Abstract
Patients with metastatic lung adenocarcinoma (mADC) harboring EGFR-activating mutations can benefit from first-line Osimertinib, but acquired resistance inevitably occurs. Different resistance mechanisms, on- and off-target, have been described. Here, we evaluated the prevalence of phenotypic transformation as a resistance mechanism in a consecutive series of EGFR-mutated mADC, diagnosed at our institution, and on the basis of literature data. A consecutive 3-year series of non-small cell lung cancer (NSCLC) was reviewed according to histological and molecular characteristics. A total of 100 mADCs harboring EGFR exon-19 deletions (61 cases) and the p.(L858R) mutation (39 cases) were selected. All cases were treated by first-line Osimertinib. The prevalence and type of phenotypic transformation were evaluated in patients with available rebiopsy at the time of first-line progression. A total of 32 mADC patients underwent rebiopsy upon first-line Osimertinib progression, and 23 cases had EGFR exon-19 in-frame deletions and 9 p.(L858R) mutations. Four cases showed a phenotypic transformation after a median of 15 months from the start of Osimertinib treatment. All these cases harbored EGFR exon-19 deletions and TP53 pathogenic mutations on diagnostic tumor tissues. Three cases switched to small cell lung cancer histology; in one case, a MET amplification was also detected on rebiopsy. One case changed to spindle cell carcinoma. All cases maintained the initial activating EGFR alteration. For three cases, liquid biopsy was performed at the time of progression: one was negative, one presented only an EGFR exon-19 deletion, and one presented only a MET amplification. In our study, phenotypic transformation had a considerable prevalence among EGFR-positive mADC patients treated by first-line Osimertinib. Different types of histological changes were detected as the only resistance mechanism except for one case with a simultaneously acquired MET amplification. Moreover, all cases harbored TP53 alterations, influencing treatment response. Despite the usefulness of liquid biopsy, rebiopsy should be executed whenever possible. Indeed, it remains the only tool for assessing histological transformation, which greatly impacts prognosis and treatment decisions.
1. Introduction
Lung cancer is one of the main causes of cancer-related deaths worldwide [1]. Non-small cell lung cancer (NSCLC) accounts for 85–90% of all lung cancers, while small cell lung cancer (SCLC) only accounts for 10–15% [2]. According to its histopathological features, NSCLC can be divided into three major types: adenocarcinoma (ADC), squamous cell carcinoma (SCC), and large cell carcinoma. Less common types of NSCLC include large cell neuroendocrine carcinoma (LCNEC), sarcomatoid carcinoma, adenosquamous carcinoma, and salivary gland carcinoma [2]. ADC is the most common histotype, representing 50–60% of all NSCLCs. About 70% of lung ADC cases are diagnosed in the advanced/metastatic stages [3]. The identification and understanding of “driver mutations” in ADC led to the development of molecularly targeted therapies, particularly for advanced/metastatic tumors. Currently, several tyrosine kinase inhibitors (TKIs) are approved for the first or subsequent treatment lines of lung ADC, requiring the molecular testing of actionable alterations within the following genes: epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), B-Raf proto-oncogene (BRAF), ROS proto-oncogene 1, receptor tyrosine kinase (ROS1), ret proto-oncogene (RET), neurotrophic tyrosine receptor kinase (NTRK1, NTRK2, and NTRK3), MET proto-oncogene (MET), Kirsten rat sarcoma (KRAS), and human epidermal growth factor receptor 2 (HER2) [4]. Approximately 20–30% of lung ADC harbor driver mutations in the EGFR gene. EGFR encodes for a growth factor receptor that induces cell differentiation and proliferation through the activation of a tyrosine kinase signaling pathway [5,6]. About 90% of reported EGFR activating mutations are represented by in-frame deletions in exon 19 and the p.(L858R) point mutation in exon 21 [7].
Among resistance mechanisms, histological transformation is a process characterized by major remodeling of the cytoskeleton by the epithelial-to-mesenchymal transition process with a key role in tumor transformation, invasion, and metastasis. Particularly, phenotypic transformation from ADC to SCLC or SCC was reported in 2–15% of patients who progressed during treatment with Osimertinib [5,8,9].
This study aims to evaluate the prevalence of phenotypic transformation in a 3-year unselected consecutive series of metastatic EGFR mutated lung ADC diagnosed at our institution and treated by first-line Osimertinib. Moreover, literature data about histological transformation of NSCLCs after TKI treatment were reviewed.
1.1. Histological Transformation: A Literature Review
1.1.1. Histological Transformation of EGFR-Mutated ADCs to SCLC
Histological transformation into SCLC is the most common type during TKI treatment [10]. SCLC is highly aggressive with a very poor prognosis accounting for 10–15% of all lung cancers [2]. Several studies reported that 3–14% of EGFR-mutated ADCs undergo phenotypic transformation into SCLC as a mechanism of resistance to EGFR-TKI (Table 1). The median transformation time from the start of treatment is about 18 months [11,12]. Histological examination and neuroendocrine markers evaluation on tumor tissue biopsy are crucial for transformed SCLC (tSCLC) diagnosis [13]. The molecular mechanisms behind this transformation are still unclear; however, two possible hypotheses have been suggested. According to the first one, transformation occurs by the trans-differentiation of primary adenocarcinoma cells into SCLC cells during EGFR-TKI treatment. Whereas in the second hypothesis, transformation occurs from pre-existing, dominant SCLC cells under the selection pressure of EGFR-TKIs [14]. Pre- and post-therapy samples were molecularly analyzed to better characterize this phenotypic change. For instance, it has been widely reported that tSCLC tumors often retain the original EGFR-activating mutation [14]. Another relevant issue is the potential impact of co-occurring gene mutations on phenotypic transformation. Among the most prevalent molecular mechanisms involved in NSCLC to SCLC transformation, there are tumor protein p53 (TP53) mutations, retinoblastoma 1 (RB1) loss, lack of EGFR expression and MYC amplification [15]. According to recent studies, concurrent TP53 and RB1 alterations are the most frequent in EGFR-mutated tSCLC [14,16,17]. To date, it has not been fully elucidated whether loss of function mutations in RB1 and TP53 are an early event in EGFR-mutant tumors or an acquired event occurring later in the histological change process [17]. Offin et al. hypothesized that EGFR-mutant lung cancers with RB1/TP53 alterations can be associated with a high risk of SCLC transformation [18]. Studies focused on the Notch signal pathway, a key player in transdifferentiating processes, have shown that alterations in its regulation cause a loss of RB1. In the presence of TP53 mutations, RB1 loss may be involved in neuroendocrine differentiation in non-neuroendocrine tumor tissues [15]. tSCLC is characterized by an unfavorable prognosis, and an interval of more than 12 months between NSCLC diagnosis and SCLC transformation has been associated with longer survival than cases with earlier SCLC transformations [19].

Table 1.
Characteristics of described EGFR-mutated lung ADCs transformed to SCLC under Osimertinib treatment.
Despite the diagnosis of histological transformation requiring a rebiopsy, liquid biopsy provides an impressive amount of data, potentially correlating to the transformation process, even if no validated biomarkers are yet available [5].
1.1.2. Histological Transformation of Pre- and Post-Therapy Samples—Mutated ADCs to SCC
Histological transformation from ADC to SCC is rare but may occur after treatment with EGFR-TKIs [26]. Pulmonary ADC and SCC have different characteristics regarding origin cells and tumor localization. Lung ADCs originate from type II pneumocytes or club cells, while lung SCCs derive from basal cells located underneath the trachea or bronchus epithelium. Moreover, ADCs are frequently located at distal bronchioles, whereas SCCs are often found at more proximal airways [27]. Squamous cell transformation (tSCC) has been reported in 1.1–14% of EGFR-positive ADCs, developing resistance to TKIs [28]. Song et al., in a retrospective cohort of 233 EGFR-mutated patients, showed that 11% of cases developed histological transformation, 42% were tSCC and transformation occurred 19–20 months after therapy start [29]. The most common mutations involved in tSCC are within EGFR/RB1/TP53 genes; but also APC/MED12/RBL2 alterations can contribute to this histological change [30]. Recent studies using a KRAS p.(G12D) mutation-positive cell line and a mouse model have indicated that the deletion of liver kinase B1 (LKB1) may be closely related to tSCC [31]. tSCC rarely responds to EGFR-TKI therapy, independently of its driver mutation, and it has a worse prognosis. The median post-transformation survival is about 12 months, while after SCC transformation, it is about 17 months [32].
1.1.3. Histological Transformation of EGFR-Mutated ADCs to LCNET
LCNET is an uncommon subtype of malignant pulmonary tumor with a prevalence of 0.3–3% [33]. Histological transformation from EGFR-mutated ADC to LCNEC, as a mechanism of resistance to EGFR-TKI, is extremely rare (about 0.1% of patients) [34]. Despite its rarity, in all reported transformed cases, the initial EGFR activating mutation is retained, suggesting an origin from a previously present EGFR-dependent clone [35,36,37,38]. In tLCNET, EGFR protein expression appears to be suppressed, especially in cases with concurrent RB1 and TP53 mutations, as for tSCLC [37]. Although further studies are necessary, the detection of RB1 and TP53 co-occurring alterations in diagnostic specimens could be useful to better monitor tumor progression and clinical outcome. [37].
1.1.4. Histological Transformation of EGFR-Mutated ADCs to Sarcomatoid Carcinoma
Sarcomatoid carcinoma is a rare subtype of NSCLC with a prevalence of about 0.4% [39]. The histological transformation from NSCLC to sarcomatoid carcinoma occurs in 2.5–4.8% of patients after failure of treatment with first/second and third-generation EGFR-TKIs [39]. TP53 mutations, RB1 inactivation, MET over-expression, and PI3K/AKT/mTOR pathway activation are frequently observed [40]. The sarcomatous transformation represents the process whereby the neoplastic cell acquires mutations responsible for the epithelial–mesenchymal transition (EMT). Most of these mutations occur within genes encoding for transcriptional factors involved in regulatory pathways, such as zinc finger E-box binding homeobox (ZEB1 and ZEB2) and snail family transcriptional repressor (SNAI1 and SNAl2) [41]. Transformation into sarcomatoid carcinoma has an extremely poor prognosis. After transformation, EGFR-TKIs are not effective, even if the original EGFR-activating mutation is retained. In some cases, MET amplification is detected and may qualify patients for targeted therapy. In contrast, high PD-L1 expression levels (even > 80% in sarcoma) usually lead to immunotherapy and chemotherapy approaches [39,40].
2. Case Description
2.1. Study Cohort
From 2020 to 2022, one hundred patients with lung ADC harboring EGFR common mutations were diagnosed at our institution and included in this study. Sixty-one percent of patients were female and 39% were male. The median age was 68 years (range 34–91 years). Molecular analyses were performed at time of tumor diagnosis using biopsy sample in 61% of cases, cytological specimens in 36%, and surgical specimens in 3%, Approximately 81% of tumor specimen sites were located in the lung, while the remaining 19% were located in other tissues (6% lymph nodes; 4% liver; 5% bones; less than 4% on heart, brain, and diaphragm). Detailed clinicopathological features of the patients are reported in Supplementary Table S2.
2.2. Molecular Data
Overall, 61 out of 100 EGFR-mutated tumors harbored exon-19 deletion, while 39 cases carried the p.(L858R) point mutation. Three cases with the p.(L858R) mutation showed also additional EGFR alterations: one case had the p.(V834L) mutation in exon 21, one had the p.(A871G) mutation in exon 21, and one case had a p.(S768I) mutation in exon 20.
Details about EGFR alterations observed in the study cohort are summarized in Table 2.

Table 2.
EGFR mutations in the study cohort.
2.3. Histological Transformation
As shown in Figure 1, 32 out of 100 cases of advanced and/or metastatic lung ADC underwent rebiopsy at the time of disease progression to first-line Osimertinib. Resistance to Osimertinib occurred after a median of 17 months and 12 months for patients with exon 19 deletions and p.(L858R) mutation, respectively. Among rebiopsied mADCs, in 41% of cases, known acquired resistance mechanisms were identified; out of these, 13% of cases underwent phenotypic transformation.
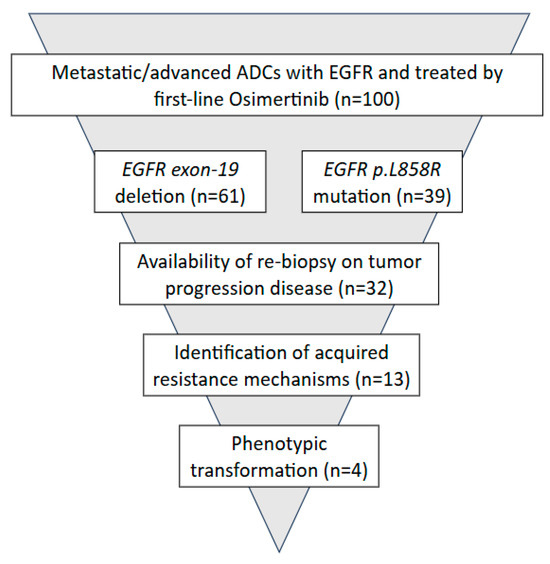
Figure 1.
Study design.
Out of the 22 rebiopsied cases for which TP53 mutational status was available, nine patients (40.9%) harbored a pathogenic TP53 variant in the diagnostic tumor specimens. Notably, TP53 mutations were more frequently detected in association with EGFR exon-19 deletions. Further details regarding the rebiopsied cases are presented in Table 3.

Table 3.
Acquired resistance mechanisms identified in this study cohort.
2.4. Transformed Cases
Below, we describe the four EGFR mutated cases showing histological transformation, hereafter named T1, T2, T3 and T4, at first-line progression to Osimertinib:
- T1: 58-year-old-woman, received Osimertinib for approximately 25 months. At progression, a histological transformation to SCLC was observed (Figure 2A–C). This case preserved EGFR exon-19 deletion on the tumor progression specimen. Furthermore, it harbored also a TP53-activating alteration c.776A>T p.(D259V) on exon 7, which was already detected at diagnosis. Our liquid biopsy, performed on the plasma sample at progression time, was negative. The patient received second-line treatment with carboplatin and etoposide chemotherapy combined with radiotherapy targeting a mediastinal metastatic lesion. After 14 months, the patient showed progression disease and was switched to topotecan chemotherapy for an additional 13 months. The patient died two months later.
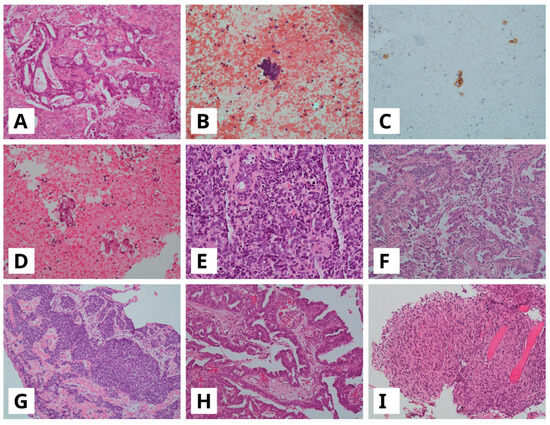 Figure 2. (A–C) Lung adenocarcinoma (ADC) in bronchial biopsy ((A), hematoxylin and eosin, H&E) showing transformation to small cell lung cancer (SCLC) on cell block from pleural effusion ((B), H&E) with strong cytoplasmatic immunoreactivity for synaptophysin (C); (D,E) ADC in CT-guided biopsy ((D), H&E) with following SCLC transformation on bronchial biopsy ((E), H&E); (F,G), ADC in pleural biopsy ((F), H&E), with SCLC transformation on bronchial biopsy ((G), H&E); (H,I), Lateral cervical lymph node with metastasis of lung ADC ((H), H&E) with following sarcomatoid carcinoma transformation on pleural biopsy ((I), H&E). All images are at a 10× magnification.
Figure 2. (A–C) Lung adenocarcinoma (ADC) in bronchial biopsy ((A), hematoxylin and eosin, H&E) showing transformation to small cell lung cancer (SCLC) on cell block from pleural effusion ((B), H&E) with strong cytoplasmatic immunoreactivity for synaptophysin (C); (D,E) ADC in CT-guided biopsy ((D), H&E) with following SCLC transformation on bronchial biopsy ((E), H&E); (F,G), ADC in pleural biopsy ((F), H&E), with SCLC transformation on bronchial biopsy ((G), H&E); (H,I), Lateral cervical lymph node with metastasis of lung ADC ((H), H&E) with following sarcomatoid carcinoma transformation on pleural biopsy ((I), H&E). All images are at a 10× magnification. - T2: A 61-year-old-woman developed histological transformation to SCLC after 14 months of Osimertinib therapy (Figure 2D,E). The tumor retained the EGFR exon 19 deletion and showed an acquired MET amplification at progression. Through examination of the diagnostic specimen, it was found that this case harbored a TP53 pathogenic alteration c.329G>C p.(R110P) on exon 4. Liquid biopsy performed on the plasma sample at progression time confirmed MET amplification as an acquired resistance mechanism. According to the presence of MET amplification, the patient received an MET inhibitor, Tepotinib, in combination with Osimertinib as second-line treatment. After disease progression, five months later, she underwent carboplatin and etoposide chemotherapy combined with the PD-L1 inhibitor Atezolizumab. She continued treatment for 7 months before her death.
- T3: A 78-year-old-man with a histological transformation to SCLC after 14 months of Osimertinib therapy (Figure 2F,G). Through examination of the diagnostic specimen, it was found that this case harbored a TP53-activating alteration c.659A>G p.(Y220C) on exon 6. Liquid biopsy was not performed. Following disease progression, the patient received palliative care and died one month later.
- T4: A 72-year-old-woman developed transformation to spindle cell carcinoma, a variant of sarcomatoid carcinoma, after approximately 11 months of treatment (Figure 2H,I). The tumor harbored a TP53-activating alteration c.602del p. (L201fs), within exon 6, on the diagnostic specimen. Liquid biopsy at progression detected only the EGFR exon 19 deletion with no additional resistance mechanisms. The patient was treated with carboplatin combined with pemetrexed as second-line treatment chemotherapy but died three months later.
Further details about these cases are reported in Table 4.

Table 4.
Details of histological transformed cases.
3. Materials and Methods
3.1. Study Cohort and Design
The study included all consecutive cases of advanced and/or metastatic lung ADC harboring EGFR mutations diagnosed at the University Hospital of Pisa from January 2020 to December 2022. Histological and cytological diagnoses were performed by expert pathologists according to the WHO 2021 histological and immunohistochemical criteria [1]. Tumors were classified as advanced stages based on the latest American Joint Commission on Cancer—Tumor Nodes Metastasis (TNM) classification [42]. This study was conducted in accordance with the principles of the 1975 Helsinki Declaration. Written informed consent was obtained from all patients prior to tumor biopsy or surgical resection. All cases were anonymized for this study, and no sensitive data were used. This study did not interfere with routine clinical management.
3.2. Molecular Analysis
Molecular analyses were performed on formalin-fixed paraffin-embedded (FFPE) tissue samples from surgical resections, biopsies, and cell-blocks or on Papanicolaou-stained smears. For each case, the specimen with the highest tumor cell content was selected to determine the status of predictive biomarkers routinely tested for advanced NSCLC. DNA extraction from FFPE samples involved three 10 μm thick unstained sections that were processed by standard deparaffinization in xylene and graded ethanol rehydration. Cytologic smears underwent coverslip removal by incubation in xylene for 48 hours, which was followed by graded ethanol rehydration (99%, 95%, 70%, and 50%, 10 min each). All samples were enriched for cancer cells by manual macrodissection. DNA purification was carried out using the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) according to the manufacturer’s protocol. DNA concentration and integrity were assessed via spectrophotometry and a real-time PCR kit (Diatech Pharmacogenetics, Jesi, Italy). Molecular testing methodologies were performed according to changing practice patterns over time. Samples collected in 2020 were analyzed by MALDI-TOF technology on the Sequenom platform (Agena Bioscience, San Diego, CA, USA) using the kit Myriapod Lung Status (Diatech Pharmacogenetics, Jesi, Italy) according to the manufacturer’s instructions [43]. For samples collected from 2021 to 2022, next-generation sequencing (NGS) was performed with the amplicon-based panel Myriapod-NGS Cancer panel DNA (Diatech Pharmacogenetics, Jesi, Italy), according to the manufacturer’s protocol. Clinically relevant gene mutations within EGFR, BRAF, KRAS and MET genes were evaluated. TP53 mutations in diagnostic tumor tissue were assessed by direct Sanger sequencing (primers listed in Supplementary Table S1).
Circulating cell-free DNA (cfDNA) was analyzed externally by digital real-time PCR from 4 mL of plasma.
Histopathological and clinical data were retrospectively reviewed and collected for each patient.
3.3. PD-L1 Evaluation
PDL-1 expression in tumor tissue was assessed by immunohistochemistry (IHC). A representative tissue block or cell block from each lesion was selected. Tissue sections of 4 μm thickness were deparaffinized in xylene, rehydrated using a graded series of ethanol solutions, and then subjected to immunohistochemical staining. IHC straining was performed using the rabbit monoclonal anti-PD-L1 antibody (clone SP263, Roche-Ventana, Oro Valley, AZ, USA) with the OptiView DAB IHC Detection Kit and OptiView Amplification Kit (Roche-Ventana, Oro Valley, AZ, USA). Immunostaining was performed as a fully automated assay using BenchMark XT automated slide stainers (Roche-Ventana, Oro Valley, AZ, USA). Negative controls were carried out by omitting the primary antibodies. Immunohistochemical evaluation was performed independently by two pathologists (G.A. and A.C. (Alessandra Celi)), blinded to clinicopathological characteristics and molecular data, as previously described. PD-L1 expression was quantified using the tumor proportional score (TPS), which was defined as the percentage of viable tumor cells exhibiting partial or complete membrane staining of any intensity among all of the viable tumor cells in the examined section [44].
3.4. Data Analysis
Within the study cohort, the prevalence and patterns of histological transformation were evaluated in EGFR-mutated patients undergoing rebiopsy at progression during first-line Osimertinib treatment. The persistence of EGFR-activating alterations and the coexistence of multiple resistance mechanisms on rebiopsy were evaluated.
4. Discussion
NSCLC is one of the most frequent and aggressive human cancers. Lung ADC is the most common histotype, and the majority of cases are diagnosed in advanced stages. In 15% of cases, lung ADCs have driver mutations in the EGFR gene, which are mainly in-frame deletions in exon 19 and the p.(L858R) point mutation in exon 21 [7]. These mutations are predictive of response to EGFR-TKIs. The third-generation EGFR-inhibitor Osimertinib, initially developed for patients with acquired resistance to 1st and 2nd-generation EGFR-TKI, is the election method for the first-line treatment [7]. However, acquired resistance inevitably occurs [8]. Mechanisms of resistance can consist of an on- target EGFR altered signaling pathway, off-target alterations and phenotypic transformation. The most common on-target resistance mechanism to first-line Osimertinib is the mutation of p.(C797S) within EGFR exon 20, which is observed in 22–44% of cases [8]. The EGFR p.(C797S) leads to drug resistance by breaking the covalent bond between the inhibitor and the mutant EGFR site [25]. On the other hand, the most frequently acquired off-target resistance mechanism is MET amplification (15% of cases). Alterations in RET, ALK, BRAF, KRAS, PIK3CA and FGFR genes have also been reported [8,45]. Another important mechanism of acquired resistance to first-line Osimertinib consists of histological transformation occurring in about 15% of cases [8], whose detection necessarily requires a tumor tissue rebiopsy. The most common type of histological transformation is represented by an ADC switch to SCLC with a prevalence of 3–14% [16]. Less common histological changes occur in 1.1–10% of cases and include transformation to SCC, sarcomatoid carcinoma and LCNEC [26,34,39].
In our study, from a consecutive series of 100 EGFR mutated ADCs, only 32 underwent tumor rebiopsy at progression time and 4 (12%) showed histological transformation. Although in agreement with the literature [8], the prevalence of histological transformation here reported could be underestimated. In fact, tumor rebiopsy at Osimertinib progression was performed in a minority of cases. In the clinical practice, tissue rebiopsy is not always feasible for several reasons, such as the accessibility of the metastatic site and the patients’ clinical conditions. Moreover, repeated rebiopsies can be associated with increased risks and costs [46].
Regarding histological transformations detected in our series, three cases switched to SCLC, and one switched to sarcomatoid carcinoma. A proper and correct histological diagnosis greatly influences each patient’s clinical outcome. For example, the transformed SCLCs are associated with a poor survival rate, and up to 60% of patients achieve consistent response rates with concurrent chemo-radiotherapy [1,47]. Several studies are evaluating the genetic background of transformed ADCs to understand whether there are molecular alterations acting as predisposing factors [10] potentially detectable also on liquid biopsy. Among the genetic alterations usually associated with phenotypic transformation, there are TP53 pathogenic mutations [18,48]. Marcoux et al. found that approximately 3% to 10% of EGFR-mutant NSCLCs undergoing SCLC transformation often present concurrent TP53 pathogenic mutations [11]. In EGFR-mutated ADCs, the co-occurrence of TP53 pathogenic alterations has been reported to increase the risk of histological changes not only in SCLC transformation but also in SCC, sarcomatoid carcinoma and LCNET [30,37,40]. TP53 plays an important role in phenotypic plasticity [24] and interestingly, in our cohort, all transformed cases harbored EGFR exon-19 deletions and TP53 co-mutations on tumor diagnosis. To deepen its role in histological transformation, ADCs characterized by this co-mutation should be further investigated both at molecular and clinical levels. In the absence of tumor tissue availability, liquid biopsy can be used to detect gene alterations in plasma; however, no molecular biomarkers suggestive of histological transformation have yet been identified [10]. Moreover, according to tumor volume, type and site, shedding levels can be different, and some molecular alterations can be missed by liquid biopsy analysis. In our study, liquid biopsies at progression time were available for three out of four transformed cases: one was negative, one presented only EGFR exon-19 deletion, and one presented only MET amplification. Probably negative cases can be due to the low amount of released cfDNA, and also intra-tumor heterogeneity can strongly impact this type of test [49].
In addition, resistance to Osimertinib can be extremely heterogeneous [9], and more molecular mechanisms can coexist. For instance, one of our reported cases harbored both MET amplification and histological transformation.
Although obtained on a limited series number, our data, in agreement with the available literature, suggest that at progression time, tumor rebiopsy should be considered whenever possible to better evaluate resistance mechanisms and to properly define therapeutic strategies.
5. Conclusions
Osimertinib is currently the preferred first-line treatment for EGFR-mutated NSCLCs; acquired resistance inevitably occurs. Histological transformation has been widely reported as an acquired, off-target resistance mechanism. While liquid biopsy represents a valuable tool for detecting EGFR resistance mechanisms, only a pathological examination of tumor rebiopsy can reliably identify phenotypic transformation, which strongly influences therapy assessment and prognosis.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms262110462/s1.
Author Contributions
Conceptualization: R.B., R.S. and G.A.; methodology: R.S. and A.C. (Alessandra Celi); investigation: R.B., R.S. and G.A.; resources: I.P., A.S., A.C. (Antonio Chella) and C.U.; formal analysis: R.S. and A.C. (Alessandra Celi); writing original draft: R.S.; writing—reviewing and editing: R.B. and G.A.; supervision: R.B. and G.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki and approved by the local Ethics Committee (protocol code 9989/2019, approval data 20 February 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The original contributions presented in this study are included in the article/Supplementary Materials. Further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| mADC | metastatic lung adenocarcinoma |
| NSCLC | non-small cell lung cancer |
| SCC | squamous cell carcinoma |
| LCNEC | large cell neuroendocrine carcinoma |
| TKI | tyrosine kinase inhibitors |
| SCLC | small cell lung cancer |
| tSCLC | transformed SCLC |
| tLCNET | transformed LCNEC |
| EMT | epithelial–mesenchymal transition |
| H&E | hematoxylin and eosin |
| FFPE | formalin-fixed paraffin-embedded |
| IHC | immunohistochemistry |
| TPS | tumor proportional score |
References
- Nicholson, A.G.; Tsao, M.S.; Beasley, M.B.; Borczuk, A.C.; Brambilla, E.; Cooper, W.A.; Dacic, S.; Jain, D.; Kerr, K.M.; Lantuejoul, S.; et al. The 2021 WHO Classification of Lung Tumors: Impact of Advances Since 2015. J. Thorac. Oncol. 2022, 17, 362–387. [Google Scholar] [CrossRef]
- Sankar, V.; Kothai, R.; Vanisri, N.; Akilandeswari, S.; Anandharaj, G. Lung cancer—A review. Int. J. Health Sci. Res. 2023, 13, 307–315. [Google Scholar] [CrossRef]
- Li, Y.; Yan, B.; He, S. Advances and challenges in the treatment of lung cancer. Biomed. Pharmacother. 2023, 169, 115891. [Google Scholar] [CrossRef] [PubMed]
- Pezzuto, F.; Hofman, V.; Bontoux, C.; Fortarezza, F.; Lunardi, F.; Calabrese, F.; Hofman, P. The significance of co-mutations in EGFR-mutated non-small cell lung cancer: Optimizing the efficacy of targeted therapies? Lung Cancer 2023, 181, 107249. [Google Scholar] [CrossRef] [PubMed]
- Fu, K.; Xie, F.; Wang, F.; Fu, L. Therapeutic strategies for EGFR-mutated non-small cell lung cancer patients with osimertinib resistance. J. Hematol. Oncol. 2022, 15, 173. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cheng, W.L.; Feng, P.H.; Lee, K.Y.; Chen, K.Y.; Sun, W.L.; Van Hiep, N.; Luo, C.S.; Wu, S.M. The Role of EREG/EGFR Pathway in Tumor Progression. Int. J. Mol. Sci. 2021, 22, 12828. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- He, J.; Huang, Z.; Han, L.; Gong, Y.; Xie, C. Mechanisms and management of 3rd-generation EGFR-TKI resistance in advanced non-small cell lung cancer (Review). Int. J. Oncol. 2021, 59, 90. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Schoenfeld, A.J.; Chan, J.M.; Kubota, D.; Sato, H.; Rizvi, H.; Daneshbod, Y.; Chang, J.C.; Paik, P.K.; Offin, M.; Arcila, M.E.; et al. Tumor Analyses Reveal Squamous Transformation and Off-Target Alterations As Early Resistance Mechanisms to First-line Osimertinib in EGFR-Mutant Lung Cancer. Clin. Cancer Res. 2020, 26, 2654–2663. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Leonetti, A.; Sharma, S.; Minari, R.; Perego, P.; Giovannetti, E.; Tiseo, M. Resistance mechanisms to osimertinib in EGFR-mutated non-small cell lung cancer. Br. J. Cancer 2019, 121, 725–737. [Google Scholar] [CrossRef] [PubMed]
- Reita, D.; Pabst, L.; Pencreach, E.; Guérin, E.; Dano, L.; Rimelen, V.; Voegeli, A.C.; Vallat, L.; Mascaux, C.; Beau-Faller, M. Molecular Mechanism of EGFR-TKI Resistance in EGFR-Mutated Non-Small Cell Lung Cancer: Application to Biological Diagnostic and Monitoring. Cancers 2021, 13, 4926. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Marcoux, N.; Gettinger, S.N.; O’Kane, G.; Arbour, K.C.; Neal, J.W.; Husain, H.; Evans, T.L.; Brahmer, J.R.; Muzikansky, A.; Bonomi, P.D.; et al. EGFR-Mutant Adenocarcinomas That Transform to Small-Cell Lung Cancer and Other Neuroendocrine Carcinomas: Clinical Outcomes. J. Clin. Oncol. 2019, 37, 278–285. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vendrell, J.A.; Quantin, X.; Serre, I.; Solassol, J. Combination of tissue and liquid biopsy molecular profiling to detect transformation to small cell lung carcinoma during osimertinib treatment. Ther. Adv. Med. Oncol. 2020, 12, 1758835920974192. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sequist, L.V.; Waltman, B.A.; Dias-Santagata, D.; Digumarthy, S.; Turke, A.B.; Fidias, P.; Bergethon, K.; Shaw, A.T.; Gettinger, S.; Cosper, A.K.; et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci. Transl. Med. 2011, 3, 75ra26. [Google Scholar] [CrossRef]
- Niederst, M.J.; Sequist, L.V.; Poirier, J.T.; Mermel, C.H.; Lockerman, E.L.; Garcia, A.R.; Katayama, R.; Costa, C.; Ross, K.N.; Moran, T.; et al. RB loss in resistant EGFR mutant lung adenocarcinomas that transform to small-cell lung cancer. Nat. Commun. 2015, 6, 6377. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dorantes-Heredia, R.; Ruiz-Morales, J.M.; Cano-García, F. Histopathological transformation to small-cell lung carcinoma in non-small cell lung carcinoma tumors. Transl. Lung Cancer Res. 2016, 5, 401–412. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Y.; He, M.; Dai, Z.; Wang, Y.; Chen, J.; Wang, X.; Dong, X.; Huang, J.; Ruan, J.; Zhang, X.; et al. Clinical and molecular profiling of EGFR-mutant lung adenocarcinomas transformation to small cell lung cancer during TKI treatment. Front. Oncol. 2023, 13, 1308313. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hao, L.; Chen, H.; Wang, L.; Zhou, H.; Zhang, Z.; Han, J.; Hou, J.; Zhu, Y.; Zhang, H.; Wang, Q. Transformation or tumor heterogeneity: Mutations in EGFR, SOX2, TP53, and RB1 persist in the histological rapid conversion from lung adenocarcinoma to small-cell lung cancer. Thorac. Cancer 2023, 14, 1036–1041. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Offin, M.; Chan, J.M.; Tenet, M.; Rizvi, H.A.; Shen, R.; Riely, G.J.; Rekhtman, N.; Daneshbod, Y.; Quintanal-Villalonga, A.; Penson, A.; et al. Concurrent RB1 and TP53 Alterations Define a Subset of EGFR-Mutant Lung Cancers at risk for Histologic Transformation and Inferior Clinical Outcomes. J. Thorac. Oncol. 2019, 14, 1784–1793. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Catania, C.; Liu, S.V.; Garassino, M.; Delmonte, A.; Scotti, V.; Cappuzzo, F.; Genova, C.; Russo, A.; Russano, M.; Bennati, C.; et al. Correlation between treatments and outcomes of patients with EGFR-mutated non-small-cell lung cancer that transitioned into small-cell lung cancer: An international retrospective study. ESMO Open 2025, 10, 105326. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lai, L.; Meng, W.; Wei, J.; Zhang, X.; Tan, Z.; Lu, Y.; Hou, E. Transformation of NSCLC to SCLC after 1st- and 3rd-generation EGFR-TKI resistance and response to EP regimen and erlotinib: 2 CARE-compliant case reports. Medicine 2021, 100, e25046. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lee, P.H.; Huang, Y.H.; Lin, H.; Hsu, K.H.; Chen, K.C.; Tseng, J.S.; Chang, G.C.; Yang, T.Y. Histological Transformation after Acquired Resistance to the Third-Generation EGFR-TKI in Patients with Advanced EGFR-Mutant Lung Adenocarcinoma. Medicina 2022, 58, 908. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhang, B.; Lewis, W.; Stewart, C.A.; Morris, B.B.; Solis, L.M.; Serrano, A.; Xi, Y.; Wang, Q.; Lopez, E.R.; Concannon, K.; et al. Brief Report: Comprehensive Clinicogenomic Profiling of Small Cell Transformation from EGFR-Mutant NSCLC Informs Potential Therapeutic Targets. JTO Clin. Res. Rep. 2023, 5, 100623. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mambetsariev, I.; Arvanitis, L.; Fricke, J.; Pharaon, R.; Baroz, A.R.; Afkhami, M.; Koczywas, M.; Massarelli, E.; Salgia, R. Small Cell Lung Cancer Transformation following Treatment in EGFR-Mutated Non-Small Cell Lung Cancer. J. Clin. Med. 2022, 11, 1429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jin, C.B.; Yang, L. Histological transformation of non-small cell lung cancer: Clinical analysis of nine cases. World J. Clin. Cases 2021, 9, 4617–4626. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ferrer, L.; Giaj Levra, M.; Brevet, M.; Antoine, M.; Mazieres, J.; Rossi, G.; Chiari, R.; Westeel, V.; Poudenx, M.; Letreut, J.; et al. A Brief Report of Transformation from NSCLC to SCLC: Molecular and Therapeutic Characteristics. J. Thorac. Oncol. 2019, 14, 130–134. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Ma, Y.; Ou, Q.; Yan, J.; Ye, B.; Li, Y. Long-Term Clinical Benefit in EGFR-Mutant Lung Adenocarcinoma with Local Squamous Cell Carcinoma Transformation After EGFR TKI Resistance: A Case Report. Front. Oncol. 2022, 12, 883367. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hou, S.; Zhou, S.; Qin, Z.; Yang, L.; Han, X.; Yao, S.; Ji, H. Evidence, Mechanism, and Clinical Relevance of the Transdifferentiation from Lung Adenocarcinoma to Squamous Cell Carcinoma. Am. J. Pathol. 2017, 187, 954–962. [Google Scholar] [CrossRef] [PubMed]
- Shiba-Ishii, A.; Takemura, N.; Kawai, H.; Matsubara, D. Histologic transformation of non-small-cell lung cancer in response to tyrosine kinase inhibitors: Current knowledge of genetic changes and molecular mechanisms. Cancer Sci. 2024, 115, 2138–2146. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Song, H.; Kim, D.; Jang, S.J.; Hwang, H.S.; Song, J.S. Clinicopathologic features of histologic transformation in lung adenocarcinoma after treatment with epidermal growth factor receptor-tyrosine kinase inhibitors. Ann. Diagn. Pathol. 2025, 77, 152478. [Google Scholar] [CrossRef] [PubMed]
- Woo, C.G.; Son, S.M.; Lee, H.C.; Han, H.S.; Lee, K.H.; Kim, D.; Kim, E.G.; Lee, O.J. Histologic Changes in Non-Small Cell Lung Cancer under Various Treatments: A Comparison of Histology and Mutation Status in Serial Samples. Cancer Res. Treat. 2022, 54, 737–743. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sato, Y.; Saito, G.; Fujimoto, D. Histologic transformation in lung cancer: When one door shuts, another opens. Ther. Adv. Med. Oncol. 2022, 14, 17588359221130503. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Xi, Y.Z.; Xie, L.; Tan, X.W.; Zeng, S.L. Transformation of adenocarcinoma to squamous cell carcinoma as a source of EGFR-TKI resistance: A case report and literature review. Front. Oncol. 2022, 12, 942084. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Yang, L.; Fan, Y.; Lu, H. Pulmonary Large Cell Neuroendocrine Carcinoma. Pathol. Oncol. Res. 2022, 28, 1610730. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santoni-Rugiu, E. Clinical outcomes provide new insights into transformation to small-cell lung cancer of pulmonary EGFR-mutant adenocarcinoma. Precis. Cancer Med. 2019, 37, 278–285. [Google Scholar] [CrossRef]
- Kogo, M.; Shimizu, R.; Uehara, K.; Takahashi, Y.; Kokubo, M.; Imai, Y.; Tomii, K. Transformation to large cell neuroendocrine carcinoma as acquired resistance mechanism of EGFR tyrosine kinase inhibitor. Lung Cancer 2015, 90, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Moriya, R.; Hokari, S.; Shibata, S.; Koizumi, T.; Tetsuka, T.; Ito, K.; Hashidate, H.; Tsukada, H. Histological Transformation to Large Cell Neuroendocrine Carcinoma from Lung Adenocarcinoma Harboring an EGFR Mutation: An Autopsy Case Report. Intern. Med. 2017, 56, 2013–2017. [Google Scholar] [CrossRef] [PubMed] [PubMed Central][Green Version]
- Pathak, R.; Villaflor, V.M. Histologic Transformation in EGFR-Mutant Lung Adenocarcinomas: Mechanisms and Therapeutic Implications. Cancers 2021, 13, 4641. [Google Scholar] [CrossRef] [PubMed]
- Baglivo, S.; Ludovini, V.; Sidoni, A.; Metro, G.; Ricciuti, B.; Siggillino, A.; Rebonato, A.; Messina, S.; Crinò, L.; Chiari, R. Large Cell Neuroendocrine Carcinoma Transformation and EGFR-T790M Mutation as Coexisting Mechanisms of Acquired Resistance to EGFR-TKIs in Lung Cancer 2017. Mayo Clin. Proc. 2017, 92, 1304–1311. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Zhuang, W.; Huang, Y.; Liao, J.; Yang, M.; Zhang, L.; Zhang, Y.; Fang, W. Rare transformation from lung adenocarcinoma to sarcomatoid carcinoma mediates resistance to inhibitors targeting different driver oncogenes. J. Natl. Cancer Cent. 2024, 5, 75–81. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hsieh, M.S.; Lin, M.W.; Lee, Y.H. Lung adenocarcinoma with sarcomatoid transformation after tyrosine kinase inhibitor treatment and chemotherapy. Lung Cancer 2019, 137, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Calabrese, F.; Pezzuto, F.; Lunardi, F.; Fortarezza, F.; Tzorakoleftheraki, S.E.; Resi, M.V.; Tiné, M.; Pasello, G.; Hofman, P. Morphologic-Molecular Transformation of Oncogene Addicted Non-Small Cell Lung Cancer. Int. J. Mol. Sci. 2022, 23, 4164. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Lababede, O.; Meziane, M.A. The Eighth Edition of TNM Staging of Lung Cancer: Reference Chart and Diagrams. Oncologist 2018, 23, 844–848. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giannini, R.; Lupi, C.; Sensi, E.; Alì, G.; Proietti, A.; Boldrini, L.; Servadio, A.; Giordano, M.; Macerola, E.; Bruno, R.; et al. EGFR and KRAS Mutational Analysis in a Large Series of Italian Non-Small Cell Lung Cancer Patients: 2387 Cases from a Single Center. Oncol. Rep. 2016, 36, 1166–1172. [Google Scholar] [CrossRef]
- Garon, E.B.; Rizvi, N.A.; Hui, R.; Leighl, N.; Balmanoukian, A.S.; Eder, J.P.; Patnaik, A.; Aggarwal, C.; Gubens, M.; Horn, L.; et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 2015, 372, 2018–2028. [Google Scholar] [CrossRef] [PubMed]
- Chmielecki, J.; Gray, J.E.; Cheng, Y.; Ohe, Y.; Imamura, F.; Cho, B.C.; Lin, M.C.; Majem, M.; Shah, R.; Rukazenkov, Y.; et al. Candidate mechanisms of acquired resistance to first-line osimertinib in EGFR-mutated advanced non-small cell lung cancer. Nat. Commun. 2023, 14, 1070, Erratum in Nat. Commun. 2023, 14, 3179. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fukui, T.; Ishihara, M.; Kasajima, M.; Hiyoshi, Y.; Nakahara, Y.; Otani, S.; Igawa, S.; Yokoba, M.; Mitsufuji, H.; Kubota, M.; et al. Questionnaire survey on patient awareness of invasive rebiopsy in advanced non-small cell lung cancer. Thorac. Cancer 2019, 10, 501–507. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rudin, C.M.; Brambilla, E.; Faivre-Finn, C.; Sage, J. Small-cell lung cancer. Nat. Rev. Dis. Primers 2021, 7, 3. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, J.; Wei, B.; Feng, J.; Wu, X.; Chang, Y.; Wang, Y.; Yang, X.; Zhang, H.; Han, S.; Zhang, C.; et al. Case report: TP53 and RB1 loss may facilitate the transformation from lung adenocarcinoma to small cell lung cancer by expressing neuroendocrine markers. Front. Endocrinol. 2022, 13, 1006480. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pizzutilo, E.G.; Pedrani, M.; Amatu, A.; Ruggieri, L.; Lauricella, C.; Veronese, S.M.; Signorelli, D.; Cerea, G.; Giannetta, L.; Siena, S.; et al. Liquid Biopsy for Small Cell Lung Cancer either De Novo or Transformed: Systematic Review of Different Applications and Meta-Analysis. Cancers 2021, 13, 2265. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).