Purine Nucleotide Precursors in Preventing Myocardial Ischemia–Reperfusion Injury
Abstract
1. Introduction
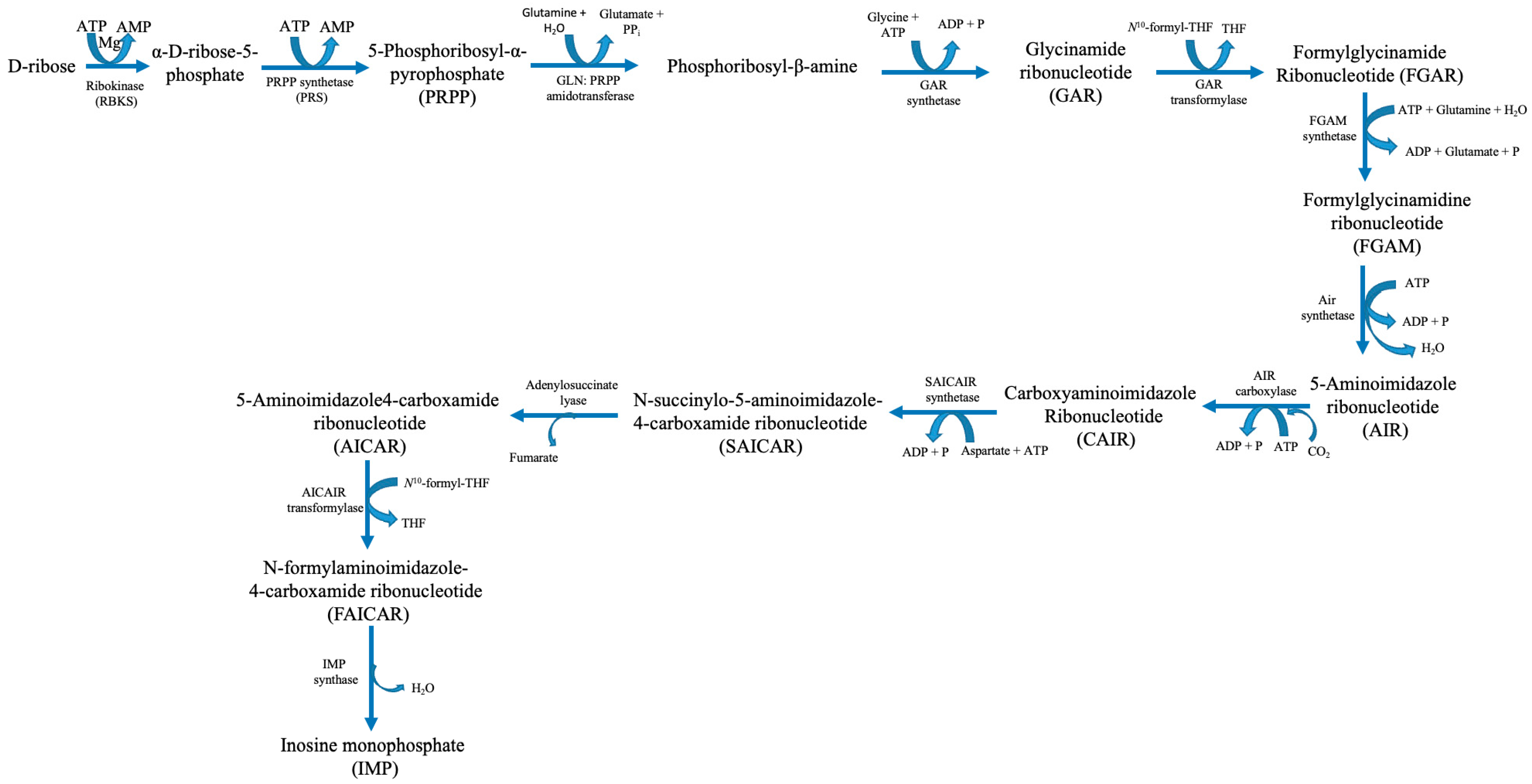
2. Human Purine Metabolism
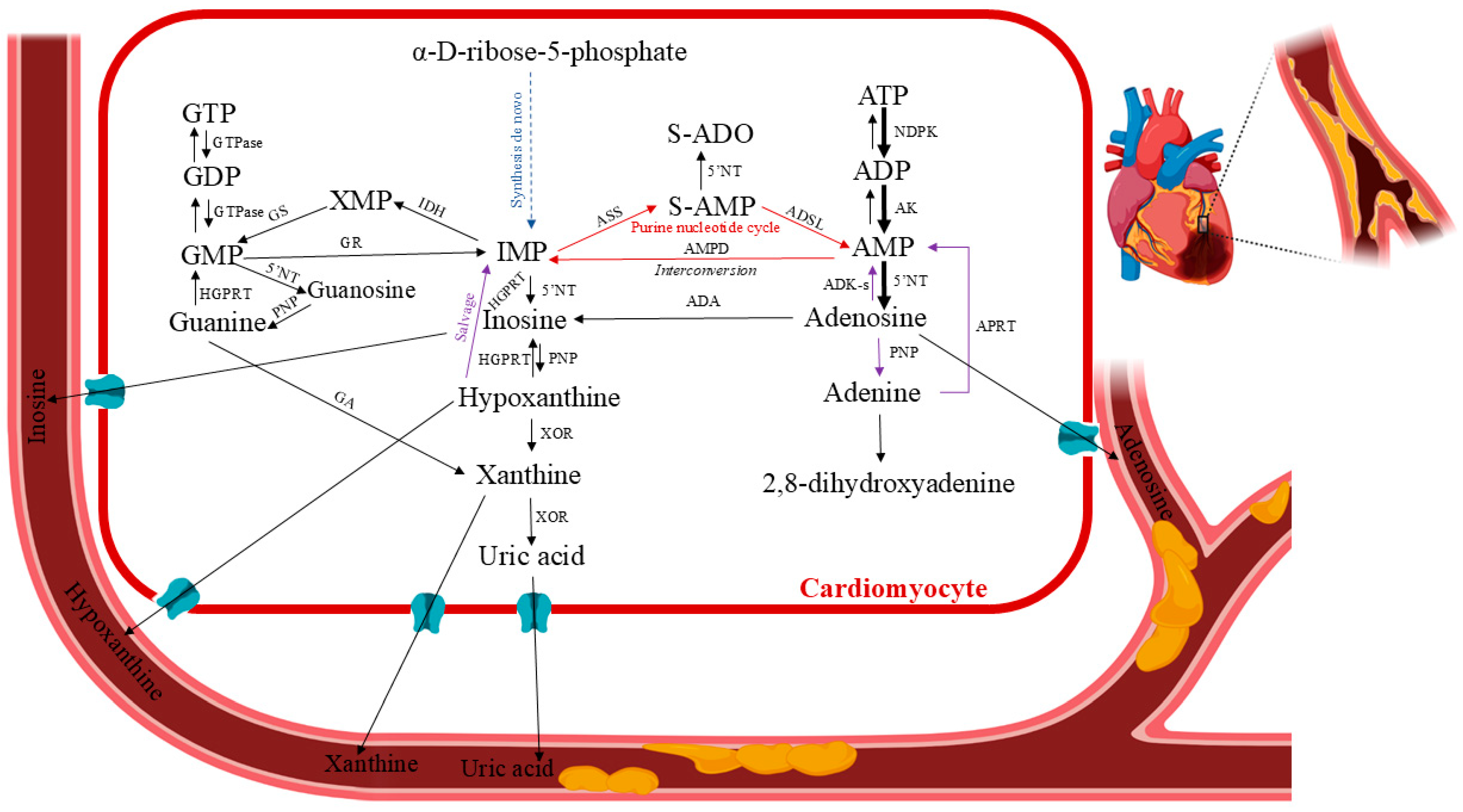
3. Human Purine Metabolism in Cardiac Ischemia
4. Purine Nucleotide Precursors Prevent Ischemia/Reperfusion Injury
4.1. D-Ribose
| Purine Nucleotide Precursor | Objectives | Significant Findings | Implications for Future Research | Study References |
|---|---|---|---|---|
| D-ribose | Isoproterenol-induced alterations in cardiac adenine nucleotides and morphology by D-ribose. | Supplementation with D-ribose decreased the incidence of isoproterenol-induced cardiac cell damage, and the diminution of adenine nucleotides was completely avoided. | Heart necrosis develops as a result of adenine nucleotide deficiency. | Zimmer, H.-G.; 1980 [28] |
| Determination of the effects of D-ribose infusion in a long-term model of global ischemia. | D-ribose infusion significantly enhanced the recovery of energy levels in the postischemic heart. | D-ribose could be a promising therapeutic agent for enhancing cardiac function after ischaemic events. Further research is necessary to investigate the underlying mechanisms | St. Cyr, J.A.; 1989 [32] | |
| A study of the effect of D-ribose on heart function and infarct size after myocardial infarction (MI). | Six hours after MI, ribose treatment dramatically decreased MI size and enhanced left ventricular function. Ribose treatment contributes to maintaining the remote myocardium’s function | Increasing myocardial energy levels enhances function and may postpone long-term alterations, such as apoptosis, in several surgically curable chronic heart failure disorders. | González, G.E.; 2009 [39] | |
| D-ribose supplementation for patients with congestive heart failure (CHF) who often reported exhaustion and dyspnoea. | Patients with class II–III CHF and left ventricular dysfunction were able to maintain their VO2max, and they increased their ventilatory efficiency. They showed a satisfactory trend in their daily quality of life assessment when they supplemented with D-ribose. | For advanced CHF, D-ribose should definitely be taken into consideration as an addition to standard therapy regimens. | Carter O.; 2005 [47] | |
| Determination of whether ubiquinol and/or d-ribose would reduce the symptoms and improve cardiac performance in patients with heart failure with preserved ejection fraction (HFpEF). | A treatment with ubiquinol or D-ribose improved EF and production of ATP while lowering HF symptoms in patients with HFpEF. The results were not further improved by adding D-ribose to ubiquinol treatment, indicating that either supplement, by itself, is adequate to enhance physiologic variables and symptoms, but requires the study dosage. | Phase 3 clinical studies, which are carried out in numerous clinics worldwide, must involve a greater number of patients. | Pierce, J.D.; 2020 [48] | |
| AICAR | Investigation of the acute effects of AICAR on adenine nucleotides, inosine monophosphate (IMP), and postischemic ventricular function | The rise in IMP indicates that AICAR was phosphorylated and incorporated in the normal and postischemic myocardium over a comparatively brief perfusion interval, but it was unable to improve function recovery or raise AMP or ATP levels. | AICAR was not a useful pharmacologic technique for evaluating the connection between the recovery of ventricular function and the postischemic adenine nucleotide pool. | Mentzer, R.M.; 1988 [49] |
| Evaluation of the effects of AICAR on myocardial ischemia, left ventricular function, myocardial infarction, heart failure, life-threatening dysrhythmias, and death in patients undergoing coronary artery bypass graft (CABG) surgery. | The study demonstrated that the administration of AICAR perioperatively is safe in patients undergoing CABG surgery. It limits the severity of post-bypass myocardial ischemia as shown by shorter ischemic duration in patients receiving high doses of AICAR. | More research was needed to evaluate whether AICAR can significantly reduce the incidence of myocardial infarction and the severity of cardiac damage. | Leung, J.M.; 1994 [50] | |
| Involvement in improving contractile dysfunction by AICAR by increasing adenosine release in ischemic myocardium. | The results indicate that reperfusion injury is unavoidable but can be mitigated. The study demonstrated that AICAR administration significantly enhanced contractile dysfunction after a short duration of myocardial ischemia through adenosine-dependent mechanisms. | Additional efforts are required for the clinical application of AICAR. | Kitakaze, M.; 1999 [51] | |
| AICAR-dependent AMPK activation involvement in improving scar formation in the aged heart in a model of MI | AICAR avoids unfavourable remodelling and enhances post-ischemic cardiac function. | A new treatment approach for preventing harmful remodelling in the ageing heart may result from these findings. | Cieslik, K.A.; 2013 [52] | |
| Inosine | Regulatory mechanisms involved in the therapeutic use of purines for the treatment of ischemic heart disease. | Inosine and hypoxanthine were incorporated into both the ATP and GTP pools in the heart. This process is stimulated after ischemia and by ribose perfusion and is thereby dependent on myocardial PRPP concentrations. | Inosine seemed to restore ATP levels, which could be beneficial after ischemia | Harmsen, E.; 1984 [31] |
| The influence of supplementation with inosine of cold cardioplegia (CPS) and recovery perfusate on the cardiac output, ATP and total adenine nucleotide content. | Nucleotide levels and cardiac output recovery were enhanced by the addition of inosine to the recovery perfusate and CPS. | These findings suggest that functional recovery from cardioplegia is hindered by the washout of nucleotide breakdown products in the cytosol or during reperfusion, which prevents their rescue for nucleotide resynthesis. | DeWitt, D.F.; 1983 [53] | |
| The effects of inosine on ischemia/reperfusion injury in a rat heart transplantation model. | Inosine enhanced myocardial and endothelial function at early reperfusion after heart transplantation with a continuing protective effect against reperfusion-induced graft coronary endothelial dysfunction. Peroxynitrite-poly(ADP-ribose) polymerase (PARP) pathway modification may be at least partially responsible for inosine’s actions. | Inosine seemed to function as a nonprofessional but rather effective inhibitor of PARP activation. To clarify the precise mechanism of action of inosine therapy, more research is required. | Szabó, G.; 2006 [54] | |
| Plasma inosine levels as a valuable diagnostic marker of pre-necrosis cardiac ischaemia. | A possible biomarker for early cardiac ischaemia could be the amount of inosine present in animals exposed to cardiac oxidative stress. | Increased inosine levels could be indicative of early cardiac ischaemia and should be determined by preliminary human investigations. | Farthing, D.; 2006 [55] | |
| Adenine | The effect of adenine on myocardial ATP content in the post-anoxic nonworking rat heart | The results demonstrated that a 50 μM dose of adenine could regulate ATP concentrations during 60 min of anoxia in the nonworking rat heart; however, increasing the adenine dosage to 1 mM resulted in a decrease in tissue ATP concentration. | Data suggested a potential dose-dependent effect of adenine on ATP metabolism, highlighting the need for further investigation into the mechanisms underlying these changes. | Halle, A.A.; 1989 [56] |
| Development of concurrent kidney and cardiovascular injury induced by chronic dietary adenine intake. | Treatment with 0.25% adenine in rats resulted in chronic renal and cardiovascular damage. Cardiovascular alterations encompassed heightened ventricular fibrosis, elevated systolic blood pressure, increased left ventricular stiffness, and compromised vascular responses. | These findings suggest a significant correlation between adenine exposure and the deterioration of both renal and cardiovascular functions. Further investigation was warranted to elucidate the underlying mechanisms contributing to these adverse effects. | Diwan, V.; 2013 [57] | |
| Endogenous adenine as a potential driver of the cardiovascular-kidney-metabolic (CKM) syndrome | Research has demonstrated that endogenous adenine has a causative role in heart failure and ischemic heart disease within the context of CKM syndrome | The importance of further exploring the biochemical pathways involved in adenine metabolism. Understanding these mechanisms could lead to novel therapeutic strategies for managing heart-related conditions associated with CKM syndrome. | Tamayo, I.; 2024 [58] | |
| Hypoxanthine | The development of the high-pressure liquid chromatographic system for the determination of purine nucleosides in the blood. | Increased ischemic heart’s synthesis of hypoxanthine was detected. | The high-pressure liquid chromatographic assay of blood hypoxanthine as a useful tool in the diagnosis of ischemic heart disease. | Harmsen, E.; 1981 [59] |
| The development of a rapid and simple chemiluminescence method was developed for screening levels of inosine and hypoxanthine in human plasma | The capacity to distinguish among total hypoxanthine levels in healthy individuals and patients presenting with non-traumatic chest pain and possible acute cardiac ischaemia was proven by the fast chemiluminescence approach. | Chemiluminescence technology may be used as a diagnostic tool to quickly check for high levels of hypoxanthine and inosine in human plasma, which may be indicators of acute myocardial ischaemia. | Farthing, D.E.; 2011 [60] |
4.2. 5-Aminoimidazole-4-Carboxamide Ribonucleotide (AICAR)
4.3. Adenine
4.4. Inosine
4.5. Hypoxanthine
5. Conclusions/Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pagliaro, B.R.; Cannata, F.; Stefanini, G.G.; Bolognese, L. Myocardial Ischemia and Coronary Disease in Heart Failure. Heart Fail. Rev. 2020, 25, 53–65. [Google Scholar] [CrossRef]
- de Groot, H.; Rauen, U. Ischemia-Reperfusion Injury: Processes in Pathogenetic Networks: A Review. Transplant. Proc. 2007, 39, 481–484. [Google Scholar] [CrossRef]
- Hearse, D.J. Ischemia, Reperfusion, Tissue Injury and the Determinants of Tissue Injury. Cardiovasc. Drugs Ther. 1990, 4, 767–776. [Google Scholar] [CrossRef]
- Baines, C.P.; Molkentin, J.D. STRESS Signaling Pathways That Modulate Cardiac Myocyte Apoptosis. J. Mol. Cell Cardiol. 2005, 38, 47–62. [Google Scholar] [CrossRef]
- Pareek, V.; Tian, H.; Winograd, N.; Benkovic, S.J. Metabolomics and Mass Spectrometry Imaging Reveal Channeled de Novo Purine Synthesis in Cells. Science 2020, 368, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Pareek, V.; Pedley, A.M.; Benkovic, S.J. Human de Novo Purine Biosynthesis. Crit. Rev. Biochem. Mol. Biol. 2021, 56, 1–16. [Google Scholar] [CrossRef]
- Braczko, A.; Kutryb-Zajac, B.; Jedrzejewska, A.; Krol, O.; Mierzejewska, P.; Zabielska-Kaczorowska, M.; Slominska, E.M.; Smolenski, R.T. Cardiac Mitochondria Dysfunction in Dyslipidemic Mice. Int. J. Mol. Sci. 2022, 23, 11488. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhang, E.Y.; Zhang, J.; Bache, R.J.; Beard, D.A. Phosphate Metabolite Concentrations and ATP Hydrolysis Potential in Normal and Ischaemic Hearts. J. Physiol. 2008, 586, 4193. [Google Scholar] [CrossRef] [PubMed]
- Carlucci, F.; Tabucchi, A.; Biagioli, B.; Simeone, F.; Scolletta, S.; Rosi, F.; Marinello, E. Cardiac Surgery: Myocardial Energy Balance, Antioxidant Status and Endothelial Function after Ischemia-Reperfusion. Biomed. Pharmacother. 2002, 56, 483–491. [Google Scholar] [CrossRef]
- Soares, R.O.S.; Losada, D.M.; Jordani, M.C.; Évora, P.; Castro-e-Silva, O. Ischemia/Reperfusion Injury Revisited: An Overview of the Latest Pharmacological Strategies. Int. J. Mol. Sci. 2019, 20, 5034. [Google Scholar] [CrossRef]
- Carden, D.L.; Granger, D.N. Pathophysiology of Ischaemia ± reperfusion Injury. J. Pathol. 2000, 190, 255–266. [Google Scholar] [CrossRef]
- Grisham, M.B.; Granger, D.N.; Lefer, D.J. Modulation of Leukocyte-Endothelial Interactions by Reactive Metabolites of Oxygen and Nitrogen: Relevance to Ischemic Heart Disease. Free Radic. Biol. Med. 1998, 25, 404–433. [Google Scholar] [CrossRef]
- Reimer, K.A.; Hill, M.L.; Jennings, R.B. Prolonged Depletion of ATP and of the Adenine Nucleotide Pool Due to Delayed Resynthesis of Adenine Nucleotides Following Reversible Myocardial Ischemic Injury in Dogs. J. Mol. Cell Cardiol. 1981, 13, 229–239. [Google Scholar] [CrossRef]
- Chandel, N.S. Nucleotide Metabolism. Cold Spring Harb. Perspect. Biol. 2021, 13, a040592. [Google Scholar] [CrossRef]
- Kutryb-Zajac, B.; Mierzejewska, P.; Slominska, E.M.; Smolenski, R.T. Therapeutic Perspectives of Adenosine Deaminase Inhibition in Cardiovascular Diseases. Molecules 2020, 25, 4652. [Google Scholar] [CrossRef]
- Kutryb-Zając, B.; Kawecka, A.; Nasadiuk, K.; Braczko, A.; Stawarska, K.; Caiazzo, E.; Koszałka, P.; Cicala, C. Drugs Targeting Adenosine Signaling Pathways: A Current View. Biomed. Pharmacother. 2023, 165, 115184. [Google Scholar] [CrossRef] [PubMed]
- Zukowska, P.; Kutryb-Zajac, B.; Jasztal, A.; Toczek, M.; Zabielska, M.; Borkowski, T.; Khalpey, Z.; Smolenski, R.T.; Slominska, E.M. Deletion of CD73 in Mice Leads to Aortic Valve Dysfunction. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Kutryb-Zajac, B.; Zukowska, P.; Toczek, M.; Zabielska, M.; Lipinski, M.; Rybakowska, I.; Chlopicki, S.; Slominska, E.M.; Smolenski, R.T. Extracellular Nucleotide Catabolism in Aortoiliac Bifurcation of Atherosclerotic ApoE/LDLr Double Knock out Mice. Nucleosides Nucleotides Nucleic Acids 2014, 33, 323–328. [Google Scholar] [CrossRef] [PubMed]
- Kutryb-Zajac, B.; Mateuszuk, L.; Zukowska, P.; Jasztal, A.; Zabielska, M.A.; Toczek, M.; Jablonska, P.; Zakrzewska, A.; Sitek, B.; Rogowski, J.; et al. Increased Activity of Vascular Adenosine Deaminase in Atherosclerosis and Therapeutic Potential of Its Inhibition. Cardiovasc. Res. 2016, 112, 590–605. [Google Scholar] [CrossRef]
- Zabielska, M.A.; Borkowski, T.; Slominska, E.M.; Smolenski, R.T. Inhibition of AMP Deaminase as Therapeutic Target in Cardiovascular Pathology. Pharmacol. Rep. 2015, 67, 682–688. [Google Scholar] [CrossRef]
- Smolenski, R.T.; Rybakowska, I.; Turyn, J.; Romaszko, P.; Zabielska, M.; Taegtmeyer, A.; Słomińska, E.M.; Kaletha, K.K.; Barton, P.J.R. AMP Deaminase 1 Gene Polymorphism and Heart Disease—A Genetic Association That Highlights New Treatment. Cardiovasc. Drugs Ther. 2014, 28, 183–189. [Google Scholar] [CrossRef]
- Zimmer, H.G.; Trendelenburg, C.; Kammermeier, H.; Gerlach, E. De Novo Synthesis of Myocardial Adenine Nucleotides in the Rat. Acceleration during Recovery from Oxygen Deficiency. Circ. Res. 1973, 32, 635–642. [Google Scholar] [CrossRef]
- Maguire, M.H.; Lukas, M.C.; Rettie, J.F. Adenine Nucleotide Salvage Synthesis in the Rat Heart; Pathways of Adnosine Salvage. Biochim. Biophys. Acta 1972, 262, 108–115. [Google Scholar] [CrossRef]
- Zimmer, H.G. Significance of the 5-Phosphoribosyl-1-Pyrophosphate Pool for Cardiac Purine and Pyrimidine Nucleotide Synthesis: Studies with Ribose, Adenine, Inosine, and Orotic Acid in Rats. Cardiovasc. Drugs Ther. 1998, 12 (Suppl. S2), 179–187. [Google Scholar] [CrossRef] [PubMed]
- Imai, S.; Riley, A.L.; Berne, R.M. Effect of Ischemia on Adenine Nucleotides in Cardiac and Skeletal Muscle. Circ. Res. 1964, 15, 443–450. [Google Scholar] [CrossRef]
- Zabielska-Kaczorowska, M.A.; Braczko, A.; Pelikant-Malecka, I.; Slominska, E.M.; Smolenski, R.T. Hidden Pool of Cardiac Adenine Nucleotides That Controls Adenosine Production. Pharmaceuticals 2023, 16, 599. [Google Scholar] [CrossRef] [PubMed]
- Zabielska-Kaczorowska, M.A.; Stawarska, K.; Kawecka, A.; Urbanowicz, K.; Smolenski, R.T.; Kutryb-Zajac, B. Nucleotide Depletion in Hypoxia Experimental Models of Mouse Myocardial Slices. Nucleosides Nucleotides Nucleic Acids 2024, 43, 770–782. [Google Scholar] [CrossRef]
- Zimmer, H.-G.; Ibel, H.; Steinkopff, G.; Korb, G. Reduction of the Isoproterenol-Induced Alterations in Cardiac Adenine Nucleotides and Morphology by Ribose. Science 1980, 207, 319–321. [Google Scholar] [CrossRef] [PubMed]
- Zimmer, H.G.; Gerlach, E. Stimulation of Myocardial Adenine Nucleotide Biosynthesis by Pentoses and Pentitols. Pflug. Arch. 1978, 376, 223–227. [Google Scholar] [CrossRef]
- Mahoney, D.E.; Hiebert, J.B.; Thimmesch, A.; Pierce, J.T.; Vacek, J.L.; Clancy, R.L.; Sauer, A.J.; Pierce, J.D. Understanding D-Ribose and Mitochondrial Function. Adv. Biosci. Clin. Med. 2018, 6, 1. [Google Scholar] [CrossRef]
- Harmsen, E.; de Tombe, P.P.; de Jong, J.W.; Achterberg, P.W. Enhanced ATP and GTP Synthesis from Hypoxanthine or Inosine after Myocardial Ischemia. Am. J. Physiol. 1984, 246, H37–H43. [Google Scholar] [CrossRef]
- St. Cyr, J.A.; Bianco, R.W.; Schneider, J.R.; Mahoney, J.R.; Tveter, K.; Einzig, S.; Foker, J.E. Enhanced High Energy Phosphate Recovery with Ribose Infusion after Global Myocardial Ischemia in a Canine Model. J. Surg. Res. 1989, 46, 157–162. [Google Scholar] [CrossRef]
- Schneider, J.R.; St. Cyr, J.; Mahoney, J.R.; Bianco, R.W.; Ring, W.S.; Foker, J.E. Recovery of ATP and Return of Function after Global Ischemia. Circulation 1985, 72, 298. [Google Scholar]
- St. Cyr, J.; Ward, H.; Kriett, J.; Alyono, D.; Einzig, S.; Bianco, R.; Anderson, R.; Foker, J. Long Term Model for Evaluation of Myocardial Metabolic Recovery Following Global Ischemia. Adv. Exp. Med. Biol. 1986, 194, 401–414. [Google Scholar] [CrossRef] [PubMed]
- Tveter, K.; St. Cyr, J.A.; Schneider, J.; Bianco, R.; Foker, J. Enhanced Recovery of Diastolic Function after Global Myocardial Ischemia in the Intact Animal. Pediatr. Res. 1988, 23, 226A. [Google Scholar]
- Shecterle, L.M.; St. Cyr, J.A. Myocardial Ischemia: Alterations in Myocardial Cellular Energy and Diastolic Function, a Potential Role for D-Ribose. In Novel Strategies in Ischemic Heart Disease; InTech: London, UK, 2012; ISBN 978-953-51-0184-0. [Google Scholar][Green Version]
- Zimmer, H.G.; Martius, P.A.; Marschner, G. Myocardial Infarction in Rats: Effects of Metabolic and Pharmacologic Interventions. Basic. Res. Cardiol. 1989, 84, 332–343. [Google Scholar] [CrossRef]
- Befera, I.N.; Rivard, A.; Gatlin, D.; Black, S.; Zhang, J.; Foker, J.E.; Radhakrishnan, P.R.S.; Xue, H.; Felix, K.; Moore-Olufemi, S.D.; et al. 13: Ribose Treatment Helps Preserve Function of the Remote Myocardium after Myocardial Infarction. J. Surg. Res. 2007, 137, 156. [Google Scholar] [CrossRef]
- González, G.E.; Rabald, S.; Briest, W.; Gelpi, R.J.; Seropian, I.; Zimmer, H.G.; Deten, A. Ribose Treatment Reduced the Infarct Size and Improved Heart Function after Myocardial Infarction in Rats. Cell Physiol. Biochem. 2009, 24, 211–218. [Google Scholar] [CrossRef]
- Zimmer, H.; Ibel, H. Ribose Accelerates the Repletion of the ATP Pool during Recovery from Reversible Ischemia of the Rat Myocardium. J. Mol. Cell Cardiol. 1984, 16, 863–866. [Google Scholar] [CrossRef]
- Wallen, W.J.; Belanger, M.P.; Wittnich, C. Preischemic Administration of Ribose to Delay the Onset of Irreversible Ischemic Injury and Improve Function: Studies in Normal and Hypertrophied Hearts. Can. J. Physiol. Pharmacol. 2003, 81, 40–47. [Google Scholar] [CrossRef]
- Shecterle, L.M.; Terry, K.R.; St. Cyr, J.A. Potential Clinical Benefits of D-Ribose in Ischemic Cardiovascular Disease. Cureus 2018, 10, e2291. [Google Scholar] [CrossRef] [PubMed]
- Kloner, R.A. Stunned and Hibernating Myocardium: Where Are We Nearly 4 Decades Later? J. Am. Heart Assoc. 2020, 9, e015502. [Google Scholar] [CrossRef]
- Perlmutter, N.S.; Wilson, R.A.; Angello, D.A.; Palac, R.T.; Lin, J.; Brown, B.G. Ribose Facilitates Thallium-201 Redistribution in Patients with Coronary Artery Disease. J. Nucl. Med. 1991, 32, 193–200. [Google Scholar] [PubMed]
- Hegewald, M.G.; Palac, R.T.; Angello, D.A.; Perlmutter, N.S.; Wilson, R.A. Ribose Infusion Accelerates Thallium Redistribution with Early Imaging Compared with Late 24-Hour Imaging without Ribose. J. Am. Coll. Cardiol. 1991, 18, 1671–1681. [Google Scholar] [CrossRef][Green Version]
- Sawada, S.G.; Lewis, S.; Kovacs, R.; Khouri, S.; Gradus-Pizlo, I.; St. Cyr, J.A.; Feigenbaum, H. Evaluation of the Anti-Ischemic Effects of D-Ribose during Dobutamine Stress Echocardiography: A Pilot Study. Cardiovasc. Ultrasound 2009, 7, 5. [Google Scholar] [CrossRef]
- Carter, O.; MacCarter, D.; Mannebach, S.; Biskupiak, J.; Stoddard, G.; Gilbert, E.M.; Munger, M.A. D-Ribose Improves Peak Exercise Capacity and Ventilatory Efficiency in Heart Failure Patients. J. Am. Coll. Cardiol. 2005, 45 (Suppl. S3), 185A. [Google Scholar]
- Pierce, J.D.; Shen, Q.; Vacek, J.; Rahman, F.K.; Krueger, K.J.; Gupta, B.; Hiebert, J.B. Potential Use of Ubiquinol and D-Ribose in Patients with Heart Failure with Preserved Ejection Fraction. Ann. Med. Surg. 2020, 55, 77–80. [Google Scholar] [CrossRef] [PubMed]
- Mentzer, R.M.; Ely, S.W.; Lasley, R.D.; Berne, R.M. The Acute Effects of AICAR on Purine Nucleotide Metabolism and Postischemic Cardiac Function. J. Thorac. Cardiovasc. Surg. 1988, 95, 286–293. [Google Scholar] [CrossRef]
- Leung, J.M.; Stanley, T.; Mathew, J.; Curling, P.; Barash, P.; Salmenpera, M.; Reves, J.G.; Hollenberg, M.; Mangano, D.T.; Browner, W.S.; et al. An Initial Multicenter, Randomized Controlled Trial on the Safety and Efficacy of Acadesine in Patients Undergoing Coronary Artery Bypass Graft Surgery. SPI Research Group. Anesth. Analg. 1994, 78, 420–434. [Google Scholar] [CrossRef]
- Kitakaze, M.; Takashima, S.; Minamino, T.; Node, K.; Shinozaki, Y.; Mori, H.; Kuzuya, T.; Hori, M. Improvement by 5-Amino-4-Imidazole Carboxamide Riboside of the Contractile Dysfunction That Follows Brief Periods of Ischemia through Increases in Ecto-5-Nucleotidase Activity and Adenosine Release in Canine Hearts. Jpn. Circ. J. 1999, 63, 542–553. [Google Scholar] [CrossRef][Green Version]
- Cieslik, K.A.; Taffet, G.E.; Crawford, J.R.; Trial, J.A.; Mejia Osuna, P.; Entman, M.L. AICAR-Dependent AMPK Activation Improves Scar Formation in the Aged Heart in a Murine Model of Reperfused Myocardial Infarction. J. Mol. Cell Cardiol. 2013, 63, 26–36. [Google Scholar] [CrossRef]
- DeWitt, D.F.; Jochim, K.E.; Behrendt, D.M. Nucleotide Degradation and Functional Impairment during Cardioplegia: Amelioration by Inosine. Circulation 1983, 67, 171–178. [Google Scholar] [CrossRef]
- Szabó, G.; Stumpf, N.; Radovits, T.; Sonnenberg, K.; Gerö, D.; Hagl, S.; Szabó, C.; Bährle, S. Effects of Inosine on Reperfusion Injury after Heart Transplantation. Eur. J. Cardiothorac. Surg. 2006, 30, 96–102. [Google Scholar] [CrossRef]
- Farthing, D.; Xi, L.; Gehr, L.; Sica, D.; Larus, T.; Karnes, H.T. High-Performance Liquid Chromatography (HPLC) Determination of Inosine, a Potential Biomarker for Initial Cardiac Ischaemia, Using Isolated Mouse Hearts. Biomarkers 2006, 11, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Halle, A.A.; Mirvis, D.M.; Sullivan, J.M.; Kang, E.S. Dose Effects of Adenine on Myocardial ATP Content in the Post-Anoxic Nonworking Rat Heart. Gen. Pharmacol. Vasc. Syst. 1989, 20, 817–820. [Google Scholar] [CrossRef] [PubMed]
- Diwan, V.; Mistry, A.; Gobe, G.; Brown, L. Adenine-Induced Chronic Kidney and Cardiovascular Damage in Rats. J. Pharmacol. Toxicol. Methods 2013, 68, 197–207. [Google Scholar] [CrossRef]
- Tamayo, I.; Lee, H.J.; Aslam, M.I.; Liu, J.-J.; Ragi, N.; Karanam, V.; Maity, S.; Saliba, A.; Treviño, E.; Zheng, H.; et al. Endogenous Adenine Is a Potential Driver of the Cardiovascular-Kidney-Metabolic Syndrome. medRxiv 2024. [Google Scholar] [CrossRef]
- Harmsen, E.; de Jong, J.W.; Serruys, P.W. Hypoxanthine Production by Ischemic Heart Demonstrated by High Pressure Liquid Chromatography of Blood Purine Nucleosides and Oxypurines. Clin. Chim. Acta 1981, 115, 73–84. [Google Scholar] [CrossRef]
- Farthing, D.E.; Sica, D.; Hindle, M.; Edinboro, L.; Xi, L.; Gehr, T.W.B.; Gehr, L.; Farthing, C.A.; Larus, T.L.; Fakhry, I.; et al. A Rapid and Simple Chemiluminescence Method for Screening Levels of Inosine and Hypoxanthine in Non-Traumatic Chest Pain Patients. Luminescence 2011, 26, 65–75. [Google Scholar] [CrossRef]
- Pliml, W.; von Arnim, T.; Stablein, A.; Erdmann, E.; Zimmer, H.-G.; Hofmann, H. Effects of Ribose on Exercise-Induced Ischaemia in Stable Coronary Artery Disease. Lancet 1992, 340, 507–510. [Google Scholar] [CrossRef] [PubMed]
- Urmaliya, V.B.; Pouton, C.W.; Ledent, C.; Short, J.L.; White, P.J. Cooperative Cardioprotection Through Adenosine A1 and A2A Receptor Agonism in Ischemia-Reperfused Isolated Mouse Heart. J. Cardiovasc. Pharmacol. 2010, 56, 379–388. [Google Scholar] [CrossRef]
- Wyatt, D.A.; Ely, S.W.; Walsh, R.; Mainwaring, R.; Berne, R.M.; Lasley, R.D.; Mentzer, R.M. Purine-Enriched Asanguineous Cardioplegia Retards Adenosine Triphosphate Degradation during Ischemia and Improves Postischemic Ventricular Function. J. Thorac. Cardiovasc. Surg. 1989, 97, 771–778. [Google Scholar] [CrossRef]
- Vance, R.; Einzig, S.; Kreisler, K.; St. Cyr, J. D-Ribose Maintained Ejection Fraction Following Aortic Valve Surgery. FASEB J. 2000, 14, A419. [Google Scholar]
- Perkowski, D.J.; Wagner, S.; Schneider, J.R.; St. Cyr, J.A. A Targeted Metabolic Protocol with D-Ribose for off-Pump Coronary Artery Bypass Procedures: A Retrospective Analysis. Ther. Adv. Cardiovasc. Dis. 2011, 5, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Omran, H.; Illien, S.; MacCarter, D.; St. Cyr, J.; Lüderitz, B. D-Ribose Improves Diastolic Function and Quality of Life in Congestive Heart Failure Patients: A Prospective Feasibility Study. Eur. J. Heart Fail. 2003, 5, 615–619. [Google Scholar] [CrossRef]
- MacCarter, D.; Vijay, N.; Washam, M.; Shecterle, L.; Sierminski, H.; St. Cyr, J.A. D-Ribose Aids Advanced Ischemic Heart Failure Patients. Int. J. Cardiol. 2009, 137, 79–80. [Google Scholar] [CrossRef]
- Krueger, K.J.; Rahman, F.K.; Shen, Q.; Vacek, J.; Hiebert, J.B.; Pierce, J.D. Mitochondrial Bioenergetics and D-Ribose in HFpEF: A Brief Narrative Review. Ann. Transl. Med. 2021, 9, 1504. [Google Scholar] [CrossRef] [PubMed]
- Pierce, J.D.; Shen, Q.; Mahoney, D.E.; Rahman, F.; Krueger, K.J.; Diaz, F.J.; Clark, L.; Smith, C.; Vacek, J.; Hiebert, J.B. Effects of Ubiquinol and/or D-Ribose in Patients with Heart Failure with Preserved Ejection Fraction. Am. J. Cardiol. 2022, 176, 79–88. [Google Scholar] [CrossRef]
- Višnjić, D.; Lalić, H.; Dembitz, V.; Tomić, B.; Smoljo, T. AICAr, a Widely Used AMPK Activator with Important AMPK-Independent Effects: A Systematic Review. Cells 2021, 10, 1095. [Google Scholar] [CrossRef]
- Corton, J.M.; Gillespie, J.G.; Hawley, S.A.; Hardie, D.G. 5-Aminoimidazole-4-Carboxamide Ribonucleoside. A Specific Method for Activating AMP-Activated Protein Kinase in Intact Cells? Eur. J. Biochem. 1995, 229, 558–565. [Google Scholar] [CrossRef]
- Longnus, S.L.; Wambolt, R.B.; Parsons, H.L.; Brownsey, R.W.; Allard, M.F. 5-Aminoimidazole-4-Carboxamide 1-Beta -D-Ribofuranoside (AICAR) Stimulates Myocardial Glycogenolysis by Allosteric Mechanisms. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2003, 284, R936–R944. [Google Scholar] [CrossRef]
- Javaux, F.; Vincent, M.F.; Wagner, D.R.; Van den Berghe, G. Cell-Type Specificity of Inhibition of Glycolysis by 5-Amino-4-Imidazolecarboxamide Riboside. Lack of Effect in Rabbit Cardiomyocytes and Human Erythrocytes, and Inhibition in FTO-2B Rat Hepatoma Cells. Biochem. J. 1995, 305 Pt 3, 913–919. [Google Scholar] [CrossRef]
- Viscomi, C.; Bottani, E.; Civiletto, G.; Cerutti, R.; Moggio, M.; Fagiolari, G.; Schon, E.A.; Lamperti, C.; Zeviani, M. In Vivo Correction of COX Deficiency by Activation of the AMPK/PGC-1α Axis. Cell Metab. 2011, 14, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Meng, R.S.; Pei, Z.H.; Yin, R.; Zhang, C.X.; Chen, B.L.; Zhang, Y.; Liu, D.; Xu, A.L.; Dong, Y.G. Adenosine Monophosphate-Activated Protein Kinase Inhibits Cardiac Hypertrophy through Reactivating Peroxisome Proliferator-Activated Receptor-α Signaling Pathway. Eur. J. Pharmacol. 2009, 620, 63–70. [Google Scholar] [CrossRef]
- Du, J.; Li, H.; Song, J.; Wang, T.; Dong, Y.; Zhan, A.; Li, Y.; Liang, G. AMPK Activation Alleviates Myocardial Ischemia-Reperfusion Injury by Regulating Drp1-Mediated Mitochondrial Dynamics. Front. Pharmacol. 2022, 13, 862204, Erratum in Front. Pharmacol. 2024, 15, 1502512. [Google Scholar] [CrossRef]
- Swain, J.L.; Hines, J.J.; Sabina, R.L.; Holmes, E.W. Accelerated Repletion of ATP and GTP Pools in Postischemic Canine Myocardium Using a Precursor of Purine de Novo Synthesis. Circ. Res. 1982, 51, 102–105. [Google Scholar] [CrossRef]
- Galiñanes, M.; Mullane, K.M.; Bullough, D.; Hearse, D.J. Acadesine and Myocardial Protection. Studies of Time of Administration and Dose-Response Relations in the Rat. Circulation 1992, 86, 598–608. [Google Scholar] [CrossRef]
- Mauser, M.; Hoffmeister, H.M.; Nienaber, C.; Schaper, W. Influence of Ribose, Adenosine, and “AICAR” on the Rate of Myocardial Adenosine Triphosphate Synthesis during Reperfusion after Coronary Artery Occlusion in the Dog. Circ. Res. 1985, 56, 220–230. [Google Scholar] [CrossRef]
- Flanagan, W.F.; Holmes, E.W.; Sabina, R.L.; Swain, J.L. Importance of Purine Nucleotide Cycle to Energy Production in Skeletal Muscle. Am. J. Physiol. 1986, 251, C795–C802. [Google Scholar] [CrossRef]
- Moopanar, T.R.; Xiao, X.H.; Jiang, L.; Chen, Z.P.; Kemp, B.E.; Allen, D.G. AICAR Inhibits the Na+/H+ Exchanger in Rat Hearts-Possible Contribution to Cardioprotection. Pflug. Arch. 2006, 453, 147–156. [Google Scholar] [CrossRef]
- Matsiukevich, D.; Piraino, G.; Klingbeil, L.R.; Hake, P.W.; Wolfe, V.; O’Connor, M.; Zingarelli, B. The AMPK Activator Aicar Ameliorates Age-Dependent Myocardial Injury in Murine Hemorrhagic Shock. Shock 2017, 47, 70–78. [Google Scholar] [CrossRef]
- Yang, C.; Xu, H.; Cai, L.; Du, X.; Jiang, Y.; Zhang, Y.; Zhou, H.; Chen, Z.K. Donor Pretreatment with Adenosine Monophosphate-Activated Protein Kinase Activator Protects Cardiac Grafts from Cold Ischaemia/Reperfusion Injury. Eur. J. Cardiothorac. Surg. 2016, 49, 1354–1360. [Google Scholar] [CrossRef]
- Mangano, D.T. Effects of Acadesine on Myocardial Infarction, Stroke, and Death Following Surgery. A Meta-Analysis of the 5 International Randomized Trials. The Multicenter Study of Perioperative Ischemia (McSPI) Research Group. JAMA 1997, 277, 325–332. [Google Scholar] [CrossRef]
- Holdright, D.R.; Sparrow, J.L.; Wright, C.L.; Steiner, J.; Fox, K.M. Effect of Acadesine, a New Metabolic Agent, on Exercise-Induced Myocardial Ischemia in Chronic Stable Angina. Cardiovasc. Drugs Ther. 1994, 8, 193–197. [Google Scholar] [CrossRef]
- Menasché, P.; Jamieson, W.R.E.; Flameng, W.; Michael, K.D. Acadesine: A New Drug That May Improve Myocardial Protection in Coronary Artery Bypass Grafting. Results of the First International Multicenter Study. Multinational Acadesine Study Group. J. Thorac. Cardiovasc. Surg. 1995, 110, 1096–1106. [Google Scholar] [CrossRef] [PubMed]
- Nakai, T.; Kano, S.; Satoh, K.; Hoshi, K.; Ichihara, K. Effects of Adenine Nucleotide Analogues on Myocardial Dysfunction during Reperfusion after Ischemia in Dogs. J. Cardiovasc. Pharmacol. 1996, 28, 264–270. [Google Scholar] [CrossRef]
- Gruber, H.E.; Hoffer, M.E.; McAllister, D.R.; Laikind, P.K.; Lane, T.A.; Schmid-Schoenbein, G.W.; Engler, R.L. Increased Adenosine Concentration in Blood from Ischemic Myocardium by AICA Riboside. Effects on Flow, Granulocytes, and Injury. Circulation 1989, 80, 1400–1411. [Google Scholar] [CrossRef]
- Mullane, K. Acadesine: The Prototype Adenosine Regulating Agent for Reducing Myocardial Ischaemic Injury. Cardiovasc. Res. 1993, 27, 43–47. [Google Scholar] [CrossRef]
- Newman, M.F.; Ferguson, T.B.; White, J.A.; Ambrosio, G.; Koglin, J.; Nussmeier, N.A.; Pearl, R.G.; Pitt, B.; Wechsler, A.S.; Weisel, R.D.; et al. Effect of Adenosine-Regulating Agent Acadesine on Morbidity and Mortality Associated with Coronary Artery Bypass Grafting: The RED-CABG Randomized Controlled Trial. JAMA 2012, 308, 157–164. [Google Scholar] [CrossRef]
- Khattri, R.B.; Thome, T.; Ryan, T.E. Tissue-Specific 1H-NMR Metabolomic Profiling in Mice with Adenine-Induced Chronic Kidney Disease. Metabolites 2021, 11, 45. [Google Scholar] [CrossRef]
- French, B.W.; Breidenbach, J.D.; Yassine, S.G.; Khatib-Shahidi, B.Z.; Kazmi, S.; Murphy, C.M.; Bashir, H.S.; Benson, E.M.; Timalsina, B.; Shrestha, U.; et al. A Simplified Model of Adenine-Induced Chronic Kidney Disease Using SKH1 Mice. Cells 2024, 13, 2117. [Google Scholar] [CrossRef]
- Oka, T.; Hikoso, S.; Yamaguchi, O.; Taneike, M.; Takeda, T.; Tamai, T.; Oyabu, J.; Murakawa, T.; Nakayama, H.; Nishida, K.; et al. Mitochondrial DNA That Escapes from Autophagy Causes Inflammation and Heart Failure. Nature 2012, 485, 251–255, Erratum in Nature 2012, 490, 292. [Google Scholar] [CrossRef]
- Viegas, T.X.; Omura, G.A.; Stoltz, R.R.; Kisicki, J. Pharmacokinetics and Pharmacodynamics of Peldesine (BCX-34), a Purine Nucleoside Phosphorylase Inhibitor, Following Single and Multiple Oral Doses in Healthy Volunteers. J. Clin. Pharmacol. 2000, 40, 410–420. [Google Scholar] [CrossRef]
- Berne, R.M. Cardiac Nucleotides in Hypoxia: Possible Role in Regulation of Coronary Blood Flow. Am. J. Physiol.-Leg. Content 1963, 204, 317–322. [Google Scholar] [CrossRef]
- Smolenski, R.T.; Yacoub, M.H.; Seymour, A.-M.L. Reduced Purine Catabolite Production in the Postischemic Rat Heart: A31P NMR Assessment of Cytosolic Metabolites. Magma Magn. Reson. Mater. Phys. Biol. Med. 1994, 2, 417–420. [Google Scholar] [CrossRef]
- Smolenski, R.T.; Jayakumar, J.; Seymour, A.-M.L.; Yacoub, M.H. The Effects of Nucleotide Pool on Purine Production in the Postischemic Heart; Springer: Boston, MA, USA, 1998; pp. 271–274. [Google Scholar]
- Jennings, R.B.; Reimer, K.A.; Hill, M.L.; Mayer, S.E. Total Ischemia in Dog Hearts, in Vitro. 1. Comparison of High Energy Phosphate Production, Utilization, and Depletion, and of Adenine Nucleotide Catabolism in Total Ischemia in Vitro vs. Severe Ischemia in Vivo. Circ. Res. 1981, 49, 892–900. [Google Scholar] [CrossRef]
- Aussedat, J.; Verdys, M.; Rossi, A. Adenine Nucleotide Synthesis from Inosine during Normoxia and after Ischaemia in the Isolated Perfused Rat Heart. Can. J. Physiol. Pharmacol. 1985, 63, 1159–1164. [Google Scholar] [CrossRef]
- Devous, M.D.; Douglas Lewandowski, E.; Douglas Lewan-devous, E. Inosine Preserves ATP During Ischemia and Enhances Recovery during Reperfusion. Am. J. Physiol.-Heart Circ. Physiol. 1987, 253, H1224–H1233. [Google Scholar] [CrossRef]
- Schneider, A.; Zimmer, H.-G. Effect of Inosine on Function and Adenine Nucleotide Content of the Isolated Working Rat Heart. J. Cardiovasc. Pharmacol. 1991, 17, 466–473. [Google Scholar] [CrossRef]
- de Jong, J.W.; Achterberg, P.W. Developmental Differences in Myocardial ATP Metabolism. Basic. Res. Cardiol. 1987, 82 (Suppl. S2), 121–126. [Google Scholar] [CrossRef] [PubMed]
- Muscari, C.; Finelli, C.; Stefanelli, C.; Flamigni, F.; Guarnieri, C.; Caldarera, C.M. Age-Dependent Differences of ATP Breakdown and ATP-Catabolite Release in Ischemic and Reperfused Hearts. Mech. Ageing Dev. 1993, 67, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Peart, J.; Matherne, G.P.; Cerniway, R.J.; Headrick, J.P. Cardioprotection with Adenosine Metabolism Inhibitors in Ischemic-Reperfused Mouse Heart. Cardiovasc. Res. 2001, 52, 120–129. [Google Scholar] [CrossRef]
- Farthing, D.; Gehr, L.; Karnes, H.T.; Sica, D.; Gehr, T.; Larus, T.; Farthing, C.; Xi, L. Effects of Salicylic Acid on Post-Ischaemic Ventricular Function and Purine Efflux in Isolated Mouse Hearts. Biomarkers 2007, 12, 623–634. [Google Scholar] [CrossRef]
- Hirai, K.; Ashraf, M. Modulation of Adenosine Effects in Attenuation of Ischemia and Reperfusion Injury in Rat Heart. J. Mol. Cell Cardiol. 1998, 30, 1803–1815. [Google Scholar] [CrossRef] [PubMed]
- Farthing, D.E.; Farthing, C.A.; Xi, L. Inosine and Hypoxanthine as Novel Biomarkers for Cardiac Ischemia: From Bench to Point-of-Care. Exp. Biol. Med. 2015, 240, 821–831. [Google Scholar] [CrossRef] [PubMed]
- Molina-Arcas, M.; Casado, F.; Pastor-Anglada, M. Nucleoside Transporter Proteins. Curr. Vasc. Pharmacol. 2009, 7, 426–434. [Google Scholar] [CrossRef]
- Oliveira, P.J.; Rolo, A.P.; Palmeira, C.M.; Moreno, A.J.M. Carvedilol Reduces Mitochondrial Damage Induced by Hypoxanthine/Xanthine Oxidase: Relevance to Hypoxia/Reoxygenation Injury. Cardiovasc. Toxicol. 2001, 1, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Jennings, R.B.; Shen, A.C.; Hill, M.L.; Ganote, C.E.; Herdson, P.B. Mitochondrial Matrix Densities in Myocardial Ischemia and Autolysis. Exp. Mol. Pathol. 1978, 29, 55–65. [Google Scholar] [CrossRef]
- Ronca-Testoni, S.; Borghini, F. Degradation of Perfused Adenine Compounds up to Uric Acid in Isolated Rat Heart. J. Mol. Cell Cardiol. 1982, 14, 177–180. [Google Scholar] [CrossRef]
- Cargnoni, A.; Ceconi, C.; Curello, S.; Benigno, M.; De Jong, J.W.; Ferrari, R. Relation between Energy Metabolism, Glycolysis, Noradrenaline Release and Duration of Ischemia. Mol. Cell Biochem. 1996, 160–161, 187–194. [Google Scholar] [CrossRef]
- Tarantola, M.; Motterlini, R.; Beretta, M.; Samaja, M. Dual Role of Hypoxanthine in the Reoxygenation of Hypoxic Isolated Rat Hearts. J. Mol. Cell Cardiol. 1991, 23, 77–82. [Google Scholar] [CrossRef][Green Version]
- Prousi, G.S.; Joshi, A.M.; Atti, V.; Addison, D.; Brown, S.A.; Guha, A.; Patel, B. Vascular Inflammation, Cancer, and Cardiovascular Diseases. Curr. Oncol. Rep. 2023, 25, 955–963. [Google Scholar] [CrossRef] [PubMed]
- Gaidai, O.; Cao, Y.; Loginov, S. Global Cardiovascular Diseases Death Rate Prediction. Curr. Probl. Cardiol. 2023, 48, 101622. [Google Scholar] [CrossRef]
- Bułdak, Ł. Cardiovascular Diseases—A Focus on Atherosclerosis, Its Prophylaxis, Complications and Recent Advancements in Therapies. Int. J. Mol. Sci. 2022, 23, 4695. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musial, P.T.; Badtke, P.A.; Zabielska-Kaczorowska, M.A. Purine Nucleotide Precursors in Preventing Myocardial Ischemia–Reperfusion Injury. Int. J. Mol. Sci. 2025, 26, 10455. https://doi.org/10.3390/ijms262110455
Musial PT, Badtke PA, Zabielska-Kaczorowska MA. Purine Nucleotide Precursors in Preventing Myocardial Ischemia–Reperfusion Injury. International Journal of Molecular Sciences. 2025; 26(21):10455. https://doi.org/10.3390/ijms262110455
Chicago/Turabian StyleMusial, Pawel Tomasz, Piotr Arkadiusz Badtke, and Magdalena Agnieszka Zabielska-Kaczorowska. 2025. "Purine Nucleotide Precursors in Preventing Myocardial Ischemia–Reperfusion Injury" International Journal of Molecular Sciences 26, no. 21: 10455. https://doi.org/10.3390/ijms262110455
APA StyleMusial, P. T., Badtke, P. A., & Zabielska-Kaczorowska, M. A. (2025). Purine Nucleotide Precursors in Preventing Myocardial Ischemia–Reperfusion Injury. International Journal of Molecular Sciences, 26(21), 10455. https://doi.org/10.3390/ijms262110455






