, A.P.D.; Alaimo, A.; Pérez, O.E. Chitosan-tripolyphosphate nanoparticles designed to encapsulate polyphenolic compounds for biomedical and pharmaceutical applications—A review. Biomed. Pharmacother. 2021, 142, 111970. [Google Scholar] [CrossRef]
- Confederat, L.G.; Tuchilus, C.G.; Dragan, M.; Sha’at, M.; Dragostin, O.M. Preparation and Antimicrobial Activity of Chitosan and Its Derivatives: A Concise Review. Molecules 2021, 26, 3694. [Google Scholar] [CrossRef] [PubMed]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef]
- Kedir, W.M.; Abdi, G.F.; Goro, M.M.; Tolesa, L.D. Pharmaceutical and drug delivery applications of chitosan biopolymer and its modified nanocomposite: A review. Heliyon 2022, 8, e10196. [Google Scholar] [CrossRef]
- Fan, X.; Chen, G.; Wang, S.; Liu, X.; Huang, S.; Feng, C.; Jiang, X. Applications of Chitosan and its Derivatives in the Treatment of Osteoarthritis. Aging Dis. 2025, 16, 3284–3290. [Google Scholar] [CrossRef]
- Jagdale, S.; Agarwal, B.; Dixit, A.; Gaware, S. Chitosan as excellent bio-macromolecule with myriad of anti-activities in biomedical applications—A review. Int. J. Biol. Macromol. 2024, 257, 128697. [Google Scholar] [CrossRef] [PubMed]
- Dambuza, A.; Mokolokolo, P.P.; Makhatha, M.E.; Sibeko, M.A. Chitosan-Based Materials as Effective Materials to Remove Pollutants. Polymers 2025, 17, 2447. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, N. Chitosan with PVC polymer for biomedical applications: A bibliometric analysis. Mater. Today Proc. 2023, 81, 894–898. [Google Scholar] [CrossRef]
- Mokhtari, H.; Bahari, M.; Yeganeh, F. Chitosan-based Biomaterials in Regenerative Medicine: Optimizing Mesenchymal Stem Cell Viability and Function. Stem Cell Rev. Rep. 2025, 21, 2010–2030. [Google Scholar] [CrossRef]
- Wang, W.; Meng, Q.; Li, Q.; Liu, J.; Zhou, M.; Jin, Z.; Zhao, K. Chitosan Derivatives and Their Application in Biomedicine. Int. J. Mol. Sci. 2020, 21, 487. [Google Scholar] [CrossRef]
- Lima, R.; Fernandes, C.; Pinto, M.M.M. Molecular modifications, biological activities, and applications of chitosan and derivatives: A recent update. Chirality 2022, 34, 1166–1190. [Google Scholar] [CrossRef]
- Mourya, V.; Inamdar, N.N. Chitosan-modifications and applications: Opportunities galore. React. Funct. Polym. 2008, 68, 1013–1051. [Google Scholar] [CrossRef]
- Pathak, K.; Misra, S.K.; Sehgal, A.; Singh, S.; Bungau, S.; Najda, A.; Gruszecki, R.; Behl, T. Biomedical Applications of Quaternized Chitosan. Polymers 2021, 13, 2514. [Google Scholar] [CrossRef]
- Biswas, U.K.; Bose, A.; Ghosh, B.; Sharma, S. An insight into chemically modified chitosan and their biological, pharmaceutical, and medical applications: A review. Int. J. Biol. Macromol. 2025, 303, 140612. [Google Scholar] [CrossRef] [PubMed]
- Pandey, R.; Mathur, G. Current Trends in Chitosan Functionalization Methods and Their Applications. Starch/Starke 2025, 77, 2300248. [Google Scholar] [CrossRef]
- Almeida, A.; Araújo, M.; Novoa-Carballal, R.; Andrade, F.; Gonçalves, H.; Reis, R.L.; Lúcio, M.; Schwartz, S.; Sarmento, B. Novel amphiphilic chitosan micelles as carriers for hydrophobic anticancer drugs. Mater. Sci. Eng. C 2020, 112, 110920. [Google Scholar] [CrossRef] [PubMed]
- Leitner, V.M.; Walker, G.F.; Bernkop-Schnürch, A. Thiolated polymers: Evidence for the formation of disulphide bonds with mucus glycoproteins. Eur. J. Pharm. Biopharm. 2003, 56, 207–214. [Google Scholar] [CrossRef] [PubMed]
- Jain, S.; Nuwal, K.; Mahmood, A.; Piplani, M.; Chander, S.; Dubey, S.K.; Singhvi, G. Chapter 10—Thiolated chitosan as an improved bioadhesive polymer in drug delivery. In Chitosan in Drug Delivery; Hasnain, M.S., Beg, S., Nayak, A.K., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 247–276. [Google Scholar] [CrossRef]
- Tekade, M.; Maheshwari, N.; Youngren-Ortiz, S.R.; Pandey, V.; Chourasiya, Y.; Soni, V.; Deb, P.K.; Sharma, M.C. Chapter 13—Thiolated-Chitosan: A Novel Mucoadhesive Polymer for Better-Targeted Drug Delivery. In Biomaterials and Bionanotechnology; Tekade, R.K., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 459–493. [Google Scholar] [CrossRef]
- Kafedjiiski, K.; Föger, F.; Werle, M.; Bernkop-Schnürch, A. Synthesis and in Vitro Evaluation of a Novel Chitosan–Glutathione Conjugate. Pharm. Res. 2005, 22, 1480–1488. [Google Scholar] [CrossRef]
- Manna, S.; Seth, A.; Gupta, P.; Nandi, G.; Dutta, R.; Jana, S.; Jana, S. Chitosan Derivatives as Carriers for Drug Delivery and Biomedical Applications. ACS Biomater. Sci. Eng. 2023, 9, 2181–2202. [Google Scholar] [CrossRef] [PubMed]
- Kurita, Y.; Isogai, A. N-Alkylations of chitosan promoted with sodium hydrogen carbonate under aqueous conditions. Int. J. Biol. Macromol. 2012, 50, 741–746. [Google Scholar] [CrossRef]
- Ma, G.; Yang, D.; Zhou, Y.; Xiao, M.; Kennedy, J.F.; Nie, J. Preparation and characterization of water-soluble N-alkylated chitosan. Carbohydr. Polym. 2008, 74, 121–126. [Google Scholar] [CrossRef]
- Yang, T.-C.; Chou, C.-C.; Li, C.-F. Antibacterial activity of N-alkylated disaccharide chitosan derivatives. Int. J. Food Microbiol. 2005, 97, 237–245. [Google Scholar] [CrossRef]
- Jin, H.; Wang, Z. Advances in Alkylated Chitosan and Its Applications for Hemostasis. Macromol 2022, 2, 346–360. [Google Scholar] [CrossRef]
- Chen, Z.; Yao, X.; Liu, L.; Guan, J.; Liu, M.; Li, Z.; Yang, J.; Huang, S.; Wu, J.; Tian, F.; et al. Blood coagulation evaluation of N -alkylated chitosan. Carbohydr. Polym. 2017, 173, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wu, L.; Yan, H.; Jiang, Z.; Li, S.; Li, W.; Bai, Y.; Wang, H.; Cheng, Z.; Kong, D.; et al. Microchannelled alkylated chitosan sponge to treat noncompressible hemorrhages and facilitate wound healing. Nat. Commun. 2021, 12, 4733. [Google Scholar] [CrossRef]
- Sun, X.; Li, J.; Shao, K.; Su, C.; Bi, S.; Mu, Y.; Zhang, K.; Cao, Z.; Wang, X.; Chen, X.; et al. A composite sponge based on alkylated chitosan and diatom-biosilica for rapid hemostasis. Int. J. Biol. Macromol. 2021, 182, 2097–2107. [Google Scholar] [CrossRef]
- Chen, Z.; Han, L.; Liu, C.; Du, Y.; Hu, X.; Du, G.; Shan, C.; Yang, K.; Wang, C.; Li, M.; et al. A rapid hemostatic sponge based on large, mesoporous silica nanoparticles and N-alkylated chitosan. Nanoscale 2018, 10, 20234–20245. [Google Scholar] [CrossRef]
- Huang, Y.; Zhang, Y.; Feng, L.; He, L.; Guo, R.; Xue, W. Synthesis of N-alkylated chitosan and its interactions with blood. Artif. Cells Nanomed. Biotechnol. 2018, 46, 544–550. [Google Scholar] [CrossRef]
- Žigrayová, D.; Mikušová, V.; Mikuš, P. Advances in Chitosan Derivatives: Preparation, Properties and Applications in Pharmacy and Medicine. Gels 2024, 10, 701. [Google Scholar] [CrossRef] [PubMed]
- Toufik, E.M.; Noukrati, H.; Rey, C.; Coppel, Y.; Charvillat, C.; Barroug, A.; Ben Youcef, H.; Combes, C. Phosphorylated chitosan as a hydrosoluble additive for bioactive calcium carbonate cements: Elaboration, setting mechanism, and handling properties. Ceram. Int. 2023, 49, 34780–34794. [Google Scholar] [CrossRef]
- Zheng, B.; Mao, C.; Gu, T.; Pan, H.; Shao, C.; Sun, J.; Chen, C.; Tang, R.; Gu, X. Phosphorylated chitosan to promote biomimetic mineralization of type I collagen as a strategy for dentin repair and bone tissue engineering. New J. Chem. 2019, 43, 2002–2010. [Google Scholar] [CrossRef]
- Almajidi, Y.Q.; Muslim, R.K.; Issa, A.A.; Al-Musawi, M.H.; Shahriari-Khalaji, M.; Mirhaj, M. Three-dimensional printed polyelectrolyte construct containing mupirocin-loaded quaternized chitosan nanoparticles for skin repair. Int. J. Biol. Macromol. 2024, 280, 136214. [Google Scholar] [CrossRef]
- Wang, L.; Zhao, Z.; Dong, J.; Li, D.; Dong, W.; Li, H.; Zhou, Y.; Liu, Q.; Deng, B. Mussel-Inspired Multifunctional Hydrogels with Adhesive, Self-Healing, Antioxidative, and Antibacterial Activity for Wound Healing. ACS Appl. Mater. Interfaces 2023, 15, 16515–16525. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Ren, Y.; Chang, R.; He, Y.; Zhang, D.; Guan, F.; Yao, M. Injectable Self-Healing Adhesive Chitosan Hydrogel with Antioxidative, Antibacterial, and Hemostatic Activities for Rapid Hemostasis and Skin Wound Healing. ACS Appl. Mater. Interfaces 2022, 14, 34455–34469. [Google Scholar] [CrossRef]
- Lazaridou, M.; Moroni, S.; Klonos, P.; Kyritsis, A.; Bikiaris, D.N.; Lamprou, D. 3D-printed hydrogels based on amphiphilic chitosan derivative loaded with levofloxacin for wound healing applications. Int. J. Polym. Mater. Polym. Biomater. 2025, 74, 67–84. [Google Scholar] [CrossRef]
- Wang, L.; Ding, X.; Li, J.; Li, M.; Ding, P.; Guo, W.; Wu, Q.; Sun, Y.; Jiang, G.; Okoro, O.V.; et al. Genipin crosslinked quaternary ammonium chitosan hydrogels for wound dressings. Biomed. Mater. 2024, 19, 045042. [Google Scholar] [CrossRef]
- Wenxiu, L.; Guojiang, H.; Liying, Q.; Wenli, D.; Baoqin, H.; Liming, J.; Yan, Y. Fabrication of bioactive glass/phosphorylated chitosan composite scaffold and its effects on MC3T3-E1 cells. Biomed. Mater. 2024, 19, 025002. [Google Scholar] [CrossRef]
- Ke, Y.; Ye, Y.; Wu, J.; Ma, Y.; Fang, Y.; Jiang, F.; Yu, J. Phosphoserine-loaded chitosan membranes promote bone regeneration by activating endogenous stem cells. Front. Bioeng. Biotechnol. 2023, 11, 1096532. [Google Scholar] [CrossRef]
- Liu, L.; Miao, Y.; Shi, X.; Gao, H.; Wang, Y. Phosphorylated Chitosan Hydrogels Inducing Osteogenic Differentiation of Osteoblasts via JNK and p38 Signaling Pathways. ACS Biomater. Sci. Eng. 2020, 6, 1500–1509. [Google Scholar] [CrossRef]
- Ji, M.; Li, F.; Li, J.; Li, J.; Wang, X.; Zhang, C.; Peng, S.; Man, J. Physical, antibacterial, blood coagulation, and healing promotion evaluations of chitosan derivative-based composite films. Int. J. Biol. Macromol. 2024, 278, 134714. [Google Scholar] [CrossRef]
- Li, Q.; Chen, Z.; Darvell, B.W.; Liu, L.; Jiang, H.; Zen, Q.; Peng, Q.; Ou, G. Chitosan-phosphorylated chitosan polyelectrolyte complex hydrogel as an osteoblast carrier. J. Biomed. Mater. Res. Part B Appl. Biomater. 2007, 82, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Ji, L.; Yu, Y.; Zhu, F.; Huang, D.; Wang, X.; Wang, J.; Liu, C. 2-N, 6-O sulfated chitosan evokes periosteal stem cells for bone regeneration. Bioact. Mater. 2024, 34, 282–297. [Google Scholar] [CrossRef] [PubMed]
- Sahraneshin-Samani, F.; Kazemi-Ashtiani, M.; Karimi, H.; Shiravandi, A.; Baharvand, H.; Daemi, H. Regioselective sulfated chitosan produces a biocompatible and antibacterial wound dressing with low inflammatory response. Mater. Sci. Eng. C 2022, 139, 213020. [Google Scholar] [CrossRef]
- Yu, Y.; Dai, K.; Gao, Z.; Tang, W.; Shen, T.; Yuan, Y.; Wang, J.; Liu, C. Sulfated polysaccharide directs therapeutic angiogenesis via endogenous VEGF secretion of macrophages. Sci. Adv. 2021, 7, eabd8217. [Google Scholar] [CrossRef]
- Shu, Y.; Yu, Y.; Zhang, S.; Wang, J.; Xiao, Y.; Liu, C. The immunomodulatory role of sulfated chitosan in BMP-2-mediated bone regeneration. Biomater. Sci. 2018, 6, 2496–2507. [Google Scholar] [CrossRef]
- Luo, P.; Liu, W.; Ye, Z.; Zhang, Y.; Zhang, Z.; Yi, J.; Zeng, R.; Yang, S.; Tu, M. 26SCS-Loaded SilMA/Col Composite Sponge with Well-Arranged Layers Promotes Angiogenesis-Based Diabetic Wound Repair by Mediating Macrophage Inflammatory Response. Molecules 2024, 29, 1832. [Google Scholar] [CrossRef]
- Zhang, Y.-X.; Miao, Y.-T.; Li, Z.-Y.; Li, M.-X.; Zou, X.-N.; Chu, L.-Q. Efficient One-Pot Synthesis of Carboxymethyl Chitosan Hydrogel for Sustained Release of Ibuprofen and Sulfadiazine. ACS Appl. Polym. Mater. 2024, 6, 15355–15365. [Google Scholar] [CrossRef]
- Sahiner, M.; Yilmaz, A.S.; Ayyala, R.S.; Sahiner, N. Carboxymethyl Chitosan Microgels for Sustained Delivery of Vancomycin and Long-Lasting Antibacterial Effects. Gels 2023, 9, 708. [Google Scholar] [CrossRef]
- Bratskaya, S.; Boroda, A.; Bogomaz, T.; Privar, Y.; Maiorova, M.; Malyshev, D.; Shindina, A.; Skatova, A.; Goncharuk, R. Antimicrobial Zn2+-Carboxymethyl Chitosan Cryogel for Controlled Loading and Release of Ciprofloxacin via Coordination Bonds. Gels 2024, 10, 841. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, S.; Cui, S.; Jing, X.; Feng, Y.; Coseri, S. Rapid self-healing carboxymethyl chitosan/hyaluronic acid hydrogels with injectable ability for drug delivery. Carbohydr. Polym. 2023, 328, 121707. [Google Scholar] [CrossRef] [PubMed]
- Xiong, M.; Chen, Y.; Hu, H.-J.; Cheng, H.; Li, W.-X.; Tang, S.; Hu, X.; Lan, L.-M.; Zhang, H.; Jiang, G.-B. Multifunctional pH-responsive hydrogel dressings based on carboxymethyl chitosan: Synthesis, characterization fostering the wound healing. Carbohydr. Polym. 2024, 341, 122348. [Google Scholar] [CrossRef]
- Samuel, S.E.; Ghosh, T.; Nayak, A.D.; Deveswaran, R.; Basavaraj, B. Exploring self-crosslinked hydrogel: Carboxymethyl chitosan and oxidized alginate for wound healing applications. J. Drug Deliv. Sci. Technol. 2024, 97, 105746. [Google Scholar] [CrossRef]
- Ghosh, T.; Rajamanickam, D.; Nayak, D.; Srinivasan, B.; BV, B. Physicochemical and in vivo evaluation of crosslinked carboxymethyl chitosan-gelatin scaffolds for wound healing application. Mater. Today Commun. 2023, 37, 107307. [Google Scholar] [CrossRef]
- Alven, S.; Aderibigbe, B.A. Chitosan and Cellulose-Based Hydrogels for Wound Management. Int. J. Mol. Sci. 2020, 21, 9656. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Liu, Y.; Wu, J.; Xu, J.; Wei, W.; Yuan, Z.; Han, H.; Bu, L.; Song, Z. Chitosan-based double-network hydrogel with synergistic photothermal/nitric oxide therapy for methicillin-resistance Staphylococcus aureus infected wound healing. J. Colloid Interface Sci. 2025, 700, 138520. [Google Scholar] [CrossRef]
- Kumi, M.; Hou, Z.; Zhang, Y.; Yang, Y.; Han, C.; Wang, T.; Li, P. 3D Printed Chitosan-Based Flexible Electrode with Antimicrobial Properties for Electrical Stimulation Therapy in Wound Healing. Supramol. Mater. 2025, 4, 100110. [Google Scholar] [CrossRef]
- Xiao, Q.; Peng, R.; Fang, T.; Zhou, M.; Liu, K.; Qiu, W.; Cen, Y.; Dai, L.; Zhou, D.; Wu, J.; et al. Gellan gum and carboxymethyl chitosan-based acid-responsive hydrogels modulate the ROS-macrophage axis to expedite burn wound healing. Carbohydr. Polym. 2025, 369, 124285. [Google Scholar] [CrossRef]
- Ren, M.; Yao, J.; Yang, D.; Zhu, J.; Dai, K.; Zhong, Y.; Zhu, J.; Tang, L.; Xu, Y.; Yu, J. Chitosan hydrogels loaded with Cu3SnS4 NSs for the treatment of second-degree burn wounds. Sci. Rep. 2025, 15, 12449. [Google Scholar] [CrossRef]
- Gao, Q.; Hu, F.; Chai, Z.; Zheng, C.; Zhang, W.; Pu, K.; Yang, Z.; Zhang, Y.; Ramrkrishna, S.; Wu, X.; et al. Multifunctional hydrogel with mild photothermal properties enhances diabetic wound repair by targeting MRSA energy metabolism. J. Nanobiotechnol. 2025, 23, 380. [Google Scholar] [CrossRef]
- Yu, L.; Dai, Z.; Huang, Y.; Tang, S.; Zhou, L.; Zhao, X.; Que, X.; Shi, R.; Zhou, J.; Dong, J.; et al. A temperature-sensitive chitosan hydrogels loaded with nano-zinc oxide and exosomes from human umbilical vein endothelial cells accelerates wound healing. Regen. Ther. 2025, 30, 63–74. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wu, H.; Zhang, L. Multifunctional nanocomposite chitosan-based hydrogel promotes healing of infected wounds through sustained release of Zn2+ and nicotinamide mononucleotide. Carbohydr. Polym. 2025, 368, 124111. [Google Scholar] [CrossRef]
- Salarizadeh, N.; Sabour, E.S.; Hushmandi, K. Two-layered sodium alginate/polyvinyl alcohol/chitosan-based nanocomposite wound dressing with Hyssopus officinalis extract for enhanced wound healing. Ind. Crops Prod. 2025, 235, 121736. [Google Scholar] [CrossRef]
- Cai, F.; Liu, B.; Jiang, T.; Li, Z.; Yang, J.; Zheng, R. Chitosan-based hemostatic bandage with in-situ formed silica nanoparticles for rapid bleeding control and wound healing promotion. Colloids Surf. B Biointerfaces 2025, 255, 114891. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Li, X.; Zhu, Y.; Hu, Y.; Zhang, S.; Tong, Z.; Zhou, Y.; Chen, Y. Fungal-derived chitosan-based hydrogels with antimicrobial properties for infectious wound healing. Carbohydr. Polym. 2025, 366, 123917. [Google Scholar] [CrossRef]
- Dai, L.; Geng, Y.; Ding, X.; Zhang, Z.; Lai, C.; Zhang, D.; Xia, C.; Lai, Y. Highly stretchable, self-adhesive, and biocompatible cellulose/chitosan based double network hydrogel for wound dressing. Carbohydr. Polym. 2025, 366, 123869. [Google Scholar] [CrossRef]
- Wang, J.; Ye, H.; He, K.; Jiang, W.; Lan, W.; Zeng, Z.; Lin, P.; Wang, H.; Gao, B.; Wang, W.; et al. Chitosan-based Janus dressing with asymmetric wettability for rapid hemostasis, antibacterial protection, and tissue regeneration in hemorrhagic wound management. Carbohydr. Polym. 2025, 366, 123891. [Google Scholar] [CrossRef]
- Long, Y.; Liu, X.; Huang, F.; Peng, L.; Duan, Y.; Bai, G.; Zhu, Z.; Su, D. Normal-pressure-prepared chitosan carbonate liquid dressing: Spontaneous transformation into a pure chitosan water-resistant film and enhanced wound repair. Carbohydr. Polym. 2025, 366, 123805. [Google Scholar] [CrossRef]
- Dong, D.; Peng, H.; Gan, C.; Hu, X.; Yu, J.; Zhao, K.; Qin, Z.; Feng, S.; Liang, J.; Liu, Y.; et al. Injectable chitosan-based hydrogels harnessing resveratrol micelles for treatment of infected wounds. Mater. Des. 2025, 258, 114559. [Google Scholar] [CrossRef]
- Witkowska, K.; Paczkowska-Walendowska, M.; Miklaszewski, A.; Plech, T.; Michniak-Kohn, B.; Swora-Cwynar, E.; Cielecka-Piontek, J. Development of 3D-Printed chitosan-based hydrogels rich in Centella asiatica extract for enhanced wound healing applications. J. Drug Deliv. Sci. Technol. 2025, 111, 107143. [Google Scholar] [CrossRef]
- Chelminiak-Dudkiewicz, D.; Dlugaszewska, J.; Wujak, M.; Smolarkiewicz-Wyczachowski, A.; Mylkie, K.; Machacek, M.; Ziegler-Borowska, M. Chitosan- and levan-based wound dressings crosslinked with dialdehyde levan: Structural and biological stability under climatic aging. Int. J. Biol. Macromol. 2025, 321, 146207. [Google Scholar] [CrossRef]
- Alexpandi, R.; Abirami, G.; Balaji, M.; Sathiyaraj, G.; Ma, X.; Lei, C.; Ravi, A.V.; Cai, Y. Proteomic insights into the multi-target mechanism and therapeutic application of citronellol-loaded carboxymethyl chitosan-based hydrogel for wound infection treatment. Int. J. Biol. Macromol. 2025, 323, 147179. [Google Scholar] [CrossRef] [PubMed]
- Tien, N.D.; Geng, T.; Coelho, D.; Reseland, J.E.; Lyngstadaas, S.P.; Blaker, J.J.; Haugen, H.J. Multilayer gradient chitosan fiber scaffolds for skin tissue regeneration with enhanced mechanical strength and cellular infiltration. React. Funct. Polym. 2025, 214, 106276. [Google Scholar] [CrossRef]
- Mathew, D.; Thomas, B.; Soumya, P.; Sudheep, N. Development of chitosan based hydrogels with marigold flower extract: An innovative, low cost, biodegradable and antimicrobial solution for enhanced wound healing applications. Results Surf. Interfaces 2025, 20, 100602. [Google Scholar] [CrossRef]
- Sharun, K.; Banu, S.A.; Mamachan, M.; Subash, A.; Karikalan, M.; Kumar, R.; Vinodhkumar, O.R.; Dhama, K.; Pawde, A. Amarpal Development and characterization of contraction-suppressed full-thickness skin wound model in rabbits. Tissue Cell 2024, 90, 102482. [Google Scholar] [CrossRef] [PubMed]
- Rössler, S.; Nischwitz, S.P.; Luze, H.; Holzer-Geissler, J.C.J.; Zrim, R.; Kamolz, L.-P. In Vivo Models for Hypertrophic Scars—A Systematic Review. Medicina 2022, 58, 736. [Google Scholar] [CrossRef]
- Zhang, N.; Tian, H.; Zong, W.; Fan, Q.; Hua, J.; Wang, J.; Tu, Q. Chitosan based cryogel loaded with zeolitic imidazolate framework-67 and glucose oxidase enabling hemostasis and diabetic wound healing. Carbohydr. Polym. 2025, 363, 123709. [Google Scholar] [CrossRef]
- Durand, A.; Pin, D.; Rita, M.; Kuznietsova, H.; Dziubenko, N.; Lysenko, V.; Tillement, A.; Legastelois, S.; Montembault, A.; David, L.; et al. Chitosan@DOTAGA-based hydrogel: A metal-chelating dressing for the treatment of complex wounds. Int. J. Biol. Macromol. 2025, 320, 145884. [Google Scholar] [CrossRef]
- Sasmal, P.K.; Samanta, S.; Dasgupta, S.; Nandi, S.K.; Chanda, A.; Datta, P. Oyster shell powder reinforced chitosan-poly(vinyl alcohol) freeze-dried composite sponge for on-site hemorrhage control. J. Biomater. Appl. 2025, 40, 487–499. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, S.; Zhou, J.; Zhang, Z.; Hu, Y.; Xu, Q.; Zhou, Y.; Xu, Y. An injectable triple-responsive chitosan-based hydrogel encapsulating QCT-Cu self-assembled nanoparticles accelerating diabetic wound healing via remodeling the local microenvironment. Carbohydr. Polym. 2025, 363, 123706. [Google Scholar] [CrossRef]
- Zhu, Y.; Ali, A.; dos Santos, G.M.; Franciscon, J.P.S.; de Molon, R.S.; Goh, C.; Ervolino, E.; Theodoro, L.H.; Shrestha, A. A Chitosan-based Hydrogel to Modulate Immune Cells and Promote Periodontitis Healing in the High-Fat Diet-induced Periodontitis Rat Model. Acta Biomater. 2025, 200, 452–463. [Google Scholar] [CrossRef]
- Chen, G.; Xun, X.; Ao, H.; Chen, Z.; Wang, D.; Wang, M.; Zhang, D.; Liu, M.; Guo, G. Quaternized chitosan-based injectable self-healing hydrogel for improving wound management in aging populations. Colloids Surf. B Biointerfaces 2025, 253, 114721. [Google Scholar] [CrossRef]
- Elbialy, N.S.; Aboushoushah, S.F.; Mohamed, N.; Abaza, S.F. Chitosan-MXene-silver nanocomposite film with improved physicochemical and biological functionalities to potentiate wound healing process. J. Drug Deliv. Sci. Technol. 2025, 111, 107133. [Google Scholar] [CrossRef]
- Iftime, M.M.; Ailiesei, G.L.; Morariu, S.; Sandu, A.-I.; Rambu, C.M.; Marin, L. Functional biocompatible chitosan hydrogels crosslinked with a vanillin isomer: Controlled antioxidant, antimicrobial, and self-healing properties. Int. J. Biol. Macromol. 2025, 319, 145411. [Google Scholar] [CrossRef]
- Zhou, Y.-Q.; Deng, X.; Zhao, Z.-X.; Wang, X.-T.; Jin, X.-Q.; Xiong, J.-B.; Chen, L.-L.; Guo, W.-H.; Zhou, R.-B.; Yin, D.-C. High-strength chitosan-sericin cryogel with synergistically reinforced networks for hemostasis. Int. J. Biol. Macromol. 2025, 318, 145038. [Google Scholar] [CrossRef]
- Lei, Y.; Wang, S.; Liu, Q.; Zeng, D.; Wang, K.; Chang, C.; Zhang, B.; Zhang, L.; Shi, Z.; Meng, Y. Construction of AgNPs-loaded oriented hydrogel based on Periostracum Cicadae chitosan by electro-assembly for rapid hemostasis and wound healing. Carbohydr. Polym. 2025, 358, 123500. [Google Scholar] [CrossRef] [PubMed]
- Yildirim, M.; Dogan, K.; Yiğin, A.; Cimentepe, M.; Necip, A.; Amangeldinova, M.; Dellal, Ö. A breakthrough in infection control: Niaouli oil–chitosan hydrogels for enhanced wound healing. Polym. Bull. 2025, 82, 7265–7288. [Google Scholar] [CrossRef]
- Silva, J.M.; Carvalho, J.P.; Teixeira, M.C.; Facchinatto, W.M.; Braz, M.; Almeida, A.; Oliveira, H.; Vilela, C.; Branco, P.C.; Martins, J.; et al. Xylan-chitosan based films with deep eutectic solvents for wound healing applications. Int. J. Biol. Macromol. 2025, 320, 145482. [Google Scholar] [CrossRef]
- Han, Q.; Zhao, F.; Wu, T.; Zhao, C.; Li, J.; Huang, S.; Zhang, T.; Xing, J. Gelatin/quaternized chitosan-based macroporous sponge inspired by food foaming for rapid hemostasis and wound healing. Int. J. Biol. Macromol. 2025, 320, 146117. [Google Scholar] [CrossRef] [PubMed]
- Saini, A.; Kumar, M.; Bhatt, S.; Saini, V.; Malik, A. Cancer causes and treatments. Int. J. Pharm. Sci. Res. 2020, 11, 3121. [Google Scholar] [CrossRef]
- Iwamoto, T. Clinical Application of Drug Delivery Systems in Cancer Chemotherapy: Review of the Efficacy and Side Effects of Approved Drugs. Biol. Pharm. Bull. 2013, 36, 715–718. [Google Scholar] [CrossRef] [PubMed]
- Salehi, F.; Behboudi, H.; Kavoosi, G.; Ardestani, S.K. Chitosan promotes ROS-mediated apoptosis and S phase cell cycle arrest in triple-negative breast cancer cells: Evidence for intercalative interaction with genomic DNA. RSC Adv. 2017, 7, 43141–43150. [Google Scholar] [CrossRef]
- Peters, G.J.; Honeywell, R.J. Drug transport and metabolism of novel anticancer drugs. Expert Opin. Drug Metab. Toxicol. 2015, 11, 661–663. [Google Scholar] [CrossRef]
- Ng, C.Y.; Chen, C.-B.; Wu, M.-Y.; Wu, J.; Yang, C.-H.; Hui, R.C.-Y.; Chang, Y.-C.; Lu, C.-W. Anticancer Drugs Induced Severe Adverse Cutaneous Drug Reactions: An Updated Review on the Risks Associated with Anticancer Targeted Therapy or Immunotherapies. J. Immunol. Res. 2018, 2018, 5376476. [Google Scholar] [CrossRef]
- Narvekar, M.; Xue, H.Y.; Eoh, J.Y.; Wong, H.L. Nanocarrier for Poorly Water-Soluble Anticancer Drugs—Barriers of Translation and Solutions. AAPS PharmSciTech 2014, 15, 822–833. [Google Scholar] [CrossRef] [PubMed]
- Ioele, G.; Chieffallo, M.; Occhiuzzi, M.A.; De Luca, M.; Garofalo, A.; Ragno, G.; Grande, F. Anticancer Drugs: Recent Strategies to Improve Stability Profile, Pharmacokinetic and Pharmacodynamic Properties. Molecules 2022, 27, 5436. [Google Scholar] [CrossRef]
- Yu, X.; Yu, H.; Yang, C.; Wu, C.; Cui, Y.; Xu, N. Gellan gum/chitosan-based bilayer scaffold for the targeted delivery of curcumin and green tea, designed to enhance breast cancer treatment paradigms. Carbohydr. Polym. 2025, 368, 124159. [Google Scholar] [CrossRef]
- Mirzaie, Z.H.; Irani, S.; Mirfakhraie, R.; Atyabi, S.M.; Dinarvand, M.; Dinarvand, R.; Varshochian, R.; Atyabi, F. Docetaxel–Chitosan nanoparticles for breast cancer treatment: Cell viability and gene expression study. Chem. Biol. Drug Des. 2016, 88, 850–858. [Google Scholar] [CrossRef]
- Payomhom, P.; Panyain, N.; Sakonsinsiri, C.; Wongtrakoongate, P.; Lertsuwan, K.; Pissuwan, D.; Katewongsa, K.P. Chitosan-Coated Poly(lactic-co-glycolic acid) Nanoparticles Loaded with Ursolic Acid for Breast Cancer Therapy. ACS Appl. Nano Mater. 2024, 7, 5383–5395. [Google Scholar] [CrossRef]
- Olaoye, L.; Sadraddin, A.; Braim, S. Smart chitosan-based microbead formulation for colon-targeted delivery of lactoferrin. Int. J. Appl. Pharm. 2024, 16, 382–387. [Google Scholar] [CrossRef]
- Ibrahim, I.A.A.; Alzahrani, A.R.; Alanazi, I.M.; Shahzad, N.; Shahid, I.; Falemban, A.H.; Azlina, M.F.N.; Arulselvan, P. Chitosan biopolymer functionalized with graphene oxide and titanium dioxide with Escin metallic nanocomposites for anticancer potential against colon cancer. Int. J. Biol. Macromol. 2023, 253, 127334. [Google Scholar] [CrossRef]
- Chang, P.-H.; Sekine, K.; Chao, H.-M.; Hsu, S.-H.; Chern, E. Chitosan promotes cancer progression and stem cell properties in association with Wnt signaling in colon and hepatocellular carcinoma cells. Sci. Rep. 2017, 8, 45751. [Google Scholar] [CrossRef] [PubMed]
- Qu, D.; Jiao, M.; Lin, H.; Tian, C.; Qu, G.; Xue, J.; Xue, L.; Ju, C.; Zhang, C. Anisamide-functionalized pH-responsive amphiphilic chitosan-based paclitaxel micelles for sigma-1 receptor targeted prostate cancer treatment. Carbohydr. Polym. 2020, 229, 115498. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bharali, D.J.; Adhami, V.M.; Siddiqui, I.A.; Cui, H.; Shabana, S.M.; Mousa, S.A.; Mukhtar, H. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis 2014, 35, 415–423. [Google Scholar] [CrossRef]
- Xu, J.; Yu, J.; Xu, X.; Wang, L.; Liu, Y.; Li, L.; Zhao, J.; He, M. Development, Characterization, and Evaluation of PSMA-Targeted Glycol Chitosan Micelles for Prostate Cancer Therapy. J. Nanomater. 2014, 2014, 462356. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Son, S.; Lee, S.J.; Gil You, D.; Yhee, J.Y.; Park, J.H.; Swierczewska, M.; Lee, S.; Kwon, I.C.; Kim, S.H.; et al. Glycol chitosan nanoparticles as specialized cancer therapeutic vehicles: Sequential delivery of doxorubicin and Bcl-2 siRNA. Sci. Rep. 2014, 4, 6878. [Google Scholar] [CrossRef] [PubMed]
- Dhas, N.L.; Ige, P.P.; Kudarha, R.R. Design, optimization and in-vitro study of folic acid conjugated-chitosan functionalized PLGA nanoparticle for delivery of bicalutamide in prostate cancer. Powder Technol. 2015, 283, 234–245. [Google Scholar] [CrossRef]
- Aghasafari, P.; George, U.; Pidaparti, R. A review of inflammatory mechanism in airway diseases. Inflamm. Res. 2019, 68, 59–74. [Google Scholar] [CrossRef]
- Mohan, S.; Gupta, D. Crosstalk of toll-like receptors signaling and Nrf2 pathway for regulation of inflammation. Biomed. Pharmacother. 2018, 108, 1866–1878. [Google Scholar] [CrossRef]
- Xu, J.; Chang, L.; Xiong, Y.; Peng, Q. Chitosan-Based Hydrogels as Antibacterial/Antioxidant/Anti-Inflammation Multifunctional Dressings for Chronic Wound Healing. Adv. Healthc. Mater. 2024, 13, e2401490. [Google Scholar] [CrossRef]
- Zhong, H.; Fang, Y.; Luo, M.; Wang, L.; Huang, J.; Dai, G.; Liu, K.; Wu, J.; Du, J. Deferoxamine-Loaded Injectable Chitosan-Grafted Chlorogenic Acid/Oxidized Hyaluronic Acid Hybrid Hydrogel with Antibacterial, Anti-inflammatory, and Angiogenesis-Promoting Properties for Diabetic Wound Repair. ACS Appl. Mater. Interfaces 2024, 16, 28209–28221. [Google Scholar] [CrossRef]
- Goyal, S.; Thirumal, D.; Rana, J.; Gupta, A.K.; Kumar, A.; Babu, M.A.; Kumar, P.; Sindhu, R.K. Chitosan based nanocarriers as a promising tool in treatment and management of inflammatory diseases. Carbohydr. Polym. Technol. Appl. 2024, 7, 100442. [Google Scholar] [CrossRef]
- Lu, Y.; Lou, X.; Jiang, J.; Wang, J.; Peng, X.; Yao, H.; Wu, J. Antioxidative, Anti-Inflammatory, Antibacterial, Photo-Cross-Linkable Hydrogel of Gallic Acid–Chitosan Methacrylate: Synthesis, In Vitro, and In Vivo Assessments. Biomacromolecules 2024, 25, 4358–4373. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Chu, Y.; Pan, S.-J.; Tan, L. Drug-loading colloidal gels assembled from polymeric nanoparticles as an anti-inflammatory platform. New J. Chem. 2021, 45, 13796–13805. [Google Scholar] [CrossRef]
- Guan, X.; Yao, H.; Wu, J. Photocrosslinkable hydrogel of ibuprofen-chitosan methacrylate modulates inflammatory response. J. Biomed. Mater. Res. Part A 2024, 112, 2001–2017. [Google Scholar] [CrossRef]
- Liu, J.; Liu, H.; Jia, Y.; Tan, Z.; Hou, R.; Lu, J.; Luo, D.; Fu, X.; Wang, L.; Wang, X. Glucose-sensitive delivery of tannic acid by a photo-crosslinked chitosan hydrogel film for antibacterial and anti-inflammatory therapy. J. Biomater. Sci. Polym. Ed. 2022, 33, 1644–1663. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Yang, Z.; Tao, X.; Ma, C.; Cao, P.; Wei, P.; Jiang, C.; Ren, H.; Li, X. Sprayable chitosan nanogel with nitric oxide to accelerate diabetic wound healing through bacteria inhibition, biofilm eradication and macrophage polarization. Int. J. Biol. Macromol. 2023, 254, 127806. [Google Scholar] [CrossRef]
- Sun, M.; Deng, Z.; Shi, F.; Zhou, Z.; Jiang, C.; Xu, Z.; Cui, X.; Li, W.; Jing, Y.; Han, B.; et al. Rebamipide-loaded chitosan nanoparticles accelerate prostatic wound healing by inhibiting M1 macrophage-mediated inflammation via the NF-κB signaling pathway. Biomater. Sci. 2020, 8, 912–925. [Google Scholar] [CrossRef]
- Russo, C.; Piccioni, M.; Lorenzini, M.L.; Catalano, C.; Ambrogi, V.; Pagiotti, R.; Pietrella, D. Bud-Poplar-Extract-Embedded Chitosan Films as Multifunctional Wound Healing Dressing. Molecules 2022, 27, 7757. [Google Scholar] [CrossRef]
- Chen, X.; Zhang, M.; Wang, X.; Chen, Y.; Yan, Y.; Zhang, L.; Zhang, L. Peptide-modified chitosan hydrogels promote skin wound healing by enhancing wound angiogenesis and inhibiting inflammation. Am. J. Transl. Res. 2017, 9, 2352–2362. [Google Scholar]
- Egan, A.M.; Dinneen, S.F. What is diabetes? Medicine 2019, 47, 1–4. [Google Scholar] [CrossRef]
- Dilworth, L.; Facey, A.; Omoruyi, F. Diabetes Mellitus and Its Metabolic Complications: The Role of Adipose Tissues. Int. J. Mol. Sci. 2021, 22, 7644. [Google Scholar] [CrossRef]
- Karadeniz, F.; Kim, S.-K. Chapter Three—Antidiabetic Activities of Chitosan and Its Derivatives: A Mini Review. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2014; Volume 73, pp. 33–44. [Google Scholar] [CrossRef]
- Priyanka, D.N.; Harish Prashanth, K.V. Low molecular weight chitosan (~20 kDa) exhibits in vivo anti-hyperglycemic effects through AKT/PI3K/FOXO pathway. Carbohydr. Polym. Technol. Appl. 2024, 8, 100534. [Google Scholar] [CrossRef]
- Wadasinghe, R.R.; Kalansuriya, P.; Attanayake, A.P. Development, Characterization, in vitro Antidiabetic Activity of Chitosan−tripolyphosphate Nanoparticles Encapsulating Gmelina arborea Roxb. and Spondias pinnata (L.f) Kurz Aqueous Extracts. ChemistrySelect 2023, 8, e202302300. [Google Scholar] [CrossRef]
- Abdel-Baky, Y.M.; Omer, A.M.; El-Fakharany, E.M.; Ammar, Y.A.; Abusaif, M.S.; Ragab, A. Developing a new multi-featured chitosan-quinoline Schiff base with potent antibacterial, antioxidant, and antidiabetic activities: Design and molecular modeling simulation. Sci. Rep. 2023, 13, 22792. [Google Scholar] [CrossRef]
- Yang, J.; Liu, Y.; Deng, G.; Feng, J.; Yu, H.; Cen, X.; Li, H.; Huang, Q.; Zhang, H. Thermosensitive and injectable chitosan-based hydrogel embedding umbilical cord mesenchymal stem cells for β-cell repairing in type 2 diabetes mellitus. Int. J. Biol. Macromol. 2024, 279, 135546. [Google Scholar] [CrossRef]
- Maanvizhi, S.; Radhakrishnan, N.; Krishnan, C.; Gnanamani, A. Pharmacological evaluation of embelin—Chitosan nanoparticles as an antidiabetic agent. Indian J. Pharmacol. 2022, 54, 126–130. [Google Scholar] [CrossRef]
- Kabir, N.; Aliyu, A.S.; Jibrin, J.I. Synthesis and Evaluation of the Antidiabetic Potentials of Chitosan Combinations with Metformin and Momordica Balsamina Leaf Extract in Alloxan-Induced Diabetic Rats. Dutse J. Pure Appl. Sci. 2022, 7, 103–112. [Google Scholar] [CrossRef]
- Dambuza, A.; Rungqu, P.; Oyedeji, A.O.; Miya, G.; Oriola, A.O.; Hosu, Y.S.; Oyedeji, O.O. Therapeutic Potential of Pectin and Its Derivatives in Chronic Diseases. Molecules 2024, 29, 896. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Zou, Y.; Chi, H. Quantitative assessment of the effects of chitosan intervention on blood pressure control. Drug Des. Dev. Ther. 2018, 12, 67–75. [Google Scholar] [CrossRef]
- Park, S.-H.; Dutta, N.K.; Baek, M.-W.; Kim, D.-J.; Na, Y.-R.; Seok, S.-H.; Lee, B.-H.; Cho, J.-E.; Cho, G.-S.; Park, J.-H. NaCl plus chitosan as a dietary salt to prevent the development of hypertension in spontaneously hypertensive rats. J. Vet. Sci. 2009, 10, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-J.; Hwang, M.-J.; Shim, W.-G.; Youn, Y.-N.; Yoon, S.-D. Sustained drug release behavior of captopril-incorporated chitosan/carboxymethyl cellulose biomaterials for antihypertensive therapy. Int. J. Biol. Macromol. 2024, 255, 128087. [Google Scholar] [CrossRef] [PubMed]
- Batista, P.; Castro, P.; Madureira, A.R.; Sarmento, B.; Pintado, M. Development and Characterization of Chitosan Microparticles-in-Films for Buccal Delivery of Bioactive Peptides. Pharmaceuticals 2019, 12, 32. [Google Scholar] [CrossRef]
- Auwal, S.M.; Zarei, M.; Tan, C.P.; Basri, M.; Saari, N. Improved In Vivo Efficacy of Anti-Hypertensive Biopeptides Encapsulated in Chitosan Nanoparticles Fabricated by Ionotropic Gelation on Spontaneously Hypertensive Rats. Nanomaterials 2017, 7, 421. [Google Scholar] [CrossRef]
- Du, M.; Kou, L.; Li, S. Water-soluble chitosan regulates vascular remodeling in hypertension via NFATc1. Eur. Heart J. Suppl. 2016, 18, F38. [Google Scholar] [CrossRef] [PubMed]
- Danish, M.K.; Gleeson, J.P.; Brayden, D.J.; Byrne, H.J.; Frías, J.M.; Ryan, S.M. Formulation, Characterisation and Evaluation of the Antihypertensive Peptides, Isoleucine-Proline-Proline and Leucine-Lysine-Proline in Chitosan Nanoparticles Coated with Zein for Oral Drug Delivery. Int. J. Mol. Sci. 2022, 23, 11160. [Google Scholar] [CrossRef]
- Jensen, D.M.; Han, P.; Mangala, L.S.; Lopez-Berestein, G.; Sood, A.K.; Liu, J.; Kriegel, A.J.; Usa, K.; Widlansky, M.E.; Liang, M. Broad-acting therapeutic effects of miR-29b-chitosan on hypertension and diabetic complications. Mol. Ther. 2022, 30, 3462–3476. [Google Scholar] [CrossRef]
- Mittal, A.; Singh, A.; Hong, H.; Nazeer, R.A.; Benjakul, S. Chitooligosaccharide and its conjugates with different polyphenols: Antihypertensive activity and blood pressure lowering effects in spontaneously hypertensive rats. J. Funct. Foods 2024, 113, 106039. [Google Scholar] [CrossRef]
- Murata, Y.; Nagaki, K.; Kofuji, K.; Kishi, T. Functions of Chitosan-Ferulic Acid Salt for Prevention of Hypertension. Food Sci. Technol. Res. 2010, 16, 437–442. [Google Scholar] [CrossRef]
- Cho, Y.; Shi, R.; Borgens, R.B. Chitosan produces potent neuroprotection and physiological recovery following traumatic spinal cord injury. J. Exp. Biol. 2010, 213, 1513–1520. [Google Scholar] [CrossRef]
- Pangestuti, R.; Kim, S.-K. Neuroprotective Properties of Chitosan and Its Derivatives. Mar. Drugs 2010, 8, 2117–2128. [Google Scholar] [CrossRef]
- Iyer, M.; Elangovan, A.; Sennimalai, R.; Babu, H.W.S.; Thiruvenkataswamy, S.; Krishnan, J.; Yadav, M.K.; Gopalakrishnan, A.V.; Narayanasamy, A.; Vellingiri, B. Chitosan—An alternative drug delivery approach for neurodegenerative diseases. Carbohydr. Polym. Technol. Appl. 2024, 7, 100460. [Google Scholar] [CrossRef]
- Khodaverdi, K.; Bakhshi, A.; Mozafari, M.; Naghib, S.M. A review of chitosan-based nanocarriers as drug delivery systems for brain diseases: Critical challenges, outlooks and promises. Int. J. Biol. Macromol. 2024, 278, 134962. [Google Scholar] [CrossRef]
- Dadkhah, M.; Afshari, S.; Samizadegan, T.; Shirmard, L.R.; Barin, S. Pegylated chitosan nanoparticles of fluoxetine enhance cognitive performance and hippocampal brain derived neurotrophic factor levels in a rat model of local demyelination. Exp. Gerontol. 2024, 195, 112533. [Google Scholar] [CrossRef]
- Lin, S.; Huang, A.P.; Hsu, S. Injectable, Micellar Chitosan Self-Healing Hydrogel for Asynchronous Dual-Drug Delivery to Treat Stroke Rats. Adv. Funct. Mater. 2023, 33, 2303853. [Google Scholar] [CrossRef]
- Wang, D.; Wang, K.; Liu, Z.; Wang, Z.; Wu, H. Valproic Acid Labeled Chitosan Nanoparticles Promote the Proliferation and Differentiation of Neural Stem Cells After Spinal Cord Injury. Neurotox. Res. 2021, 39, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Saleem, S.; Banerjee, R.; Kannan, R.R. Chrysin-Loaded Chitosan Nanoparticle-Mediated Neuroprotection in Aβ1–42-Induced Neurodegenerative Conditions in Zebrafish. ACS Chem. Neurosci. 2022, 13, 2017–2034. [Google Scholar] [CrossRef] [PubMed]
- Sierakowska-Byczek, A.; Gałuszka, A.; Janus, Ł.; Radwan-Pragłowska, J. Bioactive Three-Dimensional Chitosan-Based Scaffolds Modified with Poly(dopamine)/CBD@Pt/Au/PVP Nanoparticles as Potential NGCs Applicable in Nervous Tissue Regeneration—Preparation and Characterization. Molecules 2024, 29, 5376. [Google Scholar] [CrossRef]
- Ragusa, A.; Priore, P.; Giudetti, A.M.; Ciccarella, G.; Gaballo, A. Neuroprotective Investigation of Chitosan Nanoparticles for Dopamine Delivery. Appl. Sci. 2018, 8, 474. [Google Scholar] [CrossRef]
- Bashir, D.J.; Manzoor, S.; Khan, I.A.; Bashir, M.; Agarwal, N.B.; Rastogi, S.; Arora, I.; Samim, M. Nanonization of Magnoflorine-Encapsulated Novel Chitosan–Collagen Nanocapsules for Neurodegenerative Diseases: In Vitro Evaluation. ACS Omega 2022, 7, 6472–6480. [Google Scholar] [CrossRef]
- Liu, K.; Wu, X.; Dai, H. Citric acid cross-linked chitosan for inhibiting oxidative stress after nerve injury. J. Biomed. Mater. Res. Part B Appl. Biomater. 2022, 110, 2231–2240. [Google Scholar] [CrossRef]
- Wang, Y.-W.; Liu, C.-C.; Cherng, J.-H.; Lin, C.-S.; Chang, S.-J.; Hong, Z.-J.; Liu, C.-C.; Chiu, Y.-K.; Hsu, S.-D.; Chang, H. Biological Effects of Chitosan-Based Dressing on Hemostasis Mechanism. Polymers 2019, 11, 1906. [Google Scholar] [CrossRef]
- Ramos-Zúñiga, R.; López-González, F.; Segura-Durán, I. Bilaminar Chitosan Scaffold for Sellar Floor Repair in Transsphenoidal Surgery. Front. Bioeng. Biotechnol. 2020, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Kao, C.-C.; Huang, S.-Y.; Chiang, C.-H.; Lin, C.-H.; Chang, T.-C. Microencapsulated rhEGF to facilitate epithelial healing and prevent scar formation of cesarean wound: A randomized controlled trial. Taiwan. J. Obstet. Gynecol. 2021, 60, 468–473. [Google Scholar] [CrossRef] [PubMed]
- ElGendy, M.H.; Fetoh, S.A.; Salem, S.E.; Daihom, B.A.; Fahmy, E.M.; ElMeligie, M.M. Effectiveness of chitosan phonophoresis on ulnar nerve conduction velocity, pain relief, and functional outcomes for mild to moderate cubital tunnel syndrome: A double-blind randomized controlled trial. J. Hand Ther. 2024, 37, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.clinicaltrials.gov/search?term=Chitosan&viewType=Table (accessed on 14 September 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).