Antioxidant Activity, Total Polyphenol Content, and Mineral Composition of Milk Beverages Fortified with Spice Mixtures (Clove, Cinnamon, and Turmeric) and Natural Sweeteners (Erythritol and Stevia): Evidence of Synergistic or Antagonistic Effects of Compounds
Abstract
1. Introduction
2. Results
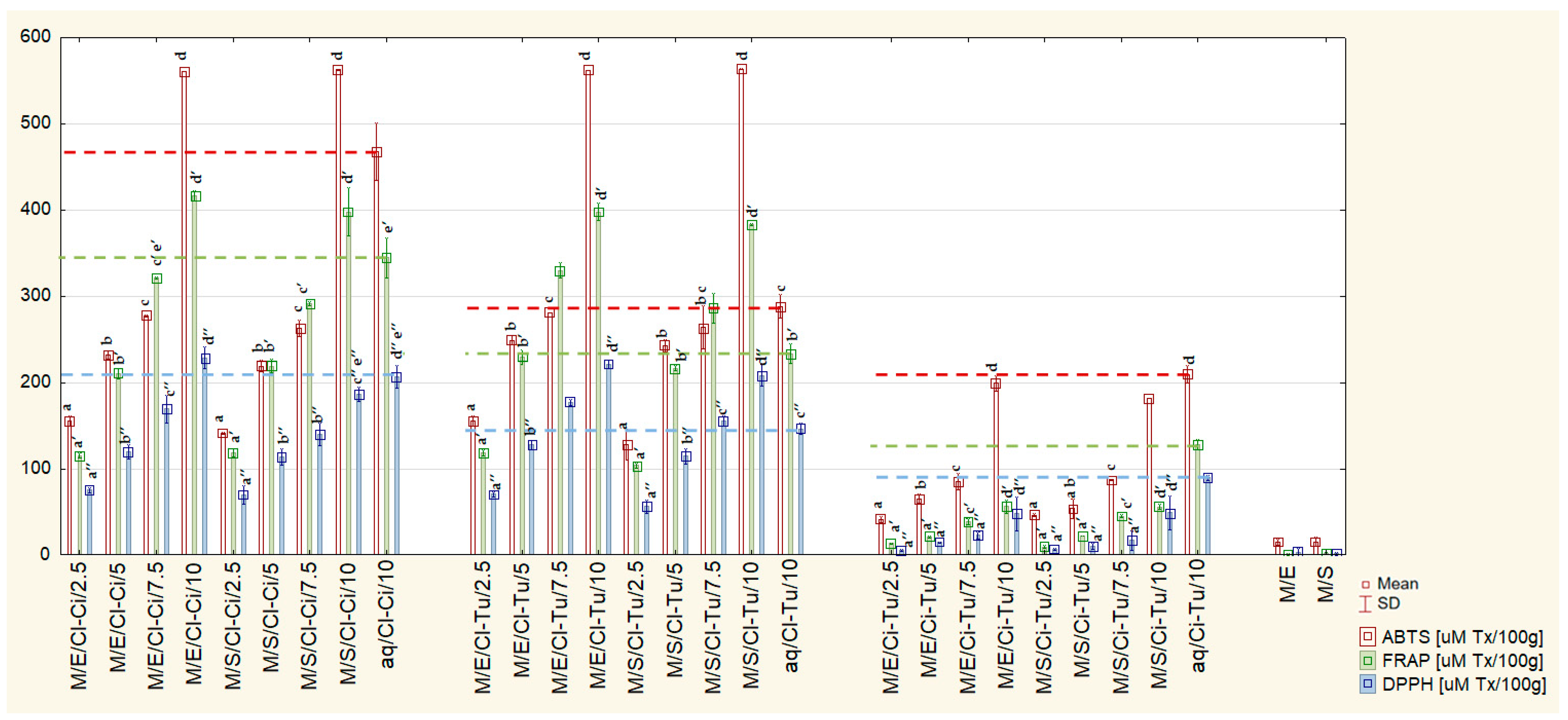
2.1. Antioxidant Capacity
2.2. Total Polyphenol Content (TPC)
2.3. Mineral Element Content
2.4. Additive, Synergistic, and Antagonistic Effect of Compounds
3. Discussion
4. Materials and Methods
4.1. Groceries
4.2. Reagents
4.3. Preparation of Beverages—Milk Beverages Fortified with Spices and Sweeteners
4.4. Initial Sample Processing
4.5. Extraction of Polyphenolic Compounds
4.6. Antioxidant Capacity
4.6.1. FRAP Assay
4.6.2. ABTS Assay
4.6.3. DPPH Assay
4.7. Total Polyphenol Content
4.8. Wet Mineralization
4.9. Mineral Element Content
4.10. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Damián, M.R.; Cortes-Perez, N.G.; Quintana, E.T.; Ortiz-Moreno, A.; Garfias Noguez, C.; Cruceño-Casarrubias, C.E.; Sánchez Pardo, M.E.; Bermúdez-Humarán, L.G. Functional foods, nutraceuticals and probiotics: A focus on human health. Microorganisms 2022, 10, 1065. [Google Scholar] [CrossRef]
- Functional Foods Market Size, Share & Trends Analysis Report by Ingredient (Carotenoids, Prebiotics & Probiotics, Fatty Acids, Dietary Fibers), by Product, by Application, by Region, and Segment Forecasts, 2022–2030. Available online: https://www.grandviewresearch.com/industry-analysis/functional-food-market (accessed on 23 May 2025).
- Sugar Substitutes Market Size, Share & Trends Analysis Report by Type (High-intensity Sweeteners, High Fructose Syrup), by Application (Food, Beverages), by Region (North America, Asia Pacific), and Segment Forecasts, 2024–2030. Available online: https://www.grandviewresearch.com/industry-analysis/sugar-substitutes-market (accessed on 23 May 2025).
- Câmara, J.S.; Albuquerque, B.R.; Aguiar, J.; Corrêa, R.C.G.; Gonçalves, J.L.; Granato, D.; Pereira, J.A.M.; Barros, L.; Ferreira, I.C.F.R. Food bioactive compounds and emerging techniques for their extraction: Polyphenols as a case study. Foods 2020, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Mackonochie, M.; Rodriguez-Mateos, A.; Mills, S.; Rolfe, V. A scoping review of the clinical evidence for the health benefits of culinary doses of herbs and spices for the prevention and treatment of metabolic syndrome. Nutrients 2023, 15, 4867. [Google Scholar] [CrossRef]
- Isbill, J.; Kandiah, J.; Kruzliakova, N. Opportunities for health promotion: Highlighting herbs and spices to improve immune support and well-being. Integr. Med. 2020, 19, 30–42. [Google Scholar]
- Jiang, T.A. Health benefits of culinary herbs and spices. J. AOAC Int. 2019, 102, 395–410. [Google Scholar] [CrossRef] [PubMed]
- Yashin, A.; Yashin, Y.; Xia, X.; Nemzer, B. Antioxidant activity of spices and their impact on human health: A review. Antioxidants 2017, 6, 70. [Google Scholar] [CrossRef] [PubMed]
- Haytowitz, D.B.; Bhagwat, S.A. USDA Database for the Oxygen Radical Capacity (ORAC) of Selected Foods, Release 2. USDA National Nutrient Database for Standard Reference. 2010. Available online: https://www.ars.usda.gov/research/publications/publication/?seqNo115=251105 (accessed on 5 September 2025).
- Jain, S.G.; Puri, S.; Misra, A.; Gulati, S.; Mani, K. Effect of oral cinnamon intervention on metabolic profile and body composition of Asian Indians with metabolic syndrome: A randomized double-blind control trial. Lipids Health Dis. 2017, 16, 113. [Google Scholar] [CrossRef]
- Askari, F.; Rashidkhani, B.; Hekmatdoost, A. Cinnamon may have therapeutic benefits on lipid profile, liver enzymes, insulin resistance, and high-sensitivity C-reactive protein in nonalcoholic fatty liver disease patients. Nutr. Res. 2014, 34, 143–148. [Google Scholar] [CrossRef]
- Adab, Z.; Eghtesadi, S.; Vafa, M.R.; Heydari, I.; Shojaii, A.; Haqqani, H.; Arablou, T.; Eghtesadi, M. Effect of turmeric on glycemic status, lipid profile, hs-CRP, and total antioxidant capacity in hyperlipidemic type 2 diabetes mellitus patients. Phytother. Res. 2019, 33, 1173–1181. [Google Scholar] [CrossRef]
- Kuroda, M.; Mimaki, Y.; Ohtomo, T.; Yamada, J.; Nishiyama, T.; Mae, T.; Kishida, H.; Kawada, T. Hypoglycemic effects of clove (Syzygium aromaticum flower buds) on genetically diabetic KK-Ay mice and identification of the active ingredients. J. Nat. Med. 2012, 66, 394–399. [Google Scholar] [CrossRef]
- Tao, G.; Irie, Y.; Li, D.J.; Keung, W.M. Eugenol and its structural analogs inhibit monoamine oxidase A and exhibit antidepressant-like activity. Bioorg. Med. Chem. 2005, 13, 4777–4788. [Google Scholar] [CrossRef]
- Halder, S.; Mehta, A.; Kar, R.; Mustafa, M.; Mediratta, P.; Sharma, K. Clove oil reverses learning and memory deficits in scopolamine-treated mice. Planta Med. 2011, 77, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Mazi, T.A.; Stanhope, K.L. Erythritol: An in-depth discussion of its potential to be a beneficial dietary component. Nutrients 2023, 15, 204. [Google Scholar] [CrossRef]
- EFSA Panel on Food Additives and Flavourings (FAF). Re-evaluation of erythritol (E968) as a food additive. EFSA J. 2023, 21, e8430. [Google Scholar]
- EFSA Panel on Food Additives and Nutrient Sources (ANS). Scientific opinion on safety of steviolglycosides for the proposed uses as a food additive. EFSA J. 2010, 8, 1537. [Google Scholar]
- Noda, K.; Nakayama, K.; Oku, T. Serum glucose and insulin levels and erythritol balance after oral administration of erythritol in healthy subjects. Eur. J. Clin. Nutr. 1994, 48, 286–292. [Google Scholar]
- Peteliuk, V.; Rybchuk, L.; Bayliak, M.; Storey, K.B.; Lushchak, O. Natural sweetener Stevia rebaudiana: Functionalities, health benefits and potential risks. EXCLI J. 2021, 20, 1412–1430. [Google Scholar]
- Wölnerhanssen, B.K.; Cajacob, L.; Keller, N.; Doody, A.; Rehfeld, J.F.; Drewe, J.; Peterli, R.; Beglinger, C.; Meyer-Gerspach, A.C. Gut hormone secretion, gastric emptying, and glycemic responses to erythritol and xylitol in lean and obese subjects. Am. J. Physiol. Endocrinol. Metab. 2016, 310, 1053–1061. [Google Scholar] [CrossRef]
- Wölnerhanssen, B.K.; Drewe, J.; Verbeure, W.; le Roux, C.W.; Dellatorre-Teixeira, L.; Rehfeld, J.F.; Holst, J.J.; Hartmann, B.; Tack, J.; Peterli, R.; et al. Gastric emptying of solutions containing the natural sweetener erythritol and effects on gut hormone secretion in humans: A pilot dose-ranging study. Diabetes Obes. Metab. 2021, 23, 1311–1321. [Google Scholar] [CrossRef] [PubMed]
- Livesey, G. Health potential of polyols as sugar replacers with emphasis on low glycaemic properties. Nutr. Res. Rev. 2003, 16, 163–191. [Google Scholar] [CrossRef] [PubMed]
- Falony, G.; Honkala, S.; Runnel, R.; Olak, J.; Nommela, R.; Russak, S.; Saag, M.; Makinen, P.L.; Makinen, K.; Vahlberg, T.; et al. Long-term effect of erythritol on dental caries development during childhood: A posttreatment survival analysis. Caries Res. 2016, 50, 579–588. [Google Scholar] [CrossRef]
- Contreras, S. Anticariogenic properties and effects on periodontal structures of Stevia rebaudiana Bertoni. Narrative review. J. Oral Res. 2013, 2, 158–166. [Google Scholar] [CrossRef]
- Gwak, M.-J.; Chung, S.-J.; Kim, Y.J.; Lim, C.S. Relative sweetness and sensory characteristics of bulk and intense sweeteners. Food Sci. Biotechnol. 2012, 21, 889–894. [Google Scholar] [CrossRef]
- Antunes, I.C.; Bexiga, R.; Pinto, C.; Roseiro, L.C.; Quaresma, M.A.G. Cow’s milk in human nutrition and the emergence of plant-based milk alternatives. Foods 2023, 12, 99. [Google Scholar] [CrossRef]
- El-Sayed, S.M.; Youssef, A.M. Potential application of herbs and spices and their effects in functional dairy products. Heliyon 2019, 5, e01989. [Google Scholar] [CrossRef] [PubMed]
- Cutrim, C.S.; Cortez, M.A.S. A review on polyphenols: Classification, beneficial effects and their application in dairy products. Int. J. Dairy Technol. 2018, 71, 564–578. [Google Scholar] [CrossRef]
- van de Langerijt, T.M.; O’Mahony, J.A.; Crowley, S.V. Structural, binding and functional properties of milk protein-polyphenol systems: A review. Molecules 2023, 28, 2288. [Google Scholar] [CrossRef]
- Kardum, N.; Glibetic, M. Polyphenols and Their Interactions with 629 Other Dietary Compounds: Implications for Human Health. In Advances in Food and Nutrition Research; Academic Press: Cambridge, MA, USA, 2018; Volume 84, pp. 103–144. [Google Scholar]
- Sun, X.; Sarteshnizi, R.A.; Udenigwe, C.C. Recent advances in protein–polyphenol interactions focusing on structural properties related to antioxidant activities. Curr. Opin. Food Sci. 2022, 45, 100840. [Google Scholar] [CrossRef]
- Lamothe, S.; Langlois, A.; Bazinet, L.; Couillard, C.; Britten, M. Antioxidant activity and nutrient release from polyphenol-enriched cheese in a simulated gastrointestinal environment. Food Funct. 2016, 7, 1634–1644. [Google Scholar] [CrossRef]
- Helal, A.; Tagliazucchi, D. Impact of in-vitro gastro-pancreatic digestion on polyphenols and cinnamaldehyde bioaccessibility and antioxidant activity in stirred cinnamon-fortified yogurt. LWT 2018, 89, 164–170. [Google Scholar] [CrossRef]
- Zhang, H.; Zheng, J.; Liu, X.; Ding, Q.; Jiang, L.; Guo, H.; Ren, F. Milk protein and fat play different roles in affecting the bioavailability and the antioxidant activity of jujube juice phenolics in rats. Mol. Nutr. Food Res. 2012, 56, 1511–1519. [Google Scholar] [CrossRef]
- Langley-Evans, S.C. Consumption of black tea elicits an increase in plasma antioxidant potential in humans. Int. J. Food Sci. Nutr. 2000, 51, 309–315. [Google Scholar] [CrossRef]
- Jakobek, L. Interactions of polyphenols with carbohydrates, lipids and proteins. Food Chem 2015, 175, 556–567. [Google Scholar] [CrossRef]
- Bohn, T. Dietary factors affecting polyphenol bioavailability. Nutr. Rev. 2014, 72, 429–452. [Google Scholar] [CrossRef]
- Chen, Z.; Świsłocka, R.; Choinska, R.; Marszaek, K.; Dąbrowska, A.; Lewandowski, W.; Lewandowska, H. Exploring the correlation between the molecular structure and biological activities of metal–phenolic compound complexes: Research and description of the role of metal ions in improving the antioxidant activities of phenolic compounds. Int. J. Mol. Sci. 2024, 25, 11775. [Google Scholar] [CrossRef]
- Granda, H.; de Pascual-Teresa, S. Interaction of polyphenols with other food components as a means for their neurological health benefits. J. Agric. Food Chem. 2018, 66, 8224–8230. [Google Scholar] [CrossRef]
- Ma, Q.; Kim, E.Y.; Lindsay, E.A.; Han, O. Bioactive dietary polyphenols inhibit heme iron absorption in a dose-dependent manner in human intestinal Caco-2 cells. J. Food Sci. 2011, 76, H143–H150. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.Y.; Pai, T.K.; Han, O. Effect of bioactive dietary polyphenols on zinc transport across the intestinal Caco-2 cell monolayers. J. Agric. Food Chem. 2011, 59, 3606–3612. [Google Scholar] [CrossRef]
- Cioanca, O.; Lungu, I.-I.; Batir-Marin, D.; Lungu, A.; Marin, G.-A.; Huzum, R.; Stefanache, A.; Sekeroglu, N.; Hancianu, M. Modulating polyphenol activity with metal ions: Insights into dermatological applications. Pharmaceutics 2025, 17, 194. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Tareq, A.M.; Das, R.; Emran, T.B.; Nainu, F.; Chakraborty, A.J.; Ahmad, I.; Tallei, T.E.; Idris, A.M.; Simal-Gandara, S. Polyphenols: A first evidence in the synergism and bioactivities. Food Rev. Int. 2023, 39, 4419–4441. [Google Scholar] [CrossRef]
- Erskine, E.; Subaşı, B.G.; Vahapoglu, B.; Capanoglu, E. Coffee phenolics and their interaction with other food phenolics: Antagonistic and synergistic effects. ACS Omega 2022, 7, 1595–1601. [Google Scholar] [CrossRef]
- Nguyen, M.M.; Karboune, S. Combinatorial interactions of essential oils enriched with individual polyphenols, polyphenol mixes, and plant extracts: Multi-antioxidant systems. Antioxidants 2023, 12, 486. [Google Scholar] [CrossRef]
- Pellegrini, N.; Chiavaro, E.; Gardana, C.; Mazzeo, T.; Contino, D.; Gallo, M.; Riso, P.; Fogliano, V.; Porrini, M. Effect of different cooking methods on color, phytochemical concentration, and antioxidant capacity of raw and frozen brassica vegetables. J. Agric. Food Chem. 2010, 58, 4310–4321. [Google Scholar] [CrossRef]
- Chohan, M.; Naughton, D.P.; Jones, L.; Opara, E.I. An investigation of the relationship between the anti-inflammatory activity, polyphenolic content, and antioxidant activities of cooked and in vitro digested culinary herbs. Oxidative Med. Cell. Longev. 2012, 2012, 627843. [Google Scholar] [CrossRef]
- Chohan, M. The Impact of Digestion and Gut Bioavailability, in Vitro, on the Polyphenolic Associated Activity of Cooked Culinary Herbs. Ph.D. Thesis, Kingston University, Kingston upon Thames, UK, 2011. [Google Scholar]
- Choi, Y.; Lee, S.M.; Chun, J.; Lee, H.B.; Lee, J. Influence of heat treatment on the antioxidant activities and polyphenolic compounds of Shiitake (Lentinus edodes) mushroom. Food Chem. 2006, 99, 381–387. [Google Scholar] [CrossRef]
- Chen, X.; Li, H.; Zhang, B.; Deng, Z. The synergistic and antagonistic antioxidant interactions of dietary phytochemical combinations. Crit. Rev. Food Sci. Nutr. 2022, 62, 5658–5677. [Google Scholar] [CrossRef]
- Manoharan, A.; Ramasamy, D.; Dhanalashmi, B.; Gnanalashmi, K.S.; Thyagarajan, D. Studies on sensory evaluation of Curcumin powder as natural color for butterscotch flavor ice cream. Indian J. Drugs Dis. 2012, 1, 2278–2958. [Google Scholar]
- Marinho, M.T.; Zielinski, A.A.F.; Demiate, I.M.; Bersot, L.D.S.; Granato, D.; Nogueira, A. Ripened semihard cheese covered with lard and dehydrated rosemary (Rosmarinus officinalis L.) leaves: Processing, characterization, and quality traits. J. Food Sci. 2015, 80, S2045–S2054. [Google Scholar] [CrossRef]
- Rathod, P.B.; Zinjarde, R.M.; Ingole, A.S.; Meshram, T.A. Utilization of ginger (Zingiber officinale) juice for preparation of flavoured milk. Int. J. Chem. Stud. 2019, 7, 2648–2651. [Google Scholar]
- Hamid, O.I.A.; Abdelrahman, N.A.M. Effect of adding cardamom, cinnamon and fenugreek to goat’s milk curd on the quality of white cheese during storage. Int. J. Dairy Sci. 2012, 7, 43–50. [Google Scholar] [CrossRef]
- Mohamed, E.-F.; Salama, H.H.; El-Sayed, S.M.; Samir, H.; Zahran, H. Utilization of natural antimicrobial and antioxidant of Moringa oleifera leaves extract in manufacture of cream cheese. J. Biol. Sci. 2018, 18, 92–106. [Google Scholar] [CrossRef]
- Paswan, V.K.; Rose, H.; Singh, C.S.; Yamini, S.; Rathaur, A. Herbs and Spices Fortified Functional Dairy Products; IntechOpen: London, UK, 2021; p. 98775. [Google Scholar]
- Singh, N.; Yadav, S.S. A review on health benefits of phenolics derived from dietary spices. Curr. Res. Food Sci. 2022, 5, 1508–1523. [Google Scholar] [CrossRef]
- Mensah, M.A. Polyphenols and Flavonoids Containing Food Spices. In Analysis of Food Spices. Identification and Authentification, 1st ed.; Nollet, L., Ahmad, J., Ahamad, J., Eds.; CRC Press: Boca Raton, FL, USA, 2023. [Google Scholar]
- den Hartog, G.J.M.; Boots, A.W.; Adam-Perrot, A.; Brouns, F.; Verkooijen, I.W.C.M.; Weseler, A.R.; Haenen, G.R.M.M.; Bast, A. Erythritol is a sweet antioxidant. Nutrition 2010, 26, 449–458. [Google Scholar] [CrossRef]
- Soetan, O.A.; Ajao, F.O.; Ajayi, A.F. Blood glucose lowering and anti-oxidant potential of erythritol: An in vitro and in vivo study. J. Diabetes Metab. Disord. 2023, 22, 1217–1229. [Google Scholar] [CrossRef]
- Wang, S.; Meckling, K.A.; Marcone, M.F.; Kakuda, Y.; Tsao, R. Synergistic, additive, and antagonistic effects of food mixtures on total antioxidant capacities. J. Agric. Food Chem. 2011, 59, 960–968. [Google Scholar] [CrossRef]
- Bayram, I.; Decker, E.A. Underlying mechanisms of synergistic antioxidant interactions during lipid oxidation. Trends Food Sci. Technol. 2023, 133, 219–230. [Google Scholar] [CrossRef]
- Rashidinejad, A.; Birch, E.J.; Sun-Waterhouse, D.; Everett, D.W. Addition of milk to tea infusions: Helpful or harmful? Evidence from in vitro and in vivo studies on antioxidant properties. Crit. Rev. Food Sci. Nutr. 2017, 57, 3188–3196. [Google Scholar] [CrossRef]
- Hossain, M.B.; Ahmed, L.; Martin-Diana, A.B.; Brunton, N.P.; Barry-Ryan, C. Individual and combined antioxidant activity of spices and spice phenolics. Antioxidants 2023, 12, 308. [Google Scholar] [CrossRef]
- Feng, J.Y.; Liu, Z.Q. Phenolic and enolic hydroxyl groups in curcumin: Which plays the major role in scavenging radicals? J. Agric. Food Chem. 2009, 57, 11041–11146. [Google Scholar] [CrossRef]
- Olszowy, M.; Dawidowicz, A.L.; Jóźwik-Dolęba, M. Are mutual interactions between antioxidants the only factors responsible for antagonistic antioxidant effect of their mixtures? Additive and antagonistic antioxidant effects in mixtures of gallic, ferulic and caffeic acids. Eur. Food Res. Technol. 2019, 245, 1473–1485. [Google Scholar] [CrossRef]
- Carey, F.A.; Sundberg, R.J. Addition, Condensation and Substitution Reactions of Carbonyl Compounds. In Advanced Organic Chemistry. Part A: Structure and Mechanisms, 5th ed.; Carey, F.A., Sundberg, R.J., Eds.; Springer: Boston, MA, USA, 2007. [Google Scholar]
- Schmittel, M.; Lal, M.; Lal, R.; Röck, M.; Langels, A.; Rappoport, Z.; Basheer, A.; Schlirf, J.; Deiseroth, H.-J.; Flörke, U.; et al. A comprehensive picture of the one-electron oxidation chemistry of enols, enolates and α-carbonyl radicals: Oxidation potentials and characterization of radical intermediates. Tetrahedron 2009, 65, 10842–10855. [Google Scholar] [CrossRef]
- Haro-González, J.N.; Castillo-Herrera, G.A.; Martínez-Velázquez, M.; Espinosa-Andrews, H. Clove essential oil (Syzygium aromaticum L. Myrtaceae): Extraction, chemical composition, food applications, and essential bioactivity for human health. Molecules 2021, 26, 6387. [Google Scholar] [CrossRef]
- Han, R.; Li, X.; Gao, X.; Lv, G. Cinnamaldehyde: Pharmacokinetics, anticancer properties and therapeutic potential (Review). Mol. Med. Rep. 2024, 30, 163. [Google Scholar] [CrossRef]
- Dhakal, S.; Chao, K.; Schmidt, W.; Qin, J.; Kim, M.; Chan, D. Evaluation of turmeric powder adulterated with metanil yellow using FT-Raman and FT-IR spectroscopy. Foods 2016, 5, 36. [Google Scholar] [CrossRef]
- Ayoade, W.G.; Gbadamosi, L.; Ajayi, M.G.; Badmus, M.O. Assessment of minerals and vitamin constituents of some commonly consumed spices. Int. J. Sci. Res. Arch. 2023, 10, 53–61. [Google Scholar] [CrossRef]
- Cimmino, F.; Catapano, A.; Petrella, L.; Villano, I.; Tudisco, R.; Cavaliere, G. Role of milk micronutrients in human health. Front. Biosci. 2023, 28, 41. [Google Scholar] [CrossRef]
- Ashique, S.; Kumar, S.; Hussain, A.; Mishra, N.; Garg, A.; Gowda, B.H.J.; Farid, A.; Gupta, G.; Dua, K.; Taghizadeh-Hesary, F. Correction: A narrative review on the role of magnesium in immune regulation, inflammation, infectious diseases, and cancer. J. Health Popul. Nutr. 2023, 42, 117, Erratum in J. Health Popul. Nutr. 2023, 42, 74. [Google Scholar] [CrossRef]
- Ragaee, S.; Abdel-Aal, E.S.M.; Noaman, M. Antioxidant activity and nutrient composition of selected cereals for food use. Food Chem. 2006, 98, 32–38. [Google Scholar] [CrossRef]
- Ciscomani-Larios, J.P.; Sánchez-Chávez, E.; Jacobo-Cuellar, J.L.; Sáenz-Hidalgo, H.K.; Orduño-Cruz, N.; Cruz-Alvarez, O.; Ávila-Quezada, G.D. Biofortification efficiency with magnesium salts on the increase of bioactive compounds and antioxidant capacity in snap beans. Ciênc. Rural. 2021, 51, e20200442. [Google Scholar] [CrossRef]
- Szentmihályi, K.; Szilágyi, M.; Balla, J.; Ujhelyi, L.; Blázovics, A. In vitro antioxidant activities of magnesium compounds used in food industry. Acta Aliment. 2014, 43, 419–425. [Google Scholar] [CrossRef]
- Zago, M.P.; Oteiza, P.I. The antioxidant properties of zinc: Interactions with iron and antioxidants. Free Radic. Biol. Med. 2001, 31, 266–274. [Google Scholar] [CrossRef] [PubMed]
- Vieira, P.A.F.; Gontijo, D.C.; Vieira, B.C.; Fontes, E.A.; de Assunção, L.S.; Leite, J.P.V.; de A. Oliveira, M.G.; Kasuya, M.C.M. Antioxidant activities, total phenolics and metal contents in Pleurotus ostreatus mushrooms enriched with iron, zinc or lithium. LWT-Food Sci. Technol. 2013, 54, 421–425. [Google Scholar] [CrossRef]
- Rumpf, J.; Burger, R.; Schulze, M. Statistical evaluation of DPPH, ABTS, FRAP, and Folin-Ciocalteu assays to assess the antioxidant capacity of lignins. Int. J. Biol. Macromol. 2023, 233, 123470. [Google Scholar] [CrossRef]
- Kiss, A.; Papp, V.A.; Pál, A.; Prokisch, J.; Mirani, S.; Toth, B.E.; Alshaal, T. Comparative study on antioxidant capacity of diverse food matrices: Applicability, suitability and inter-correlation of multiple assays to assess polyphenol and antioxidant status. Antioxidants 2025, 14, 317. [Google Scholar] [CrossRef]
- USDA. Food Data Central. Available online: https://fdc.nal.usda.gov (accessed on 8 August 2025).
- MyFoodData. Nutrition Facts for All-Natural Erythritol. Available online: https://tools.myfooddata.com/nutrition-facts/ (accessed on 8 August 2025).
- Neveu, V.; Perez-Jiménez, J.; Vos, F.; Crespy, V.; du Chaffaut, L.; Mennen, L.; Knox, C.; Eisner, R.; Cruz, J.; Wishart, D.; et al. Phenol-Explorer: An online comprehensive database on polyphenol contents in foods. Database 2010, 2010, bap024. [Google Scholar] [CrossRef]
- Sowa, P.; Tarapatskyy, M.; Puchalski, C.; Dżugan, M. Quality evaluation of cinnamon marketed in Poland on the basis of determining ratio of cinnamaldehyde-to-coumarin content. Food Sci. Technol. Qual. 2019, 26, 113–125. [Google Scholar] [CrossRef]
- Rajbhar, K.; Dawda, H.; Makundan, U. Polyphenols: Methods of extraction. Sci. Revs. Chem. Commun. 2015, 5, 1–6. [Google Scholar]
- Antony, A.; Farid, M. Effect of temperatures on polyphenols during extraction. Appl. Sci. 2022, 12, 2107. [Google Scholar] [CrossRef]
- Idowu-Adebayo, F.; Fogliano, V.; Linnemann, A. Turmeric-fortified cow and soya milk: Golden milk as a street food to support consumer health. Foods 2022, 11, 558. [Google Scholar] [CrossRef]
- 10 Benefits of Golden (Turmeric) Milk and How to Make It. Available online: https://foolproofliving.com/turmeric-golden-milk/ (accessed on 7 August 2025).
- Oszmiański, J.; Kolniak-Ostek, J.; Lachowicz, S.; Gorzelany, J.; Matłok, N. Phytochemical compounds and antioxidant activity in different cultivars of cranberry (Vaccinium Macrocarpon L). J. Food Sci. 2017, 82, 2569–2575. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Yen, G.C.; Chen, H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995, 43, 27–32. [Google Scholar] [CrossRef]
- Gao, X.; Ohlander, M.; Jeppsson, N.; Björk, L.; Trajkovski, V. Changes in antioxidant effects and their relationship to phytonutrients in fruits of Sea Buckthorn (Hippophae rhamnoides L.) during maturation. J. Agric. Food Chem. 2000, 48, 1485–1490. [Google Scholar] [CrossRef]
- Huang, L.; Bell, R.W.; Dell, B.; Woodward, J. Rapid nitric acid digestion of plant material with an open-vessel microwave system. Commun. Soil Sci. Plant Anal. 2004, 35, 427–440. [Google Scholar] [CrossRef]
- European Standard Commitee. Food Products—Determination of Trace Elements—Pressure Mineralization; European Standard Committee: Brussels, Belgium, 2014. [Google Scholar]
- SS-EN 15505:2008; Swedish Institute for Standards. Foodstuffs–Determination of Trace elements—Determination of Sodium and Magnesium By Flame Atomic Absorption Spectrometry (AAS) After Microwave Digestion. Swedish Institute for Standards: Stockholm, Sweden, 2008. Available online: https://www.sis.se/en/produkter/food-technology/general-methods-of-tests-and-analysis-for-food-products/ssen155052008/ (accessed on 5 September 2025).
- SS-EN 14082:2003; Swedish Institute for Standards. Foodstuffs—Determination of Trace Elements—Determination of Lead, Cadmium, Zinc, Copper, Iron and Chromium By Atomic Absorption Spectrometry (AAS) After Dry Ashing. Swedish Institute for Standards: Stockholm, Sweden, 2003. Available online: https://trust.sis.se/en/produkter/food-technology/general-methods-of-tests-and-analysis-for-food-products/ssen14082/ (accessed on 5 September 2025).
- Stanisz, A. The Accessible Course of Statistics with Use the STATISTICA PL for Medicine Examples. Basic Statistics; StatSoft Polska: Kraków, Poland, 2006; Volume 1. [Google Scholar]





| SS | df | MS | F | p | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ABTS | FRAP | DPPH | ABTS | FRAP | DPPH | ABTS | FRAP | DPPH | ABTS | FRAP | DPPH | ||
| Spice mixture | 925,913.45 | 1,098,966.53 | 296,869.05 | 2 | 462,956.72 | 549,483.26 | 148,434.52 | 6978.81 | 8002.66 | 1630.08 | <0.000 | <0.000 | <0.000 |
| Share of spice mixture [%] | 1,453,763.15 | 553,273.34 | 155,128.85 | 3 | 484,587.71 | 184,424.44 | 51,709.61 | 7304.88 | 2685.95 | 567.86 | <0.000 | <0.000 | <0.000 |
| Sweetener | 2014.93 | 2413.71 | 3899.39 | 1 | 2014.93 | 2413.71 | 3899.39 | 30.37 | 35.15 | 42.82 | <0.000 | <0.000 | <0.000 |
| Spice mixture * share of spice mixture [%] | 213,038.15 | 166,834.52 | 31,892.37 | 6 | 35,506.35 | 27,805.75 | 5315.39 | 535.23 | 404.96 | 58.37 | <0.000 | <0.000 | <0.000 |
| Spice mixture * sweetener | 186.96 | 2058.26 | 1485.58 | 2 | 93.48 | 1029.13 | 742.79 | 1.40 | 14.98 | 8.15 | 0.251 | <0.000 | <0.001 |
| Share of spice mixture [%] * sweetener | 182.49 | 1529.49 | 813.76 | 3 | 60.83 | 509.83 | 271.25 | 0.916 | 7.42 | 2.97 | 0.437 | <0.001 | 0.037 |
| Spice mixture * share of spice mixture [%] * sweetener | 1851.95 | 1803.66 | 1344.61 | 6 | 308.65 | 300.61 | 224.10 | 4.65 | 4.37 | 2.46 | <0.001 | <0.001 | 0.032 |
| Mean ± SD | p | |||
|---|---|---|---|---|
| ABTS [µM Tx/100 g] | FRAP [µM Tx/100 g] | DPPH [µM Tx/100 g] | ||
| Spice mixture | ||||
| Cl-Ci | 301.11 ± 159.05 a | 261.15 ± 109.90 a | 137.72 ± 52.96 a | a,b <0.000 |
| Cl-Tu | 305.35 ± 159.13 b | 258.00 ± 106.01 b | 141.32 ± 57.64 b | |
| Ci-Tu | 94.93 ± 58.63 ab | 32.63 ± 18.06 ab | 21.59 ± 18.98 ab | |
| Share of spice mixture [%] | ||||
| 2.5 | 111.16 ± 50.17 abc | 79.87 ± 49.96 abc | 47.26 ± 30.68 abc | a–f <0.000 |
| 5.0 | 176.70 ± 85.44 ade | 153.00 ± 95.37 ade | 83.31 ± 52.01 ade | |
| 7.5 | 209.38 ± 89.93 bdf | 218.56 ± 128.72 bdf | 113.51 ± 69.29 bdf | |
| 10.0 | 437.94 ± 178.91 cef | 284.27 ± 165.61 cef | 156.76 ± 80.37 cef | |
| Sweetener | ||||
| E | 238.37 ± 164.85 | 188.94 ± 144.20 | 106.58 ± 76.98 | <0.000 |
| S | 229.21 ± 167.75 | 178.91 ± 134.64 | 93.83 ± 67.72 | |
| SS | df | MS | F | p | |
|---|---|---|---|---|---|
| Spice mixture | 12,823.25 | 2 | 6411.62 | 573.76 | <0.000 |
| Share of spice mixture [%] | 10,784.40 | 3 | 3594.80 | 321.69 | <0.000 |
| Sweetener | 2312.73 | 1 | 2312.73 | 206.96 | <0.000 |
| Spice mixture * share of spice mixture [%] | 3305.92 | 6 | 550.98 | 49.30 | <0.000 |
| Spice mixture * sweetener | 691.53 | 2 | 345.76 | 30.94 | <0.000 |
| Share of spice mixture [%] * sweetener | 426.79 | 3 | 142.26 | 12.73 | <0.000 |
| Spice mixture*share of spice mixture [%] * sweetener * | 144.08 | 6 | 24.01 | 2.14 | 0.058 |
| TPC [mg GAE/100 g] Mean ± SD | ||||||||
|---|---|---|---|---|---|---|---|---|
| Spice Mixture | Share of Spice Mixture [%] | Sweetener | ||||||
| Cl-Ci | Cl-Tu | Ci-Tu | 2.5 | 5 | 7.5 | 10 | E | S |
| 27.28 ± 15.75 ab | 29.49 ± 18.24 bc | 3.94 ± 3.87 ac | 5.99 ± 4.88 abc | 15.93 ± 11.05 ade | 24.22 ± 16.80 bdf | 34.80 ± 21.52 cef | 25.15 ± 20.49 a | 15.32 ± 14.04 a |
| SS | df | MS | F | p | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ca [mg/100 g] | Mg [mg/100 g] | Fe [µg/100 g] | Zn [µg/100 g] | Cu [µg/100 g] | Ca [mg/100 g] | Mg [mg/100 g] | Fe [µg/100 g] | Zn [µg/100 g] | Cu [µg/100 g] | Ca [mg/100 g] | Mg [mg/100 g] | Fe [µg/100 g] | Zn [µg/100 g] | Cu [µg/100 g] | |||
| Spice mixture | 1275.2 | 239.7 | 310,681.7 | 14,426.6 | 25,654.9 | 2 | 637.6 | 119.8 | 155,340.8 | 7213.3 | 12,827.4 | 175.1 | 19,509.7 | 3326.3 | 505.0 | 2627.2 | <0.000 |
| Share of spice mixture [%] | 272.2 | 1678.5 | 154,226.0 | 23,328.6 | 11,328.3 | 3 | 90.7 | 559.5 | 51,408.7 | 7776.2 | 3776.1 | 24.9 | 91,095.2 | 1100.8 | 544.4 | 773.4 | <0.000 |
| Sweetener | 1524.9 | 106.4 | 9986.9 | 430.2 | 38,789.6 | 1 | 1524.9 | 106.4 | 9986.9 | 430.2 | 38,789.6 | 418.8 | 17,327.3 | 213.8 | 30.1 | 7944.4 | <0.000 |
| Spice mixture * share of spice mixture [%] | 180.7 | 61.4 | 98,116.4 | 12,900.9 | 5127.9 | 6 | 30.1 | 10.2 | 16,352.7 | 2150.1 | 854.7 | 8.3 | 1666.1 | 350.2 | 150.5 | 175.0 | <0.000 |
| Spice mixture * sweetener | 501.6 | 48.8 | 141,804.0 | 19,319.8 | 2243.6 | 2 | 250.8 | 24.4 | 70,902.0 | 9659.9 | 1121.8 | 68.9 | 3968.6 | 1518.2 | 676.3 | 229.8 | <0.000 |
| Share of spice mixture [%] * sweetener | 243.8 | 4.6 | 47,417.7 | 9332.8 | 2452.4 | 3 | 81.3 | 1.5 | 15,805.9 | 3110.9 | 817.5 | 22.3 | 250.9 | 338.4 | 217.8 | 167.4 | <0.000 |
| Spice mixture * share of spice mixture [%] * sweetener | 268.8 | 21.7 | 86,279.4 | 18,506.1 | 6819.7 | 6 | 44.8 | 3.6 | 14,379.9 | 3084.4 | 1136.6 | 12.3 | 588.0 | 307.9 | 215.9 | 232.8 | <0.000 |
| Mean ± SD | p | |||||
|---|---|---|---|---|---|---|
| Ca [mg/100 g] | Mg [mg/100 g] | Fe [µg/100 g] | Zn [µg/100 g] | Cu [µg/100 g] | ||
| Spice mixture | ||||||
| Cl-Ci | 102.85 ± 7.53 ab | 18.90 ± 3.39 ab | 784.63 ± 85.28 ab | 257.56 ± 43.01 ab | 79.87 ± 30.05 ab | a–c 0.000 |
| Cl-Tu | 105.77 ± 4.49 ac | 22.73 ± 5.46 ac | 743.49 ± 64.89 ac | 285.54 ± 15.73 ac | 57.08 ± 22.45 ac | |
| Ci-Tu | 111.61 ± 5.30 bc | 20.32 ± 4.55 bc | 648.76 ± 77.30 bc | 280.99 ± 25.30 bc | 39.96 ± 27.53 bc | |
| Share of spice mixture [%] | ||||||
| 2.5 | 109.37 ± 5.96 abc | 14.92 ± 1.34 abc | 696.99 ± 63.59 ab | 257.08 ± 21.17 abc | 48.29 ± 28.57 abc | a–f 0.000 [Ca: d 0.028] [Ca: e 0.004] |
| 5.0 | 106.27 ± 7.99 ad | 19.04 ± 1.98 ade | 696.98 ± 86.09 cd | 266.02 ± 11.32 ade | 52.53 ± 24.61 ade | |
| 7.5 | 106.63 ± 6.28 be | 22.36 ± 2.53 bdf | 714.63 ± 86.81 ace | 276.86 ± 33.87 bdf | 58.24 ± 27.99 bdf | |
| 10.0 | 104.70 ± 6.80 cde | 26.27 ± 2.98 cef | 793.92 ± 106.50 bde | 298.82 ± 39.72 cef | 76.75 ± 36.55 cef | |
| Sweetener | ||||||
| E | 102.76 ± 6.62 a | 19.60 ± 4.33 a | 735.83 ± 62.39 a | 272.58 ± 31.82 a | 79.07 ± 14.66 a | a 0.000 |
| S | 110.73 ± 4.48 a | 21.70 ± 5.00 a | 715.43 ± 118.40 a | 276.81 ± 33.03 a | 38.87 ± 30.56 a | |
| ABTS [µM Tx/100 g] | FRAP [µM Tx/100 g] | DPPH [µM Tx/100 g] | ||||
|---|---|---|---|---|---|---|
| r | p | r | p | r | p | |
| Ca [mg/100 g] | −0.471 | 0.0000 | −0.539 | 0.0000 | −0.580 | 0.0000 |
| Mg [mg/100 g] | 0.619 | 0.0000 | 0.500 | 0.0000 | 0.512 | 0.0000 |
| Fe [µg/100 g] | 0.665 | 0.0000 | 0.654 | 0.0000 | 0.643 | 0.0000 |
| Zn [µg/100 g] | 0.300 | 0.003 | 0.143 | 0.165 | 0.117 | 0.257 |
| Cu [µg/100 g] | 0.568 | 0.0000 | 0.564 | 0.0000 | 0.566 | 0.0000 |
| Nutrients | Semi-Skimmed (2% Fat) Cow’s Milk | Ground Clove | Ground Cinnamon | Ground Turmeric | Sweetener, Stevia (Glycosides) | Sweetener, Erythritol | Ref |
|---|---|---|---|---|---|---|---|
| Energy [kcal] | 50 | 274 | 247 | 312 | 0 | 0 | [83] 1 [84] 2 |
| Macronutrients | |||||||
| Protein [g] | 3.3 | 6.0 | 4.0 | 9.7 | 0 | 0 | |
| Fat [g] | 2.0 | 13.0 | 1.2 | 3.3 | 0 | 0 | |
| Carbohydrates [g] | 4.8 | 65.5 | 80.6 | 67.1 | 100 | 0 | |
| Mineral elements | |||||||
| Calcium [mg] | 120 | 632 | 1000 | 168 | 0 | nd | |
| Magnesium [mg] | 11 | 259 | 60 | 208 | 0 | nd | |
| Iron [µg] | 20 | 11,800 | 8320 | 55,000 | 0 | nd | |
| Zinc [µg] | 480 | 2320 | 1830 | 4500 | 0 | nd | |
| Copper [µg] | 6 | 368 | 339 | 1300 | 0 | nd | |
| Phenolic compounds | [85] [86] 4 | ||||||
| TPC [mg GAE] | 0 3 | 16,047.50 | 9700.00 | 2117.00 | 0 3 | 0 3 | |
| Phenolic acids [mg] | |||||||
| Hydroxybenzoic acids | |||||||
| Gallic acid | 458.19 | ||||||
| 2-hydroxybenzoic acid | 0.70 | ||||||
| Protocatechuic acid | 0.52 | 1.16 | |||||
| Syringic acid | 0.79 | 0.78 | |||||
| Hydroxycinnamic acids | |||||||
| Caffeic acid | 24.20 | ||||||
| p-Coumaric acid | 8.49 | 0.55 | |||||
| Flavonoids [mg] | |||||||
| Flavonols | |||||||
| Kaempferol | 23.80 | ||||||
| Quercetin | 28.40 | ||||||
| Curcuminoids [mg] | |||||||
| Bisdemethoxycurcumin | 1237.50 | ||||||
| Curcumin | 2213.57 | ||||||
| Demethoxycurcumin | 1982.50 | ||||||
| Other polyphenols [mg] | |||||||
| Acetyl eugenol | 2075.10 | ||||||
| Eugenol | 12,592.93 | ||||||
| Cinnamaldehyde | 749 4 |
| Parameters | Ca | Mg | Fe | Zn | Cu |
|---|---|---|---|---|---|
| Wavelength [nm] | 285.2 | 422.7 | 372.0 | 213.9 | 327.4 |
| Gap [mm] | 0.5 | 0.5 | 0.2 | 1.0 | 0.2 |
| Burner height [cm] | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Acetylene/oxygen flows [l/h] | 70/60 | 70/60 | 70/60 | 70/60 | 70/60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rak, K.; Kolniak-Ostek, J.; Gajda, R.; Marcinkiewicz, K.; Nemś, A.; Raczkowska, E. Antioxidant Activity, Total Polyphenol Content, and Mineral Composition of Milk Beverages Fortified with Spice Mixtures (Clove, Cinnamon, and Turmeric) and Natural Sweeteners (Erythritol and Stevia): Evidence of Synergistic or Antagonistic Effects of Compounds. Int. J. Mol. Sci. 2025, 26, 8813. https://doi.org/10.3390/ijms26188813
Rak K, Kolniak-Ostek J, Gajda R, Marcinkiewicz K, Nemś A, Raczkowska E. Antioxidant Activity, Total Polyphenol Content, and Mineral Composition of Milk Beverages Fortified with Spice Mixtures (Clove, Cinnamon, and Turmeric) and Natural Sweeteners (Erythritol and Stevia): Evidence of Synergistic or Antagonistic Effects of Compounds. International Journal of Molecular Sciences. 2025; 26(18):8813. https://doi.org/10.3390/ijms26188813
Chicago/Turabian StyleRak, Karolina, Joanna Kolniak-Ostek, Robert Gajda, Katarzyna Marcinkiewicz, Agnieszka Nemś, and Ewa Raczkowska. 2025. "Antioxidant Activity, Total Polyphenol Content, and Mineral Composition of Milk Beverages Fortified with Spice Mixtures (Clove, Cinnamon, and Turmeric) and Natural Sweeteners (Erythritol and Stevia): Evidence of Synergistic or Antagonistic Effects of Compounds" International Journal of Molecular Sciences 26, no. 18: 8813. https://doi.org/10.3390/ijms26188813
APA StyleRak, K., Kolniak-Ostek, J., Gajda, R., Marcinkiewicz, K., Nemś, A., & Raczkowska, E. (2025). Antioxidant Activity, Total Polyphenol Content, and Mineral Composition of Milk Beverages Fortified with Spice Mixtures (Clove, Cinnamon, and Turmeric) and Natural Sweeteners (Erythritol and Stevia): Evidence of Synergistic or Antagonistic Effects of Compounds. International Journal of Molecular Sciences, 26(18), 8813. https://doi.org/10.3390/ijms26188813







