Abstract
Cold stress reduces horticultural crop yield and postharvest quality by disrupting membrane fluidity, redox equilibrium, and the cell wall structure. This results in chilling injury, tissue softening, and loss of color. Long noncoding RNAs (lncRNAs) have emerged as key integrators of plant cold signaling pathways. LncRNAs mediate the interaction between calcium signaling systems and transcriptional cascades while coordinating hormone signaling networks, including those involving abscisic acid, jasmonic acid, ethylene, salicylic acid, and brassinosteroids. LncRNAs influence gene regulation through chromatin-based guidance, sequestration of repressive complexes, natural antisense transcriptional interference, microRNA-centered competing endogenous RNA networks, and control of RNA splicing, stability, localization, and translation. Studies in horticultural species revealed that cold-responsive lncRNAs regulate processes essential for fruit firmness, antioxidant levels, and shelf-life, including lipid modification, reactive oxygen species balance, and cell wall or cuticle remodeling. This review aims to summarize tissue- and developmental stage-specific expression patterns and highlight experimental approaches to validate RNA function, including gene editing, transcript recovery, advanced sequencing, and analysis of protein-RNA interactions. Integrating these results will facilitate the development of precise molecular markers and nodes of regulatory networks that increase cold tolerance, and improve the quality of horticultural crops.
1. Introduction
Cold stress, which is caused by temperatures below 15 °C, significantly reduces the productivity, quality, and postharvest performance of horticultural crops. During storage, cold injury (CI) is a distinctive physiological problem that has an adverse effect on market value. Recent comprehensive studies integrate knowledge of CI symptoms, underlying mechanisms, and mitigation strategies for various fruits and vegetables [1]. Plants alleviate cold stress through processes of cold acclimation, which include membrane and thermosensory perception, calcium-dependent (Ca2+) signaling pathways, kinase cascades, and transcriptional regulation. These processes are primarily coordinated by the Inducer of CBF Expression 1-C-repeat Binding Factors-Cold-Responsive genes (ICE1-CBF-COR) signaling module and by reactive oxygen species (ROS) homeostasis and hormone-dependent interactions [2].
LncRNAs have emerged as pivotal regulators of plant responses to abiotic stresses, including cold stress [3]. Cold stress significantly restricts the productivity of horticultural crops. LncRNAs modulate gene expression through transcriptional regulation, chromatin remodeling, RNA stability, and interactions with small RNAs [3]. Often, lncRNAs function by interacting with transcription factors, modifying chromatin states, or acting as molecular decoys to fine-tune stress responses [4]. Understanding how lncRNAs mediate cold stress regulation in horticultural crops is expanding.
This study aims to comprehensively review and elucidate the molecular roles of lncRNAs in the cold stress response of horticultural plants. Current knowledge on the diverse regulatory functions of lncRNAs, such as transcriptional modulation, epigenetic regulation, and interaction with signaling pathways, that underpin cold tolerance is lacking. Furthermore, this study highlights recent advances in identifying and characterizing cold-responsive lncRNAs across key horticultural species. Potential applications of lncRNAs in breeding and biotechnological strategies to increase cold resilience and improve crop productivity under adverse environmental conditions are also discussed.
2. Plant Long Non-Coding RNAs
2.1. Definition
Long non-coding RNAs are transcripts that are longer than 200 nucleotides and lack significant protein-coding potential [5]. Unlike messenger RNAs, their diverse biogenesis and structural complexity make annotation and functional prediction challenging [6,7]. Once dismissed as transcriptional noise, lncRNAs are recognized as essential regulators of gene expression that modulate chromatin conformation and transcription, both locally and distally [8,9]. They also affect RNA processing, stability, translation, and nuclear architecture. Plant lncRNAs originate from intergenic, intronic, antisense, promoter-, and enhancer-proximal regions. This reflects their diverse regulatory functions [10,11]. LncRNAs often exhibit low sequence conservation across species. However, their involvement in stress responses, development, and epigenetic regulation is well documented in plants [12,13]. This opens the door for the identification of potential targets to improve plant resilience and productivity.
2.2. Biogenesis and Genomic Context
In plants, most lncRNAs are transcribed by RNA polymerase II and undergo processing similar to that of messenger RNAs (mRNAs), including 5′ capping, alternative splicing, and 3′ polyadenylation [14]. RNA polymerase III transcribes shorter, more stable, and more abundant lncRNAs, which undergo polyadenylation less frequently. These transcripts are involved in stress responses and immune regulation [15]. Plants possess two additional RNA polymerases IV (Pol IV) and V (Pol V), which produce lncRNAs involved in RNA-directed DNA methylation (RdDM), a process that is critical for gene silencing and genome stability [11,16]. Pol IV-derived lncRNAs are precursors of small interfering RNAs. Pol V transcripts serve as scaffolds that guide silencing complexes to chromatin, modulating its architecture and often exhibiting tissue specificity [16,17]. Thus, plant lncRNA biogenesis involves a complex interplay of multiple polymerases and posttranscriptional modifications that underpin their diverse regulatory roles in development and environmental responses.
2.3. Classification
Plant lncRNAs are classified according to their genomic context concerning protein-coding genes. This classification is crucial for understanding the origin and regulatory roles of lncRNAs. Types of lncRNAs include natural antisense transcripts (NATs), sense lncRNAs, bidirectional lncRNAs, intergenic lncRNAs (lincRNAs), and intronic lncRNAs (incRNAs) (Figure 1) [6,15]. NATs overlap with antisense genes and regulate gene expression transcriptionally and posttranscriptionally. Sense lncRNAs originate from the same strand and modulate nearby gene activity. Bidirectional lncRNAs originate near transcription start sites, but are transcribed from the opposite strand of the DNA template. LincRNAs are abundant in species such as tomato (Solanum lycopersicum L.) and cucumber (Cucumis sativus L.) and exhibit tissue specificity and diverse regulatory functions. IncRNAs originate entirely from introns and influence splicing and host gene expression [18]. These classifications reflect the dynamic and varied functions of lncRNAs in the development and stress responses of horticultural plants.
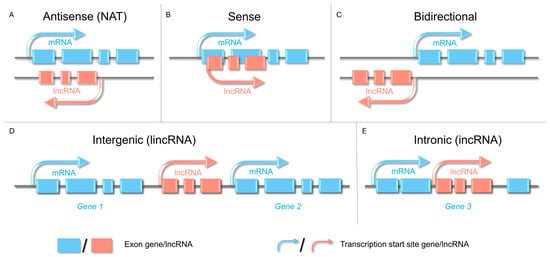
Figure 1.
Classification of long non-coding RNAs (lncRNAs) based on the genomic context concerning protein-coding genes. (A) Natural antisense transcript (NAT) lncRNAs are transcribed from the strand opposite a nearby protein-coding gene. The resulting NAT overlaps exons and/or introns and can modulate sense transcription through transcriptional interference or RNA-RNA-chromatin interactions. (B) Sense-overlapping-lncRNAs transcribed from the same strand as a protein-coding gene, overlapping part of its exonic/intronic sequence, may therefore, interfere with the splicing, elongation, or transcript turnover rate of the mRNA. Interference may influence splicing, elongation, or RNA turnover. (C) Bidirectional-lncRNAs initiated from a promoter region that drives a protein-coding gene in the opposite direction (head-to-head, typically within ~1 kb of the coding TSS), enabling coordinated, strand-specific regulation. (D) Intergenic lncRNAs (lincRNAs) arise from an intergenic locus and do not overlap with any annotated coding genes. Regulation is conferred by its own promoter/enhancers and distal chromatin contacts. (E) Intronic lncRNAs are wholly contained within an intron of a host gene, do not overlap with its exons, and may be transcribed or processed independently of host transcripts.
2.4. Mechanisms of Action
Despite rapid sequence divergence, lncRNAs in plants function through conserved modes of interaction. These RNAs act as signals, decoys, guides, or scaffolds, linking structural classes (lincRNAs, NATs, and incRNAs) to regulatory roles (Figure 2) [19,20]. Signal lncRNAs respond to environmental cues by modulating transcription via promoter remodeling and alternative RNA processing. This phenomenon has been observed in tomato and grapevine (Vitis vinifera L.) [21,22]. Decoy lncRNAs sequester microRNAs (miRNAs) or proteins, thereby regulating crop nutrient homeostasis and pathogen defense responses [23]. Guide lncRNAs direct chromatin modifiers to target loci, thereby modulating gene expression. COLDAIR exemplifies this process in Arabidopsis (Arabidopsis thaliana L.) and its homologs in horticultural species [24,25]. As demonstrated in rice (Oryza sativa L.) and Arabidopsis, scaffold lncRNAs organize protein complexes to facilitate chromatin remodeling [26,27]. These functional archetypes are essential for development and stress adaptation in horticultural plants.
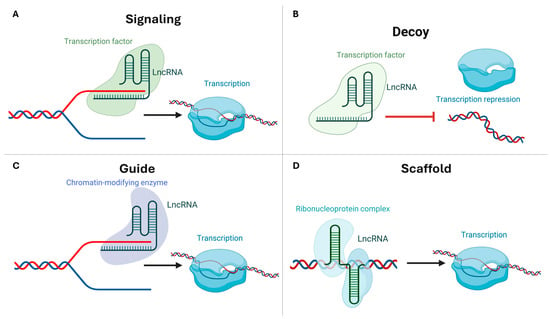
Figure 2.
Mechanisms of action of plant long non-coding RNAs. LncRNAs regulate gene expression through four conserved modes of action: (A) Signaling—in response to a stimulus, such as cold, an induced long non-coding RNA (lncRNA) is transcribed. This lncRNA functions as a molecular signal that engages enhancer- or promoter-proximal regulators. This process controls the timing and location of the assembly of an active transcription complex. It also initiates the transcription of a target gene. (B) Decoys—lncRNAs act as decoys by sequestering a transcription factor, RNA-binding protein, or small RNA component away from its cognate DNA/RNA site. This prevents the factor from occupying the promoter and consequently modulates transcriptional output. (C) Guide—lncRNAs guide regulatory proteins to specific genomic locations through RNA—protein and/or RNA-DNA/RNA base-pairing interactions. This process concentrates chromatin modifiers or transcription factors at target promoters, thereby activating or repressing transcription. (D) Scaffolds–lncRNAs serve as modular scaffolds that carry multiple binding surfaces. These surfaces allow lncRNAs to nucleate higher-order regulatory assemblies, such as transcription factors with chromatin enzymes, at the locus. This stabilizes interactions that fine-tune transcription. This schematic diagram was edited with BioRender.
3. Sensing and Signaling of Cold: Integration Nodes for lncRNAs
3.1. ICE1–CBF–COR Module and Coregulators
The ICE1-CBF-COR cascade forms the basis of transcriptional regulation in response to cold. ICE1 initiates the rapid induction of dehydration-responsive element-binding (DREB)/CBF transcription factors, which subsequently activate COR genes while undergoing complex multilayer regulation (Figure 3) [28]. After cold exposure, Snf1-related kinase 2.6 (SnRK2.6)/OST1 (open stomata 1) becomes activated and phosphorylates ICE1. This increases ICE1 stability and simultaneously inhibits HOS1-dependent degradation. Thus, CBF expression increases during the early stages of acclimation [29]. In turn, the MAP kinases MPK3 and MPK6 phosphorylate ICE1, promoting its destabilization and creating a delayed negative feedback loop that attenuates the response [30]. Temporal regulation is further enhanced by differential ubiquitination. Early attachment of K63-linked ubiquitin chains by PUB25/26 stabilizes ICE1 and inhibits MYB15. Later, K48-linked ubiquitination attenuates the signal [31]. Calcium ion (Ca2+) influx activates calmodulin-binding transcription activator (CAMTA) transcription factors that bind to CBF promoters, integrating Ca2+-mediated signals with light and diurnally regulating CBF expression [28]. Similar regulatory networks are evident in horticultural systems. For example, multiomic analyses in banana (Musa spp.) revealed a mitogen-activated protein kinase Kinase 1 (MEKK)1-MAPK-ICE1-COR signaling axis coupled to lipid remodeling that contributes to cold tolerance [32].
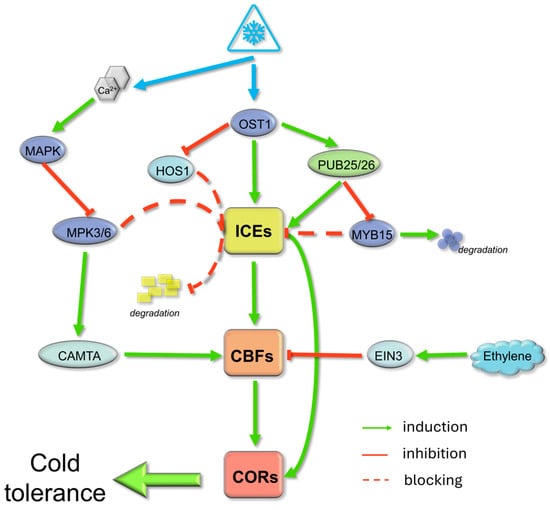
Figure 3.
The canonical ICE1-CBF-COR regulatory cascade is the basis of plant responses to cold acclimation. Upon exposure to cold, ICE (Inducer of CBF Expression) transcription factors are activated, triggering the rapid induction of C-repeat Binding Factors (CBFs). These CBFs then upregulate Cold Responsive (COR) genes, enhancing cold tolerance. OST1 kinase phosphorylates ICEs, stabilizing them and promoting CBF transcription. HOS1-mediated ubiquitination facilitates ICE degradation, modulating response intensity. PUB25/26-mediated ubiquitination stabilizes ICEs and inhibits MYB15, which is a negative regulator of CBFs. The MAP kinases MPK3/6 further fine-tune ICE stability by promoting destabilization through phosphorylation-dependent mechanisms, thereby creating negative feedback. Calcium influx activates CAMTA transcription factors, which can also induce CBFs. Ethylene and its signaling component EIN3 act as negative regulators by inhibiting CBFs. Induction, inhibition, and signal blocking are represented by green, red, and dashed lines, respectively, integrating various signaling components into a multilayered cold response regulatory network.
3.2. Hormonal and Metabolic Interactions
Phytohormones integrate the ICE1 node into physiological networks throughout the plant. Abscisic acid (ABA) generally enhances frost tolerance by interacting with the OST1-ICE1 pathway. Ethylene signaling, which is mediated by ethylene-insensitive 3 (EIN3), often opposes CBF transcription. On the other hand, jasmonic acid (JA) exerts a context-dependent effect by modulating the balance of growth and defense. Brassinosteroids (BRs) can promote cold tolerance. The overexpression of SIBRI1 in tomato elevates ICE1/CBF transcripts and stimulates ROS detoxification pathways [33]. From a metabolic point of view, cold stress disrupts membrane fluidity and redox homeostasis. Tolerant genotypes typically exhibit increased unsaturation of glycerolipids and increased antioxidant capacity. In bananas, the accumulation of specific lipid forms coincides with the activity of the MAPK-ICE1-COR47 axis [32]. These hormonal and metabolic processes converge on the ICE1-CBF pathway and its parallel pathways to fine-tune the amplitude and duration of the cold response.
3.3. Hypothesized and Documented lncRNA Points of Action
LncRNAs are involved in various layers of cold signaling integration, including chromatin targeting and decoy functions involving Polycomb complexes. For example, the antisense lncRNA SVALKA from Arabidopsis restricts CBF1 expression through transcriptional interference and potentially modulates Polycomb-mediated chromatin states at the CBF locus [34]. Transcriptional interference is also mediated by NATs, as demonstrated in chrysanthemum (Chrysanthemum morifolium Ramat.). NATs increase DgTCP1 expression, increasing cold tolerance and confirming this mode of regulation in ornamental plants [35]. Competing endogenous RNAs (ceRNAs) mimic miRNA target receptors. Specifically, lncRNAs act as sponges for miRNAs, alleviating the repression of stress-responsive transcription factors. Although cold-specific modules are still being elucidated, this molecular mechanism has been established in tomato and suggested for cucurbits subjected to cold stress, highlighting its importance for horticultural adaptation to cold [36,37]. The scaffolding role in the RdDM and small-interfering RNA (siRNA) pathways involves Pol V-linked lncRNAs that recruit Argonaute (AGO)-siRNA complexes and the DNA methyltransferases Domains Rearranged Methyltransferase 1/2 (DRM1/2) to deposit cytosine methylation in regulatory regions. This enables epigenetic tuning of inducible promoters [7,38,39]. Additionally, posttranscriptional regulation affects the splicing and stability of cold-responsive transcripts [3]. Together, these pathways establish lncRNAs as versatile modulators capable of recalibrating the ICE1-CBF cascade and its hormonal and redox interfaces in vegetable, fruit, and ornamental plant species.
4. LncRNA Mechanisms of Action in Cold Stress
4.1. Epigenetic and Transcription Regulation
Acclimation to cold requires the rapid elimination of Polycomb repression of specific genes. In Arabidopsis, under short-term chilling, many cold-induced loci translocate histone H3 lysine 27 trimethylation (H3K27me3) under controlled conditions and accumulate histone H3 lysine 4 trimethylation (H3K4me3) when exposed to cold. This is consistent with the stabilized chromatin architecture and the recruitment or translocation of lncRNA-mediated chromatin modifiers [40]. In plants, lincRNAs can engage Polycomb repressive complex 2 (PRC2)/LHP1 and DNA methylation readers for chromatin reprogramming. The Arabidopsis lncRNA APOLO forms R-loops on target promoters, binds LHP1 and VIM1 (a UHRF1 homolog), and coordinates H3K27me3 with DNA methylation to regulate genes in response to the environment. This establishes a general RNA scaffold that can then guide the response of cold-induced modules [41].
The biology of plant enhancers introduces a vital nuance: unlike mammals, unstable bidirectional enhancer RNAs (eRNAs) are rare in plants. The Interspecies Transcription Atlas revealed that distal bidirectional unstable transcription, a characteristic of vertebrate eRNAs, is uncommon. Instead, many distal plant regulatory elements produce stable RNAs, often lncRNAs, and exhibit more vigorous enhancer activity in Self-Transcribing Active Regulatory Region sequencing (STARR-seq) than unstable transcripts do [42]. Therefore, “enhancer-like” lncRNA mechanisms in plants should be inferred based on functional enhancer assays (and 3D contact data), not just bidirectional instability. Finally, NATs can fine-tune transcription through polymerase interference. In chrysanthemum. For example, the NAT controlling DgTCP1 modulates cold tolerance, illustrating the role of NATs in horticulture [35].
4.2. Crosstalk with Small RNAs
LncRNAs bind to miRNA pathways as endogenous target imitators or ceRNAs. In cucumber, CsLncRNA94 forms a cold-responsive module with miR156f and Squamosa Promoter Binding Protein-like (SPL) mRNAs; transient overexpression alters miR156f/SPL expression patterns and reduces cold tolerance, which is consistent with a miRNA titration mechanism affecting developmental/stress centers [43]. Robust inference of ceRNAs in plants requires plant-trained target predictors and degradome support for the miRNA-mRNA cleavage site [44,45]. Horticultural syntheses catalog cold-coupled lncRNAs, which are predicted to sponge miRNAs that target desaturases or antioxidant enzymes, linking membrane fluidity and ROS detoxification to the CBF regulon—hypotheses that can now be tested via Parallel Analysis of RNA Ends (PARE)-supported target maps and genetic epistasis [4].
4.3. Posttranscriptional Control
Cold stress alters alternative splicing (AS), mRNA stability, and translation. Plant lncRNAs can bind splicing regulators to reprogram alternative splicing (AS) programs. Recent reviews of plants integrate such lncRNA-AS interactions with stress phenotypes, suggesting a pathway for tuning signaling and metabolic isoforms during periods of reduced temperature [7]. At the stability/translation interface, plants undergo cotranslational mRNA decay, which links ribosome transit to mRNA degradation and the stress response. LncRNAs incorporated into ribonucleoproteins (RNPs) or associated with polysomes can disrupt the selective stability or subcellular route of cold-responsive mRNAs [46]. Since some annotated lncRNAs contain translated small open reading frames (sORFs), the assignment of a cold phenotype must exclude micropeptide effects by integrating Ribo-seq (triplet periodicity), ORF-specific mutagenesis (start-codon disruption), and proteomics. Conversely, if a micropeptide is produced, gain or loss at the peptide level should phenocopy the effect [47]. Emerging publications also include lncRNAs among the RNAs that organize biomolecular condensates (Liquid-Liquid Phase Separation (LLPS)), a likely mechanism for rapid acclimation to cold temperatures. However, direct evidence in plants remains limited and should include LLPS markers and lncRNA disruption [7].
5. Case Studies: Horticultural Plants
5.1. Grapevine (Vitis vinifera L.)
The sequencing of RNA specific to grapevine leaves exposed to cold temperatures revealed hundreds of cold-responsive (CR)-lncRNAs (Figure 4). Many of these RNAs, such as C-repeat Binding Factor 4 (CBF4), Late Embryogenesis Abundant 14 (LEA14), and WRKY41, are cis-linked to proximal cold pathway genes. Coexpression analyses combined with RNAplex-based pairing have confirmed functional relationships, both cis- and trans-, between these transcripts [48]. The evidence supporting their regulatory role is primarily computational and suggests the cis-activation of the CBF and LEA gene modules. However, the experimental validation of their enhancer-like functions remains inconclusive [48]. The predicted mRNA targets include those encoding CBF/DREB transcription factors, LEA proteins, calcium ATPases, and UDP-glucosyltransferases (UGTs). Several CR-lncRNAs have been found to overlap with genomic regions associated with chloroplast and mitochondrial genes. This suggests that communication between organelles and the cell nucleus occurs during cold acclimation [48,49]. There are no reports of phenotypes resulting from molecular manipulation strategies for grapevine CR-lncRNAs; however, the modules with which they bind are enriched for cold-tolerant genotypes in interspecific crosses and correspond to physiological processes, such as cell membrane stabilization and osmoprotection, observed during acclimation [48,49]. Potential applications include marker-assisted selection via CR-lncRNA-CBF/LEA haplotypes, editing the promoters of cis-acting CR-lncRNAs to modulate ICE-CBF signaling with minimal pleiotropic effects, and predicting cultivar-specific responses to low-temperature storage on the basis of network analyses of horticultural crops [49].
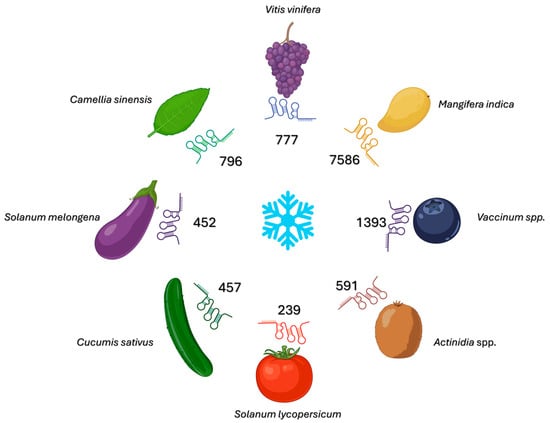
Figure 4.
Cold-responsive long non-coding RNAs (lncRNAs) across horticultural species. This radial summary illustrates the number of lncRNAs identified as cold-responsive (i.e., differentially expressed under chilling/freezing conditions versus control conditions) in Vitis vinifera, Mangifera indica, Vaccinium spp., Actinidia spp., Solanum lycopersicum, Cucumis sativus, Solanum melongena and Camellia sinensis. The central snowflake denotes the low-temperature stimulus. The colored “RNA” symbols are schematic markers of lncRNAs and are illustrative only. The numeric labels provide the quantitative information. The counts compile the totals reported in the cited RNA-seq studies for each species and may reflect study-specific pipelines, tissues, developmental stages, and stress regimes. Therefore, cross-species comparisons should be interpreted cautiously. This schematic diagram was edited with BioRender.
5.2. Kiwifruit (Actinidia spp.)
Storage of kiwifruit at low temperatures after harvest activates lncRNA programs involved in starch and sucrose metabolism and cell wall remodeling. These processes are associated with maintaining fruit firmness and mitigating frost damage. These findings stem from integrated multiomics studies of fruits and vegetables [37]. Network analyses in kiwifruit revealed that enhancer-like lncRNAs and ceRNA motifs may participate in the regulation of carbohydrate metabolism by influencing the expression of genes such as expansins, pectin methylesterase/lyase (PME/PG) enzymes, and sugar transporters. The full-length transcriptomes of kiwifruit under cold conditions revealed that lncRNAs are coexpressed with cold-responsive transcription factors [50]. Full-length transcriptome sequencing under cold conditions further showed coexpression of lncRNAs with cold-responsive transcription factors. Analysis of molecular interaction networks indicates that lncRNAs, miRNAs, and mRNAs can be interconnected within modules that regulate the expression of these target genes. Transcription factors from the CBF and DOF/MYB families appear to be coregulated in cold datasets [37]. These correlations link these lncRNA modules to phenotypic traits such as firmness preservation and reduced chilling damage. However, functional perturbations at the gene level have yet to be described. Potential applications include designing storage regimes based on lncRNA expression profiles associated with cell wall turnover and pretreatment with MeJa or ABA before harvesting to activate lncRNA–ceRNA networks and stabilize cell wall integrity and reactive oxygen species homeostasis [37].
5.3. Citrus (Citrus spp.)
Citrus species undergo robust transcriptomic reprogramming under cold stress. Although direct functional characterization of CR-lncRNAs remains limited, lncRNA atlases have been generated for lemon (Citrus × limon) and other species [51,52,53]. Cold-induced activation of anthocyanin biosynthesis programs through transcription factors, such as Ruby1, which is regulated by CBF-coupled factors, is a key pathway that lncRNAs can modulate through interactions with ceRNA or chromatin-mediated mechanisms. This is analogous to data from other fruit crops [54]. The documented cold-responsive networks in Citrus include those involving transcription factors and ROS-scavenging enzymes. LncRNAs have been functionally mapped for secondary metabolism and disease resistance, suggesting a transferable framework for cold-response research [51]. Existing lncRNA catalogs can be translated into a reanalysis of cold response datasets by comparing NATs and coexpression modules with metabolomic and pigment data. NATs that resemble those in the CBF or ICE1 loci and those that affect anthocyanin accumulation and antioxidant protection could be prioritized as regulatory hubs that link abiotic tolerance to fruit quality traits. Such integrative analyses can reveal lncRNA–miRNA–mRNA interaction axes that regulate cell wall remodeling, osmolite metabolism, or the fine-tuning of ROS, using degradome and small RNA datasets. These data analyses can enable marker-assisted selection or CRISPR-based modulation of key NAT loci associated with cold tolerance and high nutritional quality. The targeted manipulation of these lncRNAs or their associated promoters is an easily applicable method for identifying the epiballistic and transcriptional variants that underlie adaptive cold tolerance. Breeding high-quality, cold-tolerant citrus varieties that combine stress resistance with optimal pigmentation and antioxidants could be made easier by translating the signatures of long non-coding RNA-based regulatory systems into a breeding framework.
5.4. Mango (Mangifera indica)
A computational analysis of various mango cultivars revealed over 31,000 candidate lncRNAs, of which more than 7500 exhibited significant expression changes at chilling temperatures (5–12 °C) (Figure 4) [55]. These cold-responsive lncRNAs target genes involved in critical pathways, such as metabolic regulation, stress response, and development. These pathways include Alcohol Dehydrogenase 1 (ADH1) and 3-ketoacyl-CoA thiolase 2 (KAT2), which play a role in fruit ripening and abscisic acid signaling. Network analysis revealed their interactions with WRKY transcription factors that are central to cold tolerance. Functional enrichment analysis links these lncRNAs to photosynthesis, sugar metabolism, flavonoid biosynthesis, and membrane processes. Some lncRNAs exhibit conserved sequences in related species, indicating that their regulatory roles are conserved [55]. Mango lncRNAs form complex networks that regulate physiological and metabolic adaptations to cold. This makes them promising targets for breeding cold-tolerant mango varieties.
5.5. Blueberries (Vaccinium spp.)
Transcriptomic and proteomic analyses of blueberries under cold stress provide fundamental insights into the regulatory circuits underlying acclimation. Although lncRNA and their regulatory layers have not been fully characterized in existing cold-stress datasets, their abundance during fruit development in related systems suggests the value of retrospective exploration of lncRNAs within existing RNA-seq data [56,57]. Translational efforts should focus on reannotation of available cold stress libraries via lncRNA identification and ceRNA network methods, followed by validation of strong lncRNA candidates that correlate with key phenotypes, such as cell membrane integrity and antioxidant defense, associated with reduced fruit pitting and softening.
5.6. Tomato (Solanum lycopersicum)
Strand-specific RNA sequencing of chilled tomatoes revealed more than 1400 lncRNAs, 239 of which were differentially expressed during chilling injury (Figure 4). Co-expression analyses revealed modules associated with cell membrane structure, ROS homeostasis, cell wall-modifying enzymes, heat and cold shock proteins, and salicylic acid (SA)/ABA metabolism [58]. Integrated analyses revealed a network of competing endogenous RNAs at the lncRNA-miRNA-mRNA level that respond to cold injury. Degradome data confirmed that ceRNAs and target mimicry occur via miRNA targeting and anti-correlated expression profiles. These modules focus on lipid remodeling, ROS detoxification, and defense hormone regulation [58]. The predicted interactions of miRNA families target AGO1, antioxidant genes such as copper/zinc superoxide dismutase (CSD), and cell wall enzymes. Stress-related transcription factors such as DREB/CBF and NAC domain transcription factors are also localized in these networks [59,60]. Network rearrangement is consistent with physiological tolerance indicators, such as reduced electrolyte leakage, malondialdehyde content, and pitting, which are observed in tolerant lines or after elicitor treatment. Although the gene-specific functional validation of cold-responsive lncRNAs (CR-lncRNAs) in tomato remains limited, several established molecular approaches can be employed. These include overexpression and RNA interference (RNAi) assays, virus-induced gene silencing (VIGS), and antisense or hairpin constructs for the transient or stable suppression of lncRNA activity. These routine functional genomics tools provide direct phenotypic evidence and can be complemented by allele-specific CRISPR interference (CRISPRi) or promoter editing expression fine-tuning is needed. Their practical application extends beyond genome editing. It also includes transgenic complementation, promoter–reporter assays, and overexpression in tolerant genetic backgrounds. Together, these offer a versatile framework for identifying and manipulating lncRNA nodes. These nodes predict cold tolerance and post-harvest quality stability [61,62].
5.7. Eggplant (Solanum melongena)
LncRNAs play a key role in regulating the cold stress response in eggplant, and genotype-specific expression patterns have been linked to variations in tolerance. Transcriptomic analyses of the cold-tolerant “CGN22911” and cold-sensitive “Chengdumoqie” varieties revealed 452 differentially expressed lncRNAs under low-temperature conditions (Figure 4) [63]. These lncRNAs modulate target genes involved in metabolic and cellular processes essential for cold adaptation, such as acyl-CoA dehydrogenase and pseudouridine synthase activities. The enriched pathways included oxidative phosphorylation, peroxisome function, endoplasmic reticulum protein processing, and ubiquitin-dependent proteolysis, which emphasize cellular homeostasis during cold stress. The marked enrichment of pathways between genotypes reveals the mechanisms underlying differential tolerance. Many lncRNAs regulate genes involved in ROS metabolism, cell membrane stability, and protein quality control through cis- and trans-interactions, thereby coordinating complex networks of stress responses [63]. Notably, the two varieties presented different enrichment patterns, suggesting the existence of specific lncRNA regulatory circuits that may underlie the variation in cold tolerance. The dataset provides prioritized lncRNA candidates for functional validation through cis/trans target analyses and ceRNA interaction studies, offering promising entry points for regulatory genetic improvement and breeding of cold-tolerant eggplant cultivars.
5.8. Cucumber (Cucumis sativus)
The responses of cucumber to coding regulators such as basic pentacysteine 2 (CsBPC2), HRS1 homolog 2 (CsHHO2), and galactinol synthase 1 (CsGolS1) are well understood [64,65,66]. Recently, cucumber lncRNA repertoires exhibiting temperature-sensitive expression profiles, including the downregulation of specific lncRNA expression under cold stress, have been described [43,67]. While a direct functional role for cold-sensitive lncRNAs has yet to be confirmed experimentally, promoter analyses and transcriptome dynamics have identified candidate lncRNAs associated with abscisic acid-, jasmonic acid-, and ROS-related subnetworks involved in cold tolerance. These predicted ceRNA associations link lncRNAs to genes involved in hormone signaling and cell membrane integrity, with several cold-induced transcription factors (e.g., BPC, HHO, and MYB families) thought to interact [43,64,65,66,67]. Combining the potential lncRNAs identified via the omics method with confirmed cold tolerance effectors offers a multilayered improvement strategy to reduce fruit cold damage in the production and supply chain phases.
5.9. Chrysanthemum (Chrysanthemum morifolium)
The natural antisense transcript DglncTCP1, which is transcribed antisensely to the teosinte branched1/cycloidea/proliferating 1 (DgTCP1) gene, has been characterized in chrysanthemum. This lncRNA acts as a scaffold that recruits trithorax-like H3K4 methyltransferase (DgATX), which antagonizes the Polycomb 2 repressive complex. This increases the number of H3K4me3 marks at the DgTCP1 locus, thereby increasing DgTCP1 expression [35]. RNA immunoprecipitation and chromatin isolation confirmed this model. The transcription factor DgTCP1 directly activates DgPOD, which facilitates the removal of ROS. The overexpression of DglncTCP1 or DgTCP1 increases freezing tolerance. In contrast, CRISPR-mediated knockout of DgTCP1 decreases tolerance. These results prove that RNA-mediated chromatin modulation improves cold acclimation in ornamental species [35]. This NAT-mediated chromatin recruitment strategy is promising for enhancing frost hardiness in ornamental plants while minimizing its impact on growth.
5.10. Tea (Camellia sinensis)
In tea, the gradually expanding catalog of non-coding RNAs, including lncRNAs, has been linked to secondary metabolism and responses to temperature stress [68,69]. Transcriptomic analyses revealed that many lncRNAs that are differentially expressed under abiotic stress conditions share signaling components with those under cold stress, such as the regulation of ROS and calcium signaling pathways. For example, the coexpression of specific lncRNAs with calcium-transporting ATPases suggests their involvement in Ca2+ signaling cascades, which are crucial for acclimation to cold [68]. Functional predictions and network analyses suggest that tea lncRNAs modulate gene expression by acting as ceRNAs for microRNAs and interacting with transcription factors to influence hormone signaling pathways relevant to cold tolerance. While direct experimental verification of the function of lncRNAs in tea-induced cold stress is limited, the conserved role of identified lncRNAs in regulating stress-related transcription factors, ROS homeostasis, and metabolic pathways suggests their importance in the cold response [68]. These studies highlight the potential of using tea lncRNA profiles for molecular breeding and improving cold stress resistance. Translational approaches will likely benefit from integrated multimaps and functional assays that elucidate the mechanisms of lncRNA regulation under cold stress.
6. Prospects for Application and Challenges for lncRNA-Based Cold Stress Applications in Horticultural Crops
In light of recent studies, lncRNAs can be key regulators of adaptation to cold stress, coordinating the transcriptional and physiological processes underlying crop resilience. An increasing number of studies from horticultural species suggest that lncRNAs play a role in post-harvest cold tolerance and storage quality by influencing ROS removal pathways and hormonal interactions [70]. The broad regulatory network highlights the function of lncRNAs as dynamic molecular switches, coordinating the multifactorial responses essential for cold acclimation and stress memory. Characterizing them has a dual benefit: it deepens our understanding of cold tolerance and allows for the definition of specific molecular targets for breeding climate-resilient horticultural crops [71].
6.1. Application of Molecular Tools
In horticultural crops, cold-responsive lncRNAs can be organized into RT-qPCR panels and embedded in genetic designs that treat lncRNA abundance as a quantitative trait [72]. Expression quantitative trait loci (eQTLs) and related association frameworks, which are already routine for ripening and texture, can be extended to lncRNAs to identify loci whose expression covaries with chilling injury indices, firmness decay, and antioxidant balance, especially when long reads and allele-aware mapping resolve isoforms [73,74]. Recent resources and studies have explicitly incorporated noncoding transcripts into eQTL/Transcriptome-Wide Association Studies (TWAS) analyses, underscoring the feasibility of this strategy. For candidates with supportive evidence, cis-regulatory edits (promoter or enhancer motifs using base/prime editors) allow dosage tuning without altering the protein-coding sequence. Moreover, CRISPR activation (CRISPRa) systems provide potent transcriptional upregulation from native loci. The CRISPR-Act3.0 platform achieves robust, multiplex activation across Arabidopsis, rice, and tomato and can be coupled to PAM-relaxed nucleases to broaden the target space [75]. Similarly, CRISPRi repression using dCas9-based repressors has matured to reversible gene-circuit toolkits and improved repressor designs, enabling systematic down-tuning and orthogonal controls in planta [76]. Together, these platforms support causal testing of lncRNA-centered hypotheses in relevant tissues and developmental windows. Multiple biostimulant and edible-coating regimes alleviate cold-storage deterioration: MeJA reduces chilling injury across species according to a quantitative meta-analysis; combinations of MeJA or SA with 1-Methylcyclopropene (1-MCP) improve firmness and limit weight loss; and chitosan-based coatings (including nanoformulations) help maintain quality in tomato, mandarin (Citrus reticulata Blanco) and other fruits [77,78,79,80,81]. While direct cause–effect links between these treatments and specific lncRNA modules remain limited, lncRNA expression panels are well-suited for monitoring readouts to evaluate intervention efficacy across cultivars and storage regimens.
6.2. Critical Biological Limitations
Robust inference requires RNA-centered designs that leave local DNA context intact. In plants, CRISPRa can uptune transcription from native promoters (e.g., CRISPR-Act3.0 enables multiplex activation across Arabidopsis, rice, and tomato), whereas CRISPRi using improved dCas9 repressors provides stable downtuning; together, they permit bidirectional tests of function without altering the underlying locus. Trans-rescue from an ectopic site should restore the candidate RNA to confirm specificity because specific transcripts annotated as lncRNAs harbor short open reading frames (sORFs) producing bioactive micropeptides, Ribo-seq and deep proteomics, along with start-codon/frameshift-mutant rescues that preserve RNA structure, are necessary to exclude peptide-driven phenotypes [75,82,83]. Plant lncRNA loci frequently express multiple isoforms with distinct ends, structures, and interactomes. Long-read RNA-seq (PacBio Iso-Seq or ONT DRS) is indispensable in defining the operative isoform(s) before perturbation. Functional tests should target isoform-unique features, splice junctions, polyadenylation signal (PAS)/cleavage sites, or isoform-specific exons, and pair loss-of-function with isoform-matched rescue. Given potential redundancy among paralogous noncoding transcripts or nearby cis-regulatory elements, tiling approaches and multiplex perturbations may be needed to reveal phenotypes of modest effect size [84,85]. Many validated lncRNA effects are organ- and stage-restricted. The signals detected in the leaf during cold acclimation often fail to reproduce in fruit during post-harvest storage. Therefore, experimental designs should consider different time points in ontogeny, tissue types, and genotypes. For hybrids or grafted pairs, allele-aware mapping is necessary to assign signals unambiguously. Under stress conditions, the movement of mRNA from the rootstock to the scion was demonstrated in studies of Cucurbits, implicating mobile transcripts in cold-related responses [86,87]. However, a comprehensive meta-analysis indicated that the global extent of long-distance mRNA communication has likely been overestimated. It emphasizes mapping biases, inadequate genotype controls, and the limited use of reciprocal grafts [88]. Consequently, claims of mobility and exceptionally functional long-distance lncRNA movement should satisfy stringent criteria. These include SNP-aware pipelines, reciprocal grafts, vascular time series, strict k-mer/UMI-based chimera filters, and donor tissue perturbation yielding concordant receiver tissue changes.
6.3. Technological and Translational Barriers
The number of elite cultivars and woody perennials remains difficult to transform. Two classes of regeneration boosters have changed the landscape: morphogenic factors WUSCHEL (WUS) and BABY BOOM (BBM), which promote somatic embryogenesis and markedly increase transformation efficiency in cereals; and Growth-Regulating Factor (GRF)–GRF-Interacting Factor (GIF) chimeras, which accelerate shoot formation and broaden the scope of genotypes in cereals and citrus. Despite these gains, responses remain genotype-dependent and can entail pleiotropic growth effects that must be mitigated by inducible or transient expression [89,90,91]. Plant-tailored CRISPRa/CRISPRi systems now enable the reversible modulation of native loci, for example, CRISPR-Act3.0 for the multiplexed activation of genes, and new dCas9-based repressors that are effective in Arabidopsis and cucumber [75]. These systems support the testing of hypotheses without altering the underlying DNA sequence. Near-PAMless SpRY increases the range of targets in rice and other plants, but can also increase the risk of off-target effects, necessitating high-fidelity variants and careful guide design [82,92,93]. Prime editing continues to improve through the tuning of DNA repair and design rules; however, efficiencies vary across species [94]. Virus-based delivery can bypass recalcitrant tissue culture. It can increase HDR. This is achieved using TRV/PVX/FoMV vectors and rhabdovirus/geminivirus platforms. However, cargo limitations, host range, and inheritance remain key constraints [95,96]. Progress is slowed by inconsistent naming and by overreliance on primary-sequence similarity in cross-species analyses. Plant lncRNAs show low sequence conservation yet often retain positional (syntenic) conservation; comparative inferences should therefore prioritize genomic position, promoter context, and conserved RNA structures over raw nucleotide identity [97]. Adopting coordinate-based identifiers (including strand and isoform tags), aligned with curated resources (PLncDB, GreeNC), would improve portability across studies [98,99]. In parallel, functional annotation should incorporate RNA-binding protein (RBP) interaction maps using plant-adapted ribonomics (e.g., HyperTRIBE, RIP/eCLIP-like workflows) and dsRNA-binding protein profiling, which provide orthogonal evidence that a candidate engages specific protein partners under stress [100,101]. Addressing these barriers requires pairing context-controlled regeneration (WUS/BBM, GRF–GIF) with precision modulation and delivery (CRISPRa/CRISPRi, SpRY, prime editing, viral/replicon systems), while enforcing naming standards that reflect how plant lncRNAs evolve and function. Such alignment will enable rigorous, comparable studies of lncRNAs in cold-stress biology across species and genotypes of horticultural plants [90].
6.4. Outlook and Knowledge Gaps
Three limitations currently constrain robust inference for lncRNAs in the cold-stress response of horticultural plants. First, causality in plants is limited. RNA-centered perturbations that leave the locus intact, i.e., CRISPR activation/repression at native promoters, complemented by antisense or dCas13 approaches, should be coupled to trans-rescue and to the systematic exclusion of peptide effects via Ribo-seq/proteomics and start-codon/frameshift controls. The Arabidopsis SVALKA/SVALNA case illustrates how multi-layer evidence is needed even for canonical cold-induced loci [75,102]. Second, isoform complexity and redundancy are underaddressed. Plant lncRNA loci frequently express multiple isoforms with distinct ends and interaction profiles. Long-read RNA-seq improves the recovery of major isoforms and should precede perturbation; functional tests should target isoform-unique junctions or 3′ ends and pair loss-of-function with isoform-matched rescue. Community-level benchmarks confirm the superior isoform resolution of long reads and highlight the need for consistent reporting standards in plants [85]. Third, portability across organs and the reality of long-distance RNA movement remain uncertain. The signals detected in the leaf during cold acclimation rarely extrapolate to the fruit pericarp during storage; designs should integrate time-resolved sampling and tissue stratification, with allele-aware mapping in hybrids or grafts. For graft systems, convincing long-distance movement of lncRNAs in horticultural species has not been established [88]. Standardization is a cross-cutting need. Because primary-sequence conservation is low, cross-species inference should weight genomic position and synteny, promoter context, and RBP interaction data over raw sequence identity. Adopting coordinate-based, strand- and isoform-aware identifiers and linking them to curated repositories would improve portability and reduce study ambiguity. Differential expression catalogs, qRT-PCR panels, co-expression modules, initial VIGS/RNAi assays, CRISPRa/CRISPRi in model and select crops, and enhancer assessment with STARR-seq are routinely used today. However, isoform-resolved, RNA-centered causality with rescue; multienvironment validation in fruit tissues; rigorous mobility pipelines; harmonized nomenclature/metadata; and broader transformation/delivery solutions in recalcitrant horticultural genotypes still require further development.
7. Conclusions and Perspectives
LncRNAs have emerged as integrative regulators within plant cold signaling networks. These genes link calcium/CAMTA inputs and the ICE1-CBF-COR transcriptional cascade with hormonal pathways, chromatin remodeling, sRNA-mediated interactions, and posttranscriptional regulation. In horticultural species, cold-responsive lncRNAs are consistently associated with modules that control membrane lipid remodeling, ROS homeostasis, and cell wall and cuticle dynamics. These processes collectively influence key quality attributes, such as firmness, coloration, antioxidant capacity, and shelf-life [7,37]. Comprehensive analyses of cold signaling pathways highlight how they intersect and can be fine-tuned during acclimation. This defines regulatory nodes through which lncRNAs modulate the intensity and duration of responses [103,104].
From an applied perspective, two avenues are promising. First, profiling lncRNA expression across tolerant and sensitive cultivars provides quantitative information that complements morphological and eQTL-based trait analyses, offering predictive insights into cold adaptability [37]. Second, transcriptional engineering strategies enable fine-tuned modulation of gene dosage without altering protein-coding capacity. CRISPR activation systems, for example, have been applied to induce CBF4 transcription in grapevine, resulting in reduced electrolyte leakage and increased cold tolerance [105]. These results underscore not only technical feasibility but also the regulatory flexibility of activating key endogenous cold-response hubs. By coupling CRISPRa or CRISPR interference with the manipulation of non-coding loci, such as promoters harboring lncRNA–miRNA interaction sites, it becomes possible to modify stress response kinetics rather than structural gene output. This targeted dosage control provides an experimental framework for testing hypotheses on network resilience and adaptive transcriptional plasticity.
Future progress relies on integrating isoform-resolved transcriptome profiling via long-read RNA sequencing with targeted, RNA-centric perturbation assays and standardized data reporting. This will advance long non-coding RNAs from descriptive indicators toward actionable components in the breeding of cold-resilient, quality-focused horticultural crops [2,7,103,104].
Author Contributions
Conceptualization M.B.; writing—original draft preparation M.W. and M.B.; visualization Y.K. and M.B.; writing—review and editing A.R. and Y.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded in whole by the National Science Centre, Poland, 2021/43/O/NZ9/03108.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Yuan, Q.; Jiang, Y.; Yang, Q.; Li, W.; Gan, G.; Cai, L.; Li, W.; Qin, C.; Yu, C.; Wang, Y. Mechanisms and control measures of low temperature storage-induced chilling injury to solanaceous vegetables and fruits. Front. Plant Sci. 2024, 15, 1488666. [Google Scholar] [CrossRef]
- Qian, Z.; He, L.; Li, F. Understanding cold stress response mechanisms in plants: An overview. Front. Plant Sci. 2024, 15, 1443317. [Google Scholar] [CrossRef]
- Jin, X.; Wang, Z.; Li, X.; Ai, Q.; Wong, D.C.J.; Zhang, F.; Yang, J.; Zhang, N.; Si, H. Current perspectives of lncRNAs in abiotic and biotic stress tolerance in plants. Front. Plant Sci. 2024, 14, 1334620. [Google Scholar] [CrossRef] [PubMed]
- Imaduwage, I.; Hewadikaram, M. Predicted roles of long non-coding RNAs in abiotic stress tolerance responses of plants. Mol. Hortic. 2024, 4, 20. [Google Scholar] [CrossRef]
- Saha, C.; Saha, S.; Bhattacharyya, N.P. LncRNAOmics: A Comprehensive Review of Long Non-Coding RNAs in Plants. Genes 2025, 16, 765. [Google Scholar] [CrossRef]
- Ma, L.; Bajic, V.B.; Zhang, Z. On the classification of long non-coding RNAs. RNA Biol. 2013, 10, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Palos, K.; Yu, L.a.; Railey, C.E.; Nelson Dittrich, A.C.; Nelson, A.D. Linking discoveries, mechanisms, and technologies to develop a clearer perspective on plant long noncoding RNAs. Plant Cell 2023, 35, 1762–1786. [Google Scholar] [CrossRef]
- Ponting, C.P.; Oliver, P.L.; Reik, W. Evolution and functions of long noncoding RNAs. Cell 2009, 136, 629–641. [Google Scholar] [CrossRef]
- Statello, L.; Guo, C.-J.; Chen, L.-L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118, Correction in Nat. Rev. Mol. Cell Biol. 2021, 22, 159. [Google Scholar] [CrossRef]
- Ariel, F.; Romero-Barrios, N.; Jegu, T.; Benhamed, M.; Crespi, M. Battles and hijacks: Noncoding transcription in plants. Trends Plant Sci. 2015, 20, 362–371. [Google Scholar] [CrossRef] [PubMed]
- Chekanova, J.A. Long non-coding RNAs and their functions in plants. Curr. Opin. Plant Biol. 2015, 27, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Kapusta, A.; Feschotte, C. Volatile evolution of long noncoding RNA repertoires: Mechanisms and biological implications. Trends Genet. 2014, 30, 439–452. [Google Scholar] [CrossRef]
- Deng, P.; Liu, S.; Nie, X.; Weining, S.; Wu, L. Conservation analysis of long non-coding RNAs in plants. Sci. China Life Sci. 2018, 61, 190–198. [Google Scholar] [CrossRef]
- Wang, H.V.; Chekanova, J.A. Long Noncoding RNAs in Plants. Adv. Exp. Med. Biol. 2017, 1008, 133–154. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Q.-H.; Kaufmann, K. Long non-coding RNAs in plants: Emerging modulators of gene activity in development and stress responses. Planta 2020, 252, 92. [Google Scholar] [CrossRef]
- Budak, H.; Kaya, S.B.; Cagirici, H.B. Long Non-coding RNA in Plants in the Era of Reference Sequences. Front. Plant Sci. 2020, 11, 276. [Google Scholar] [CrossRef] [PubMed]
- Bhat, S.A.; Najar, M.A.; Wani, A.A.; Qadir, S.; John, R. The Long-noncoding RNAs: Effective players in plant development and stress responses. J. Plant Biochem. Biotechnol. 2025, 34, 35–61. [Google Scholar] [CrossRef]
- Dominguez-Rosas, E.; Hernandez-Onate, M.A.; Fernandez-Valverde, S.L.; Tiznado-Hernandez, M.E. Plant long non-coding RNAs: Identification and analysis to unveil their physiological functions. Front. Plant Sci. 2023, 14, 1275399. [Google Scholar] [CrossRef]
- Gonzales, L.R.; Blom, S.; Henriques, R.; Bachem, C.W.; Immink, R.G. LncRNAs: The art of being influential without protein. Trends Plant Sci. 2024, 29, 770–785. [Google Scholar] [CrossRef]
- Chorostecki, U.; Bologna, N.G.; Ariel, F. The plant noncoding transcriptome: A versatile environmental sensor. EMBO J. 2023, 42, e114400. [Google Scholar] [CrossRef]
- Li, T.; Yuan, L.; Yin, X.; Jiang, X.; Wei, Y.; Tang, X.; Li, N.; Liu, Q. The Grapevine Transcription Factor VvTGA8 Enhances Resistance to White Rot via the Salicylic Acid Signaling Pathway in Tomato. Agronomy 2023, 13, 3054. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Gao, Z.; Wang, F.; Xu, T.; Qi, M.; Liu, Y.; Li, T. MicroRNA162 regulates stomatal conductance in response to low night temperature stress via abscisic acid signaling pathway in tomato. Front. Plant Sci. 2023, 14, 1045112. [Google Scholar] [CrossRef]
- Chowdhury, M.; Chatterjee, C.; Ghosh, D.; Mukherjee, J.; Shaw, S.; Basak, J. Deciphering miRNA-lncRNA-mRNA interaction through experimental validation of miRNAs, lncRNAs, and miRNA targets on mRNAs in Cajanus cajan. Plant Biol. 2024, 26, 560–567. [Google Scholar] [CrossRef]
- Wang, J.; Yu, W.; Yang, Y.; Li, X.; Chen, T.; Liu, T.; Ma, N.; Yang, X.; Liu, R.; Zhang, B. Genome-wide analysis of tomato long non-coding RNAs and identification as endogenous target mimic for microRNA in response to TYLCV infection. Sci. Rep. 2015, 5, 16946, Correction in Sci. Rep. 2016, 6, 32828. [Google Scholar] [CrossRef]
- Calixto, C.P.; Tzioutziou, N.A.; James, A.B.; Hornyik, C.; Guo, W.; Zhang, R.; Nimmo, H.G.; Brown, J.W. Cold-dependent expression and alternative splicing of Arabidopsis long non-coding RNAs. Front. Plant Sci. 2019, 10, 235. [Google Scholar] [CrossRef]
- Wang, Y.; Luo, X.; Sun, F.; Hu, J.; Zha, X.; Su, W.; Yang, J. Overexpressing lncRNA LAIR increases grain yield and regulates neighbouring gene cluster expression in rice. Nat. Commun. 2018, 9, 3516. [Google Scholar] [CrossRef]
- Mattick, J.S.; Amaral, P.P.; Carninci, P.; Carpenter, S.; Chang, H.Y.; Chen, L.-L.; Chen, R.; Dean, C.; Dinger, M.E.; Fitzgerald, K.A. Long non-coding RNAs: Definitions, functions, challenges and recommendations. Nat. Rev. Mol. Cell Biol. 2023, 24, 430–447. [Google Scholar] [CrossRef] [PubMed]
- Białoskórska, M.; Rucińska, A.; Boczkowska, M. Molecular mechanisms underlying freezing tolerance in plants: Implications for cryopreservation. Int. J. Mol. Sci. 2024, 25, 10110. [Google Scholar] [CrossRef]
- Ding, Y.; Li, H.; Zhang, X.; Xie, Q.; Gong, Z.; Yang, S. OST1 kinase modulates freezing tolerance by enhancing ICE1 stability in Arabidopsis. Dev. Cell 2015, 32, 278–289. [Google Scholar] [CrossRef]
- Li, H.; Ding, Y.; Shi, Y.; Zhang, X.; Zhang, S.; Gong, Z.; Yang, S. MPK3-and MPK6-mediated ICE1 phosphorylation negatively regulates ICE1 stability and freezing tolerance in Arabidopsis. Dev. Cell 2017, 43, 630–642.e634. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, X.; Song, C.-P.; Gong, Z.; Yang, S.; Ding, Y. PUB25 and PUB26 dynamically modulate ICE1 stability via differential ubiquitination during cold stress in Arabidopsis. Plant Cell 2023, 35, 3585–3603. [Google Scholar] [CrossRef]
- Wu, S.; Hu, C.; Zhang, S.; Deng, G.; Sheng, O.; Dou, T.; Bi, F.; He, W.; Dong, T.; Li, C. Lipid metabolism and MAPK-ICE1 cascade play crucial roles in cold tolerance of banana. Hortic. Adv. 2024, 2, 8. [Google Scholar] [CrossRef]
- Zhang, L.; Jiang, X.; Liu, Q.; Ahammed, G.J.; Lin, R.; Wang, L.; Shao, S.; Yu, J.; Zhou, Y. The HY5 and MYB15 transcription factors positively regulate cold tolerance in tomato via the CBF pathway. Plant Cell Environ. 2020, 43, 2712–2726. [Google Scholar] [CrossRef]
- Kiger, N.M.; Schroeder, S.J. SVALKA: A Long Noncoding Cis-Natural Antisense RNA That Plays a Role in the Regulation of the Cold Response of Arabidopsis thaliana. Non-Coding RNA 2024, 10, 59. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, Q.; Liao, X.; Tian, Y.; Zhang, F.; Zhang, L.; Liu, Q. A natural antisense RNA improves chrysanthemum cold tolerance by regulating the transcription factor DgTCP1. Plant Physiol. 2022, 190, 605–620. [Google Scholar] [CrossRef]
- Jiang, N.; Cui, J.; Shi, Y.; Yang, G.; Zhou, X.; Hou, X.; Meng, J.; Luan, Y. Tomato lncRNA23468 functions as a competing endogenous RNA to modulate NBS-LRR genes by decoying miR482b in the tomato-Phytophthora infestans interaction. Hortic. Res. 2019, 6, 28. [Google Scholar] [CrossRef]
- Zhao, X.; Li, F.; Ali, M.; Li, X.; Fu, X.; Zhang, X. Emerging roles and mechanisms of lncRNAs in fruit and vegetables. Hortic. Res. 2024, 11, uhae046. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, R.M.; Picard, C.L. RNA-directed DNA methylation. PLoS Genet. 2020, 16, e1009034. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Huang, H.; Bradai, M.; Zhao, C.; You, Y.; Ma, J.; Zhao, L.; Lozano-Durán, R.; Zhu, J.-K. DNA methylation-free Arabidopsis reveals crucial roles of DNA methylation in regulating gene expression and development. Nat. Commun. 2022, 13, 1335. [Google Scholar] [CrossRef]
- Faivre, L.; Kinscher, N.-F.; Kuhlmann, A.B.; Xu, X.; Kaufmann, K.; Schubert, D. Cold stress induces rapid gene-specific changes in the levels of H3K4me3 and H3K27me3 in Arabidopsis thaliana. Front. Plant Sci. 2024, 15, 1390144. [Google Scholar] [CrossRef]
- Fonouni-Farde, C.; Christ, A.; Blein, T.; Legascue, M.F.; Ferrero, L.; Moison, M.; Lucero, L.; Ramírez-Prado, J.S.; Latrasse, D.; Gonzalez, D. The Arabidopsis APOLO and human UPAT sequence-unrelated long noncoding RNAs can modulate DNA and histone methylation machineries in plants. Genome Biol. 2022, 23, 181. [Google Scholar] [CrossRef]
- McDonald, B.R.; Picard, C.L.; Brabb, I.M.; Savenkova, M.I.; Schmitz, R.J.; Jacobsen, S.E.; Duttke, S.H. Enhancers associated with unstable RNAs are rare in plants. Nat. Plants 2024, 10, 1246–1257. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.-W.; Song, C.-J.; Zheng, X.-F.; Zhang, Y.; Anwar, W.; Dissanayaka, D.D.; Zhu, P.-Z.; Liu, J.-L.; Lai, Y.-S. Identification and characterization of long non-coding RNA (lncRNA) in wild and semi-wild cucumbers. BMC Plant Biol. 2025, 25, 748. [Google Scholar]
- Ajila, V.; Colley, L.; Ste-Croix, D.T.; Nissan, N.; Golshani, A.; Cober, E.R.; Mimee, B.; Samanfar, B.; Green, J.R. P-TarPmiR accurately predicts plant-specific miRNA targets. Sci. Rep. 2023, 13, 332. [Google Scholar] [CrossRef]
- Thody, J.; Moulton, V.; Mohorianu, I. PAREameters: A tool for computational inference of plant miRNA–mRNA targeting rules using small RNA and degradome sequencing data. Nucleic Acids Res. 2020, 48, 2258–2270. [Google Scholar] [CrossRef] [PubMed]
- Deragon, J.-M.; Merret, R. Co-Translational mRNA Decay in Plants: Recent advances and future directions. J. Exp. Bot. 2025, eraf146. [Google Scholar] [CrossRef]
- Xiao, Y.; Ren, Y.; Hu, W.; Paliouras, A.R.; Zhang, W.; Zhong, L.; Yang, K.; Su, L.; Wang, P.; Li, Y. Long non-coding RNA-encoded micropeptides: Functions, mechanisms and implications. Cell Death Discov. 2024, 10, 450. [Google Scholar] [CrossRef]
- Wang, P.; Dai, L.; Ai, J.; Wang, Y.; Ren, F. Identification and functional prediction of cold-related long non-coding RNA (lncRNA) in grapevine. Sci. Rep. 2019, 9, 6638. [Google Scholar] [CrossRef]
- Ma, W.; Ma, L.; Ma, Z.; Li, W.; Lu, S.; Gou, H.; Mao, J.; Chen, B. Profiling the lncRNA–miRNA–mRNA interaction network in the cold-resistant exercise period of grape (Vitis amurensis Rupr.). Chem. Biol. Technol. Agric. 2024, 11, 143. [Google Scholar] [CrossRef]
- Lai, R.; Wu, X.; Feng, X.; Gao, M.; Long, Y.; Wu, R.; Cheng, C.; Chen, Y. Identification and characterization of long non-coding RNAs: Implicating insights into their regulatory role in kiwifruit ripening and softening during low-temperature storage. Plants 2023, 12, 1070. [Google Scholar] [CrossRef]
- Bordoloi, K.S.; Baruah, P.M.; Das, M.; Agarwala, N. Unravelling lncRNA mediated gene expression as potential mechanism for regulating secondary metabolism in Citrus limon. Food Biosci. 2022, 46, 101448. [Google Scholar] [CrossRef]
- Ke, L.; Zhou, Z.; Xu, X.W.; Wang, X.; Liu, Y.; Xu, Y.; Huang, Y.; Wang, S.; Deng, X.; Chen, L.L. Evolutionary dynamics of linc RNA transcription in nine citrus species. Plant J. 2019, 98, 912–927. [Google Scholar] [CrossRef]
- Hu, X.-L.; You, C.; Zhu, K.; Li, X.; Gong, J.; Ma, H.; Sun, X. Nanopore long-read RNAseq reveals transcriptional variations in citrus species. Front. Plant Sci. 2023, 13, 1077797. [Google Scholar] [CrossRef] [PubMed]
- Primo-Capella, A.; Martínez-Cuenca, M.-R.; Forner-Giner, M.Á. Cold stress in Citrus: A molecular, physiological and biochemical perspective. Horticulturae 2021, 7, 340. [Google Scholar] [CrossRef]
- Moh, N.M.M.; Zhang, P.; Chen, Y.; Chen, M. Computational identification of miRNAs and temperature-responsive lncRNAs from mango (Mangifera indica L.). Front. Genet. 2021, 12, 607248. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Wang, H.; Guo, D.; Wang, B.; Zhang, X.; Wang, J.; Liu, Y.; Wang, X.; Liu, C.; Dong, W. Integrated Transcriptomic and Proteomic Analysis Reveals Molecular Mechanisms of the Cold Stress Response during the Overwintering Period in Blueberries (Vaccinium spp.). Plants 2024, 13, 1911. [Google Scholar] [CrossRef]
- Li, S.; Zhang, J.; Zhang, L.; Fang, X.; Luo, J.; An, H.; Zhang, X. Genome-wide identification and comprehensive analysis reveal potential roles of long non-coding RNAs in fruit development of southern highbush blueberry (Vaccinium corymbosum L.). Front. Plant Sci. 2022, 13, 1078085. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, L.; Zhu, B.; Zhu, H.; Luo, Y.; Wang, Q.; Zuo, J. Integrative analysis of long non-coding RNA acting as ceRNAs involved in chilling injury in tomato fruit. Gene 2018, 667, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Wang, Q.; Han, C.; Ju, Z.; Cao, D.; Zhu, B.; Luo, Y.; Gao, L. SRNAome and degradome sequencing analysis reveals specific regulation of sRNA in response to chilling injury in tomato fruit. Physiol. Plant. 2017, 160, 142–154. [Google Scholar] [CrossRef]
- Cao, X.; Wu, Z.; Jiang, F.; Zhou, R.; Yang, Z. Identification of chilling stress-responsive tomato microRNAs and their target genes by high-throughput sequencing and degradome analysis. BMC Genom. 2014, 15, 1130. [Google Scholar] [CrossRef]
- Guo, M.; Yang, F.; Zhu, L.; Wang, L.; Li, Z.; Qi, Z.; Fotopoulos, V.; Yu, J.; Zhou, J. Loss of cold tolerance is conferred by absence of the WRKY34 promoter fragment during tomato evolution. Nat. Commun. 2024, 15, 6667, Correction in Nat. Commun. 2024, 15, 8267. [Google Scholar] [CrossRef]
- Li, N.; Wang, Z.; Wang, B.; Wang, J.; Xu, R.; Yang, T.; Huang, S.; Wang, H.; Yu, Q. Identification and characterization of long non-coding RNA in tomato roots under salt stress. Front. Plant Sci. 2022, 13, 834027. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhang, J.; Jun, L.; Xiaohui, Z.; Liang, S.; Songyu, L.; Zhuang, Y. Global identification and functional prediction of cold-related lncRNAs in eggplant. Not. Bot. Horti Agrobot. Cluj-Napoca 2022, 50, 12931. [Google Scholar]
- Meng, D.; Li, S.; Feng, X.; Di, Q.; Zhou, M.; Yu, X.; He, C.; Yan, Y.; Wang, J.; Sun, M. CsBPC2 is essential for cucumber survival under cold stress. BMC Plant Biol. 2023, 23, 566. [Google Scholar] [CrossRef]
- Wang, B.; Wang, G.; Wang, Y.; Jiang, Y.; Zhu, Y.; He, J.; Zhu, S. A cold-inducible MYB like transcription factor, CsHHO2, positively regulates chilling tolerance of cucumber fruit by enhancing CsGR-RBP3 expression. Postharvest Biol. Technol. 2024, 218, 113172. [Google Scholar]
- Dai, H.; Zhu, Z.; Wang, Z.; Zhang, Z.; Kong, W.; Miao, M. Galactinol synthase 1 improves cucumber performance under cold stress by enhancing assimilate translocation. Hortic. Res. 2022, 9, uhab063. [Google Scholar] [CrossRef]
- He, X.; Guo, S.; Wang, Y.; Wang, L.; Shu, S.; Sun, J. Systematic identification and analysis of heat-stress-responsive lncRNAs, circRNAs and miRNAs with associated co-expression and ceRNA networks in cucumber (Cucumis sativus L.). Physiol. Plant. 2020, 168, 736–754. [Google Scholar] [CrossRef]
- Baruah, P.M.; Agarwala, N.; Bordoloi, K.S.; Regon, P.; Tanti, B. Long Non-Coding RNAs Responsive to Temperature Stress Conditions in Tea Plants. J. Plant Growth Regul. 2024, 44, 1728–1752. [Google Scholar] [CrossRef]
- Zhou, C.; Tian, C.; Zhu, C.; Lai, Z.; Lin, Y.; Guo, Y. Hidden players in the regulation of secondary metabolism in tea plant: Focus on non-coding RNAs. Beverage Plant Res. 2022, 2, 1–12. [Google Scholar] [CrossRef]
- Huo, C.; Zhang, B.; Wang, R. Research progress on plant noncoding RNAs in response to low-temperature stress. Plant Signal. Behav. 2022, 17, 2004035. [Google Scholar] [CrossRef] [PubMed]
- Lal, M.K.; Kumar, R.; Tiwari, R.K.; Kumar, A.; Ghorbani, A.; Pehlivan, N.; Zargar, M. Mechanisms of stress tolerance in horticultural crops: Physiological and molecular insights. Front. Plant Sci. 2023, 16, 1664603. [Google Scholar]
- Yang, Y.; Wang, D.; Miao, Y.-R.; Wu, X.; Luo, H.; Cao, W.; Yang, W.; Yang, J.; Guo, A.-Y.; Gong, J. lncRNASNP v3: An updated database for functional variants in long non-coding RNAs. Nucleic Acids Res. 2023, 51, D192–D198. [Google Scholar] [CrossRef]
- Zhao, S.; Bai, H.; Fan, Z.; Zhu, M.; Qiu, Z. A long non-coding RNA lncRNA18313 regulates resistance against cadmium stress in wheat. Front. Plant Sci. 2025, 16, 1583758. [Google Scholar] [CrossRef]
- Chen, X.; Chen, Z.; Watts, R.; Luo, H. Non-coding RNAs in plant stress responses: Molecular insights and agricultural applications. Plant Biotechnol. J. 2025, 23, 3195–3233. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Wu, X.; Markel, K.; Malzahn, A.A.; Kundagrami, N.; Sretenovic, S.; Zhang, Y.; Cheng, Y.; Shih, P.M.; Qi, Y. CRISPR–Act3. 0 for highly efficient multiplexed gene activation in plants. Nat. Plants 2021, 7, 942–953. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.; Herring, G.; Zhu, J.Y.; Oliva, M.; Fourie, E.; Johnston, B.; Zhang, Z.; Potter, J.; Pineda, L.; Pflueger, J. CRISPRi-based circuits to control gene expression in plants. Nat. Biotechnol. 2025, 43, 416–430. [Google Scholar] [CrossRef] [PubMed]
- Min, D.; Li, F.; Ali, M.; Zhang, X.; Liu, Y. Application of methyl jasmonate to control chilling tolerance of postharvest fruit and vegetables: A meta-analysis and eliciting metabolism review. Crit. Rev. Food Sci. Nutr. 2024, 64, 12878–12891. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, Y.; Chen, Q.; Yin, F.; Song, M.; Cai, W.; Shuai, L. Methyl jasmonate treatment alleviates chilling injury and improves antioxidant system of okra pod during cold storage. Food Sci. Nutr. 2023, 11, 2049–2060. [Google Scholar] [CrossRef]
- Sen, F.; Yilmaz, E.; Ozturk, B. Effects of 1-methylcyclopropene, methyl jasmonate and salicylic acid on physicochemical properties and wooliness of nectarine fruit during cold storage. BMC Plant Biol. 2024, 24, 1205. [Google Scholar] [CrossRef]
- Lorente-Mento, J.M.; Serrano, M.; Martínez-Romero, D.; Ruiz-Aracil, M.C.; Valero, D.; Guillén, F. The simultaneous use of 1-methylcyclopropene and methyl jasmonate vapor as an innovative strategy for reducing chilling injury and maintaining pomegranate fruit quality at suboptimal temperatures. Foods 2023, 13, 60. [Google Scholar] [CrossRef]
- Al Zahrani, N.A.; Gad, M.M.; Fikry, A.M.; Ahmed, A.E.; El-Tarabily, K.A.; Elakkad, H.A.; Elesawi, I.E. Efficacy of chitosan nanoparticles and wax coatings on maintaining post-harvest quality of “Murcott” mandarins. Saudi J. Biol. Sci. 2024, 31, 103894. [Google Scholar] [CrossRef]
- Selma, S. Crisp and quiet: A novel programmable transcriptional repressor in plants. Plant Physiol. 2024, 195, 1748–1750. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.-Y.L.; Ai, Q.; Teixeira, R.T.; Nguyen, P.H.; Song, G.; Montes, C.; Elmore, J.M.; Walley, J.W.; Hsu, P.Y. Improved super-resolution ribosome profiling reveals prevalent translation of upstream ORFs and small ORFs in Arabidopsis. Plant Cell 2024, 36, 510–539. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.-X.; Xiang, L.; Zhao, H.-M.; Yang, L.-Q.; Chen, Z.-C.; Pu, Y.-Q.; Li, Y.-W.; Luo, B.; Cai, Q.-Y.; Liu, B.-L. High-throughput single-molecule long-read RNA sequencing analysis of tissue-specific genes and isoforms in lettuce (Lactuca sativa L.). Commun. Biol. 2024, 7, 920. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Davidson, N.M.; Wan, Y.K.; Yao, F.; Su, Y.; Gamaarachchi, H.; Sim, A.; Patel, H.; Low, H.M.; Hendra, C. A systematic benchmark of Nanopore long-read RNA sequencing for transcript-level analysis in human cell lines. Nat. Methods 2025, 22, 801–812. [Google Scholar] [CrossRef]
- Liu, W.; Xiang, C.; Li, X.; Wang, T.; Lu, X.; Liu, Z.; Gao, L.; Zhang, W. Identification of long-distance transmissible mRNA between scion and rootstock in cucurbit seedling heterografts. Int. J. Mol. Sci. 2020, 21, 5253. [Google Scholar] [CrossRef]
- Davoudi, M.; Song, M.; Zhang, M.; Chen, J.; Lou, Q. Long-distance control of the scion by the rootstock under drought stress as revealed by transcriptome sequencing and mobile mRNA identification. Hortic. Res. 2022, 9, uhab033. [Google Scholar] [CrossRef]
- Paajanen, P.; Tomkins, M.; Hoerbst, F.; Veevers, R.; Heeney, M.; Thomas, H.R.; Apelt, F.; Saplaoura, E.; Gupta, S.; Frank, M. Re-analysis of mobile mRNA datasets raises questions about the extent of long-distance mRNA communication. Nat. Plants 2025, 11, 977–984. [Google Scholar] [CrossRef]
- Aesaert, S.; Impens, L.; Coussens, G.; Van Lerberge, E.; Vanderhaeghen, R.; Desmet, L.; Vanhevel, Y.; Bossuyt, S.; Wambua, A.N.; Van Lijsebettens, M. Optimized transformation and gene editing of the B104 public maize inbred by improved tissue culture and use of morphogenic regulators. Front. Plant Sci. 2022, 13, 883847. [Google Scholar] [CrossRef]
- Jiang, Y.; Liu, S.; An, X. Functional Mechanisms and the Application of Developmental Regulators for Improving Genetic Transformation in Plants. Plants 2024, 13, 2841. [Google Scholar] [CrossRef]
- Debernardi, J.M.; Tricoli, D.M.; Ercoli, M.F.; Hayta, S.; Ronald, P.; Palatnik, J.F.; Dubcovsky, J. A GRF–GIF chimeric protein improves the regeneration efficiency of transgenic plants. Nat. Biotechnol. 2020, 38, 1274–1279. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Kuang, Y.; Ren, B.; Yan, D.; Yan, F.; Spetz, C.; Sun, W.; Wang, G.; Zhou, X.; Zhou, H. SpRY greatly expands the genome editing scope in rice with highly flexible PAM recognition. Genome Biol. 2021, 22, 6. [Google Scholar] [CrossRef]
- Asano, Y.; Yamashita, K.; Hasegawa, A.; Ogasawara, T.; Iriki, H.; Muramoto, T. Knock-in and precise nucleotide substitution using near-PAMless engineered Cas9 variants in Dictyostelium discoideum. Sci. Rep. 2021, 11, 11163. [Google Scholar] [CrossRef]
- Vats, S.; Kumar, J.; Sonah, H.; Zhang, F.; Deshmukh, R. Prime editing in plants: Prospects and challenges. J. Exp. Bot. 2024, 75, 5344–5356. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.; Li, X.; Zhang, R.; Li, J. Virus-induced gene editing and its applications in plants. Int. J. Mol. Sci. 2022, 23, 10202. [Google Scholar] [CrossRef]
- Shen, Y.; Ye, T.; Li, Z.; Kimutai, T.H.; Song, H.; Dong, X.; Wan, J. Exploiting viral vectors to deliver genome editing reagents in plants. Abiotech 2024, 5, 247–261. [Google Scholar] [CrossRef]
- Szcześniak, M.W.; Kubiak, M.R.; Wanowska, E.; Makałowska, I. Comparative genomics in the search for conserved long noncoding RNAs. Essays Biochem. 2021, 65, 741–749. [Google Scholar] [CrossRef]
- Jin, J.; Lu, P.; Xu, Y.; Li, Z.; Yu, S.; Liu, J.; Wang, H.; Chua, N.-H.; Cao, P. PLncDB V2. 0: A comprehensive encyclopedia of plant long noncoding RNAs. Nucleic Acids Res. 2021, 49, D1489–D1495. [Google Scholar] [CrossRef] [PubMed]
- Di Marsico, M.; Paytuvi Gallart, A.; Sanseverino, W.; Aiese Cigliano, R. GreeNC 2.0: A comprehensive database of plant long non-coding RNAs. Nucleic Acids Res. 2022, 50, D1442–D1447. [Google Scholar] [CrossRef]
- Yin, S.; Chen, Y.; Chen, Y.; Xiong, L.; Xie, K. Genome-wide profiling of rice Double-stranded RNA-Binding Protein 1–associated RNAs by targeted RNA editing. Plant Physiol. 2023, 192, 805–820. [Google Scholar] [CrossRef] [PubMed]
- Haroon, M.; Afzal, R.; Zafar, M.M.; Zhang, H.; Li, L. Ribonomics approaches to identify RBPome in plants and other eukaryotes: Current progress and future prospects. Int. J. Mol. Sci. 2022, 23, 5923. [Google Scholar] [CrossRef] [PubMed]
- Zacharaki, V.; Meena, S.K.; Kindgren, P. The non-coding RNA SVALKA locus produces a cis-natural antisense transcript that negatively regulates the expression of CBF1 and biomass production at normal temperatures. Plant Commun. 2023, 4, 100551. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Shi, Y.; Yang, S. Regulatory networks underlying plant responses and adaptation to cold stress. Annu. Rev. Genet. 2024, 58. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-S.; Kidokoro, S.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Regulatory networks in plant responses to drought and cold stress. Plant Physiol. 2024, 195, 170–189. [Google Scholar] [CrossRef]
- Ren, C.; Li, H.; Liu, Y.; Li, S.; Liang, Z. Highly efficient activation of endogenous gene in grape using CRISPR/dCas9-based transcriptional activators. Hortic. Res. 2022, 9, uhab037. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).