N-Terminal Metal-Binding Domain of Arabidopsis IBR5 Is Important for Its in Planta Functions
Abstract
1. Introduction
2. Results
2.1. AtIBR5 Contains a Putative Metal-Binding Rubredoxin-like Sequence in Its N-Terminal Region
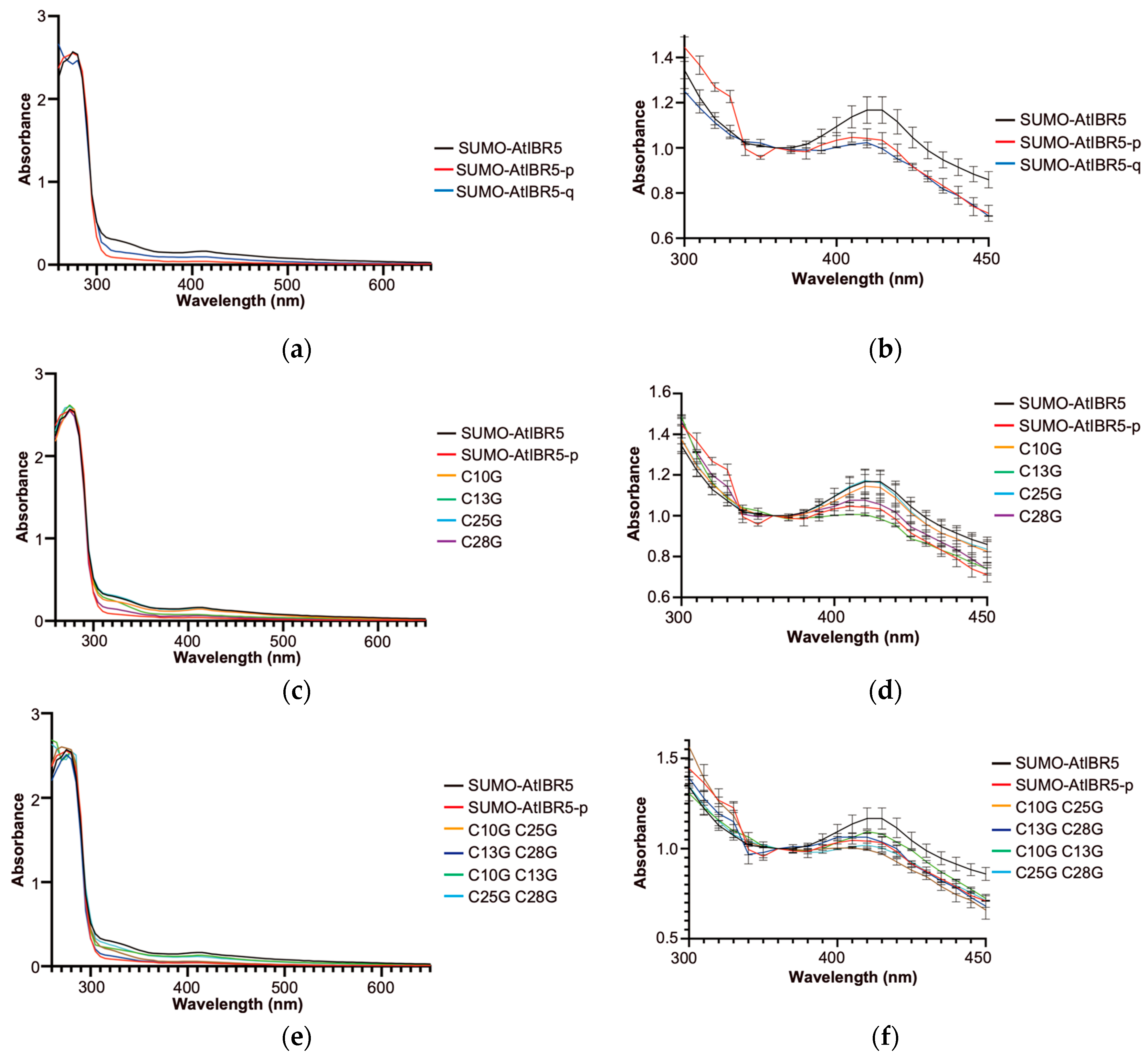
2.2. The Four Cysteine Residues in the Rubredoxin-like Domain Are Essential for the Characteristic Absorption Spectrum of AtIBR5
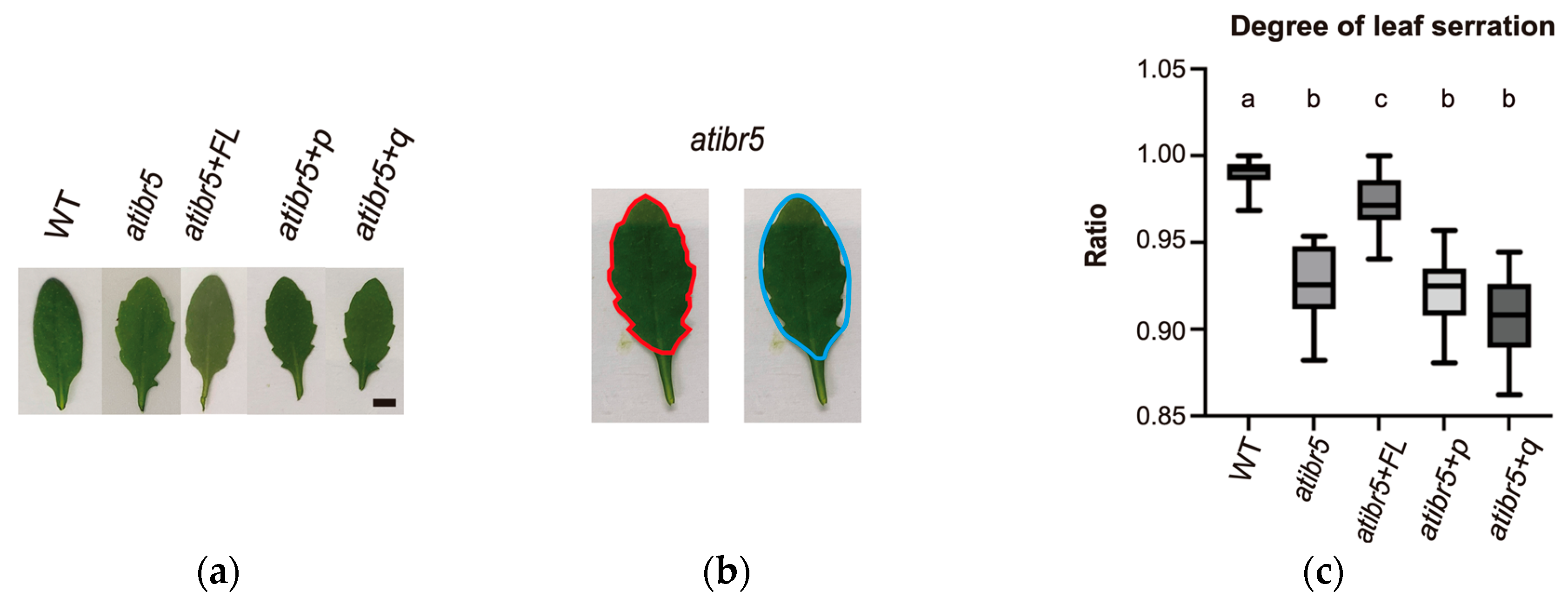
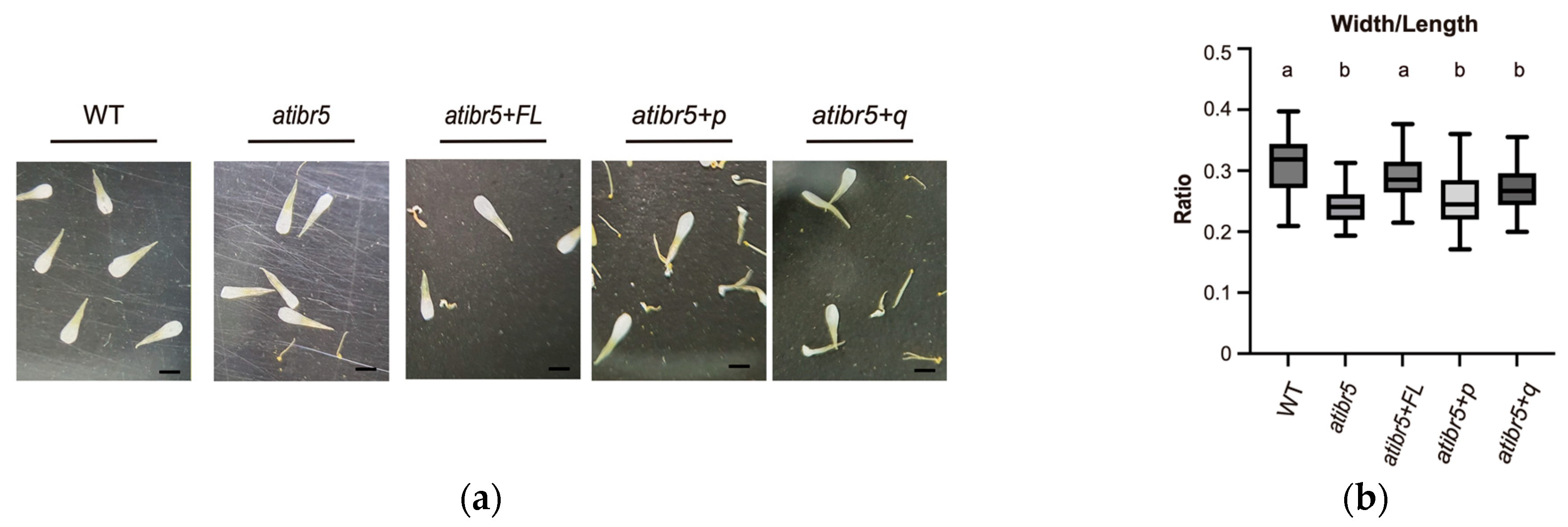
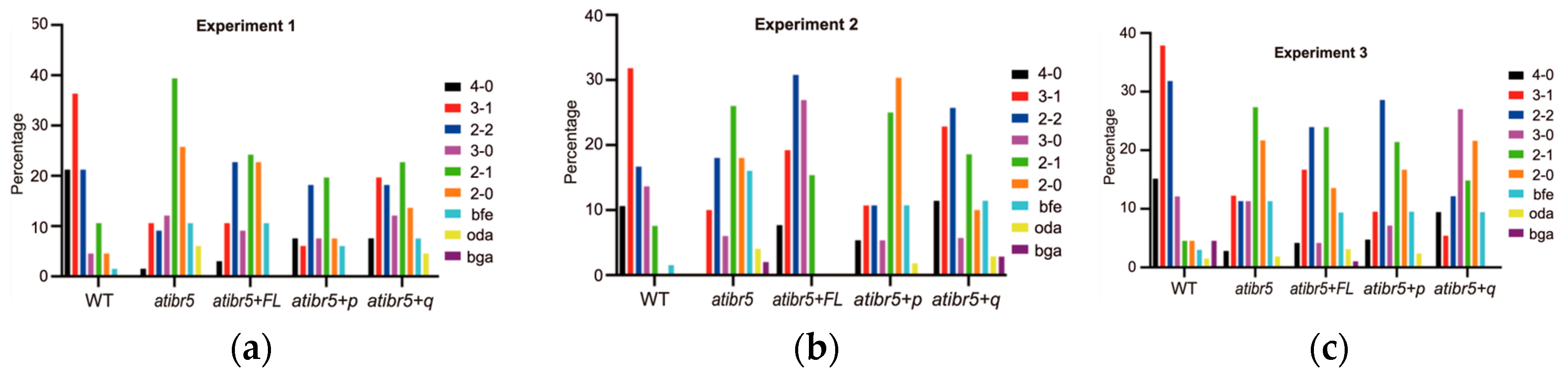
2.3. The Rubredoxin-like Domain in AtIBR5 Plays a Crucial Role in Various Auxin Responses
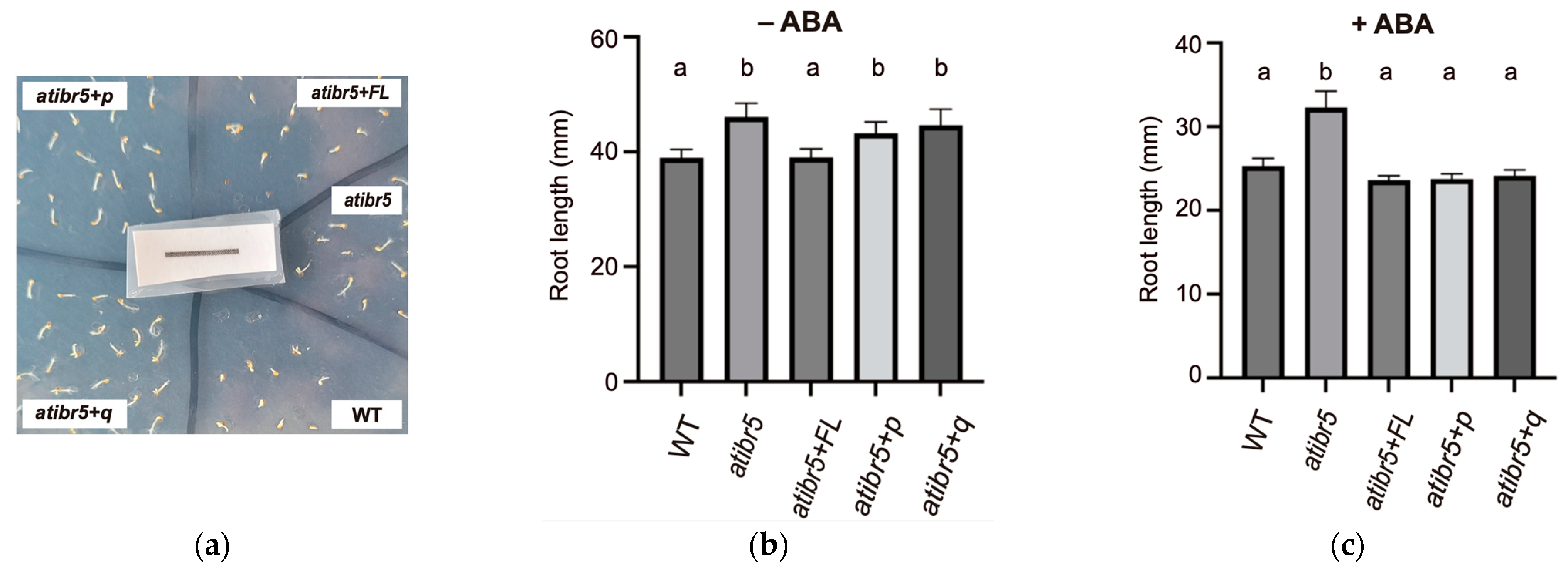
2.4. The Rubredoxin-like Domain in AtIBR5 Is Required for ABA-Mediated Suppression of Germination but Dispensable for ABA-Mediated Root Elongation
3. Discussion
3.1. Orthologs of AtIBR5 Are Found Exclusively in the Green Plant Lineage, Including Chlamydomonas Reinhardtii and Physcomitrium Patens
3.2. Zinc Binds to AtIBR5 Through Four Cysteine Residues in Its N-Terminal Rubredoxin-like Domain, When Expressed in E. coli
3.3. Rubredoxin-like Domain in AtIBR5 Is Required for Complementation of Most atibr5 Mutant Phenotypes
4. Materials and Methods
4.1. Plant Materials and Arabidopsis Transformation
4.2. Cloning and Mutagenesis
4.3. Expression and Affinity Purification of Recombinant Proteins
4.4. Size Exclusion Chromatography (SEC) and Size Prediction of Proteins
4.5. Measurement of Absorption Spectrum
4.6. Sequence Alignment and Structural Prediction of Proteins
4.7. Inductively Coupled Plasma–Mass Spectrometry (ICP-MS)
4.8. Immunoprecipitation and Immunoblot Experiment
4.9. Measurement of Hypocotyl Length, Primary Root Length, Leaf Serration, and Petal Size
4.10. Observation of Cotyledon Vein Pattern
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Arabidopsis | Arabidopsis thaliana |
| Arabidopsis thaliana AtIBR5 | Arabidopsis thaliana indole-3-butyric acid response 5 |
| IAA | indole-3-acetic acid |
| DSP | dual-specificity phosphatase |
| IBA | indole-3-butyric acid |
| ABA | abscisic acid |
| WT | wild type |
| SEC | size exclusion chromatography |
| ICP-MS | inductively coupled plasma mass spectrometry |
References
- Gao, J.; Zhuang, S.; Zhang, W. Advances in Plant Auxin Biology: Synthesis, Metabolism, Signaling, Interaction with Other Hormones, and Roles under Abiotic Stress. Plants 2024, 13, 2523. [Google Scholar] [CrossRef]
- Ikeuchi, M.; Favero, D.S.; Sakamoto, Y.; Iwase, A.; Coleman, D.; Rymen, B.; Sugimoto, K. Molecular Mechanisms of Plant Regeneration. Annu. Rev. Plant Biol. 2019, 29, 377–406. [Google Scholar] [CrossRef]
- Weijers, D.; Wagner, D. Transcriptional Responses to the Auxin Hormone. Annu. Rev. Plant Biol. 2016, 67, 539–574. [Google Scholar] [CrossRef]
- Calatrava, V.; Hom, E.F.Y.; Guan, Q.; Llamas, A.; Fernández, E.; Galván, A. Genetic evidence for algal auxin production in Chlamydomonas and its role in algal-bacterial mutualism. iScience 2023, 27, 108762. [Google Scholar] [CrossRef]
- Cox, C.E.; Brandl, M.T.; de Moraes, M.H.; Gunasekera, S.; Teplitski, M. Production of the plant hormone auxin by Salmonella and its role in the interactions with plants and animals. Front. Microbiol. 2018, 8, 2668. [Google Scholar] [CrossRef]
- Dubey, S.M.; Serre, N.B.C.; Oulehlová, D.; Vittal, P.; Fendrych, M. No Time for Transcription—Rapid Auxin Responses in Plants. Cold Spring Harb. Perspect. Biol. 2021, 13, a039891. [Google Scholar] [CrossRef]
- Leyser, O. Auxin Signaling. Plant Physiol. 2018, 176, 465–479. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Carrasco, V.P.; Hernandez-Garcia, J.; Mutte, S.K.; Weijers, D. The birth of a giant: Evolutionary insights into the origin of auxin responses in plants. EMBO J. 2023, 42, e113018. [Google Scholar] [CrossRef] [PubMed]
- Mutte, S.K.; Kato, H.; Rothfels, C.; Melkonian, M.; Wong, G.K.; Weijers, D. Origin and evolution of the nuclear auxin response system. eLife 2018, 7, e33399. [Google Scholar] [CrossRef] [PubMed]
- Monroe-Augustus, M.; Zolman, B.K.; Bartel, B. IBR5, a dual-specificity phosphatase-like protein modulating auxin and abscisic acid responsiveness in Arabidopsis. Plant Cell 2003, 15, 2979–2991. [Google Scholar] [CrossRef]
- Zolman, B.K.; Martinez, N.; Millius, A.; Adham, A.R.; Bartel, B. Identification and characterization of Arabidopsis indole-3-butyric acid response mutants defective in novel peroxisomal enzymes. Genetics 2008, 180, 237–251. [Google Scholar] [CrossRef] [PubMed]
- Strader, L.C.; Monroe-Augustus, M.; Bartel, B. The IBR5 phosphatase promotes Arabidopsis auxin responses through a novel mechanism distinct from TIR1-mediated repressor degradation. BMC Plant Biol. 2008, 8, 41. [Google Scholar] [CrossRef]
- Kong, X.; Huang, G.; Xiong, Y.; Zhao, C.; Wang, J.; Song, X.; Giri, J.; Zuo, K. IBR5 regulates leaf serrations development via modulation of the expression of PIN1. Int. J. Mol. Sci. 2019, 20, 4429. [Google Scholar] [CrossRef]
- Johnson, K.L.; Ramm, S.; Kappel, C.; Ward, S.; Leyser, O.; Sakamoto, T.; Kurata, T.; Bevan, M.W.; Lenhard, M. The Tinkerbell (Tink) mutation identifies the dual-specificity MAPK phosphatase indole-3-butyric acid-response5 (IBR5) as a novel regulator of organ size in Arabidopsis. PLoS ONE 2015, 10, e0131103, Erratum in PLoS ONE 2015, 10, e0131103. [Google Scholar]
- Lee, J.S.; Wang, S.; Sritubtim, S.; Chen, J.G.; Ellis, B.E. Arabidopsis mitogen-activated protein kinase MPK12 interacts with the MAPK phosphatase IBR5 and regulates auxin signaling. Plant J. 2009, 57, 975–985. [Google Scholar] [CrossRef]
- DalCorso, G.; Manara, A.; Piasentin, S.; Furini, A. Nutrient metal elements in plants. Metallomics 2014, 6, 1770–1788. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Hüner, N.P.A. Introduction to Plant Physiology, 4th ed.; Wiley: Hoboken, NJ, USA, 2008; pp. 39–59. [Google Scholar]
- Hänsch, R.; Mendel, R.R. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl). Curr. Opin. Plant Biol. 2009, 12, 259–266. [Google Scholar] [CrossRef]
- Vigani, G.; Murgia, I. Iron-Requiring Enzymes in the Spotlight of Oxygen. Trends Plant Sci. 2018, 23, 874–882. [Google Scholar] [CrossRef]
- Ciftci-Yilmaz, S.; Mittler, R. The zinc finger network of plants. Cell. Mol. Life Sci. 2008, 65, 1150–1160. [Google Scholar] [CrossRef] [PubMed]
- Stanton, C.; Sanders, D.; Krämer, U.; Podar, D. Zinc in plants: Integrating homeostasis and biofortification. Mol. Plant 2022, 15, 65–85. [Google Scholar] [CrossRef] [PubMed]
- Koksal, A.C.; Cingolani, G. Dimerization of Vaccinia virus VH1 is essential for dephosphorylation of STAT1 at tyrosine 701. J. Biol. Chem. 2011, 286, 14373–14382. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G.S.; Liu, Y.; Baskerville, C.; Charbonneau, H. The activity of Cdc14p, an oligomeric dual specificity protein phosphatase from Saccharomyces cerevisiae, is required for cell cycle progression. J. Biol. Chem. 1997, 272, 24054–24063. [Google Scholar] [CrossRef] [PubMed]
- Koropatkin, N.; Randich, A.M.; Bhattacharyya-Pakrasi, M.; Pakrasi, H.B.; Smith, T.J. The structure of the iron-binding protein, FutA1, from Synechocystis 6803. J. Biol. Chem. 2007, 282, 27468–27477. [Google Scholar] [CrossRef]
- Yi, H.; Juergens, M.; Jez, J.M. Structure of soybean β-cyanoalanine synthase and the molecular basis for cyanide detoxification in plants. Plant Cell 2012, 24, 2696–2706. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Derbyshire, M.K.; Yamashita, R.A.; Marchler-Bauer, A. NCBI’s Conserved Domain Database and Tools for Protein Domain Analysis. Curr. Protoc. Bioinform. 2020, 69, e90. [Google Scholar] [CrossRef]
- Adman, E.; Watenpaugh, K.D.; Jensen, L.H. NH---S hydrogen bonds in Peptococcus aerogenes ferredoxin, Clostridium pasteurianum rubredoxin, and Chromatium high potential iron protein. Proc. Natl. Acad. Sci. USA 1975, 72, 4854–4858. [Google Scholar] [CrossRef]
- Calderon, R.H.; García-Cerdán, J.G.; Malnoë, A.; Cook, R.; Russell, J.J.; Gaw, C.; Dent, R.M.; de Vitry, C.; Niyogi, K.K. A conserved rubredoxin is necessary for photosystem II accumulation in diverse oxygenic photoautotrophs. J. Biol. Chem. 2013, 288, 26688–26696. [Google Scholar] [CrossRef]
- Zhu, J.; Fu, X.; Koo, Y.D.; Zhu, J.K.; Jenney, F.E., Jr.; Adams, M.W.; Zhu, Y.; Shi, H.; Yun, D.J.; Hasegawa, P.M.; et al. An enhancer mutant of Arabidopsis salt overly sensitive 3 mediates both ion homeostasis and the oxidative stress response. Mol. Cell. Biol. 2007, 27, 5214–5224. [Google Scholar] [CrossRef]
- Kelley, L.A.; Mezulis, S.; Yates, C.M.; Wass, M.N.; Sternberg, M.J. The Phyre2 web portal for protein modeling, prediction and analysis. Nat. Protoc. 2015, 10, 845–858. [Google Scholar] [CrossRef]
- Dillard, B.D.; Demick, J.M.; Adams, M.W.; Lanzilotta, W.N. A cryo-crystallographic time course for peroxide reduction by rubrerythrin from Pyrococcus furiosus. J. Biol. Inorg. Chem. 2011, 16, 949–959. [Google Scholar] [CrossRef]
- Iyer, R.B.; Silaghi-Dumitrescu, R.; Kurtz, D.M.; Lanzilotta, W.N. High-resolution crystal structures of Desulfovibrio vulgaris (Hildenborough) nigerythrin: Facile, redox-dependent iron movement, domain interface variability, and peroxidase activity in the rubrerythrins. J. Biol. Inorg. Chem. 2005, 10, 407–416. [Google Scholar] [CrossRef]
- Frey, M.; Sieker, L.; Payan, F.; Haser, R.; Bruschi, M.; Pepe, G.; LeGall, J. Rubredoxin from Desulfovibrio gigas. A molecular model of the oxidized form at 1.4 Å resolution. J. Mol. Biol. 1987, 197, 525–541. [Google Scholar] [CrossRef] [PubMed]
- Ragsdale, S.W.; Ljungdahl, L.G. Characterization of ferredoxin, flavodoxin, and rubredoxin from Clostridium formicoaceticum grown in media with high and low iron contents. J. Bacteriol. 1984, 157, 1–6. [Google Scholar] [CrossRef]
- García-Cerdán, J.G.; Furst, A.L.; McDonald, K.L.; Schünemann, D.; Francis, M.B.; Niyogi, K.K. A thylakoid membrane-bound and redox-active rubredoxin (RBD1) functions in de novo assembly and repair of photosystem II. Proc. Natl. Acad. Sci. USA 2019, 116, 16631–16640. [Google Scholar] [CrossRef]
- Liu, J.; Yang, H.; Bao, F.; Ao, K.; Zhang, X.; Zhang, Y.; Yang, S. IBR5 Modulates Temperature-Dependent, R Protein CHS3-Mediated Defense Responses in Arabidopsis. PLoS Genet. 2015, 11, e1005584, Erratum in PLoS Genet. 2016, 12, e1005795. [Google Scholar] [CrossRef]
- Blanco-Touriñán, N.; Hardtke, C.S. Connecting emerging with existing vasculature above and below ground. Curr. Opin. Plant Biol. 2023, 76, 102461. [Google Scholar] [CrossRef]
- Yanagisawa, M.; Poitout, A.; Otegui, M.S. Arabidopsis vascular complexity and connectivity controls PIN-FORMED1 dynamics and lateral vein patterning during embryogenesis. Development 2021, 148, dev197210. [Google Scholar] [CrossRef] [PubMed]
- Kerk, D.; Templeton, G.; Moorhead, G.B. Evolutionary radiation pattern of novel protein phosphatases revealed by analysis of protein data from the completely sequenced genomes of humans, green algae, and higher plants. Plant Physiol. 2008, 146, 351–363. [Google Scholar] [CrossRef] [PubMed]
- Clark, J.W.; Donoghue, P.C.J. Whole-Genome Duplication and Plant Macroevolution. Trends Plant Sci. 2018, 23, 933–945. [Google Scholar] [CrossRef]
- Jagodzik, P.; Tajdel-Zielinska, M.; Ciesla, A.; Marczak, M.; Ludwikow, A. Mitogen-Activated Protein Kinase Cascades in Plant Hormone Signaling. Front. Plant Sci. 2018, 9, 1387. [Google Scholar] [CrossRef]
- Uji, T.; Mizuta, H. The role of plant hormones on the reproductive success of red and brown algae. Front. Plant Sci. 2022, 13, 1019334. [Google Scholar] [CrossRef] [PubMed]
- Chino, M.; Di Costanzo, L.F.; Leone, L.; La Gatta, S.; Famulari, A.; Chiesa, M.; Lombardi, A.; Pavone, V. Designed Rubredoxin miniature in a fully artificial electron chain triggered by visible light. Nat. Commun. 2023, 14, 2368. [Google Scholar] [CrossRef]
- Dauter, Z.; Wilson, K.S.; Sieker, L.C.; Moulis, J.M.; Meyer, J. Zinc- and iron-rubredoxins from Clostridium pasteurianum at atomic resolution: A high-precision model of a ZnS4 coordination unit in a protein. Proc. Natl. Acad. Sci. USA 1996, 93, 8836–8840. [Google Scholar] [CrossRef] [PubMed]
- Maher, M.; Cross, M.; Wilce, M.C.; Guss, J.M.; Wedd, A.G. Metal-substituted derivatives of the rubredoxin from Clostridium pasteurianum. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 298–303, Erratum in Acta Crystallogr. D Biol. Crystallogr. 2004, 60 Pt 4, 800. [Google Scholar] [CrossRef]
- Outten, C.E.; O’Halloran, T.V. Femtomolar sensitivity of metalloregulatory proteins controlling zinc homeostasis. Science 2001, 292, 2488–2492. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef]
- Hyun, K.G.; Lee, Y.; Yoon, J.; Yi, H.; Song, J.J. Crystal structure of Arabidopsis thaliana SNC1 TIR domain. Biochem. Biophys. Res. Commun. 2016, 481, 146–152. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Maheshwari, S.; Brylinski, M. Predicting protein interface residues using easily accessible on-line resources. Brief. Bioinform. 2025, 16, 1025–1034. [Google Scholar] [CrossRef]
- Meng, E.C.; Goddard, T.D.; Pettersen, E.F.; Couch, G.S.; Pearson, Z.J.; Morris, J.H.; Ferrin, T.E. UCSF ChimeraX: Tools for structure building and analysis. Protein Sci. 2023, 32, e4792. [Google Scholar] [CrossRef] [PubMed]
- Yeon, J.; Lee, Y.; Kang, B.; Lim, J.; Yi, H. TIR Domains in Arabidopsis thaliana Suppressor of npr1-1, Constitutive 1 and Its Closely Related Disease Resistance Proteins Form Intricate Interaction Networks. J. Plant Biol. 2023, 66, 439–453. [Google Scholar] [CrossRef]



| Ion | SUMO-AtIBR5 | SUMO-AtIBR5-p | SUMO-AtIBR5-q | GmDES1 |
|---|---|---|---|---|
| Zn | 3874 ± 452.14 (48.36) | 271 ± 27.57 (5.07) | 347 ± 67 (7.14) | 554 ± 15.0 (10.96) |
| Ni | 3603 ± 400.30 (44.98) | 4553 ± 559.97 (85.10) | 4122 ± 396.37 (84.40) | 4113 ± 541.44 (80.63) |
| Cu | 460 ± 79.2 (5.74) | 471 ± 127.89 (8.72) | 359 ± 90.51 (7.42) | 377 ± 60.53 (7.37) |
| Mg | 53 ± 26.03 (0.66) | 38 ± 19.84 (0.73) | 30 ± 0.35 (0.63) | 33 ± 10.41 (0.65) |
| Fe | <10 (0.12) | <10 (0.19) | <10 (0.21) | <10 (0.2) |
| Cd | <10 (0.12) | <10 (0.19) | <10 (0.21) | <10 (0.2) |
| Ion | MBP-AtIBR5 | MBP-AtIBR5-q |
|---|---|---|
| Zn | 3034 (75.74) | 439 (28.07) |
| Mg | 357 (8.91) | 494 (31.59) |
| Ni | 279 (6.96) | 298 (19.05) |
| Cu | 181 (4.52) | 174 (11.13) |
| Fe | 148 (3.69) | 151 (9.65) |
| Mn | 7 (0.17) | 8 (0.51) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yeon, J.; Lim, J.; Song, S.-K.; Yi, H. N-Terminal Metal-Binding Domain of Arabidopsis IBR5 Is Important for Its in Planta Functions. Int. J. Mol. Sci. 2025, 26, 9315. https://doi.org/10.3390/ijms26199315
Yeon J, Lim J, Song S-K, Yi H. N-Terminal Metal-Binding Domain of Arabidopsis IBR5 Is Important for Its in Planta Functions. International Journal of Molecular Sciences. 2025; 26(19):9315. https://doi.org/10.3390/ijms26199315
Chicago/Turabian StyleYeon, Jinouk, Jaebeom Lim, Sang-Kee Song, and Hankuil Yi. 2025. "N-Terminal Metal-Binding Domain of Arabidopsis IBR5 Is Important for Its in Planta Functions" International Journal of Molecular Sciences 26, no. 19: 9315. https://doi.org/10.3390/ijms26199315
APA StyleYeon, J., Lim, J., Song, S.-K., & Yi, H. (2025). N-Terminal Metal-Binding Domain of Arabidopsis IBR5 Is Important for Its in Planta Functions. International Journal of Molecular Sciences, 26(19), 9315. https://doi.org/10.3390/ijms26199315






