Early Renal Remission Is Associated with Increased Likelihood of Subsequent Remission in Lupus Nephritis: Single-Centre Observational Study in Australia
Abstract
1. Introduction
2. Results
2.1. Patient Characteristics at Baseline
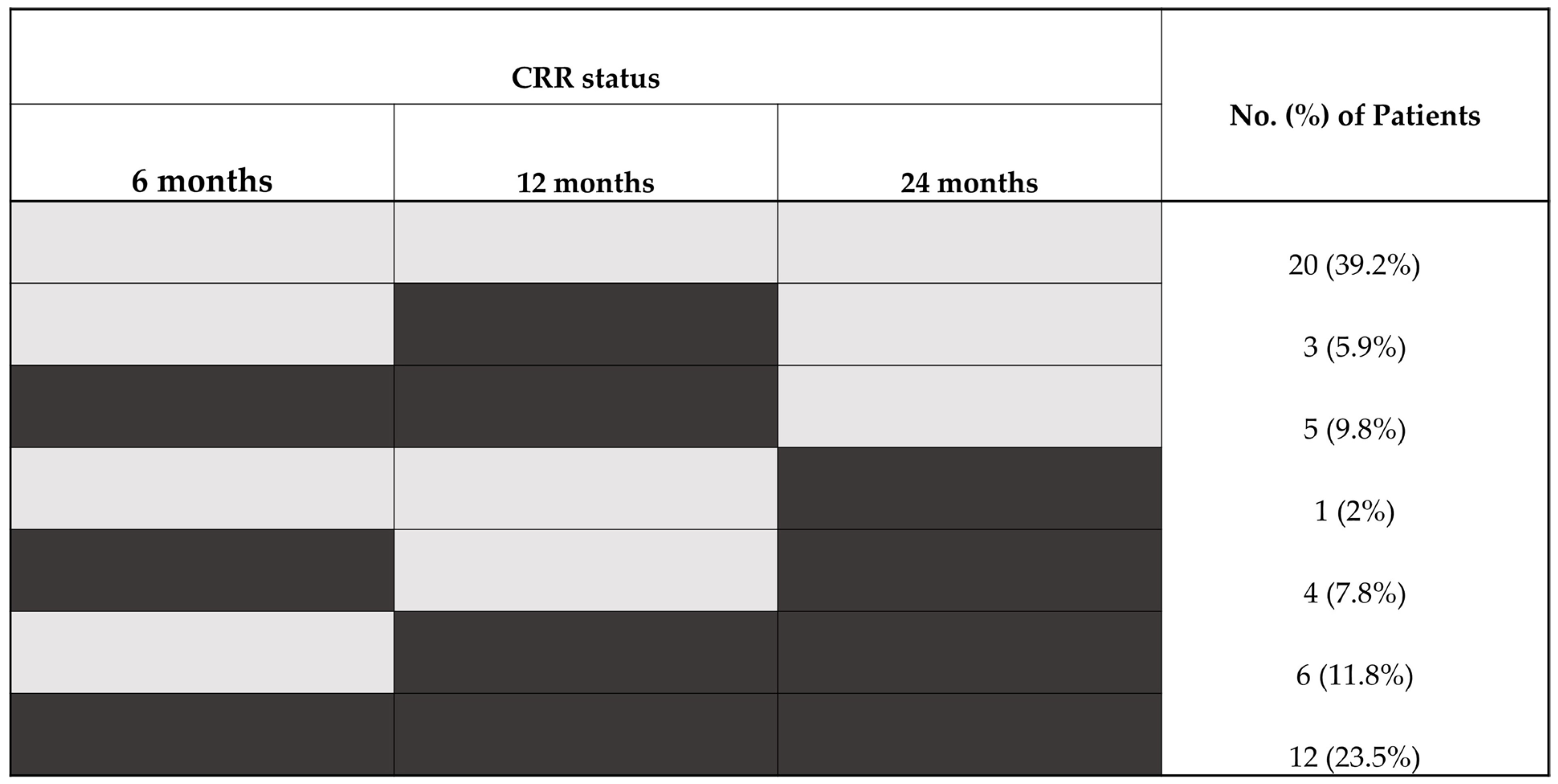
2.2. CRR Attainment at 6, 12, and 24 Months
2.3. Factors Associated with CRR Attainment at 24 Months
3. Discussion
4. Methods
4.1. Study Design and Participants
4.2. Variables and Definitions
4.3. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACR | American College of Rheumatology |
| ALRB | Australian Lupus Registry and Biobank |
| CI | confidence interval |
| CRR | complete renal remission |
| CYC | cyclophosphamide |
| eGFR | estimated glomerular filtration rate |
| ESKD | end-stage kidney disease |
| GC | Glucocorticoids |
| IQR | Interquartile range |
| LN | lupus nephritis |
| MMF | mycophenolate mofetil |
| mPERR | modified primary efficacy renal response |
| OR | odds ratio |
| RTX | Rituximab |
| SLE | Systemic Lupus Erythematosus |
| SLEDAI-2K | SLE Disease Activity Index 2000 |
| TAC | Tacrolimus |
| UPCR | urine protein/creatinine ratio |
References
- Hanly, J.G.; O’Keeffe, A.G.; Su, L.; Urowitz, M.B.; Romero-Diaz, J.; Gordon, C.; Bae, S.C.; Bernatsky, S.; Clarke, A.E.; Wallace, D.J.; et al. The frequency and outcome of lupus nephritis: Results from an international inception cohort study. Rheumatology 2016, 55, 252–262. [Google Scholar] [CrossRef]
- Farinha, F.; Pepper, R.J.; Oliveira, D.G.; McDonnell, T.; Isenberg, D.A.; Rahman, A. Outcomes of membranous and proliferative lupus nephritis—Analysis of a single-centre cohort with more than 30 years of follow-up. Rheumatology 2020, 59, 3314–3323. [Google Scholar] [CrossRef]
- Mok, C.C.; Ho, L.Y.; Ying, S.K.Y.; Leung, M.C.; To, C.H.; Ng, W.L. Long-term outcome of a randomised controlled trial comparing tacrolimus with mycophenolate mofetil as induction therapy for active lupus nephritis. Ann. Rheum. Dis. 2020, 79, 1070–1076. [Google Scholar] [CrossRef]
- Moroni, G.; Gatto, M.; Tamborini, F.; Quaglini, S.; Radice, F.; Saccon, F.; Frontini, G.; Alberici, F.; Sacchi, L.; Binda, V.; et al. Lack of EULAR/ERA-EDTA response at 1 year predicts poor long-term renal outcome in patients with lupus nephritis. Ann. Rheum. Dis. 2020, 79, 1077–1083. [Google Scholar] [CrossRef]
- Urowitz, M.; Georgiou, M.E.; Touma, Z.; Su, J.; Diaz-Martinez, J.P.; Fu, Q.; Levy, R.A.; Gairy, K.; MacKinnon, A.; Anderson, N.; et al. Renal response status to predict long-term renal survival in patients with lupus nephritis: Results from the Toronto Lupus Cohort. Lupus Sci. Med. 2024, 11, e001264. [Google Scholar] [CrossRef]
- Yap, D.Y.H.; Xu, X.; Juliao, P.C.; Tang, C.S.O.; Ng, L.; Milea, D.; Chan, T.M. Long-Term Kidney Outcome of Lupus Nephritis by Renal Response Status. Kidney Int. Rep. 2024, 9, 3532–3541. [Google Scholar] [CrossRef]
- Furie, R.; Rovin, B.H.; Houssiau, F.; Malvar, A.; Teng, Y.K.O.; Contreras, G.; Amoura, Z.; Yu, X.; Mok, C.C.; Santiago, M.B.; et al. Two-Year, Randomised, Controlled Trial of Belimumab in Lupus Nephritis. N. Engl. J. Med. 2020, 383, 1117–1128. [Google Scholar] [CrossRef]
- Saxena, A.; Ginzler, E.M.; Gibson, K.; Satirapoj, B.; Santillán, A.E.Z.; Levchenko, O.; Navarra, S.; Atsumi, T.; Yasuda, S.; Chavez-Perez, N.N.; et al. Safety and Efficacy of Long-Term Voclosporin Treatment for Lupus Nephritis in the Phase 3 AURORA 2 Clinical Trial. Arthritis Rheumatol. 2024, 76, 59–67. [Google Scholar] [CrossRef]
- Sammaritano, L.R.; Askanase, A.; Bermas, B.L.; Dall’Era, M.; Duarte-Garcia, A.; Hiraki, L.T.; Rovin, B.H.; Son, M.B.F.; Alvarado, A.; Aranow, C.; et al. 2024 American College of Rheumatology (ACR) Guideline for the Screening, Treatment, and Management of Lupus Nephritis. Arthritis Rheumatol. 2025, 77, 1115–1135. [Google Scholar] [CrossRef]
- Gatto, M.; Frontini, G.; Furlan, C.; Calatroni, M.; Cruciani, C.; Reggiani, F.; Bellis, E.; Iaccarino, L.; Sinico, R.A.; Moroni, G.; et al. Three years is the minimal effective duration of sustained clinical remission which prevents impaired kidney function and damage accrual in lupus nephritis. Ann. Rheum. Dis. 2025, 84, 594–600. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes Lupus Nephritis Work Group. KDIGO 2024 Clinical Practice Guideline for the management of LUPUS NEPHRITIS. Kidney Int. 2024, 105, S1–S69. [Google Scholar] [CrossRef]
- Condon, M.B.; Ashby, D.; Pepper, R.J.; Cook, H.T.; Levy, J.B.; Griffith, M.; Cairns, T.D.; Lightstone, L. Prospective observational single-centre cohort study to evaluate the effectiveness of treating lupus nephritis with rituximab and mycophenolate mofetil but no oral steroids. Ann. Rheum. Dis. 2013, 72, 1280–1286. [Google Scholar] [CrossRef]
- Kikuchi, J.; Hanaoka, H.; Saito, S.; Oshige, T.; Hiramoto, K.; Takeuchi, T.; Kaneko, Y. Deep remission within 12 months prevents renal flare and damage accrual in lupus nephritis. Clin. Exp. Rheumatol. 2023, 41, 1500–1506. [Google Scholar] [CrossRef]
- Davidson, J.E.; Fu, Q.; Ji, B.; Rao, S.; Roth, D.; Magder, L.S.; Petri, M. Renal Remission Status and Longterm Renal Survival in Patients with Lupus Nephritis: A Retrospective Cohort Analysis. J. Rheumatol. 2018, 45, 671–677. [Google Scholar] [CrossRef]
- Hanaoka, H.; Iida, H.; Kiyokawa, T.; Takakuwa, Y.; Kawahata, K. Early achievement of deep remission predicts low incidence of renal flare in lupus nephritis class III or IV. Arthritis Res. Ther. 2018, 20, 86. [Google Scholar] [CrossRef]
- Pirson, V.; Enfrein, A.; Houssiau, F.A.; Tamirou, F. Absence of renal remission portends poor long-term kidney outcome in lupus nephritis. Lupus Sci. Med. 2021, 8, e000533. [Google Scholar] [CrossRef]
- Korbet, S.M.; Schwartz, M.M.; Evans, J.; Lewis, E.J.; Collaborative Study Group. Severe lupus nephritis: Racial differences in presentation and outcome. J. Am. Soc. Nephrol. 2007, 18, 244–254. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Kostopoulou, M.; Cheema, K.; Anders, H.J.; Aringer, M.; Bajema, I.; Boletis, J.; Frangou, E.; Houssiau, F.A.; Hollis, J.; et al. 2019 Update of the Joint European League Against Rheumatism and European Renal Association-European Dialysis and Transplant Association (EULAR/ERA-EDTA) recommendations for the management of lupus nephritis. Ann. Rheum. Dis. 2020, 79, 713–723. [Google Scholar] [CrossRef]
- Luis, M.S.F.; Bultink, I.E.M.; da Silva, J.A.P.; Voskuyl, A.E.; Ines, L.S. Early predictors of renal outcome in patients with proliferative lupus nephritis: A 36-month cohort study. Rheumatology 2021, 60, 5134–5141. [Google Scholar] [CrossRef]
- De Vriese, A.S.; Sethi, S.; Fervenza, F.C. Lupus nephritis: Redefining the treatment goals. Kidney Int. 2025, 107, 198–211. [Google Scholar] [CrossRef]
- Pakchotanon, R.; Gladman, D.D.; Su, J.; Urowitz, M.B. Sustained complete renal remission is a predictor of reduced mortality, chronic kidney disease and end-stage renal disease in lupus nephritis. Lupus 2018, 27, 468–474. [Google Scholar] [CrossRef]
- Parodis, I.; Adamichou, C.; Aydin, S.; Gomez, A.; Demoulin, N.; Weinmann-Menke, J.; Houssiau, F.A.; Tamirou, F. Per-protocol repeat kidney biopsy portends relapse and long-term outcome in incident cases of proliferative lupus nephritis. Rheumatology 2020, 59, 3424–3434. [Google Scholar] [CrossRef]
- Pennesi, M.; Benvenuto, S. Lupus Nephritis in Children: Novel Perspectives. Medicina 2023, 59, 1841. [Google Scholar] [CrossRef]
- Hochberg, M.C. Updating the American College of Rheumatology revised criteria for the classification of systemic lupus erythematosus. Arthritis Rheum. 1997, 40, 1725. [Google Scholar] [CrossRef]
- Petri, M.; Orbai, A.M.; Alarcon, G.S.; Gordon, C.; Merrill, J.T.; Fortin, P.R.; Bruce, I.N.; Isenberg, D.; Wallace, D.J.; Nived, O.; et al. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012, 64, 2677–2686. [Google Scholar] [CrossRef]
- Weening, J.J.; D’Agati, V.D.; Schwartz, M.M.; Seshan, S.V.; Alpers, C.E.; Appel, G.B.; Balow, J.E.; Bruijn, J.A.; Cook, T.; Ferrario, F.; et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J. Am. Soc. Nephrol. 2004, 15, 241–250. [Google Scholar] [CrossRef]
- Touma, Z.; Urowitz, M.B.; Ibanez, D.; Gladman, D.D. SLEDAI-2K 10 days versus SLEDAI-2K 30 days in a longitudinal evaluation. Lupus 2011, 20, 67–70. [Google Scholar] [CrossRef]
- National Health and Medical Research Council; Australian Research Council and Universities Australia. National Statement on Ethical Conduct in Human Research. Canberra: National Health and Medical Research Council. 2023. Available online: www.nhmrc.gov.au/about-us/publications/national-statement-ethical-conduct-human-research-2023 (accessed on 30 September 2025).
| Study Cohort | |
|---|---|
| Total Number (n) of Patients = 60 | |
| n (%) or Median [IQR] | |
| Demographics | |
| Female | 49 (82%) |
| Age at LN diagnosis | 27 [19, 39] |
| SLE disease duration prior to biopsy-proven Class III or IV ± V LN (months) | 4.5 [0, 10.5] |
| Ethnicity | |
| Caucasian | 21 (35%) |
| Asian | 31 (52%) |
| Others | 8 (13%) |
| Current or ex-smoker 1 | 11 (19%) |
| Hypertension | 12 (20%) |
| Baseline Serological profile | |
| Anti-dsDNA positive 2 | 53 (93%) |
| Hypocomplementaemia (low C3/C4) 3 | 47 (80%) |
| Kidney Function at LN diagnosis | |
| Estimated glomerular filtration rate (eGFR, mL/min/1.73 m2) 4 | 80.5 [58, 90] |
| Urinary protein/creatinine ratio (UPCR, g/mmol) | 0.3 [0.2, 0.5] |
| Medications | |
| Glucocorticoids (GC) exposure at least once (ever) | 60 (100%) |
| Daily GC dose (mg) at: | |
| Baseline 5 | 17.5 [7.5, 38.8] |
| 6 months 6 | 10.0 [7.5, 15.0] |
| 12 months 7 | 5.0 [1.2, 10.0] |
| 24 months 8 | 5.0 [1.0, 7.5] |
| Induction therapy 3 | 59 (100%) |
| MMF | 41 (69%) |
| CYC | 11 (19%) |
| RTX | 2 (3%) |
| MMF/RTX | 3 (5%) |
| MMF/TAC | 1 (2%) |
| CYC/RTX | 1 (2%) |
| Antimalarial therapy 1 | 49 (84%) |
| Class of LN (ISN/RPS Classification) | |
| III | 12 (20%) |
| IV | 26 (43%) |
| III or IV ± V | 22 (37%) |
| Complete renal remission (CRR) | |
| CRR at least once (CRR-ever) 9 | 31 (61%) |
| CRR never 9 | 20 (39%) |
| CRR at 6 months 1 | 23 (40%) |
| CRR at 12 months 10 | 26 (47%) |
| CRR at 24 months 10 | 24 (44%) |
| SLE Disease Activity Index (SLEDAI)-2K score | |
| Baseline 11 | 14.5 [8.0, 19.0] |
| 6 months 12 | 6.0 [4.0, 10.0] |
| 12 months 13 | 6.0 [4.0, 8.0] |
| 24 months 14 | 4.0 [4.0, 8.0] |
| CRR at 6 Months | CRR at 12 Months | CRR at 24 Months | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Non-Responder | Responder | Non-Responder | Responder | Non-Responder | Responder | ||||
| n = 35 | n = 23 | n = 29 | n = 26 | n = 31 | n = 24 | ||||
| n (%) or Median [IQR] | p-Value | n (%) or Median [IQR] | p-Value | n (%) or Median [IQR] | p-Value | ||||
| Demographics | |||||||||
| Female | 27 (77%) | 20 (87%) | 0.35 | 19 (66%) | 25 (96%) | 0.005 | 23 (74%) | 23 (96%) | 0.031 |
| Age at LN diagnosis | 28 [17, 40] | 27 [19, 39] | 0.60 | 23 [15, 31] | 30 [24, 39] | 0.088 | 24 [15, 30] | 28.5 [21.5, 41.5] | 0.064 |
| Pre-biopsy SLE duration (months) | 4 [0, 12] | 5 [0, 9] | 0.90 | 5 [1, 12] | 4.5 [0.0, 9.0] | 0.30 | 7 [1, 13] | 3 [0, 7.5] | 0.083 |
| Asian ethnicity | 21 (60%) | 11 (48%) | 0.49 | 16 (55%) | 13 (50%) | 0.70 | 16 (52%) | 15 (62%) | 0.85 |
| Current or ex-smoker 1 | 7 (21%) | 4 (17%) | 0.72 | 7 (25%) | 3 (12%) | 0.20 | 6 (20%) | 3 (12%) | 0.46 |
| Serological profile | |||||||||
| Anti-dsDNA positive 2 | 32(97%) | 20(87%) | 0.15 | 26 (96%) | 23 (88%) | 0.28 | 26 (93%) | 22 (92%) | 0.87 |
| Hypocomplementaemia 3 | 26(76.5%) | 19(82.6%) | 0.57 | 22 (79%) | 20 (77%) | 0.84 | 23 (77%) | 19 (79%) | 0.83 |
| LN histological Class (ISN/RPS 2003 classification) | 0.64 | 0.17 | 0.82 | ||||||
| III | 6 (17%) | 6 (26%) | 5 (17%) | 7 (27%) | 7 (23%) | 4 (17%) | |||
| IV | 15 (43%) | 10 (43%) | 9 (31%) | 12 (46%) | 12 (39%) | 11 (46%) | |||
| III/IV + V | 14 (40%) | 7 (30%) | 15 (52%) | 7 (27%) | 12 (39%) | 9 (38%) | |||
| eGFR (mL/min/1.73 m2) 4 | 90 [59, 90] | 77 [60, 90] | 0.45 | 84 [57, 90] | 82 [60, 90] | 0.98 | 90 [57, 90] | 77 [60, 90] | 0.92 |
| UPCR (g/mmol) | 0.42 [0.27, 0.61] | 0.18 [0.12, 0.30] | 0.003 | 0.30 [0.14, 0.59] | 0.27 [0.15, 0.48] | 0.55 | 0.41 [0.20, 0.61] | 0.23 [0.13, 0.43] | 0.058 |
| Medications | |||||||||
| Glucocorticoids (GC) ever | 35 (100%) | 23 (100%) | 29 (100%) | 26 (100%) | 31 (100%) | 24 (100%) | |||
| Daily GC dose (mg) at: | |||||||||
| Baseline 5 | 15 [7, 40] | 20 [8, 38] | 0.8 | 15 [5, 40] | 20 [8, 40] | 0.57 | 20 [10, 25] | 15 [5, 40] | 0.59 |
| 6 months 6 | 10 [5, 15] | 10 [9, 15] | 0.54 | 10 [5, 15] | 10 [8, 15] | 0.66 | 10 [5, 15] | 11.2 [9, 15] | 0.29 |
| 12 months 7 | 5 [0, 10] | 5 [1, 10] | 0.88 | 5 [4, 13] | 5 [1, 10] | 0.27 | 7.5 [2, 15] | 5 [1, 5] | 0.11 |
| 24 months 8 | 5 [3, 10] | 3.2 [0, 5] | 0.014 | 5 [1, 7.5] | 2.5 [0, 5] | 0.079 | 5 [2.5, 10.0] | 4 [0, 5.] | 0.038 |
| Induction therapy 3 | 0.59 | 0.84 | 0.70 | ||||||
| MMF | 23 (68%) | 16 (70%) | 20 (69%) | 18 (69%) | 21 (68%) | 18 (75%) | |||
| CYC | 6 (18%) | 5 (22%) | 6 (21%) | 5 (19%) | 7 (23%) | 3 (12%) | |||
| RTX | 2 (6%) | 0 (0%) | 1 (3.4%) | 1 (3.8%) | 1 (3%) | 1 (4%) | |||
| MMF/RTX | 2 (6%) | 1 (4%) | 1 (3.4%) | 1 (3.8%) | 1 (3%) | 1 (4%) | |||
| MMF/Tac | 1 (3%) | 0 (0%) | 1 (3.4%) | 0 (0%) | 1 (3%) | 0 (0%) | |||
| CYC/RTX | 0 (0%) | 1 (4%) | 0 (0%) | 1 (3.8%) | 0 (0%) | 1 (4%) | |||
| Antimalarial therapy 1 | 22 (67%) | 12 (52%) | 0.27 | 24 (86%) | 23 (88%) | 0.76 | 20 (65%) | 13 (54%) | 0.44 |
| Complete renal remission (CRR) | |||||||||
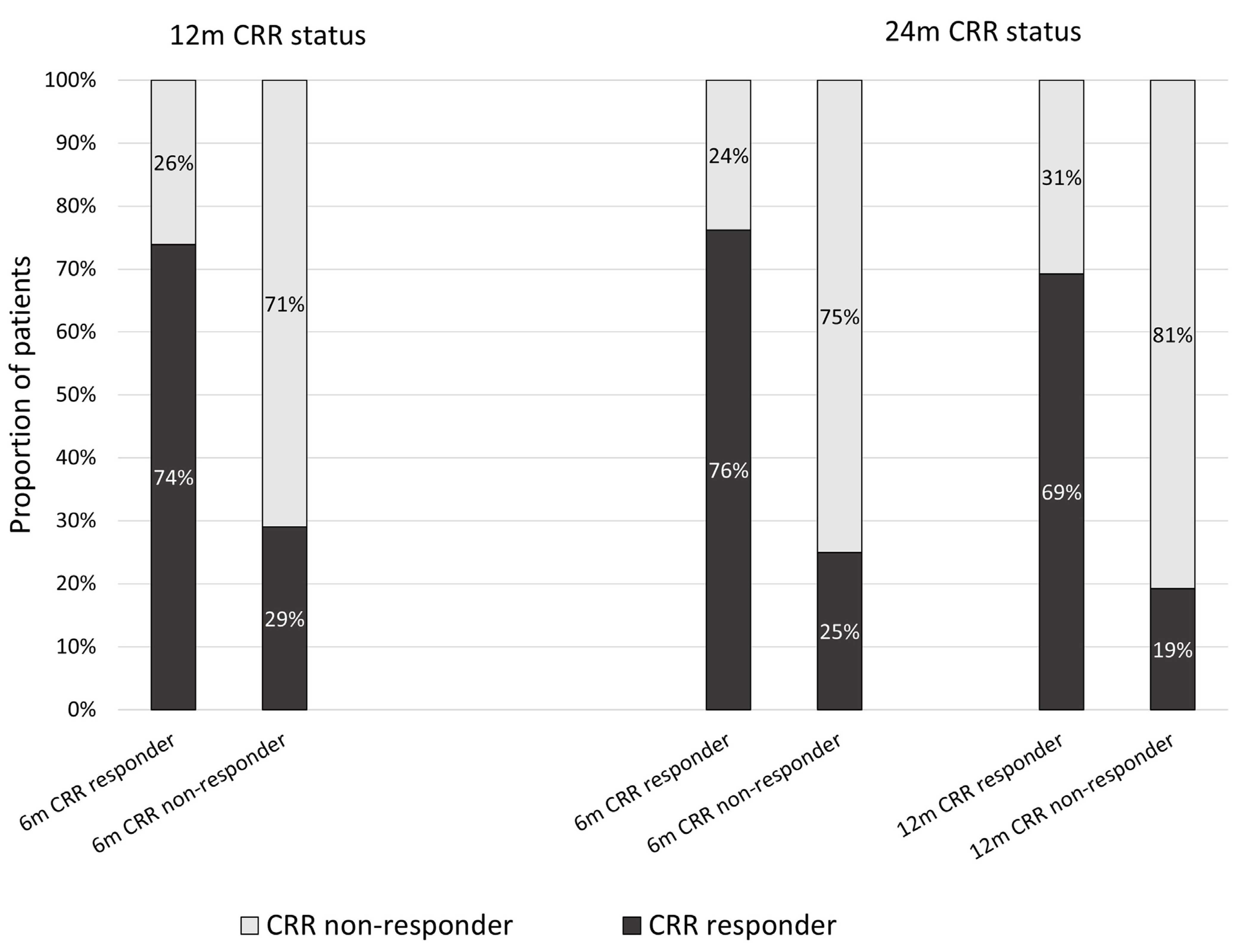
| CRR at 6 months 1 | 6 (21%) | 17 (65%) | 0.001 | 5 (17%) | 16 (67%) | <0.001 | |||
| CRR at 12 months 9 | 9 (29%) | 17 (74%) | 0.001 | 8 (28%) | 18 (78%) | <0.001 | |||
| CRR at 24 months 9 | 8 (25%) | 16 (76%) | <0.001 | 5 (19%) | 18 (69%) | <0.001 | |||
| SLEDAI-2K score | |||||||||
| Baseline 10 | 10 [8, 16] | 14.5 [10, 19] | 0.41 | 12.5 [8, 16] | 13 [8, 18] | 0.95 | 10 [8, 16] | 13 [8, 20] | 0.37 |
| 6 months 11 | 8 [6, 14] | 4 [2, 4] | <0.001 | 8 [5, 10] | 4 [2, 6] | 0.008 | 7 [4, 12] | 4 [2, 8] | 0.040 |
| 12 months 12 | 8 [6, 10] | 4 [2, 4] | 0.011 | 8 [6.5, 10] | 4 [2, 4] | <0.001 | 8 [6, 10] | 4 [2, 4] | 0.002 |
| 24 months 13 | 6 [4, 10] | 4 [4, 4] | 0.005 | 7 [4, 12] | 4 [4, 4] | 0.012 | 8 [6, 10] | 4 [3, 4] | <0.001 |
| 12 m CRR Achievement | 24 m CRR Achievement | |||
|---|---|---|---|---|
| Univariable Logistic Regression Analysis | Multivariable Logistic Regression Analysis | Univariable Logistic Regression Analysis | Multivariable Logistic Regression Analysis | |
| OR (95%CI), p-Value | OR 1 (95%CI), p-Value | OR (95%CI), p-Value | OR 1 (95%CI), p-Value | |
| 6 months non-responder | 1.00 | 1.00 | 1.00 | 1.00 |
| 6 months responder | 6.93 (2.06, 23.26), 0.002 | 9.72 (2.19, 43.03), 0.003 | 9.60 (2.66, 34.67), 0.001 | 11.23(2.53, 49.88), 0.001 |
| 12 months non-responder | 1.00 | 1.00 | ||
| 12 months responder | 9.45 (2.62, 34.07), 0.001 | 11.39 (2.41, 53.80), 0.002 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakagawa, S.; Yeung, E.K.; Hoi, A.; Morand, E.F.; Kent, J.R.; Kandane-Rathnayake, R. Early Renal Remission Is Associated with Increased Likelihood of Subsequent Remission in Lupus Nephritis: Single-Centre Observational Study in Australia. Int. J. Mol. Sci. 2025, 26, 9634. https://doi.org/10.3390/ijms26199634
Nakagawa S, Yeung EK, Hoi A, Morand EF, Kent JR, Kandane-Rathnayake R. Early Renal Remission Is Associated with Increased Likelihood of Subsequent Remission in Lupus Nephritis: Single-Centre Observational Study in Australia. International Journal of Molecular Sciences. 2025; 26(19):9634. https://doi.org/10.3390/ijms26199634
Chicago/Turabian StyleNakagawa, Shiori, Emily K. Yeung, Alberta Hoi, Eric F. Morand, Joanna R. Kent, and Rangi Kandane-Rathnayake. 2025. "Early Renal Remission Is Associated with Increased Likelihood of Subsequent Remission in Lupus Nephritis: Single-Centre Observational Study in Australia" International Journal of Molecular Sciences 26, no. 19: 9634. https://doi.org/10.3390/ijms26199634
APA StyleNakagawa, S., Yeung, E. K., Hoi, A., Morand, E. F., Kent, J. R., & Kandane-Rathnayake, R. (2025). Early Renal Remission Is Associated with Increased Likelihood of Subsequent Remission in Lupus Nephritis: Single-Centre Observational Study in Australia. International Journal of Molecular Sciences, 26(19), 9634. https://doi.org/10.3390/ijms26199634






