Using the Mechanisms of Action Involved in the Pathogenesis of Androgenetic Alopecia to Treat Hair Loss
Abstract
1. Introduction
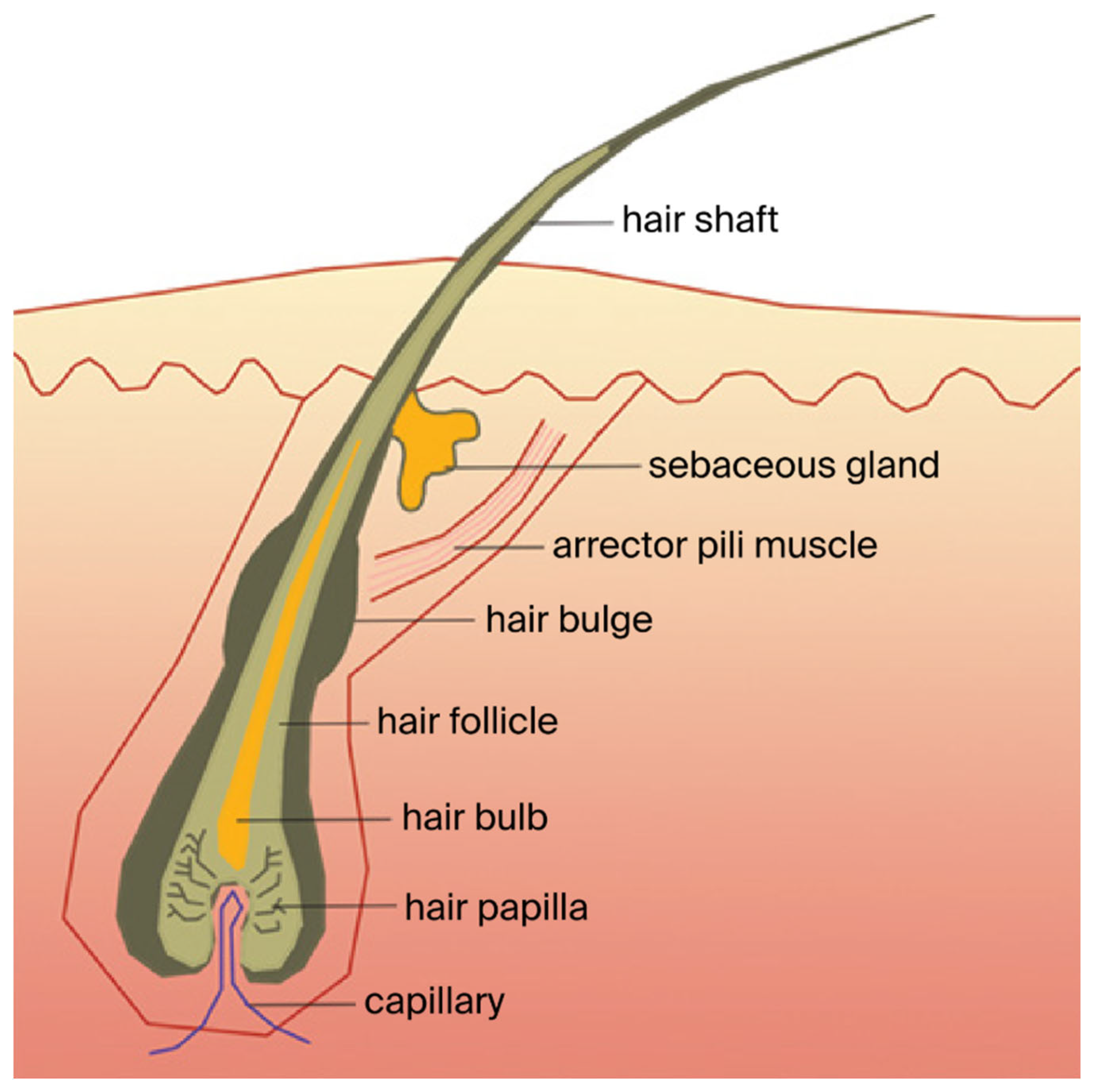
2. Androgenetic Alopecia
2.1. AGA Pathophysiology
2.2. Current Treatments of AGA
3. Exploiting Different Molecular Mechanisms of Hair Growth Induced by Pharmacological Therapies for the Treatment of AGA
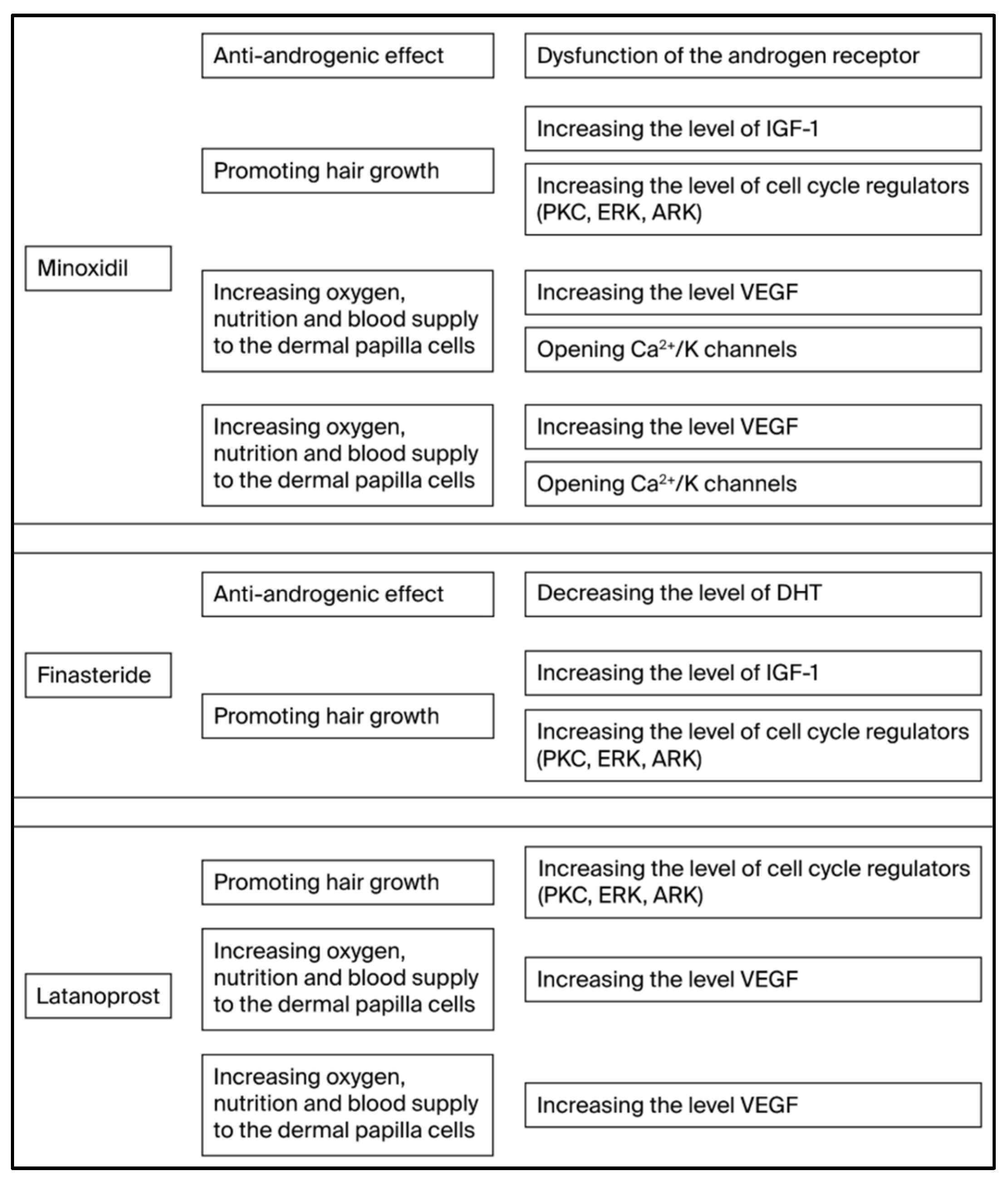
3.1. Finasteride
3.2. Minoxidil
3.3. Latanoprost
3.4. Additional Drugs Aimed at Targeting the Various Mechanisms of AGA Pathogenesis
4. Combined Topical Treatments for AGA
4.1. Combination of Finasteride + Minoxidil in the Treatment of AGA
4.2. Combination of Latanoprost + Minoxidil in the Treatment of AGA
5. Additional Combined Topical Treatments for AGA
5.1. Combined Topical Treatment for AGA with a Combination of Minoxidil, Finasteride, and Latanoprost
5.2. Additional Topical Drug Combinations for the Treatment of AGA
5.3. Satisfaction of Patients with AGA from Topical Treatment with TH07
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Jablonski, N.G.; Chaplin, G. Colloquium Paper: Human Skin Pigmentation as an Adaptation to UV Radiation. Proc. Natl. Acad. Sci. USA 2010, 107 (Suppl. 2), 8962–8968. [Google Scholar] [CrossRef] [PubMed]
- Iyengar, B. The Hair Follicle: A Specialised UV Receptor in the Human Skin? Biol. Signals Recept. 1998, 7, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Moattari, C.R.; Jafferany, M. Psychological Aspects of Hair Disorders: Consideration for Dermatologists, Cosmetologists, Aesthetic, and Plastic Surgeons. Ski. Appendage Disord. 2022, 8, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Krause, K.; Foitzik, K. Biology of the Hair Follicle: The Basics. Semin. Cutan. Med. Surg. 2006, 25, 2–10. [Google Scholar] [CrossRef]
- Koch, S.L.; Tridico, S.R.; Bernard, B.A.; Shriver, M.D.; Jablonski, N.G. The Biology of Human Hair: A Multidisciplinary Review. Am. J. Hum. Biol. 2020, 32, e23316. [Google Scholar] [CrossRef]
- Ji, S.; Zhu, Z.; Sun, X.; Fu, X. Functional Hair Follicle Regeneration: An Updated Review. Signal Transduct. Target. Ther. 2021, 6, 66. [Google Scholar] [CrossRef]
- Erdoğan, B. Anatomy and Physiology of Hair. In Hair and Scalp Disorders; IntechOpen: London, UK, 2017. [Google Scholar]
- Courtois, M.; Loussouarn, G.; Hourseau, C.; Grollier, J.F. Ageing and Hair Cycles. Br. J. Dermatol. 1995, 132, 86–93. [Google Scholar] [CrossRef]
- Pratt, C.H.; King, L.E.J.; Messenger, A.G.; Christiano, A.M.; Sundberg, J.P. Alopecia Areata. Nat. Rev. Dis. Primers 2017, 3, 17011. [Google Scholar] [CrossRef]
- Goodier, M.; Hordinsky, M. Normal and Aging Hair Biology and Structure “Aging and Hair”. Curr. Probl. Dermatol. 2015, 47, 1–9. [Google Scholar] [CrossRef]
- Whiting, D.A. Possible Mechanisms of Miniaturization during Androgenetic Alopecia or Pattern Hair Loss. J. Am. Acad. Dermatol. 2001, 45, S81–S86. [Google Scholar] [CrossRef]
- Dainichi, T.; Iwata, M.; Kaku, Y. Alopecia Areata: What’s New in the Epidemiology, Comorbidities, and Pathogenesis? J. Dermatol. Sci. 2023, 112, 120–127. [Google Scholar] [CrossRef] [PubMed]
- Nestor, M.S.; Ablon, G.; Gade, A.; Han, H.; Fischer, D.L. Treatment Options for Androgenetic Alopecia: Efficacy, Side Effects, Compliance, Financial Considerations, and Ethics. J. Cosmet. Dermatol. 2021, 20, 3759–3781. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Diaz Duran, R.; Martinez-Ledesma, E.; Garcia-Garcia, M.; Bajo Gauzin, D.; Sarro-Ramírez, A.; Gonzalez-Carrillo, C.; Rodríguez-Sardin, D.; Fuentes, A.; Cardenas-Lopez, A. The Biology and Genomics of Human Hair Follicles: A Focus on Androgenetic Alopecia. Int. J. Mol. Sci. 2024, 25, 2542. [Google Scholar] [CrossRef]
- Courtois, M.; Loussouarn, G.; Hourseau, C.; Grollier, J.F. Hair Cycle and Alopecia. Ski. Pharmacol. 1994, 7, 84–89. [Google Scholar] [CrossRef]
- Choi, B.Y. Targeting Wnt/β-Catenin Pathway for Developing Therapies for Hair Loss. Int. J. Mol. Sci. 2020, 21, 4915. [Google Scholar] [CrossRef]
- Kawano, M.; Komi-Kuramochi, A.; Asada, M.; Suzuki, M.; Oki, J.; Jiang, J.; Imamura, T. Comprehensive Analysis of FGF and FGFR Expression in Skin: FGF18 Is Highly Expressed in Hair Follicles and Capable of Inducing Anagen from Telogen Stage Hair Follicles. J. Investig. Dermatol. 2005, 124, 877–885. [Google Scholar] [CrossRef]
- Suraj Singh, N.S. Does Hair Loss Impact Mood, Self-esteem, Body Image, and Quality of Life in Patients of Androgenetic Alopecia? Ann. Indian Psychiatry 2025. [Google Scholar] [CrossRef]
- Yu, L.; Moorthy, S.; Peng, L.; Shen, L.; Han, Y.; Zhang, Z.; Li, Y.; Huang, X. Valuation of Anxiety and Depression in Patients with Androgenetic Alopecia in Shanghai: A Cross-Sectional Study. Dermatol. Ther. 2023, 2023, 1–9. [Google Scholar] [CrossRef]
- Muhaidat, J.; Alshwayyat, S.; Kamal, H.; Haddadin, Z.; Qablan, A.; Qeyam, H.; Al-Qarqaz, F. Impact of Male Androgenetic Alopecia on a Jordanian Cohort Measured Using the Dermatology Life Quality Index. J. Int. Med. Res. 2025, 53, 3000605251332799. [Google Scholar] [CrossRef]
- Frith, H.; Jankowski, G.S. Psychosocial Impact of Androgenetic Alopecia on Men: A Systematic Review and Meta-Analysis. Psychol. Health Med. 2024, 29, 822–842. [Google Scholar] [CrossRef]
- York, K.; Meah, N.; Bhoyrul, B.; Sinclair, R. A Review of the Treatment of Male Pattern Hair Loss. Expert Opin. Pharmacother. 2020, 21, 603–612. [Google Scholar] [CrossRef]
- Devjani, S.; Ezemma, O.; Kelley, K.J.; Stratton, E.; Senna, M. Androgenetic Alopecia: Therapy Update. Drugs 2023, 83, 701–715. [Google Scholar] [CrossRef] [PubMed]
- Lolli, F.; Pallotti, F.; Rossi, A.; Fortuna, M.C.; Caro, G.; Lenzi, A.; Sansone, A.; Lombardo, F. Androgenetic Alopecia: A Review. Endocrine 2017, 57, 9–17. [Google Scholar] [CrossRef] [PubMed]
- Sato-Miyaoka, M.; Hisatsune, C.; Ebisui, E.; Ogawa, N.; Takahashi-Iwanaga, H.; Mikoshiba, K. Regulation of Hair Shedding by the Type 3 IP3 Receptor. J. Investig. Dermatol. 2012, 132, 2137–2147. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Gao, Y.; Wang, A.; Sun, Y.; Wang, J.; Kwok, H.H.M.; Wu, S.; Lam, C.K.; Tao, E.D.; Jiao, J.J.; et al. Effectiveness of an Adapted Physical Activity Intervention for Weight Management in Adolescents with Intellectual Disability: A Randomized Controlled Trial. Pediatr. Obes. 2022, 17, e12882. [Google Scholar] [CrossRef]
- Xie, Y.; Chen, D.; Jiang, K.; Song, L.; Qian, N.; Du, Y.; Yang, Y.; Wang, F.; Chen, T. Hair Shaft Miniaturization Causes Stem Cell Depletion through Mechanosensory Signals Mediated by a Piezo1-Calcium-TNF-α Axis. Cell Stem Cell 2022, 29, 70–85.e6. [Google Scholar] [CrossRef]
- Rathnayake, D.; Sinclair, R. Male Androgenetic Alopecia. Expert Opin. Pharmacother. 2010, 11, 1295–1304. [Google Scholar] [CrossRef]
- Leirós, G.J.; Ceruti, J.M.; Castellanos, M.L.; Kusinsky, A.G.; Balañá, M.E. Androgens Modify Wnt Agonists/Antagonists Expression Balance in Dermal Papilla Cells Preventing Hair Follicle Stem Cell Differentiation in Androgenetic Alopecia. Mol. Cell. Endocrinol. 2017, 439, 26–34. [Google Scholar] [CrossRef]
- Inui, S.; Itami, S. Molecular Basis of Androgenetic Alopecia: From Androgen to Paracrine Mediators through Dermal Papilla. J. Dermatol. Sci. 2011, 61, 1–6. [Google Scholar] [CrossRef]
- Ceruti, J.M.; Leirós, G.J.; Balañá, M.E. Androgens and Androgen Receptor Action in Skin and Hair Follicles. Mol. Cell. Endocrinol. 2018, 465, 122–133. [Google Scholar] [CrossRef]
- Inui, S.; Itami, S. Androgen Actions on the Human Hair Follicle: Perspectives. Exp. Dermatol. 2013, 22, 168–171. [Google Scholar] [CrossRef]
- Leirós, G.J.; Attorresi, A.I.; Balañá, M.E. Hair Follicle Stem Cell Differentiation Is Inhibited through Cross-Talk between Wnt/β-Catenin and Androgen Signalling in Dermal Papilla Cells from Patients with Androgenetic Alopecia. Br. J. Dermatol. 2012, 166, 1035–1042. [Google Scholar] [CrossRef] [PubMed]
- Panchaprateep, R.; Asawanonda, P. Insulin-like Growth Factor-1: Roles in Androgenetic Alopecia. Exp. Dermatol. 2014, 23, 216–218. [Google Scholar] [CrossRef] [PubMed]
- Trüeb, R.M. Further Clinical Evidence for the Effect of IGF-1 on Hair Growth and Alopecia. Ski. Appendage Disord. 2018, 4, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Batch, J.A.; Mercuri, F.A.; Werther, G.A. Identification and Localization of Insulin-like Growth Factor-Binding Protein (IGFBP) Messenger RNAs in Human Hair Follicle Dermal Papilla. J. Investig. Dermatol. 1996, 106, 471–475. [Google Scholar] [CrossRef]
- Li, L.-F.; Guo, J.; Gao, Z.-F. Overexpression of Skin Protein Kinase C-Alpha in Anagen Hair Follicles during Induced Growth of Mouse Hair. Clin. Exp. Dermatol. 2003, 28, 429–433. [Google Scholar] [CrossRef]
- Li, W.; Man, X.-Y.; Li, C.-M.; Chen, J.-Q.; Zhou, J.; Cai, S.-Q.; Lu, Z.-F.; Zheng, M. VEGF Induces Proliferation of Human Hair Follicle Dermal Papilla Cells through VEGFR-2-Mediated Activation of ERK. Exp. Cell Res. 2012, 318, 1633–1640. [Google Scholar] [CrossRef]
- Kwon, O.S.; Han, J.H.; Yoo, H.G.; Lee, S.R.; Kim, K.H.; Eun, H.C.; Cho, K.H.; Sim, Y.C. Expression of Androgen Receptor, Estrogen Receptor Alpha and Beta in the Dermal Papilla of Human Hair Follicles in Vivo. J. Dermatol. Sci. 2004, 36, 176–179. [Google Scholar] [CrossRef]
- Mattioli, M.; Barboni, B.; Turriani, M.; Galeati, G.; Zannoni, A.; Castellani, G.; Berardinelli, P.; Scapolo, P.A. Follicle Activation Involves Vascular Endothelial Growth Factor Production and Increased Blood Vessel Extension. Biol. Reprod. 2001, 65, 1014–1019. [Google Scholar] [CrossRef]
- Yano, K.; Brown, L.F.; Detmar, M. Control of Hair Growth and Follicle Size by VEGF-Mediated Angiogenesis. J. Clin. Investig. 2001, 107, 409–417. [Google Scholar] [CrossRef]
- Hou, C.; Miao, Y.; Wang, J.; Wang, X.; Chen, C.-Y.; Hu, Z.-Q. Collagenase IV Plays an Important Role in Regulating Hair Cycle by Inducing VEGF, IGF-1, and TGF-β Expression. Drug Des. Dev. Ther. 2015, 9, 5373–5383. [Google Scholar] [CrossRef]
- Slominski, R.M.; Raman, C.; Jetten, A.M.; Slominski, A.T. Neuro-Immuno-Endocrinology of the Skin: How Environment Regulates Body Homeostasis. Nat. Rev. Endocrinol. 2025, 21, 495–509. [Google Scholar] [CrossRef]
- Paus, R. Stress, Hair Growth Control, and the Neuro-Endocrine-Immune Connection. Allergo J. Int. 2000, 9, 411–420. [Google Scholar] [CrossRef]
- Fischer, T.W.; Slominski, A.; Tobin, D.J.; Paus, R. Melatonin and the Hair Follicle. J. Pineal Res. 2008, 44, 1–15. [Google Scholar] [CrossRef]
- Fischer, T.W.; Burmeister, G.; Schmidt, H.W.; Elsner, P. Melatonin Increases Anagen Hair Rate in Women with Androgenetic Alopecia or Diffuse Alopecia: Results of a Pilot Randomized Controlled Trial. Br. J. Dermatol. 2004, 150, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Garza, L.A.; Liu, Y.; Yang, Z.; Alagesan, B.; Lawson, J.A.; Norberg, S.M.; Loy, D.E.; Zhao, T.; Blatt, H.B.; Stanton, D.C.; et al. Prostaglandin D2 Inhibits Hair Growth and Is Elevated in Bald Scalp of Men with Androgenetic Alopecia. Sci. Transl. Med. 2012, 4, 126ra34. [Google Scholar] [CrossRef] [PubMed]
- Yuen, K.W.; Leung, K.W.; Lai, Q.W.S.; Guo, M.S.S.; Gao, A.X.; Ho, J.Y.M.; Chu, H.C.T.; Tsim, K.W.K. The Muscarinic Acetylcholine Receptor in Dermal Papilla Cells Regulates Hair Growth. bioRxiv 2024. [Google Scholar] [CrossRef]
- Nejati, R.; Kovacic, D.; Slominski, A. Neuro-Immune-Endocrine Functions of the Skin: An Overview. Expert Rev. Dermatol. 2013, 8, 581–583. [Google Scholar] [CrossRef]
- Zubair, Z.; Kantamaneni, K.; Jalla, K.; Renzu, M.; Jena, R.; Jain, R.; Muralidharan, S.; Yanamala, V.L.; Alfonso, M. Prevalence of Low Serum Vitamin D Levels in Patients Presenting With Androgenetic Alopecia: A Review. Cureus 2021, 13, e20431. [Google Scholar] [CrossRef]
- Rossi, A.; Cantisani, C.; Scarnò, M.; Trucchia, A.; Fortuna, M.C.; Calvieri, S. Finasteride, 1 Mg Daily Administration on Male Androgenetic Alopecia in Different Age Groups: 10-Year Follow-Up. Dermatol. Ther. 2011, 24, 455–461. [Google Scholar] [CrossRef]
- Roberts, J.; Desai, N.; McCoy, J.; Goren, A. Sulfotransferase Activity in Plucked Hair Follicles Predicts Response to Topical Minoxidil in the Treatment of Female Androgenetic Alopecia. Dermatol. Ther. 2014, 27, 252–254. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J.L. Enhancing the Growth of Natural Eyelashes: The Mechanism of Bimatoprost-Induced Eyelash Growth. Dermatol. Surg. 2010, 36, 1361–1371. [Google Scholar] [CrossRef] [PubMed]
- Barrón-Hernández, Y.L.; Tosti, A. Bimatoprost for the Treatment of Eyelash, Eyebrow and Scalp Alopecia. Expert Opin. Investig. Drugs 2017, 26, 515–522. [Google Scholar] [CrossRef] [PubMed]
- Jo, S.J.; Shin, H.; Park, Y.W.; Paik, S.H.; Park, W.S.; Jeong, Y.S.; Shin, H.J.; Kwon, O. Topical Valproic Acid Increases the Hair Count in Male Patients with Androgenetic Alopecia: A Randomized, Comparative, Clinical Feasibility Study Using Phototrichogram Analysis. J. Dermatol. 2014, 41, 285–291. [Google Scholar] [CrossRef]
- Wilt, T.; Ishani, A.; Mac Donald, R. Serenoa Repens for Benign Prostatic Hyperplasia. Cochrane Database Syst. Rev. 2002, 6, CD001423. [Google Scholar] [CrossRef]
- Adil, A.; Godwin, M. The Effectiveness of Treatments for Androgenetic Alopecia: A Systematic Review and Meta-Analysis. J. Am. Acad. Dermatol. 2017, 77, 136–141.e5. [Google Scholar] [CrossRef]
- Smith, N.; Shin, D.B.; Brauer, J.A.; Mao, J.; Gelfand, J.M. Use of Complementary and Alternative Medicine among Adults with Skin Disease: Results from a National Survey. J. Am. Acad. Dermatol. 2009, 60, 419–425. [Google Scholar] [CrossRef]
- Gupta, A.K.; Mays, R.R.; Versteeg, S.G.; Shear, N.H.; Piguet, V.; Piraccini, B.M. Efficacy of Off-Label Topical Treatments for the Management of Androgenetic Alopecia: A Review. Clin. Drug Investig. 2019, 39, 233–239. [Google Scholar] [CrossRef]
- Kaufman, K.D.; Girman, C.J.; Round, E.M.; Johnson-Levonas, A.O.; Shah, A.K.; Rotonda, J. Progression of Hair Loss in Men with Androgenetic Alopecia (Male Pattern Hair Loss): Long-Term (5-Year) Controlled Observational Data in Placebo-Treated Patients. Eur. J. Dermatol. EJD 2008, 18, 407–411. [Google Scholar]
- Jimenez, J.J.; Wikramanayake, T.C.; Bergfeld, W.; Hordinsky, M.; Hickman, J.G.; Hamblin, M.R.; Schachner, L.A. Efficacy and Safety of a Low-Level Laser Device in the Treatment of Male and Female Pattern Hair Loss: A Multicenter, Randomized, Sham Device-Controlled, Double-Blind Study. Am. J. Clin. Dermatol. 2014, 15, 115–127. [Google Scholar] [CrossRef]
- Xiao, R.; Lee, L.N. Updated Review of Treatment of Androgenetic Alopecia. Facial Plast. Surg. Clin. N. Am. 2024, 32, 417–423. [Google Scholar] [CrossRef]
- FDA. Alerts Health Care Providers, Compounders and Consumers of Potential Risks Associated with Compounded Topical Finasteride Products. Available online: https://www.fda.gov/drugs/human-drug-compounding/fda-alerts-health-care-providers-compounders-and-consumers-potential-risks-associated-compounded (accessed on 25 October 2025).
- Piraccini, B.M.; Blume-Peytavi, U.; Scarci, F.; Jansat, J.M.; Falqués, M.; Otero, R.; Tamarit, M.L.; Galván, J.; Tebbs, V.; Massana, E. Efficacy and Safety of Topical Finasteride Spray Solution for Male Androgenetic Alopecia: A Phase III, Randomized, Controlled Clinical Trial. J. Eur. Acad. Dermatol. Venereol. JEADV 2022, 36, 286–294. [Google Scholar] [CrossRef]
- Tang, L.; Bernardo, O.; Bolduc, C.; Lui, H.; Madani, S.; Shapiro, J. The Expression of Insulin-like Growth Factor 1 in Follicular Dermal Papillae Correlates with Therapeutic Efficacy of Finasteride in Androgenetic Alopecia. J. Am. Acad. Dermatol. 2003, 49, 229–233. [Google Scholar] [CrossRef]
- Rattanachitthawat, N.; Pinkhien, T.; Opanasopit, P.; Ngawhirunpat, T.; Chanvorachote, P. Finasteride Enhances Stem Cell Signals of Human Dermal Papilla Cells. Vivo 2019, 33, 1209–1220. [Google Scholar] [CrossRef]
- Gubelin Harcha, W.; Barboza Martínez, J.; Tsai, T.-F.; Katsuoka, K.; Kawashima, M.; Tsuboi, R.; Barnes, A.; Ferron-Brady, G.; Chetty, D. A Randomized, Active- and Placebo-Controlled Study of the Efficacy and Safety of Different Doses of Dutasteride versus Placebo and Finasteride in the Treatment of Male Subjects with Androgenetic Alopecia. J. Am. Acad. Dermatol. 2014, 70, 489–498.e3. [Google Scholar] [CrossRef]
- Dinh, Q.Q.; Sinclair, R. Female Pattern Hair Loss: Current Treatment Concepts. Clin. Interv. Aging 2007, 2, 189–199. [Google Scholar] [PubMed]
- Hsu, C.-L.; Liu, J.-S.; Lin, A.-C.; Yang, C.-H.; Chung, W.-H.; Wu, W.-G. Minoxidil May Suppress Androgen Receptor-Related Functions. Oncotarget 2014, 5, 2187–2197. [Google Scholar] [CrossRef] [PubMed]
- Park, K.S.; Park, D.H. Comparison of Saccharina Japonica-Undaria Pinnatifida Mixture and Minoxidil on Hair Growth Promoting Effect in Mice. Arch. Plast. Surg. 2016, 43, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.Y.; Park, M.A.; Kim, Y.C. Peppermint Oil Promotes Hair Growth without Toxic Signs. Toxicol. Res. 2014, 30, 297–304. [Google Scholar] [CrossRef]
- Han, J.H.; Kwon, O.S.; Chung, J.H.; Cho, K.H.; Eun, H.C.; Kim, K.H. Effect of Minoxidil on Proliferation and Apoptosis in Dermal Papilla Cells of Human Hair Follicle. J. Dermatol. Sci. 2004, 34, 91–98. [Google Scholar] [CrossRef]
- Lachgar, S.; Charveron, M.; Gall, Y.; Bonafe, J.L. Minoxidil Upregulates the Expression of Vascular Endothelial Growth Factor in Human Hair Dermal Papilla Cells. Br. J. Dermatol. 1998, 138, 407–411. [Google Scholar] [CrossRef]
- Dastan, M.; Najafzadeh, N.; Abedelahi, A.; Sarvi, M.; Niapour, A. Human Platelet Lysate versus Minoxidil Stimulates Hair Growth by Activating Anagen Promoting Signaling Pathways. Biomed. Pharmacother. 2016, 84, 979–986. [Google Scholar] [CrossRef]
- Davies, G.C.; Thornton, M.J.; Jenner, T.J.; Chen, Y.-J.; Hansen, J.B.; Carr, R.D.; Randall, V.A. Novel and Established Potassium Channel Openers Stimulate Hair Growth in Vitro: Implications for Their Modes of Action in Hair Follicles. J. Investig. Dermatol. 2005, 124, 686–694. [Google Scholar] [CrossRef]
- Goren, A.; Naccarato, T.; Situm, M.; Kovacevic, M.; Lotti, T.; McCoy, J. Mechanism of Action of Minoxidil in the Treatment of Androgenetic Alopecia Is Likely Mediated by Mitochondrial Adenosine Triphosphate Synthase-Induced Stem Cell Differentiation. J. Biol. Regul. Homeost. Agents 2017, 31, 1049–1053. [Google Scholar]
- Li, M.; Marubayashi, A.; Nakaya, Y.; Fukui, K.; Arase, S. Minoxidil-Induced Hair Growth Is Mediated by Adenosine in Cultured Dermal Papilla Cells: Possible Involvement of Sulfonylurea Receptor 2B as a Target of Minoxidil. J. Investig. Dermatol. 2001, 117, 1594–1600. [Google Scholar] [CrossRef]
- Husain, S.; Jafri, F.; Crosson, C.E. Acute Effects of PGF2alpha on MMP-2 Secretion from Human Ciliary Muscle Cells: A PKC- and ERK-Dependent Process. Investig. Ophthalmol. Vis. Sci. 2005, 46, 1706–1713. [Google Scholar] [CrossRef]
- Sabaner, M.C.; Duman, R.; Vurmaz, A.; Ertekin, T. Effects of Topical Prostaglandin Drops on Angiogenesis in an in Ovo Chick Chorioallantoic Membrane Model. Cutan. Ocul. Toxicol. 2021, 40, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Wu, F. Effects of Latanoprost on Vascular Endothelial Growth Factor Protein Synthesis in Human Dermal Papilla Cells. J. Clin. Dermatol. 2006, 35, 283–285. [Google Scholar]
- Zaky, M.S.; Abo Khodeir, H.; Ahmed, H.-A.; Elsaie, M.L. Therapeutic Implications of Topical Cetirizine 1% in Treatment of Male Androgenetic Alopecia: A Case-Controlled Study. J. Cosmet. Dermatol. 2021, 20, 1154–1159. [Google Scholar] [CrossRef] [PubMed]
- Biosplice Therapeutics, Inc. A Study of SM04554 Applied Topically to the Scalp of Male Subjects With Androgenetic Alopecia Analyzed by Biopsy of the Scalp Prior To and Post Dosing; Biosplice Therapeutics, Inc.: San Diego, CA, USA, 2020. [Google Scholar]
- JW Pharmaceutical. Hair Loss Treatment by JW0061-Mediated Wnt Modulation for “Growth in Hair Length & Follicular Number; JW Pharmaceutical: Seoul, Republic of Korea, 2022. [Google Scholar]
- Ryu, Y.C.; Lee, D.-H.; Shim, J.; Park, J.; Kim, Y.-R.; Choi, S.; Bak, S.S.; Sung, Y.K.; Lee, S.-H.; Choi, K.-Y. KY19382, a Novel Activator of Wnt/β-Catenin Signalling, Promotes Hair Regrowth and Hair Follicle Neogenesis. Br. J. Pharmacol. 2021, 178, 2533–2546. [Google Scholar] [CrossRef]
- Pozo-Pérez, L.; Tornero-Esteban, P.; López-Bran, E. Clinical and Preclinical Approach in AGA Treatment: A Review of Current and New Therapies in the Regenerative Field. Stem Cell Res. Ther. 2024, 15, 260. [Google Scholar] [CrossRef]
- Hausauer, A.K.; Jones, D.H. Evaluating the Efficacy of Different Platelet-Rich Plasma Regimens for Management of Androgenetic Alopecia: A Single-Center, Blinded, Randomized Clinical Trial. Dermatol. Surg. 2018, 44, 1191–1200. [Google Scholar] [CrossRef]
- Gentile, P.; Scioli, M.G.; Bielli, A.; De Angelis, B.; De Sio, C.; De Fazio, D.; Ceccarelli, G.; Trivisonno, A.; Orlandi, A.; Cervelli, V.; et al. Platelet-Rich Plasma and Micrografts Enriched with Autologous Human Follicle Mesenchymal Stem Cells Improve Hair Re-Growth in Androgenetic Alopecia. Biomolecular Pathway Analysis and Clinical Evaluation. Biomedicines 2019, 7, 27. [Google Scholar] [CrossRef]
- Rafi, A.W.; Katz, R.M. Pilot Study of 15 Patients Receiving a New Treatment Regimen for Androgenic Alopecia: The Effects of Atopy on AGA. ISRN Dermatol. 2011, 2011, 241953. [Google Scholar] [CrossRef]
- Tanglertsampan, C. Efficacy and Safety of 3% Minoxidil versus Combined 3% Minoxidil/0.1% Finasteride in Male Pattern Hair Loss: A Randomized, Double-Blind, Comparative Study. J. Med. Assoc. Thail. 2012, 95, 1312–1316. [Google Scholar]
- Chandrashekar, B.S.; Nandhini, T.; Vasanth, V.; Sriram, R.; Navale, S. Topical Minoxidil Fortified with Finasteride: An Account of Maintenance of Hair Density after Replacing Oral Finasteride. Indian Dermatol. Online J. 2015, 6, 17–20. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, S.; Ahmad, A.; Ali, S.M.; Ahmad, M.U.; Paithankar, M.; Saptarshi, D.; Kale, P.; Maheshwari, K.; Hemant, V. A New Topical Formulation of Minoxidil and Finasteride Improves Hair Growth in Men with Androgenetic Alopecia. J. Clin. Exp. 2015, 6, 6–11. [Google Scholar] [CrossRef]
- Suchonwanit, P.; Srisuwanwattana, P.; Chalermroj, N.; Khunkhet, S. A Randomized, Double-Blind Controlled Study of the Efficacy and Safety of Topical Solution of 0.25% Finasteride Admixed with 3% Minoxidil vs. 3% Minoxidil Solution in the Treatment of Male Androgenetic Alopecia. J. Eur. Acad. Dermatol. Venereol. JEADV 2018, 32, 2257–2263. [Google Scholar] [CrossRef]
- Suchonwanit, P.; Iamsumang, W.; Rojhirunsakool, S. Efficacy of Topical Combination of 0.25% Finasteride and 3% Minoxidil Versus 3% Minoxidil Solution in Female Pattern Hair Loss: A Randomized, Double-Blind, Controlled Study. Am. J. Clin. Dermatol. 2019, 20, 147–153. [Google Scholar] [CrossRef]
- Marotta, J.C.; Patel, G.; Carvalho, M.; Blakeney, S. Clinical Efficacy of a Topical Compounded Formulation in Male Androgenetic Alopecia: Minoxidil 10%, Finasteride 0.1%, Biotin 0.2%, and Caffeine Citrate 0.05% Hydroalcoholic Solution. Int. J. Pharm. Compd. 2020, 24, 69–76. [Google Scholar]
- Bloch, L.D.; Escudeiro, C.C.; Sarruf, F.D.; Valente, N.Y.S. Latanoprost and minoxidil: Comparative double-blind, placebo-controlled study for the treatment of hair loss. Surg. Cosmet. Dermatol. 2018, 10, 39–43. [Google Scholar] [CrossRef]
- Sekhavat, H.; Ford, P.; Lepage, A.; Nateghi, A.; Bar Yehuda, S.; Bourgeois, M. TH07–A New Novel Topical Treatment for Androgenic Alopecia. Int. J. Trichology 2023, 15, 241–247. [Google Scholar]
- Abdi, P.; Awad, C.; Anthony, M.R.; Farkouh, C.; Kenny, B. Efficacy and safety of combinational therapy using topical minoxidil and microneedling for the treatment of androgenetic alopecia: A systematic review and meta-analysis. Arch. Dermatol. Res. 2023, 315, 2775–2785. [Google Scholar] [CrossRef]
- Sekhavat, H.; Ford, P.; Nateghi, A.; Bar Yehuda, S. Satisfaction of Patients with Androgenetic Alopecia from a Topical Treatment with TH07—A New Novel Combination of Substances that Safely Encourage Hair Growth. JOJ Dermatol. Cosmet. 2024, 5, 5. [Google Scholar]
| Androgen Receptors | Activated by 5-Dihydrotestosterone | Induces Progressive Hair Follicle Miniaturization and Hair Follicle Abnormalities |
|---|---|---|
| Growth factors and/or extracellular matrix factors | Insulin-like growth factor 1 (IGF-1) | Promotes proliferation, survival, and migration of hair follicle cell, leading to hair growth |
| PKC Family | Promotes androgenic receptor desensitization, mediating immune responses and regulating dermal papilla cell growth | |
| ERK and Akt | Regulate the survival of dermal papilla cells | |
| VEGF | Regulates perifollicular vascularization of dermal papilla cells |
| Origin | No. of Patients | Treatments | Assessments | Study Outcomes | Adverse Events |
|---|---|---|---|---|---|
| Rafi et al. (2011) [88] | 15 Males | A lotion including Finasteride, Dutasteride, and Minoxidil with Minoxidil Foam 5% or Finasteride tablet 1 mg or ketoconazole shampoo for 9 months. | Hair growth. | All patients demonstrated significant growth of hair. In those patients who utilized all 4 components, significant growth was achieved in as little as 30 days. In those patients who choose only to utilize combination of Finasteride, Dutasteride, and Minoxidil, significant growth was demonstrated after 3 months. | No side effects were reported. |
| Tanglertsampan et al. (2012) [89] | 40 Males | Minoxidil lotion 3% vs. combined Minoxidil 3% and Finasteride lotion 0.1% for 6 months. | Hair counts and global photographic assessment. | No statistical difference between two groups with respect to change in hair counts at 6 months from baseline. Global photographic assessment revealed that Minoxidil 3% and Finasteride lotion 0.1% group demonstrated a significantly higher efficacy than Minoxidil 3% group. | No significant difference in side effects between both groups. |
| Chandrashekar et al. (2015) [90] | 50 Males | Topical Minoxidil 5% and topical Finasteride 0.1% for 12 months, after initial treatment with topical Minoxidil and oral Finasteride for two years. | Hair density. | Patients who were shifted from oral to topical Finasteride maintained their hair density. Five patients stopped all treatment for a year, and a decrease in hair density over a period of 8–12 months after stopping treatment was noted, but improvement was observed upon treatment with topical Minoxidil and topical Finasteride. | Combination of drugs was well tolerated. |
| Sheikh et al. (2015) [91] | 50 Males | Topical Minoxidil 5% and Finasteride solution 0.1% vs. Minoxidil 5% for 6 months. | Investigators’ assessment, global photographic assessment, and patients’ self-assessment. | Patients who were shifted from oral to topical Finasteride maintained their hair density. Five patients stopped all treatment for a year, and a decrease in hair density over a period of 8–12 months after stopping treatment was noted, but improvement was observed upon treatment with topical Minoxidil and topical Finasteride. | Majority of patients did not have any adverse events during trial. |
| Suchonwanit et al. (2018) [92] | 40 Males | Topical Finasteride 0.25% and Minoxidil solution 3% vs. topical Minoxidil solution 3% for 6 months. | Hair density, hair diameter, and global photographic assessment. | Combined solution of Finasteride and Minoxidil was significantly superior to Minoxidil alone in improvements in hair density, hair diameter, and global photographic assessment by patients and by investigators. | There were also no systemic adverse events reported by patients in both groups. |
| Suchonwanit et al. (2019) [93] | 30 Females | Topical Finasteride 0.25% and Minoxidil solution 3% vs. topical Minoxidil solution 3% for 6 months. | Hair density and hair diameter. | Finasteride and Minoxidil group was significantly superior to Minoxidil group in terms of hair diameter. | No systemic side effects were reported. |
| Marotta et al. (2020) [94] | 5 Males | Topical Minoxidil 10%, Finasteride 0.1%, biotin 0.2%, and caffeine citrate 0.05%, hydroalcoholic solutions, for 6 months. | Hair thickness, vellus hair counts, scalp coverage, general hair appearance, photographic assessment, patients’ self-assessment. | Most patients had thicker, more voluminous hair, improved scalp coverage, and improved general hair appearance. Increase of +1.05 in patients’ hair density was observed. According to patients’ self-assessment, topical compounded formulation was effective. | No side effects were reported. |
| Origin | No of Patients | Treatments | Assessments | Study Outcomes |
|---|---|---|---|---|
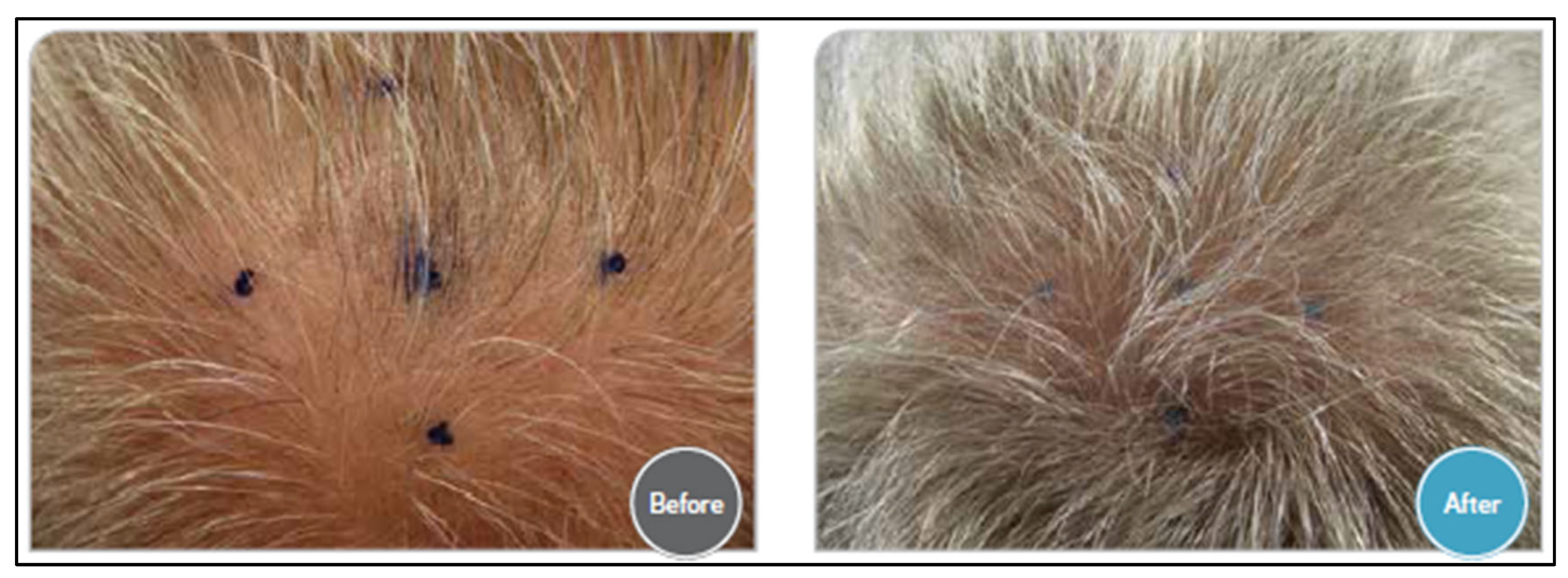
| Bloch et al. (2018) [95] | 98 participants | Placebo; 5% Minoxidiltopical lotion; 5% Minoxidil + 0.005% Latanoprost; 0.005% Latanoprost; 5% Minoxidil + 0.010% Latanoprost; and 0.010% Latanoprost. | Comparative visual analysis of the macro images. |
|
| Sekhavat et al. (2023) [96] | 40 men | Topical solution, a mixture of Minoxidil 5%, Finasteride 0.1%, and Latanoprost 0.03%, defined as TH07. | Investigators’ assessment, global photographic assessment, and patients’ self-assessment. | Most of the patients treated with TH07 were satisfied with their hair appearance.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sekhavat, H.; Bar Yehuda, S.; Asotra, S. Using the Mechanisms of Action Involved in the Pathogenesis of Androgenetic Alopecia to Treat Hair Loss. Int. J. Mol. Sci. 2025, 26, 10712. https://doi.org/10.3390/ijms262110712
Sekhavat H, Bar Yehuda S, Asotra S. Using the Mechanisms of Action Involved in the Pathogenesis of Androgenetic Alopecia to Treat Hair Loss. International Journal of Molecular Sciences. 2025; 26(21):10712. https://doi.org/10.3390/ijms262110712
Chicago/Turabian StyleSekhavat, Houfar, Sara Bar Yehuda, and Satish Asotra. 2025. "Using the Mechanisms of Action Involved in the Pathogenesis of Androgenetic Alopecia to Treat Hair Loss" International Journal of Molecular Sciences 26, no. 21: 10712. https://doi.org/10.3390/ijms262110712
APA StyleSekhavat, H., Bar Yehuda, S., & Asotra, S. (2025). Using the Mechanisms of Action Involved in the Pathogenesis of Androgenetic Alopecia to Treat Hair Loss. International Journal of Molecular Sciences, 26(21), 10712. https://doi.org/10.3390/ijms262110712






