Abstract
6-Prenylnaringenin (6-PN) is a natural compound which occurs in some plants, but the primary dietary source for humans is beer. This compound exhibits broad and potent antimicrobial, anticancer, and neuroactive properties, and weak estrogenic effects. Currently, hop extracts standardized for 6-PN content (relative to other prenylflavonoids) are commercially available and utilized in the non-hormonal treatment of menopause. It is probable that in the future, 6-PN will be employed in the prevention or treatment of non-hormone-dependent cancers and infectious diseases, as well as a sedative, hypnotic, and analgesic agent. Further research is essential to precisely determine the exact mechanisms of action of 6-PN and, critically, to leverage its unique therapeutic profile. This review synthesizes current evidence, highlighting that 6-PN warrants priority investigation as a core scaffold for novel drug development, particularly as a GABAA positive allosteric modulator and a synergistic antimicrobial agent, potentially offering a safer alternative to more potent phytoestrogens found in hops.
1. Introduction
C-prenylated flavonoids are a group of compounds where the flavonoid backbone is linked to a lipophilic prenyl side chain. This prenylation typically occurs at the C-6 and/or C-8 positions in the A ring, or at the C-3′ and/or C-5′ positions in the B ring [1].
Prenylation is known to enhance the antibacterial, anti-inflammatory, antioxidant, cytotoxic, insecticidal, and estrogenic activities of flavonoids [2]. The prenyl side chain can increase the binding affinity of flavonoids to P-glycoprotein, significantly improving their biological activity [3], including their anticancer properties [4]. Furthermore, prenylated flavonoids often act selectively, exhibiting a higher cytotoxic effect against cancer cells than against normal cells. However, the presence of a non-polar prenyl group in the flavonoid molecule has the adverse effect of reducing their water solubility, which in turn negatively impacts their bioavailability and reduces their absorption [2]. Consequently, this limits the therapeutic potential of these valuable biologically active compounds.
6-Prenylnaringenin (6-PN) (1) (2,3-dihydro-5,7-dihydroxy-2-(4-hydroxyphenyl)-6-(3-methyl-2-butenyl)-4H-1-benzopyran-4-one) is classified as a prenylated flavanone with three hydroxyl groups located at C-5, C-7, and C-4′, and the prenyl group at C-6 (Figure 1).
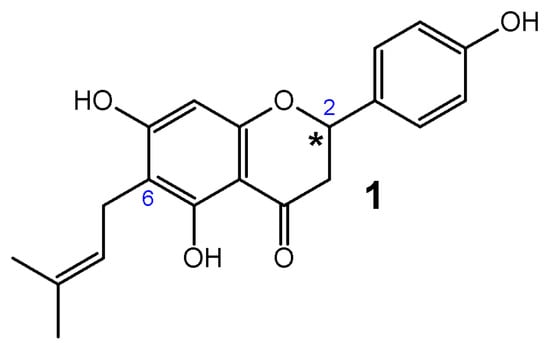
Figure 1.
Structure of 6-Prenylnaringenin (6-PN) (1). An asterisk indicates the chiral center in the compounds.
In its pure form, compound 1 presents as a crystalline pale yellow solid with a melting point of 209–209.5 °C. Due to the additional prenyl group, it exhibits poor solubility in water (1.55 mg/L) [5] but is readily soluble in solvents such as chloroform, dichloromethane, ethyl acetate, DMSO, and acetone [6].
Dhooghe et al. developed an accessible and highly accurate HPLC-DAD method for determining prenylflavonoids in hop extract and capsules, which employed quercetin and naringenin as secondary standards. The use of secondary standards is a valuable solution when quantifying components that are either commercially unavailable or prohibitively expensive [7].
6-PN (1) is a chiral compound (2R, 2S) and can be quantified using enantiospecific LC-ESI-MS on a Chiralpak® AD-RH column using isocratic elution. Quantitative MS data were obtained by monitoring selected [M-H] ions for both enantiomers of 6-PN (1) and the internal standard, 4-acetamidobenzoic acid. This approach allows for the precise enantiospecific quantification of compound 1 [8].
1.1. Occurrence
6-PN (1) was initially described in Sophora tomentosa L. [9]. It has also been identified in various other plants, including hops (Humulus lupulus L.) [10], Wyethia glabra [11], W. invenusta [12], Lupinus luteus [13], Glycyrrhiza glabra [14], and Psoralea corylifolia [15]. The primary natural source of 6-PN (1) in the human diet is hops. Hop cones contain a small amount of 6-PN (1) (approximately 0.004% of dry weight) [16], while hop extracts used in brewing are much richer in 6-PN (1) (Table 1).

Table 1.
The content of 6-Prenylnaringenin (1) in different plant materials.
Hops traditionally used in beer production have been valued as a medicinal herb since ancient Egyptian times [21,22]. The earliest recorded medicinal use of hops dates back to an 11th-century book, where the Arab physician Mesue described the anti-inflammatory properties of this perennial herb [23].
Subsequently, the German Commission E, which is a scientific advisory board of the Federal Institute for Drugs and Medical Devices, also approved hops for addressing “mood disorders such as anxiety and restlessness, and sleep disorders” [24,25]. Currently, Humulus lupulus L. (hops) is recognized as a source of many valuable compounds with notable antibacterial activity [26,27,28] and significant pharmaceutical potential [29,30]. The main source of 6-PN (1) in the human diet is beer. However, the presence of 6-PN (1) in beer is not solely due to its content in hops, but also as a result of the isomerization of desmethylxanthohumol (3) to 6-PN (1) during the hop wort boiling process. Desmethylxanthohumol (DXN) (3), a prenylated chalcone found in hops, is transformed into a mixture of phytoestrogens: 6-prenylnaringenin (1) and 8-prenylnaringenin (8-PN) (2). This conversion is facilitated by the presence of two free hydroxyl groups at the C-2′ and C-6′ positions of desmethylxanthohumol. While 6-PN (1) and 8-PN (2) are formed in small quantities during the drying, storage, and extraction of hops, the conversion process from DXN (3) to 6-PN (1) and 8-PN (2) is particularly rapid when the wort is boiled in a brewhouse [16,31] (Figure 2).
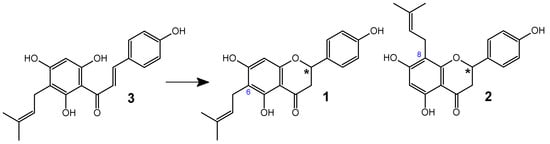
Figure 2.
Transformations of desmethylxanthohumol (3) to 6-PN (1) and 8-PN (2) during the beer production process. Asterisks indicate the chiral center in the compounds.
The 6-PN (1) content in beer varies significantly, influenced by both the brewing process and the specific beer brand [32,33]. The concentration of 6-PN (1) can differ widely, also depending on the analytical method employed. For instance, research by Stevens and Page demonstrated a range from 1 µg/L in European lager to 240 µg/L in American porter [32]. While the aforementioned concentrations of 6-PN (1) might appear to have marginal effects (Table 2), they are nonetheless believed to offer beneficial health effects, a point that warrants further investigation. Simultaneously, it is important to acknowledge the known harmful side effects associated with alcohol consumption in beer [34,35,36]. Moreover, per capita beer consumption varies significantly by country, for instance, with the highest levels observed in the Czech Republic—152.1 L in 2023 [37].

Table 2.
Content of 6-prenylnaringenin (1) and total content of prenylflavonoids in various types of beers and herb tea [32,38].
1.2. Pharmacokinetics of 6-PN (1)
The metabolism and mode of action of all prenylated flavonoids are not yet fully understood, particularly regarding the biotransformation of these compounds, which are present in small quantities in hops. Only a few studies have assessed the absorption, metabolism, and excretion of 6-prenylnaringenin (1) or its structural isomer 8-prenylnaringenin (2), both derived from hop extracts, in humans. There are individual differences in the biotransformation of compound 1, and its absorption is notably weaker than that of 8-PN (2) [39,40,41,42,43].
Van Breemen et al. observed that 6-PN (1) and 8-PN (2) exhibit low oral bioavailability and significant inter-individual variability. Following a single oral dose of 500 mg of either compound, both were rapidly absorbed and extensively metabolized in both sexes. Notably, 6-PN (1) was found to be four to five times less bioavailable than its isomer, 8-PN (2). Despite this lower bioavailability, with mean Cmax values for 6-PN (1) ranging from 483 to 602 nmol/L compared to 2250–3418 nmol/L for 8-PN (2), 6-PN (1) demonstrated similar bioactivity to 8-PN (2). Pharmacokinetic and biotransformation studies of pure 6-PN (1) revealed its rapid conjugation with glucuronic acid. Furthermore, both prenylflavonoids exhibited promising ex vivo immunostimulatory effects [39]. Calvo-Castro et al. also investigated the bioavailability and safety of orally administered 6-PN (1) and 8-PN (2) in sixteen healthy young subjects, as well as their effects on peripheral blood mononuclear cells (PBMC). Their experiments confirmed previous findings, demonstrating that both compounds were equally effective in increasing PBMC viability [44].
The significant differences observed in the absorption, metabolism, elimination, and biological activity between these two structurally very similar positional isomers warrant further comprehensive study.
2. Biological Activity
Prenylflavonoids are a fascinating class of naturally occurring substances known for their diverse and desired biological properties [45,46,47,48,49]. While prenylation often enhances certain bioactivities of flavonoids, particularly their estrogenic and anticancer effects, it also tends to reduce their bioavailability and increase their bioaccumulation in tissues [49,50]. Despite these challenges, their promising biological activity and favorable safety profiles suggest that prenylated flavonoids hold significant potential for use as nutraceuticals or drugs [51,52,53].
While numerous reviews exist for prenylflavonoids generally, research on 6-PN (1) often lacks a unified focus due to its presence alongside the highly potent phytoestrogen 8-PN (2). This review aims to critically evaluate the current literature on 6-PN’s non-hormonal properties—including its potent neuroactive, antimicrobial activities as well as other potentially beneficial health effects—to demonstrate its potential as a safer therapeutic agent compared to both conventional hormone replacement therapy (HRT) and more potent phytoestrogens.
2.1. Antioxidant Activity
The antioxidant effect of flavonoids stems, in part, from the presence of -OH functional groups in the molecule, which can scavenge free radicals and chelate transition metal ions. This property makes these compounds a subject of significant research regarding their potential anticancer effects [54,55,56]. 6-PN (1), like other prenylflavonoids, positively impacts human health, largely attributed to its antioxidant properties. However, relatively few studies on this specific aspect have been published, likely due to its low dietary content [57]. The conducted research indicates that most analyzed compounds exhibit high levels of antioxidant activity, though inhibitory effects and structure-dependence have also been observed [2,17,47]. Direct comparisons between compounds are challenging due to the variety of measurement methods and positive controls used by different research groups. Implementing validation in future studies could yield more reliable information. For instance, studies on antioxidant activity have shown that 8-PN (2) was identified as an antioxidant, while 6-PN (1) appeared to be a mild pro-oxidant in certain assays, such as the DMPD chemiluminescence assay (based on DMPD•+ scavenging activity) and the LDL oxidation inhibition assay [58,59]. Therefore, the precise mechanism of the antioxidant activity of these compounds still requires further elucidation.
2.2. Phytoestrogen Activity
Estrogens have been shown to offer several beneficial effects on human health, including protection against menopausal symptoms, osteoporosis, cardiovascular disease, and some potentially neurodegenerative disorders. However, estrogens are also identified as one of the most significant risk factors for breast cancer [60]. Phytoestrogens are non-steroidal compounds derived from plants that elicit a biological response similar to that produced by the primary human estrogen, 17β-estradiol, in hormonal assays. Flavonoids represent the main group of phytoestrogens associated with this physiological effect [61].
During hop research, it was found that desmethylxanthohumol (3) is a pro-estrogenic substance [62]. Hänsel and Schulz were the first to accurately describe the chemical structure of desmethylxanthohumol (3) derivatives. However, they concluded that while the mixture of 8-prenylnaringenin (2) and 6-prenylnaringenin (1) exhibited an estrogenic effect, 6-PN (1) alone did not [63]. Milligan et al. isolated and characterized 8-PN (2) as the major estrogenic substance in hops, considering it one of the most potent plant estrogens known [64].
In vitro studies have shown that 8-PN (2) mimics the action of 17β-estradiol, although its potency as an estrogen is considerably lower (10–20,000-fold) [65,66,67,68,69]. The estrogenicity of other structurally related hop flavonoids was less than 1% compared to 8-PN (2), decreasing in the following order: 8-prenylnaringenin (2) >> 6-prenylnaringenin (1) > 8-geranylnaringenin > 6,8-diprenylnaringenin [65].
Menopause is defined as the permanent cessation of menstruation, directly resulting from the termination of ovarian follicle activity [70]. The standard treatment for menopause involves hormone replacement therapy (HRT), the use of selective estrogen receptor modulators [71], and other medications, such as selective serotonin reuptake inhibitors, which help improve vasomotor symptoms [72].
The use of botanical dietary supplements as an alternative to hormone replacement therapy (HRT) has recently increased [73,74]. This trend is partly driven by findings from the Women’s Health Initiative (WHI), which showed a relationship between long-term HRT use and an increased risk of developing hormone-dependent cancers [75,76].
Effenberger et al. demonstrated that 6-PN (1) might serve as an alternative to conventional hormone replacement therapy for preventing osteoporosis due to its preference for ERβ (estrogen receptor beta). However, its safety is dose-dependent, with cytotoxic effects observed at high concentrations (≥10−4 M) [77]. The clinical utility of 6-PN (1) in managing menopausal symptoms and osteopenia is primarily derived from its preferential activity toward ERβ. Crucially, its significantly weaker estrogenic effect compared to 8-PN (2) [65] makes it a particularly attractive and safer candidate for alternative hormone therapy, reducing the potential risks associated with stimulating estrogen-receptor alpha (ERα)-positive tissue proliferation—a known concern with potent phytoestrogens in susceptible individuals.
Humulus lupulus L. (hops) is a popular plant source for dietary supplements, particularly used by women to alleviate postmenopausal symptoms. Despite its popularity, the benefit–risk ratio and the precise composition of hop extract remain an area of ongoing research (Table 3).

Table 3.
Natural health products and dietary supplements containing 6-PN (1) for managing menopausal symptoms or as an alternative to hormone replacement therapy [8].
Clinical trials are crucial for evaluating the effectiveness of 6-PN (1) in alleviating menopause symptoms and ensuring its safe use. A comprehensive understanding of 6-PN’s (1) impact on the human body will enable its most beneficial application in treating postmenopausal symptoms while minimizing side effects. This knowledge will also facilitate the optimal selection of dosage and therapy duration.
2.3. Anticancer Activity
Cancer stands as the second leading cause of death globally. In 2022, an estimated 20 million new cancer cases and 9.7 million deaths were reported [78]. Among all cancers, the most frequently diagnosed types include lung (12.7%), breast (10.9%), colorectal (9.7%), and gastric cancer (7.81%). Cancer cells are distinct from normal cells due to their uncontrolled proliferation, dedifferentiation, and loss of function, invasiveness, and metastasis [79,80].
Although numerous therapies currently exist, significant emphasis is placed on naturally derived compounds. This is due to their often high bioavailability, cost-effectiveness, and minimal side effects. Flavonoids, for instance, demonstrate anticancer effects by regulating various molecular targets. These include cell cycle blocking, DNA repair, anti-inflammatory effects, activation of tumor suppressor genes and oncogene suppression, regulation of hormone levels and growth factors, inhibition of invasion, proliferation, angiogenesis, and metastasis, and induction of apoptosis [81].
Some prenylflavonoids can directly inhibit the growth of cancer cells while exhibiting low toxicity towards healthy tissues. Due to their antioxidant effects, anti-inflammatory properties, and ability to modulate the metabolism of carcinogenic substances, some of these compounds may even be utilized in cancer prevention [82,83,84]. Interestingly, certain compounds can affect cancer cells even when classical chemotherapy fails. Among these is 6-PN (1), which acts as both a substrate and inhibitor of the efflux transporter breast cancer resistance protein (BCRP/ABCG2). BCRP/ABCG2 is a membrane-bound multidrug transporter often overexpressed in cancer cells, contributing to their resistance to conventional treatments [85]. Although not as active as chalcone xanthohumol (4), a major prenylflavonoid found in hops, 6-PN (1) demonstrates anticancer activity through multiple pathways. Its anti-cancer properties are summarized in Table 4.

Table 4.
In vitro activity of 6-prenylnaringenin (1) as a potential anticancer agent.
Currently, the impact of prenylnaringenin 6-PN (1) on the risk of developing estrogen-dependent cancers remains insufficiently explained and documented. Phytoestrogens are thought to be linked to the etiology of breast cancer and are being evaluated as either potential chemopreventive agents or cancer promoters [95]. For example, the highly estrogenic 8-PN (2) has been shown to increase uterine wet weight in rat experiments, which suggests that it may promote hormone-dependent tumors [96,97].
Phytoestrogens may act as chemopreventive agents, while also potentially promoting the growth of cancer cells via activation of the estrogen receptor. Furthermore, they can exert estrogenic effects via both receptor-dependent and receptor-independent mechanisms. Activation of ERα is associated with proliferative responses in the mammary gland and uterus. These findings suggest that phytoestrogen intake might not be suitable for patients at an increased risk of hormone-dependent cancers or for cancer survivors [98]. Molecular studies have demonstrated that almost all popular herbal supplements contain phytochemicals capable of binding to the human estrogen receptor. Phytoestrogens could be effective growth promoters for estrogen receptor-positive tumors and may also pose a risk to patients with ER-positive tumors who are undergoing antiestrogen treatment [99].
In contrast to 8-PN (2), neither 6-PN (1) nor hop extract produced such a tumor-promoting effect. Wang et al. explained that 6-PN (1), acting as an aryl hydrocarbon receptor (AHR) agonist, and hop extract enhance the non-toxic estrogen 2-hydroxylation pathway by increasing P450 1A1 expression via AHR in MCF-10A and MCF-7 cell lines [94]. 6-PN (1) increased the expression of the tumor suppressor gene (AHRR) and genes involved in estrogen metabolism (CYP1A1, CYP1B1). Although 6-PN (1) can activate both the detoxification and genotoxic pathways of estrogen metabolism, the hop extract as a whole only modulates the genotoxic pathway by increasing CYP1B1 mRNA expression.
These data highlight the significant role of 6-PN (1), found in hop extract, as a potential modulator of estrogen metabolism. This is attributed to its agonist effects on both ERα and AHR [100]. Hitzman et al. further confirmed that 6-PN (1) from hops interferes with the regulation of CYP1A1 via ERα, thereby facilitating estrogen detoxification [101]. While hop extracts primarily modulate the genotoxic pathway of estrogen metabolism, they also contain phytoestrogens like 8-PN (2). Since hop supplements are frequently used by women to alleviate postmenopausal symptoms, caution and further research are warranted regarding the use of hop preparations. This is particularly important due to the role of estrogenic compounds in the development of estrogen-dependent cancers, including endometrial cancer.
The results gathered thus far highlight the significant role of 6-PN (1), present in hop extract, as a potential modulator of estrogen metabolism. This suggests a potentially protective role in reducing the risk of breast cancer and underscores the importance of standardizing botanical extracts for safe use. Therefore, hop extracts intended for use should contain precisely defined and adjusted doses of desired bioactive phytochemicals, including the chemopreventive xanthohumol (4). Crucially, they must not contain phytochemicals that could expose individuals to adverse side effects. However, for medicinal purposes, using pure compounds appears to be a safer approach.
The identification of 6-PN’s (1) broad potential against both estrogenic and non-estrogenic cancer cells offers promising clinical prospects for its use to enhance the effects of pharmacological drugs. However, caution is advised when using even weak phytoestrogens in individuals at risk of hormone-dependent cancers.
Chemical modifications to compounds based on the initial 6-PN (1) structure could potentially lead to enhanced pharmacological effects.
In summary, the presented data suggest that 6-PN (1) is a promising candidate for use as an active substance in chemoprevention. It could also serve as a valuable model structure for developing novel epigenetic prevention and therapy strategies for various cancers, including melanoma.
2.4. Antimicrobial Activity
Antimicrobial properties of flavonoids strictly depend on the compound’s structure [102,103]. Literature data indicate that the saturation of the double bond at the C2–C3 position, the presence and position of hydroxyl groups (at C-5, C-7, and C-4′) [104,105,106], as well as the presence of additional substituents such as prenyl groups (primarily at the C6 position, and also at C8) [107,108] are crucial for the antibacterial properties of flavonoids.
2.4.1. Antibacterial Activity
Multidrug-resistant microorganisms are a leading cause of infectious diseases globally [109]. The most recent global estimates on antibiotic resistance highlight three human pathogens as particularly concerning worldwide due to their association with nosocomial and community-acquired infections: Staphylococcus aureus, Escherichia coli, and Klebsiella pneumoniae [110]. Approximately 50% of patients hospitalized with diabetic foot infections suffer from osteomyelitis, which is often linked to MRSA (Methicillin-resistant Staphylococcus aureus) [111]. The poor diffusion of antibiotics into necrotic tissues, primarily due to persistent biofilm formation, poses significant challenges in clinical practice [112]. A promising strategy for addressing the problem of antibiotic resistance in certain bacterial strains is to exploit the synergy between natural compounds, including flavonoids and traditional antibiotics [113]. Hop phenolic compounds, with their dual antibacterial and anti-biofilm effects, may offer a new perspective in treating MRSA-caused infections [114].
The antimicrobial activity of 6-prenylnaringenin (1) was first demonstrated by Mizobuchi and Sato [10]. 6-PN (1) inhibited the growth of dermatophytes Trichophyton mentagrophytes and T. rubrum more effectively than the positive control griseofulvin (MIC: 3.13 µg/mL) [10] (Table 5).

Table 5.
Antimicrobial, antiviral, antifungal, and antiparasitic activities of 6-prenylnaringenin (1).
Shirataki et al. studied the activity of 6-PN (1) against Gram-positive bacteria of the genera Bacillus and Staphylococcus as well as Gram-negative bacteria of the genera Escherichia, Helicobacter, Klebsiella, Providencia, Shigella, and Vibrio, obtaining results ranging from strong to moderate antibacterial activity [89]. While Osorio et al. demonstrated that 6-PN (1) is active against Methicillin-resistant Staphylococcus aureus bacteria (MRSA). It exhibits a strong synergistic effect when combined with commonly used antibiotics such as vancomycin, ciprofloxacin, and methicillin, enhancing their effectiveness by 10 to 100 times [90] (Table 5). The finding that 6-PN (1) exhibits a strong synergistic effect with conventional antibiotics is highly significant. This synergy represents a clear, actionable pathway for future drug development. It is therefore crucial that future research prioritize the development of topical formulations of 6-PN (1) for localized infections, such as those caused by MRSA, where its dual action (direct antibacterial and antibiotic-enhancing) can be leveraged without the systemic bioavailability challenges.
2.4.2. Antiviral Activity
Recurrent viral epidemics, particularly the recent COVID-19 pandemic, have driven scientists to search intensively for health-promoting supplements and drugs. Numerous studies have demonstrated the antiviral effects of polyphenols, particularly prenylflavonoids, on a variety of viruses. These include several RNA viruses, such as BVDV and HCV [116], as well as CMV and DNA viruses like HSV-1 and HSV-2 [117]. Additionally, phenolic compounds show antiviral activity against HIV-1 by inhibiting HIV-1 reverse transcriptase in vitro [118].
Bouback et al. investigated the effect of a crude extract from H. lupulus (hops) on the inhibition of Middle East respiratory syndrome coronavirus (MERS-CoV) and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) replication in vitro, using Vero E6 cell lines (cells isolated from the kidney of an African green monkey). Hops extract showed very low toxicity on Vero E6 cells, with a CC50 of 23.25 µg/µL, while it demonstrated significant antiviral activity against MERS-CoV and SARS-CoV-2, with IC50 values of 0.18 µg/µL and 0.9 µg/µL, respectively. The crude extract achieved an inhibition rate of 84.6% for MERS-CoV and 80% for SARS-CoV-2. An in silico analysis revealed the presence of 6-PN (1) in the extract. This compound was found to inhibit the process of viral invasion into host cells by interfering with the viral spike protein’s ability to recognize the host cell receptor [119]. Herzog et al. reported that 6-PN (1), while not as active as xanthohumol (4), also targets SARS-CoV-2 PLpro, which is a promising therapeutic target because it contributes to both viral replication and the modulation of the immune system. This study found that 6-PN (1) exhibits marked antiviral activity against SARS-CoV-2. These findings support the possibility of developing new antiviral drugs [120]. Osorio et al. tested 6-PN (1) against SARS-CoV-2, obtaining antiviral activity IC50 = 7.3 μg/mL [90], while Morimoto et al. showed anti-Influenza activity of 6-PN (1) [53] (Table 5).
2.4.3. Other Antimicrobial Activities
Additionally, 6-PN (1) has demonstrated antiparasitic activity against Trypanosoma brucei [115]. Human African trypanosomiasis, caused by this protozoan, is considered a neglected tropical disease that has a significant impact on human health, as it is fatal if left untreated [121]. Antifungal studies conducted on numerous filamentous fungi have shown rather low activity of 6-PN (1), with the exception of fungi of the genera Microsporum, Mucor, and Trichophyton, where growth inhibitory activity ranged from strong to moderate [10,90] (Table 5).
2.5. Impact on the Nervous System
Neurodegenerative diseases represent a growing burden on aging societies, making them a significant area of interest. Neurogenesis, the process by which new neurons are generated from neural stem cells in the adult brain, is partly regulated by sex hormones like estradiol. In animals, stimulation of neurogenesis by estradiol correlates with improved neurological function. Urmann et al. explored the effects of 8-PN (2), 6-PN (1), and related compounds on the in vitro differentiation of neuronal precursor cells. While prenylated flavanones 1 and 2 exhibit differing estrogenic activities, they both have the same effect on inducing differentiation in neural precursor cells [122].
GABA type A (GABAA) receptors for γ-aminobutyric acid are the primary inhibitory neurotransmitter receptors responsible for rapid inhibition in the basal part of the brain. These receptors belong to the ion channel family. They mediate rapid synaptic transmission and serve as important pharmacological targets for drugs, including anxiolytics and hypnotics [123]. In 1980, Hänsel et al. identified that 2-methyl-3-buten-2-ol, a degradation product of humulone and lupulone from hops, is responsible for the plant’s sedative and hypnotic properties by increasing the activity of γ-aminobutyric acid (GABA) [124]. Later, it was shown that 8-prenylnaringenin (2) also has a therapeutic effect, but this effect depends on the specific enantiomer (2R or 2S). The 2R-8-PN (2) enantiomer shows a higher affinity (meaning a lower inhibition constant) for all tested transporters—serotonin, noradrenaline, and dopamine—than the 2S-8-PN (2) enantiomer [125]. Since 8-PN (2) naturally occurs in hops as the 2S enantiomer, these findings suggest that the synthesis of optically pure enantiomers of prenylated flavanones, such as 8-PN (2) or 6-PN (1), could be crucial for the pharmaceutical industry to develop more effective treatments.
Benkherouf et al. examined humulone interactions with other active hops compounds: 6-PN (1) and isoxanthohumol (IXN) on GABA-induced displacement of [3H]EBOB binding to native GABAA receptors in rat brain membranes. They confirmed that humulone exhibits sedative/hypnotic effects and acts as a positive allosteric modulator of GABAA receptors. In the presence of 3 mM GABA, 1 mM of humulone led to an additive potentiation of GABA-induced [3H]EBOB displacement in rat forebrain. The same results were obtained when testing 6-PN (1) and isoxanthohumol. Moreover, co-incubation of humulone with 6-PN (1) or isoxanthohumol, and with a combination of both 6-PN (1) and isoxanthohumol, significantly increased this potentiation [126]. These findings suggest that the neuroactivity of hops may result from the presence and interaction of other hop compounds, such as 6-PN (1) and/or isoxanthohumol, but not humulone alone. The results demonstrate a synergistic action of hop flavanones in stimulating the GABAA receptor, emphasizing the role of 6-PN (1) and isoxanthohumol in enhancing the action of humulone on this receptor [126]. Other studies have shown that among the prenylflavonoids found in hops, 6-prenylnaringenin (1) is the most active positive allosteric modulator of GABAA receptors. In comparison to xanthohumol (4), isoxanthohumol, and 8-prenylnaringenin (2), 6-PN (1) was found to be 8, 3, and 2 times more active, respectively [127,128].
An open-field rotary rod study in mice demonstrated that 6-PN (1) can cross the blood–brain barrier (BBB) without affecting locomotor activity. Literature data suggest that both 2R and 2S enantiomers of 6-PN (1), along with their derivatives, act as blockers of T-type calcium channels (T channels) Cav3.2. The blocking of these T channels has been shown to alleviate visceral and neuropathic pain without causing cardiovascular or behavioral side effects [129]. 6-PN (1) also shows promise for pain treatment [130,131].
The superior potency of 6-PN (1) as a GABAA receptor positive allosteric modulator, being 2 to 8 times more active than its congeners, positions it as a prime therapeutic lead for conditions requiring CNS modulation (anxiety, insomnia, pain). Furthermore, its capacity to block T-type calcium channels (Cav3.2) to alleviate visceral and neuropathic pain provides a second, distinct mechanism for analgesic application. Future studies should focus on synthesizing optically pure enantiomers and derivatives to optimize both their GABAA and T-channel activity, aiming for clinical trials to confirm their behavioral effects in vivo.
3. Synthesis
Although 6-prenylnaringenin (1) has attractive biological properties, its low concentration in plant extracts makes large-scale isolation economically impractical [132]. The various synthetic approaches developed have often suffered from low efficiency and multiple steps. While some methods have been modified to improve yields, the complex three-ring structure of flavonoids allows for prenylation at different positions, which complicates synthesis.
6-PN (1) was first synthesized in 1978 [133,134,135]. However, the descriptions of the NMR spectra from that time contain discrepancies that have raised doubts about the reported structure [10]. One early method involved the condensation of naringenin with 2-methyl-but-3-en-2-ol in the presence of boron trifluoride etherate, which produced a mixture of three main fractions, one of which contained 6-PN (1) [135]. The total synthesis of 6-PN (1) has also been reported using an acetophenone derivative as a key intermediate [136]. A later pathway, reported by Tischer and Metz in 2006, aimed for complete regioselectivity at the C-6 position using a europium(III)-catalyzed Claisen rearrangement [137] followed by cross-metathesis [138,139], using relatively cheap and readily available naringenin (4′,5,7-trihydroxyflavanone) (5) as substrate for synthesis (Figure 3a). Despite these efforts, the yields of these early synthetic methods were not satisfactory. More recently, Urmann and Riepl developed a more efficient method using the design of experiment (DOE) and microwave synthesis. They achieved a high total yield of 76% by demethylating xanthohumol (4) with lithium chloride in dimethylformamide, which produced both 8-prenylnaringenin (2) (yield 38%) and 6-prenylnaringenin (1) (yield 38%) (Figure 3b) [140].
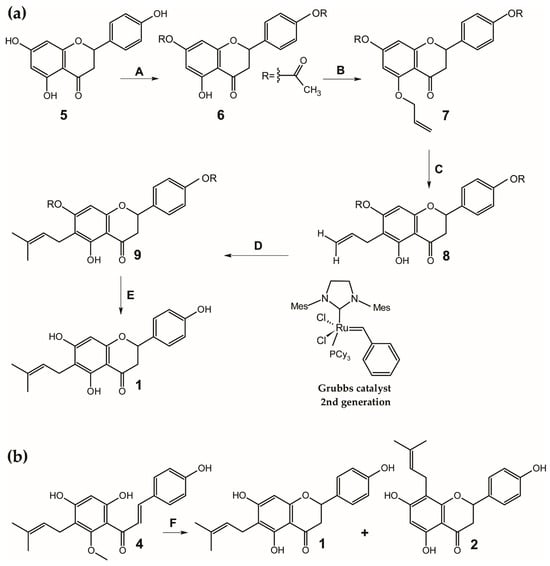
Figure 3.
Synthesis of 6-prenylnaringenin (1) via: (a) europium(III)-catalyzed Claisen rearrangement and cross-metathesis [139] and (b) microwave irradiation [140]. Reagents, conditions and yields: (A) Ac2O 2 equivs, pyridine, room temperature, 89%; (B) allyl alcohol, PPh3, diethyl azodicarboxylate, THF, 0 °C to room temperature, 73%; (C) 10 mol% Eu(fod)3, CHCl3, 70 °C, 16.5 h, 74%; (D) isobutylene, 1 mol% Grubbs catalyst 2nd generation, benzene, room temperature 2 days, 78%; (E) MeOH, K2CO3 0.3 equivs., 40 °C, 2 h, 88%; (F) lithium chloride, dimethylforamide, microwave irradiation, 198 °C, 9 min, 38%. 1—6-prenylnaringenin (4′,5,7-trihydroxy-6-prenylflavanone), 2—8-prenylnaringenin (4′,5,7-trihydroxy-8-prenylflavanone), 4—xanthohumol (2′,4′,4-trihydroxy-6′-methoxy-5′-prenylchalcone), 5—naringenin (4′,5,7-trihydroxyflavanone), 6—4′,7,-diacetylnaringenin (4′,7-diacetoxy-5-hydroxyflavanone), 7—4′,7-diacetyl-5-O-allylnaringenin (4′,7-diacetoxy-5-allyloxyflavanone), 8—4′,7-diacetyl-6-allylnaringenin (4′,7-diacetoxy-6-allyl-5-hydroxyflavanone), 9—7,4′-diacetyl-6-prenylnaringenin (4′,7-diacetoxy-5-hydroxy-6-prenylflavanone). Total yields: 5→1 29%; 4→1 38%.
Another promising approach is through genetic engineering. Researchers successfully transferred the prenyltransferase gene (ShFPT) from Streptomyces sp. NT11 into Escherichia coli. The enzyme showed high selectivity for prenylating naringenin at the C-6 position. Under optimal conditions (pH 6.0 and 55 °C), this bioconversion method achieved a peak yield of 69.9 mg/L and an average yield of 4.0 mg/L/h after 16 h of incubation, demonstrating an effective biotechnological way to produce 6-PN (1) [141].
4. Conclusions
This review establishes 6-PN (1) as a multifaceted, high-priority therapeutic candidate distinguished by its potent neuroactive, synergistic antimicrobial, and unique chemopreventive profile.
6-PN (1) demonstrates a range of antimicrobial activities, including antibacterial, antiviral (e.g., against SARS-CoV-2), and antiparasitic effects. 6-PN (1) could serve as a core scaffold for developing new antimicrobials, especially through structural modifications such as halogenation of ring B. Research on the simultaneous administration of 6-PN (1) and antibiotics is also worth pursuing. Combining them could potentially reduce the required drug dosage, lower the risk of systemic toxicity, and increase treatment effectiveness, particularly when applied topically to avoid toxicity. Given the promising results from therapies using lupulone when antibiotic therapy fails, the antibacterial properties of 6-PN (1) are of significant interest.
6-PN (1) exhibits cytotoxic and antiproliferative effects on various cancer cell lines. It is considered a candidate for chemoprevention of non-hormone-dependent tumors and as a model structure for new therapies. However, to determine the usefulness of 6-PN (1) for cancer treatment, several key areas need further investigation. It is necessary to understand its potential toxic side effects on healthy tissues, detail its molecular mechanisms, and, most importantly, confirm its effects in vivo. Current laboratory data are insufficient to provide clinicians with clear guidelines for using 6-PN (1). While early short-term studies suggest that phytoestrogens might have a stimulatory effect in women at high risk for or with breast cancer, long-term studies are crucial to confirm their effects and to avoid potential side effects related to their weak estrogenic activity in patients with hormone-dependent cancers. Crucially, researchers need to verify whether 6-PN (1) acts as a chemopreventive agent or if it can produce a synergistic effect when combined with conventional therapies. Such an effect could potentially shorten the duration of therapy, limit side effects, and help prevent further cancer mutations.
The most promising avenue for 6-prenylnaringenin (1) lies in its capacity as a positive allosteric modulator of GABAA receptors and its synergistic effect with traditional antibiotics. To translate these findings into clinical reality, research efforts must be directed toward the chemical modification of the 6-PN (1) core—leveraging modern synthetic techniques to improve the water solubility and oral bioavailability that currently limit its therapeutic potential. Furthermore, targeted in vivo studies are urgently needed to confirm its behavioral effects (anxiolytic, sedative, analgesic) and establish its long-term safety profile, particularly concerning its anti-cancer chemopreventive role and non-proliferative effects on ER-positive tumors. Concurrently, the accelerated development of topical or localized and targeted delivery systems should be pursued to capitalize on the powerful antibiotic synergy demonstrated against drug-resistant pathogens like MRSA. Given the amount of ongoing research and modern technological capabilities, the future for 6-prenylnaringenin (1) looks promising, provided that research shifts focus from mere characterization to targeted development based on its distinct non-hormonal therapeutic advantages.
Author Contributions
Conceptualization, A.B. and T.T.; writing—original draft preparation, A.B. and T.T.; writing—review and editing, A.B., T.T., D.Ł. and J.P.; visualization, A.B. and T.T.; supervision, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Karel, Š. Cytotoxic potential of C-prenylated flavonoids. Phytochem. Rev. 2014, 13, 245–275. [Google Scholar]
- Chen, X.; Mukwaya, E.; Wong, M.-S.; Zhang, Y. A systematic review on biological activities of prenylated flavonoids. Pharm. Biol. 2014, 52, 655–660. [Google Scholar] [CrossRef]
- Nguyen, V.S.; Dong, L.P.; Wang, S.C.; Wang, Q. The first total synthesis of sophoflavescenol, flavenochromane C, and citrusinol. Eur. J. Org. Chem. 2015, 2015, 2297–2302. [Google Scholar] [CrossRef]
- Shi, S.; Li, J.; Zhao, X.; Liu, Q.; Song, S.-J. A comprehensive review: Biological activity, modification and synthetic methodologies of prenylated flavonoids. Phytochemistry 2021, 191, 112895. [Google Scholar] [CrossRef]
- TGSC Information System. Available online: http://www.thegoodscentscompany.com/data/rw1859521.html (accessed on 6 August 2025).
- ChemFaces. Available online: https://www.chemfaces.com/natural/6-prenylnaringenin-CFN92017.html (accessed on 6 August 2025).
- Dhooghe, L.; Naessens, T.; Heyerick, A.; De Keukeleire, D.; Vlietinck, A.J.; Pieters, L.; Apers, S. Quantification of xanthohumol, isoxanthohumol, 8-prenylnaringenin, and 6-prenylnaringenin in hop extracts and derived capsules using secondary standards. Talanta 2010, 83, 448–456. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.; Davies, N. Stereospecific quantitation of 6-prenylnaringenin in commercially available H. lupulus-containing natural health products and dietary supplements. Res. Pharm. Sci. 2015, 10, 182–191. [Google Scholar] [PubMed]
- Komatsu, M.; Yokoe, I.; Shirataki, Y. Studies on the constituents of Sophora species. XIII. Constituents of the aerial parts of Sophora tomentosa L. (2). Chem. Pharm. Bull. 1978, 26, 3863–3870. [Google Scholar] [CrossRef]
- Mizobuchi, S.; Sato, Y. A new flavanone with antifungal activity isolated from hops. Agric. Biol. Chem. 1984, 48, 2771–2775. [Google Scholar]
- McCormick, S.; Robson, K.; Bohm, B. Flavonoids from Wyethia glabra. Phytochemistry 1985, 24, 1614–1616. [Google Scholar] [CrossRef]
- McCormick, S.P.; Robson, K.A.; Maze, J.; Bohm, B.A. Flavonoids of wyethia section agnorhiza. Phytochemistry 1987, 26, 2421–2422. [Google Scholar] [CrossRef]
- Tahara, S.; Katagiri, Y.; Ingham, J.L.; Mizutani, J. Prenylated flavonoids in the roots of yellow lupin. Phytochemistry 1994, 36, 1261–1271. [Google Scholar] [CrossRef]
- Hayashi, H.; Yasuma, M.; Hiraoka, N.; Ikeshiro, Y.; Yamamoto, H.; Yeşilada, E.; Sezik, E.; Honda, G.; Tabata, M. Flavonoid variation in the leaves of Glycyrrhiza glabra. Phytochemistry 1996, 42, 701–704. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Lee, H.-N.; Park, E.-H.; Shim, S.-H. Inhibition of human 20S proteasome by compounds from seeds of Psoralea corylifolia. Bull. Korean Chem. Soc. 2009, 30, 1867–1869. [Google Scholar] [CrossRef]
- Chadwick, L.R.; Nikolic, D.; Burdette, J.E.; Overk, C.R.; Bolton, J.L.; van Breemen, R.B.; Fröhlich, R.; Fong, H.H.; Farnsworth, N.R.; Pauli, G.F. Estrogens and Congeners from Spent Hops (Humulus lupulus). J. Nat. Prod. 2004, 67, 2024–2032. [Google Scholar] [CrossRef]
- Shirataki, Y.; Motohashi, N.; Tani, S.; Sakagami, H.; Satoh, K.; Nakashima, H.; Mahapatra, S.K.; Ganguly, K.; Dastidar, S.G.; Chakrabarty, A.N. In vitro biological activity of prenylflavanones. Anticancer Res. 2001, 21, 275–280. [Google Scholar]
- Shirataki, Y.; Motohashi, N. Flavonoids in Sophora species. In Bioactive Heterocycles VII: Flavonoids and Anthocyanins in Plants, and Latest Bioactive Heterocycles II; Springer: Berlin/Heidelberg, Germany, 2009; pp. 41–91. [Google Scholar]
- McCormick, S.; Robson, K.; Bohm, B. Flavonoids of Wyethia angustifolia and W. helenioides. Phytochemistry 1986, 25, 1723–1726. [Google Scholar] [CrossRef]
- Limper, C.; Wang, Y.; Ruhl, S.; Wang, Z.; Lou, Y.; Totzke, F.; Kubbutat, M.H.; Chovolou, Y.; Proksch, P.; Wätjen, W. Compounds isolated from Psoralea corylifolia seeds inhibit protein kinase activity and induce apoptotic cell death in mammalian cells. J. Pharm. Pharmacol. 2013, 65, 1393–1408. [Google Scholar] [CrossRef] [PubMed]
- Zanoli, P.; Zavatti, M. Pharmacognostic and pharmacological profile of Humulus lupulus L. J. Ethnopharmacol. 2008, 116, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Moir, M. Hops—A millennium review. J. Am. Soc. Brew. Chem. 2000, 58, 131–146. [Google Scholar] [CrossRef]
- Dostálek, P.; Karabín, M.; Jelínek, L. Hop phytochemicals and their potential role in metabolic syndrome prevention and therapy. Molecules 2017, 22, 1761. [Google Scholar] [CrossRef]
- Nathan, M.; Scholten, R. The complete German Commission E monographs: Therapeutic guide to herbal medicines. Ann. Intern. Med. 1999, 130, 459. [Google Scholar] [CrossRef]
- Borrás, S.; Martínez-Solís, I.; Ríos, J.L. Medicinal plants for insomnia related to anxiety: An updated review. Planta Medica 2021, 87, 738–753. [Google Scholar] [CrossRef]
- Teuber, M.; Schmalreck, A.F. Membrane leakage in Bacillus subtilis 168 induced by the hop constituents lupulone, humulone, isohumulone and humulinic acid. Arch. Microbiol. 1973, 94, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Rozalski, M.; Micota, B.; Sadowska, B.; Stochmal, A.; Jedrejek, D.; Wieckowska-Szakiel, M.; Rozalska, B. Antiadherent and antibiofilm activity of Humulus lupulus L. derived products: New pharmacological properties. BioMed Res. Int. 2013, 2013, 101089. [Google Scholar] [CrossRef] [PubMed]
- Dresel, M.; Dunkel, A.; Hofmann, T. Sensomics analysis of key bitter compounds in the hard resin of hops (Humulus lupulus L.) and their contribution to the bitter profile of Pilsner-type beer. J. Agric. Food Chem. 2015, 63, 3402–3418. [Google Scholar] [CrossRef]
- Liu, M.; Hansen, P.; Wang, G.; Qiu, L.; Dong, J.; Yin, H.; Qian, Z.; Yang, M.; Miao, J. Pharmacological Profile of Xanthohumol, a Prenylated Flavonoid from Hops (Humulus lupulus). Molecules 2015, 20, 754. [Google Scholar] [CrossRef]
- Olsovska, J.; Bostikova, V.; Dusek, M.; Jandovska, V.; Bogdanova, K.; Cermak, P.; Bostik, P.; Mikyska, A.; Kolar, M. Humulus lupulus L.(hops)–a valuable source of compounds with bioactive effects for future therapies. Mil. Med. Sci. Lett 2016, 85, 19–30. [Google Scholar] [CrossRef]
- Stevens, J.F.; Page, J.E. Xanthohumol and related prenylflavonoids from hops and beer: To your good health! Phytochemistry 2004, 65, 1317–1330. [Google Scholar] [CrossRef]
- Stevens, J.F.; Taylor, A.W.; Deinzer, M.L. Quantitative analysis of xanthohumol and related prenylflavonoids in hops and beer by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 1999, 832, 97–107. [Google Scholar] [CrossRef]
- Rothwell, J.A.; Perez-Jimenez, J.; Neveu, V.; Medina-Remon, A.; M’hiri, N.; García-Lobato, P.; Manach, C.; Knox, C.; Eisner, R.; Wishart, D.S. Phenol-Explorer 3.0: A major update of the Phenol-Explorer database to incorporate data on the effects of food processing on polyphenol content. Database 2013, 2013, bat070. [Google Scholar] [CrossRef]
- De Gaetano, G.; Costanzo, S.; Di Castelnuovo, A.; Badimon, L.; Bejko, D.; Alkerwi, A.; Chiva-Blanch, G.; Estruch, R.; La Vecchia, C.; Panico, S. Effects of moderate beer consumption on health and disease: A consensus document. Nutr. Metab. Cardiovasc. Dis. 2016, 26, 443–467. [Google Scholar] [CrossRef]
- Wigg, S.; Stafford, L.D. Health warnings on alcoholic beverages: Perceptions of the health risks and intentions towards alcohol consumption. PLoS ONE 2016, 11, e0153027. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Llamas, A.; De la Cruz-Sánchez, E. Moderate beer consumption is associated with good physical and mental health status and increased social support. Nutrients 2023, 15, 1519. [Google Scholar] [CrossRef] [PubMed]
- Kirin Holdings. Available online: https://www.kirinholdings.com/en/newsroom/release/2024/1219_01.html (accessed on 6 August 2025).
- Buckett, L.; Schinko, S.; Urmann, C.; Riepl, H.; Rychlik, M. Stable isotope dilution analysis of the major prenylated flavonoids found in beer, hop tea, and hops. Front. Nutr. 2020, 7, 619921. [Google Scholar] [CrossRef]
- van Breemen, R.B.; Yuan, Y.; Banuvar, S.; Shulman, L.P.; Qiu, X.; Alvarenga, R.F.R.; Chen, S.N.; Dietz, B.M.; Bolton, J.L.; Pauli, G.F. Pharmacokinetics of prenylated hop phenols in women following oral administration of a standardized extract of hops. Mol. Nutr. Food Res. 2014, 58, 1962–1969. [Google Scholar] [CrossRef]
- Bolca, S.; Possemiers, S.; Maervoet, V.; Huybrechts, I.; Heyerick, A.; Vervarcke, S.; Depypere, H.; De Keukeleire, D.; Bracke, M.; De Henauw, S. Microbial and dietary factors associated with the 8-prenylnaringenin producer phenotype: A dietary intervention trial with fifty healthy post-menopausal Caucasian women. Br. J. Nutr. 2007, 98, 950–959. [Google Scholar] [CrossRef] [PubMed]
- Bolca, S.; Wyns, C.; Possemiers, S.; Depypere, H.; De Keukeleire, D.; Bracke, M.; Verstraete, W.; Heyerick, A. Cosupplementation of isoflavones, prenylflavonoids, and lignans alters human exposure to phytoestrogen-derived 17β-estradiol equivalents. J. Nutr. 2009, 139, 2293–2300. [Google Scholar] [CrossRef]
- Schaefer, O.; Bohlmann, R.; Schleuning, W.-D.; Schulze-Forster, K.; Hümpel, M. Development of a radioimmunoassay for the quantitative determination of 8-prenylnaringenin in biological matrices. J. Agric. Food Chem. 2005, 53, 2881–2889. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Nikolić, D.; Chen, S.; Alvarenga, R.R.; Pauli, G.; van Breemen, R. Structure Determination of Isoxanthohumol and 8-Prenylnaringenin Glucuronides Formed by Human Liver Microsomes. Planta Medica 2013, 79, PR4. [Google Scholar] [CrossRef]
- Calvo-Castro, L.A.; Burkard, M.; Sus, N.; Scheubeck, G.; Leischner, C.; Lauer, U.M.; Bosy-Westphal, A.; Hund, V.; Busch, C.; Venturelli, S. The oral bioavailability of 8-prenylnaringenin from hops (Humulus Lupulus L.) in healthy women and men is significantly higher than that of its positional isomer 6-prenylnaringenin in a randomized crossover trial. Mol. Nutr. Food Res. 2018, 62, 1700838. [Google Scholar] [CrossRef]
- Ullah, A.; Munir, S.; Badshah, S.L.; Khan, N.; Ghani, L.; Poulson, B.G.; Emwas, A.-H.; Jaremko, M. Important flavonoids and their role as a therapeutic agent. Molecules 2020, 25, 5243. [Google Scholar] [CrossRef]
- Nešpor, J.; Hanko, V.; Karabín, M.; Jelínek, L.; Dostálek, P. Prenylated flavonoids as valuable biologically active compounds from hops. Kvas. Prum. 2017, 63, 164–172. [Google Scholar] [CrossRef][Green Version]
- Santos, C.M.; Silva, A.M. The antioxidant activity of prenylflavonoids. Molecules 2020, 25, 696. [Google Scholar] [CrossRef]
- Schmandke, H. Prenylflavonoids in hops and beer-biochemical and biological activities. Ernährungs-Umschau 2006, 53, 225–229, 210. [Google Scholar]
- Yang, X.; Jiang, Y.; Yang, J.; He, J.; Sun, J.; Chen, F.; Zhang, M.; Yang, B. Prenylated flavonoids, promising nutraceuticals with impressive biological activities. Trends Food Sci. Technol. 2015, 44, 93–104. [Google Scholar] [CrossRef]
- Mukai, R. Prenylation enhances the biological activity of dietary flavonoids by altering their bioavailability. Biosci. Biotechnol. Biochem. 2018, 82, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Lv, H.-W.; Wang, Q.-L.; Luo, M.; Zhu, M.-D.; Liang, H.-M.; Li, W.-J.; Cai, H.; Zhou, Z.-B.; Wang, H.; Tong, S.-Q. Phytochemistry and pharmacology of natural prenylated flavonoids. Arch. Pharmacal Res. 2023, 46, 207–272. [Google Scholar] [CrossRef]
- Bartmańska, A.; Tronina, T.; Popłonski, J.; Huszcza, E. Biotransformations of prenylated hop flavonoids for drug discovery and production. Curr. Drug Metab. 2013, 14, 1083–1097. [Google Scholar] [CrossRef] [PubMed]
- Morimoto, R.; Matsubara, C.; Hanada, A.; Omoe, Y.; Ogata, T.; Isegawa, Y. Effect of Structural Differences in Naringenin, Prenylated Naringenin, and Their Derivatives on the Anti-Influenza Virus Activity and Cellular Uptake of Their Flavanones. Pharmaceuticals 2022, 15, 1480. [Google Scholar] [CrossRef]
- Cotelle, N.; Bernier, J.-L.; Catteau, J.-P.; Pommery, J.; Wallet, J.-C.; Gaydou, E.M. Antioxidant properties of hydroxy-flavones. Free Radic. Biol. Med. 1996, 20, 35–43. [Google Scholar] [CrossRef]
- Jovanovic, S.V.; Steenken, S.; Tosic, M.; Marjanovic, B.; Simic, M.G. Flavonoids as antioxidants. J. Am. Chem. Soc. 1994, 116, 4846–4851. [Google Scholar] [CrossRef]
- Grigalius, I.; Petrikaite, V. Relationship between antioxidant and anticancer activity of trihydroxyflavones. Molecules 2017, 22, 2169. [Google Scholar] [CrossRef]
- Ambrož, M.; Lněničková, K.; Matoušková, P. Antiproliferative effects of hop-derived prenylflavonoids and their influence on the efficacy of oxaliplatine, 5-fluorouracil and irinotecan in human colorectalC cells. Nutrients 2019, 11, 879. [Google Scholar] [CrossRef]
- Ng, K.R.; Lyu, X.; Mark, R.; Chen, W.N. Antimicrobial and antioxidant activities of phenolic metabolites from flavonoid-producing yeast: Potential as natural food preservatives. Food Chem. 2019, 270, 123–129. [Google Scholar] [CrossRef]
- Miranda, C.L.; Stevens, J.F.; Ivanov, V.; McCall, M.; Frei, B.; Deinzer, M.L.; Buhler, D.R. Antioxidant and prooxidant actions of prenylated and nonprenylated chalcones and flavanones in vitro. J. Agric. Food Chem. 2000, 48, 3876–3884. [Google Scholar] [CrossRef]
- Galluzzo, P.; Marino, M. Nutritional flavonoids impact on nuclear and extranuclear estrogen receptor activities. Genes Nutr. 2006, 1, 161–176. [Google Scholar] [CrossRef]
- Kiyama, R. Estrogenic flavonoids and their molecular mechanisms of action. J. Nutr. Biochem. 2023, 114, 109250. [Google Scholar] [CrossRef] [PubMed]
- Nastainczyk, W. Untersuchung über die Östrogene Wirkung des Hopfens und des Bieres. Ph.D. Thesis, Universitat des Saarlandes, Saarbrucken, Germany, 1972. [Google Scholar]
- Hänsel, R.; Schulz, J. Desmethylxanthohumol: Isolierung aus hopfen und cyclisierung zu flavanonen. Arch. Pharm. 1988, 321, 37–40. [Google Scholar] [CrossRef]
- Milligan, S.R.; Kalita, J.C.; Heyerick, A.; Rong, H.; De Cooman, L.; De Keukeleire, D. Identification of a potent phytoestrogen in hops (Humulus lupulus L.) and beer. J. Clin. Endocrinol. Metab. 1999, 84, 2249–2252. [Google Scholar] [CrossRef] [PubMed]
- Milligan, S.; Kalita, J.; Pocock, V.; Van De Kauter, V.; Stevens, J.; Deinzer, M.; Rong, H.; De Keukeleire, D. The endocrine activities of 8-prenylnaringenin and related hop (Humulus lupulus L.) flavonoids. J. Clin. Endocrinol. Metab. 2000, 85, 4912–4915. [Google Scholar] [CrossRef]
- Milligan, S.; Kalita, J.; Pocock, V.; Heyerick, A.; De Cooman, L.; Rong, H.; De Keukeleire, D. Oestrogenic activity of the hop phyto-oestrogen, 8-prenylnaringenin. Reprod. Camb. 2002, 123, 235–242. [Google Scholar] [CrossRef][Green Version]
- Rong, H.; Boterberg, T.; Maubach, J.; Stove, C.; Depypere, H.; Van Slambrouck, S.; Serreyn, R.; De Keukeleire, D.; Mareel, M.; Bracke, M. 8-Prenylnaringenin, the phytoestrogen in hops and beer, upregulates the function of the E-cadherin/catenin complex in human mammary carcinoma cells. Eur. J. Cell Biol. 2001, 80, 580–585. [Google Scholar] [CrossRef]
- Coldham, N.G.; Sauer, M.J. Identification, quantitation and biological activity of phytoestrogens in a dietary supplement for breast enhancement. Food Chem. Toxicol. 2001, 39, 1211–1224. [Google Scholar] [CrossRef]
- Zierau, O.; Gester, S.; Schwab, P.; Metz, P.; Kolba, S.; Wulf, M.; Vollmer, G. Estrogenic activity of the phytoestrogens naringenin, 6-(1, 1-dimethylallyl) naringenin and 8-prenylnaringenin. Planta Medica 2002, 68, 449–451. [Google Scholar] [CrossRef]
- Berek, J.S.; Berek, D.L.; Hengst, T.C. Berek & Novak’s Gynecology, 15th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012. [Google Scholar]
- Davis, S.R.; Dinatale, I.; Rivera-Woll, L.; Davison, S. Postmenopausal hormone therapy: From monkey glands to transdermal patches. J. Endocrinol. 2005, 185, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.S.; Nakajima, S.T. Hormonal and nonhormonal treatment of vasomotor symptoms. Obstet. Gynecol. Clin. 2015, 42, 163–179. [Google Scholar] [CrossRef]
- Dietz, B.M.; Hajirahimkhan, A.; Dunlap, T.L.; Bolton, J.L. Botanicals and their bioactive phytochemicals for women’s health. Pharmacol. Rev. 2016, 68, 1026–1073. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.; Kawa, K.; Eckl, V.; Morton, C.; Stredney, R. Herbal supplement sales in US increased 8.5% in 2017, topping $8 billion. HerbalGram 2018, 119, 62–71. [Google Scholar]
- Holmberg, L.; Anderson, H. HABITS (hormonal replacement therapy after breast cancer—Is it safe?), a randomised comparison: Trial stopped. Lancet 2004, 363, 453–455. [Google Scholar] [CrossRef]
- D’Alonzo, M.; Bounous, V.E.; Villa, M.; Biglia, N. Current evidence of the oncological benefit-risk profile of hormone replacement therapy. Medicina 2019, 55, 573. [Google Scholar] [CrossRef] [PubMed]
- Effenberger, K.E.; Johnsen, S.A.; Monroe, D.G.; Spelsberg, T.C.; Westendorf, J.J. Regulation of osteoblastic phenotype and gene expression by hop-derived phytoestrogens. J. Steroid Biochem. Mol. Biol. 2005, 96, 387–399. [Google Scholar] [CrossRef]
- Global cancer burden growing, amidst mounting need for services. Saudi Med. J. 2024, 45, 326–327.
- Rang, H.P.; Dale, M.M.; Ritter, J.M.; Flower, R.J.; Henderson, G. Rang & Dale’s Pharmacology; Elsevier Health Sciences: Amsterdam, The Netherlands, 2011. [Google Scholar]
- Le Marchand, L. Cancer preventive effects of flavonoids-a review. Biomed. Pharmacother. 2002, 56, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Afrin, S.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Cianciosi, D.; Reboredo-Rodriguez, P.; Zhang, J.; Manna, P.P.; Daglia, M.; Atanasov, A.G. Dietary phytochemicals in colorectal cancer prevention and treatment: A focus on the molecular mechanisms involved. Biotechnol. Adv. 2020, 38, 107322. [Google Scholar] [CrossRef]
- Venturelli, S.; Burkard, M.; Biendl, M.; Lauer, U.M.; Frank, J.; Busch, C. Prenylated chalcones and flavonoids for the prevention and treatment of cancer. Nutrition 2016, 32, 1171–1178. [Google Scholar] [CrossRef]
- Molčanová, L.; Janošíková, D.; Dall’ Acqua, S.; Šmejkal, K. C-prenylated flavonoids with potential cytotoxic activity against solid tumor cell lines. Phytochem. Rev. 2019, 18, 1051–1100. [Google Scholar] [CrossRef]
- Tronina, T.; Bartmańska, A.; Popłoński, J.; Rychlicka, M.; Sordon, S.; Filip-Psurska, B.; Milczarek, M.; Wietrzyk, J.; Huszcza, E. Prenylated flavonoids with selective toxicity against human cancers. Int. J. Mol. Sci. 2023, 24, 7408. [Google Scholar] [CrossRef]
- Tan, K.W.; Cooney, J.; Jensen, D.; Li, Y.; Paxton, J.W.; Birch, N.P.; Scheepens, A. Hop-derived prenylflavonoids are substrates and inhibitors of the efflux transporter breast cancer resistance protein (BCRP/ABCG2). Mol. Nutr. Food Res. 2014, 58, 2099–2110. [Google Scholar] [CrossRef]
- Delmulle, L.; Bellahcène, A.; Dhooge, W.; Comhaire, F.; Roelens, F.; Huvaere, K.; Heyerick, A.; Castronovo, V.; De Keukeleire, D. Anti-proliferative properties of prenylated flavonoids from hops (Humulus lupulus L.) in human prostate cancer cell lines. Phytomedicine 2006, 13, 732–734. [Google Scholar] [CrossRef]
- Bartmańska, A.; Tronina, T.; Popłoński, J.; Milczarek, M.; Filip-Psurska, B.; Wietrzyk, J. Highly cancer selective antiproliferative activity of natural prenylated flavonoids. Molecules 2018, 23, 2922. [Google Scholar] [CrossRef]
- Busch, C.; Noor, S.; Leischner, C.; Burkard, M.; Lauer, U.M.; Venturelli, S. Anti-proliferative activity of hop-derived prenylflavonoids against human cancer cell lines. Wien. Med. Wochenschr. (1946) 2015, 165, 258–261. [Google Scholar] [CrossRef]
- Dastidar, S.G.; Mahapatra, S.K.; Ganguly, K.; Chakrabarty, A.N.; Shirataki, Y.; Motohashi, N. Antimicrobial activity of prenylflavanones. In Vivo 2001, 15, 519–523. [Google Scholar]
- Osorio, M.; Carvajal, M.; Vergara, A.; Butassi, E.; Zacchino, S.; Mascayano, C.; Montoya, M.; Mejías, S.; Martín, M.C.-S.; Vásquez-Martínez, Y. Prenylated flavonoids with potential antimicrobial activity: Synthesis, biological activity, and in silico study. Int. J. Mol. Sci. 2021, 22, 5472. [Google Scholar] [CrossRef] [PubMed]
- Venturelli, S.; Niessner, H.; Sinnberg, T.; Berger, A.; Burkard, M.; Urmann, C.; Donaubauer, K.; Böcker, A.; Leischner, C.; Riepl, H. 6-and 8-Prenylnaringenin, novel natural histone deacetylase inhibitors found in hops, exert antitumor activity on melanoma cells. Cell. Physiol. Biochem. 2018, 51, 543–556. [Google Scholar] [CrossRef] [PubMed]
- Delmulle, L.; Vanden Berghe, T.; De Keukeleire, D.; Vandenabeele, P. Treatment of PC-3 and DU145 prostate cancer cells by prenylflavonoids from hop (Humulus lupulus L.) induces a caspase-independent form of cell death. Phytother. Res. 2008, 22, 197–203. [Google Scholar] [CrossRef]
- Miranda, C.L.; Aponso, G.L.M.; Stevens, J.F.; Deinzer, M.L.; Buhler, D.R. Prenylated chalcones and flavanones as inducers of quinone reductase in mouse Hepa 1c1c7 cells. Cancer Lett. 2000, 149, 21–29. [Google Scholar] [CrossRef]
- Wang, S.; Dunlap, T.L.; Howell, C.E.; Mbachu, O.C.; Rue, E.A.; Phansalkar, R.; Chen, S.-N.; Pauli, G.F.; Dietz, B.M.; Bolton, J.L. Hop (Humulus lupulus L.) extract and 6-prenylnaringenin induce P450 1A1 catalyzed estrogen 2-hydroxylation. Chem. Res. Toxicol. 2016, 29, 1142–1150. [Google Scholar] [CrossRef]
- Stubert, J.; Gerber, B. Isoflavones–mechanism of action and impact on breast cancer risk. Breast Care 2009, 4, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Diel, P.; Thomae, R.B.; Caldarelli, A.; Zierau, O.; Kolba, S.; Schmidt, S.; Schwab, P.; Metz, P.; Vollmer, G. Regulation of gene expression by 8-prenylnaringenin in uterus and liver of Wistar rats. Planta Medica 2004, 70, 39–44. [Google Scholar] [CrossRef]
- Zierau, O.; Kretzschmar, G.; Möller, F.; Weigt, C.; Vollmer, G. Time dependency of uterine effects of naringenin type phytoestrogens in vivo. Mol. Cell. Endocrinol. 2008, 294, 92–99. [Google Scholar] [CrossRef][Green Version]
- Piersen, C.E. Phytoestrogens in botanical dietary supplements: Implications for cancer. Integr. Cancer Ther. 2003, 2, 120–138. [Google Scholar] [CrossRef]
- Powers, C.N.; Setzer, W.N. A molecular docking study of phytochemical estrogen mimics from dietary herbal supplements. In Silico Pharmacol. 2015, 3, 1–63. [Google Scholar] [CrossRef] [PubMed]
- Zanardi, M.V.; Gastiazoro, M.P.; Kretzschmar, G.; Wober, J.; Vollmer, G.; Varayoud, J.; Durando, M.; Zierau, O. AHR agonistic effects of 6-PN contribute to potential beneficial effects of Hops extract. Mol. Cell. Endocrinol. 2022, 543, 111540. [Google Scholar] [CrossRef] [PubMed]
- Hitzman, R.T.; Dunlap, T.L.; Howell, C.E.; Chen, S.-N.; Vollmer, G.n.; Pauli, G.F.; Bolton, J.L.; Dietz, B.M. 6-Prenylnaringenin from hops disrupts ERα-mediated downregulation of CYP1A1 to facilitate estrogen detoxification. Chem. Res. Toxicol. 2020, 33, 2793–2803. [Google Scholar] [CrossRef]
- Gerhäuser, C. Broad spectrum antiinfective potential of xanthohumol from hop (Humulus lupulus L.) in comparison with activities of other hop constituents and xanthohumol metabolites. Mol. Nutr. Food Res. 2005, 49, 827–831. [Google Scholar] [CrossRef]
- Bartmańska, A.; Wałecka-Zacharska, E.; Tronina, T.; Popłoński, J.; Sordon, S.; Brzezowska, E.; Bania, J.; Huszcza, E. Antimicrobial properties of spent hops extracts, flavonoids isolated therefrom, and their derivatives. Molecules 2018, 23, 2059. [Google Scholar] [CrossRef] [PubMed]
- Tsuchiya, H.; Sato, M.; Miyazaki, T.; Fujiwara, S.; Tanigaki, S.; Ohyama, M.; Tanaka, T.; Iinuma, M. Comparative study on the antibacterial activity of phytochemical flavanones against methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 1996, 50, 27–34. [Google Scholar] [CrossRef]
- Sato, M.; Tanaka, H.; Tani, N.; Nagayama, M.; Yamaguchi, R. Different antibacterial actions of isoflavones isolated from Erythrina poeppigiana against methicillin-resistant Staphylococcus aureus. Lett. Appl. Microbiol. 2006, 43, 243–248. [Google Scholar] [CrossRef]
- das Chagas Almeida, A.; Azevedo Rodrigues, L.; dos Santos Paulino, G.; Pereira Aguilar, A.; Andrade Almeida, A.; Olavo Ferreira, S.; Brandão, G.C.; Viana Leite, J.P.; de Oliveira Barros Ribon, A. Prenylated flavonoid-enriched fraction from Maclura tinctoria shows biological activity against Staphylococcus aureus and protects Galleria mellonella larvae from bacterial infection. BMC Complement. Altern. Med. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Zuo, G.-Y.; Yang, C.-X.; Han, J.; Li, Y.-Q.; Wang, G.-C. Synergism of prenylflavonoids from Morus alba root bark against clinical MRSA isolates. Phytomedicine 2018, 39, 93–99. [Google Scholar] [CrossRef]
- Sohn, H.-Y.; Son, K.; Kwon, C.-S.; Kwon, G.-S.; Kang, S. Antimicrobial and cytotoxic activity of 18 prenylated flavonoids isolated from medicinal plants: Morus alba L., Morus mongolica Schneider, Broussnetia papyrifera (L.) Vent, Sophora flavescens Ait and Echinosophora koreensis Nakai. Phytomedicine 2004, 11, 666–672. [Google Scholar] [CrossRef]
- World Health Organization. Global Antimicrobial Resistance Surveillance System (GLASS) Report: Early Implementation 2017–2018; World Health Organization: Geneva, Switzerland, 2018. [Google Scholar]
- One Health Trust. Available online: https://onehealthtrust.org/wp-content/uploads/2015/09/the-state-of-the-worlds-antibiotics-_2015.pdf (accessed on 7 August 2025).
- Nicodème, J.; Paulin, E.N.; Zingg, M.; Uçkay, I.; Malacarne, S.; Suva, D. Pied diabétique infecté. Rev. Médicale Suisse 2015, 11, 1238–1241. [Google Scholar] [CrossRef]
- Lipsky, B.A.; Hoey, C. Topical antimicrobial therapy for treating chronic wounds. Clin. Infect. Dis. 2009, 49, 1541–1549. [Google Scholar] [CrossRef]
- Cheesman, M.J.; Ilanko, A.; Blonk, B.; Cock, I.E. Developing new antimicrobial therapies: Are synergistic combinations of plant extracts/compounds with conventional antibiotics the solution? Pharmacogn. Rev. 2017, 11, 57. [Google Scholar] [CrossRef]
- Bocquet, L.; Sahpaz, S.; Bonneau, N.; Beaufay, C.; Mahieux, S.; Samaillie, J.; Roumy, V.; Jacquin, J.; Bordage, S.; Hennebelle, T. Phenolic compounds from Humulus lupulus as natural antimicrobial products: New weapons in the fight against methicillin resistant Staphylococcus aureus, Leishmania mexicana and Trypanosoma brucei strains. Molecules 2019, 24, 1024. [Google Scholar] [CrossRef]
- Omar, R.M.; Igoli, J.; Gray, A.I.; Ebiloma, G.U.; Clements, C.; Fearnley, J.; Ebel, R.A.; Zhang, T.; De Koning, H.P.; Watson, D.G. Chemical characterisation of Nigerian red propolis and its biological activity against Trypanosoma brucei. Phytochem. Anal. PCA 2016, 27, 107–115. [Google Scholar] [CrossRef]
- Zhang, N.; Liu, Z.; Han, Q.; Chen, J.; Lv, Y. Xanthohumol enhances antiviral effect of interferon α-2b against bovine viral diarrhea virus, a surrogate of hepatitis C virus. Phytomedicine 2010, 17, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Buckwold, V.E.; Wilson, R.J.H.; Nalca, A.; Beer, B.B.; Voss, T.G.; Turpin, J.A.; Buckheit, R.W.; Wei, J.; Wenzel-Mathers, M.; Walton, E.M.; et al. Antiviral activity of hop constituents against a series of DNA and RNA viruses. Antivir. Res. 2004, 61, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Ding, Z.-H.; Liu, J.-K.; Zheng, Y.-T. Xanthohumol, a novel anti-HIV-1 agent purified from hops Humulus lupulus. Antivir. Res. 2004, 64, 189–194. [Google Scholar] [CrossRef]
- Bouback, T.A.; Aljohani, A.M.; Albeshri, A.; Al-Talhi, H.; Moatasim, Y.; GabAllah, M.; Badierah, R.; Albiheyri, R.; Al-Sarraj, F.; Ali, M.A. Antiviral activity of Humulus lupulus (HOP) aqueous extract against MERS-CoV and SARS-CoV-2: In-vitro and in-silico study. Biotechnol. Biotechnol. Equip. 2023, 37, 167–179. [Google Scholar] [CrossRef]
- Herzog, A.-M.; Göbel, K.; Marongiu, L.; Ruetalo, N.; Alonso, M.C.; Leischner, C.; Busch, C.; Burkard, M.; Lauer, U.M.; Geurink, P.P. Compounds derived from Humulus lupulus inhibit SARS-CoV-2 papain-like protease and virus replication. Phytomedicine 2024, 123, 155176. [Google Scholar] [CrossRef]
- Büscher, P.; Cecchi, G.; Jamonneau, V.; Priotto, G. Human african trypanosomiasis. Lancet 2017, 390, 2397–2409. [Google Scholar] [CrossRef]
- Urmann, C.; Oberbauer, E.; Couillard-Després, S.; Aigner, L.; Riepl, H. Neurodifferentiating potential of 8-prenylnaringenin and related compounds in neural precursor cells and correlation with estrogen-like activity. Planta Medica 2015, 81, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Goetz, T.; Arslan, A.; Wisden, W.; Wulff, P. GABAA receptors: Structure and function in the basal ganglia. Prog. Brain Res. 2007, 160, 21–41. [Google Scholar]
- Hänsel, R.; Wohlfart, R.; Coper, H. Versuche, sedativ-hypnotische Wirkstoffe im Hopfen nachzuweisen, II/Narcotic Action of 2-Methyl-3-butene-2-ol Contained in the Exhalation of Hops. Z. Für Naturforschung C 1980, 35, 1096–1097. [Google Scholar] [CrossRef]
- Bagatin, M.C.; Tozatti, C.S.S.; Abiko, L.A.; dos Santos Yamazaki, D.A.; Silva, P.R.A.; Perego, L.M.; Audi, E.A.; Seixas, F.A.V.; Basso, E.A.; de Freitas Gauze, G. Molecular docking and panicolytic effect of 8-Prenylnaringenin in the elevated T-Maze. Chem. Pharm. Bull. 2014, 62, 1231–1237. [Google Scholar] [CrossRef]
- Benkherouf, A.Y.; Eerola, K.; Soini, S.L.; Uusi-Oukari, M. Humulone modulation of GABAA receptors and its role in hops sleep-promoting activity. Front. Neurosci. 2020, 14, 594708. [Google Scholar] [CrossRef]
- Benkherouf, A.Y.; Logrén, N.; Somborac, T.; Kortesniemi, M.; Soini, S.L.; Yang, B.; Salo-Ahen, O.M.; Laaksonen, O.; Uusi-Oukari, M. Hops compounds modulatory effects and 6-prenylnaringenin dual mode of action on GABAA receptors. Eur. J. Pharmacol. 2020, 873, 172962. [Google Scholar] [CrossRef]
- Benkherouf, A.Y.; Soini, S.L.; Stompor, M.; Uusi-Oukari, M. Positive allosteric modulation of native and recombinant GABAA receptors by hops prenylflavonoids. Eur. J. Pharmacol. 2019, 852, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Sekiguchi, F.; Fujita, T.; Deguchi, T.; Yamaoka, S.; Tomochika, K.; Tsubota, M.; Ono, S.; Horaguchi, Y.; Ichii, M.; Ichikawa, M. Blockade of T-type calcium channels by 6-prenylnaringenin, a hop component, alleviates neuropathic and visceral pain in mice. Neuropharmacology 2018, 138, 232–244. [Google Scholar] [CrossRef] [PubMed]
- Du Nguyen, H.; Okada, T.; Kitamura, S.; Yamaoka, S.; Horaguchi, Y.; Kasanami, Y.; Sekiguchi, F.; Tsubota, M.; Yoshida, S.; Nishikawa, H. Design and synthesis of novel anti-hyperalgesic agents based on 6-prenylnaringenin as the T-type calcium channel blockers. Bioorganic Med. Chem. 2018, 26, 4410–4427. [Google Scholar] [CrossRef]
- Nguyen, H.D.; Okada, T.; Sekiguchi, F.; Tsubota, M.; Nishikawa, H.; Kawabata, A.; Toyooka, N. Prenylflavanones as novel T-type calcium channel blockers useful for pain therapy. Nat. Prod. Commun. 2019, 14, 1934578X19873441. [Google Scholar] [CrossRef]
- Botta, B.; Vitali, A.; Menendez, P.; Misiti, D.; Monache, G.D. Prenylated Flavonoids: Pharmacology and Biotechnology. Curr. Med. Chem. 2005, 12, 713–739. [Google Scholar] [CrossRef]
- Jain, A.; Gupta, R.; Sarpal, P. Synthesis of (±) lupinifoun, di-O-methyl xanthohumol and isoxanthohumol and related compounds. Tetrahedron 1978, 34, 3563–3567. [Google Scholar] [CrossRef]
- Jain, A.C.; Gupta, R.C.; Sarpal, P.D. SYNTHESIS OF RACEMIC 8-C-PRENYL-6″,6″-DIMETHYLPYRANO(2″, 3″:7,6)NARINGENIN. Chem. Lett. 1978, 7, 995–998. [Google Scholar] [CrossRef]
- Nagar, A.; Gujral, V.K.; Gupta, S.R. Synthesis of lupinifolin. Tetrahedron Lett. 1978, 19, 2031–2034. [Google Scholar] [CrossRef]
- Bu, X.; Zhao, L.; Li, Y. A facile synthesis of 6-C-prenylflavanones. Synthesis 1997, 1997, 1246–1248. [Google Scholar] [CrossRef]
- Trost, B.M.; Toste, F.D. Asymmetric O- and C-Alkylation of Phenols. J. Am. Chem. Soc. 1998, 120, 815–816. [Google Scholar] [CrossRef]
- Chatterjee, A.K.; Sanders, D.P.; Grubbs, R.H. Synthesis of symmetrical trisubstituted olefins by cross metathesis. Org. Lett. 2002, 4, 1939–1942. [Google Scholar] [CrossRef]
- Tischer, S.; Metz, P. Selective C-6 Prenylation of Flavonoids via Europium(III)-Catalyzed Claisen Rearrangement and Cross-Metathesis. Adv. Synth. Catal. 2007, 349, 147–151. [Google Scholar] [CrossRef]
- Urmann, C.; Riepl, H. Semi-Synthetic Approach Leading to 8-Prenylnaringenin and 6-Prenylnaringenin: Optimization of the Microwave-Assisted Demethylation of Xanthohumol Using Design of Experiments. Molecules 2020, 25, 4007. [Google Scholar] [CrossRef] [PubMed]
- Qiu, C.; Liu, Y.; Wu, Y.; Zhao, L.; Pei, J. Biochemical characterization of a novel prenyltransferase from Streptomyces sp. NT11 and development of a recombinant strain for the production of 6-prenylnaringenin. J. Agric. Food Chem. 2021, 69, 14231–14240. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).