Role of Myeloid Cell Glucose Transporter 1 in the Host Response During Pneumonia Caused by Streptococcus pneumoniae
Abstract
1. Introduction
2. Results
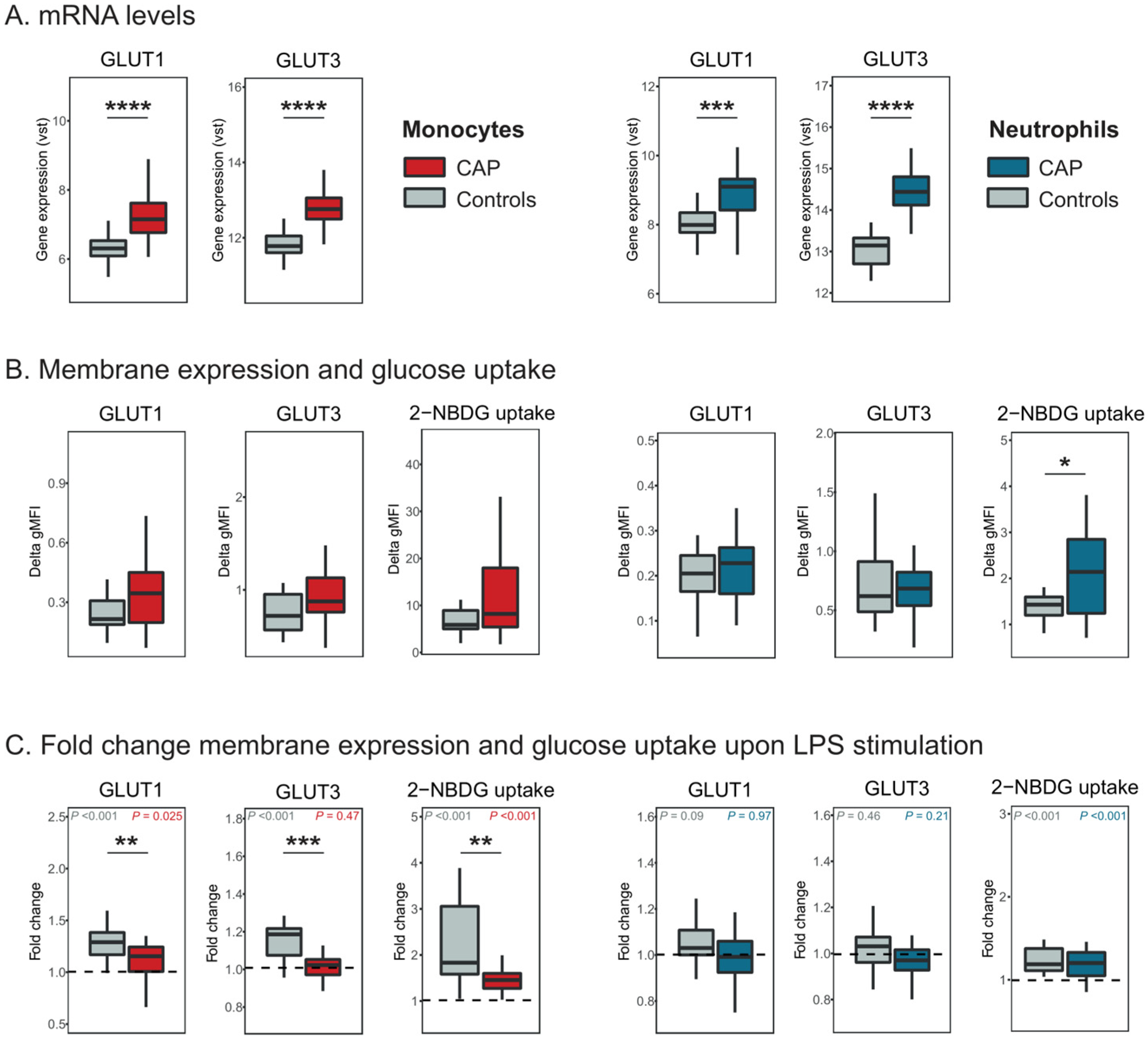
2.1. Monocyte and Neutrophil GLUT1 Expression and Glucose Uptake in CAP Patients
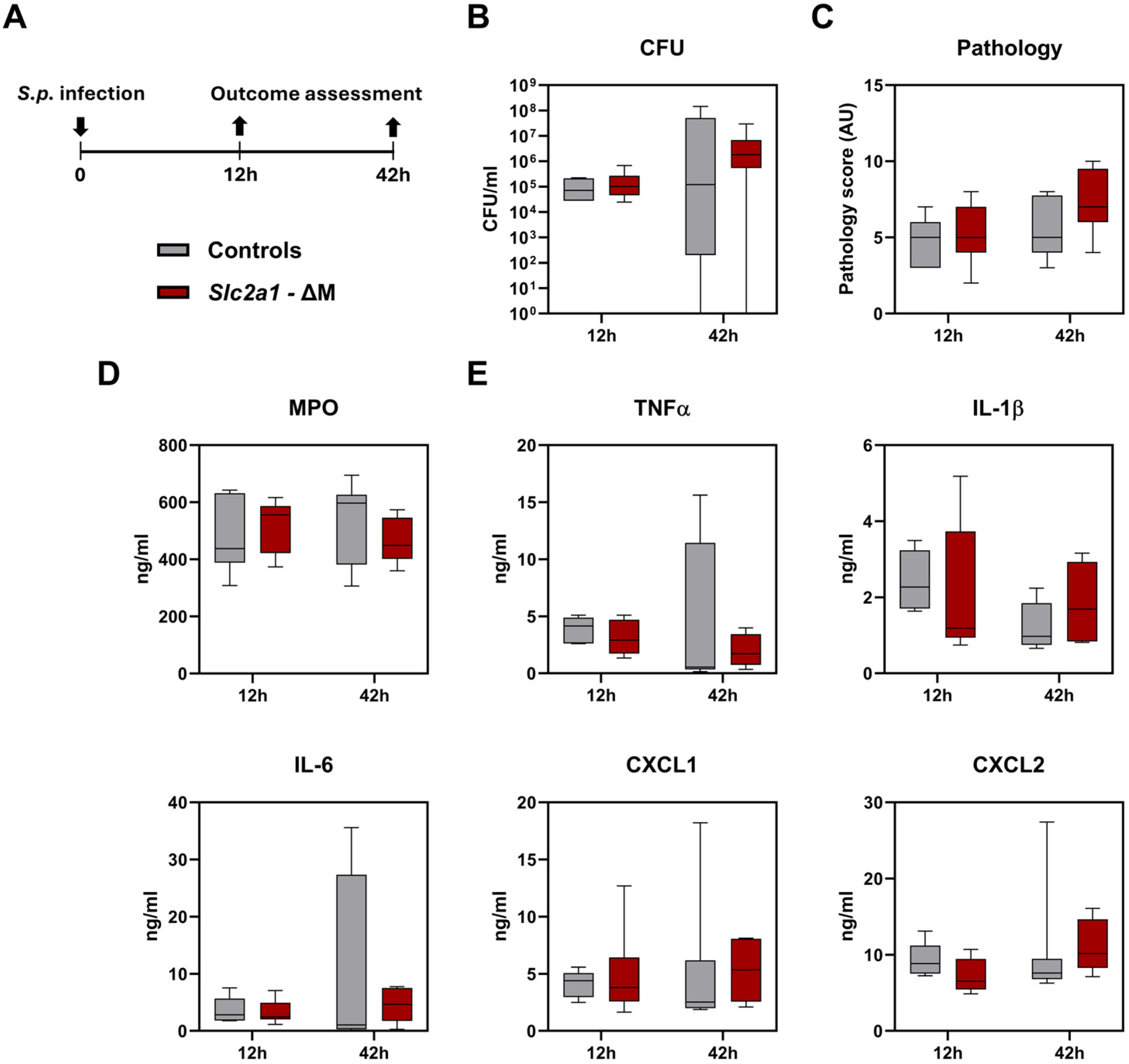
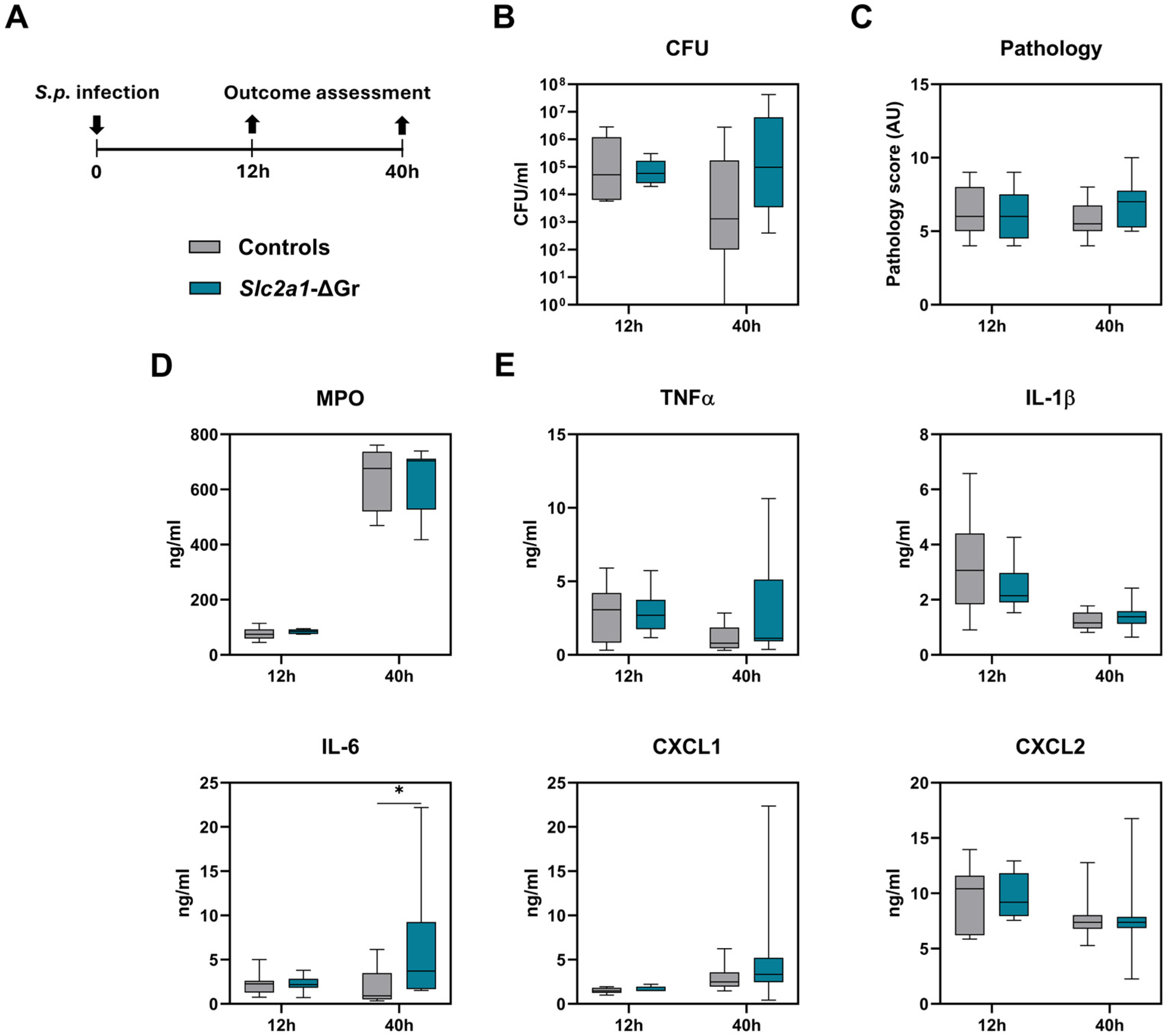
2.2. Role of GLUT1 in Myeloid Cells During Pneumonia Caused by S. pneumoniae
3. Discussion
4. Materials and Methods
4.1. Study Population and Sample Collection
4.2. Monocyte and Neutrophil Isolation
4.3. RNA Isolation, Sequencing, and Transcription Analysis
4.4. Whole Blood Stimulation
4.5. Flow Cytometry
4.6. Mice
4.7. Mouse Infection Model
4.8. Histopathology
4.9. Assays
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- O’Neill, L.A.; Kishton, R.J.; Rathmell, J. A guide to immunometabolism for immunologists. Nat. Rev. Immunol. 2016, 16, 553–565. [Google Scholar] [CrossRef]
- Arts, R.J.; Gresnigt, M.S.; Joosten, L.A.; Netea, M.G. Cellular metabolism of myeloid cells in sepsis. J. Leukoc. Biol. 2017, 101, 151–164. [Google Scholar] [CrossRef]
- Van den Bossche, J.; O’Neill, L.A.; Menon, D. Macrophage immunometabolism: Where are we (going)? Trends Immunol. 2017, 38, 395–406. [Google Scholar] [CrossRef]
- Mueckler, M.; Thorens, B. The SLC2 (GLUT) family of membrane transporters. Mol. Aspects Med. 2013, 34, 121–138. [Google Scholar]
- Kasahara, M.; Hinkle, P.C. Reconstitution and purification of the D-glucose transporter from human erythrocytes. J. Biol. Chem. 1977, 252, 7384–7390. [Google Scholar] [CrossRef]
- Gorga, F.R.; Lienhard, G.E. Changes in the intrinsic fluorescence of the human erythrocyte monosaccharide transporter upon ligand binding. Biochemistry 1982, 21, 1905–1908. [Google Scholar] [CrossRef] [PubMed]
- Klepper, J.; Akman, C.; Armeno, M.; Auvin, S.; Cervenka, M.; Cross, H.J.; De Giorgis, V.; Della Marina, A.; Engelstad, K.; Heussinger, N.; et al. Glut1 deficiency syndrome (Glut1DS): State of the art in 2020 and recommendations of the international Glut1DS study group. Epilepsia Open 2020, 5, 354–365. [Google Scholar] [CrossRef] [PubMed]
- Moley, K.H. Hyperglycemia and apoptosis: Mechanisms for congenital malformations and pregnancy loss in diabetic women. Trends Endocrinol. Metab. 2001, 12, 78–82. [Google Scholar] [CrossRef] [PubMed]
- Moley, K.H.; Chi, M.M.; Knudson, C.M.; Korsmeyer, S.J.; Mueckler, M.M. Hyperglycemia induces apoptosis in pre-implantation embryos through cell death effector pathways. Nat. Med. 1998, 4, 1421–1424, Correction in Nat. Med. 2002, 8, 303. [Google Scholar] [CrossRef]
- Wang, D.; Pascual, J.M.; Yang, H.; Engelstad, K.; Mao, X.; Cheng, J.; Yoo, J.; Noebels, J.L.; De Vivo, D.C. A mouse model for Glut-1 haploinsufficiency. Hum. Mol. Genet. 2006, 15, 1169–1179. [Google Scholar] [CrossRef]
- Ancey, P.B.; Contat, C.; Meylan, E. Glucose transporters in cancer—From tumor cells to the tumor microenvironment. FEBS J. 2018, 285, 2926–2943. [Google Scholar] [PubMed]
- Calder, P.C.; Dimitriadis, G.; Newsholme, P. Glucose metabolism in lymphoid and inflammatory cells and tissues. Curr. Opin. Clin. Nutr. Metab. Care 2007, 10, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Maianu, L.; Melbert, B.R.; Garvey, W.T. Facilitative glucose transporter gene expression in human lymphocytes, monocytes, and macrophages: A role for GLUT isoforms 1, 3, and 5 in the immune response and foam cell formation. Blood Cells Mol. Dis. 2004, 32, 182–190. [Google Scholar] [CrossRef]
- Maratou, E.; Dimitriadis, G.; Kollias, A.; Boutati, E.; Lambadiari, V.; Mitrou, P.; Raptis, S.A. Glucose transporter expression on the plasma membrane of resting and activated white blood cells. Eur. J. Clin. Investig. 2007, 37, 282–290. [Google Scholar] [CrossRef]
- Simpson, I.A.; Dwyer, D.; Malide, D.; Moley, K.H.; Travis, A.; Vannucci, S.J. The facilitative glucose transporter GLUT3: 20 years of distinction. Am. J. Physiol. Endocrinol. Metab. 2008, 295, E242–E253. [Google Scholar] [CrossRef]
- Torres, A.; Cilloniz, C.; Niederman, M.S.; Menendez, R.; Chalmers, J.D.; Wunderink, R.G.; van der Poll, T. Pneumonia. Nat. Rev. Dis. Primers 2021, 7, 25. [Google Scholar] [CrossRef] [PubMed]
- Musher, D.M.; Abers, M.S.; Bartlett, J.G. Evolving understanding of the causes of pneumonia in adults, with special attention to the role of pneumococcus. Clin. Infect. Dis. 2017, 65, 1736–1744. [Google Scholar] [CrossRef]
- Freemerman, A.J.; Zhao, L.; Pingili, A.K.; Teng, B.; Cozzo, A.J.; Fuller, A.M.; Johnson, A.R.; Milner, J.J.; Lim, M.F.; Galanko, J.A.; et al. Myeloid Slc2a1-deficient murine model revealed macrophage activation and metabolic phenotype are fueled by GLUT1. J. Immunol. 2019, 202, 1265–1286. [Google Scholar] [CrossRef]
- Jose, R.J.; Williams, A.E.; Mercer, P.F.; Sulikowski, M.G.; Brown, J.S.; Chambers, R.C. Regulation of neutrophilic inflammation by proteinase-activated receptor 1 during bacterial pulmonary infection. J. Immunol. 2015, 194, 6024–6034. [Google Scholar] [CrossRef]
- De Porto, A.P.; Liu, Z.; de Beer, R.; Florquin, S.; Roelofs, J.; De Boer, O.J.; Den Haan, J.M.M.; Hendriks, R.W.; Van’t Veer, C.; Van der Poll, T.; et al. Bruton’s tyrosine kinase-mediated signaling in myeloid cells is required for protective innate immunity during pneumococcal pneumonia. Front. Immunol. 2021, 12, 723967. [Google Scholar] [CrossRef]
- Abram, C.L.; Roberge, G.L.; Hu, Y.; Lowell, C.A. Comparative analysis of the efficiency and specificity of myeloid-Cre deleting strains using ROSA-EYFP reporter mice. J. Immunol. Methods 2014, 408, 89–100. [Google Scholar] [CrossRef]
- Csepregi, J.Z.; Orosz, A.; Zajta, E.; Kasa, O.; Nemeth, T.; Simon, E.; Fodor, S.; Csonka, K.; Baratki, B.L.; Kovesdi, D.; et al. Myeloid-specific deletion of Mcl-1 yields severely neutropenic mice that survive and breed in homozygous form. J. Immunol. 2018, 201, 3793–3803. [Google Scholar] [CrossRef]
- Van Ziffle, J.A.; Lowell, C.A. Neutrophil-specific deletion of Syk kinase results in reduced host defense to bacterial infection. Blood 2009, 114, 4871–4882. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadis, G.; Maratou, E.; Boutati, E.; Psarra, K.; Papasteriades, C.; Raptis, S.A. Evaluation of glucose transport and its regulation by insulin in human monocytes using flow cytometry. Cytom. Part A 2005, 64, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Kipmen-Korgun, D.; Bilmen-Sarikcioglu, S.; Altunbas, H.; Demir, R.; Korgun, E.T. Type-2 diabetes down-regulates glucose transporter proteins and genes of the human blood leukocytes. Scand. J. Clin. Lab. Investig. 2009, 69, 350–358. [Google Scholar] [CrossRef]
- Korgun, E.T.; Demir, R.; Sedlmayr, P.; Desoye, G.; Arikan, G.; Puerstner, P.; Haeusler, M.; Dohr, G.; Skofitsch, G.; Hahn, T. Physiological leukocytosis during pregnancy is associated with changes in glucose transporter expression of maternal peripheral blood granulocytes and monocytes. Am. J. Reprod. Immunol. 2002, 48, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Korgun, E.T.; Demir, R.; Sedlmayr, P.; Desoye, G.; Arikan, G.M.; Puerstner, P.; Haeusler, M.; Dohr, G.; Skofitsch, G.; Hahn, T. Sustained hypoglycemia affects glucose transporter expression of human blood leukocytes. Blood Cells Mol. Dis. 2002, 28, 152–159. [Google Scholar] [CrossRef]
- Holman, G.D.; Kozka, I.J.; Clark, A.E.; Flower, C.J.; Saltis, J.; Habberfield, A.D.; Simpson, I.A.; Cushman, S.W. Cell surface labeling of glucose transporter isoform GLUT4 by bis-mannose photolabel. Correlation with stimulation of glucose transport in rat adipose cells by insulin and phorbol ester. J. Biol. Chem. 1990, 265, 18172–18179. [Google Scholar] [CrossRef]
- McMillin, S.L.; Schmidt, D.L.; Kahn, B.B.; Witczak, C.A. GLUT4 is not necessary for overload-induced glucose uptake or hypertrophic growth in mouse skeletal muscle. Diabetes 2017, 66, 1491–1500. [Google Scholar] [CrossRef]
- Zeng, W.; Xing, Z.; Tan, M.; Wu, Y.; Zhang, C. Propofol regulates activated macrophages metabolism through inhibition of ROS-mediated GLUT1 expression. Inflamm. Res. 2021, 70, 473–481. [Google Scholar] [CrossRef]
- Lee, M.K.S.; Al-Sharea, A.; Shihata, W.A.; Bertuzzo Veiga, C.; Cooney, O.D.; Fleetwood, A.J.; Flynn, M.C.; Claeson, E.; Palmer, C.S.; Lancaster, G.I.; et al. Glycolysis is required for LPS-induced activation and adhesion of human CD14+CD16− monocytes. Front. Immunol. 2019, 10, 2054. [Google Scholar] [CrossRef]
- Lee, W.; Ryu, J.; Spangler, R.A.; Jung, C.Y. Modulation of GLUT4 and GLUT1 recycling by insulin in rat adipocytes: Kinetic analysis based on the involvement of multiple intracellular compartments. Biochemistry 2000, 39, 9358–9366. [Google Scholar] [CrossRef] [PubMed]
- Mamun, A.A.; Hayashi, H.; Yamamura, A.; Nayeem, M.J.; Sato, M. Hypoxia induces the translocation of glucose transporter 1 to the plasma membrane in vascular endothelial cells. J. Physiol. Sci. 2020, 70, 44. [Google Scholar] [CrossRef] [PubMed]
- Brands, X.; Haak, B.W.; Klarenbeek, A.M.; Butler, J.; Uhel, F.; Qin, W.; Otto, N.A.; Jakobs, M.E.; Faber, D.R.; Lutter, R.; et al. An epigenetic and transcriptomic signature of immune tolerance in human monocytes through multi-omics integration. Genome Med. 2021, 13, 131. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Kunz, S.; Crompton, N.E.; Volk, H.D.; Sabat, R. Multiple mechanisms of reduced major histocompatibility complex class II expression in endotoxin tolerance. J. Biol. Chem. 2003, 278, 18030–18036. [Google Scholar] [CrossRef]
- Van Raam, B.J.; Verhoeven, A.J.; Kuijpers, T.W. Mitochondria in neutrophil apoptosis. Int. J. Hematol. 2006, 84, 199–204. [Google Scholar] [CrossRef]
- Kirchner, T.; Moller, S.; Klinger, M.; Solbach, W.; Laskay, T.; Behnen, M. The impact of various reactive oxygen species on the formation of neutrophil extracellular traps. Mediat. Inflamm. 2012, 2012, 849136. [Google Scholar] [CrossRef]
- Rodriguez-Espinosa, O.; Rojas-Espinosa, O.; Moreno-Altamirano, M.M.; Lopez-Villegas, E.O.; Sanchez-Garcia, F.J. Metabolic requirements for neutrophil extracellular traps formation. Immunology 2015, 145, 213–224. [Google Scholar] [CrossRef]
- Caruana, B.T.; Byrne, F.L.; Knights, A.J.; Quinlan, K.G.R.; Hoehn, K.L. Characterization of glucose transporter 6 in lipopolysaccharide-induced bone marrow-derived macrophage function. J. Immunol. 2019, 202, 1826–1832. [Google Scholar] [CrossRef]
- Li, K.J.; Wu, C.H.; Hsieh, S.C.; Lu, M.C.; Tsai, C.Y.; Yu, C.L. Deranged bioenergetics and defective redox capacity in T lymphocytes and neutrophils are related to cellular dysfunction and increased oxidative stress in patients with active systemic lupus erythematosus. Clin. Dev. Immunol. 2012, 2012, 548516. [Google Scholar] [CrossRef]
- Mavros, Y.; Simar, D.; Singh, M.A. Glucose tranporter-4 expression in monocytes: A systematic review. Diabetes Res. Clin. Pract. 2009, 84, 123–131. [Google Scholar] [CrossRef]
- Rodrigues, R.S.; Bozza, F.A.; Hanrahan, C.J.; Wang, L.M.; Wu, Q.; Hoffman, J.M.; Zimmerman, G.A.; Morton, K.A. 18F-fluoro-2-deoxyglucose PET informs neutrophil accumulation and activation in lipopolysaccharide-induced acute lung injury. Nucl. Med. Biol. 2017, 48, 52–62. [Google Scholar] [CrossRef]
- Ercoli, G.; Fernandes, V.E.; Chung, W.Y.; Wanford, J.J.; Thomson, S.; Bayliss, C.D.; Straatman, K.; Crocker, P.R.; Dennison, A.; Martinez-Pomares, L.; et al. Intracellular replication of Streptococcus pneumoniae inside splenic macrophages serves as a reservoir for septicaemia. Nat. Microbiol. 2018, 3, 600–610. [Google Scholar] [CrossRef]
- Gil, E.; Noursadeghi, M.; Brown, J.S. Streptococcus pneumoniae interactions with the complement system. Front. Cell Infect. Microbiol. 2022, 12, 929483. [Google Scholar] [CrossRef]
- Cho, S.J.; Moon, J.S.; Nikahira, K.; Yun, H.S.; Harris, R.; Hong, K.S.; Huang, H.; Choi, A.M.K.; Stout-Delgado, H. GLUT1-dependent glycolysis regulates exacerbation of fibrosis via AIM2 inflammasome activation. Thorax 2020, 75, 227–236. [Google Scholar] [CrossRef]
- De Vos, A.F.; Dessing, M.C.; Lammers, A.J.; De Porto, A.P.; Florquin, S.; De Boer, O.J.; De Beer, R.; Terpstra, S.; Bootsma, H.J.; Hermans, P.W.; et al. The polysaccharide capsule of Streptococcus pneumonia partially impedes MyD88-mediated immunity during pneumonia in mice. PLoS ONE 2015, 10, e0118181. [Google Scholar] [CrossRef]
- Brands, X.; Haak, B.W.; Klarenbeek, A.M.; Otto, N.A.; Faber, D.R.; Lutter, R.; Scicluna, B.P.; Wiersinga, W.J.; Van der Poll, T. Concurrent immune suppression and hyperinflammation in patients with community-acquired pneumonia. Front. Immunol. 2020, 11, 796. [Google Scholar] [CrossRef] [PubMed]
- Anders, S.; Pyl, P.T.; Huber, W. HTSeq—A Python framework to work with high-throughput sequencing data. Bioinformatics 2015, 31, 166–169. [Google Scholar] [CrossRef] [PubMed]
- Young, C.D.; Lewis, A.S.; Rudolph, M.C.; Ruehle, M.D.; Jackman, M.R.; Yun, U.J.; Ilkun, O.; Pereira, R.; Abel, E.D.; Anderson, S.M. Modulation of glucose transporter 1 (GLUT1) expression levels alters mouse mammary tumor cell growth in vitro and in vivo. PLoS ONE 2011, 6, e23205. [Google Scholar] [CrossRef] [PubMed]
- Clausen, B.E.; Burkhardt, C.; Reith, W.; Renkawitz, R.; Forster, I. Conditional gene targeting in macrophages and granulocytes using LysMcre mice. Transgenic Res. 1999, 8, 265–277. [Google Scholar] [CrossRef]
- Passegue, E.; Wagner, E.F.; Weissman, I.L. JunB deficiency leads to a myeloproliferative disorder arising from hematopoietic stem cells. Cell 2004, 119, 431–443. [Google Scholar] [CrossRef] [PubMed]
- Hoogendijk, A.J.; Roelofs, J.J.; Duitman, J.; van Lieshout, M.H.; Blok, D.C.; van der Poll, T.; Wieland, C.W. R-roscovitine reduces lung inflammation induced by lipoteichoic acid and Streptococcus pneumoniae. Mol. Med. 2012, 18, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereverzeva, L.; Léopold, V.; Saris, A.; Schuurman, A.R.; Butler, J.M.; Reijnders, T.D.Y.; Roelofs, J.J.T.H.; Faber, D.R.; Wiersinga, W.J.; van’t Veer, C.; et al. Role of Myeloid Cell Glucose Transporter 1 in the Host Response During Pneumonia Caused by Streptococcus pneumoniae. Int. J. Mol. Sci. 2025, 26, 10461. https://doi.org/10.3390/ijms262110461
Pereverzeva L, Léopold V, Saris A, Schuurman AR, Butler JM, Reijnders TDY, Roelofs JJTH, Faber DR, Wiersinga WJ, van’t Veer C, et al. Role of Myeloid Cell Glucose Transporter 1 in the Host Response During Pneumonia Caused by Streptococcus pneumoniae. International Journal of Molecular Sciences. 2025; 26(21):10461. https://doi.org/10.3390/ijms262110461
Chicago/Turabian StylePereverzeva, Liza, Valentine Léopold, Anno Saris, Alex R. Schuurman, Joe M. Butler, Tom D. Y. Reijnders, Joris J. T. H. Roelofs, Daniël R. Faber, W. Joost Wiersinga, Cornelis van’t Veer, and et al. 2025. "Role of Myeloid Cell Glucose Transporter 1 in the Host Response During Pneumonia Caused by Streptococcus pneumoniae" International Journal of Molecular Sciences 26, no. 21: 10461. https://doi.org/10.3390/ijms262110461
APA StylePereverzeva, L., Léopold, V., Saris, A., Schuurman, A. R., Butler, J. M., Reijnders, T. D. Y., Roelofs, J. J. T. H., Faber, D. R., Wiersinga, W. J., van’t Veer, C., de Vos, A. F., & van der Poll, T. (2025). Role of Myeloid Cell Glucose Transporter 1 in the Host Response During Pneumonia Caused by Streptococcus pneumoniae. International Journal of Molecular Sciences, 26(21), 10461. https://doi.org/10.3390/ijms262110461





