Neurobiological and Existential Profiles in Posttraumatic Stress Disorder: The Role of Serotonin, Cortisol, Noradrenaline, and IL-12 Across Chronicity and Age
Abstract
1. Introduction
2. Results
2.1. Demographic, Biomarker, and Psychological Profiles of Study Participants According to PTSD Status
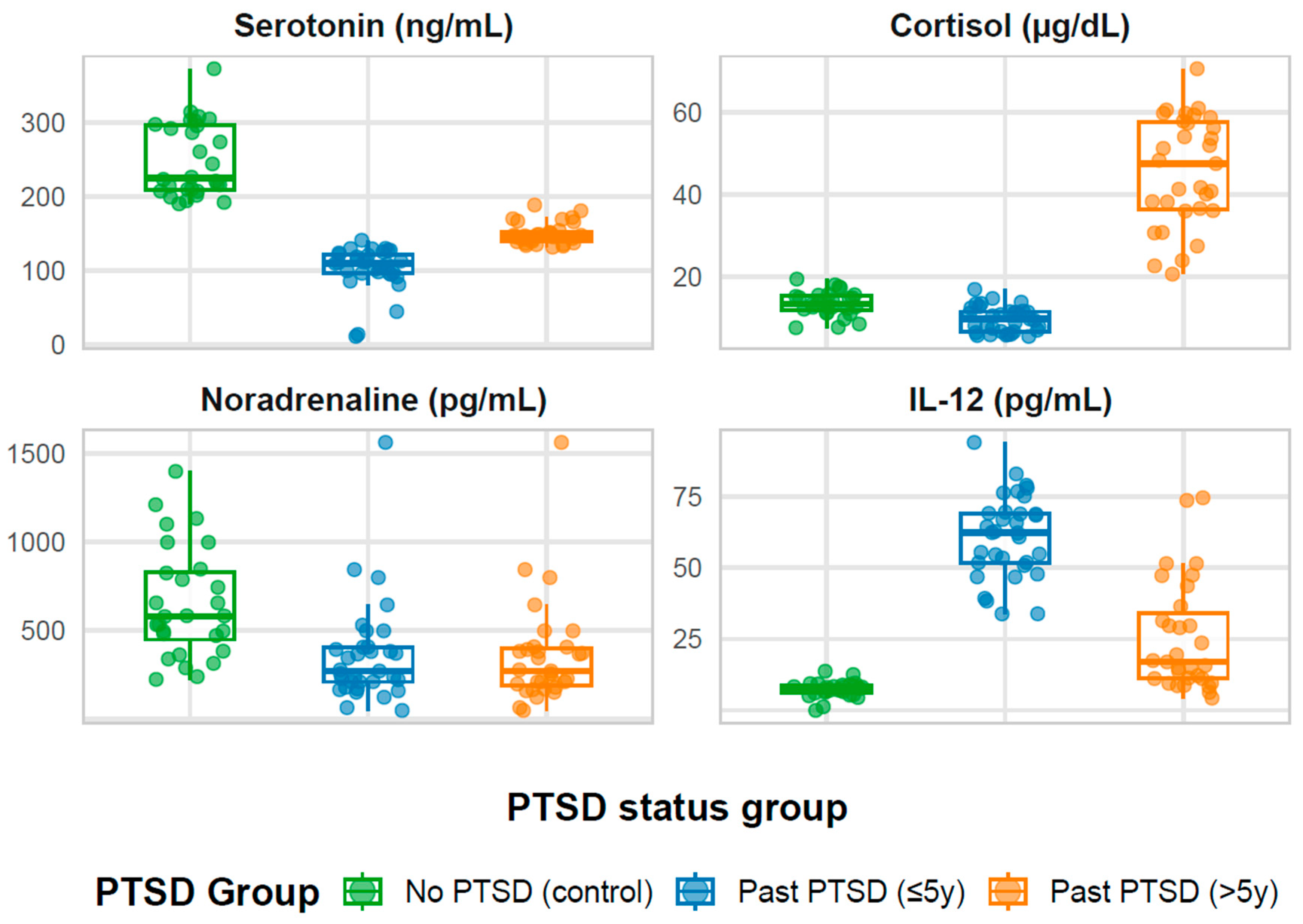
2.1.1. The Overall Sample
2.1.2. Stratification by Group Status
2.1.3. Overall Patterns
2.2. Effect of Age on Biomarker and Psychological Profiles Across PTSD Status
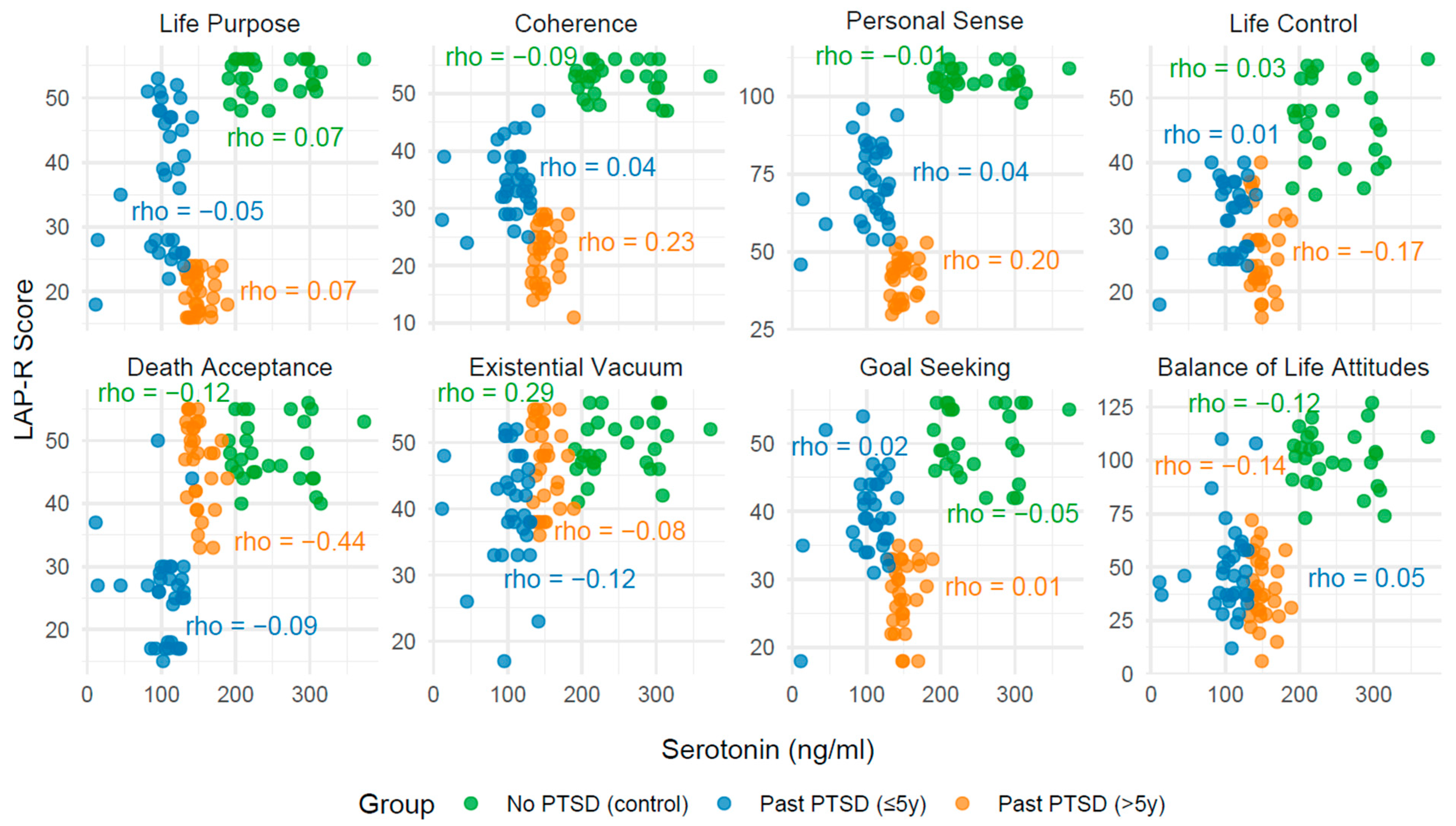
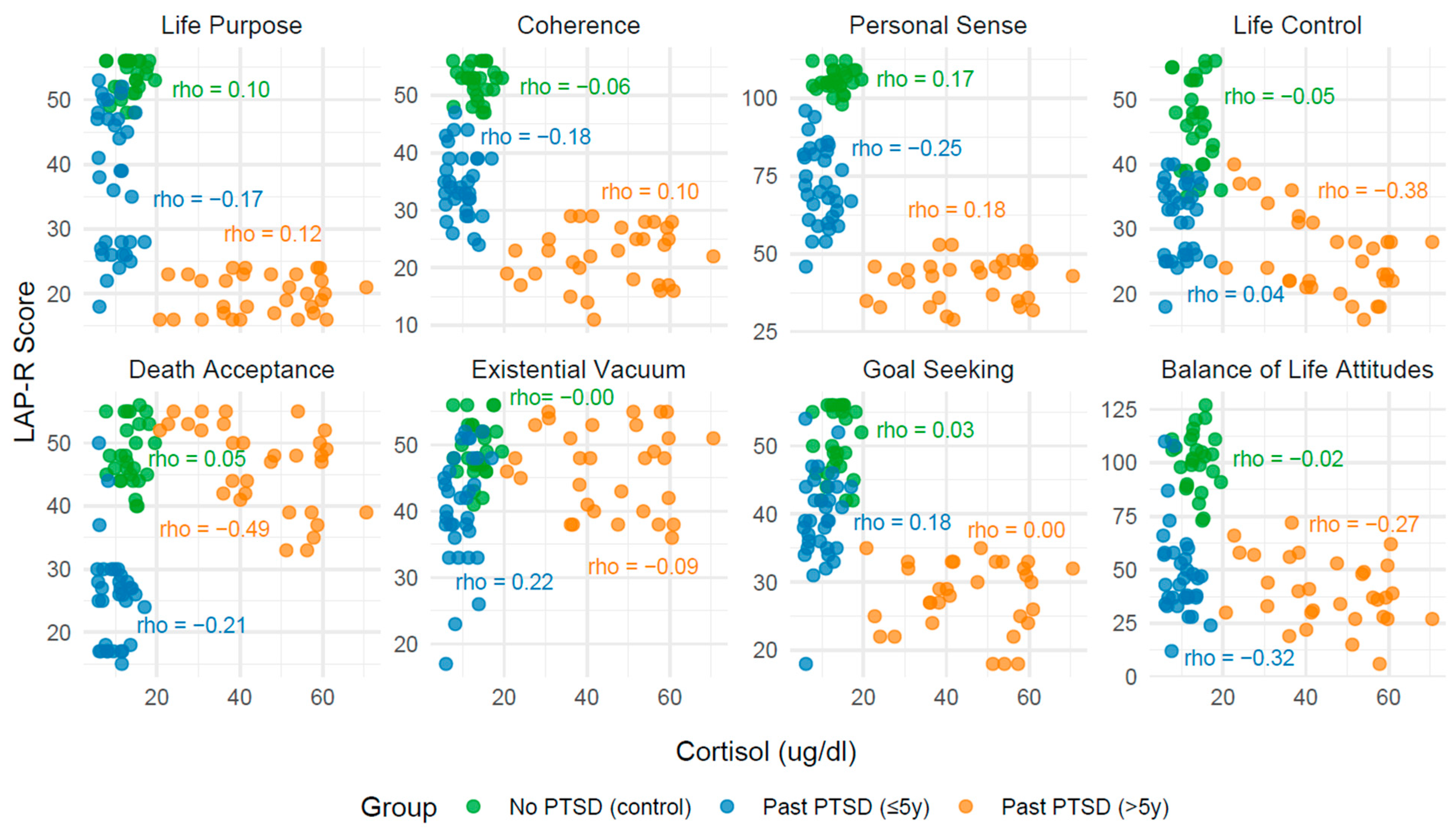
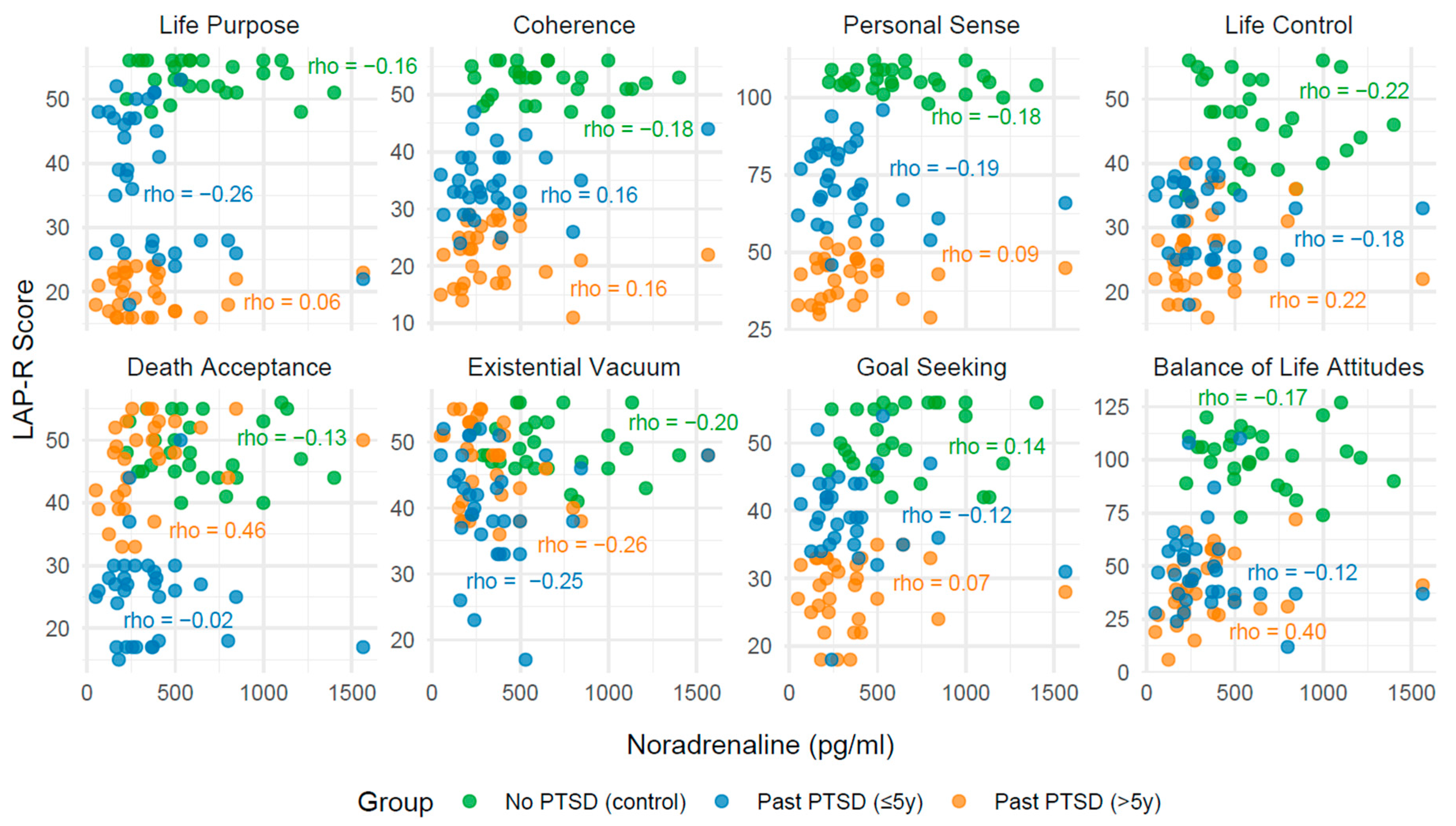
2.3. Analysis of Correlation Between Biomarkers and LAP-R Scales Across PTSD Status
2.3.1. Effect of PTSD Status on Biomarker–LAP-R Associations
2.3.2. Influence of PTSD Duration on Associations
2.3.3. Convergence Toward Control Group Patterns
2.3.4. Clinical Narrative
| LAP-R Scale | No PTSD (Control) | PAST PTST (≤5y) | PAST PTST (>5y) | |||
|---|---|---|---|---|---|---|
| Rho | p | Rho | p | Rho | p | |
| Life Purpose | 0.07 | 0.718 | −0.05 | 0.768 | 0.07 | 0.699 |
| Coherence | −0.09 | 0.636 | 0.04 | 0.828 | 0.23 | 0.210 |
| Personal Sense | −0.01 | 0.969 | 0.04 | 0.816 | 0.20 | 0.276 |
| Life Control | 0.03 | 0.879 | 0.01 | 0.967 | −0.17 | 0.361 |
| Death acceptance | −0.12 | 0.538 | −0.09 | 0.629 | −0.44 | 0.014 |
| Existential Vacuum | 0.29 | 0.129 | −0.12 | 0.510 | −0.08 | 0.650 |
| Goal Seeking | −0.05 | 0.795 | 0.02 | 0.933 | 0.01 | 0.945 |
| Balance of Life Attitudes | −0.12 | 0.549 | 0.05 | 0.884 | −0.14 | 0.459 |
| LAP-R Scale | No PTSD (Control) | PAST PTST (≤5y) | PAST PTST (>5y) | |||
|---|---|---|---|---|---|---|
| Rho | p | Rho | p | Rho | p | |
| Life purpose | 0.10 | 0.617 | −0.17 | 0.344 | 0.12 | 0.524 |
| Coherence | −0.06 | 0.756 | −0.18 | 0.327 | 0.10 | 0.598 |
| Personal Sense | 0.17 | 0.389 | −0.25 | 0.156 | 0.18 | 0.328 |
| Life Control | −0.05 | 0.789 | 0.04 | 0.833 | −0.38 | 0.036 |
| Death acceptance | 0.05 | 0.817 | −0.21 | 0.240 | −0.49 | 0.005 |
| Existential Vacuum | 0.00 | 0.989 | 0.22 | 0.210 | −0.09 | 0.645 |
| Goal Seeking | 0.03 | 0.887 | 0.18 | 0.330 | 0.00 | 0.979 |
| Balance of Life Attitudes | −0.02 | 0.921 | −0.32 | 0.07 | −0.27 | 0.146 |
| LAP-R Scale | No PTSD (Control) | PAST PTST (≤5y) | PAST PTST (>5y) | |||
|---|---|---|---|---|---|---|
| Rho | p | Rho | p | Rho | p | |
| Life purpose | −0.16 | 0.417 | −0.26 | 0.147 | 0.06 | 0.768 |
| Coherence | −0.18 | 0.359 | 0.16 | 0.364 | 0.16 | 0.399 |
| Personal Sense | −0.18 | 0.359 | 0.19 | 0.281 | 0.09 | 0.643 |
| Life Control | −0.22 | 0.268 | 0.18 | 0.317 | 0.22 | 0.240 |
| Death acceptance | −0.13 | 0.501 | −0.02 | 0.902 | 0.46 | 0.009 |
| Existential Vacuum | −0.20 | 0.296 | −0.25 | 0.165 | −0.26 | 0.153 |
| Goal Seeking | 0.14 | 0.473 | −0.12 | 0.506 | 0.07 | 0.711 |
| Balance of Life Attitudes | −0.17 | 0.396 | −0.12 | 0.498 | 0.40 | 0.028 |
| LAP-R Scale | No PTSD (Control) | PAST PTST (≤5y) | PAST PTST (>5y) | |||
|---|---|---|---|---|---|---|
| Rho | p | Rho | p | Rho | p | |
| Life purpose | 0.32 | 0.098 | −0.10 | 0.592 | −0.18 | 0.340 |
| Coherence | 0.04 | 0.942 | 0.22 | 0.211 | −0.13 | 0.488 |
| Personal Sense | 0.14 | 0.470 | −0.01 | 0.935 | −0.20 | 0.272 |
| Life Control | 0.43 | 0.023 | 0.09 | 0.636 | −0.28 | 0.127 |
| Death acceptance | 0.53 | 0.004 | −0.23 | 0.203 | −0.32 | 0.083 |
| Existential Vacuum | 0.05 | 0.792 | −0.26 | 0.142 | 0.01 | 0.943 |
| Goal Seeking | 0.15 | 0.451 | 0.12 | 0.519 | 0.05 | 0.792 |
| Balance of Life Attitudes | 0.43 | 0.022 | −0.04 | 0.813 | −0.29 | 0.110 |
| PTSD Group | Biomarker | LAP-R Scale | Direction | Rho | p-Value |
|---|---|---|---|---|---|
| No PTSD (Control) | IL-12 | Life Control (LC) | Positive | 0.43 | 0.023 |
| No PTSD (Control) | IL-12 | Death Acceptance (DA) | Positive | 0.53 | 0.004 |
| No PTSD (Control) | IL-12 | Balance of Life Attitudes (BLA) | Positive | 0.43 | 0.022 |
| PTSD ≤ 5y | No significant associations with LAP-R scale | ||||
| PTSD > 5y | Serotonin | Death Acceptance (DA) | Negative | −0.44 | 0.014 |
| PTSD > 5y | Cortisol | Life Control (LC) | Negative | −0.38 | 0.036 |
| PTSD > 5y | Cortisol | Death Acceptance (DA) | Negative | −0.49 | 0.005 |
| PTSD > 5y | Noradrenaline | Death Acceptance (DA) | Positive | 0.46 | 0.009 |
| PTSD > 5y | Noradrenaline | Balance of Life Attitudes (BLA) | Positive | 0.40 | 0.028 |
3. Discussion
4. Materials and Methods
4.1. Characteristics of the Participants
- 1.
- group with diagnosed PTSD with a duration ≤5 years from the traumatic event;
- 2.
- group with PTSD > 5 years from the traumatic event;
- 3.
- control group—healthy individuals with no history of PTSD, age-matched.
- –
- age 18–50 years,
- –
- male gender,
- –
- having experienced psychological trauma consistent with DSM-5 criterion A,
- –
- diagnosis of PTSD in the past (≤5 years or >5 years from trauma, depending on the group).
- –
- presence of other mental disorders (including psychotic episodes, affective disorders, addictions),
- –
- chronic somatic diseases,
- –
- current use of psychotropic, anti-inflammatory, or hormonal medications,
- –
- nicotine, alcohol, medication, or psychoactive substance addiction,
- –
- legal incapacitation status,
- –
- employment as a uniformed service officer (military, police).
4.2. Life Attitude Profile (Revised) (LAP-R) Questionnaire
- 1.
- Life Purpose—assesses the sense of direction and meaning in existence,
- 2.
- Coherence—relates to internal consistency and understanding of life;
- 3.
- Existential Vacuum—measures the degree of experiencing meaninglessness, boredom, and apathy;
- 4.
- Death Acceptance—refers to readiness for the inevitability of death;
- 5.
- Goal Seeking—examines the tendency to set and achieve goals;
- 6.
- Life Control—assesses the sense of agency and influence over one’s own decisions.
- 7.
- Personal Sense (PS)—Relates to the perception of self-integrity, authenticity, identity coherence, and living in accordance with one’s own values.
- 8.
- Balance of Life Attitudes (BLA)—Refers to harmony between different existential dimensions, integrating purpose, coherence, control, death acceptance, and goal seeking.
4.3. Blood Sampling and Serum Preparation and Biomarker Analysis Procedure
4.4. Statistical Analysis
4.4.1. Power Analysis
4.4.2. Statistical Tool
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PTSD | Post-Traumatic Stress Disorder |
| IL-12 | Interleukin 12 |
| LAP-R | Life Attitude Profile-Revised |
| y/yrs | Years |
| n | Number (sample size) |
| Kruskal–Wallis | Kruskal–Wallis test (nonparametric Analysis of Variance–ANOVA by ranks) |
| Mann–Whitney U | Mann–Whitney U test (nonparametric test for two groups) |
| DSM-5 | Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition |
| HPA axis | Hypothalamic–Pituitary–Adrenal axis |
| GR | Glucocorticoid Receptor |
| 5-HT | 5-Hydroxytryptamine (Serotonin) |
| NA | Noradrenaline |
| NMDA receptor | N-Methyl-D-Aspartate receptor |
| STAT4 | Signal Transducer and Activator of Transcription 4 |
| NK cells | Natural Killer cells |
| Th1 response | T helper 1 response |
| LF | Life Purpose |
| C | Coherence |
| PS | Personal Sense |
| LC | Life Control |
| DA | Death Acceptance |
| EV | Existential Vacuum |
| GS | Goal Seeking |
| BLA | Balance of Life Attitudes |
| IQR | Interquartile Range |
| p-value | Probability value |
| CLD | Compact Letter Display |
| 4-PL | Four-Parameter Logistic |
| CAPS-5 | Clinician-Administered PTSD Scale for DSM-5 |
| CE/IVD | Conformité Européenne/In Vitro Diagnostic |
| DMN | Default Mode Network |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| HRP | Horseradish Peroxidase |
| Rho | Spearman’s rank correlation coefficient |
| TMB | 3,3′,5,5′-Tetramethylbenzidine |
References
- Bhatt, S.; Hillmer, A.T.; Girgenti, M.J.; Rusowicz, A.; Kapinos, M.; Nabulsi, N.; Huang, Y.; Matuskey, D.; Angarita, G.A.; Esterlis, I.; et al. PTSD is associated with neuroimmune suppression: Evidence from PET imaging and postmortem transcriptomic studies. Nat. Commun. 2020, 11, 2360. [Google Scholar] [CrossRef] [PubMed]
- Serdiuk, O.; Burlaka, V.; Markovska, A.; Smith, C.; Panok, V.; Klochkov, V.; Javanbakht, A. Trauma exposure and risk of post-traumatic stress disorder among youth and young adults during the Russia-Ukraine war. J. Affect. Disord. 2025, 391, 119944. [Google Scholar] [CrossRef]
- Traina, G.; Tuszyński, J.A. The Neurotransmission Basis of Post-Traumatic Stress Disorders by the Fear Conditioning Paradigm. Int. J. Mol. Sci. 2023, 24, 16327. [Google Scholar] [CrossRef]
- Ogłodek, E.A.; Just, M.J.; Szromek, A.R.; Araszkiewicz, A. Assessing the serum concentration levels of NT-4/5, GPX-1, TNF-α, and l-arginine as biomediators of depression severity in first depressive episode patients with and without posttraumatic stress disorder. Pharmacol. Rep. 2017, 69, 1049–1058. [Google Scholar] [CrossRef]
- Ogłodek, E.A. Evaluation of ADMA, carbonyl groups, CAT and NKA in depressed patients with and without posttraumatic stress disorder. Pharmacol. Rep. 2017, 69, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Al Jowf, G.I.; Ahmed, Z.T.; Reijnders, R.A.; de Nijs, L.; Eijssen, L.M.T. To Predict, Prevent, and Manage Post-Traumatic Stress Disorder (PTSD): A Review of Pathophysiology, Treatment, and Biomarkers. Int. J. Mol. Sci. 2023, 24, 5238. [Google Scholar] [CrossRef] [PubMed]
- Ogłodek, E.A.; Szota, A.M.; Just, M.J.; Szromek, A.R.; Araszkiewicz, A. A study of chemokines, chemokine receptors and interleukin-6 in patients with panic disorder, personality disorders and their co-morbidity. Pharmacol. Rep. 2016, 68, 756–763. [Google Scholar] [CrossRef]
- Govindula, A.; Ranadive, N.; Nampoothiri, M.; Rao, C.M.; Arora, D.; Mudgal, J. Emphasizing the Crosstalk Between Inflammatory and Neural Signaling in Post-traumatic Stress Disorder (PTSD). J. Neuroimmune Pharmacol. 2023, 18, 248–266. [Google Scholar] [CrossRef]
- Ogłodek, E.A. Changes in the Serum Concentration Levels of Serotonin, Tryptophan and Cortisol among Stress-Resilient and Stress-Susceptible Individuals after Experiencing Traumatic Stress. Int. J. Environ. Res. Public Health 2022, 19, 16517. [Google Scholar] [CrossRef]
- Morse, J.L.; Wooldridge, J.S.; Afari, N.; Angkaw, A.C.; Schnurr, P.P.; Lang, A.J.; Capone, C.; Norman, S.B. Associations among meaning in life, coping, and distress in trauma-exposed U.S. military veterans. Psychol. Serv. 2024, 21, 247–253. [Google Scholar] [CrossRef]
- Lacagnina, A.F.; Brockway, E.T.; Crovetti, C.R.; Shue, F.; McCarty, M.J.; Sattler, K.P.; Lim, S.C.; Santos, S.L.; Denny, C.A.; Drew, M.R. Distinct hippocampal engrams control extinction and relapse of fear memory. Nat. Neurosci. 2019, 22, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Mocle, A.J.; Ramsaran, A.I.; Jacob, A.D.; Rashid, A.J.; Luchetti, A.; Tran, L.M.; Richards, B.A.; Frankland, P.W.; Josselyn, S.A. Excitability mediates allocation of pre-configured ensembles to a hippocampal engram supporting contextual conditioned threat in mice. Neuron 2024, 112, 1487–1497.e6. [Google Scholar] [CrossRef] [PubMed]
- Dixon, R.; Malave, L.; Thompson, R.; Wu, S.; Li, Y.; Sadik, N.; Anacker, C. Sex-specific and developmental effects of early life adversity on stress reactivity are rescued by postnatal knockdown of 5-HT1A autoreceptors. Neuropsychopharmacology 2025, 50, 507–518. [Google Scholar] [CrossRef]
- Rhodes, J.R.; Tedeschi, R.G.; Moore, B.A.; Alldredge, C.T.; Elkins, G.R. Posttraumatic growth-oriented peer-based training among U.S. veterans: Evaluation of post-intervention and long-term follow-up outcomes. Front. Psychol. 2024, 14, 1322837. [Google Scholar] [CrossRef]
- Zhou, Y.; Guo, M.; Jiang, X.; Li, L.; Zhang, Y.; Zheng, L.; He, X.; Tan, Q.; Wang, R.; Liu, F.; et al. Correlation study of neurotransmitter and immune levels in pre-hospital emergency nurses with PTSD. J. Behav. Brain Sci. 2024, 14, 12–22. [Google Scholar] [CrossRef]
- Zuo, C.; Zhuang, Z.; Yang, P.; Zhang, H.; Li, X.; Huang, T.; Ahluwalia, T.S. Dissecting the causal association between inflammation and risk of PTSD: A Mendelian randomization study. J. Affect. Disord. 2023, 333, 436–445. [Google Scholar] [CrossRef]
- Bam, M.; Yang, X.; Zhou, J.; Ginsberg, J.P.; Leyden, Q.; Nagarkatti, P.S.; Nagarkatti, M. Evidence for Epigenetic Regulation of Pro-Inflammatory Cytokines, Interleukin-12 and Interferon Gamma, in Peripheral Blood Mononuclear Cells from PTSD Patients. J. Neuroimmune Pharmacol. 2016, 11, 168–181. [Google Scholar] [CrossRef]
- Cordero, M.I.; Moser, D.A.; Manini, A.; Suardi, F.; Sancho-Rossignol, A.; Torrisi, R.; Rossier, M.F.; Ansermet, F.; Dayer, A.G.; Rusconi-Serpa, S.; et al. Effects of interpersonal violence-related post-traumatic stress disorder (PTSD) on mother and child diurnal cortisol rhythm and cortisol reactivity to a laboratory stressor involving separation. Horm. Behav. 2017, 90, 15–24. [Google Scholar] [CrossRef]
- Sterina, E.; Michopoulos, V.; Linnstaedt, S.D.; Neylan, T.C.; Clifford, G.D.; Ethun, K.F.; Lori, A.; Wingo, A.P.; Rothbaum, B.O.; Ressler, K.J.; et al. Time of trauma prospectively affects PTSD symptom severity: The impact of circadian rhythms and cortisol. Psychoneuroendocrinology 2022, 141, 105729. [Google Scholar] [CrossRef]
- Inslicht, S.S.; Niles, A.N.; Metzler, T.J.; Lipshitz, S.L.; Otte, C.; Milad, M.R.; Orr, S.P.; Marmar, C.R.; Neylan, T.C. Randomized controlled experimental study of hydrocortisone and D-cycloserine effects on fear extinction in PTSD. Neuropsychopharmacology 2022, 47, 1945–1952. [Google Scholar] [CrossRef] [PubMed]
- Florido, A.; Velasco, E.R.; Monari, S.; Cano, M.; Cardoner, N.; Sandi, C.; Andero, R.; Perez-Caballero, L. Glucocorticoid-based pharmacotherapies preventing PTSD. Neuropharmacology 2023, 224, 109344. [Google Scholar] [CrossRef] [PubMed]
- Cabrera, Y.; Koymans, K.J.; Poe, G.R.; Kessels, H.W.; Van Someren, E.J.W.; Wassing, R. Overnight neuronal plasticity and adaptation to emotional distress. Nat. Rev. Neurosci. 2024, 25, 253–271. [Google Scholar] [CrossRef]
- Zhang, E.T.; Saglimbeni, G.S.; Feng, J.; Li, Y.; Bruchas, M.R. Dentate gyrus norepinephrine ramping facilitates aversive contextual processing. Nat. Commun. 2025, 16, 454. [Google Scholar] [CrossRef]
- İlhan, Ç.F.; Kışlal, S. Memory Impairing Effect of Propranolol on Consolidation and Reconsolidation for Various Learning Tasks. Noro Psikiyatr. Ars. 2023, 60, 271–282. [Google Scholar] [CrossRef]
- Mallet, C.; Chick, C.F.; Maatoug, R.; Fossati, P.; Brunet, A.; Millet, B. Memory reconsolidation impairment using the β-adrenergic receptor blocker propranolol reduces nightmare severity in patients with posttraumatic stress disorder: A preliminary study. J. Clin. Sleep Med. 2022, 18, 1847–1855. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Chen, J.; Ling, L.; Wang, H.; Gong, X.; Xu, H.; Wu, L.; Yan, C. Environmental enrichment modulates HPA axis reprogramming in adult male rats exposed to early adolescent stress. Neurosci. Res. 2021, 172, 63–72. [Google Scholar] [CrossRef]
- Ketenci, S.; Acet, N.G.; Sarıdoğan, G.E.; Aydın, B.; Cabadak, H.; Gören, M.Z. The Neurochemical Effects of Prazosin Treatment on Fear Circuitry in a Rat Traumatic Stress Model. Clin. Psychopharmacol. Neurosci. 2020, 18, 219–230. [Google Scholar] [CrossRef]
- Tang, Z.; Ye, G.; Chen, X.; Pan, M.; Fu, J.; Fu, T.; Liu, Q.; Gao, Z.; Baldwin, D.S.; Hou, R. Peripheral proinflammatory cytokines in Chinese patients with generalised anxiety disorder. J. Affect. Disord. 2018, 225, 593–598. [Google Scholar] [CrossRef] [PubMed]
- Hou, R.; Ye, G.; Liu, Y.; Chen, X.; Pan, M.; Zhu, F.; Fu, J.; Fu, T.; Liu, Q.; Gao, Z.; et al. Effects of SSRIs on peripheral inflammatory cytokines in patients with Generalized Anxiety Disorder. Brain Behav. Immun. 2019, 81, 105–110. [Google Scholar] [CrossRef]
- Kinney, K.L.; Rao, U.; Bailey, B.; Hellman, N.; Kelly, C.; McAfee, N.W.; Morris, M.C. Dynamics of diurnal cortisol and alpha-amylase secretion and their associations with PTSD onset in recent interpersonal trauma survivors. Psychol. Med. 2023, 53, 2263–2273. [Google Scholar] [CrossRef]
- Yao, J.; Zhang, L.; Zhang, C.; Chen, X.; Bao, K.; Hou, S.; Yin, Y.; Liu, K.; Wen, Q.; Huang, X.; et al. Rhythmic gamma frequency light flickering ameliorates stress-related behaviors and cognitive deficits by modulating neuroinflammatory response through IL-12-Mediated cytokines production in chronic stress-induced mice. Brain Behav. Immun. 2024, 121, 213–228. [Google Scholar] [CrossRef]
- La Porta, C.; Plum, T.; Palme, R.; Mack, M.; Tappe-Theodor, A. Repeated social defeat stress differently affects arthritis-associated hypersensitivity in male and female mice. Brain Behav. Immun. 2024, 119, 572–596. [Google Scholar] [CrossRef]
- Wojno, E.D.T.; Hunter, C.A.; Stumhofer, J.S. The Immunobiology of the Interleukin-12 Family: Room for Discovery. Immunity 2019, 50, 851–870. [Google Scholar] [CrossRef] [PubMed]
- Hildenbrand, K.; Aschenbrenner, I.; Franke, F.C.; Devergne, O.; Feige, M.J. Biogenesis and engineering of interleukin 12 family cytokines. Trends Biochem. Sci. 2022, 47, 936–949. [Google Scholar] [CrossRef]
- Li, Y.; Yang, Z.; Liu, B.; Valdez, C.; Chavko, M.; Cancio, L.C. Low-Level Primary Blast Induces Neuroinflammation and Neurodegeneration in Rats. Mil. Med. 2019, 184 (Suppl. 1), 265–272. [Google Scholar] [CrossRef] [PubMed]
- Frodl, T.; Amico, F. Is there an association between peripheral immune markers and structural/functional neuroimaging findings? Prog. Neuropsychopharmacol. Biol. Psychiatry 2014, 48, 295–303. [Google Scholar] [CrossRef]
- Harsanyi, S.; Kupcova, I.; Danisovic, L.; Klein, M. Selected Biomarkers of Depression: What Are the Effects of Cytokines and Inflammation? Int. J. Mol. Sci. 2022, 24, 578. [Google Scholar] [CrossRef]
- Katrinli, S.; Oliveira, N.C.S.; Felger, J.C.; Michopoulous, V.; Smith, A.K. The role of the immune system in posttraumatic stress disorder. Transl. Psychiatry 2022, 12, 313. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Majumdar, S.; Murari, S.; MachhindraVadak, K.; Krishnamurthy, S. Neurochemical, Neurocircuitry, and Psychopathological Mechanisms of PTSD: Emerging Pharmacotherapies and Clinical Perspectives. ACS Chem. Neurosci. 2025, 16, 2355–2370. [Google Scholar] [CrossRef]
- Heyn, S.A.; Schmit, C.; Keding, T.J.; Wolf, R.; Herringa, R.J. Neurobehavioral correlates of impaired emotion recognition in pediatric PTSD. Dev. Psychopathol. 2022, 34, 946–956. [Google Scholar] [CrossRef]
- Wolf, E.J.; Hawn, S.E.; Sullivan, D.R.; Miller, M.W.; Sanborn, V.; Brown, E.; Neale, Z.; Fein-Schaffer, D.; Zhao, X.; Logue, M.W.; et al. Neurobiological and genetic correlates of the dissociative subtype of posttraumatic stress disorder. J. Psychopathol. Clin. Sci. 2023, 132, 409–427. [Google Scholar] [CrossRef]
- Girgenti, M.J.; Wang, J.; Ji, D.; Cruz, D.A.; Traumatic Stress Brain Research Group; Stein, M.B.; Gelernter, J.; Young, K.A.; Huber, B.R.; Williamson, D.E.; et al. Transcriptomic organization of the human brain in post-traumatic stress disorder. Nat. Neurosci. 2021, 24, 24–33. [Google Scholar] [CrossRef]
- Erci, B. Meaning in life for patients with cancer: Validation of the Life Attitude Profile-Revised Scale. J. Adv. Nurs. 2008, 62, 704–711. [Google Scholar] [CrossRef]
- Xie, L.; Li, Y.; Ge, W.; Lin, Z.; Xing, B.; Miao, Q. The relationship between death attitude and professional identity in nursing students from mainland China. Nurse Educ. Today 2021, 107, 105150. [Google Scholar] [CrossRef]
- Mehnert, A.; Koch, U. Psychometric evaluation of the German version of the Life Attitude Profile-Revised (LAP-R) in prostate cancer patients. Palliat. Support. Care 2008, 6, 119–124. [Google Scholar] [CrossRef]
- Kéri, S. Stress Responses Among Individuals with Spiritual Struggles in Hungary: An Experimental Study. J. Relig. Health 2024, 63, 185–201. [Google Scholar] [CrossRef]
- Zefferino, R.; Di Gioia, S.; Conese, M. Molecular links between endocrine, nervous and immune system during chronic stress. Brain Behav. 2021, 11, e01960. [Google Scholar] [CrossRef]
- Liu, W.; Su, Y.-J.; Zhou, S.-J.; Deng, W.-H.; Hu, H.-Y.; Cui, Q.; Fang, A.-S.; Peng, Y.-M.; Luo, W.-X. Death coping ability, death attitude, and professional quality of life among geriatric nurses: A multicentre cross-sectional study. BMC Palliat. Care 2025, 24, 117. [Google Scholar] [CrossRef] [PubMed]
- Zahran, Z.; Hamdan, K.M.; Hamdan-Mansour, A.M.; Allari, R.S.; Alzayyat, A.A.; Shaheen, A.M. Nursing students’ attitudes towards death and caring for dying patients. Nurs. Open 2022, 9, 614–623. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.W.; Wolf, E.J.; Zhao, X.; Logue, M.W.; Hawn, S.E. An EWAS of dementia biomarkers and their associations with age, African ancestry, and PTSD. Clin. Epigenetics 2024, 16, 38. [Google Scholar] [CrossRef] [PubMed]
- Ben-Zion, Z.; Zeevi, Y.; Keynan, N.J.; Admon, R.; Kozlovski, T.; Sharon, H.; Halpern, P.; Liberzon, I.; Shalev, A.Y.; Benjamini, Y.; et al. Multi-domain potential biomarkers for post-traumatic stress disorder (PTSD) severity in recent trauma survivors. Transl. Psychiatry 2020, 10, 208. [Google Scholar] [CrossRef] [PubMed]
- Marchese, S.; Cancelmo, L.; Diab, O.; Cahn, L.; Aaronson, C.; Daskalakis, N.P.; Schaffer, J.; Horn, S.R.; Johnson, J.S.; Schechter, C.; et al. Altered gene expression and PTSD symptom dimensions in World Trade Center responders. Mol. Psychiatry 2022, 27, 2225–2246. [Google Scholar] [CrossRef]
- Daneshvar, S.; Shafiei, M.; Basharpoor, S. Group-based Compassion-focused Therapy on Experiential Avoidance, Meaning-in-life, and Sense of Coherence in Female Survivors of Intimate Partner Violence with PTSD: A Randomized Controlled Trial. J. Interpers. Violence 2022, 37, NP4187–NP4211. [Google Scholar] [CrossRef]
- Meis, L.A.; Polusny, M.A.; Kehle-Forbes, S.M.; Erbes, C.R.; O’Dougherty, M.; Erickson, E.P.G.; Orazem, R.J.; Burmeister, L.B.; Spoont, M.R. Making sense of poor adherence in PTSD treatment from the perspectives of veterans and their therapists. Psychol. Trauma 2023, 15, 715–725. [Google Scholar] [CrossRef] [PubMed]
- Sbisa, A.; Graham, K.; Lawrence-Wood, E.; McFarlane, A.C.; Toben, C. PTSD biomarkers: Neuroendocrine signaling to epigenetic variants. Adv. Clin. Chem. 2024, 122, 209–260. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, B.; Allgulander, C.; Baldwin, D.S.; Costa, D.L.D.C.; Denys, D.; Dilbaz, N.; Domschke, K.; Eriksson, E.; Fineberg, N.A.; Hättenschwiler, J.; et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for treatment of anxiety, obsessive-compulsive and posttraumatic stress disorders—Version 3. Part I: Anxiety disorders. World J. Biol. Psychiatry 2023, 24, 79–117. [Google Scholar] [CrossRef]
- Riedel, B.; Horen, S.R.; Reynolds, A.; Jahromi, A.H. Mental Health Disorders in Nurses During the COVID-19 Pandemic: Implications and Coping Strategies. Front. Public Health 2021, 9, 707358. [Google Scholar] [CrossRef]
- Sun, Y.; Qu, Y.; Zhou, X. Relationship between inflammation and post-traumatic stress disorder. Front. Psychiatry 2021, 12, 707543. [Google Scholar] [CrossRef]
- Hoskins, M.D.; Bridges, J.; Sinnerton, R.; Nakamura, A.; Underwood, J.F.G.; Slater, A.; Lee, M.R.D.; Clarke, L.; Lewis, C.; Roberts, N.P.; et al. Pharmacological therapy for post-traumatic stress disorder: A systematic review and meta-analysis of monotherapy, augmentation and head-to-head approaches. Eur. J. Psychotraumatol. 2021, 12, 1802920. [Google Scholar] [CrossRef]
- Ogłodek, E.A.; Szota, A.M.; Just, M.J.; Araszkiewicz, A.; Szromek, A.R. Sense of alexithymia in patients with anxiety disorders comorbid with recurrent urticaria. Neuropsychiatr. Dis. Treat. 2016, 12, 995–1004. [Google Scholar] [CrossRef]
- Keenan, E.L.; Granstein, R.D. Proinflammatory cytokines and neuropeptides in psoriasis, depression, and anxiety. Acta Physiol. 2025, 241, e70019. [Google Scholar] [CrossRef]
- Merians, A.N.; Spiller, T.; Harpaz-Rotem, I.; Krystal, J.H.; Pietrzak, R.H. Post-traumatic Stress Disorder. Med. Clin. N. Am. 2023, 107, 85–99. [Google Scholar] [CrossRef]
- Williamson, J.B.; Jaffee, M.S.; Jorge, R.E. Posttraumatic Stress Disorder and Anxiety-Related Conditions. Continuum 2021, 27, 1738–1763. [Google Scholar] [CrossRef]
- Pinna, G.; Ponomareva, O.; Stalcup, G.L.; Rasmusson, A.M. Neuroactive steroids and the pathophysiology of PTSD: Biomarkers for treatment targeting. Neurosci. Biobehav. Rev. 2025, 172, 106085. [Google Scholar] [CrossRef]
- Georgiou, P.; Farmer, C.A.; Medeiros, G.C.; Yuan, P.; Johnston, J.; Kadriu, B.; Gould, T.D.; Zarate, C.A., Jr. Associations between hypothalamic-pituitary-adrenal (HPA) axis hormone levels, major depression features and antidepressant effects of ketamine. J. Affect. Disord. 2025, 373, 126–132. [Google Scholar] [CrossRef]
- Martalek, A.; Dubertret, C.; Fovet, T.; Le Strat, Y.; Tebeka, S. Distressing memories: A continuum from wellness to PTSD. J. Affect. Disord. 2024, 363, 198–205. [Google Scholar] [CrossRef]
- Purnell, L.; Graham, A.; Chiu, K.; Trickey, D.; Meiser-Stedman, R. A systematic review and meta-analysis of PTSD symptoms at mid-treatment during trauma-focused treatment for PTSD. J. Anxiety Disord. 2024, 107, 102925. [Google Scholar] [CrossRef]
- Yadav, M.K.; Singh, S.P.; Egwuagu, C.E. IL-6/IL-12 superfamily of cytokines and regulatory lymphocytes play critical roles in the etiology and suppression of CNS autoimmune diseases. Front. Immunol. 2025, 16, 1514080. [Google Scholar] [CrossRef] [PubMed]
- Schneeberger, S.; Kim, S.J.; Geesdorf, M.N.; Friebel, E.; Eede, P.; Jendrach, M.; Boltengagen, A.; Braeuning, C.; Ruhwedel, T.; Hülsmeier, A.J.; et al. Interleukin-12 signaling drives Alzheimer’s disease pathology through disrupting neuronal and oligodendrocyte homeostasis. Nat. Aging 2025, 5, 622–641. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Liu, W.; Jiang, H.; Zhao, Q.; Hu, Y.; Tang, X.; Liu, X.; Dai, H.; Rui, H.; Liu, B. IL-12 family cytokines and autoimmune diseases: A potential therapeutic target? J. Transl. Autoimmun. 2024, 10, 100263. [Google Scholar] [CrossRef] [PubMed]
- Alshabani, N.; Haws, J.K.; Zlotnick, C.; Johnson, D.M. PTSD symptom networks during treatment among residents in domestic violence shelters. J. Couns. Psychol. 2025, 72, 1–14. [Google Scholar] [CrossRef]
- Yuan, M.; Li, L.; Zhu, H.; Zheng, B.; Lui, S.; Zhang, W. Cortical morphological changes and associated transcriptional signatures in post-traumatic stress disorder and psychological resilience. BMC Med. 2024, 22, 431. [Google Scholar] [CrossRef]
- Liddell, B.J.; Das, P.; Malhi, G.S.; Jobson, L.; Lau, W.; Felmingham, K.L.; Nickerson, A.; Askovic, M.; Aroche, J.; Coello, M.; et al. Self-construal modulates default mode network connectivity in refugees with PTSD. J. Affect. Disord. 2024, 361, 268–276. [Google Scholar] [CrossRef]
- Patriat, R.; Birn, R.M.; Keding, T.J.; Herringa, R.J. Default-mode network abnormalities in pediatric posttraumatic stress disorder. J. Am. Acad. Child Adolesc. Psychiatry 2016, 55, 319–327. [Google Scholar] [CrossRef]
- Lieberman, J.M.; Rabellino, D.; Densmore, M.; Frewen, P.A.; Steyrl, D.; Scharnowski, F.; Théberge, J.; Neufeld, R.W.J.; Schmahl, C.; Jetly, R.; et al. Posterior cingulate cortex targeted real-time fMRI neurofeedback recalibrates functional connectivity with the amygdala, posterior insula, and default-mode network in PTSD. Brain Behav. 2023, 13, e2883. [Google Scholar] [CrossRef]
- Rubio-Belmonte, C.; Mayordomo-Rodríguez, T.; García-Alandete, J. Psychometric properties of the Purpose In Life-Short Form in the Spanish population. J. Clin. Psychol. 2023, 79, 1099–1112. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Belmonte, C.; Mayordomo-Rodríguez, T.; Marco-Ahullo, A.; Aragonés-Barberá, I. Psychometric validation of the Purpose in Life Test-Short Form (PIL-SF) in individuals diagnosed with severe mental illness. Healthcare 2024, 12, 2082. [Google Scholar] [CrossRef] [PubMed]
- Jin, Y.; Xu, S.; Shao, Z.; Luo, X.; Wilson, A.; Li, J.; Wang, Y. The lasting effects of childhood trauma on developing psychiatric symptoms: A population-based, large-scale comparison study. Glob. Ment. Health 2024, 11, e98. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Cejudo, J.; Genfi, A.; Abu-Amara, D.; Debure, L.; Qian, M.; Laska, E.; Siegel, C.; Milton, N.; Newman, J.; Blessing, E.; et al. CRF serum levels differentiate PTSD from healthy controls and TBI in military veterans. Psychiatr. Res. Clin. Pract. 2021, 3, 153–162. [Google Scholar] [CrossRef]
- von Majewski, K.; Rohleder, N.; Ehring, T. Peripheral inflammation over the course of a cognitive behavioral intervention in PTSD. Brain Behav. Immun. Health 2023, 30, 100620. [Google Scholar] [CrossRef]
- Castro-Vale, I.; Carvalho, D. The pathways between cortisol-related regulation genes and PTSD psychotherapy. Healthcare 2020, 8, 376. [Google Scholar] [CrossRef]
- Feng, J.; Wu, Y.; Meng, M.; Zeng, R.; Ma, Y.; Luo, D.; Zhang, L.; Zhang, Y.; Li, Y.; Huang, W.; et al. The mediating effect of blood biomarkers in the associations between inflammatory bowel disease and incident psychiatric disorders: A prospective cohort study. Int. J. Surg. 2024, 110, 7738–7748. [Google Scholar] [CrossRef]
- Gupta, S.; Guleria, R.S. Involvement of Nuclear Factor-κB in Inflammation and Neuronal Plasticity Associated with Post-Traumatic Stress Disorder. Cells 2022, 11, 2034. [Google Scholar] [CrossRef]
- Mojtabavi, H.; Saghazadeh, A.; van den Heuvel, L.; Bucker, J.; Rezaei, N. Peripheral blood levels of brain-derived neurotrophic factor in patients with post-traumatic stress disorder (PTSD): A systematic review and meta-analysis. PLoS ONE 2020, 15, e0241928. [Google Scholar] [CrossRef] [PubMed]
- Belrose, C.; Duffaud, A.; Rakotoarison, E.; Faget, C.; Raynaud, P.; Dutheil, F.; Boyer, L.; Billaud, J.B.; Trousselard, M. Neurological soft signs and post-traumatic stress disorder: A biomarker of severity? Front. Psychiatry 2020, 11, 533662. [Google Scholar] [CrossRef] [PubMed]
- Deiber, M.P.; Perizzolo, V.C.P.; Moser, D.A.; Vital, M.; Serpa, S.R.; Ros, T.; Schechter, D.S. A biomarker of brain arousal mediates the intergenerational link between maternal and child post-traumatic stress disorder. J. Psychiatr. Res. 2024, 177, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Klamut, R. Manual to the Polish Adaptation of the Life Attitudes Profile–Revised (LAP-R); Reker, G.T., Ed.; Psychological Testing Laboratory of the Polish Psychological Society: Warsaw, Poland, 2010. [Google Scholar]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Hillsdale, NJ, USA, 1988. [Google Scholar]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2024; Available online: https://www.R-project.org/ (accessed on 13 July 2025).
- Aphalo, P. Ggpmisc: Miscellaneous Extensions to ‘ggplot2’, R package version 0.6.1. 2024. Available online: https://CRAN.R-project.org/package=ggpmisc (accessed on 22 July 2025).
- Aphalo, P. Ggpp: Grammar Extensions to ‘ggplot2’, R package version 0.5.8-1. 2024. Available online: https://CRAN.R-project.org/package=ggpp (accessed on 28 June 2025).
- Kassambara, A. Ggpubr: ‘ggplot2’ Based Publication Ready Plots, R package version 0.6.0. 2023. Available online: https://CRAN.R-project.org/package=ggpubr (accessed on 27 June 2025).
- Makowski, D.; Lüdecke, D.; Patil, I.; Thériault, R.; Ben-Shachar, M.; Wiernik, B. Automated Results Reporting as a Practical Tool to Improve Reproducibility and Methodological Best Practices Adoption; CRAN: Wien, Austria, 2023; Available online: https://easystats.github.io/report/ (accessed on 27 June 2025).
- Makowski, D.; Wiernik, B.; Patil, I.; Lüdecke, D.; Ben-Shachar, M. Correlation: Methods for Correlation Analysis, Version 0.8.3. 2022. Available online: https://CRAN.R-project.org/package=correlation (accessed on 27 June 2025).
- Sjoberg, D.; Whiting, K.; Curry, M.; Lavery, J.; Larmarange, J. Reproducible summary tables with the gtsummary package. R J. 2021, 13, 570–580. [Google Scholar] [CrossRef]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer-Verlag: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. Available online: https://ggplot2.tidyverse.org (accessed on 27 June 2025).
- Wickham, H.; François, R.; Henry, L.; Müller, K.; Vaughan, D. Dplyr: A Grammar of Data Manipulation, R package version 1.1.4. 2023. Available online: https://CRAN.R-project.org/package=dplyr (accessed on 27 June 2025).
- Wickham, H.; Vaughan, D.; Girlich, M. Tidyr: Tidy Messy Data, R package version 1.3.1. 2024. Available online: https://CRAN.R-project.org/package=tidyr (accessed on 27 June 2025).

| Characteristic | Overall (N = 92) | No PTSD (Control) (N = 28) | Past PTSD (≤5y) (N = 33) | Past PTSD (>5y) (N = 31) | p-Value |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, median (IQR) | 34.0 (28.8, 41.0) | 33.5 (24.2, 41.5) | 34.0 (31.0, 41.0) | 36.0 (29.5, 41.0) | 0.524 |
| Biomarkers, median (IQR) | |||||
| Serotonin (ng/mL) | 144.8 (120.2, 203.4) | 225.2 a (209.6, 296.7) | 109.9 c (96.6, 122.2) | 147.4 b (139.6, 153.2) | <0.001 |
| Cortisol (µg/dL) | 14.0 (10.9, 36.2) | 13.5 b (12.0, 15.4) | 9.8 b (6.8, 11.6) | 47.5 a (36.4, 57.6) | <0.001 |
| Noradrenaline (pg/mL) | 371.3 (220.3, 544.8) | 580.2 a (449.1, 830.2) | 271.7 b (210.7, 406.3) | 271.7 a (189.0, 399.7) | <0.001 |
| IL-12 (pg/mL) | 21.6 (8.5, 54.7) | 7.7 c (6.3, 8.6) | 62.4 a (51.8, 69.1) | 17.0 b (11.2, 33.9) | <0.001 |
| LAP-R questionnaire, median (IQR) | |||||
| Life Purpose (LF) | 37.0 (22.0, 51.2) | 54.0 a (51.8, 56.0) | 39.0 b (27.0, 47.0) | 20.0 c (17.0, 23.0) | <0.001 |
| Coherence (C) | 33.0 (25.0, 48.2) | 53.0 a (50.8, 54.2) | 34.0 b (31.0, 39.0) | 23.0 c (17.5, 26.0) | <0.001 |
| Personal Sense (PS) | 69.5 (46.0, 103.2) | 105.0 a (104.0, 109.0) | 70.0 b (62.0, 82.0) | 44.0 c (35.5, 46.5) | <0.001 |
| Life Control (LC) | 34.0 (25.0, 40.0) | 47.5 a (41.5, 53.0) | 33.0 b (26.0, 37.0) | 24.0 c (22.0, 29.5) | <0.001 |
| Death Acceptance (DA) | 44.0 (28.0, 50.0) | 47.5 a (44.8, 53.0) | 26.0 c (18.0, 29.0) | 48.0 a (41.5, 52.0) | <0.001 |
| Existential Vacuum (EV) | 47.0 (40.0, 51.0) | 48.5 a (46.8, 52.2) | 42.0 b (37.0, 48.0) | 48.0 a (40.5, 52.0) | <0.001 |
| Goal Seeking (GS) | 39.0 (32.0, 47.0) | 50.0 a (46.8, 55.2) | 39.0 b (35.0, 44.0) | 29.0 c (24.5, 32.5) | <0.001 |
| Balance of Life Attitudes (BLA) | 54.0 (37.0, 90.2) | 102.5 b (90.8, 111.0) | 46.0 b (37.0, 58.0) | 37.0 b (29.0, 52.5) | <0.001 |
| Parameter | Control vs. ≤5y r | Control vs. >5y r | ≤5y vs. >5y r |
|---|---|---|---|
| Biomarkers | |||
| Serotonin | 0.86 | 0.86 | 0.84 |
| Cortisol | 0.54 | 0.86 | 0.86 |
| Noradrenaline | 0.53 | 0.53 | 0.03 |
| IL-12 | 0.86 | 0.69 | 0.72 |
| LAP-R questionnaire | |||
| Life Purpose | 0.78 | 0.86 | 0.81 |
| Coherence | 0.85 | 0.86 | 0.79 |
| Personal Sense | 0.86 | 0.86 | 0.84 |
| Life Control | 0.79 | 0.83 | 0.41 |
| Death Acceptance | 0.81 | 0.10 | 0.80 |
| Existential Vacuum | 0.55 | 0.20 | 0.36 |
| Goal Seeking | 0.71 | 0.86 | 0.75 |
| Balance of Life Attitudes | 0.77 | 0.86 | 0.22 |
| Characteristic | No PTSD (Control) (N = 28) | PTSD ≤ 5y (N = 33) | PTSD > 5y (N = 31) |
|---|---|---|---|
| Serotonin (ng/mL) | |||
| 18–35 yrs | 219.1 (211.6, 266.8) | 111.6 (95.3, 122.7) | 143.8 (136.4, 158.8) |
| 36–50 yrs | 273.9 (208.3, 303.3) | 104.6 (97.7, 120.8) | 148.6 (142.8, 152.6) |
| p-value (adjusted) | 1.000 | 0.829 | 0.374 |
| Cortisol (ug/dL) | |||
| 18–35 yrs | 12.7 (11.5, 14.2) | 11.1 (7.8, 12.8) | 36.1 (29.1, 39.2) |
| 36–50 yrs | 14.9 (12.7, 15.8) | 8.2 (6.1, 11.3) | 57.6 (53.2, 59.7) |
| p-value (adjusted) | 0.294 | 0.378 | 0.003 |
| Noradrenaline (pg/mL) | |||
| 18–35 yrs | 427.2 (320.8, 582.8) | 369.2 (220.3, 534.6) | 371.3 (225.7, 571.2) |
| 36–50 yrs | 765.7 (544.8, 960.4) | 239.7 (179.7, 381.0) | 211.3 (176.3, 354.3) |
| p-value (adjusted) | 0.021 | 0.480 | 0.165 |
| IL-12 (pg/mL) | |||
| 18–35 yrs | 8.4 (7.4, 9.2) | 68.0 (51.9, 77.1) | 15.7 (10.3, 31.6) |
| 36–50 yrs | 6.5 (5.4, 7.7) | 55.4 (46.8, 65.9) | 26.3 (11.9, 32.7) |
| p-value (adjusted) | 0.033 | 0.174 | 1.000 |
| LF (Life Purpose) | |||
| 18–35 yrs | 56.0 (50.8, 56.0) | 27.5 (26.0, 29.8) | 18.0 (16.0, 22.5) |
| 36–50 yrs | 53.0 (52.0, 54.8) | 47.0 (45.0, 50.0) | 20.5 (18.8, 23.0) |
| p-value (adjusted) | 0.678 | < 0.001 | 0.648 |
| C (Coherence) | |||
| 18–35 yrs | 53.5 (50.5, 56.0) | 35.5 (32.0, 39.0) | 21.0 (18.0, 24.0) |
| 36–50 yrs | 53.0 (51.0, 53.0) | 33.0 (29.0, 35.0) | 24.5 (17.8, 27.3) |
| p-value (adjusted) | 0.504 | 0.909 | 0.909 |
| PS (Personal Sense) | |||
| 18–35 yrs | 105.5 (104.0, 109.0) | 63.0 (59.0, 67.5) | 42.0 (34.0, 45.0) |
| 36–50 yrs | 105.0 (104.0, 106.8) | 82.0 (73.0, 85.0) | 46.0 (36.8, 48.0) |
| p-value (adjusted) | 1.000 | 0.001 | 0.265 |
| LC (Life Control) | |||
| 18–35 yrs | 53.0 (48.0, 54.8) | 30.0 (25.0, 34.3) | 31.0 (22.0, 35.0) |
| 36–50 yrs | 42.5 (39.3, 46.0) | 35.0 (27.0, 37.0) | 23.0 (20.3, 28.0) |
| p-value (adjusted) | 0.006 | 0.474 | 0.147 |
| DA (Death Acceptance) | |||
| 18–35 yrs | 49.0 (47.3, 53.0) | 24.5 (17.0, 26.3) | 52.0 (46.0, 53.0) |
| 36–50 yrs | 44.5 (44.0, 49.0) | 28.0 (26.0, 30.0) | 44.5 (38.5, 48.3) |
| p-value (adjusted) | 0.111 | 0.027 | 0.021 |
| EV (Existential Vacuum) | |||
| 18–35 yrs | 48.0 (46.3, 52.8) | 40.5 (36.8, 48.0) | 46.0 (42.0, 49.5) |
| 36–50 yrs | 49.0 (47.0, 51.8) | 42.0 (37.0, 45.0) | 48.5 (39.5, 53.0) |
| p-value (adjusted) | 1.000 | 1.000 | 1.000 |
| GS (Goal Seeking) | |||
| 18–35 yrs | 50.0 (47.3, 55.0) | 43.0 (35.0, 44.5) | 28.0 (26.0, 32.5) |
| 36–50 yrs | 50.5 (44.3, 56.0) | 39.0 (37.0, 41.0) | 30.0 (23.5, 32.3) |
| p-value (adjusted) | 1.000 | 1.000 | 1.000 |
| BLA (Balance of Life Attitudes) | |||
| 18–35 yrs | 106.5 (102.0, 111.0) | 37.0 (31.8, 38.0) | 41.0 (32.0, 57.5) |
| 36–50 yrs | 93.5 (86.5, 102.8) | 57.0 (48.0, 66.0) | 36.5 (27.0, 48.3) |
| p-value (adjusted) | 0.033 | 0.001 | 0.466 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paraniak-Gieszczyk, B.; Ogłodek, E.A. Neurobiological and Existential Profiles in Posttraumatic Stress Disorder: The Role of Serotonin, Cortisol, Noradrenaline, and IL-12 Across Chronicity and Age. Int. J. Mol. Sci. 2025, 26, 9636. https://doi.org/10.3390/ijms26199636
Paraniak-Gieszczyk B, Ogłodek EA. Neurobiological and Existential Profiles in Posttraumatic Stress Disorder: The Role of Serotonin, Cortisol, Noradrenaline, and IL-12 Across Chronicity and Age. International Journal of Molecular Sciences. 2025; 26(19):9636. https://doi.org/10.3390/ijms26199636
Chicago/Turabian StyleParaniak-Gieszczyk, Barbara, and Ewa Alicja Ogłodek. 2025. "Neurobiological and Existential Profiles in Posttraumatic Stress Disorder: The Role of Serotonin, Cortisol, Noradrenaline, and IL-12 Across Chronicity and Age" International Journal of Molecular Sciences 26, no. 19: 9636. https://doi.org/10.3390/ijms26199636
APA StyleParaniak-Gieszczyk, B., & Ogłodek, E. A. (2025). Neurobiological and Existential Profiles in Posttraumatic Stress Disorder: The Role of Serotonin, Cortisol, Noradrenaline, and IL-12 Across Chronicity and Age. International Journal of Molecular Sciences, 26(19), 9636. https://doi.org/10.3390/ijms26199636





