Manganese Complexes Bearing Bulky DAB Ligands as Efficient Catalysts for the Solvent-Free Hydroboration of Ketones
Abstract
1. Introduction
2. Results and Discussion
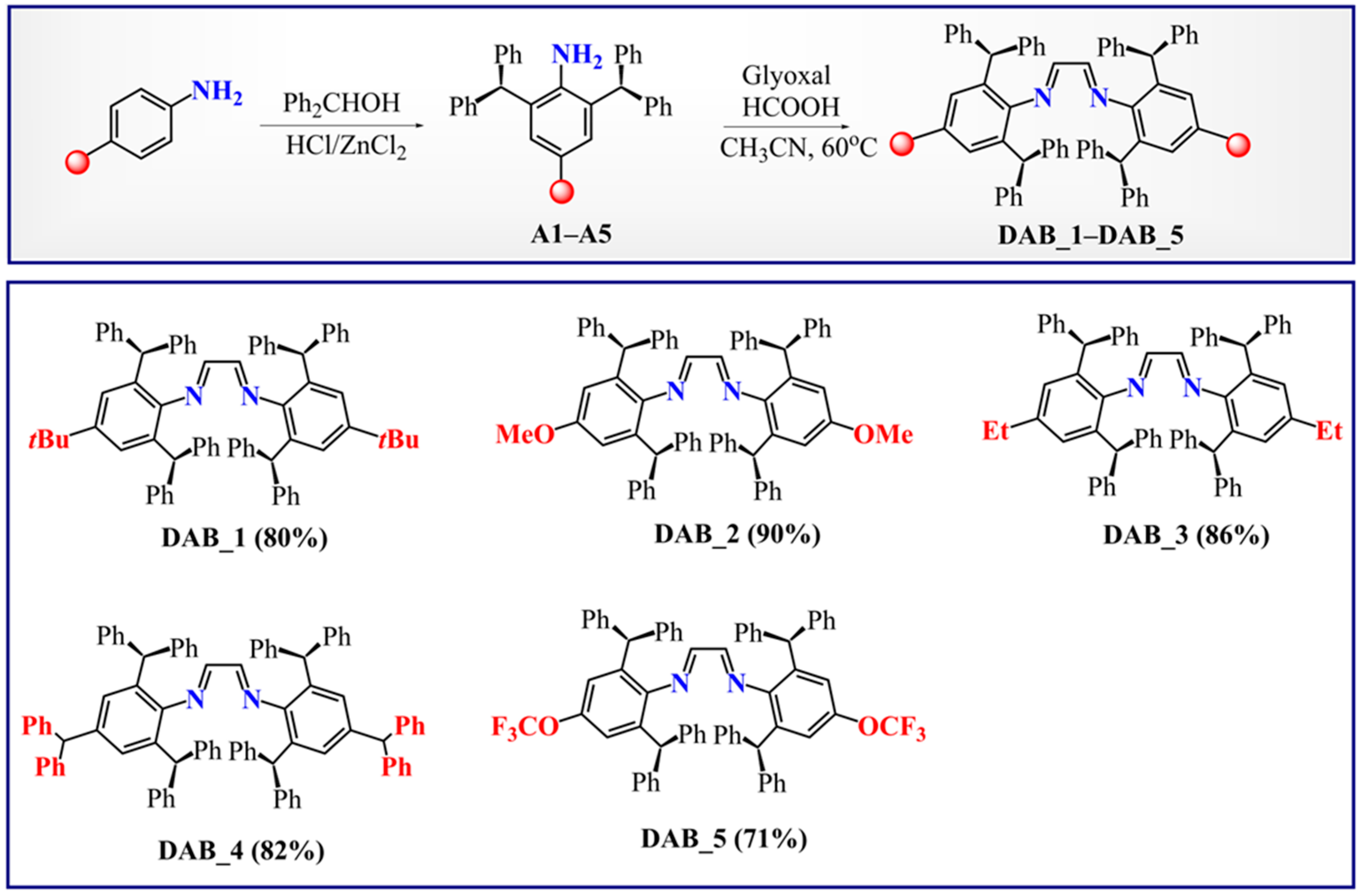
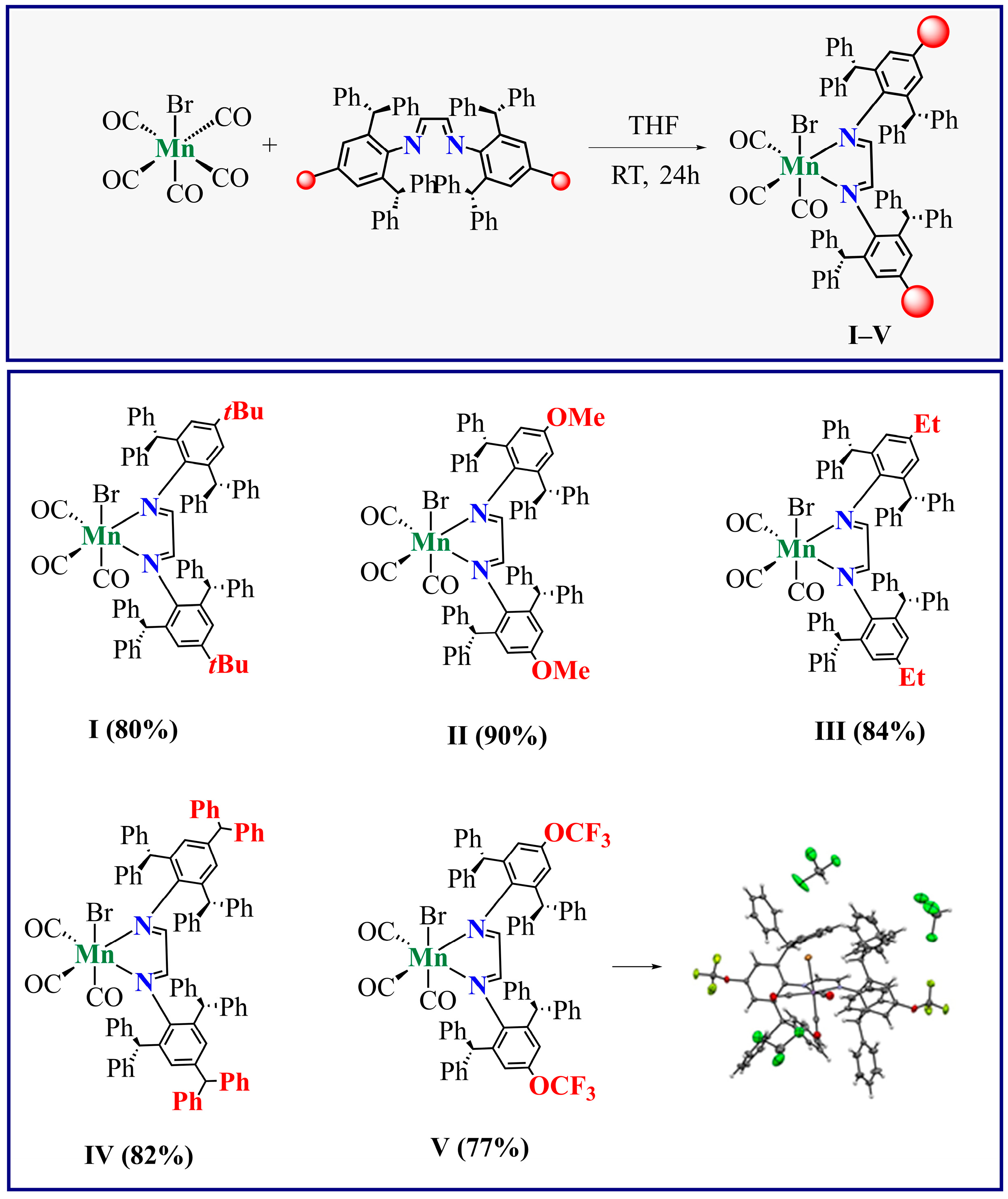
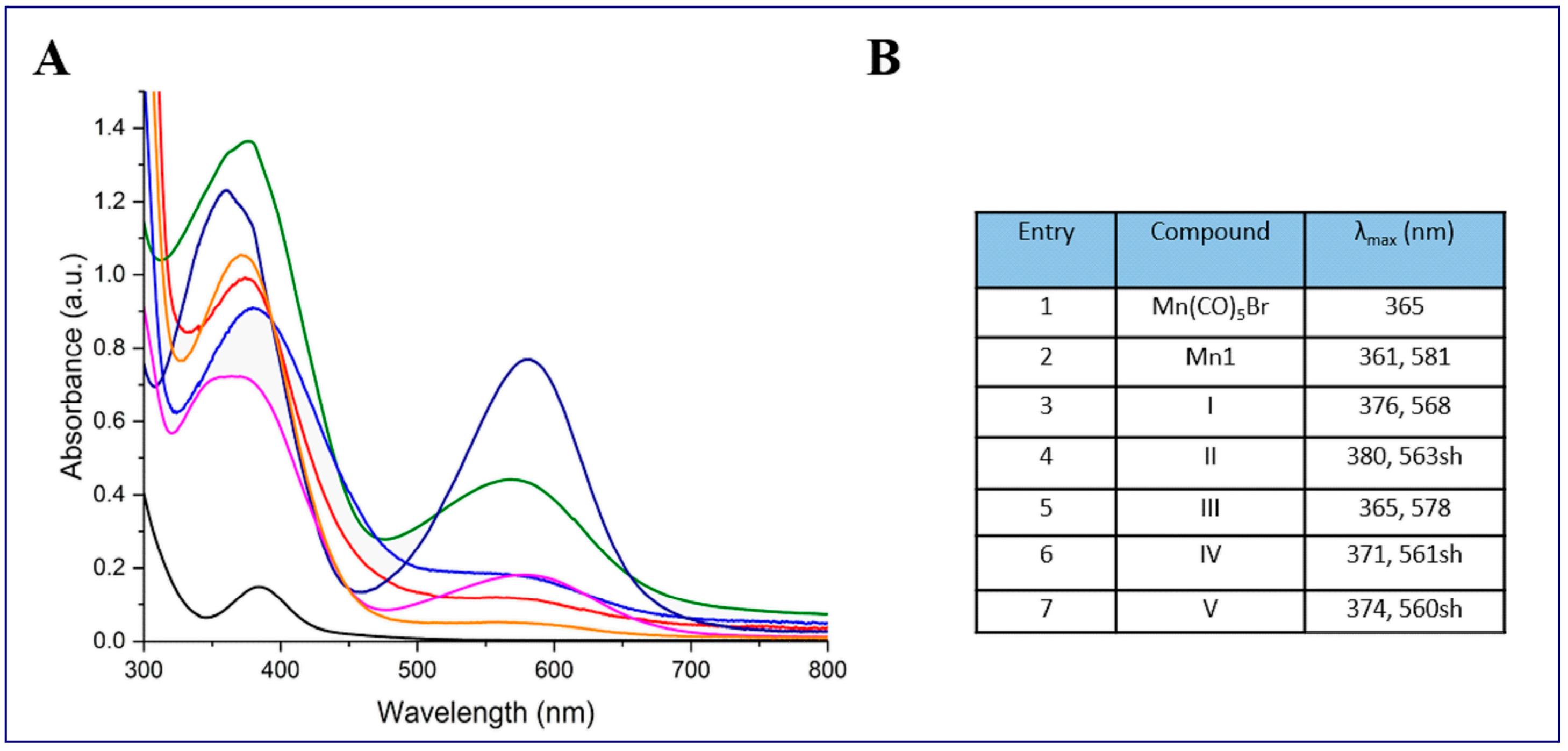
2.1. Synthesis and Characterization of Mn-Complexes
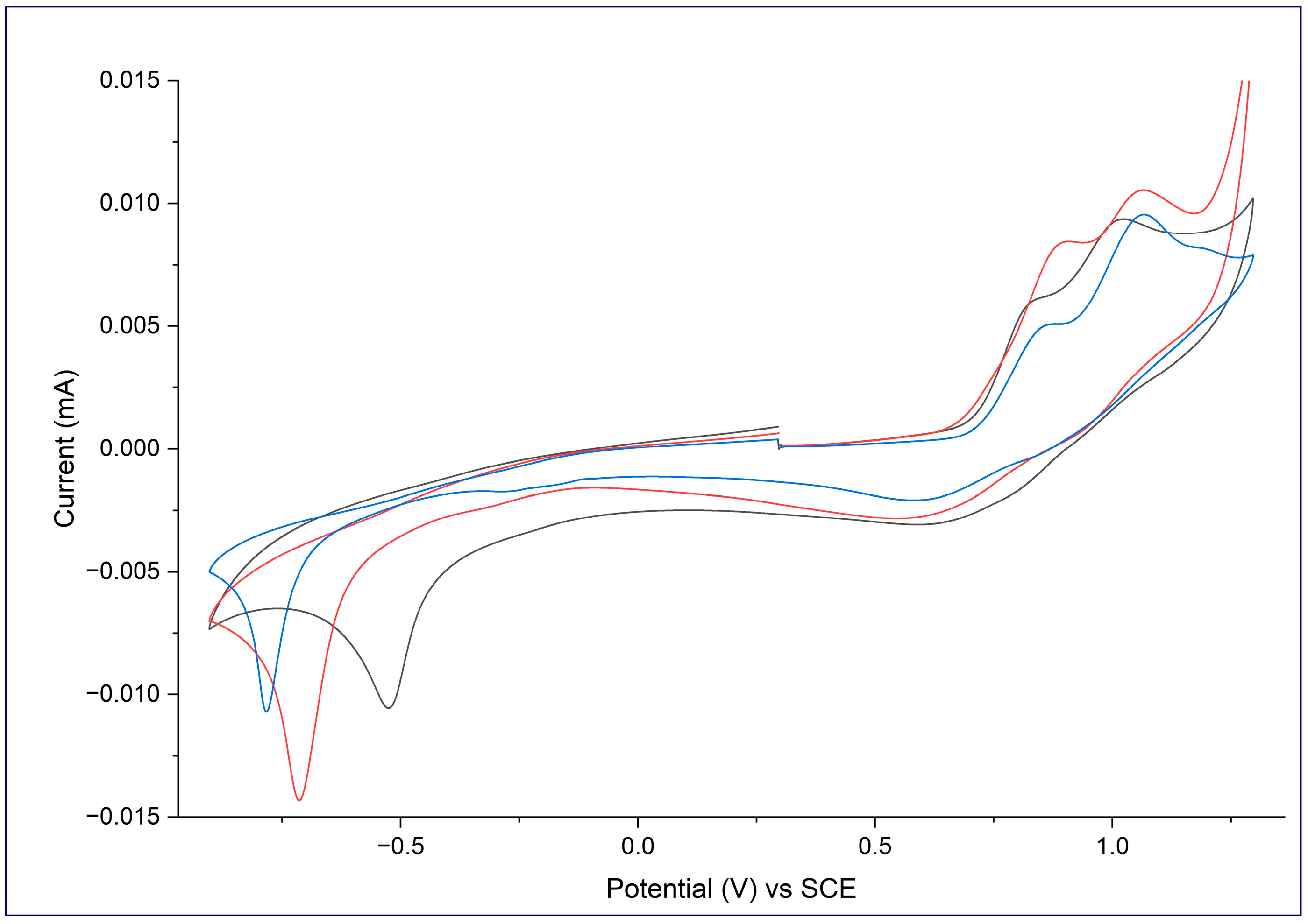
2.2. Electrochemistry
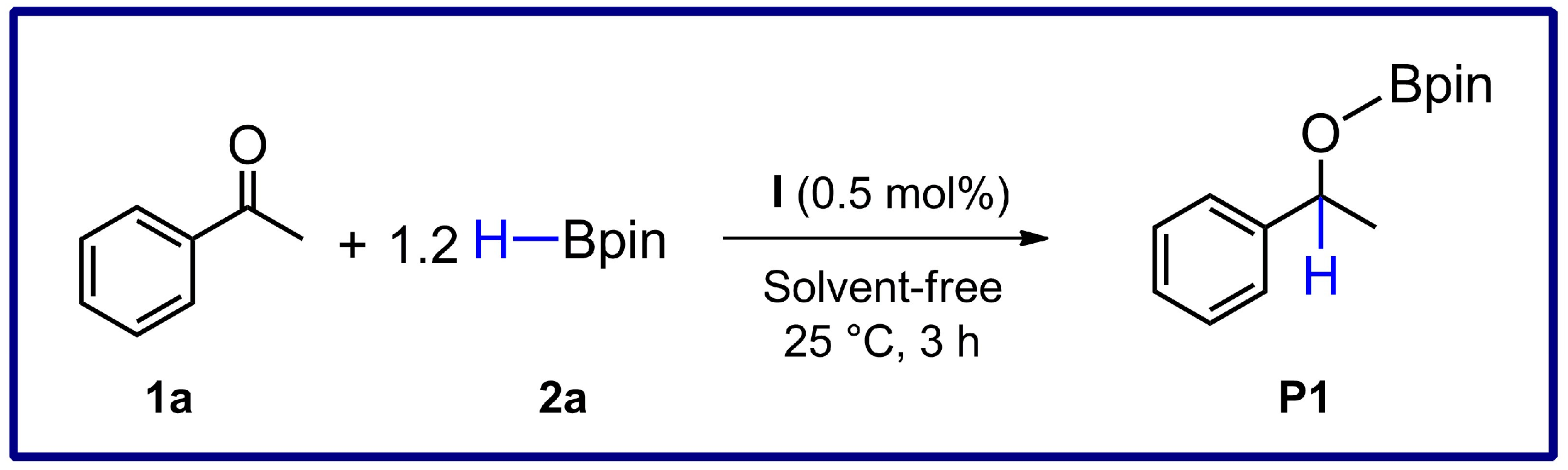
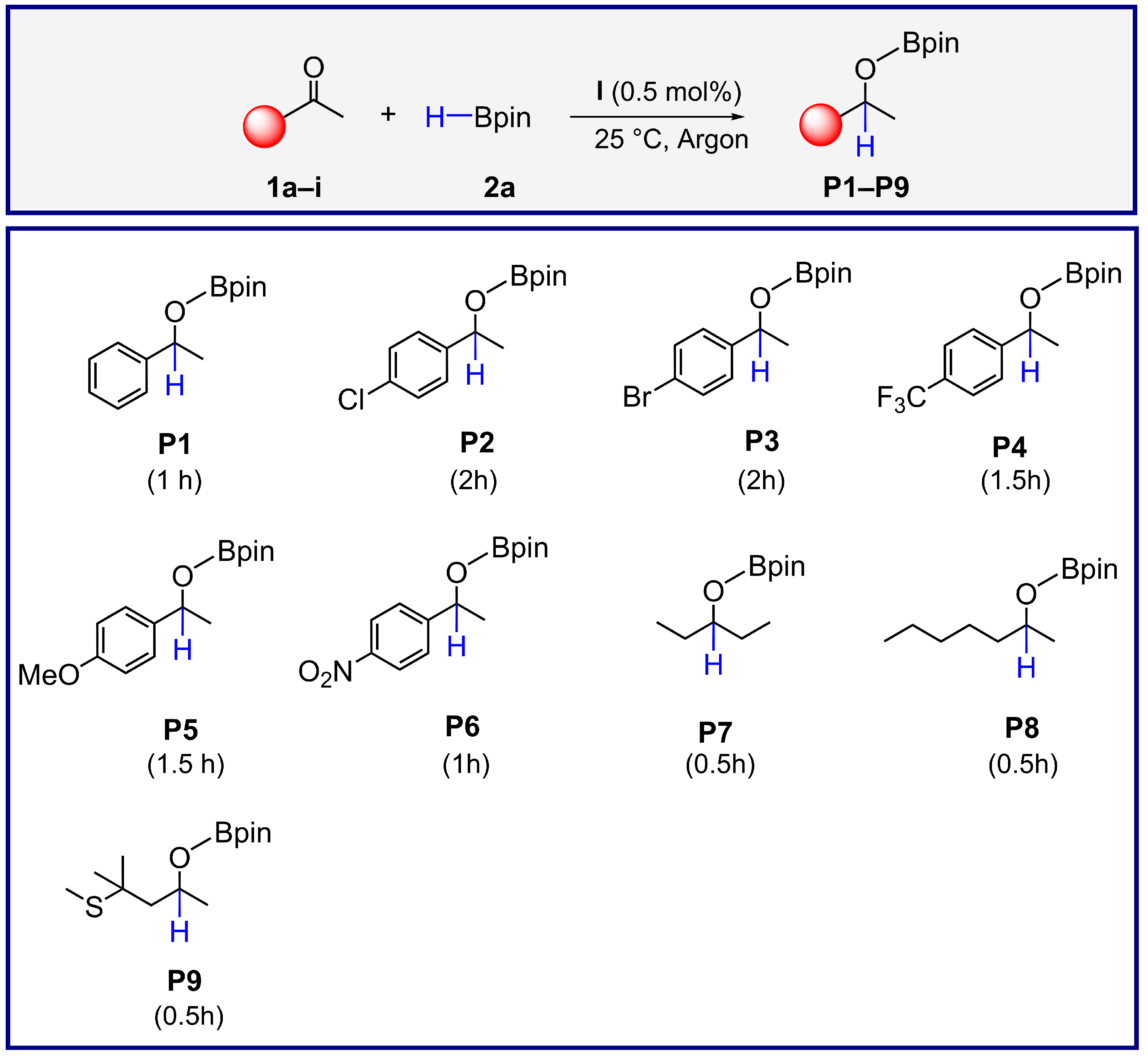
2.3. Catalytic Investigations
3. Materials and Methods
3.1. General Methods and Chemicals
3.2. General Procedure for the Synthesis of Amines
3.3. General Procedure for the Synthesis of 1,4-Diaza-1,3-Butadienes
3.4. General Procedure for the Synthesis of Mn-Complexes
3.5. General Procedure for Catalytic Tests
3.6. General Procedure for the Synthesis of Boronic Esters (P1–P9)
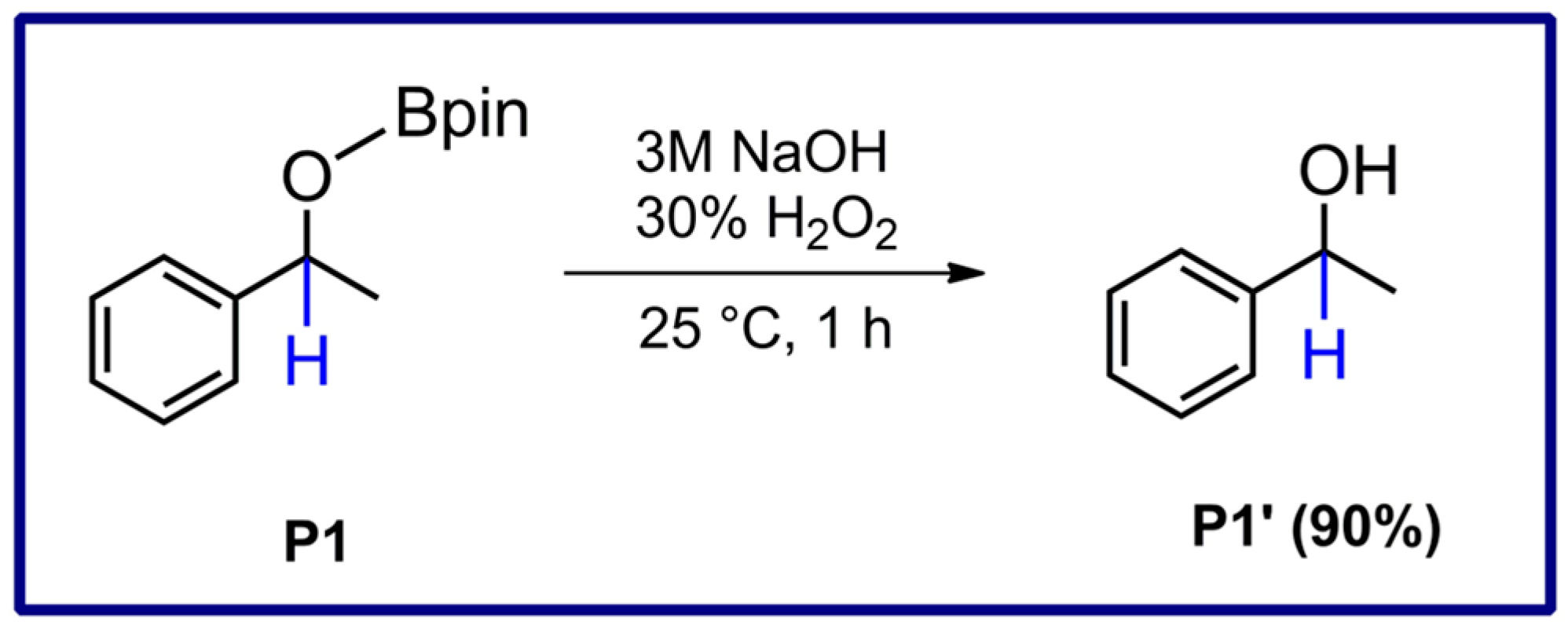
3.7. Procedure for Hydrolysis of P1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krumholz, P. Studies on the Coördinate Bond. II. Ferrous Complexes of α-Diimines. J. Am. Chem. Soc. 1953, 75, 2163–2166. [Google Scholar] [CrossRef]
- Cruz-Galván, A.; Bucio-Ortega, J.; Olguín, J. Complexes of Fe(II), Ni(II), and Zn(II) with a Tetradentate Naphthalene Diimine-Dipyrazole Ligand: Influence of a Metal Ion and Anions on the Luminescence Properties. Cryst. Growth Des. 2025, 25, 4466–4480. [Google Scholar] [CrossRef]
- de Zwart, F.J.; Reus, B.; Laporte, A.A.; Sinha, V.; de Bruin, B. Metrical oxidation states of 1, 4-diazadiene-derived ligands. Inorg. Chem. 2021, 60, 3274–3281. [Google Scholar] [CrossRef] [PubMed]
- Nikolaevskaya, E.N.; Druzhkov, N.O.; Syroeshkin, M.A.; Egorov, M.P. Chemistry of diazadiene type ligands with extra coordination groups. Prospects of reactivity. Coord. Chem. Rev. 2020, 417, 213353. [Google Scholar] [CrossRef]
- Yempally, V.; Shahbaz, A.; Fan, W.Y.; Madrahimov, S.T.; Bengali, A.A. Hydrosilylation of Aldehydes by a Manganese α-Diimine Complex. Inorganics 2020, 8, 61. [Google Scholar] [CrossRef]
- Kumar, A.; Sun, S.-S.; Lees, A.J. Photophysic and Photochemistry of Organometallic Rhenium Diimine Complexes. In Photophysics of Organometallics; Springer: Heidelberg, Germany, 2010; Volume 29, pp. 1–35. [Google Scholar]
- Zhang, R.; Wang, Y.; Zhao, Y.; Redshaw, C.; Fedushkin, I.L.; Wu, B.; Yang, X.-J. Main-group metal complexes of α-diimine ligands: Structure, bonding and reactivity. Dalton Trans. 2021, 50, 13634–13650. [Google Scholar] [CrossRef]
- Wu, R.; Wu, W.K.; Stieglitz, L.; Gaan, S.; Rieger, B.; Heuberger, M. Recent advances on α-diimine Ni and Pd complexes for catalyzed ethylene (Co)polymerization: A comprehensive review. Coord. Chem. Rev. 2023, 474, 214844. [Google Scholar] [CrossRef]
- Klimashevskaya, V.; Arsenyeva, K.V.; Maleeva, A.V.; Druzhkov, N.O.; Arsenyev, M.V.; Cherkasov, A.V.; Yakushev, I.A.; Aysin, R.R.; Piskunov, A.V. Donor-Acceptor Tin(IV) Complexes with α-Diimine and Catecholate Ligands. Eur. J. Inorg. Chem. 2023, 26, e202300540. [Google Scholar] [CrossRef]
- Yambulatov, D.S.; Lutsenko, I.A.; Nikolaevskii, S.A.; Petrov, P.P.; Smolyaninov, I.V.; Malyants, I.K.; Shender, V.O.; Kiskin, M.A.; Sidorov, A.A.; Berberova, N.T.; et al. α-Diimine Cisplatin Derivatives: Synthesis, Structure, Cyclic Voltammetry and Cytotoxicity. Molecules 2022, 27, 8565. [Google Scholar] [CrossRef]
- Mealli, C.; Ienco, A.; Philiipis, A.D.; Galindo, A. A Critical Review of Electronic Effects in Enediamido and α-Diimino Complexes of the Group 4 Metals. Eur. J. Inorg. Chem. 2007, 2007, 2556–2568. [Google Scholar] [CrossRef]
- Bart, S.C.; Hawrelak, E.J.; Lobkovsky, E.; Chirik, P.J. Low-valent α-diimine iron complexes for catalytic olefin hydrogenation. Organometallics 2005, 24, 5518–5527. [Google Scholar] [CrossRef]
- Mashima, K. Redox-Active α-Diimine Complexes of Early Transition Metals: From Bonding to Catalysis. Bull. Chem. Soc. Jpn. 2020, 93, 799–820. [Google Scholar] [CrossRef]
- Yempally, V.; Kyran, S.J.; Raju, R.K.; Fan, W.Y.; Brothers, E.N.; Darensbourg, D.J.; Bengali, A.A. Thermal and photochemical reactivity of manganese tricarbonyl and tetracarbonyl complexes with a bulky diazabutadiene ligand. Inorg. Chem. 2014, 53, 4081–4088. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, M.; Ali, R.; Jain, V.; Ballal, S.; Abosaoda, M.K.; Singh, A.; Krithiga, T.; Joshi, K.K.; Javahershenas, R. A comprehensive review on magnetic manganese as catalysts in organic synthesis. RSC Adv. 2025, 15, 23054–23088. [Google Scholar] [CrossRef]
- Coghi, P.S.; Zhu, Y.; Xie, H.; Hosmane, N.S.; Zhang, Y. Organoboron Compounds: Effective Antibacterial and Antiparasitic Agents. Molecules 2021, 26, 3309. [Google Scholar] [CrossRef]
- Jäkle, F. Recent Advances in the Synthesis and Applications of Organoborane Polymers. In Synthesis and Application of Organoboron Compounds; Topics in Organometallic Chemistry; Fernández, E., Whiting, A., Eds.; Wiley-VCH Verlag GmbH & Company KGaA and John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; Volume 49. [Google Scholar]
- Suzuki, A. Boronic Acids: Preparation and Applications in Organic Synthesis, Medicine and Materials; Wiley-VCH Verlag GmbH & Company KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef]
- Jana, R.; Pathak, T.P.; Sigman, M.S. Advances in Transition Metal (Pd, Ni, Fe)-Catalyzed Cross-Coupling Reactions Using Alkyl-organometallics as Reaction Partners. Chem. Rev. 2011, 111, 1417–1492. [Google Scholar] [CrossRef]
- Suzuki, A. Cross-Coupling Reactions of Organoboranes: An Easy Way to Construct C-C Bonds (Nobel Lecture). Angew. Chem. Int. Ed. 2011, 30, 6722–6737. [Google Scholar] [CrossRef]
- Zhang, G.; Zeng, H.; Wu, J.; Yin, Z.; Zheng, S.; Fettinger, J.C. Highly Selective Hydroboration of Alkenes, Ketones and Aldehydes Catalyzed by a Well-Defined Manganese Complex. Angew. Chem. 2016, 128, 14581–14584. [Google Scholar] [CrossRef]
- Vasilenko, V.; Blasius, C.K.; Gade, L.H. One-pot sequential kinetic profiling of a highly reactive manganese catalyst for ketone hydroboration: Leveraging σ-bond metathesis via alkoxide exchange steps. J. Am. Chem. Soc. 2018, 140, 9244–9254. [Google Scholar] [CrossRef]
- Vijjamarri, S.; O’Denius, T.M.; Yao, B.; Kubátová, A.; Du, G. Highly selective hydroboration of carbonyls by a manganese catalyst: Insight into the reaction mechanism. Organometallics 2020, 39, 3375–3383. [Google Scholar] [CrossRef]
- Zhang, G.; Zeng, H.; Li, S.; Johnson, J.; Mo, Z.; Neary, M.C.; Zheng, S. 1-D manganese (II)-terpyridine coordination polymers as precatalysts for hydrofunctionalisation of carbonyl compounds. Dalton Trans. 2020, 49, 2610–2615. [Google Scholar] [CrossRef]
- Elsby, M.R.; Son, M.; Oh, C.; Martin, J.; Baik, M.-H.; Baker, R.T. Mechanistic Study of Metal-Ligand Cooperativity in Mn(II)-Catalyzed Hydroborations: Hemilabile SNS Ligand Enables Metal Hydride-Free Reaction Pathway. ACS Catal. 2021, 11, 9043–9051. [Google Scholar] [CrossRef]
- Ghosh, P.; Jacobi von Wangelin, A. Manganese-Catalyzed Hydroborations with Broad Scope. Angew. Chem. 2021, 133, 16171–16179. [Google Scholar] [CrossRef]
- Thenarukandiyil, R.; Satheesh, V.; Shimon, L.J.W.; de Ruiter, G. Hydroboration of Nitriles, Esters, and Carbonates Catalyzed by Simple Earth-Abundant Metal Triflate Salts. Chem. Asian J. 2021, 16, 999–1006. [Google Scholar] [CrossRef]
- Erken, C.; Kaithal, A.; Sen, S.; Weyhermüller, T.; Hölscher, M.; Werlé, C.; Leitner, W. Manganese-catalyzed hydroboration of carbon dioxide and other challenging carbonyl groups. Nat. Commun. 2018, 9, 4521. [Google Scholar] [CrossRef]
- Barman, M.K.; Das, K.; Maji, B. Selective hydroboration of carboxylic acids with a homogeneous manganese catalyst. J. Org. Chem. 2019, 84, 1570–1579. [Google Scholar] [CrossRef] [PubMed]
- Kostera, S.; Peruzzini, M.; Kirchner, D.K.; Gonsalvi, L. Mild and selective carbon dioxide hydroboration to methoxyboranes catalyzed by Mn (I) PNP pincer complexes. ChemCatChem 2020, 12, 4625–4631. [Google Scholar] [CrossRef]
- Arroyo, V.D.; Arevalo, R. Tandem manganese catalysis for the chemo-, regio-, and stereoselective hydroboration of terminal alkynes: In situ precatalyst activation as a key to enhanced chemoselectivity. RSC Adv. 2024, 14, 5514–5523. [Google Scholar] [CrossRef] [PubMed]
- Szymańska, A.; Nowicki, M.; Dea, S.; Krupa, B.; Szyling, J.; Walkowiak, J. Catalytic Hydroboration of Ketones Using Mn(CO)5Br and Mn2(CO)10. Adv. Synth. Catal. 2025, 367, 7354590. [Google Scholar] [CrossRef]
- Meiries, S.; Speck, K.; Cordes, D.B.; Slawin, A.M.Z.; Nolan, S.P. [Pd(IPr*OMe)(acac)Cl]: Tuning the N-Heterocyclic Carbene in Catalytic C-N Bond Formation. Organometallics 2013, 32, 330–339. [Google Scholar] [CrossRef]
- Hans, M.; Lorkowski, J.; Demonceau, A.; Delaude, L. Efficient synthetic protocols for the preparation of common N-heterocyclic carbene precursors. Beilstein J. Org. Chem. 2015, 11, 2318–2325. [Google Scholar] [CrossRef]
- Henke, W.C.; Kerr, T.A.; Scheridan, T.R.; Henling, L.M.; Takase, M.K.; Day, V.W.; Gray, H.B.; Blakemore, J.D. Synthesis, structural studies, and redox chemistry of bimetallic [Mn(CO)3] and [Re(CO)3] complexes. Dalton Trans. 2021, 50, 2746–2756. [Google Scholar] [CrossRef]
- Kanno, T.; Takaseb, T.; Oyamab, D. Synthesis and crystal structures of manganese(I) carbonyl complexes bearing ester-substituted a-diimine ligands. Acta Cryst. 2020, E76, 1433–1436. [Google Scholar] [CrossRef] [PubMed]
- Singh, K.K.; Gerke, C.S.; Saund, S.S.; Zito, A.M.; Siegler, M.A.; Thoi, V.S. CO2 Activation with Manganese Tricarbonyl Complexes through an H-Atom Responsive Benzimidazole Ligand. Chem. Eur. J. 2023, 29, e202300796. [Google Scholar] [CrossRef]
- Jimenez, J.; Chakraborty, I.; Mascharak, P.K. Synthesis and Assessment of CO-Release Capacity of Manganese Carbonyl Complexes Derived from Rigid α-Diimine Ligands of Varied Complexity. Eur. J. Inorg. Chem. 2015, 30, 5021–5026. [Google Scholar] [CrossRef]
- Chakraborty, I.; Carrington, S.J.; Mascharak, P.K. Design Strategies To Improve the Sensitivity of Photoactive Metal Carbonyl Complexes (photoCORMs) to Visible Light and Their Potential as CO-Donors to Biological Targets. Acc. Chem. Res. 2014, 47, 2603–2611. [Google Scholar] [CrossRef]
- Hernández, J.G.; Butler, I.S.; Friščić, T. Multi-step and multi-component organometallic synthesis in one pot using orthogonal Mechanochemical properties. Chem. Sci. 2014, 5, 3576–3582. [Google Scholar] [CrossRef]
- Vielhaber, T.; Topf, C. Manganese-catalyzed homogeneous hydrogenation of ketones and conjugate reduction of α,β-unsaturated carboxylic acid derivatives: A chemoselective, robust, and phosphine-free in situ-protocol. Appl. Catal. A-Gen. 2021, 623, 118280. [Google Scholar] [CrossRef]
- Paqui, M.S.S.; Glitz, V.A.; Durigon, D.C.; Amorim, A.L.; Caramori, G.F.; Parreira, R.L.T.; Bortoluzzi, A.J.; Xavier, F.R.; Peralta, R.A. Spectroscopical and Molecular Studies of Four Manganese(I) PhotoCORMs with Bioinspired Ligands Containing Non-Coordinated Phenol Groups. Molecules 2023, 28, 3439. [Google Scholar] [CrossRef]
- SNapierała, S.; Kubicki, M.; Wałęsa-Chorab, M. Toward electrochromic metallopolymers: Synthesis and properties of polyazomethines based on complexes of transition-metal ions. Inorg. Chem. 2021, 60, 14011–14021. [Google Scholar] [CrossRef] [PubMed]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies, 3rd ed.; John Wiley & Sons, Ltd.: West Sussex, UK, 2001. [Google Scholar]
- Ossinger, S.; Prescimone, A.; Häussinger, D.; Wenger, O.S. Manganese(I) Complex with Monodentate Arylisocyanide Ligands Shows Photodissociation Instead of Luminescence. Inorg. Chem. 2022, 61, 10533–10547. [Google Scholar] [CrossRef]
- Wałęsa-Chorab, M.; Banasz, R.; Marcinkowski, D.; Kubicki, M.; Patroniak, V. Electrochromism and electrochemical properties of complexes of transition metal ions with benzimidazole-based ligand. RSC Adv. 2017, 7, 50858–50867. [Google Scholar] [CrossRef]
- Napierała, S.; Wałęsa-Chorab, M. On-substrate postsynthetic metal ion exchange as a tool for tuning electrochromic properties of materials. Eur. J. Polym. 2020, 140, 110052. [Google Scholar] [CrossRef]
- GCanard, G.; Ponce-Vargas, M.; Jacquemin, D.; Le Guennic, B.; Felouat, A.; Rivoal, M.; Zaborova, E.; D’ALéo, A.; Fages, F. Influence of the electron donor groups on the optical and electrochemical properties of borondifluoride complexes of curcuminoid derivatives: A joint theoretical and experimental study. RSC Adv. 2017, 7, 10132–10142. [Google Scholar] [CrossRef]
- Guo, C.; Li, C.; Wang, W.; Zhang, B.; Hou, Y.; Wang, X.; Zhou, Q. Electronic effects on polypyridyl Co complex-based water reduction catalysts. RSC Adv. 2021, 11, 24359–24365. [Google Scholar] [CrossRef] [PubMed]
- Fyfe, J.W.; Watson, A.J. Recent developments in organoboron chemistry: Old dogs, new tricks. Chem 2017, 3, 31–55. [Google Scholar] [CrossRef]
- Galardon, E.; Ramdeehul, S.; Brown, J.M.; Cowley, A.; Hii, K.K.; Jutand, A. Profound Steric Control of Reactivity in Aryl Halide Addition to Bisphosphane Palladium(0) Complexes. Angew. Chem. Int. Ed. 2002, 41, 1760–1763. [Google Scholar] [CrossRef]
- Altenhoff, G.; Goddard, R.; Lehmann, C.W.; Glorius, F. An N-Heterocyclic Carbene Ligand with Flexible Steric Bulk Allows Suzuki Cross-Coupling of Sterically Hindered Aryl Chlorides at Room Temperature. Angew. Chem. Int. Ed. 2003, 42, 3690–3693. [Google Scholar] [CrossRef] [PubMed]
- Hua, D.; Xu, P. Recent advances in biotechnological production of 2-phenylethanol. Biotechnol. Adv. 2011, 29, 654–660. [Google Scholar] [CrossRef]
- Bernardino, A.R.S.; Torres, C.A.V.; Crespo, J.G.; Reis, M.A.M. Biotechnological 2-Phenylethanol Production: Recent Developments. Molecules 2024, 29, 5761. [Google Scholar] [CrossRef]
- Williams, D.B.G.; Lawton, M. Drying of Organic Solvents: Quantitative Evaluation of the Efficiency of Several Desiccants. J. Org. Chem. 2010, 75, 8351–8354. [Google Scholar] [CrossRef]
- Connelly, N.G.; Geiger, W.E. Chemical Redox Agents for Organometallic Chemistry. Chem. Rev. 1996, 96, 877–910. [Google Scholar] [CrossRef]
- Cook, A.; Newman, S.G. Alcohols as Substrates in Transition-Metal-Catalyzed Arylation, Alkylation, and Related Reactions. Chem. Rev. 2024, 124, 6078–6144. [Google Scholar] [CrossRef]
- Willcox, D.; Carden, J.L.; Ruddy, A.J.; Newman, P.D.; Melen, R.L. Asymmetric ketone hydroboration catalyzed by alkali metal complexes derived from BINOL ligands. Dalton Trans. 2020, 49, 2417–2420. [Google Scholar] [CrossRef] [PubMed]
- CrysAlisPro, version 1.171.44.108a; Rigaku: Tokyo, Japan, 2025.
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge structural database. Acta Crystallogr. 2016, B72, 171–179. [Google Scholar] [CrossRef]
- Bruker, SAINT, V8.41; Bruker AXS Inc.: Madison, WI, USA, 2025.
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Cryst. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Kratzert, D. FinalCif, (Bruker Edition). Available online: https://dkratzert.de/finalcif.html (accessed on 22 September 2025).


| Complex | ν(C=O) | ν(C=N) | δ(Mn-CO) | ν(Mn-C) | ν(Mn-Br) |
|---|---|---|---|---|---|
| Mn(CO)5Br | 2081, 2035, 1985 | - | 621 | 405 | 218 |
| Mn1 | 2025, 1952, 1931 | 1626 | 623 | 405 | 220 |
| I | 2027, 1959, 1909 | 1622 | 621 | 405 | 224 |
| II | 2029, 1958, 1905 | 1619 | 625 | 407 | 218 |
| III | 2031, 1963, 1911 | 1620 | 621 | 405 | 220 |
| IV | 2027, 1959, 1906 | 1621 | 622 | 405 | 218 |
| V | 2025, 1948, 1917 | 1626 | 623 | 405 | 222 |
| Entry | Catalyst | [Catalyst] (mol%) | Time (h) | Yield of P1 (%) [a] |
|---|---|---|---|---|
| 1 | Mn(CO)5Br | 0.5 | 3 | 97 |
| 2 | Mn(CO)5Br | 0.5 | 2 | 77 |
| 3 | I | 0.5 | 3 | 100 |
| 4 | I | 0.5 | 1 | 100 |
| 5 | I | 0.5 | 0.5 | 94 |
| 6 | I | 0.2 | 8 | 100 |
| 7 | I | 0.1 | 24 | 64 |
| 8 | II | 0.5 | 1 | 100 |
| 9 | III | 0.5 | 1.5 | 99 |
| 10 | IV | 0.5 | 1 | 100 |
| 11 | V | 0.5 | 1 | 100 |
| 12 | Mn1 | 0.5 | 1 | 40 |
| 13 | Mn1 | 0.5 | 5 | 61 |
| 14 | Mn1 | 0.5 | 12 | 82 |
| 15 | - | - | 72 | 0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mermela, A.; Duch, A.; Wałęsa-Chorab, M.; Żak, P. Manganese Complexes Bearing Bulky DAB Ligands as Efficient Catalysts for the Solvent-Free Hydroboration of Ketones. Int. J. Mol. Sci. 2025, 26, 10454. https://doi.org/10.3390/ijms262110454
Mermela A, Duch A, Wałęsa-Chorab M, Żak P. Manganese Complexes Bearing Bulky DAB Ligands as Efficient Catalysts for the Solvent-Free Hydroboration of Ketones. International Journal of Molecular Sciences. 2025; 26(21):10454. https://doi.org/10.3390/ijms262110454
Chicago/Turabian StyleMermela, Aleksandra, Agata Duch, Monika Wałęsa-Chorab, and Patrycja Żak. 2025. "Manganese Complexes Bearing Bulky DAB Ligands as Efficient Catalysts for the Solvent-Free Hydroboration of Ketones" International Journal of Molecular Sciences 26, no. 21: 10454. https://doi.org/10.3390/ijms262110454
APA StyleMermela, A., Duch, A., Wałęsa-Chorab, M., & Żak, P. (2025). Manganese Complexes Bearing Bulky DAB Ligands as Efficient Catalysts for the Solvent-Free Hydroboration of Ketones. International Journal of Molecular Sciences, 26(21), 10454. https://doi.org/10.3390/ijms262110454






