Distinct Molecular Mechanisms in Oral Mucosal Wound Healing: Translational Insights and Future Directions
Abstract
1. Introduction
2. Histological and Functional Characteristics of Oral Mucosa
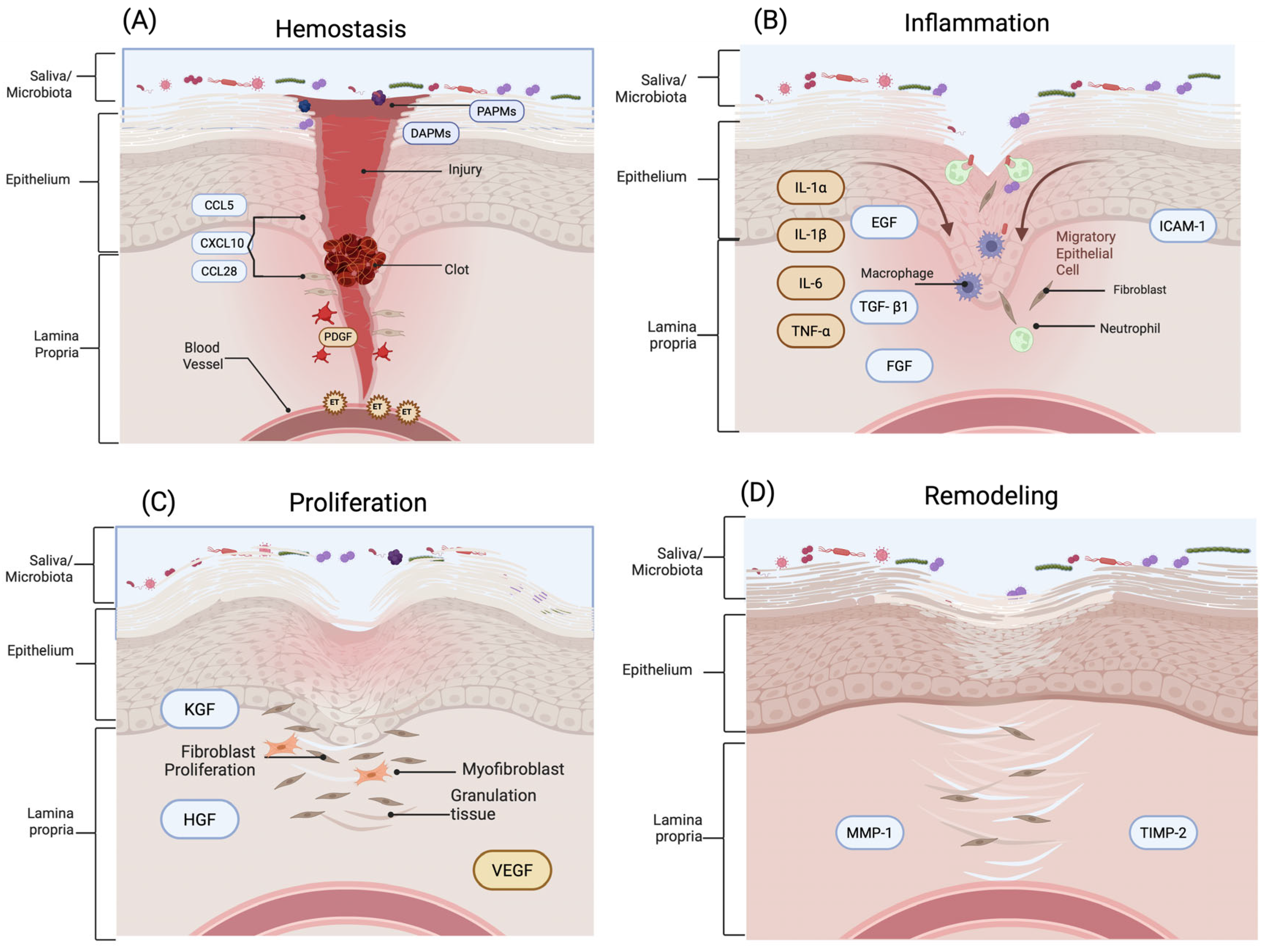
3. Phases of Oral Mucosal Wound Healing
3.1. Hemostasis
3.2. Inflammatory
3.3. Proliferation
3.4. Remodeling
4. Cellular and Molecular Mechanisms in Oral Healing
4.1. Role of Immune Cells
4.2. Tissue-Resident Cell Dynamics in Oral Mucosal Repair
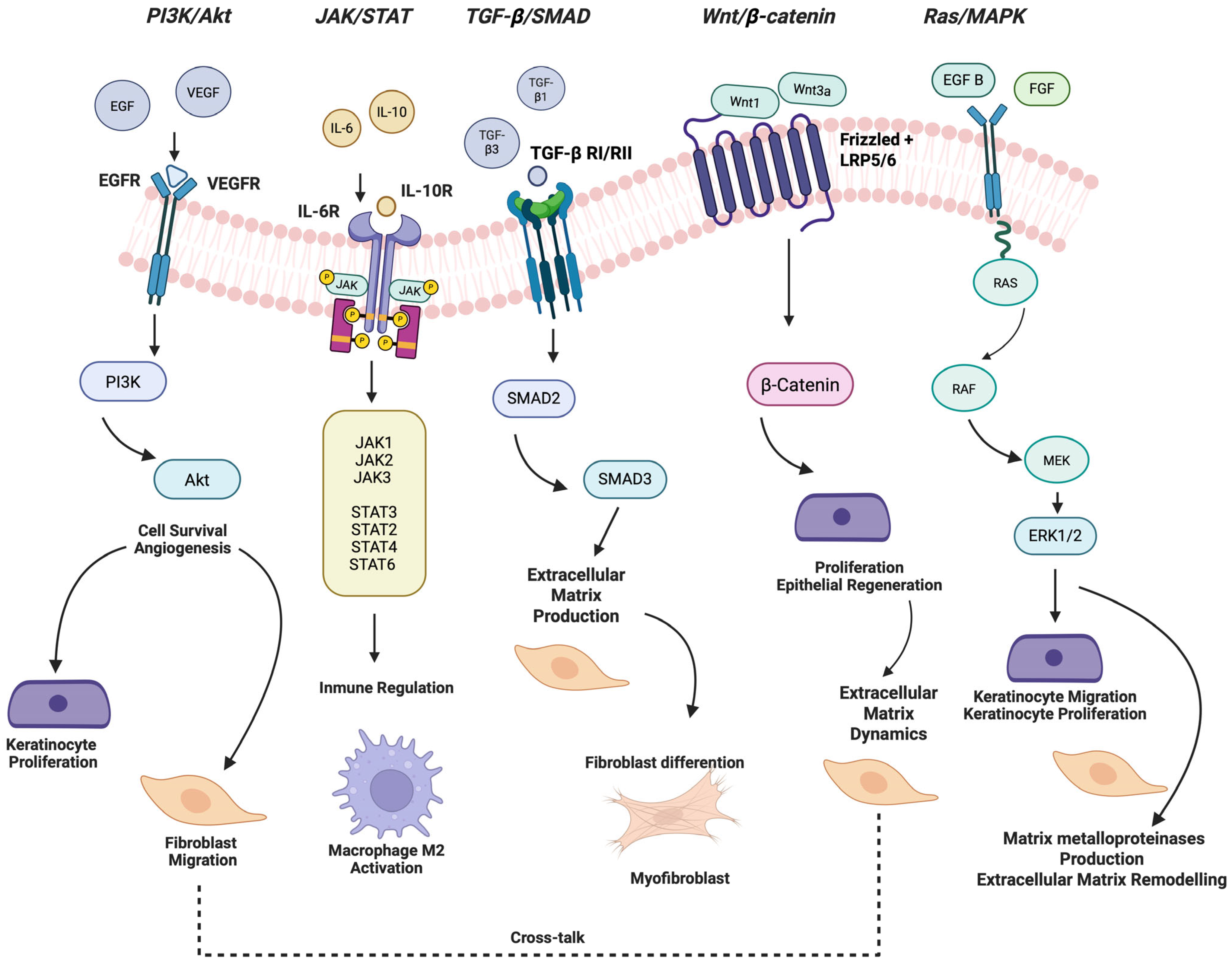
5. Key Signaling Pathways and Growth Factors
5.1. PI3K/Akt Signaling Pathway
5.2. JAK/STAT Signaling Pathway
5.3. Ras/MAPK Signaling Pathway
5.4. TGF-β1 Signaling Pathway
5.5. Wnt/β-Catenin Signaling Pathway
5.6. Growth Factors
5.6.1. Fibroblast Growth Factor (FGF)
5.6.2. Epidermal Growth Factor (EGF)
5.6.3. Platelet-Derived Growth Factor (PDGF)
5.6.4. Vascular Endothelial Growth Factor (VEGF)
5.6.5. Transforming Growth Factor-Beta (TGF-β)
5.6.6. Keratinocyte Growth Factor (KGF)
6. Modulating Factors in Oral Wound Healing
6.1. Microbiota
6.2. Saliva
6.3. Vascularization
6.4. Age
6.5. Diabetes Mellitus
6.6. Tobacco Use
7. Clinical Implications and Future Directions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Akt | Protein Kinase B |
| EGF | Epidermal Growth Factor |
| EGFR | Epidermal Growth Factor Receptor |
| ECM | Extracellular Matrix |
| FGF | Fibroblast Growth Factor |
| FGF-1 | Fibroblast Growth Factor 1 |
| FGF-2 | Fibroblast Growth Factor 2 |
| FGF-7 | Fibroblast Growth Factor 7 (Keratinocyte Growth Factor) |
| FGF21 | Fibroblast Growth Factor 21 |
| GSH | Glutathione |
| IL-1β | Interleukin-1 Beta |
| ITGA5 | Integrin Alpha-5 |
| JAK | Janus Kinase |
| JAK/STAT | Janus Kinase/Signal Transducer and Activator of Transcription Pathway |
| KGF | Keratinocyte Growth Factor |
| LLLT | Low-Level Light Therapy |
| MAPK | Mitogen-Activated Protein Kinase |
| MEK | Mitogen-Activated Protein Kinase Kinase |
| MEVs | Milk-Derived Extracellular Vesicles |
| NF-κB | Nuclear Factor Kappa B |
| PI3K | Phosphatidylinositol 3-Kinase |
| STAT | Signal Transducer and Activator of Transcription |
| TGF-β | Transforming Growth Factor Beta |
| VEGF | Vascular Endothelial Growth Factor |
| VEGFR2 | Vascular Endothelial Growth Factor Receptor 2 |
References
- Toma, A.I.; Fuller, J.M.; Willett, N.J.; Goudy, S.L. Oral wound healing models and emerging regenerative therapies. Transl. Res. 2021, 236, 17–34. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Bartolome, R.; Uchiyama, A.; Molinolo, A.A.; Abusleme, L.; Brooks, S.R.; Callejas-Valera, J.L.; Edwards, D.; Doci, C.; Asselin-Labat, M.-L.; Onaitis, M.W.; et al. Transcriptional signature primes human oral mucosa for rapid wound healing. Sci. Transl. Med. 2018, 10, eaap8798. [Google Scholar] [CrossRef] [PubMed]
- Waasdorp, M.; Waasdorp, M.; Krom, B.P.; Bikker, F.J.; van Zuijlen, P.P.M.; Niessen, F.B.; Gibbs, S. The bigger picture: Why oral mucosa heals better than skin. Biomolecules 2021, 11, 1165. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira Gonzalez, A.C.; Costa, T.F.; de Araújo Andrade, Z.; Medrado, A.R.A.P. Wound healing—A literature review. An. Bras. Dermatol. 2016, 91, 614–620. [Google Scholar] [CrossRef]
- Velnar, T.; Bailey, T.; Smrkolj, V. The wound healing process: An overview of the cellular and molecular mechanisms. J. Int. Med. Res. 2009, 37, 1528–1542. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound healing: A cellular perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Ko, K.I.; Sculean, A.; Graves, D.T. Diabetic wound healing in soft and hard oral tissues. Transl. Res. 2021, 236, 72–86. [Google Scholar] [CrossRef]
- Griffin, M.F.; Fahy, E.J.; King, M.; Guardino, N.; Chen, K.; Abbas, D.B.; Lavin, C.V.; Diaz Deleon, N.M.; Lorenz, H.P.; Longaker, M.T.; et al. Understanding scarring in the oral mucosa. Adv. Wound Care 2022, 11, 537–547. [Google Scholar] [CrossRef]
- Senel, S. An Overview of Physical, Microbiological and Immune Barriers of Oral Mucosa. Int. J. Mol. Sci. 2021, 22, 7821. [Google Scholar] [CrossRef]
- Trinh, X.T.; Long, N.V.; Van Anh, L.T.; Nga, P.T.; Giang, N.N.; Chien, P.N.; Nam, S.Y.; Heo, C.Y. A Comprehensive Review of Natural Compounds for Wound Healing: Targeting Bioactivity Perspective. Int. J. Mol. Sci. 2022, 23, 9573. [Google Scholar] [CrossRef]
- Jongjitaree, S.; Koontongkaew, S.; Niyomtham, N.; Yingyongnarongkul, B.E.; Utispan, K. The oral wound healing potential of Thai propolis based on its antioxidant activity and stimulation of oral fibroblast migration and proliferation. Evidence-Based Complement. Altern. Med. 2022, 2022, 3503164. [Google Scholar] [CrossRef]
- Hakim, R.F.; Idroes, R.; Hanafiah, O.A.; Ginting, B.; Kemala, P.; Fakhrurrazi, F.; Putra, N.I.; Shafira, G.A.; Romadhoni, Y.; Destiana, K.; et al. Characterization of red algae (Gracilaria verrucosa) on potential application for topical treatment of oral mucosa wounds in Rattus norvegicus. Narra J. 2023, 3, e422. [Google Scholar] [CrossRef]
- Pereira, D.; Sequeira, I. A scarless healing tale: Comparing homeostasis and wound healing of oral mucosa with skin and oesophagus. Front. Cell Dev. Biol. 2021, 9, 682143. [Google Scholar] [CrossRef] [PubMed]
- Overmiller, A.M.; Sawaya, A.P.; Hope, E.D.; Morasso, M.I. Intrinsic Networks Regulating Tissue Repair: Comparative Studies of Oral and Skin Wound Healing. Cold Spring Harb. Perspect. Biol. 2022, 14, a041244. [Google Scholar] [CrossRef] [PubMed]
- Basso, F.G.; Soares, D.G.; Pansani, T.N.; Cardoso, L.M.; Scheffel, D.L.; de Souza Costa, C.A.; Hebling, J. Proliferation, migration, and expression of oral-mucosal-healing-related genes by oral fibroblasts receiving low-level laser therapy after inflammatory cytokines challenge. Lasers Surg. Med. 2016, 48, 1006–1014. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues Neves, C.; Buskermolen, J.; Roffel, S.; Waaijman, T.; Thon, M.; Veerman, E.; Gibbs, S. Human saliva stimulates skin and oral wound healing in vitro. J. Tissue Eng. Regen. Med. 2019, 13, 1079–1092. [Google Scholar] [CrossRef]
- Seeger, M.A.; Paller, A.S. The Roles of Growth Factors in Keratinocyte Migration. Adv. Wound Care 2015, 4, 213–224. [Google Scholar] [CrossRef]
- Konyaeva, A.D.; Varakuta, E.Y.; Leiman, A.E.; Bolbasov, E.N.; Chernova, U.V. The Specifics of Neovascularization of Wound Defects in the Oral Mucosa during Its Regeneration under a Piezoelectric Polymer Membrane. Bull. Exp. Biol. Med. 2023, 174, 801–805. [Google Scholar] [CrossRef]
- Yadu, N.; Singh, M.; Singh, D.; Keshavkant, S. Mechanistic insights of diabetic wound: Healing process, associated pathways and microRNA-based delivery systems. Int. J. Pharm. 2025, 670, 125117. [Google Scholar] [CrossRef]
- Wang, L.; Yang, K.; Xie, X.; Wang, S.; Gan, H.; Wang, X.; Wei, H. Macrophages as Multifaceted Orchestrators of Tissue Repair: Bridging Inflammation, Regeneration, and Therapeutic Innovation. J. Inflamm. Res. 2025, 18, 8945–8959. [Google Scholar] [CrossRef]
- Basso, F.G.; Pansani, T.N.; Turrioni, A.P.; Soares, D.G.; de Souza Costa, C.A.; Hebling, J. Tumor Necrosis Factor-α and Interleukin (IL)-1β, IL-6, and IL-8 Impair In Vitro Migration and Induce Apoptosis of Gingival Fibroblasts and Epithelial Cells, Delaying Wound Healing. J. Periodontol. 2016, 87, 990–996. [Google Scholar] [CrossRef] [PubMed]
- Moretti, L.; Stalfort, J.; Barker, T.H.; Abebayehu, D. The interplay of fibroblasts, the extracellular matrix, and inflammation in scar formation. J. Biol. Chem. 2022, 298, 101530. [Google Scholar] [CrossRef] [PubMed]
- Castilho, R.M.; Squarize, C.H.; Gutkind, J.S. Exploiting PI3K/mTOR signaling to accelerate epithelial wound healing. Oral Dis. 2013, 19, 551–558. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Jia, L.; Su, Y.; Du, J.; Guo, L.; Luo, Z.; Liu, Y. Lactobacillus reuteri extracts promoted wound healing via PI3K/AKT/β-catenin/TGFβ1 pathway. Stem Cell Res. Ther. 2019, 10, 243. [Google Scholar] [CrossRef]
- Zhang, Y.; Zouboulis, C.C.; Xiao, Z. Exosomes from adipose-derived stem cells activate sebocytes through the PI3K/AKT/SREBP1 pathway to accelerate wound healing. Cell Tissue Res. 2024, 396, 329–342. [Google Scholar] [CrossRef]
- Fan, L.; Ma, X.; Liu, B.; Yang, Y.; Yang, Y.; Ren, T.; Li, Y. Antioxidant engineered milk-derived extracellular vesicles for accelerating wound healing via regulation of the PI3K/Akt signaling pathway. Adv. Healthc. Mater. 2023, 12, 2301865. [Google Scholar] [CrossRef]
- Li, Z.; Lin, K.; Wang, Y.; Mao, J.; Yin, Y.; Li, Z.; Wang, F.; Zeng, X.; Li, Q.; Wang, X.; et al. Garcinol promotes wound healing in diabetic mice by regulating inflammation and NLRP3 inflammasome mediated pyroptosis via the PI3K/Akt/NFκB pathway. Int. Immunopharmacol. 2025, 151, 114352. [Google Scholar] [CrossRef]
- Sun, J.; Zhao, H.; Shen, C.; Li, S.; Zhang, W.; Ma, J.; Li, Z.; Zhang, M.; Yang, J. Tideglusib promotes wound healing in aged skin by activating PI3K/Akt pathway. Stem Cell Res. Ther. 2022, 13, 269. [Google Scholar] [CrossRef]
- Jere, S.W.; Abrahamse, H.; Houreld, N.N. The JAK/STAT signaling pathway and photobiomodulation in chronic wound healing. Cytokine Growth Factor. Rev. 2017, 38, 73–79. [Google Scholar] [CrossRef]
- Ociepa, K.; Danilewicz, M.; Wągrowska-Danilewicz, M.; Peterson-Jęckowska, R.; Wójcicka-Rubin, A.; Lewkowicz, N.; Zajdel, R.; Żebrowska, A. Expression of the selected proteins of JAK/STAT signaling pathway in diseases with oral mucosa involvement. Int. J. Mol. Sci. 2023, 24, 323. [Google Scholar] [CrossRef]
- Xie, J.; Huang, Y.; Hu, X.; Wu, X.; Luo, X.; Wei, P.; Jing, W.; Zhao, B.; Su, J. A constant Filgotinib delivery adhesive platform based on polyethylene glycol (PEG) hydrogel for accelerating wound healing via restoring macrophage mitochondrial homeostasis. Small 2025, 21, 2408791. [Google Scholar] [CrossRef]
- Chu, L.; Shen, J.-M.; Xu, Z.; Huang, J.; Ning, L.; Feng, Z.; Jiang, Y.; Wu, P.; Gao, C.; Wang, W.; et al. Stimuli responsive hydrogel with spatiotemporal co-delivery of FGF21 and H2S for synergistic diabetic wound repair. J. Control. Release 2025, 382, 113749. [Google Scholar] [CrossRef] [PubMed]
- Farooq, M.; Khan, A.W.; Kim, M.S.; Choi, S. The Role of Fibroblast Growth Factor (FGF) Signaling in Tissue Repair and Regeneration. Cells 2021, 10, 3242. [Google Scholar] [CrossRef] [PubMed]
- Phimnuan, P.; Dirand, Z.; Tissot, M.; Worasakwutiphong, S.; Sittichokechaiwut, A.; Grandmottet, F.; Viyoch, J.; Viennet, C. Beneficial Effects of a Blended Fibroin/Aloe Gel Extract Film on the Biomolecular Mechanism(s) via the MAPK/ERK Pathway Relating to Diabetic Wound Healing. ACS Omega 2023, 8, 6813–6824. [Google Scholar] [CrossRef] [PubMed]
- Fang, C.Y.; Yu, C.C.; Liao, Y.W.; Hsieh, P.L.; Lu, M.Y.; Lin, K.C.; Wu, C.Z.; Tsai, L.L. LncRNA LINC00974 Activates TGF-β/Smad Signaling to Promote Oral Fibrogenesis. J. Oral Pathol. Med. 2019, 48, 151–158. [Google Scholar] [CrossRef]
- Zhang, S.; Elbs-Glatz, Y.; Tao, S.; Li, Y.; Wu, Y.; Ma, Y.; Lu, Q.; Wang, Z. Probiotics Promote Cellular Wound Healing Responses by Modulating the PI3K and TGF-β/Smad Signaling Pathways. Cell Commun. Signal. 2025, 23, 195. [Google Scholar] [CrossRef]
- Zhang, T.; Wang, X.F.; Wang, Z.C.; Lou, D.; Fang, Q.Q.; Hu, Y.Y.; Zhao, W.Y.; Zhang, L.Y.; Wu, L.H.; Tan, W.Q. Current Potential Therapeutic Strategies Targeting the TGF-β/Smad Signaling Pathway to Attenuate Keloid and Hypertrophic Scar Formation. Biomed. Pharmacother. 2020, 129, 110287. [Google Scholar] [CrossRef]
- Thompson, T.; Flanagan, S.; Ortega-Gonzalez, D.; Zhu, T.; Yuan, X. Immediate but Temporal Response: The Role of Distal Epithelial Cells in Wound Healing. Stem Cell Rev. Rep. 2024, 20, 1587–1598. [Google Scholar] [CrossRef]
- Barrientos, S.; Stojadinovic, O.; Golinko, M.S.; Brem, H.; TomicCanic, M. Perspective article: Growth factors and cytokines in wound healing. Wound Repair. Regen. 2008, 16, 585–601. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, Q.; Zhang, X.; Van Brunt, L.A.; Ticha, P.; Helms, J.A. Wnt-Responsive Stem Cell Fates in the Oral Mucosa. iScience 2019, 21, 84–94. [Google Scholar] [CrossRef]
- Gumede, D.B.; Abrahamse, H.; Houreld, N.N. Targeting Wnt/β-catenin signaling and its interplay with TGFβ and Notch signaling pathways for the treatment of chronic wounds. Cell Commun. Signal. 2024, 22, 244. [Google Scholar] [CrossRef]
- Takaya, K.; Aramaki Hattori, N.; Sakai, S.; Okabe, K.; Asou, T.; Kishi, K. Fibroblast Growth Factor 7 suppresses fibrosis and promotes epithelialization during wound healing in mouse fetuses. Int. J. Mol. Sci. 2022, 23, 7087. [Google Scholar] [CrossRef]
- Koike, Y.; Yozaki, M.; Utani, A.; Murota, H. Fibroblast Growth Factor 2 accelerates the epithelial–mesenchymal transition in keratinocytes during wound healing process. Sci. Rep. 2020, 10, 18545. [Google Scholar] [CrossRef]
- Ortega, S.; Ittmann, M.; Tsang, S.H.; Ehrlich, M.; Basilico, C. Neuronal defects and delayed wound healing in mice lacking fibroblast growth factor 2. Proc. Natl. Acad. Sci. USA 1998, 95, 5672–5677. [Google Scholar] [CrossRef]
- Jansen, R.G.; van Kuppevelt, T.H.; Daamen, W.F.; Kuijpers Jagtman, A.M.; Von den Hoff, J.W. FGF2 loaded collagen scaffolds attract cells and blood vessels in rat oral mucosa. J. Oral Pathol. Med. 2009, 38, 630–638. [Google Scholar] [CrossRef]
- Zakrzewska, M.; Marcinkowska, E.; Wiedlocha, A. FGF 1: From biology through engineering to potential medical applications. Crit. Rev. Clin. Lab. Sci. 2008, 45, 91–135. [Google Scholar] [CrossRef]
- Liu, N.; Guan, S.; Wang, H.; Li, C.; Cheng, J.; Yu, H.; Lin, L.; Pan, Y. The antimicrobial peptide Nal P 113 exerts a reparative effect by promoting cell proliferation, migration, and cell cycle progression. BioMed Res. Int. 2018, 2018, 7349351. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.H.; Koh, Y.G.; Lee, W.G.; Seok, J.; Park, K.Y. The use of epidermal growth factor in dermatological practice. Int. Wound J. 2023, 20, 2414–2423. [Google Scholar] [CrossRef]
- Wang, X.; Ji, X. Application of recombinant human epidermal growth factor in oral and maxillofacial trauma and its impact on healing time. J. Stomatol. Oral Maxillofac. Surg. 2025, 126, 102326. [Google Scholar] [CrossRef] [PubMed]
- Tekin, G.G.; Deveci, B.; Deveci, E. Ellagic acid protected the gingival tissue via fibroblast and epidermal growth factors in rats. Acta Cir. Bras. 2024, 39, e391224. [Google Scholar] [CrossRef] [PubMed]
- Sabbah, D.A.; Hajjo, R.; Sweidan, K. Review on Epidermal Growth Factor Receptor (EGFR) Structure, Signaling Pathways, Interactions, and Recent Updates of EGFR Inhibitors. Curr. Top. Med. Chem. 2020, 20, 815–834. [Google Scholar] [CrossRef]
- Rujirachotiwat, A.; Suttamanatwong, S. Curcumin promotes Collagen Type I, Keratinocyte Growth Factor 1, and Epidermal Growth Factor Receptor expressions in the in vitro wound healing model of human gingival fibroblasts. Eur. J. Dent. 2021, 15, 63–70. [Google Scholar] [CrossRef]
- Meizarini, A.; Aryati, A.; Rianti, D.; Riawan, W.; Puteri, A. Effectivity of zinc oxide turmeric extract dressing in stimulating the re-epithelization phase of wound healing. Vet. World 2020, 13, 2221–2225. [Google Scholar] [CrossRef]
- Tang, L.; Cai, S.; Lu, X.; Wu, D.; Zhang, Y.; Li, X.; Qin, X.; Guo, J.; Zhang, X.; Liu, C. Platelet-Derived Growth Factor Nanocapsules with Tunable Controlled Release for Chronic Wound Healing. Small 2024, 20, e2310743. [Google Scholar] [CrossRef] [PubMed]
- Illescas-Montes, R.; González-Acedo, A.; Melguizo-Rodríguez, L.; García-Recio, E.; Ruiz, C.; García-Martínez, O.; Ramos-Torrecillas, J. Modulation of Gene Expression in Human Fibroblasts by Punicalagin and Ellagic Acid: An In Vitro Study. Mol. Nutr. Food Res. 2025, e70237. [Google Scholar] [CrossRef] [PubMed]
- Piccin, A.; Di Pierro, A.M.; Canzian, L.; Primerano, M.; Corvetta, D.; Negri, G.; Mazzoleni, G.; Gastl, G.; Steurer, M.; Gentilini, I.; et al. Platelet gel: A new therapeutic tool with great potential. Blood Transfus. 2016, 14, 373–379. [Google Scholar] [CrossRef]
- Tavelli, L.; Ravidà, A.; Barootchi, S.; Chambrone, L.; Giannobile, W.V. Recombinant human platelet-derived growth factor: A systematic review of clinical findings in oral regenerative procedures. JDR Clin. Trans. Res. 2021, 6, 161–173. [Google Scholar] [CrossRef]
- Ahmad, A.; Nawaz, M.I. Molecular mechanism of VEGF and its role in pathological angiogenesis. J. Cell Biochem. 2022, 123, 1938–1965. [Google Scholar] [CrossRef]
- Bao, P.; Kodra, A.; Tomic Canic, M.; Golinko, M.S.; Ehrlich, H.P.; Brem, H. The role of vascular endothelial growth factor in wound healing. J. Surg. Res. 2009, 153, 347–358. [Google Scholar] [CrossRef]
- Shi, Z.; Yao, C.; Shui, Y.; Li, S.; Yan, H. Research progress on the mechanism of angiogenesis in wound repair and regeneration. Front. Physiol. 2023, 14, 1284981. [Google Scholar] [CrossRef]
- Keswani, S.G.; Balaji, S.; Le, L.D.; Leung, A.; Parvadia, J.K.; Frischer, J.; Yamano, S.; Taichman, N.; Crombleholme, T.M. Role of salivary vascular endothelial growth factor (VEGF) in palatal mucosal wound healing. Wound Repair. Regen. 2013, 21, 554–562. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, S.; Yasuda, T.; Hamada, Y.; Kawaguchi, N.; Fujishita, Y.; Mori, S.; Yokoyama, Y.; Yamamoto, H.; Kogo, M. Synthetic peptide SVVYGLR upregulates cell motility and facilitates oral mucosal wound healing. Peptides 2020, 134, 170405. [Google Scholar] [CrossRef] [PubMed]
- Yamano, S.; Kuo, W.P.; Sukotjo, C. Downregulated gene expression of TGFβs in diabetic oral wound healing. J. Craniomaxillofac. Surg. 2013, 41, e42–e48. [Google Scholar] [CrossRef] [PubMed]
- Bártolo, I.; Reis, R.L.; Marques, A.P.; Cerqueira, M.T. Keratinocyte Growth Factor-Based Strategies for Wound Re-Epithelialization. Tissue Eng. Part. B Rev. 2022, 28, 665–676. [Google Scholar] [CrossRef]
- Zheng, D.; Liwinski, T.; Elinav, E. Interaction between microbiota and immunity in health and disease. Cell Res. 2020, 30, 492–506. [Google Scholar] [CrossRef]
- Yuan, H.; Chlipala, G.E.; Bangash, H.I.; Meenakshi, R.; Chen, D.; Trivedi, H.M.; DiPietro, L.A.; Gajendrareddy, P.; Chen, L. Dynamics of Human Palatal Wound Healing and the Associated Microbiome. J. Dent. Res. 2025, 104, 97–105. [Google Scholar] [CrossRef]
- Engen, S.A.; Rørvik, G.H.; Schreurs, O.; Blix, I.J.; Schenck, K. The oral commensal Streptococcus mitis activates the aryl hydrocarbon receptor in human oral epithelial cells. Int. J. Oral Sci. 2017, 9, 145–150. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Sig Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Vanlancker, E.; Vanhoecke, B.; Sieprath, T.; Bourgeois, J.; Beterams, A.; De Moerloose, B.; De Vos, W.H.; Van de Wiele, T. Oral microbiota reduce wound healing capacity of epithelial monolayers, irrespective of the presence of 5-fluorouracil. Exp. Biol. Med. 2018, 243, 350–360. [Google Scholar] [CrossRef]
- Harasani, Z.; Ferdosi-Shahandashti, E.; Najafzadehvarzi, H.; Bakhshandeh, B.; Rajabzadeh, A.; Javadi, K. In Vivo Evaluation of Lacticaseibacillus reuteri and Lacticaseibacillus rhamnosus Lysates for Oral Wound Healing. Probiotics Antimicrob. Proteins 2025, 17, 1–8. [Google Scholar] [CrossRef]
- Caselli, E.; Fabbri, C.; D’Accolti, M.; Soffritti, I.; Bassi, C.; Mazzacane, S.; Franchi, M. Defining the Oral Microbiome by Whole-Genome Sequencing and Resistome Analysis: The Complexity of the Healthy Picture. BMC Microbiol. 2020, 20, 120. [Google Scholar] [CrossRef]
- Han, N.; Jia, L.; Guo, L.; Su, Y.; Luo, Z.; Du, J.; Mei, S.; Liu, Y. Balanced Oral Pathogenic Bacteria and Probiotics Promoted Wound Healing via Maintaining Mesenchymal Stem Cell Homeostasis. Stem Cell Res. Ther. 2020, 11, 61. [Google Scholar] [CrossRef] [PubMed]
- Oudhoff, M.J.; van den Keijbus, P.A.; Kroeze, K.L.; Nazmi, K.; Gibbs, S.; Bolscher, J.G.; Veerman, E.C. Histatins enhance wound closure with oral and non-oral cells. J. Dent. Res. 2009, 88, 846–850. [Google Scholar] [CrossRef] [PubMed]
- Castro, M.; Torres, P.; Solano, L.; Córdova, L.A.; Torres, V.A. Histatin-1 counteracts the cytotoxic and antimigratory effects of zoledronic acid in endothelial and osteoblast-like cells. J. Periodontol. 2019, 90, 766–774. [Google Scholar] [CrossRef] [PubMed]
- Torres, P.; Díaz, J.; Arce, M.; Silva, P.; Mendoza, P.; Lois, P.; Molina-Berríos, A.; Owen, G.I.; Palma, V.; Torres, V.A. The salivary peptide histatin-1 promotes endothelial cell adhesion, migration, and angiogenesis. FASEB J. 2017, 31, 4946–4958. [Google Scholar] [CrossRef]
- Okuyama, K.; Yanamoto, S. Saliva in balancing oral and systemic health, oral cancer, and beyond: A narrative review. Cancers 2024, 16, 4276. [Google Scholar] [CrossRef]
- Ngeow, W.C.; Tan, C.C.; Goh, Y.C.; Deliberador, T.M.; Cheah, C.W. A narrative review on means to promote oxygenation and angiogenesis in oral wound healing. Bioengineering 2022, 9, 636. [Google Scholar] [CrossRef]
- Li, L.; Ma, Q.; Mou, J.; Wang, M.; Ye, J.; Sun, G. Basic fibroblast growth factor gel preparation induces angiogenesis during wound healing. Int. J. Artif. Organs. 2023, 46, 171–181. [Google Scholar] [CrossRef]
- Pansani, T.N.; Basso, F.G.; Soares, D.G.; Hebling, J.; Costa, C.A. Functional differences in gingival fibroblasts obtained from young and elderly individuals. Braz. Dent. J. 2016, 27, 485–491. [Google Scholar] [CrossRef]
- Pansani, T.N.; Basso, F.G.; Turrioni, A.P.; Soares, D.G.; Hebling, J.; de Souza Costa, C.A. Effects of lowlevel laser therapy and epidermal growth factor on the activities of gingival fibroblasts obtained from young or elderly individuals. Lasers Med. Sci. 2017, 32, 45–52. [Google Scholar] [CrossRef]
- Tariq, M.; Tahir, H.M.; Butt, S.A.; Ali, S.; Ahmad, A.B.; Raza, C.; Summer, M.; Hassan, A.; Nadeem, J. Silk-derived formulations for accelerated wound healing in diabetic mice. PeerJ 2021, 9, e10232. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Chen, T.; Zhao, B.; Fan, W.; Shen, Y.; Wei, H.; Zhang, M.; Zheng, W.; Peng, J.; Wang, J.; et al. Acceleration of oral wound healing under diabetes mellitus conditions using bioadhesive hydrogel. ACS Appl. Mater. Interfaces 2023, 15, 416–431. [Google Scholar] [CrossRef] [PubMed]
- Radović, K.; Brković, B.; Roganović, J.; Ilić, J.; Milić Lemić, A.; Jovanović, B. Salivary VEGF and postextraction wound healing in type 2 diabetic immediate denture wearers. Acta Odontol. Scand. 2022, 80, 9–14. [Google Scholar] [CrossRef] [PubMed]
- Morishita, Y.; Hasegawa, S.; Koie, S.; Nakaya, S.; Goto, M.; Miyachi, H.; Naruse, K.; Nakamura, N.; Hayashi, T.; Kawai, T.; et al. Effects of heated tobacco products and conventional cigarettes on dental implant wound healing: Experimental research. Ann. Med. Surg. 2023, 85, 1366–1370. [Google Scholar] [CrossRef]
- Rathinavelu, A. Tumor Angiogenesis and Novel Vascular Endothelial Receptor (VEGFR) Specific Small Molecule Inhibitors. In Biopharmaceutical Drug Design and Development; Aneed, M.A., Ed.; Springer: Cham, Switzerland, 2015; pp. 245–262. [Google Scholar] [CrossRef]
- Lassig, A.A.D.; Bechtold, J.E.; Lindgren, B.R.; Pisansky, A.; Itabiyi, A.; Yueh, B.; Joseph, A.M. Tobacco exposure and wound healing in head and neck surgical wounds. Laryngoscope 2018, 128, 618–625. [Google Scholar] [CrossRef]
- Yang, L.; Song, Y.; Jiao, Y.; Liu, S.; Liu, Y.; Guo, L.; Liu, Y. An Innovative Compound That Promotes Oral Wound Healing via Mobilizing Gingival Mesenchymal Stem Cell Homing. Biochem. Biophys. Rep. 2025, 43, 102154. [Google Scholar] [CrossRef]
- Budi, H.S.; Handajani, J.; Amir, L.R.; Soekanto, S.A.; Ulfa, N.M.; Wulansari, S.A.; Shen, Y.K.; Yamada, S. Nanoemulgel Development of Stem Cells from Human Exfoliated Deciduous Teeth-Derived Conditioned Medium as a Novel Nanocarrier Growth Factors. Eur. J. Dent. 2025. [Google Scholar] [CrossRef]
- de Farias, G.A.; Wagner, V.P.; Correa, C.; Webber, L.P.; Pilar, E.F.S.; Curra, M.; Carrard, V.C.; Martins, M.A.T.; Martins, M.D. Photobiomodulation therapy modulates epigenetic events and NF-κB expression in oral epithelial wound healing. Lasers Med. Sci. 2019, 34, 1465–1472. [Google Scholar] [CrossRef]
- Etemadi, A.; Taghavi Namin, S.; Hodjat, M.; Kosarieh, E.; Hakimiha, N. Assessment of the Photobiomodulation Effect of a Blue Diode Laser on the Proliferation and Migration of Cultured Human Gingival Fibroblast Cells: A Preliminary In Vitro Study. J. Lasers Med. Sci. 2020, 11, 491–496. [Google Scholar] [CrossRef]

| Feature | Oral Mucosa | Skin |
|---|---|---|
| Epithelial Turnover | Rapid (5–12 days); high regenerative potential | Slower (28–40 days); limited regenerative capacity |
| Inflammatory Response | Attenuated and shorter; rapid macrophage M1 to M2 transition | Prolonged and intense; higher risk of chronic inflammation |
| Myofibroblast Presence | Reduced; low α-SMA expression; minimal fibrosis | Abundant; promotes scar formation |
| Angiogenesis Dynamics | Rapid, transient, VEGF-regulated; early vascular regression | Delayed; persistent neovascularization |
| Matrix Remodeling | Balanced MMP/TIMP activity; efficient ECM restoration | Imbalanced; risk of excessive matrix deposition |
| Microbiota Interaction | Commensals modulate immunity; high microbial diversity | Lower microbial load; less direct influence |
| Scarring | Minimal to none | Common; visible fibrotic scarring |
| Clinical Healing Outcome | Faster recovery, improved aesthetics and function | Slower healing, higher risk of complications |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuhuaicura, P.; Rodríguez-Niklitschek, C.; Oporto, G.H.; Salazar, L.A. Distinct Molecular Mechanisms in Oral Mucosal Wound Healing: Translational Insights and Future Directions. Int. J. Mol. Sci. 2025, 26, 10660. https://doi.org/10.3390/ijms262110660
Chuhuaicura P, Rodríguez-Niklitschek C, Oporto GH, Salazar LA. Distinct Molecular Mechanisms in Oral Mucosal Wound Healing: Translational Insights and Future Directions. International Journal of Molecular Sciences. 2025; 26(21):10660. https://doi.org/10.3390/ijms262110660
Chicago/Turabian StyleChuhuaicura, Priscila, Cynthia Rodríguez-Niklitschek, Gonzalo H. Oporto, and Luis A. Salazar. 2025. "Distinct Molecular Mechanisms in Oral Mucosal Wound Healing: Translational Insights and Future Directions" International Journal of Molecular Sciences 26, no. 21: 10660. https://doi.org/10.3390/ijms262110660
APA StyleChuhuaicura, P., Rodríguez-Niklitschek, C., Oporto, G. H., & Salazar, L. A. (2025). Distinct Molecular Mechanisms in Oral Mucosal Wound Healing: Translational Insights and Future Directions. International Journal of Molecular Sciences, 26(21), 10660. https://doi.org/10.3390/ijms262110660









