Cannabidiol Lipid Nanoparticles Stabilize Gut–Brain–Bone Axis Integrity and Enhance Neuroplasticity in Stressed Rats: A Comparison with Atomoxetine and Escitalopram
Abstract
1. Introduction
2. Results
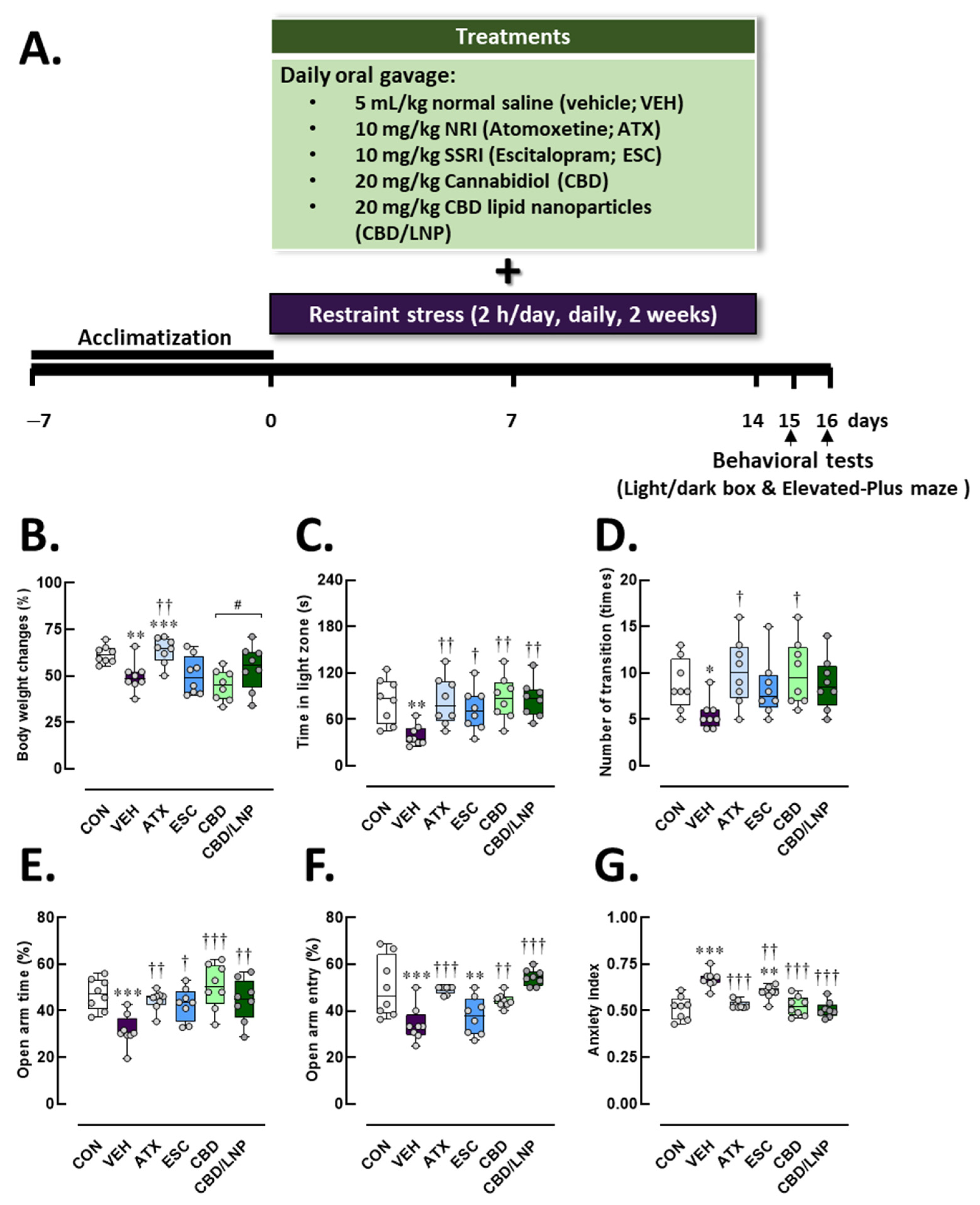
2.1. Restraint-Stressed Male Rats Treated with CBD/LNPs Showed Modulated Body Weight Loss and Reduced Anxiety-like Behaviors, Comparable to the Effects Observed with Monoaminergic Modulators
2.2. Restraint-Stressed Male Rats Treated with CBD/LNPs Showed Improved Serum Biological Markers Related to Systemic Inflammation, Gut Permeability, Intestinal Metabolites, and Bone Remodeling, Comparable to the Effects Observed with Monoaminergic Modulators
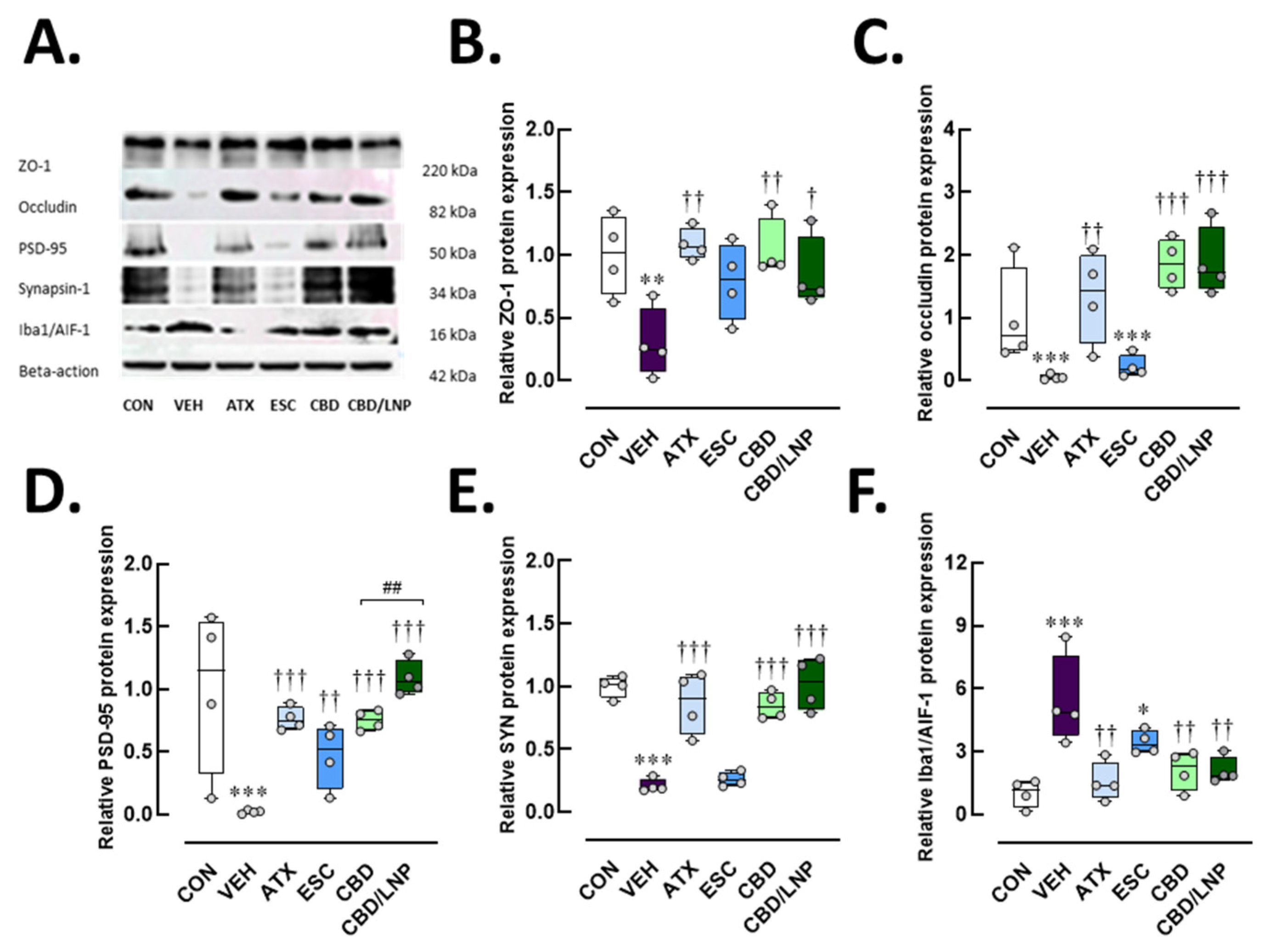
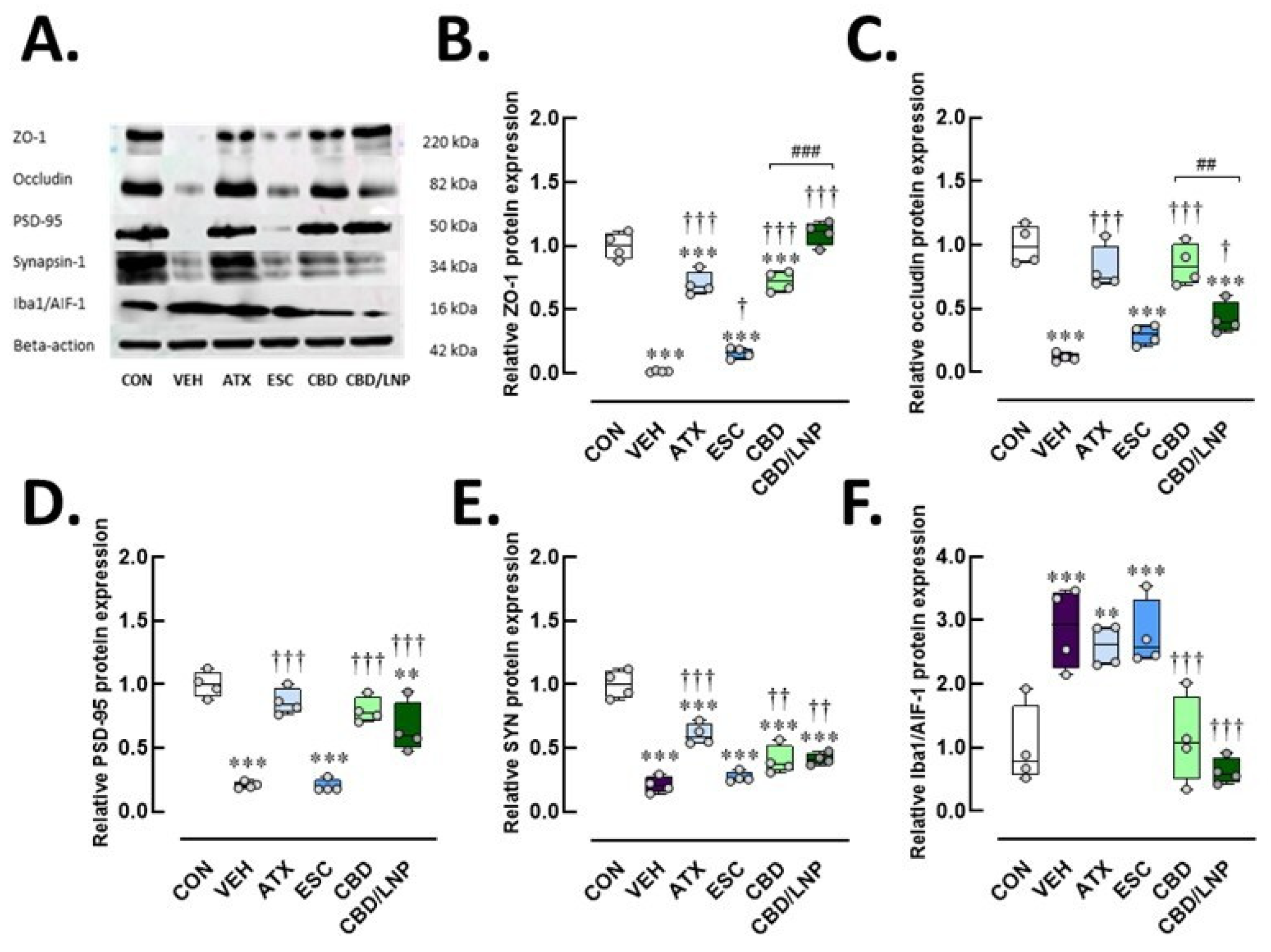
2.3. Restraint-Stressed Male Rats Treated with CBD/LNPs Exhibited Improved Expression of Proteins Associated with Hippocampal and Colonic Barrier Integrity, Synaptic Plasticity, and Neuroimmune Inflammation, Comparable to the Effects Observed with Monoaminergic Modulators
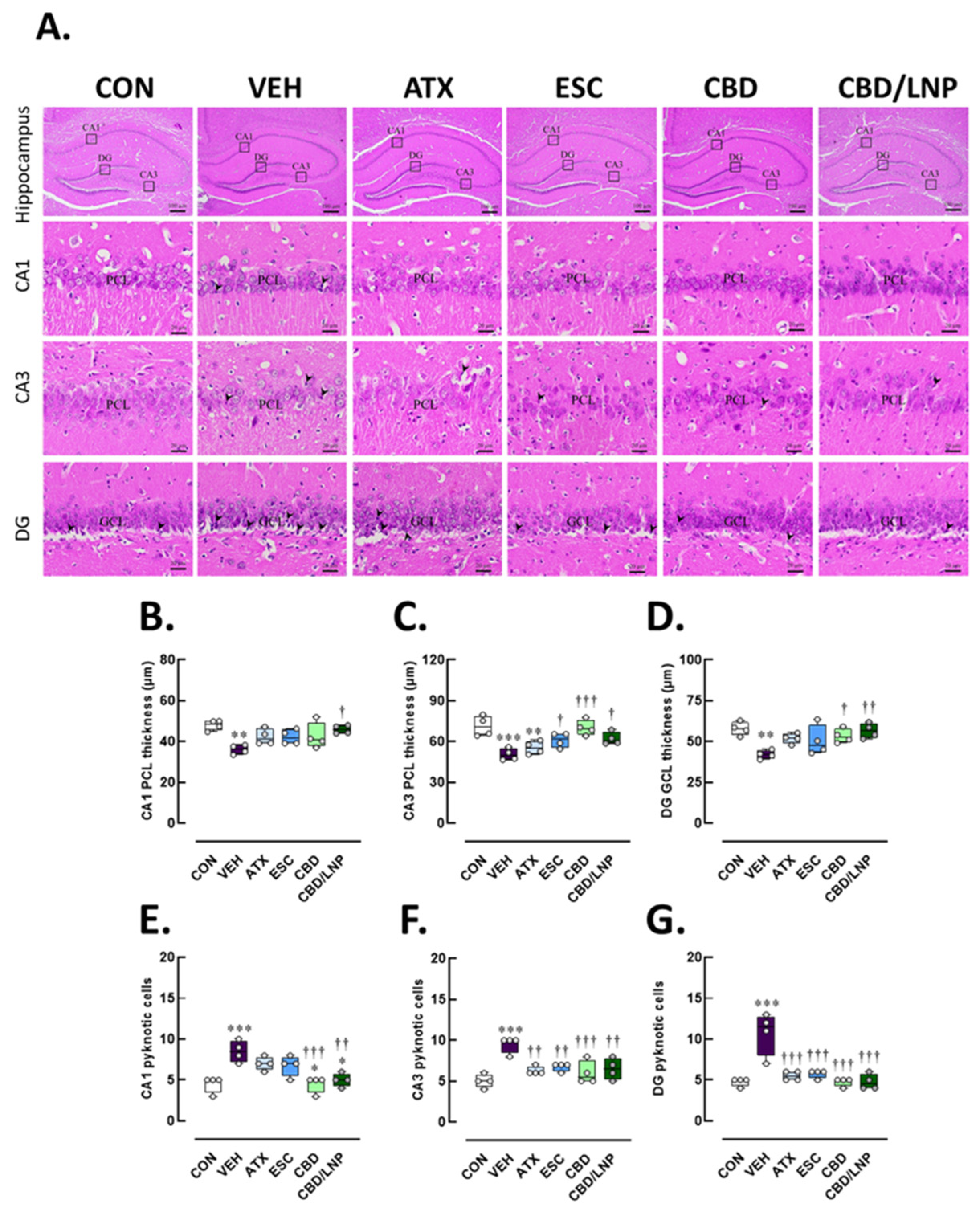
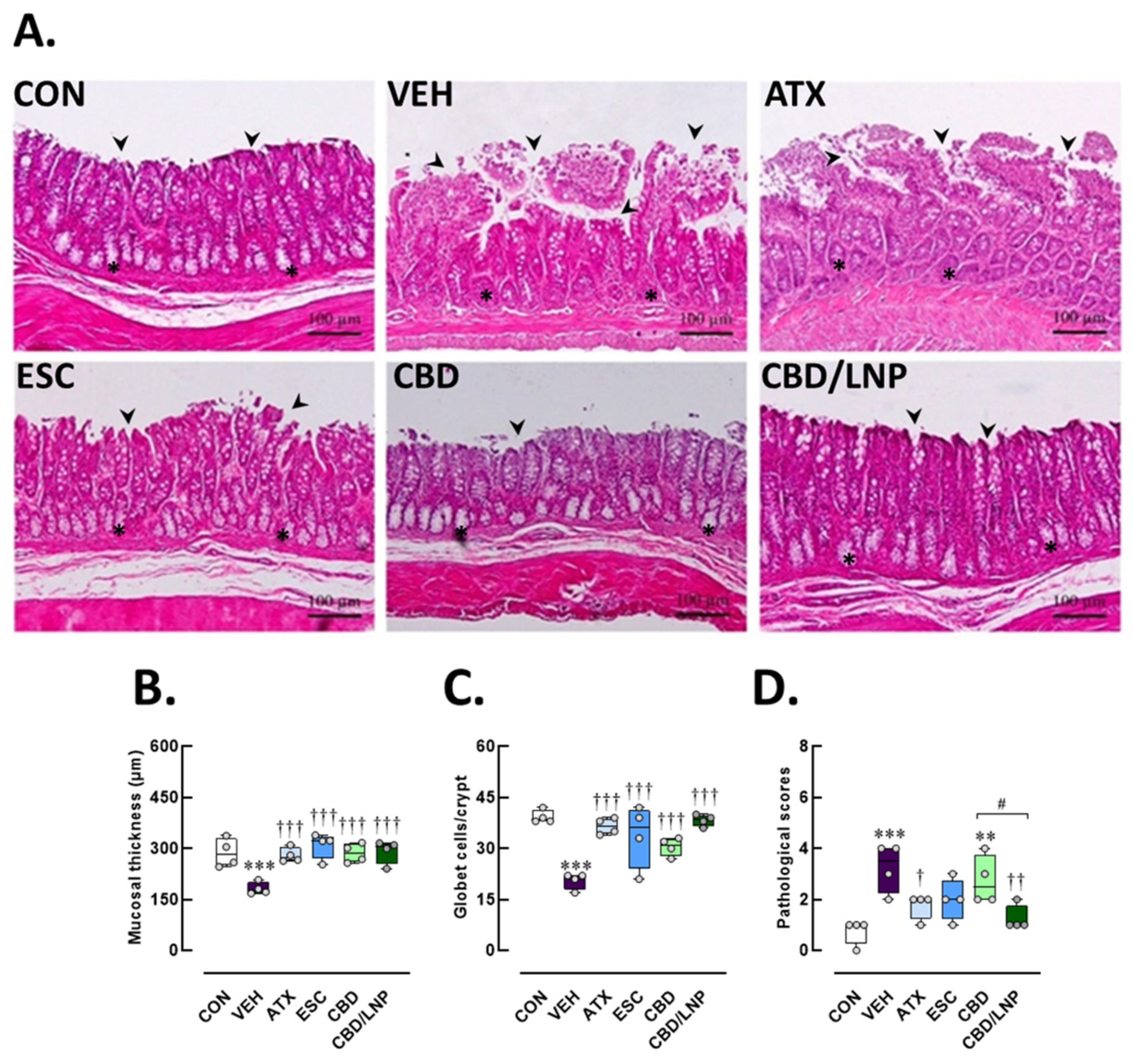
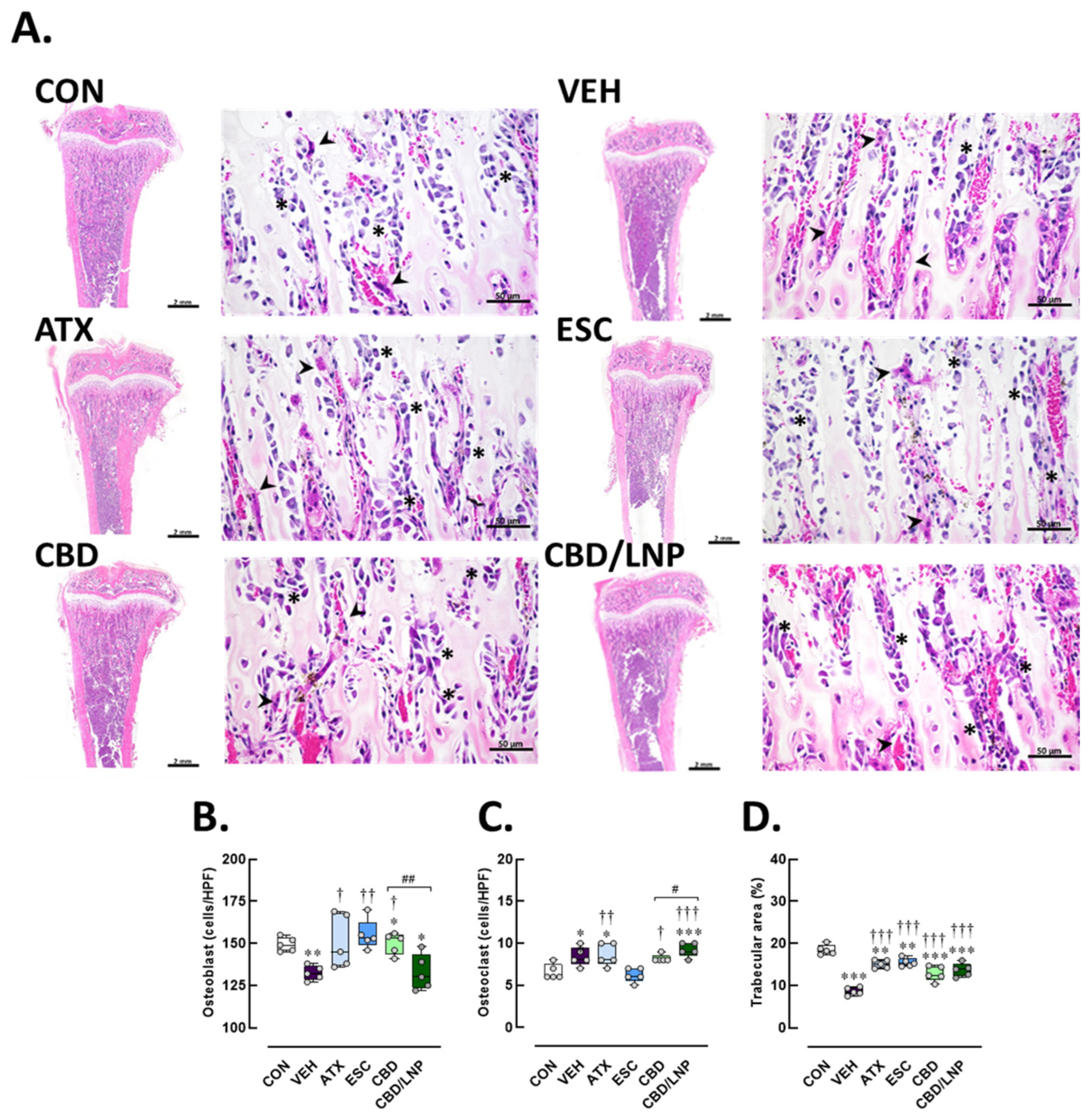
2.4. Restraint-Stressed Male Rats Treated with CBD/LNPs Exhibited Improved Histomorphological Changes in the Hippocampus, Colon, and Tibia, Comparable to the Effects Observed with Monoaminergic Modulators
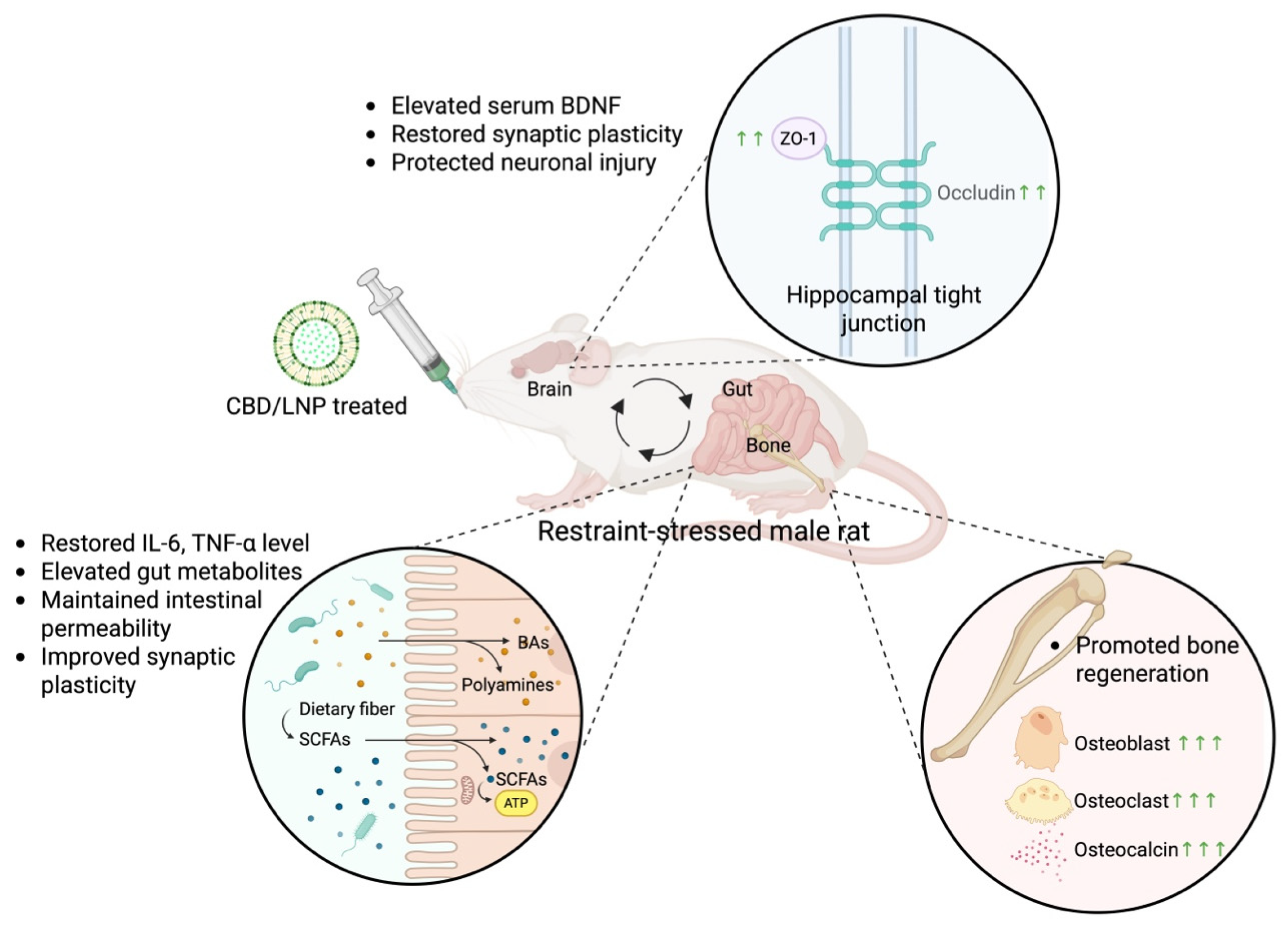
3. Discussion
4. Materials and Methods
4.1. Cannabidiol-Loaded Lipid Nanoparticles (CBD/LNPs) Preparation
4.2. Animal
4.3. Experimental Design
4.4. Stress Induction
4.5. Antipsychotic Administration
4.6. Body Weight Changes Evaluation
4.7. Anxiety-like Behavioral Change Evaluation
4.7.1. Light/Dark Test
4.7.2. Elevated-Plus Maze (EPM) Test
4.8. Tissue Collection and Sample Preparation
4.9. Serum Biochemical Markers Evaluation
4.10. Intestinal Permeability Evaluation
4.11. Western Blot Analysis
4.12. Histological Evaluations of the Hippocampus, Colon, and Tibia
4.13. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AIF-1 | Allograft inflammatory factor 1 |
| ATX | Atomoxetine |
| BA | Bile acid |
| BDNF | Brain-derived neurotrophic factor |
| CA | Cornu ammonis |
| CBD | Cannabidiol |
| CBD/LNP | Cannabidiol-loaded lipid nanoparticles |
| CON | Control |
| DG | Dentate gyrus |
| ECS | Escitalopram |
| ELISA | Enzyme-linked immunosorbent assay |
| FICT | Fluorescein isothiocyanate |
| GCL | Granule cell layer |
| H&E | Hematoxylin and eosin |
| HPA | Hypothalamic–Pituitary–Adrenal axis |
| HRP | Horseradish peroxidase |
| Iba1 | Ionized calcium-binding adapter molecule 1 |
| IL | Interleukin |
| NRI | Norepinephrine reuptake inhibitor |
| PBS | Phosphate-buffered saline |
| PFA | Paraformaldehyde |
| PCL | Pyramidal cell layer |
| PSD-95 | Postsynaptic density protein 95 |
| SCFA | Short-chain fatty acid |
| SEM | Standard error of the mean |
| SSRI | Selective serotonin reuptake inhibitor |
| SYN | Synapsin-1 |
| TNF-α | Tumor necrosis factor-alpha |
| VEH | Vehicle |
| ZO-1 | Zonula occludens-1 |
References
- James, K.A.; Stromin, J.I.; Steenkamp, N.; Combrinck, M.I. Understanding the Relationships between Physiological and Psychosocial Stress, Cortisol and Cognition. Front. Endocrinol. 2023, 14, 1085950. [Google Scholar] [CrossRef]
- Hsu, C.H.; Hsu, C.L.; Langley, A.; Wojcik, C.; Iraganje, E.; Grygiel-Górniak, B. Glucocorticoid-Induced Osteoporosis—From Molecular Mechanism to Clinical Practice. Drugs Ther. Perspect. 2024, 40, 315–329. [Google Scholar] [CrossRef]
- Lapmanee, S.; Supkamonseni, N.; Bhubhanil, S.; Treesaksrisakul, N.; Sirithanakorn, C.; Khongkow, M.; Namdee, K.; Surinlert, P.; Tipbunjong, C.; Wongchitrat, P. Stress-Induced Changes in Cognitive Function and Intestinal Barrier Integrity Can Be Ameliorated by Venlafaxine and Synbiotic Supplementations. PeerJ 2024, 12, e17033. [Google Scholar] [CrossRef] [PubMed]
- Leigh, S.-J.; Uhlig, F.; Wilmes, L.; Sanchez-Diaz, P.; Gheorghe, C.E.; Goodson, M.S.; Kelley-Loughnane, N.; Hyland, N.P.; Cryan, J.F.; Clarke, G.; et al. The Impact of Acute and Chronic Stress on Gastrointestinal Physiology and Function: A Microbiota-Gut-Brain Axis Perspective the Stress Response: From Adaptation to Disease. J. Physiol. 2023, 601, 4491–4538. [Google Scholar] [CrossRef]
- Li, R.; Miao, Z.; Liu, Y.; Chen, X.; Wang, H.; Su, J.; Chen, J. The Brain–Gut–Bone Axis in Neurodegenerative Diseases: Insights, Challenges, and Future Prospects. Adv. Sci. 2024, 11, e2307971. [Google Scholar] [CrossRef] [PubMed]
- Cho, D.E.; Hong, J.P.; Kim, Y.; Sim, J.Y.; Kim, H.S.; Kim, S.R.; Lee, B.; Cho, H.S.; Cho, I.H.; Shin, S.; et al. Role of Gut-Derived Bacterial Lipopolysaccharide and Peripheral TLR4 in Immobilization Stress-Induced Itch Aggravation in a Mouse Model of Atopic Dermatitis. Sci. Rep. 2024, 14, 6263. [Google Scholar] [CrossRef]
- Saleri, R.; Borghetti, P.; Ravanetti, F.; Cavalli, V.; Ferrari, L.; De Angelis, E.; Andrani, M.; Martelli, P. Effects of Different Short-Chain Fatty Acids (SCFA) on Gene Expression of Proteins Involved in Barrier Function in IPEC-J2. Porc. Health Manag. 2022, 8, 21. [Google Scholar] [CrossRef]
- Kasahara, N.; Teratani, T.; Yokota, S.; Sakuma, Y.; Sasanuma, H.; Fujimoto, Y.; Ijichi, T.; Urahashi, T.; Yoshitomi, H.; Kitayama, J.; et al. Dietary Polyamines Promote Intestinal Adaptation in an Experimental Model of Short Bowel Syndrome. Sci. Rep. 2024, 14, 4605. [Google Scholar] [CrossRef]
- Zeng, H.; Umar, S.; Rust, B.; Lazarova, D.; Bordonaro, M. Secondary Bile Acids and Short Chain Fatty Acids in the Colon: A Focus on Colonic Microbiome, Cell Proliferation, Inflammation, and Cancer. Int. J. Mol. Sci. 2019, 20, 1214. [Google Scholar] [CrossRef]
- Wang, Y.L.; Han, Q.Q.; Gong, W.Q.; Pan, D.H.; Wang, L.Z.; Hu, W.; Yang, M.; Li, B.; Yu, J.; Liu, Q. Microglial Activation Mediates Chronic Mild Stress-Induced Depressive- and Anxiety-like Behavior in Adult Rats. J. Neuroinflamm. 2018, 15, 21. [Google Scholar] [CrossRef]
- Breßer, M.; Siemens, K.D.; Schneider, L.; Lunnebach, J.E.; Leven, P.; Glowka, T.R.; Oberländer, K.; De Domenico, E.; Schultze, J.L.; Schmidt, J.; et al. Macrophage-Induced Enteric Neurodegeneration Leads to Motility Impairment during Gut Inflammation. EMBO Mol. Med. 2025, 17, 301–335. [Google Scholar] [CrossRef] [PubMed]
- Robinson, S.; Mogul, A.S.; Taylor-Yeremeeva, E.M.; Khan, A.; Tirabassi, A.D.; Wang, H.Y. Stress Diminishes BDNF-Stimulated TrkB Signaling, TrkB-NMDA Receptor Linkage and Neuronal Activity in the Rat Brain. Neuroscience 2021, 473, 142–158. [Google Scholar] [CrossRef] [PubMed]
- Berger, J.M.; Singh, P.; Khrimian, L.; Morgan, D.A.; Chowdhury, S.; Arteaga-Solis, E.; Horvath, T.L.; Domingos, A.I.; Marsland, A.L.; Yadav, V.K.; et al. Mediation of the Acute Stress Response by the Skeleton. Cell Metab. 2019, 30, 890–902.e8. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Valenzuela, C.; Gárate-Pérez, M.F.; Sotomayor-Zárate, R.; Delano, P.H.; Dagnino-Subiabre, A. Reboxetine Improves Auditory Attention and Increases Norepinephrine Levels in the Auditory Cortex of Chronically Stressed Rats. Front. Neural Circuits 2016, 10, 108. [Google Scholar] [CrossRef]
- Collins, H.M.; Gullino, L.S.; Fuller, C.; Pinacho, R.; Bannerman, D.M.; Sharp, T. Increased C-Fos Immunoreactivity in Anxiety-Related Brain Regions Following Paroxetine Discontinuation. Neuropharmacology 2025, 278, 110541. [Google Scholar] [CrossRef]
- Klöbl, M.; Seiger, R.; Vanicek, T.; Handschuh, P.; Reed, M.B.; Spurny-Dworak, B.; Ritter, V.; Godbersen, G.M.; Gryglewski, G.; Kraus, C.; et al. Escitalopram Modulates Learning Content-Specific Neuroplasticity of Functional Brain Networks. Neuroimage 2022, 247, 118829. [Google Scholar] [CrossRef]
- Eyzaguirre-Velásquez, J.; González-Toro, M.P.; González-Arancibia, C.; Escobar-Luna, J.; Beltrán, C.J.; Bravo, J.A.; Julio-Pieper, M. Sertraline and Citalopram Actions on Gut Barrier Function. Dig. Dis. Sci. 2021, 66, 3792–3802. [Google Scholar] [CrossRef]
- Üneri, Ö.Ş.; Çopur, M.; Tanidir, C.; Güneş, H.; Erdoǧan, A. Liver Enzymes and Bilirubin Levels during Atomoxetine Treatment in Children and Adolescents. Dusunen Adam J. Psychiatry Neurol. Sci. 2013, 26, 22–27. [Google Scholar] [CrossRef]
- Yu, G.; Li, G.F.; Markowitz, J.S. Atomoxetine: A Review of Its Pharmacokinetics and Pharmacogenomics Relative to Drug Disposition. J. Child Adolesc. Psychopharmacol. 2016, 26, 314–326. [Google Scholar] [CrossRef]
- Songphaeng, T.; Lapmanee, S.; Bhubhanil, S.; Momdee, K.; Rojviriya, C.; Kitsahawong, K.; Chailertvanitkul, P.; Welbat, J.U.; Morkmued, S. Atomoxetine and escitalopram migrate the derangement of the temporomandibular joint morphologic and histologic changes in rats exposed to stress-induced depression. J. Oral Sci. 2023, 65, 219–225. [Google Scholar] [CrossRef]
- Bolamperti, S.; Villa, I.; Rubinacci, A. Bone Remodeling: An Operational Process Ensuring Survival and Bone Mechanical Competence. Bone Res. 2022, 10, 48. [Google Scholar] [CrossRef]
- Lam, R.W.; Wong, H.K.; Kumarsing, R.A.; Chua, A.N.; Ho, R.C.; McIntyre, R.S.; Ho, C.S. Fluoxetine Improves Bone Microarchitecture and Mechanical Properties in Rodents Undergoing Chronic Mild Stress—An Animal Model of Depression. Transl. Psychiatry 2022, 12, 339. [Google Scholar] [CrossRef]
- Donat, A.; Jiang, S.; Xie, W.; Knapstein, P.R.; Albertsen, L.C.; Kokot, J.L.; Sevecke, J.; Augustin, R.; Jahn, D.; Yorgan, T.A.; et al. The Selective Norepinephrine Reuptake Inhibitor Reboxetine Promotes Late-Stage Fracture Healing in Mice. iScience 2023, 26, 107761. [Google Scholar] [CrossRef]
- Singh, K.; Bhushan, B.; Chanchal, D.K.; Sharma, S.K.; Rani, K.; Yadav, M.K.; Porwal, P.; Kumar, S.; Sharma, A.; Virmani, T.; et al. Emerging Therapeutic Potential of Cannabidiol (CBD) in Neurological Disorders: A Comprehensive Review. Behav. Neurol. 2023, 2023, 8825358. [Google Scholar] [CrossRef] [PubMed]
- Saad, N.; Raviv, D.; Mizrachi Zer-Aviv, T.; Akirav, I. Cannabidiol Modulates Emotional Function and Brain-Derived Neurotrophic Factor Expression in Middle-Aged Female Rats Exposed to Social Isolation. Int. J. Mol. Sci. 2023, 24, 15492. [Google Scholar] [CrossRef] [PubMed]
- Stein, L.; Vollstaedt, M.L.; Amasheh, S. Cannabidiol Strengthening of Gastric Tight Junction Complexes Analyzed in an Improved Xenopus Oocyte Assay. Membranes 2024, 14, 18. [Google Scholar] [CrossRef] [PubMed]
- Kamsrijai, U.; Charoensup, R.; Jaidee, W.; Hawiset, T.; Thaweethee-Sukjai, B.; Praman, S. Cannabidiol/Cannabidiolic Acid-Rich Hemp (Cannabis sativa L.) Extract Attenuates Cognitive Impairments and Glial Activations in Rats Exposed to Chronic Stress. J. Ethnopharmacol. 2025, 338, 119113. [Google Scholar] [CrossRef]
- Gehring, R.; Merino, G.; Conti, M.B. Pharmacokinetics of Cannabidiol Following Single Oral and Oral Transmucosal Administration in Dogs. Front. Vet. Sci. 2023, 9, 1104152. [Google Scholar] [CrossRef]
- Lapmanee, S.; Bhubhanil, S.; Wongchitrat, P.; Charoenphon, N.; Inchan, A.; Ngernsutivorakul, T.; Dechbumroong, P.; Khongkow, M.; Namdee, K. Assessing the Safety and Therapeutic Efficacy of Cannabidiol Lipid Nanoparticles in Alleviating Metabolic and Memory Impairments and Hippocampal Histopathological Changes in Diabetic Parkinson’s Rats. Pharmaceutics 2024, 16, 514. [Google Scholar] [CrossRef]
- Lapmanee, S.; Bhubhanil, S.; Charoenphon, N.; Inchan, A.; Bunwatcharaphansakun, P.; Khongkow, M.; Namdee, K. Cannabidiol-Loaded Lipid Nanoparticles Incorporated in Polyvinyl Alcohol and Sodium Alginate Hydrogel Scaffold for Enhancing Cell Migration and Accelerating Wound Healing. Gels 2024, 10, 843. [Google Scholar] [CrossRef]
- Aydin, S.; Ozkul, C.; Yucel, N.T.; Karaca, H. Gut Microbiome Alteration after Reboxetine Administration in Type-1 Diabetic Rats. Microorganisms 2021, 9, 1948. [Google Scholar] [CrossRef]
- Pawluski, J.L.; Murail, P.; Grudet, F.; Bys, L.; Golubeva, A.V.; Bastiaanssen, T.; Oberlander, T.F.; Cryan, J.F.; O’Mahony, S.M.; Charlier, T.D. Gestational Stress and Perinatal SSRIs Differentially Impact the Maternal and Neonatal Microbiome-Gut-Brain Axis. J. Neuroendocrinol. 2023, 35, e13261. [Google Scholar] [CrossRef]
- Chen, S.; Lee, Y.B.; Song, M.Y.; Lim, C.; Cho, H.; Shim, H.J.; Kim, J.S.; Park, B.H.; Kim, J.K.; Bae, E.J. Cannabidiol Reshapes the Gut Microbiome to Promote Endurance Exercise in Mice. Exp. Mol. Med. 2025, 57, 489–500. [Google Scholar] [CrossRef]
- Lapmanee, S.; Bhubhanil, S.; Sriwong, S.; Khongkow, M.; Namdee, K.; Wongchitrat, P.; Pongkorpsakol, P. Venlafaxine and Synbiotic Attenuated Learned Fear-like Behavior and Recognition Memory Impairment in Immobilized-Stressed Rats. Physiol. Pharmacol. 2023, 27, 171–181. [Google Scholar] [CrossRef]
- Moreno-Martínez, S.; Tendilla-Beltrán, H.; Sandoval, V.; Flores, G.; Terrón, J.A. Chronic Restraint Stress Induces Anxiety-like Behavior and Remodeling of Dendritic Spines in the Central Nucleus of the Amygdala. Behav. Brain Res. 2022, 416, 113523. [Google Scholar] [CrossRef] [PubMed]
- Rohleder, N. Stimulation of Systemic Low-Grade Inflammation by Psychosocial Stress. Biopsychosoc. Sci. Med. 2014, 76, 181–189. [Google Scholar] [CrossRef]
- Miller, E.S.; Apple, C.G.; Kannan, K.B.; Funk, Z.M.; Plazas, J.M.; Efron, P.A.; Mohr, A.M. Chronic Stress Induces Persistent Low-Grade Inflammation. Am. J. Surg. 2019, 218, 677–683. [Google Scholar] [CrossRef]
- Lapmanee, S.; Charoenphandhu, J.; Teerapornpuntakit, J.; Krishnamra, N.; Charoenphandhu, N. Agomelatine, Venlafaxine, and Running Exercise Effectively Prevent Anxiety- and Depression-like Behaviors and Memory Impairment in Restraint Stressed Rats. PLoS ONE 2017, 12, e0187671. [Google Scholar] [CrossRef]
- Parrott, J.M.; Porter, G.A.; Redus, L.; O’Connor, J.C. Brain Derived Neurotrophic Factor Deficiency Exacerbates Inflammation-Induced Anhedonia in Mice. Psychoneuroendocrinology 2021, 134, 105404. [Google Scholar] [CrossRef]
- Hu, X.; Zhao, H.L.; Kurban, N.; Qin, Y.; Chen, X.; Cui, S.Y.; Zhang, Y.H. Reduction of BDNF Levels and Biphasic Changes in Glutamate Release in the Prefrontal Cortex Correlate with Susceptibility to Chronic Stress-Induced Anhedonia. eNeuro 2023, 10, ENEURO.0406-23.2023. [Google Scholar] [CrossRef]
- Gabanyi, I.; Muller, P.A.; Feighery, L.; Oliveira, T.Y.; Costa-Pinto, F.A.; Mucida, D. Neuro-Immune Interactions Drive Tissue Programming in Intestinal Macrophages. Cell 2016, 164, 378–391. [Google Scholar] [CrossRef]
- Meena, A.S.; Shukla, P.K.; Rao, R.; Canelas, C.; Pierre, J.F.; Rao, R.K. TRPV6 Deficiency Attenuates Stress and Corticosterone-Mediated Exacerbation of Alcohol-Induced Gut Barrier Dysfunction and Systemic Inflammation. Front. Immunol. 2023, 14, 1093584. [Google Scholar] [CrossRef]
- Sun, N.; Zhang, J.; Wang, J.; Liu, Z.; Wang, X.; Kang, P.; Yang, C.; Liu, P.; Zhang, K. Abnormal Gut Microbiota and Bile Acids in Patients with First-Episode Major Depressive Disorder and Correlation Analysis. Psychiatry Clin. Neurosci. 2022, 76, 321–328. [Google Scholar] [CrossRef]
- Skonieczna-żydecka, K.; Grochans, E.; Maciejewska, D.; Szkup, M.; Schneider-Matyka, D.; Jurczak, A.; Łoniewski, I.; Kaczmarczyk, M.; Marlicz, W.; Czerwińska-Rogowska, M.; et al. Faecal Short Chain Fatty Acids Profile Is Changed in Polish Depressive Women. Nutrients 2018, 10, 1939. [Google Scholar] [CrossRef] [PubMed]
- Ney, L.M.; Wipplinger, M.; Grossmann, M.; Engert, N.; Wegner, V.D.; Mosig, A.S. Short Chain Fatty Acids: Key Regulators of the Local and Systemic Immune Response in Inflammatory Diseases and Infections. Open Biol. 2023, 13, 230014. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, A.; Matsumoto, M. Role of Polyamines in Intestinal Mucosal Barrier Function. Semin. Immunopathol. 2025, 47, 9. [Google Scholar] [CrossRef]
- Hofer, S.J.; Simon, A.K.; Bergmann, M.; Eisenberg, T.; Kroemer, G.; Madeo, F. Mechanisms of Spermidine-Induced Autophagy and Geroprotection. Nat. Aging 2022, 2, 1112–1129. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, E.; Corr, S.C. Lactobacillus Spp. for Gastrointestinal Health: Current and Future Perspectives. Front. Immunol. 2022, 13, 840245. [Google Scholar] [CrossRef]
- Torres, H.M.; Arnold, K.M.; Oviedo, M.; Westendorf, J.J.; Weaver, S.R. Inflammatory Processes Affecting Bone Health and Repair. Curr. Osteoporos. Rep. 2023, 21, 842–853. [Google Scholar] [CrossRef]
- 50 Silva, B.C.; Bilezikian, J.P. Parathyroid Hormone: Anabolic and Catabolic Actions on the Skeleton. Curr. Opin. Pharmacol. 2015, 22, 41–50. [Google Scholar] [CrossRef]
- Xiaotong, L.; Jiazhi, Y.; Xiaoguang, L.; Gang, Z. PLC, PTH and NF-ΚB Increased during Orthodontic Bone Remodeling in Chronic Stress Rats. Stress 2022, 25, 357–365. [Google Scholar] [CrossRef]
- De Crescenzo, F.; Ziganshina, L.E.; Yudina, E.V.; Kaplan, Y.C.; Ciabattini, M.; Wei, Y.; Hoyle, C.H.V. Noradrenaline Reuptake Inhibitors (NRIs) for Attention Deficit Hyperactivity Disorder (ADHD) in Adults. Cochrane Database Syst. Rev. 2018, 2018, CD013044. [Google Scholar] [CrossRef]
- Edinoff, A.N.; Akuly, H.A.; Hanna, T.A.; Ochoa, C.O.; Patti, S.J.; Ghaffar, Y.A.; Kaye, A.D.; Viswanath, O.; Urits, I.; Boyer, A.G.; et al. Selective Serotonin Reuptake Inhibitors and Adverse Effects: A Narrative Review. Neurol. Int. 2021, 13, 387–401. [Google Scholar] [CrossRef]
- Atalay, S.; Jarocka-karpowicz, I.; Skrzydlewskas, E. Antioxidative and Anti-Inflammatory Properties of Cannabidiol. Antioxidants 2020, 9, 21. [Google Scholar] [CrossRef]
- Gáll, Z.; Farkas, S.; Albert, Á.; Ferencz, E.; Vancea, S.; Urkon, M.; Kolcsár, M. Effects of Chronic Cannabidiol Treatment in the Rat Chronic Unpredictable Mild Stress Model of Depression. Biomolecules 2020, 10, 801. [Google Scholar] [CrossRef] [PubMed]
- Sabbag, T.; Kritman, M.; Akirav, I. Cannabidiol Effects on Depressive-like Behavior and Neuroinflammation in Female Rats Exposed to High-Fat Diet and Unpredictable Chronic Mild Stress. Cells 2025, 14, 938. [Google Scholar] [CrossRef]
- Nong, J.; Glassman, P.M.; Reyes-Esteves, S.; Descamps, H.C.; Shuvaev, V.V.; Kiseleva, R.Y.; Papp, T.E.; Alameh, M.-G.; Tam, Y.K.; Mui, B.L.; et al. Targeting Lipid Nanoparticles to the Blood Brain Barrier to Ameliorate Acute Ischemic Stroke. Mol. Ther. 2024, 32, 1344–1358. [Google Scholar] [CrossRef]
- Ibrahim, I.; Syamala, S.; Ayariga, J.A.; Xu, J.; Robertson, B.K.; Meenakshisundaram, S.; Ajayi, O.S. Modulatory Effect of Gut Microbiota on the Gut-Brain, Gut-Bone Axes, and the Impact of Cannabinoids. Metabolites 2022, 12, 1247. [Google Scholar] [CrossRef]
- Crowley, K.; Kiraga, Ł.; Miszczuk, E.; Skiba, S.; Banach, J.; Latek, U.; Mendel, M.; Chłopecka, M. Effects of Cannabinoids on Intestinal Motility, Barrier Permeability, and Therapeutic Potential in Gastrointestinal Diseases. Int. J. Mol. Sci. 2024, 25, 6682. [Google Scholar] [CrossRef]
- Sui, K.; Tveter, K.M.; Bawagan, F.G.; Buckendahl, P.; Martinez, S.A.; Jaffri, Z.H.; MacDonell, A.T.; Wu, Y.; Duran, R.M.; Shapses, S.A.; et al. Cannabidiol-Treated Ovariectomized Mice Show Improved Glucose, Energy, and Bone Metabolism With a Bloom in Lactobacillus. Front. Pharmacol. 2022, 13, 900667. [Google Scholar] [CrossRef]
- Kim, S.W.; Shrestha, S.K.; Chuluunbaatar, B.A.; Soh, Y. Combination of Cannabidiol with Taurine Synergistically Treated Periodontitis in Rats. Biomol. Ther. 2025, 33, 203–209. [Google Scholar] [CrossRef] [PubMed]
- Burdușel, A.C.; Andronescu, E. Lipid nanoparticles and liposomes for bone diseases treatment. Biomedicines 2022, 10, 3158. [Google Scholar] [CrossRef] [PubMed]
- Zamansky, M.; Yariv, D.; Feinshtein, V.; Ben-Shabat, S.; Sintov, A.C. Cannabidiol-Loaded Lipid-Stabilized Nanoparticles Alleviate Psoriasis Severity in Mice: A New Approach for Improved Topical Drug Delivery. Molecules 2023, 28, 6907. [Google Scholar] [CrossRef]
- Grifoni, L.; Vanti, G.; Bilia, A.R. Nanostructured Lipid Carriers Loaded with Cannabidiol Enhance Its Bioaccessibility to The Small Intestine. Nutraceuticals 2023, 3, 210–221. [Google Scholar] [CrossRef]
- Taha, I.E.; ElSohly, M.A.; Radwan, M.M.; Elkanayati, R.M.; Wanas, A.; Joshi, P.H.; Ashour, E.A. Enhancement of Cannabidiol Oral Bioavailability Through The Development of Nanostructured Lipid Carriers: In vitro and in vivo Evaluation Studies. Drug Deliv. Transl. Res. 2025, 15, 2722–2732. [Google Scholar] [CrossRef]
- Sales, A.J.; Crestani, C.C.; Guimarães, F.S.; Joca, S.R.L. Antidepressant-Like Effect Induced by Cannabidiol is Dependent on Brain Serotonin Levels. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 86, 255–261. [Google Scholar] [CrossRef]
- Sartim, A.G.; Marques, J.; Silveira, K.M.; Gobira, P.H.; Guimarães, F.S.; Wegener, G.; Joca, S.R. Co-administration of Cannabidiol and Ketamine Induces Antidepressant-Like Effects Devoid of Hyperlocomotor Side-Effects. Neuropharmacology 2021, 195, 108679. [Google Scholar] [CrossRef]
- Gillette, R.; Reilly, M.P.; Topper, V.Y.; Thompson, L.M.; Crews, D.; Gore, A.C. Anxiety-like Behaviors in Adulthood Are Altered in Male but Not Female Rats Exposed to Low Dosages of Polychlorinated Biphenyls in Utero. Horm. Behav. 2017, 87, 8–15. [Google Scholar] [CrossRef]
- Plongniras, P.; Lapmanee, S.; Thonapan, N.; Thangsombat, P.; Janthaphim, P.; Lertkarnvijai, C.; Chailertvanitkul, P.; Morkmued, S. Stress-Induced Depression and Its Effects on Tooth Wear in Rats: A 3D Dental Scan Imaging Perspective. Life 2025, 15, 712. [Google Scholar] [CrossRef]
- Potrebić, M.S.; Pavković, Ž.Z.; Srbovan, M.M.; Đmura, G.M.; Pešić, V.T. Changes in the Behavior and Body Weight of Mature, Adult Male Wistar Han Rats after Reduced Social Grouping and Social Isolation. J. Am. Assoc. Lab. Anim. Sci. 2022, 61, 615–623. [Google Scholar] [CrossRef]
- Marrocco, J.; Reynaert, M.-L.; Gatta, E.; Gabriel, C.; Mocaër, E.; Di Prisco, S.; Merega, E.; Pittaluga, A.; Nicoletti, F.; Maccari, S.; et al. The Effects of Antidepressant Treatment in Prenatally Stressed Rats Support the Glutamatergic Hypothesis of Stress-Related Disorders. J. Neurosci. 2014, 34, 2015. [Google Scholar] [CrossRef]
- Serchov, T.; van Calker, D.; Biber, K. Light/Dark Transition Test to Assess Anxiety-like Behavior in Mice. Bio Protoc. 2016, 6, e1957. [Google Scholar] [CrossRef]
- Reamtong, O.; Lapmanee, S.; Tummatorn, J.; Palavong, N.; Thongsornkleeb, C.; Ruchirawat, S. Synthesis of Benzoazepine Derivatives via Azide Rearrangement and Evaluation of Their Antianxiety Activities. ACS Med. Chem. Lett. 2021, 12, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Lapmanee, S.; Bhubhanil, S.; Sriwong, S.; Yuajit, C.; Wongchitrat, P.; Teerapornpuntakit, J.; Suntornsaratoon, P.; Charoenphandhu, J.; Charoenphandhu, N. Oral Calcium and Vitamin D Supplements Differentially Alter Exploratory, Anxiety-like Behaviors and Memory in Male Rats. PLoS ONE 2023, 18, e0290106. [Google Scholar] [CrossRef] [PubMed]
- Ghouzali, I.; Lemaitre, C.; Bahlouli, W.; Azhar, S.; Bôle-Feysot, C.; Meleine, M.; Ducrotté, P.; Déchelotte, P.; Coëffier, M. Targeting Immunoproteasome and Glutamine Supplementation Prevent Intestinal Hyperpermeability. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2017, 1861, 3278–3288. [Google Scholar] [CrossRef]
- Bahlouli, W.; Breton, J.; Lelouard, M.; L’Huillier, C.; Tirelle, P.; Salameh, E.; Amamou, A.; Atmani, K.; Goichon, A.; Bôle-Feysot, C.; et al. Stress-Induced Intestinal Barrier Dysfunction Is Exacerbated during Diet-Induced Obesity. J. Nutr. Biochem. 2020, 81, 108382. [Google Scholar] [CrossRef] [PubMed]
- Pabón, M.M.; Acosta, S.; Guedes, V.A.; Tajiri, N.; Kaneko, Y.; Borlongan, C.V. Brain Region-Specific Histopathological Effects of Varying Trajectories of Controlled Cortical Impact Injury Model of Traumatic Brain Injury. CNS Neurosci. Ther. 2016, 22, 200–211. [Google Scholar] [CrossRef]
- Wu, Y.; Li, S.; Lv, L.; Jiang, S.; Xu, L.; Chen, H.; Li, L. Protective Effect of Pediococcus pentosaceus Li05 on Diarrhea-Predominant Irritable Bowel Syndrome in Rats. Food Funct. 2024, 15, 3692–3708. [Google Scholar] [CrossRef]
- Ding, F.; Wu, J.; Liu, C.; Bian, Q.; Qiu, W.; Ma, Q.; Li, X.; Long, M.; Zou, X.; Chen, J. Effect of Xiaoyaosan on Colon Morphology and Intestinal Permeability in Rats with Chronic Unpredictable Mild Stress. Front. Pharmacol. 2020, 11, 1069. [Google Scholar] [CrossRef]
- Liu, H.; Wu, J.; Wu, H.; Wang, T.; Zhou, H.; Liu, M. Autologous ADSCs with Exogenous NPY Promotes Fracture Healing in Ovariectomized Rats. Heliyon 2024, 10, e38297. [Google Scholar] [CrossRef]
- Minami, M.; Ikoma, K.; Onishi, O.; Horii, M.; Itoh, K.; Takahashi, K. Histological Assessment of Cortical Bone Changes in Diabetic Rats. J. Orthop. Surg. Res. 2022, 17, 568. [Google Scholar] [CrossRef]
- Fan, Y.; Leape, C.P.; Hugard, S.; McCanne, M.; Thomson, A.; Wojtkiewicz, G.R.; Weaver, M.J.; Collins, J.E.; Randolph, M.; Oral, E. A Longitudinal Rat Model for Assessing Postoperative Recovery and Bone Healing Following Tibial Osteotomy and Plate Fixation. BMC Musculoskelet. Disord. 2023, 24, 854. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lapmanee, S.; Lumsutti, J.; Charoenphon, N.; Inchan, A.; Boonmuen, N.; Wongchitrat, P.; Thonapan, N.; Yuajit, C.; Surinlert, P.; Tipbunjong, C.; et al. Cannabidiol Lipid Nanoparticles Stabilize Gut–Brain–Bone Axis Integrity and Enhance Neuroplasticity in Stressed Rats: A Comparison with Atomoxetine and Escitalopram. Int. J. Mol. Sci. 2025, 26, 9318. https://doi.org/10.3390/ijms26199318
Lapmanee S, Lumsutti J, Charoenphon N, Inchan A, Boonmuen N, Wongchitrat P, Thonapan N, Yuajit C, Surinlert P, Tipbunjong C, et al. Cannabidiol Lipid Nanoparticles Stabilize Gut–Brain–Bone Axis Integrity and Enhance Neuroplasticity in Stressed Rats: A Comparison with Atomoxetine and Escitalopram. International Journal of Molecular Sciences. 2025; 26(19):9318. https://doi.org/10.3390/ijms26199318
Chicago/Turabian StyleLapmanee, Sarawut, Jitpatima Lumsutti, Natthawut Charoenphon, Anjaree Inchan, Nittaya Boonmuen, Prapimpun Wongchitrat, Natchayaporn Thonapan, Chaowalit Yuajit, Piyaporn Surinlert, Chittipong Tipbunjong, and et al. 2025. "Cannabidiol Lipid Nanoparticles Stabilize Gut–Brain–Bone Axis Integrity and Enhance Neuroplasticity in Stressed Rats: A Comparison with Atomoxetine and Escitalopram" International Journal of Molecular Sciences 26, no. 19: 9318. https://doi.org/10.3390/ijms26199318
APA StyleLapmanee, S., Lumsutti, J., Charoenphon, N., Inchan, A., Boonmuen, N., Wongchitrat, P., Thonapan, N., Yuajit, C., Surinlert, P., Tipbunjong, C., Khongkow, M., Namdee, K., & Sirithanakorn, C. (2025). Cannabidiol Lipid Nanoparticles Stabilize Gut–Brain–Bone Axis Integrity and Enhance Neuroplasticity in Stressed Rats: A Comparison with Atomoxetine and Escitalopram. International Journal of Molecular Sciences, 26(19), 9318. https://doi.org/10.3390/ijms26199318





