Biomolecular Fingerprints of Sirtuin Activity in Senescent Fibroblasts Identified via Synchrotron-Based FTIR
Abstract
1. Introduction
2. Results
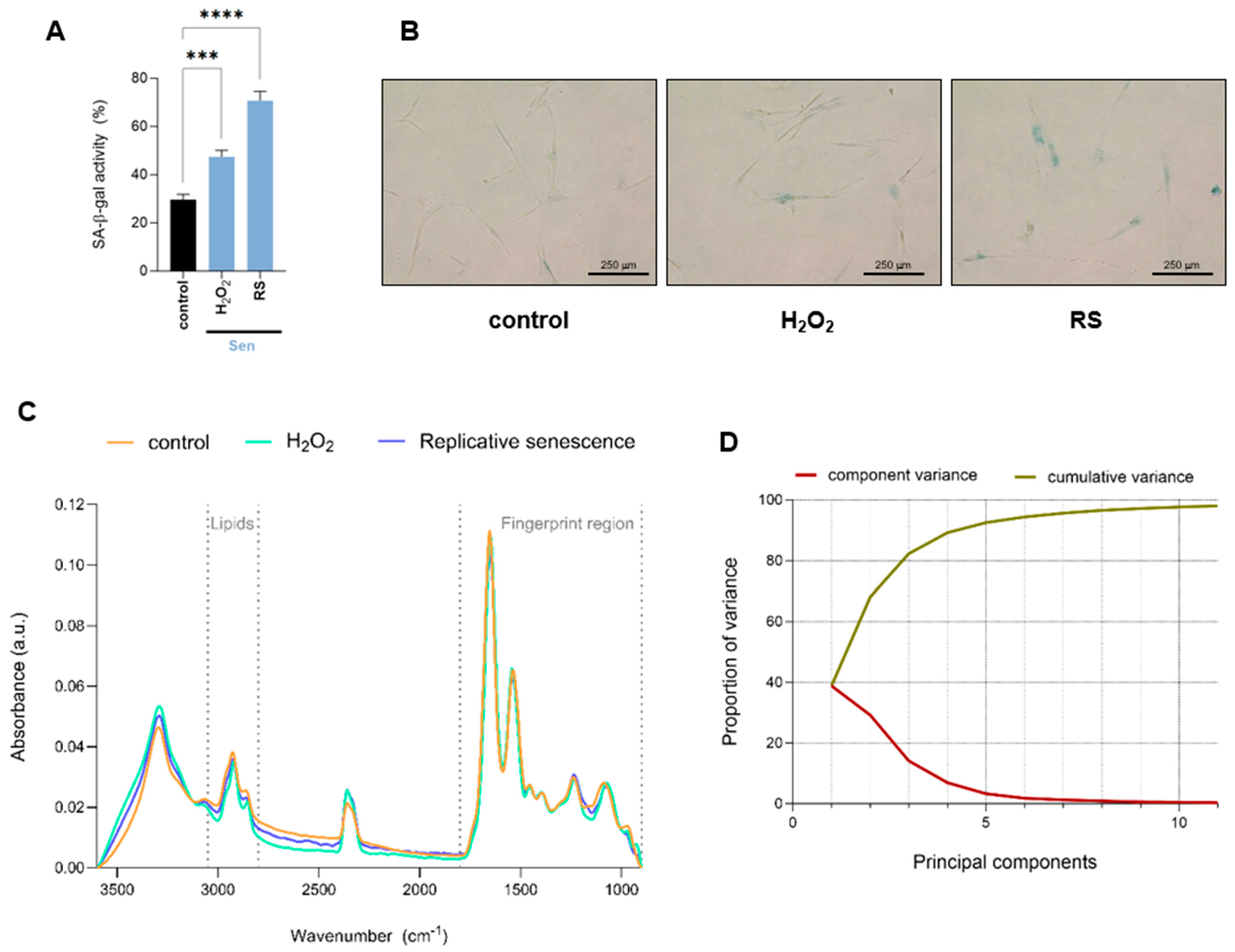
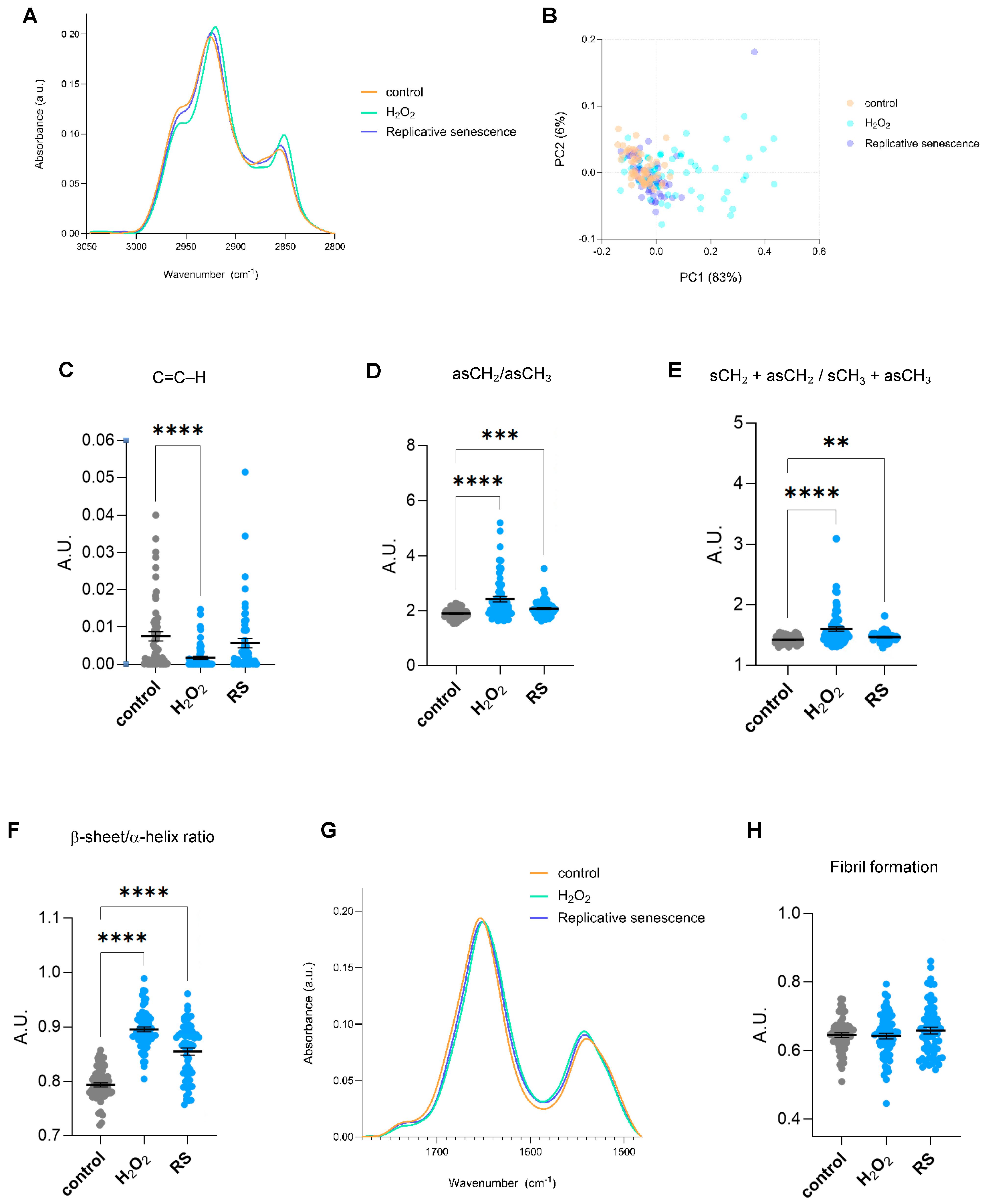
2.1. FTIR Global Spectra of Human IMR90 Fibroblasts Undergoing Cellular Senescence
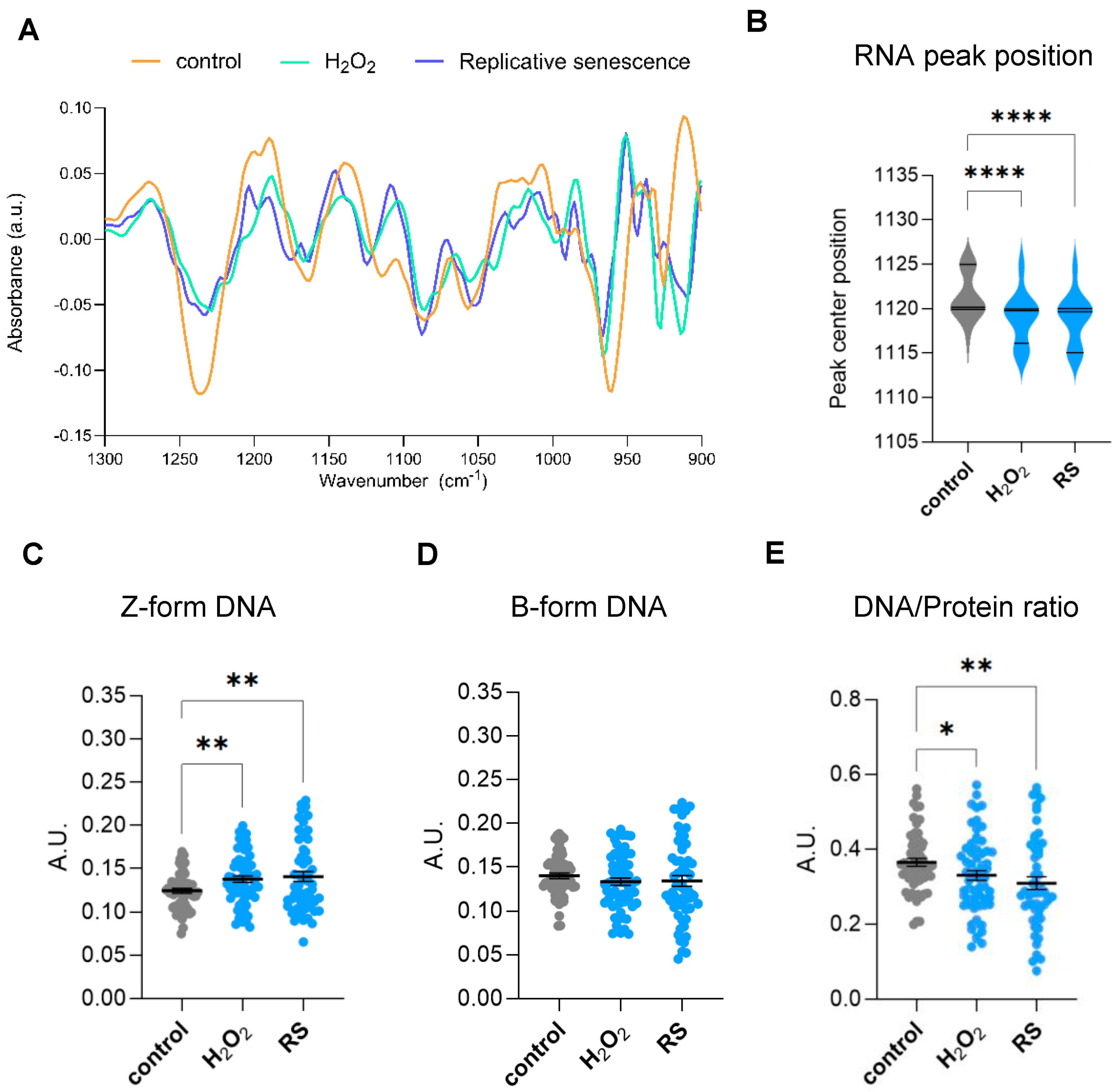
2.2. Genomic and Epigenetic Profiling by FTIR of Human IMR90 Fibroblasts Undergoing Cellular Senescence
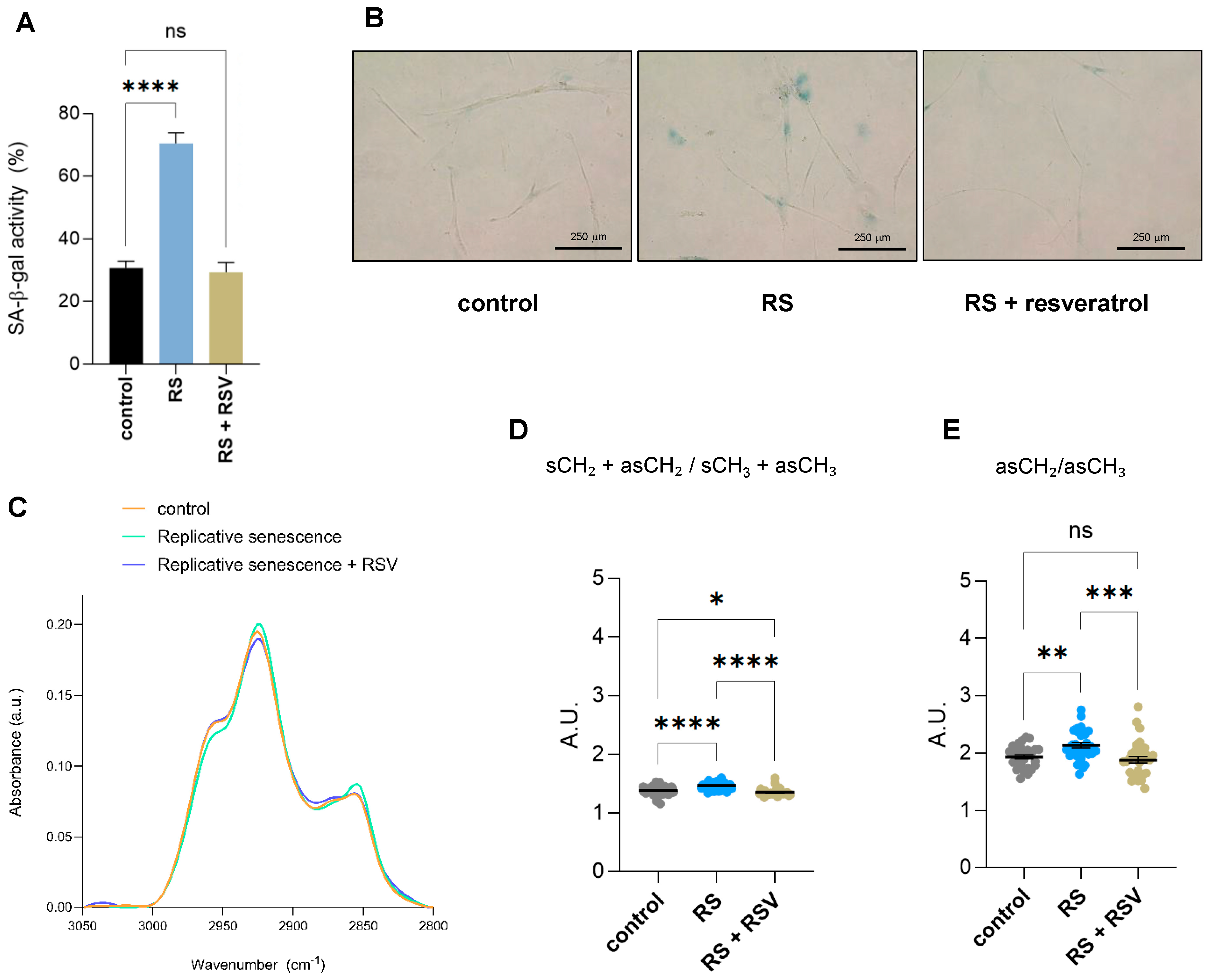
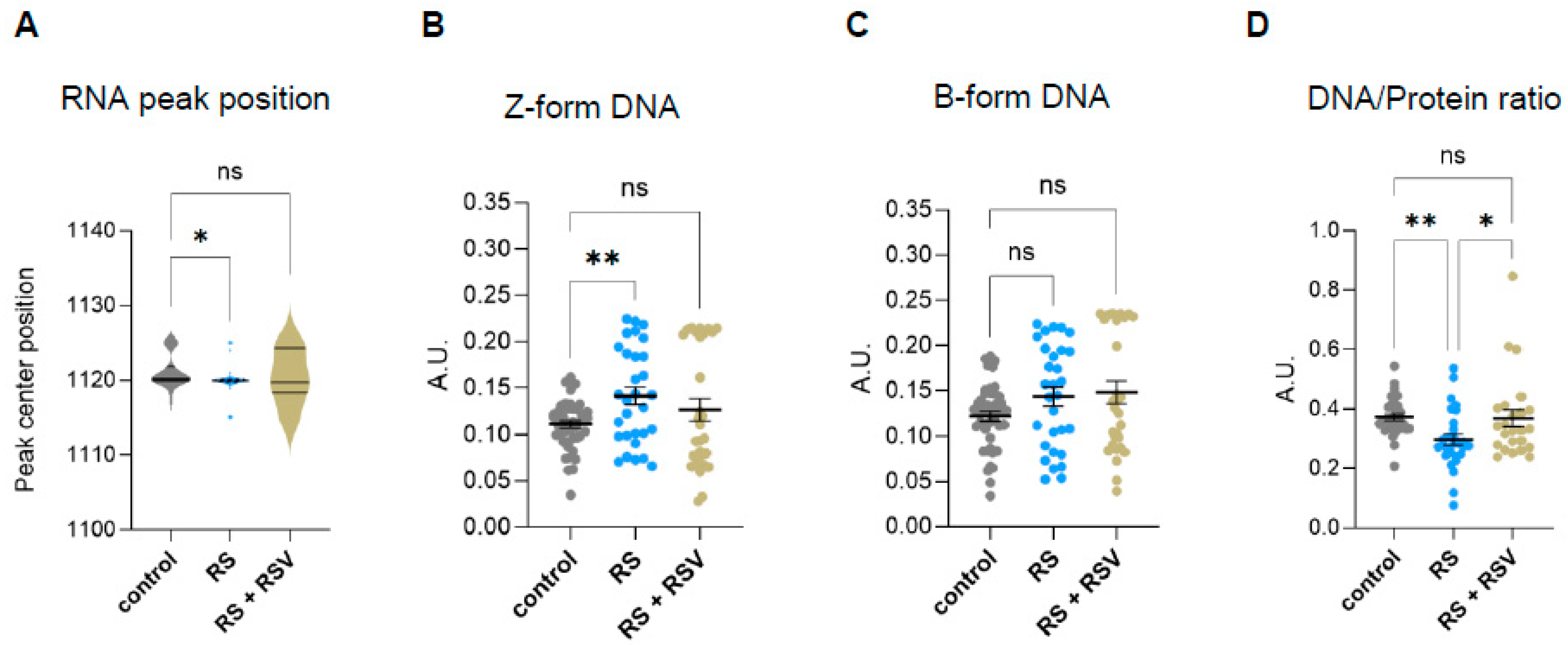
2.3. Effect of Resveratrol on the Biomolecular Composition and Structure of Fibroblasts Undergoing Replicative Senescence Analyzed by Single-Cell FTIR
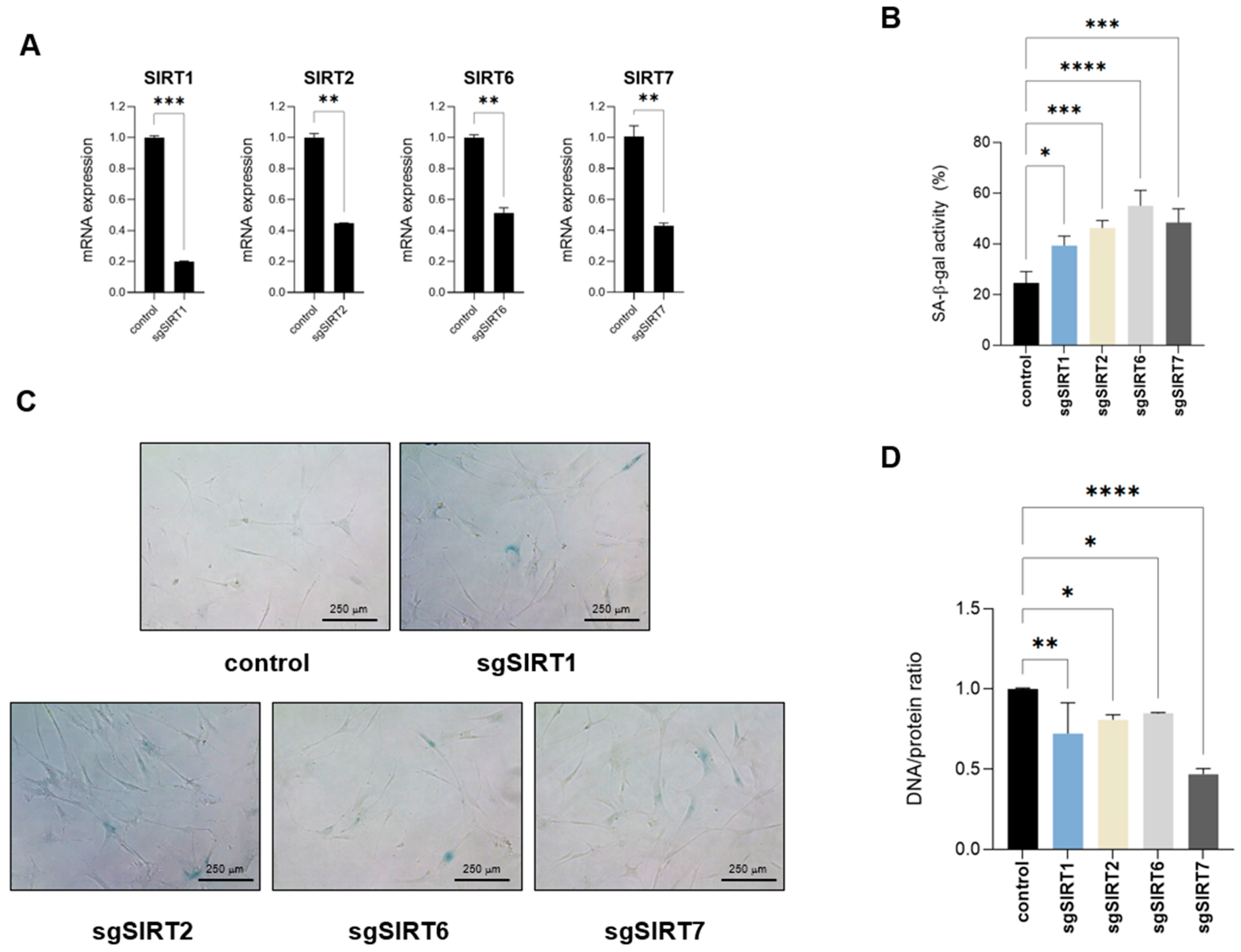
2.4. Targeting Nuclear Sirtuins Impairs Cellular DNA/Protein Ratios
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Treatments
4.2. Senescence-Associated-β-Galactosidase Staining
4.3. Lentiviral Infection and Production
4.4. Synchrotron-Based Single-Cell FTIR Acquisition Conditions and Spectral Analysis Procedures
4.5. RNA Isolation and Quantitative PCR Was Performed
4.6. Genomic DNA Extraction
4.7. Protein Extraction
4.8. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hernandez-Segura, A.; Nehme, J.; Demaria, M. Hallmarks of Cellular Senescence. Trends Cell Biol. 2018, 28, 436–453. [Google Scholar] [CrossRef]
- McHugh, D.; Gil, J. Senescence and aging: Causes, consequences, and therapeutic avenues. J. Cell Biol. 2017, 217, 65–77. [Google Scholar] [CrossRef]
- Shaban, H.A.; Gasser, S.M. Dynamic 3D genome reorganization during senescence: Defining cell states through chromatin. Cell Death Differ. 2023, 32, 9–15. [Google Scholar] [CrossRef]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. Hallmarks of aging: An expanding universe. Cell 2023, 186, 243–278. [Google Scholar] [CrossRef]
- Dučić, T.; Koch, J.C. Synchrotron-Based Fourier-Transform Infrared Micro-Spectroscopy of Cerebrospinal Fluid from Amyotrophic Lateral Sclerosis Patients Reveals a Unique Biomolecular Profile. Cells 2023, 12, 1451. [Google Scholar] [CrossRef]
- Wiley, C.D.; Campisi, J. The metabolic roots of senescence: Mechanisms and opportunities for intervention. Nat. Metab. 2021, 3, 1290–1301. [Google Scholar] [CrossRef] [PubMed]
- Chaib, S.; Tchkonia, T.; Kirkland, J.L. Cellular senescence and senolytics: The path to the clinic. Nat. Med. 2022, 28, 1556–1568. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, W.; Sikora, E.; Bielak-Zmijewska, A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology 2017, 18, 447–476. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gutiérrez, A.; Fernández-Duran, I.; Marazuela-Duque, A.; Simonet, N.G.; Yousef, I.; Martínez-Rovira, I.; Martínez-Hoyos, J.; Vaquero, A. Shikimic acid protects skin cells from UV-induced senescence through activation of the NAD+-dependent deacetylase SIRT1. Aging 2021, 13, 12308–12333. [Google Scholar] [CrossRef]
- Covarrubias, A.J.; Perrone, R.; Grozio, A.; Verdin, E. NAD+ metabolism and its roles in cellular processes during ageing. Nat. Rev. Mol. Cell Biol. 2020, 22, 119–141. [Google Scholar] [CrossRef]
- Ungurianu, A.; Zanfirescu, A.; Margină, D. Sirtuins, resveratrol and the intertwining cellular pathways connecting them. Ageing Res. Rev. 2023, 88, 101936. [Google Scholar] [CrossRef]
- Magalhães, S.; Almeida, I.; Martins, F.; Camões, F.; Soares, A.R.; Goodfellow, B.J.; Rebelo, S.; Nunes, A. FTIR Spectroscopy as a Tool to Study Age-Related Changes in Cardiac and Skeletal Muscle of Female C57BL/6J Mice. Molecules 2021, 26, 6410. [Google Scholar] [CrossRef]
- Millner, A.; Atilla-Gokcumen, G.E. Lipid Players of Cellular Senescence. Metabolites 2020, 10, 339. [Google Scholar] [CrossRef]
- Abdelrazzak, A.B.; Hezma, A.; El-Bahy, G.S. ATR-FTIR spectroscopy probing of structural alterations in the cellular membrane of abscopal liver cells. Biochim. Biophys. Acta (BBA) Biomembr. 2021, 1863, 183726. [Google Scholar] [CrossRef] [PubMed]
- Kreuzer, M.; Stamenković, S.; Chen, S.; Andjus, P.; Dučić, T. Lipids status and copper in a single astrocyte of the rat model for amyotrophic lateral sclerosis: Correlative synchrotron-based X-ray and infrared imaging. J. Biophotonics 2020, 13, e202000069. [Google Scholar] [CrossRef]
- Magalhães, S.; Almeida, I.; Pereira, C.D.; Rebelo, S.; Goodfellow, B.J.; Nunes, A. The Long-Term Culture of Human Fibroblasts Reveals a Spectroscopic Signature of Senescence. Int. J. Mol. Sci. 2022, 23, 5830. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Huang, Q.; Yan, J.; Chen, Z. Histone Acetylation Induced Transformation of B-DNA to Z-DNA in Cells Probed through FT-IR Spectroscopy. Anal. Chem. 2016, 88, 4179–4182. [Google Scholar] [CrossRef] [PubMed]
- Veronezi, G.M.B.; Felisbino, M.B.; Gatti, M.S.V.; Mello, M.L.S.; Vidal, B.D.C. DNA Methylation Changes in Valproic Acid-Treated HeLa Cells as Assessed by Image Analysis, Immunofluorescence and Vibrational Microspectroscopy. PLoS ONE 2017, 12, e0170740. [Google Scholar] [CrossRef]
- Praja, R.K.; Wongwattanakul, M.; Tippayawat, P.; Phoksawat, W.; Jumnainsong, A.; Sornkayasit, K.; Leelayuwat, C. Attenuated Total Reflectance-Fourier Transform Infrared (ATR-FTIR) Spectroscopy Discriminates the Elderly with a Low and High Percentage of Pathogenic CD4+ T Cells. Cells 2022, 11, 458. [Google Scholar] [CrossRef]
- Malek, K.; Wood, B.R.; Bambery, K.R. Optical Spectroscopy and Computational Methods in Biology and Medicine; Springer: Dordrecht, The Netherlands, 2013; pp. 419–473. [Google Scholar]
- Alerasool, N.; Segal, D.; Lee, H.; Taipale, M. An efficient KRAB domain for CRISPRi applications in human cells. Nat. Methods 2020, 17, 1093–1096. [Google Scholar] [CrossRef] [PubMed]
- González-Gualda, E.; Baker, A.G.; Fruk, L.; Muñoz-Espín, D. A guide to assessing cellular senescence in vitro and in vivo. FEBS J. 2020, 288, 56–80. [Google Scholar] [CrossRef]
- Zeng, Q.; Gong, Y.; Zhu, N.; Shi, Y.; Zhang, C.; Qin, L. Lipids and lipid metabolism in cellular senescence: Emerging targets for age-related diseases. Ageing Res. Rev. 2024, 97, 102294. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, L.; Wang, K.; An, Y.; Wang, Y.; Zheng, Y.; Zhou, Y. Relationship between fatty acid intake and aging: A Mendelian randomization study. Aging 2024, 16, 5711–5739. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, S.; Inoko, A.; Oka, Y.; Leproux, P.; Kano, H. Coherent Raman microscopy visualizes ongoing cellular senescence through amide I peak shifts originating from β sheets in disordered nucleolar proteins. Sci. Rep. 2024, 14, 27584. [Google Scholar] [CrossRef] [PubMed]
- Shan, P.; Fan, G.; Sun, L.; Liu, J.; Wang, W.; Hu, C.; Zhang, X.; Zhai, Q.; Song, X.; Cao, L.; et al. SIRT1 Functions as a Negative Regulator of Eukaryotic Poly(A)RNA Transport. Curr. Biol. 2017, 27, 2271–2284.e5. [Google Scholar] [CrossRef] [PubMed]
- Tong, Z.; Wang, M.; Wang, Y.; Kim, D.D.; Grenier, J.K.; Cao, J.; Sadhukhan, S.; Hao, Q.; Lin, H. SIRT7 Is an RNA-Activated Protein Lysine Deacylase. ACS Chem. Biol. 2016, 12, 300–310. [Google Scholar] [CrossRef]
- Ravichandran, S.; Subramani, V.K.; Kim, K.K. Z-DNA in the genome: From structure to disease. Biophys. Rev. 2019, 11, 383–387. [Google Scholar] [CrossRef]
- Radak, M.; Fallahi, H. Zbp1 gene: A modulator of multiple aging hallmarks as potential therapeutic target for age-related diseases. Biogerontology 2023, 24, 831–844. [Google Scholar] [CrossRef]
- Klein, B.; Reynolds, M.B.; Xu, B.; Gharaee-Kermani, M.; Gao, Y.; Berthier, C.C.; Henning, S.; Tsoi, L.C.; Loftus, S.N.; McNeely, K.E.; et al. Epidermal ZBP1 stabilizes mitochondrial Z-DNA to drive UV-induced IFN signaling in autoimmune photosensitivity. Sci. Immunol. 2025, 10, eado1710. [Google Scholar] [CrossRef]
- Song, Z.; Xie, X.; Chen, Y.; Zhang, B.; Li, X.; Yang, Y.; Mo, W.; Zhang, J.; Xu, D. Innate immune sensing of Z-nucleic acids by ZBP1-RIPK1 axis drives neuroinflammation in Alzheimer’s disease. Immunity 2025, 58, 2574–2592.e9. [Google Scholar] [CrossRef]
- Khurana, A.; Chadha, Y.; Schmoller, K.M. Too big not to fail: Different paths lead to senescence of enlarged cells. Mol. Cell 2023, 83, 3946–3947. [Google Scholar] [CrossRef] [PubMed]
- Neurohr, G.E.; Terry, R.L.; Lengefeld, J.; Bonney, M.; Brittingham, G.P.; Moretto, F.; Miettinen, T.P.; Vaites, L.P.; Soares, L.M.; Paulo, J.A.; et al. Excessive Cell Growth Causes Cytoplasm Dilution and Contributes to Senescence. Cell 2019, 176, 1083–1097.e18. [Google Scholar] [CrossRef] [PubMed]
- De Cecco, M.; Jeyapalan, J.; Zhao, X.; Tamamori-Adachi, M.; Sedivy, J.M. Nuclear protein accumulation in cellular senescence and organismal aging revealed with a novel single-cell resolution fluorescence microscopy assay. Aging 2011, 3, 955–967. [Google Scholar] [CrossRef]
- Basisty, N.; Kale, A.; Jeon, O.H.; Kuehnemann, C.; Payne, T.; Rao, C.; Holtz, A.; Shah, S.; Sharma, V.; Ferrucci, L.; et al. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020, 18, e3000599. [Google Scholar] [CrossRef] [PubMed]
- Walther, D.M.; Kasturi, P.; Zheng, M.; Pinkert, S.; Vecchi, G.; Ciryam, P.; Morimoto, R.I.; Dobson, C.M.; Vendruscolo, M.; Mann, M.; et al. Widespread Proteome Remodeling and Aggregation in Aging C. elegans. Cell 2015, 161, 919–932, Erratum in Cell 2017, 168, 944. https://doi.org/10.1016/j.cell.2016.12.041. [Google Scholar] [CrossRef]
- Laberge, R.-M.; Sun, Y.; Orjalo, A.V.; Patil, C.K.; Freund, A.; Zhou, L.; Curran, S.C.; Davalos, A.R.; Wilson-Edell, K.A.; Liu, S.; et al. MTOR regulates the pro-tumorigenic senescence-associated secretory phenotype by promoting IL1A translation. Nat. Cell Biol. 2015, 17, 1049–1061, Erratum in Nat. Cell Biol. 2021, 23, 564–565. https://doi.org/10.1038/s41556-021-00655-4. [Google Scholar] [CrossRef]
- Herranz, N.; Gallage, S.; Mellone, M.; Wuestefeld, T.; Klotz, S.; Hanley, C.J.; Raguz, S.; Acosta, J.C.; Innes, A.J.; Banito, A.; et al. mTOR regulates MAPKAPK2 translation to control the senescence-associated secretory phenotype. Nat. Cell Biol. 2015, 17, 1205–1217, Erratum in Nat. Cell Biol. 2015, 17, 1370. https://doi.org/10.1038/ncb3243. Correction in Nat. Cell Biol. 2024, 26, 1019. https://doi.org/10.1038/s41556-024-01443-6. [Google Scholar] [CrossRef]
- Morrish, R.B.; Hermes, M.; Metz, J.; Stone, N.; Pagliara, S.; Chahwan, R.; Palombo, F. Single Cell Imaging of Nuclear Architecture Changes. Front. Cell Dev. Biol. 2019, 7, 141. [Google Scholar] [CrossRef]
- Kiran, S.; Chatterjee, N.; Singh, S.; Kaul, S.C.; Wadhwa, R.; Ramakrishna, G. Intracellular distribution of human SIRT7 and mapping of the nuclear/nucleolar localization signal. FEBS J. 2013, 280, 3451–3466. [Google Scholar] [CrossRef]
- de Carvalho, L.A.E.B.; Cinque, G.; de Carvalho, A.L.M.B.; Marques, J.; Frogley, M.D.; Vondracek, H.; Marques, M.P.M. Synchrotron nano-FTIR spectroscopy for probing anticancer drugs at subcellular scale. Sci. Rep. 2024, 14, 17166. [Google Scholar] [CrossRef]
- Álvarez-Marimon, E.; Castillo-Michel, H.; Reyes-Herrera, J.; Seira, J.; Aso, E.; Carmona, M.; Ferrer, I.; Cladera, J.; Benseny-Cases, N. Synchrotron X-ray Fluorescence and FTIR Signatures for Amyloid Fibrillary and Nonfibrillary Plaques. ACS Chem. Neurosci. 2021, 12, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández-Duran, I.; Dučić, T.; Vaquero, A. Biomolecular Fingerprints of Sirtuin Activity in Senescent Fibroblasts Identified via Synchrotron-Based FTIR. Int. J. Mol. Sci. 2025, 26, 10495. https://doi.org/10.3390/ijms262110495
Fernández-Duran I, Dučić T, Vaquero A. Biomolecular Fingerprints of Sirtuin Activity in Senescent Fibroblasts Identified via Synchrotron-Based FTIR. International Journal of Molecular Sciences. 2025; 26(21):10495. https://doi.org/10.3390/ijms262110495
Chicago/Turabian StyleFernández-Duran, Irene, Tanja Dučić, and Alejandro Vaquero. 2025. "Biomolecular Fingerprints of Sirtuin Activity in Senescent Fibroblasts Identified via Synchrotron-Based FTIR" International Journal of Molecular Sciences 26, no. 21: 10495. https://doi.org/10.3390/ijms262110495
APA StyleFernández-Duran, I., Dučić, T., & Vaquero, A. (2025). Biomolecular Fingerprints of Sirtuin Activity in Senescent Fibroblasts Identified via Synchrotron-Based FTIR. International Journal of Molecular Sciences, 26(21), 10495. https://doi.org/10.3390/ijms262110495






