3D Bioprinting Functional Engineered Heart Tissues
Abstract
1. Introduction
2. Three-Dimensional Bioprinting in Cardiac Tissue Engineering
3. Bioprinting Techniques
3.1. Jestting-Based Bioprinting
3.1.1. Inkjet-Based Bioprinting
3.1.2. Laser-Assisted Bioprinting
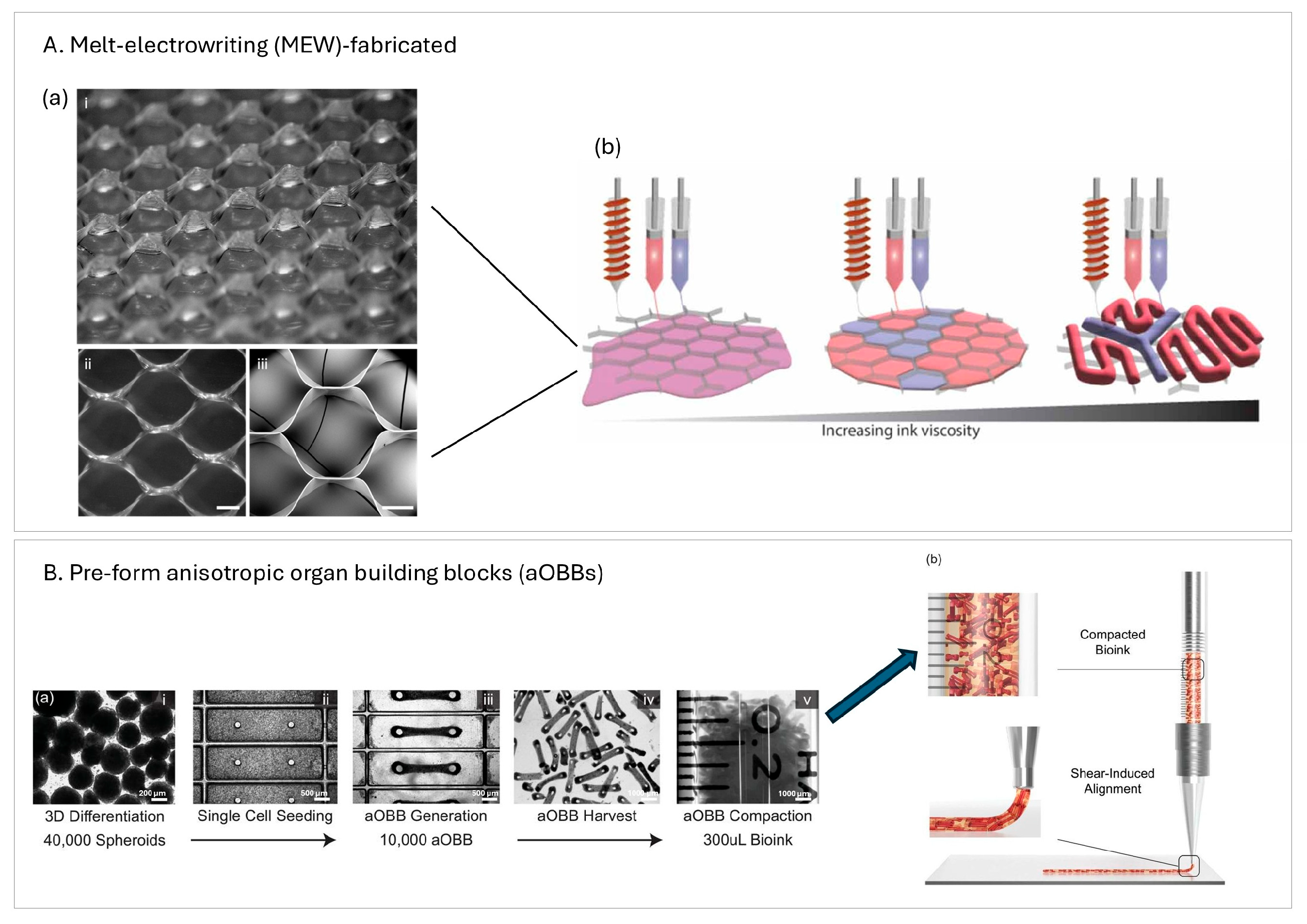
3.1.3. Electrohydrodynamic Jet
3.2. Stereolithography and Digital Light Processing (DLP)
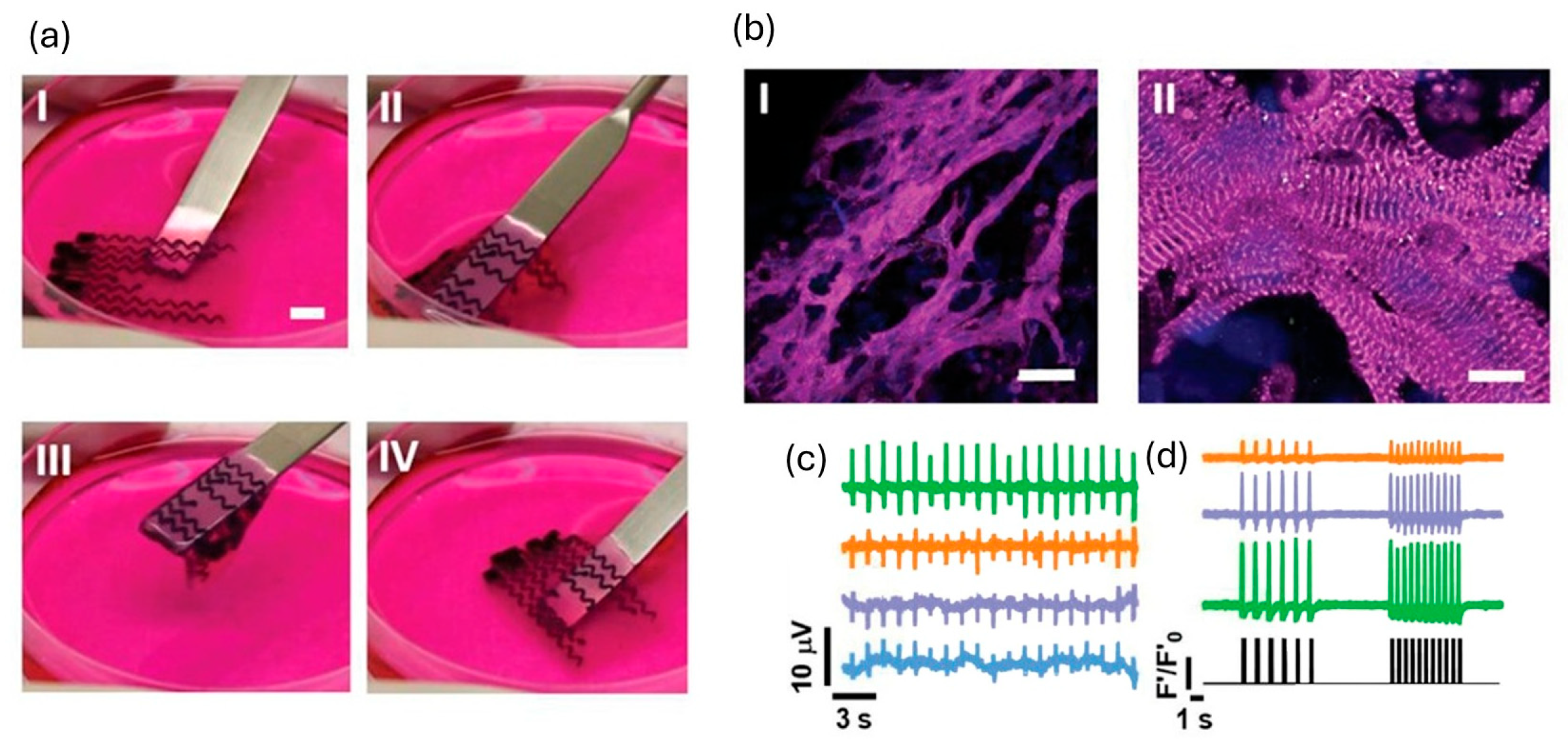
3.3. Extrusion-Based Bioprinting
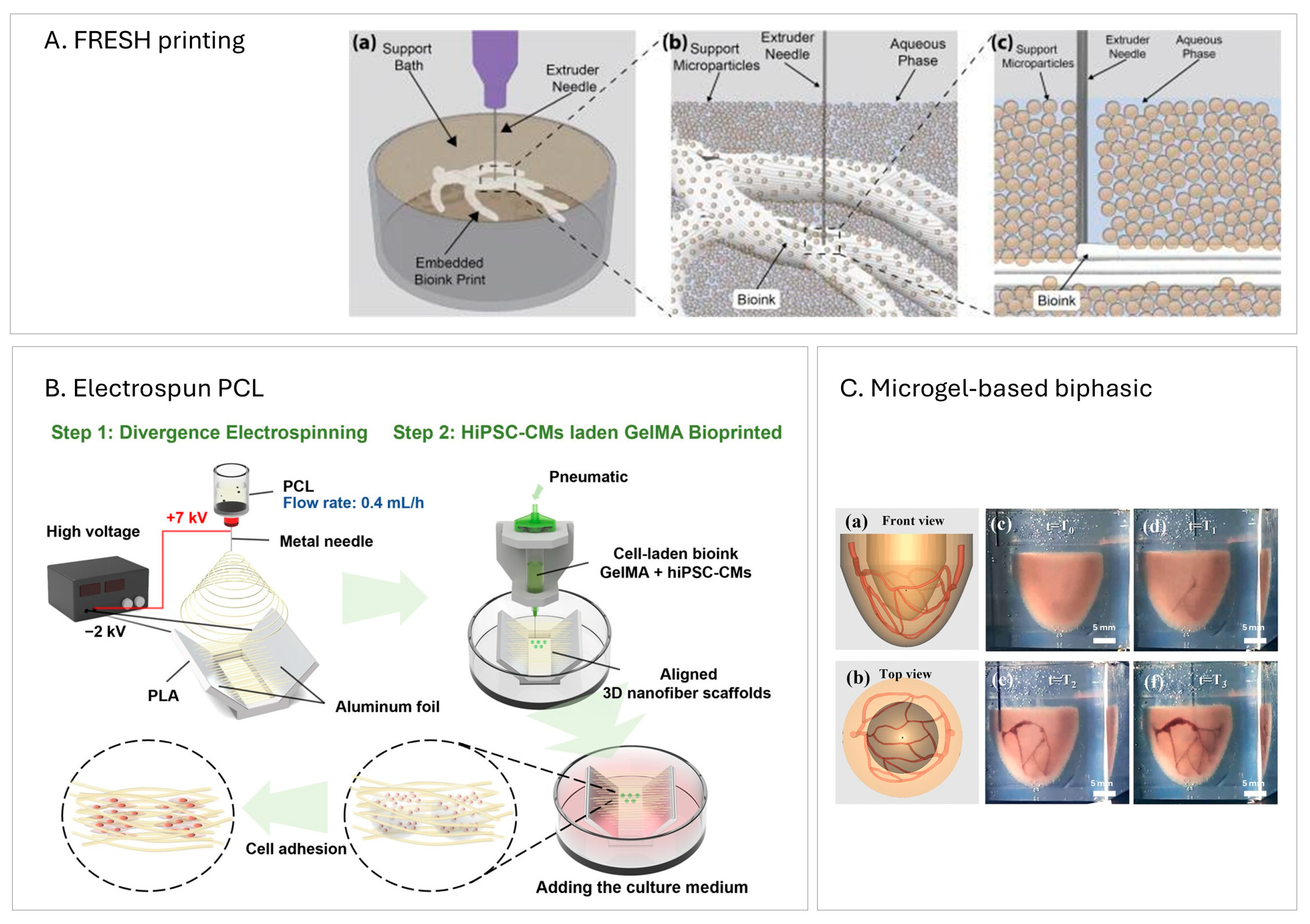
Suspended/Embedding Bioprinting
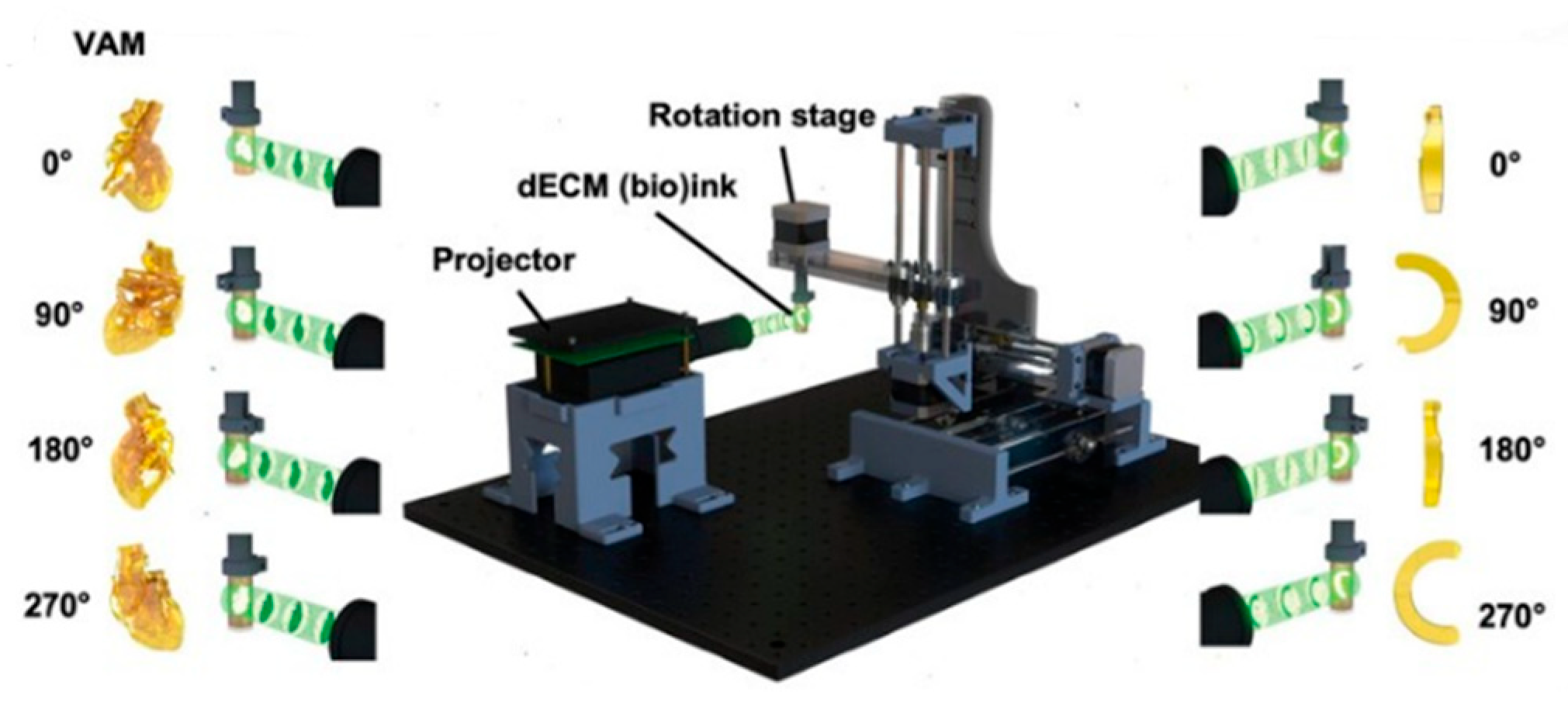
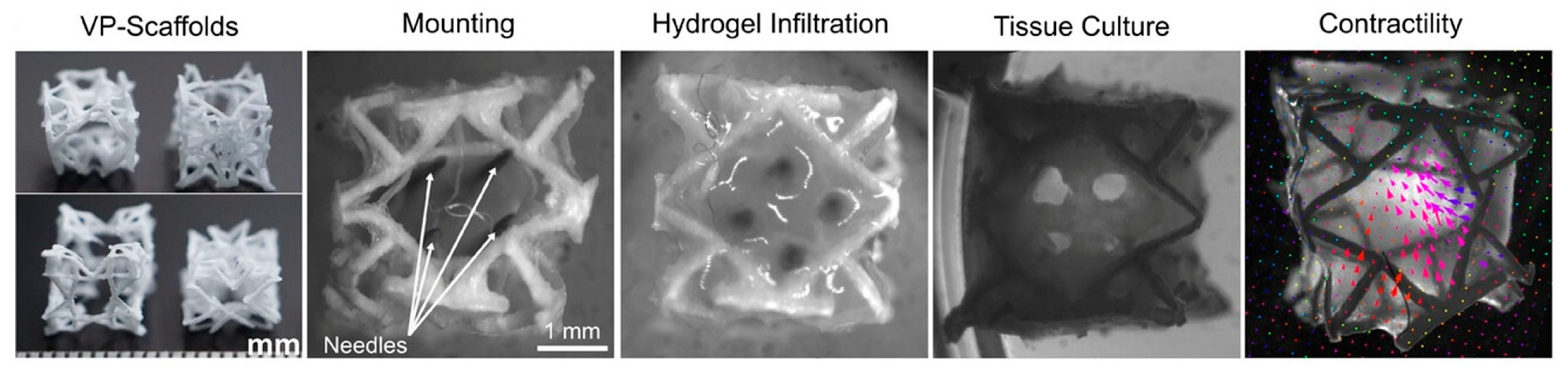
3.4. Volumetric Bioprinting
| Technique | Resolution [μm] | Bioink Viscosity Range [mPa·s] | Cell Concentration Range | |
|---|---|---|---|---|
| Jetting-based [21] | Inkjet | ~20–100 | ~3–10 | ~107 cells/mL |
| LIFT | ~10–50 | 10–300 | ~108 cells/mL | |
| EHD | <10 | ~1–100 | ~107–108 cells/mL | |
| Stereolithography [29] | 150–200 | Not provided | ~106 cells/mL | |
| DLP [32,33] | ~30–50 (XY-axis) | Not provided | ~5 × 106 cells/mL | |
| Extrusion-based | ~250–300 [11,53] | 100–105 [5,53] | ~106 cells/mL [5,38] | |
| FRESH printing | ~10–25 [43] | ~10–100 [39,41] | ~107–108 cells/mL [39,43] | |
| Volumetric | ~30–200 [50,51] | Not provided | ~107 cells/mL [51] | |
4. Bioinks and Biomaterial Inks
4.1. Bioink Characteristics
4.2. Hybrid Hydrogel
4.3. Conductive Composite Hydrogel
4.3.1. Carbon-Based Nanomaterials
4.3.2. Metallic Particles
4.3.3. Conductive Polymer
| Conductive Material | Other Composites in Bioink/Biomaterial | Conductivity [mS cm−1] | Cell Viability | |
|---|---|---|---|---|
| Jalilinejad et al. [61] | Native adult myocardium | - | 1.6 along; 0.05 across | - |
| Mousavi et al. [53] | Reduced graphene oxide | GelMA/AlgMA | 1.0 | >85% by day 7 |
| Tsui et al. [62] | dECM | ~0.9–3.1 | >90% after 35 days | |
| Mehrotra et al. [36] | Carbon nanotubes | nSF/PEGDMA/GelMA | Resistance ~93.6 kΩ (conductivity not reported) | Not provided |
| Basara et al. [63] | MXene | PEG | ~1.1 × 107 | >90% by day 7 |
| Zhu et al. [65] | Gold nanorods | GelMA | Resistance ~50 kΩ (conductivity not reported) | >70% after day 1 |
| Ramirez et al. [66] | Gold nanoparticles | Alginate/gelatin | Not provided | >80% by day 2 |
| Testore et al. [67] | PEDOT:PSS | PEGDA/gelatin | 65.3 ± 4.8 | >80% after day 1 |
| Roshanbinfar et al. [68] | PEDOT:PSS | collagen | ~0.00013 ± 0.00003 | >85% at day 40 |
4.4. Shape-Morphing Bionks
4.5. Bioinks in Volumetric Bioprinting
5. Cell Types and Co-Culture Strategies
5.1. Cardiomyocytes
5.2. Co-Culture
6. Applications
6.1. Cardiac Patches
6.1.1. Recent Bioprinting Techniques
6.1.2. Cell Type
6.1.3. Bioinks Functionalization
6.2. Cardiac Tissues
6.2.1. Structural Patterning
6.2.2. Vascularization
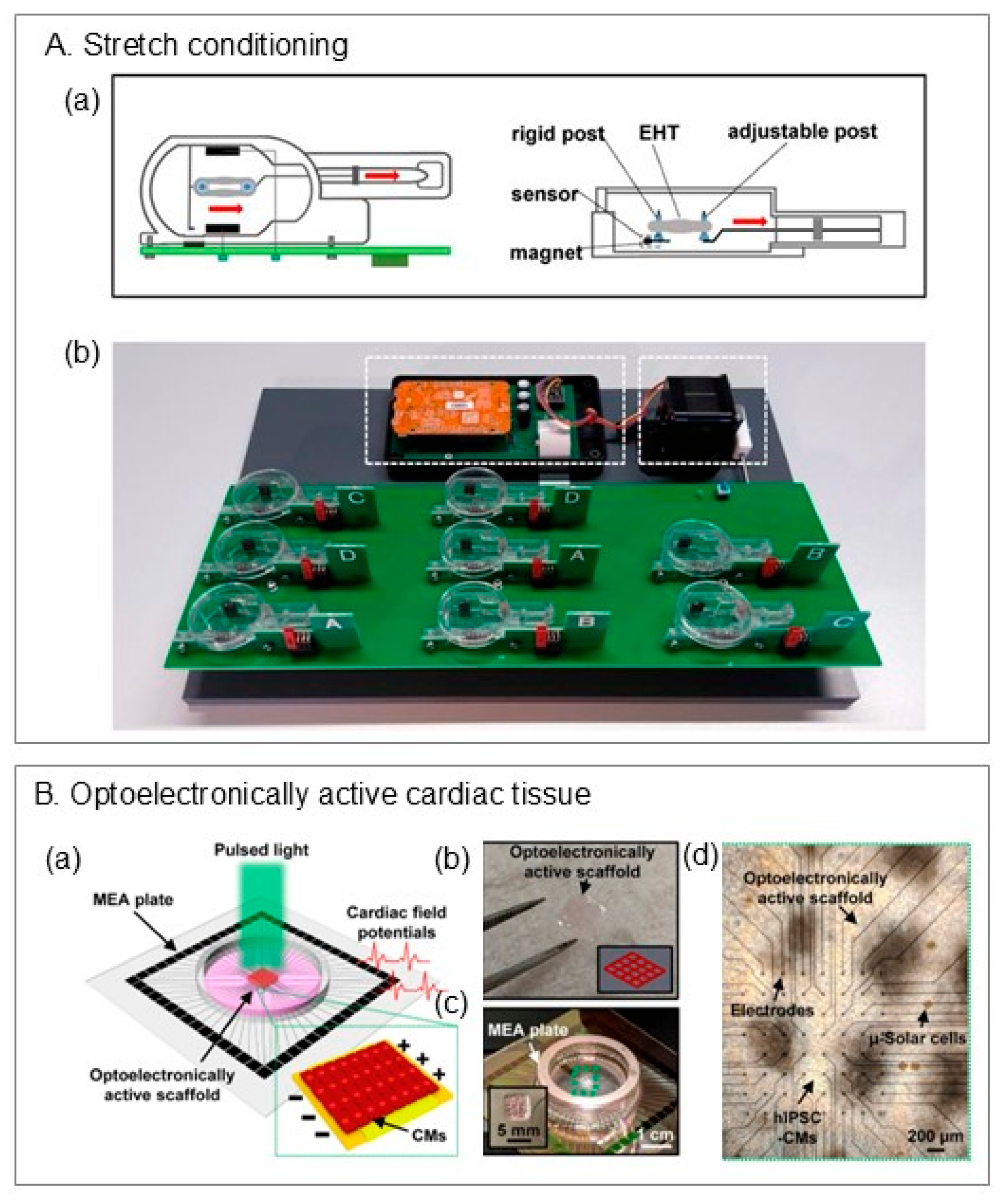
6.2.3. Stimulations
| Materials | Printing Method or Technique | Sarcomere Alignment | Sarcomere Length | Beat Rate | Contractile Force | Conduction Velocity | Expressions | Other Observations | |
|---|---|---|---|---|---|---|---|---|---|
| Mature adult cardiomyocytes (CMs) | - | - | - | ~2.2 µm [32] | ~60–100 beats/min (bpm) | 40–80 mN/mm2 [105] | ~0.3–1 m/s [105] | ↑ Sarcomere/contractile: Myh6, Myl2/Myl3, Tnni3, Tnnt2, Ttn; ↑ Calcium handling and coupling: Ryr2, Casq2 (Csq2), Atp2a2/SERCA2a, Pln, Gja1 (Connexin-43); ↑ Mito/FAO metabolism: Fabp3, Cox6c, Uqcr11, Cpt1b, Cox8b (adult isoform); ↑ S100a2 ↓ immature contractile and glycolysis: Myh7 (fetal myosin; mouse ventricle), Pgam1, Ldha, Cox8a (prenatal isoform); ↓ Proliferation/cell cycle: Mki67, Ccna2, Ccnb1/2, Top2a, Prc1; ↓ Transient CM–ECM (CME+) program: Col1a1/Col3a1 [106,107] | |
| Ahrens et al. [38] | hiPSC-CMs, fibroblasts, collagen-based | Extrusion-based printing | ~0.4 (1 = all orientation identical, 0 = orientations random) | sarcomere alignment measured by α-actinin positive cell area, not quantified | ~0.8 Hz (=48 bpm, spontaneous) | ~2 mN | ~0.042 m/s | connexin 43 (CX43), N-cadherin, cTnT | - |
| Wu et al. [39] | hiPSC-CMs, PCL, GelMA | Electrospinning and extrusion-based printing | - | ~2.2 µm | 2 beats/230 s (=0.5 bpm, spontaneous) | - | - | Sarcomeric α-actinin, CX43 | More elongated nuclei |
| Mao et al. [14] | hiPSC-CMs, ECs, PCL, fibrin | Melt-electrohydrodynamic | Clustered ~0° and 180° | ~2 µm | ~30 bpm (spontaneous) | - | ~10–14 pixels/s | Sarcomeric α-actinin, CX43, CD31; sarcomeric markers: MYOM2, MYL2; excitation–contraction: S100A1, CASQ2, GJA5, SCN5A; metabolic: CKMT2, PDK4 | More synchronized calcium waves, ~2.5 V/cm excitation thresholds |
| Lee et al. [41] | CMs, CF, ECs, MeTro/GelMA | Extrusion-based printing | - | - | ~43 ± 3 bpm (spontaneous) | - | - | Sarcomeric α-actinin, CD31 | Permeability coefficient of 0.8 × 10−3 cm/s; |
| Fabres et al. [90] | CMs, SMC, ECs, FBs, fibrin + Matrigel | microfluidics | - | - | ~2.2 Hz (=132 bpm, spontaneous) | ~0.12 mN | ~1 mm/s | ↑ Junction/EC barrier: CDH5, CLDN5, TJP1/2, PTPRB, ESAM; ↑ ECM remodel: MMP2/9/16, COL1/3/12, ITGA5; ↑ Connexins: GJA1/5/4; ↑ FA uptake/FAO: LPL–GPIHBP1–CD36, CPT1B/2, ACADVL, ACAT1; ↓ Glucose transport: GLUT2/4, HK1; | APD90 (stimulated at 2 Hz): ~180 ms; Permeability assay: Dextran-40 kDa FITC influx |
| Lu et al. [12] | hiPSC-CMs | Casting | - | 2.19 ± 0.1 µm (under high stretch) | 1 Hz (=60 bpm, after 1 week stimulation at 1 Hz) | ~11.3 mN/mm2 (under high stretch) | - | ↑ Adult phenotype: MYH7; ↑ excitation–contraction coupling, ↑ oxidative phosphorylation, and ↑ β-oxidation genes | Resting membrane potential: ~−72 mV; action potential (AP) amplitude: ~100 mV; AP upstroke velocity: ~13.5 V/s |
| Ershad et al. [104] | hiPSC-CMs | Extrusion-based printing | - | - | 72 bpm (under light) | - | - | Sarcomeric α-actinin, connexin-43, cTnT | Cell viability >96% under light stimulation (no phototoxicity). |
6.2.4. Prolonged 3D Culture
6.2.5. Disease Modeling
6.3. Cardiac Organoids
7. Limitations and Future Directions
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shadrin, I.Y.; Allen, B.W.; Qian, Y.; Jackman, C.P.; Carlson, A.L.; Juhas, M.E.; Bursac, N. Cardiopatch Platform Enables Maturation and Scale-up of Human Pluripotent Stem Cell-Derived Engineered Heart Tissues. Nat. Commun. 2017, 8, 1825. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, T.; Fortunato, G.M.; Hann, S.Y.; Ayan, B.; Vajanthri, K.Y.; Presutti, D.; Cui, H.; Chan, A.H.P.; Costantini, M.; Onesto, V.; et al. Recent Advances in Bioprinting Technologies for Engineering Cardiac Tissue. Mater. Sci. Eng. C 2021, 124, 112057. [Google Scholar] [CrossRef]
- Heinrich, M.A.; Liu, W.; Jimenez, A.; Yang, J.; Akpek, A.; Liu, X.; Pi, Q.; Mu, X.; Hu, N.; Schiffelers, R.M.; et al. 3D Bioprinting: From Benches to Translational Applications. Small 2019, 15, 1805510. [Google Scholar] [CrossRef]
- Cho, S.; Discher, D.E.; Leong, K.W.; Vunjak-Novakovic, G.; Wu, J.C. Challenges and Opportunities for the next Generation of Cardiovascular Tissue Engineering. Nat. Methods 2022, 19, 1064–1071. [Google Scholar] [CrossRef]
- Budharaju, H.; Sundaramurthi, D.; Sethuraman, S. Efficient Dual Crosslinking of Protein–in–Polysaccharide Bioink for Biofabrication of Cardiac Tissue Constructs. Biomater. Adv. 2023, 152, 213486. [Google Scholar] [CrossRef] [PubMed]
- Ainsworth, M.J.; Chirico, N.; de Ruijter, M.; Hrynevich, A.; Dokter, I.; Sluijter, J.P.G.; Malda, J.; van Mil, A.; Castilho, M. Convergence of Melt Electrowriting and Extrusion-Based Bioprinting for Vascular Patterning of a Myocardial Construct. Biofabrication 2023, 15, 035025. [Google Scholar] [CrossRef]
- Nuñez Bernal, P.; Delrot, P.; Loterie, D.; Li, Y.; Malda, J.; Moser, C.; Levato, R.; Bernal, P.N.; Li, Y.; Malda, J.; et al. Volumetric Bioprinting of Complex Living-Tissue Constructs within Seconds. Adv. Mater. 2019, 31, 1904209. [Google Scholar] [CrossRef] [PubMed]
- Vettori, L.; Tran, H.A.; Mahmodi, H.; Filipe, E.C.; Wyllie, K.; Liu Chung Ming, C.; Cox, T.R.; Tipper, J.; Kabakova, I.V.; Rnjak-Kovacina, J.; et al. Silk Fibroin Increases the Elasticity of Alginate-Gelatin Hydrogels and Regulates Cardiac Cell Contractile Function in Cardiac Bioinks. Biofabrication 2024, 16, 035025. [Google Scholar] [CrossRef]
- Park, S.H.; Park, J.Y.; Ji, Y.B.; Ju, H.J.; Min, B.H.; Kim, M.S. An Injectable Click-Crosslinked Hyaluronic Acid Hydrogel Modified with a BMP-2 Mimetic Peptide as a Bone Tissue Engineering Scaffold. Acta Biomater. 2020, 117, 108–120. [Google Scholar] [CrossRef]
- Elkhoury, K.; Patel, D.; Gupta, N.; Vijayavenkataraman, S. Nanocomposite GelMA Bioinks: Toward Next-Generation Multifunctional 3D-Bioprinted Platforms. Small 2025, e05968. [Google Scholar] [CrossRef]
- Bera, A.K.; Rizvi, M.S.; Kn, V.; Pati, F. Engineering Anisotropic Tissue Analogues: Harnessing Synergistic Potential of Extrusion-Based Bioprinting and Extracellular Matrix-Based Bioink. Biofabrication 2024, 17, 015003. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.; Seidel, T.; Cao-Ehlker, X.; Dorn, T.; Batcha, A.M.N.; Schneider, C.M.; Semmler, M.; Volk, T.; Moretti, A.; Dendorfer, A.; et al. Progressive Stretch Enhances Growth and Maturation of 3D Stem-Cell-Derived Myocardium. Theranostics 2021, 11, 6138–6153. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, C.; Ren, J.; Pope, L.; Li, Y.; Mohandas, A.; Blanchard, R.; Duchi, S.; Onofrillo, C. Characterizing Bioinks for Extrusion Bioprinting: Printability and Rheology. Methods Mol. Biol. 2020, 2140, 111–133. [Google Scholar] [CrossRef]
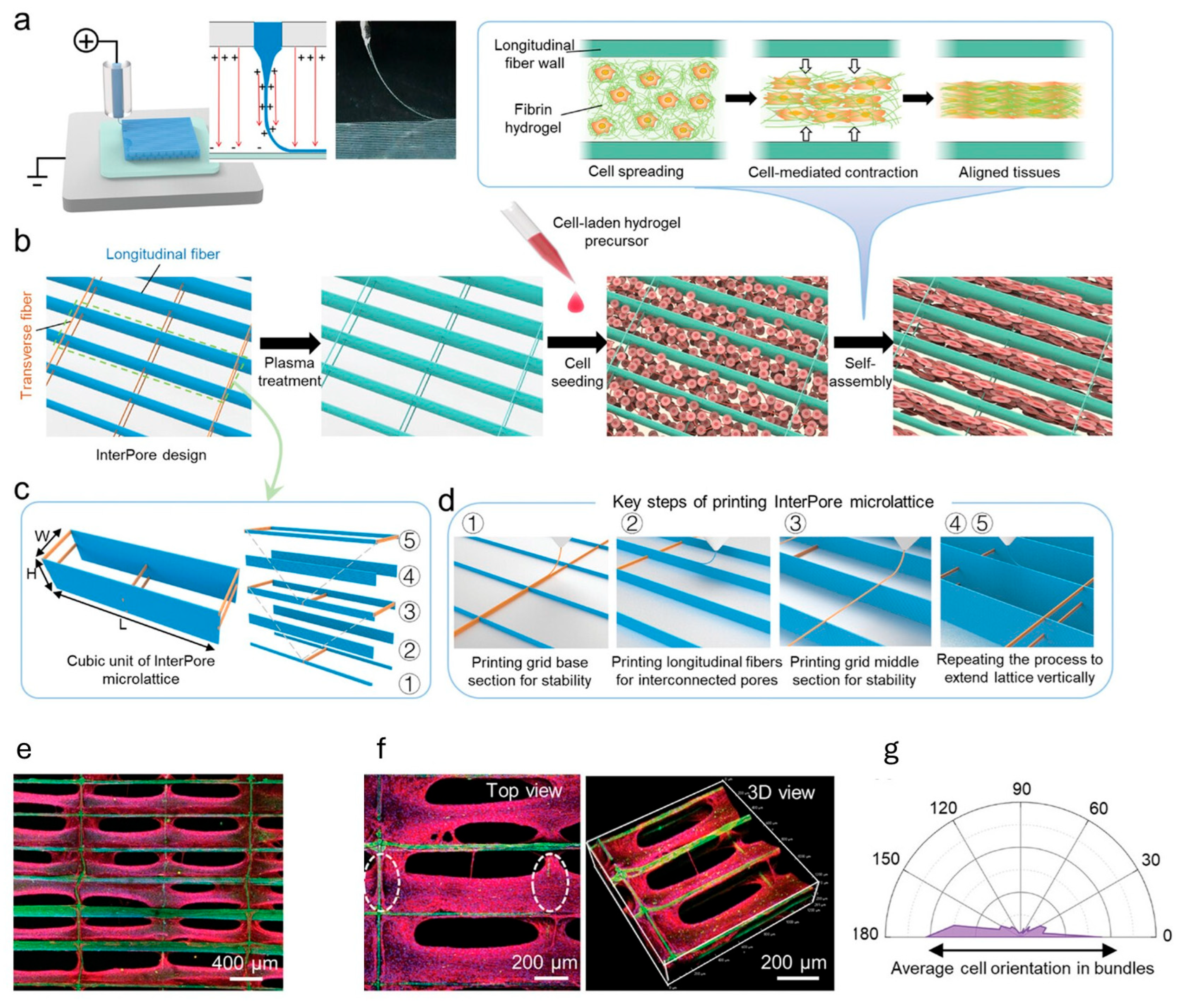
- Mao, M.; Han, K.; Gao, J.; Ren, Z.; Zhang, Y.; He, J.; Li, D. Engineering Highly Aligned and Densely Populated Cardiac Muscle Bundles via Fibrin Remodeling in 3D-Printed Anisotropic Microfibrous Lattices. Adv. Mater. 2025, 37, 2419380. [Google Scholar] [CrossRef]
- Liu, N.; Ye, X.; Yao, B.; Zhao, M.; Wu, P.; Liu, G.; Zhuang, D.; Jiang, H.; Chen, X.; He, Y.; et al. Advances in 3D Bioprinting Technology for Cardiac Tissue Engineering and Regeneration. Bioact. Mater. 2021, 6, 1388–1401. [Google Scholar] [CrossRef]
- Derakhshanfar, S.; Mbeleck, R.; Xu, K.; Zhang, X.; Zhong, W.; Xing, M. 3D Bioprinting for Biomedical Devices and Tissue Engineering: A Review of Recent Trends and Advances. Bioact. Mater. 2018, 3, 144–156. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef]
- Deo, K.A.; Singh, K.A.; Peak, C.W.; Alge, D.L.; Gaharwar, A.K. Bioprinting 101: Design, Fabrication, and Evaluation of Cell-Laden 3D Bioprinted Scaffolds. Tissue Eng. Part A 2020, 26, 318–338. [Google Scholar] [CrossRef]
- Wu, C.A.; Zhu, Y.; Woo, Y.J. Advances in 3D Bioprinting: Techniques, Applications, and Future Directions for Cardiac Tissue Engineering. Bioengineering 2023, 10, 842. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wan, L.Q.; Xiong, Z.; Marsano, A.; Maidhof, R.; Park, M.; Yan, Y.; Vunjak-Novakovic, G. Channelled Scaffolds for Engineering Myocardium with Mechanical Stimulation. J. Tissue Eng. Regen. Med. 2012, 6, 748–756. [Google Scholar] [CrossRef]
- Ng, W.L.; Shkolnikov, V. Jetting-Based Bioprinting: Process, Dispense Physics, and Applications. Biodes. Manuf. 2024, 7, 771–799, Erratum in Biodes. Manuf. 2024, 7, 823. [Google Scholar] [CrossRef]
- Pu, X.; Wu, Y.; Liu, J.; Wu, B. 3D Bioprinting of Microbial-Based Living Materials for Advanced Energy and Environmental Applications. Chem. Bio Eng. 2024, 1, 568–592. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Zhao, W.; Zhu, J.M.; Albanna, M.Z.; Yoo, J.J.; Atala, A. Complex Heterogeneous Tissue Constructs Containing Multiple Cell Types Prepared by Inkjet Printing Technology. Biomaterials 2013, 34, 130–139. [Google Scholar] [CrossRef]
- Zhu, H.; Li, R.; Li, S.; Guo, K.; Ji, C.; Gao, F.; Zheng, Y.; Zhu, R.; Wang, H.; Zhang, L.; et al. Multi-Physical Field Control Piezoelectric Inkjet Bioprinting for 3D Tissue-like Structure Manufacturing. Int. J. Bioprinting 2024, 10, 2120. [Google Scholar] [CrossRef]
- Yang, Z.; Tian, H.; Wang, C.; Li, X.; Chen, X.; Chen, X.; Shao, J. Actuation Waveform Optimization via Multi-Pulse Crosstalk Modulation for Stable Ultra-High Frequency Piezoelectric Drop-on-Demand Printing. Addit. Manuf. 2022, 60, 103165. [Google Scholar] [CrossRef]
- Koch, L.; Deiwick, A.; Soriano, J.; Chichkov, B. Laser Bioprinting of Human IPSC-Derived Neural Stem Cells and Neurons: Effect on Cell Survival, Multipotency, Differentiation, and Neuronal Activity. Int. J. Bioprinting 2023, 9, 672. [Google Scholar] [CrossRef]
- Ji, P.; Heinle, J.S.; Birla, R.K. Development of a Novel Method to Fabricate Highly Functional Human Purkinje Networks. bioRxiv 2024. [Google Scholar] [CrossRef]
- Cho, Y.H.; Kang, J.W.; Choi, S.H.; Yang, D.H.; Anh, T.T.X.; Shin, E.S.; Kim, Y.H. Reference Parameters for Left Ventricular Wall Thickness, Thickening, and Motion in Stress Myocardial Perfusion CT: Global and Regional Assessment. Clin. Imaging 2019, 56, 81–87. [Google Scholar] [CrossRef]
- Cui, H.; Liu, C.; Esworthy, T.; Huang, Y.; Yu, Z.X.; Zhou, X.; San, H.; Lee, S.J.; Hann, S.Y.; Boehm, M.; et al. 4D Physiologically Adaptable Cardiac Patch: A 4-Month in Vivo Study for the Treatment of Myocardial Infarction. Sci. Adv. 2020, 6, eabb5067. [Google Scholar] [CrossRef]
- Miao, S.; Cui, H.; Nowicki, M.; Lee, S.J.; Almeida, J.; Zhou, X.; Zhu, W.; Yao, X.; Masood, F.; Plesniak, M.W.; et al. Photolithographic-Stereolithographic-Tandem Fabrication of 4D Smart Scaffolds for Improved Stem Cell Cardiomyogenic Differentiation. Biofabrication 2018, 10, 035007. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cui, H.; Wang, Y.; Xu, C.; Esworthy, T.J.; Hann, S.Y.; Boehm, M.; Shen, Y.L.; Mei, D.; Zhang, L.G. 4D Printed Cardiac Construct with Aligned Myofibers and Adjustable Curvature for Myocardial Regeneration. ACS Appl. Mater. Interfaces 2021, 13, 12746–12758. [Google Scholar] [CrossRef]
- Yu, C.; Ma, X.; Zhu, W.; Wang, P.; Miller, K.L.; Stupin, J.; Koroleva-Maharajh, A.; Hairabedian, A.; Chen, S. Scanningless and Continuous 3D Bioprinting of Human Tissues with Decellularized Extracellular Matrix. Biomaterials 2019, 194, 1–13. [Google Scholar] [CrossRef]
- Liu, J.; Miller, K.; Ma, X.; Dewan, S.; Lawrence, N.; Whang, G.; Chung, P.; McCulloch, A.D.; Chen, S. Direct 3D Bioprinting of Cardiac Micro-Tissues Mimicking Native Myocardium. Biomaterials 2020, 256, 120204. [Google Scholar] [CrossRef] [PubMed]
- Grigoryan, B.; Paulsen, S.J.; Corbett, D.C.; Sazer, D.W.; Fortin, C.L.; Zaita, A.J.; Greenfield, P.T.; Calafat, N.J.; Gounley, J.P.; Ta, A.H.; et al. Multivascular Networks and Functional Intravascular Topologies Within Biocompatible Hydrogels. Science 2019, 364, 458–464. [Google Scholar] [CrossRef] [PubMed]
- Tijore, A.; Irvine, S.A.; Sarig, U.; Mhaisalkar, P.; Baisane, V.; Venkatraman, S. Contact Guidance for Cardiac Tissue Engineering Using 3D Bioprinted Gelatin Patterned Hydrogel. Biofabrication 2018, 10, 025003. [Google Scholar] [CrossRef]
- Mehrotra, S.; Singh, R.D.; Bandyopadhyay, A.; Janani, G.; Dey, S.; Mandal, B.B. Engineering Microsphere-Loaded Non-Mulberry Silk-Based 3D Bioprinted Vascularized Cardiac Patches with Oxygen-Releasing and Immunomodulatory Potential. ACS Appl. Mater. Interfaces 2021, 13, 50744–50759. [Google Scholar] [CrossRef]
- Maiullari, F.; Costantini, M.; Milan, M.; Pace, V.; Chirivì, M.; Maiullari, S.; Rainer, A.; Baci, D.; Marei, H.E.S.; Seliktar, D.; et al. A Multi-Cellular 3D Bioprinting Approach for Vascularized Heart Tissue Engineering Based on HUVECs and IPSC-Derived Cardiomyocytes. Sci. Rep. 2018, 8, 13532. [Google Scholar] [CrossRef] [PubMed]
- Ahrens, J.H.; Uzel, S.G.M.; Skylar-Scott, M.; Mata, M.M.; Lu, A.; Kroll, K.T.; Lewis, J.A. Programming Cellular Alignment in Engineered Cardiac Tissue via Bioprinting Anisotropic Organ Building Blocks. Adv. Mater. 2022, 34, 2200217. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Xu, F.; Jin, H.; Xue, M.; Zhang, W.; Yang, J.; Huang, J.; Jiang, Y.; Qiu, B.; Lin, B.; et al. 3D Nanofiber-Assisted Embedded Extrusion Bioprinting for Oriented Cardiac Tissue Fabrication. ACS Biomater. Sci. Eng. 2024, 10, 7256–7265. [Google Scholar] [CrossRef] [PubMed]
- Boularaoui, S.; Al Hussein, G.; Khan, K.A.; Christoforou, N.; Stefanini, C. An Overview of Extrusion-Based Bioprinting with a Focus on Induced Shear Stress and Its Effect on Cell Viability. Bioprinting 2020, 20, e00093. [Google Scholar] [CrossRef]
- Lee, S.; Sani, E.S.; Spencer, A.R.; Guan, Y.; Weiss, A.S.; Annabi, N. Human-Recombinant-Elastin-Based Bioinks for 3D Bioprinting of Vascularized Soft Tissues. Adv. Mater. 2020, 32, 2003915. [Google Scholar] [CrossRef] [PubMed]
- McCormack, A.; Highley, C.B.; Leslie, N.R.; Melchels, F.P.W. 3D Printing in Suspension Baths: Keeping the Promises of Bioprinting Afloat. Trends Biotechnol. 2020, 38, 584–593. [Google Scholar] [CrossRef]
- Lee, A.; Hudson, A.R.; Shiwarski, D.J.; Tashman, J.W.; Hinton, T.J.; Yerneni, S.; Bliley, J.M.; Campbell, P.G.; Feinberg, A.W. 3D Bioprinting of Collagen to Rebuild Components of the Human Heart. Science 2019, 365, 482–487. [Google Scholar] [CrossRef]
- Fang, Y.; Guo, Y.; Wu, B.; Liu, Z.; Ye, M.; Xu, Y.; Ji, M.; Chen, L.; Lu, B.; Nie, K.; et al. Expanding Embedded 3D Bioprinting Capability for Engineering Complex Organs with Freeform Vascular Networks. Adv. Mater. 2023, 35, 2205082. [Google Scholar] [CrossRef] [PubMed]
- Shiwarski, D.J.; Hudson, A.R.; Tashman, J.W.; Feinberg, A.W. Emergence of FRESH 3D Printing as a Platform for Advanced Tissue Biofabrication. APL Bioeng. 2021, 5, 010904. [Google Scholar] [CrossRef]
- Koti, P.; Muselimyan, N.; Mirdamadi, E.; Asfour, H.; Sarvazyan, N.A. Use of GelMA for 3D Printing of Cardiac Myocytes and Fibroblasts. J. 3D Print. Med. 2019, 3, 11–22. [Google Scholar] [CrossRef]
- Bonatti, A.F.; Vozzi, G.; Chua, C.K.; De Maria, C. A Deep Learning Quality Control Loop of the Extrusion-Based Bioprinting Process. Int. J. Bioprint 2022, 8, 307–320. [Google Scholar] [CrossRef]
- Sergis, V.; Kelly, D.; Pramanick, A.; Britchfield, G.; Mason, K.; Daly, A.C. In-Situ Quality Monitoring During Embedded Bioprinting Using Integrated Microscopy and Classical Computer Vision. Biofabrication 2025, 17, 025004. [Google Scholar] [CrossRef]
- Bernal, P.N.; Bouwmeester, M.; Madrid-Wolff, J.; Falandt, M.; Florczak, S.; Rodriguez, N.G.; Li, Y.; Größbacher, G.; Samsom, R.A.; van Wolferen, M.; et al. Volumetric Bioprinting of Organoids and Optically Tuned Hydrogels to Build Liver-like Metabolic Biofactories. Adv. Mater. 2022, 34, 2110054. [Google Scholar] [CrossRef]
- Lian, L.; Xie, M.; Luo, Z.; Zhang, Z.; Maharjan, S.; Mu, X.; Garciamendez-Mijares, C.E.; Kuang, X.; Sahoo, J.K.; Tang, G.; et al. Rapid Volumetric Bioprinting of Decellularized Extracellular Matrix Bioinks. Adv. Mater. 2024, 36, 2304846. [Google Scholar] [CrossRef] [PubMed]
- Jones, L.S.; Filippi, M.; Michelis, M.Y.; Balciunaite, A.; Yasa, O.; Aviel, G.; Narciso, M.; Freedrich, S.; Generali, M.; Tzahor, E.; et al. Multidirectional Filamented Light Biofabrication Creates Aligned and Contractile Cardiac Tissues. Adv. Sci. 2024, 11, 2404509. [Google Scholar] [CrossRef]
- Ribezzi, D.; Zegwaart, J.P.; Van Gansbeke, T.; Tejo-Otero, A.; Florczak, S.; Aerts, J.; Delrot, P.; Hierholzer, A.; Fussenegger, M.; Malda, J.; et al. Multi-Material Volumetric Bioprinting and Plug-and-Play Suspension Bath Biofabrication via Bioresin Molecular Weight Tuning and via Multiwavelength Alignment Optics. Adv. Mater. 2025, 37, 2409355. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, A.; Hedayatnia, A.; van Vliet, P.P.; Dartora, D.R.; Wong, N.; Rafatian, N.; Nuyt, A.M.; Moraes, C.; Ajji, A.; Andelfinger, G.; et al. Development of Photocrosslinkable Bioinks with Improved Electromechanical Properties for 3D Bioprinting of Cardiac BioRings. Appl. Mater. Today 2024, 36, 102035. [Google Scholar] [CrossRef]
- Groll, J.; Burdick, J.A.; Cho, D.W.; Derby, B.; Gelinsky, M.; Heilshorn, S.C.; Jüngst, T.; Malda, J.; Mironov, V.A.; Nakayama, K.; et al. A Definition of Bioinks and Their Distinction from Biomaterial Inks. Biofabrication 2018, 11, 013001. [Google Scholar] [CrossRef]
- Chopin-Doroteo, M.; Mandujano-Tinoco, E.A.; Krötzsch, E. Tailoring of the Rheological Properties of Bioinks to Improve Bioprinting and Bioassembly for Tissue Replacement. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2021, 1865, 129782. [Google Scholar] [CrossRef] [PubMed]
- Shin, Y.J.; Shafranek, R.T.; Tsui, J.H.; Walcott, J.; Nelson, A.; Kim, D.H. 3D Bioprinting of Mechanically Tuned Bioinks Derived from Cardiac Decellularized Extracellular Matrix. Acta Biomater. 2021, 119, 75–88. [Google Scholar] [CrossRef]
- Diamantides, N.; Wang, L.; Pruiksma, T.; Siemiatkoski, J.; Dugopolski, C.; Shortkroff, S.; Kennedy, S.; Bonassar, L.J. Correlating Rheological Properties and Printability of Collagen Bioinks: The Effects of Riboflavin Photocrosslinking and PH. Biofabrication 2017, 9, 034102. [Google Scholar] [CrossRef]
- Basara, G.; Ozcebe, S.G.; Ellis, B.W.; Zorlutuna, P. Tunable Human Myocardium Derived Decellularized Extracellular Matrix for 3D Bioprinting and Cardiac Tissue Engineering. Gels 2021, 7, 70. [Google Scholar] [CrossRef]
- Stola, G.P.; Paoletti, C.; Nicoletti, L.; Paul, G.; Cassino, C.; Marchese, L.; Chiono, V.; Marcello, E. Internally-Crosslinked Alginate Dialdehyde/Alginate/Gelatin-Based Hydrogels as Bioinks for Prospective Cardiac Tissue Engineering Applications. Int. J. Bioprinting 2024, 10, 4014. [Google Scholar] [CrossRef]
- Ketabat, F.; Maris, T.; Duan, X.; Yazdanpanah, Z.; Kelly, M.E.; Badea, I.; Chen, X. Optimization of 3D Printing and In Vitro Characterization of Alginate/Gelatin Lattice and Angular Scaffolds for Potential Cardiac Tissue Engineering. Front. Bioeng. Biotechnol. 2023, 11, 1161804. [Google Scholar] [CrossRef]
- Jalilinejad, N.; Rabiee, M.; Baheiraei, N.; Ghahremanzadeh, R.; Salarian, R.; Rabiee, N.; Akhavan, O.; Zarrintaj, P.; Hejna, A.; Saeb, M.R.; et al. Electrically Conductive Carbon-Based (Bio)-Nanomaterials for Cardiac Tissue Engineering. Bioeng. Transl. Med. 2023, 8, e10347. [Google Scholar] [CrossRef]
- Tsui, J.H.; Leonard, A.; Camp, N.D.; Long, J.T.; Nawas, Z.Y.; Chavanachat, R.; Smith, A.S.T.; Choi, J.S.; Dong, Z.; Ahn, E.H.; et al. Tunable Electroconductive Decellularized Extracellular Matrix Hydrogels for Engineering Human Cardiac Microphysiological Systems. Biomaterials 2021, 272, 120764. [Google Scholar] [CrossRef]
- Basara, G.; Saeidi-Javash, M.; Ren, X.; Bahcecioglu, G.; Wyatt, B.C.; Anasori, B.; Zhang, Y.; Zorlutuna, P. Electrically Conductive 3D Printed Ti3C2Tx MXene-PEG Composite Constructs for Cardiac Tissue Engineering. Acta Biomater. 2022, 139, 179–189. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, M.; Pereira, C.; Calmeiro, T.; Jana, S.; Fernandes, S.; Inácio, J.; Belo, J.; Matela, N.; Borges, J.P.; Fortunato, E.; et al. Biofabricated Electroactive Hydrogel Platforms for Cardiac Cell Stimulus with Alginate-Gelatin-Cnt/Mxene Matrices. Mxene Matrices 2025. [Google Scholar] [CrossRef]
- Zhu, K.; Shin, S.R.; van Kempen, T.; Li, Y.C.; Ponraj, V.; Nasajpour, A.; Mandla, S.; Hu, N.; Liu, X.; Leijten, J.; et al. Gold Nanocomposite Bioink for Printing 3D Cardiac Constructs. Adv. Funct. Mater. 2017, 27, 1605352. [Google Scholar] [CrossRef]
- Ramirez, S.P.; Hernandez, I.; Balcorta, H.V.; Kumar, P.; Kumar, V.; Poon, W.; Joddar, B. Microcomputed Tomography for the Microstructure Evaluation of 3D Bioprinted Scaffolds. ACS Appl. Bio Mater. 2024, 7, 7799–7808. [Google Scholar] [CrossRef] [PubMed]
- Testore, D.; Zoso, A.; Kortaberria, G.; Sangermano, M.; Chiono, V. Electroconductive Photo-Curable PEGDA-Gelatin/PEDOT:PSS Hydrogels for Prospective Cardiac Tissue Engineering Application. Front. Bioeng. Biotechnol. 2022, 10, 897575. [Google Scholar] [CrossRef] [PubMed]
- Roshanbinfar, K.; Schiffer, M.; Carls, E.; Angeloni, M.; Koleśnik-Gray, M.; Schruefer, S.; Schubert, D.W.; Ferrazzi, F.; Krstić, V.; Fleischmann, B.K.; et al. Electrically Conductive Collagen-PEDOT:PSS Hydrogel Prevents Post-Infarct Cardiac Arrhythmia and Supports HiPSC-Cardiomyocyte Function. Adv. Mater. 2024, 36, 2403642. [Google Scholar] [CrossRef]
- Gionet-Gonzales, M.; Gathman, G.; Rosas, J.; Kunisaki, K.Y.; Inocencio, D.G.P.; Hakami, N.; Milburn, G.N.; Pitenis, A.A.; Campbell, K.S.; Pruitt, B.L.; et al. Stress Relaxation Rates of Myocardium from Failing and Non-Failing Hearts. bioRxiv 2024. [Google Scholar] [CrossRef]
- Wang, J.; Gao, H.; Hu, Y.; Zhang, N.; Zhou, W.; Wang, C.; Binks, B.P.; Yang, Z. 3D Printing of Pickering Emulsion Inks to Construct Poly(D,L-lactide-co-trimethylene carbonate)-Based Porous Bioactive Scaffolds with Shape Memory Effect. J. Mater. Sci. 2021, 56, 731–745. [Google Scholar] [CrossRef]
- Cui, H.; Miao, S.; Esworthy, T.; Lee, S.J.; Zhou, X.; Hann, S.Y.; Webster, T.J.; Harris, B.T.; Zhang, L.G. A Novel Near-Infrared Light Responsive 4D Printed Nanoarchitecture with Dynamically and Remotely Controllable Transformation. Nano Res. 2019, 12, 1381–1388. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.D.; Ashok, A.; Kanwar, R.K.; Kanwar, J.R.; Kouzani, A.Z. Integrated 3D Printed Scaffolds and Electrical Stimulation for Enhancing Primary Human Cardiomyocyte Cultures. Bioprinting 2017, 6, 18–24. [Google Scholar] [CrossRef]
- Zhou, B.; Shi, X.; Tang, X.; Zhao, Q.; Wang, L.; Yao, F.; Hou, Y.; Wang, X.; Feng, W.; Wang, L.; et al. Functional Isolation, Culture and Cryopreservation of Adult Human Primary Cardiomyocytes. Signal Transduct. Target. Ther. 2022, 7, 254. [Google Scholar] [CrossRef]
- Wang, Z.; Lee, S.J.; Cheng, H.J.; Yoo, J.J.; Atala, A. 3D Bioprinted Functional and Contractile Cardiac Tissue Constructs. Acta Biomater. 2018, 70, 48–56. [Google Scholar] [CrossRef]
- Das, S.; Kim, S.W.; Choi, Y.J.; Lee, S.; Lee, S.H.; Kong, J.S.; Park, H.J.; Cho, D.W.; Jang, J. Decellularized Extracellular Matrix Bioinks and the External Stimuli to Enhance Cardiac Tissue Development In Vitro. Acta Biomater. 2019, 95, 188–200. [Google Scholar] [CrossRef]
- Tang, J.; Cui, X.; Caranasos, T.G.; Hensley, M.T.; Vandergriff, A.C.; Hartanto, Y.; Shen, D.; Zhang, H.; Zhang, J.; Cheng, K. Heart Repair Using Nanogel-Encapsulated Human Cardiac Stem Cells in Mice and Pigs with Myocardial Infarction. ACS Nano 2017, 11, 9738–9749. [Google Scholar] [CrossRef]
- Du, B.; Dai, Z.; Wang, H.; Ren, Z.; Li, D. Advances and Prospects in Using Induced Pluripotent Stem Cells for 3D Bioprinting in Cardiac Tissue Engineering. Rev. Cardiovasc. Med. 2025, 26, 26697. [Google Scholar] [CrossRef]
- Karbassi, E.; Fenix, A.; Marchiano, S.; Muraoka, N.; Nakamura, K.; Yang, X.; Murry, C.E. Cardiomyocyte Maturation: Advances in Knowledge and Implications for Regenerative Medicine. Nat. Rev. Cardiol. 2020, 17, 341–359. [Google Scholar] [CrossRef] [PubMed]
- Dhahri, W.; Sadikov Valdman, T.; Wilkinson, D.; Pereira, E.; Ceylan, E.; Andharia, N.; Qiang, B.; Masoudpour, H.; Wulkan, F.; Quesnel, E.; et al. In Vitro Matured Human Pluripotent Stem Cell-Derived Cardiomyocytes Form Grafts with Enhanced Structure and Function in Injured Hearts. Circulation 2022, 145, 1412–1426. [Google Scholar] [CrossRef]
- Lundy, S.D.; Zhu, W.Z.; Regnier, M.; Laflamme, M.A. Structural and Functional Maturation of Cardiomyocytes Derived from Human Pluripotent Stem Cells. Stem Cells Dev. 2013, 22, 1991–2002. [Google Scholar] [CrossRef] [PubMed]
- Piccini, I.; Rao, J.; Seebohm, G.; Greber, B. Human Pluripotent Stem Cell-Derived Cardiomyocytes: Genome-Wide Expression Profiling of Long-Term In Vitro Maturation in Comparison to Human Heart Tissue. Genom. Data 2015, 4, 69–72. [Google Scholar] [CrossRef]
- Tiburcy, M.; Hudson, J.E.; Balfanz, P.; Schlick, S.; Meyer, T.; Liao, M.L.C.; Levent, E.; Raad, F.; Zeidler, S.; Wingender, E.; et al. Defined Engineered Human Myocardium with Advanced Maturation for Applications in Heart Failure Modeling and Repair. Circulation 2017, 135, 1832–1847. [Google Scholar] [CrossRef] [PubMed]
- Ronaldson-Bouchard, K.; Ma, S.P.; Yeager, K.; Chen, T.; Song, L.; Sirabella, D.; Morikawa, K.; Teles, D.; Yazawa, M.; Vunjak-Novakovic, G. Advanced Maturation of Human Cardiac Tissue Grown from Pluripotent Stem Cells. Nature 2018, 556, 239–243, Erratum in Nature 2019, 572, E16–E17. [Google Scholar] [CrossRef]
- Ellis, M.E.; Harris, B.N.; Hashemi, M.; Harvell, B.J.; Bush, M.Z.; Hicks, E.E.; Finklea, F.B.; Wang, E.M.; Nataraj, R.; Young, N.P.; et al. Human Induced Pluripotent Stem Cell Encapsulation Geometry Impacts Three-Dimensional Developing Human Engineered Cardiac Tissue Functionality. Tissue Eng. Part A 2022, 28, 990–1000. [Google Scholar] [CrossRef]
- Kupfer, M.E.; Lin, W.H.; Ravikumar, V.; Qiu, K.; Wang, L.; Gao, L.; Bhuiyan, D.B.; Lenz, M.; Ai, J.; Mahutga, R.R.; et al. In Situ Expansion, Differentiation, and Electromechanical Coupling of Human Cardiac Muscle in a 3D Bioprinted, Chambered Organoid. Circ. Res. 2020, 127, 207–224. [Google Scholar] [CrossRef]
- Deidda, V.; Ventisette, I.; Langione, M.; Giammarino, L.; Pioner, J.M.; Credi, C.; Carpi, F. 3D-Printable Gelatin Methacrylate-Xanthan Gum Hydrogel Bioink Enabling Human Induced Pluripotent Stem Cell Differentiation into Cardiomyocytes. J. Funct. Biomater. 2024, 15, 297. [Google Scholar] [CrossRef]
- Anil Kumar, S.; Alonzo, M.; Allen, S.C.; Abelseth, L.; Thakur, V.; Akimoto, J.; Ito, Y.; Willerth, S.M.; Suggs, L.; Chattopadhyay, M.; et al. A Visible Light-Cross-Linkable, Fibrin-Gelatin-Based Bioprinted Construct with Human Cardiomyocytes and Fibroblasts. ACS Biomater. Sci. Eng. 2019, 5, 4551–4563. [Google Scholar] [CrossRef] [PubMed]
- Khoury, R.E.; Nagiah, N.; Mudloff, J.A.; Thakur, V.; Chattopadhyay, M.; Joddar, B. 3D Bioprinted Spheroidal Droplets for Engineering the Heterocellular Coupling Between Cardiomyocytes and Cardiac Fibroblasts. Cyborg Bionic Syst. 2021, 2021, 9864212. [Google Scholar] [CrossRef]
- Howard, C.M.; Baudino, T.A. Dynamic Cell-Cell and Cell-ECM Interactions in the Heart. J. Mol. Cell Cardiol. 2014, 70, 19–26. [Google Scholar] [CrossRef]
- Cofiño-Fabres, C.; Boonen, T.; Rivera-Arbeláez, J.M.; Rijpkema, M.; Blauw, L.; Rensen, P.C.N.; Schwach, V.; Ribeiro, M.C.; Passier, R.; Cofiño-Fabres, C.; et al. Micro-Engineered Heart Tissues On-Chip with Heterotypic Cell Composition Display Self-Organization and Improved Cardiac Function. Adv. Healthc. Mater. 2024, 13, 2303664. [Google Scholar] [CrossRef] [PubMed]
- Brady, E.L.; Prado, O.; Johansson, F.; Mitchell, S.N.; Martinson, A.M.; Karbassi, E.; Reinecke, H.; Murry, C.E.; Davis, J.; Stevens, K.R. Engineered Tissue Vascularization and Engraftment Depends on Host Model. Sci. Rep. 2023, 13, 1973. [Google Scholar] [CrossRef]
- Han, K.; He, J.; Fu, L.; Mao, M.; Kang, Y.; Li, D. Engineering Highly-Aligned Three-Dimensional (3D) Cardiac Constructs for Enhanced Myocardial Infarction Repair. Biofabrication 2022, 15, 015003. [Google Scholar] [CrossRef]
- Roche, C.D.; Lin, H.; Huang, Y.; de Bock, C.E.; Beck, D.; Xue, M.; Gentile, C. 3D Bioprinted Alginate-Gelatin Hydrogel Patches Containing Cardiac Spheroids Recover Heart Function in a Mouse Model of Myocardial Infarction. Bioprinting 2023, 30, e00263. [Google Scholar] [CrossRef]
- Jones, L.S.; Rodriguez, H.; Biefer, C.; Mekkattu, M.; Thijssen, Q.; Amicone, A.; Bock, A.; Weisskopf, M.; Zorndt, D.; Meier, D.; et al. Volumetric 3D Printing and Melt-Electrowriting to Fabricate Implantable Reinforced Cardiac Tissue Patches. Adv. Mater. 2025, 2504765. [Google Scholar] [CrossRef] [PubMed]
- Guesdon, R.; Santoro, S.; Cras, A.; Pagin, E.; Serteyn, D.; Ceusters, J.; Guillemot, F.; Hagège, A.; Menasché, P. Repair of Infarcted Myocardium by Skeletal Muscle-Derived Mesenchymal Stromal Cells Delivered by a Bioprinted Collagen Patch. Stem Cell Res. Ther. 2025, 16, 427. [Google Scholar] [CrossRef]
- Wang, Z.; Qin, C.; Liao, Z.; Zhang, H.; Lu, H.; Xiao, Y.; Wu, C. Inorganic Biomaterials Inducing Scaffolds Pre-Neuralization for Infarcted Myocardium Repair. Adv. Mater. 2025, 37, 2419765. [Google Scholar] [CrossRef]
- Asulin, M.; Michael, I.; Shapira, A.; Dvir, T. One-Step 3D Printing of Heart Patches with Built-In Electronics for Performance Regulation. Adv. Sci. 2021, 8, 2004205. [Google Scholar] [CrossRef]
- Bar, A.; Kryukov, O.; Etzion, S.; Cohen, S. Engineered Extracellular Vesicle-Mediated Delivery of MiR-199a-3p Increases the Viability of 3D-Printed Cardiac Patches. Int. J. Bioprint 2023, 9, 670. [Google Scholar] [CrossRef]
- Gil, C.J.; Allphin, A.J.; Jin, L.; Amoli, M.S.; Rezapourdamanab, S.; Tomov, M.L.; Hwang, B.; Sridhar, V.; El Shammas, L.R.; Wu, Y.; et al. Image-Guided Cardiac Regeneration via a 3D Bioprinted Vascular Patch with Built-in CT Visibility. Chem. Eng. J. 2025, 520, 165926. [Google Scholar] [CrossRef] [PubMed]
- Jebran, A.F.; Seidler, T.; Tiburcy, M.; Daskalaki, M.; Kutschka, I.; Fujita, B.; Ensminger, S.; Bremmer, F.; Moussavi, A.; Yang, H.; et al. Engineered Heart Muscle Allografts for Heart Repair in Primates and Humans. Nature 2025, 639, 503–511. [Google Scholar] [CrossRef] [PubMed]
- Lu, K. Maturation of Human Induced Pluripotent Stem Cell Based Myocardium by Biomechanical Stimulation of Three-Dimensional Tissue Cultures. Ph.D. Thesis, Ludwig Maximilian University of Munich, Munich, Germany, 2023. [Google Scholar] [CrossRef]
- Dellaquila, A.; Le Bao, C.; Letourneur, D.; Simon-Yarza, T.; Dellaquila, A.; Le Bao, C.; Letourneur, D.; Simon-Yarza, T.; Dellaquila Biomolecular Photonics, A. In Vitro Strategies to Vascularize 3D Physiologically Relevant Models. Adv. Sci. 2021, 8, 2100798. [Google Scholar] [CrossRef] [PubMed]
- Maihemuti, W.; Murata, K.; Abulaiti, M.; Minatoya, K.; Masumoto, H. Simultaneous Electro-Dynamic Stimulation Accelerates Maturation of Engineered Cardiac Tissues Generated by Human IPS Cells. Biochem. Biophys. Res. Commun. 2024, 733, 150605. [Google Scholar] [CrossRef] [PubMed]
- Ershad, F.; Rao, Z.; Maharajan, S.; Mesquita, F.C.P.; Ha, J.; Gonzalez, L.; Haideri, T.; Da Costa, E.C.; Moctezuma-Ramirez, A.; Wang, Y.; et al. Bioprinted Optoelectronically Active Cardiac Tissues. Sci. Adv. 2025, 11, 7210. [Google Scholar] [CrossRef]
- Schwach, V.; Passier, R. Native Cardiac Environment and Its Impact on Engineering Cardiac Tissue. Biomater. Sci. 2019, 7, 3566–3580. [Google Scholar] [CrossRef] [PubMed]
- DeLaughter, D.M.; Bick, A.G.; Wakimoto, H.; McKean, D.; Gorham, J.M.; Kathiriya, I.S.; Hinson, J.T.; Homsy, J.; Gray, J.; Pu, W.; et al. Single-Cell Resolution of Temporal Gene Expression during Heart Development. Dev. Cell 2016, 39, 480–490. [Google Scholar] [CrossRef]
- Quaife-Ryan, G.A.; Sim, C.B.; Ziemann, M.; Kaspi, A.; Rafehi, H.; Ramialison, M.; El-Osta, A.; Hudson, J.E.; Porrello, E.R. Multicellular Transcriptional Analysis of Mammalian Heart Regeneration. Circulation 2017, 136, 1123–1139. [Google Scholar] [CrossRef]
- Alonzo, M.; El Khoury, R.; Nagiah, N.; Thakur, V.; Chattopadhyay, M.; Joddar, B. 3D Biofabrication of a Cardiac Tissue Construct for Sustained Longevity and Function. ACS Appl. Mater. Interfaces 2022, 14, 21800–21813. [Google Scholar] [CrossRef]
- Maas, R.G.C.; Beekink, T.; Chirico, N.; Snijders Blok, C.J.B.; Dokter, I.; Sampaio-Pinto, V.; van Mil, A.; Doevendans, P.A.; Buikema, J.W.; Sluijter, J.P.G.; et al. Generation, High-Throughput Screening, and Biobanking of Human-Induced Pluripotent Stem Cell-Derived Cardiac Spheroids. J. Vis. Exp. 2023, 2023, e64365. [Google Scholar] [CrossRef]
- Daly, A.C.; Davidson, M.D.; Burdick, J.A. 3D Bioprinting of High Cell-Density Heterogeneous Tissue Models through Spheroid Fusion within Self-Healing Hydrogels. Nat. Commun. 2021, 12, 753. [Google Scholar] [CrossRef]
- Basara, G.; Celebi, L.E.; Ronan, G.; Discua Santos, V.; Zorlutuna, P. 3D Bioprinted Aged Human Post-Infarct Myocardium Tissue Model. Health Sci. Rep. 2024, 7, e1945. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.L.; Sit, I.; Xiang, Y.; Wu, J.; Pustelnik, J.; Tang, M.; Kiratitanaporn, W.; Grassian, V.; Chen, S. Evaluation of CuO Nanoparticle Toxicity on 3D Bioprinted Human IPSC-Derived Cardiac Tissues. Bioprinting 2023, 32, e00284. [Google Scholar] [CrossRef]
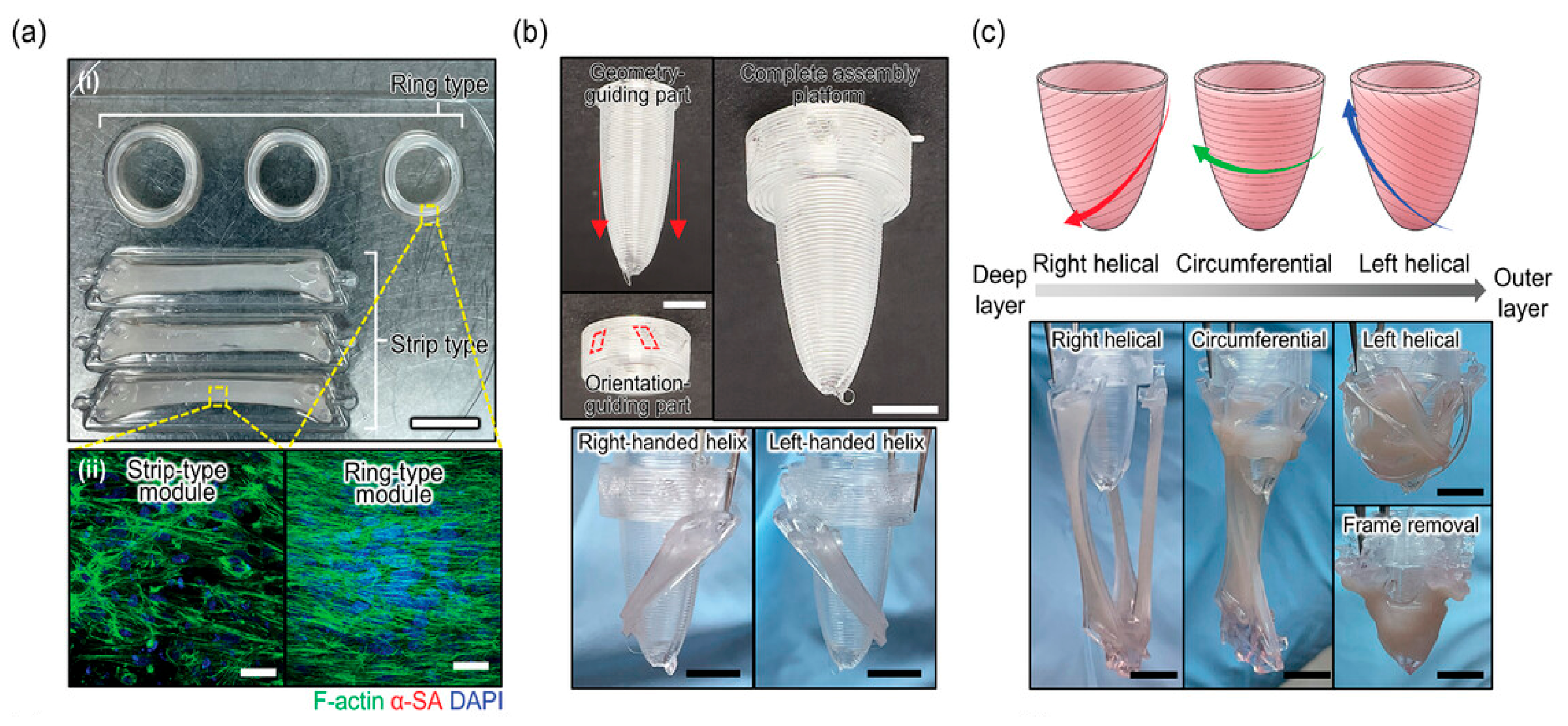
- Hwang, D.G.; Choi, H.; Yong, U.; Kim, D.; Kang, W.; Park, S.M.; Jang, J. Bioprinting-Assisted Tissue Assembly for Structural and Functional Modulation of Engineered Heart Tissue Mimicking Left Ventricular Myocardial Fiber Orientation. Adv. Mater. 2024, 36, 2400364. [Google Scholar] [CrossRef]
- Zeng, Q.; Yang, Y.; Wang, H.; Ye, T.; Wang, Z.; Chai, M.; Lu, Z.; He, S.; Yang, H.; Zhang, J.Z.; et al. 3D Printing of Structural Bionic and Functionalized Hydrogels for the Construction of Macroscale Human Cardiac Tissues. Biomaterials 2026, 325, 123573. [Google Scholar] [CrossRef]
- Bliley, J.M.; Stang, M.A.; Behre, A.; Feinberg, A.W. Advances in 3D Bioprinted Cardiac Tissue Using Stem Cell-Derived Cardiomyocytes. Stem Cells Transl. Med. 2024, 13, 425–435. [Google Scholar] [CrossRef]
- Ho, D.L.L.; Lee, S.; Du, J.; Weiss, J.D.; Tam, T.; Sinha, S.; Klinger, D.; Devine, S.; Hamfeldt, A.; Leng, H.T.; et al. Large-Scale Production of Wholly Cellular Bioinks via the Optimization of Human Induced Pluripotent Stem Cell Aggregate Culture in Automated Bioreactors. Adv. Healthc. Mater. 2022, 11, 2201138. [Google Scholar] [CrossRef]
- da Silva, V.A.; Leung, M.C.; Clayton, M.; Oommen, L.; Madrigal, H.; Laksman, Z.; Yu, B.; Willerth, S.M. Building the Framework for Bioprinted Human Heart Tissue: Recent Developments and Future Prospects. Regen. Med. 2025, 20, 409–430. [Google Scholar] [CrossRef]


| Bioink Composition | Bioprinting Technique | Young’s Modulus (Compressive) | Shear Elastic Modulus (G′) Post-Crosslink | Shear Viscous Modulus (G″) Post-Crosslink | Print Fidelity | Swelling Ratio | Degradation Resistance | Physiological Results | |
|---|---|---|---|---|---|---|---|---|---|
| Native tissues | - | - | ~10–50 kPa [8] | ~2.5–2.9 kPa (porcine) | ~0.6–0.8 kPa (porcine) [69] | - | - | - | - |
| Shin et al. [56] | dECM/Laponite-XLG/PEG-DA | extrusion | ~13.4 ± 0.4 kPa (healthy myocardium) | ~0.761 kPa | Not provided | ~2.86 (filament width/nozzle ID) | ~0 (after 7 days) | Not provided | >94% cell viability after 7 days; Beating velocity: 8.0 ± 2 µm/s; Beating frequency: 0.65 ± 0.26 Hz; Better sarcomeric α-actinin striation and connexin 43 expression with dhECM. |
| Basara et al. [58] | dECM/MeHA/GelMA | extrusion | ~2.8 kPa (healthy myocardium) | ~4.7 kPa | ~2.3 kPa | ~0.8 (line spacing target/measured) | 12 ± 3% after 24 h | ~degraded in 5 h (enzymatic digestion with collagenase) | Beating velocity: 8.0 ± 2 µm/s; Beating frequency: 0.65 ± 0.26 Hz; Better sarcomeric α-actinin striation and connexin 43 expression with dECM. |
| Stola et al. [59] | ADA/alginate/gelatin | extrusion | ~2–6.8 kPa | ~0.65–1.3 kPa | Not provided | ~1.3–2.5 (filament width/nozzle ID) | ~7–21% increase in weight over 21 days (for 0–20% gelatin content) | ~30–80% weight loss after 24 h (dependent on gelatin concentration) | cell viability: >50%; cell adhesion observed in 25% gelatin content |
| Budharaju et al. [5] | Alginate/fibrin | Cardiac tissue | 64.81 ± 21.89 kPa | ~123 ± 10 kPa @100 rads | Not provided | 2.33 ± 0.2 (filament width/nozzle ID) | ~1783 ± 187% after 6 h | 48.0 ± 4.1% mass loss at day 14 | cell viability: >80%; high proliferation observed; shown sarcomeric α-actinin and connexin-43 cardiac marker expression |
| Vettori et al. [8] | Silk fibroin/alginate/gelatin | extrusion | 38 kPa | ~8 kPa | ~0.7 kPa | ~0.6 (filament width/nozzle ID) | Not reported | ~91% intact at day 28 | cell viability: ~2 toxicity ratio; immunostaining confirmed CD31+, vimentin+, and troponin T+ cells; ~0.02 Hz contraction frequency |
| Mousavi et al. [53] | rGO/GelMA/AlgMA | extrusion | 29.9 ± 2.6 kPa | ~50 kPa @0.1–10% rad/s | ~10 kPa @0.1–10% strain | Not provided | ~800% after 24 h (mass gain relative to dry mass) | 59% mass loss in 28 days | cell viability: >85%; confirmed cTnT, α-actinin, connexin-43, and F-actin staining in cells; confirmed spontaneous beating in cells at ~36 ± 5 BPM by day 7 |
| Tsui et al. [62] | rGO/dECM | extrusion | 17.5 ± 0.5 kPa | ~0.5 kPa @0.1–100% rad/s | ~0.12 kPa @0.1–100% rad/s | Not provided | Not provided | Not provided | Twitch force: ~45 µN by day 28–35; sarcomere length up to 2.11 µm; Conduction velocity: 35.4 ± 2.3 cm/s at Day 35 |
| Mehrotra et al. [36] | CNTs/nSF/PEGDMA/GelMA | extrusion | 51.4 ± 4.5 kPa | ~0.05 kPa @0.01–1% rad/s | ~0.005 kPa @0.01–1 rad/s | ~0.88 (filament width/nozzle ID) | 80% | Not provided | Gene expression upregulated; Beating rate: 102 ± 5 bpm (CNT) vs. 76 ± 6 bpm (no CNT); Sarcomere z-lines: 1.5–2.5 µm |
| Basara et al. [63] | MXene (Ti3C2Tx)/PEG | Aerosol jet printing | 17.5 ± 5.5 kPa | Not provided | Not provided | Not provided | Not provided | Not provided | cell viability: 93%; gene expressed: MYH7, TNNT2, SERCA2 and connexin 43; conduction velocity: 6.5 cm/s |
| Zhu et al. [65] | GNRs/GelMA | Extrusion | ~4.2–4.7 ± 0.3 kPa | Not provided | Not provided | Not provided | Not provided | ~100% intact at day 5 (shown by no GNRs leaking) | gene expressed: Cx-43 and troponin I; synchronous beating from day 2; enhanced cardiomyocytes adhesion |
| Ramirez et al. [66] | Au-NPs/alginate/gelatin | Extrusion | ~18.5 kPa | 6.15 ± 0.19 kPa | 0.32 ± 0.08 kPa | Not provided | Not provided | Not provided, but biodegradation observed | cell viability: >90% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leung, M.C.; Laksman, Z. 3D Bioprinting Functional Engineered Heart Tissues. Int. J. Mol. Sci. 2025, 26, 10707. https://doi.org/10.3390/ijms262110707
Leung MC, Laksman Z. 3D Bioprinting Functional Engineered Heart Tissues. International Journal of Molecular Sciences. 2025; 26(21):10707. https://doi.org/10.3390/ijms262110707
Chicago/Turabian StyleLeung, Man Chi, and Zachary Laksman. 2025. "3D Bioprinting Functional Engineered Heart Tissues" International Journal of Molecular Sciences 26, no. 21: 10707. https://doi.org/10.3390/ijms262110707
APA StyleLeung, M. C., & Laksman, Z. (2025). 3D Bioprinting Functional Engineered Heart Tissues. International Journal of Molecular Sciences, 26(21), 10707. https://doi.org/10.3390/ijms262110707






