Neurotransmitter Genes in the Nucleus Accumbens That Are Involved in the Development of a Behavioral Pathology After Positive Fighting Experiences and Their Deprivation: A Conceptual Paradigm for Data Analysis
Abstract
1. Introduction
2. Results
2.1. The Winners’ Behavior During Agonistic Interactions
2.2. Detecting DEGs in Pairwise Comparisons
2.3. DEGs of Neurotransmitter Systems
2.3.1. CAergic Genes
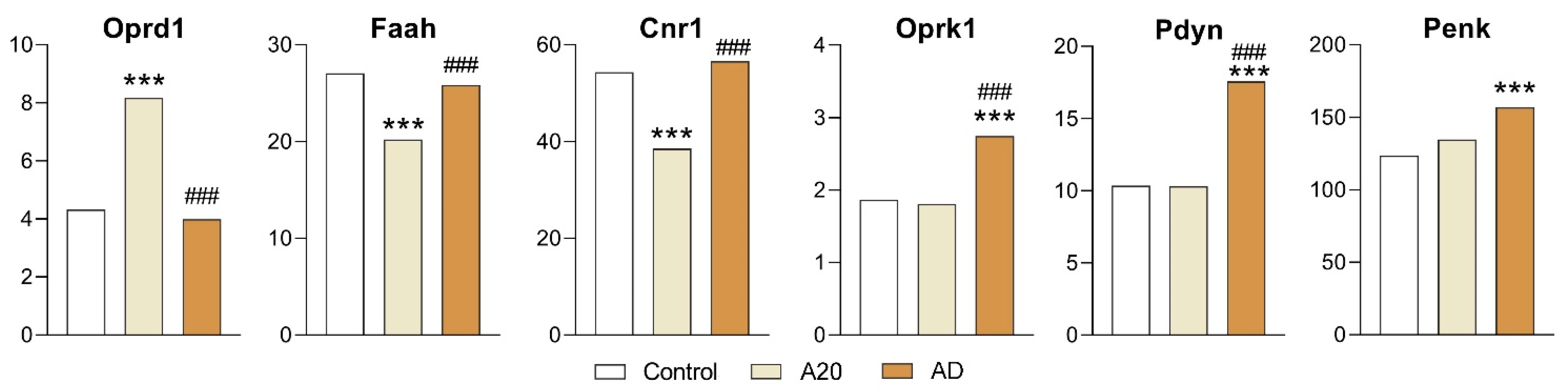
2.3.2. Opioidergic and Cannabinoidergic Genes
2.3.3. Serotonergic Genes
2.3.4. GABAergic Genes
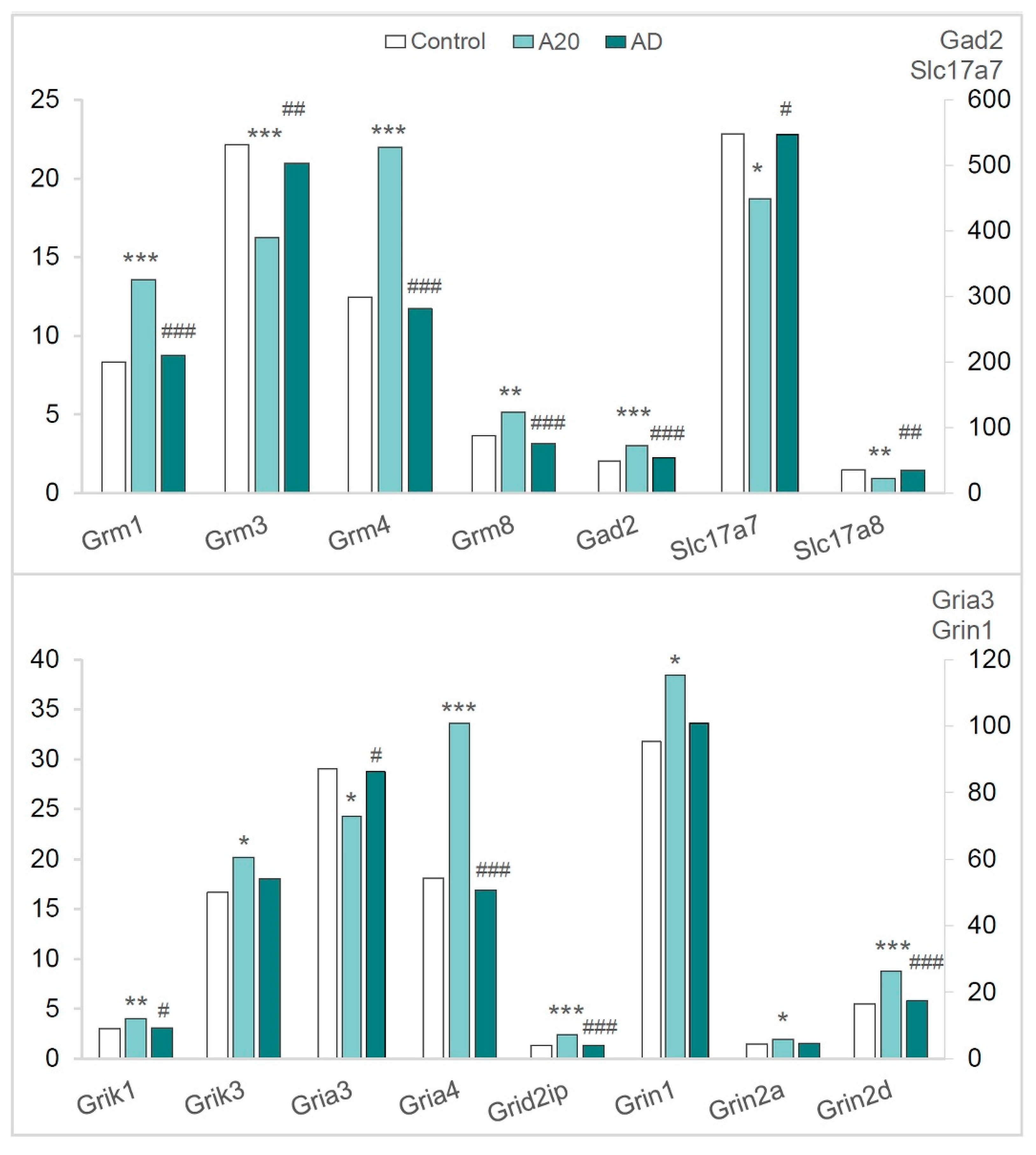
2.3.5. The Glutamatergic System
2.4. DEGs Associated with the Observed Pathology
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Implementation of the Repeated Aggressive Experience in Male Mice
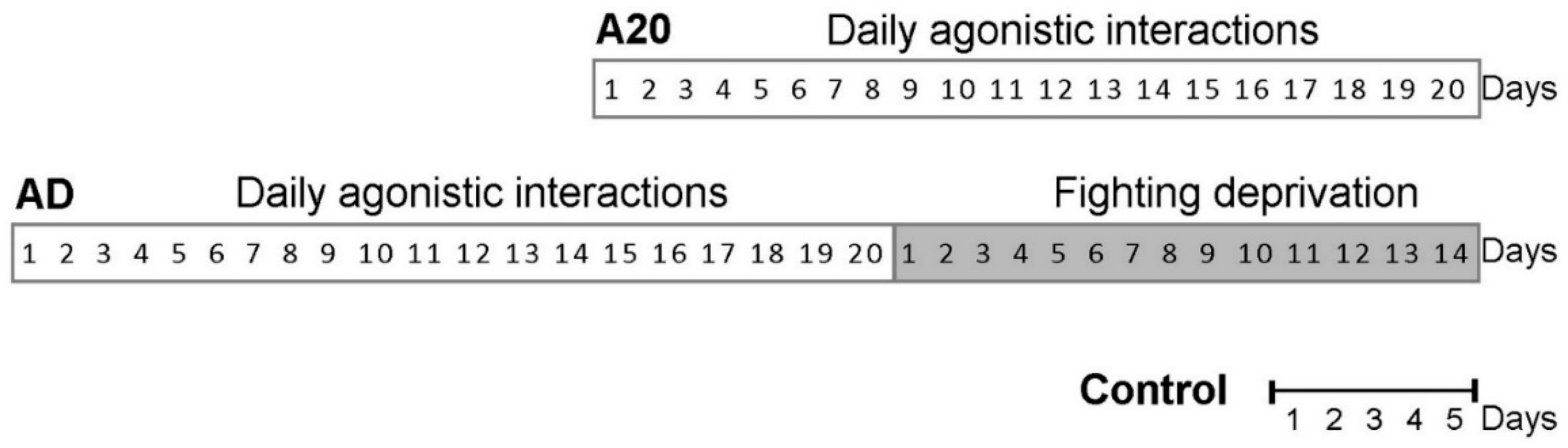
4.3. Experimental Scheme
4.4. Behavioral Tests for Aggressive Motivation and Hostile Behavior
4.5. The NAcc in the Control of Aggressive Behavior
4.6. Statistical Analysis
4.7. RNA-Seq Data Analysis and Processing
4.8. The Genes That Were Analyzed in the NAcc Before and After the Deprivation Period
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| C | control |
| A20 | groups of intermittently daily aggressive mice during 20 days |
| AD | A20 mice after 14 days of deprivation (no-fight period without agonistic interactions) |
| 5-HT | serotonin |
| 5-HIAA | 5-hydroxyindolacetic acid |
| DA | dopamine |
| DOPAC | 3,4-dihydroxyphenyleacetic acid |
| DEGs | differentially expressed genes |
| FPKM | fragments per kilobase of transcript per million mapped reads |
| CAergic | catecholaminergic |
| GABA | γ-aminobutyric acid |
| MRN | midbrain raphe nuclei |
| MSNs | medium spiny neurons |
| NAcc | nucleus accumbens |
| STR | dorsal striatum |
| VTA | ventral tegmental area |
References
- Scott, J.P. Agonistic behavior of mice and rats: A review. Am. Zool. 1966, 6, 683–701. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.P. Theoretical issues concerning the origin and causes of fighting. In The Physiology of Aggression and Defeat; Eleftheriou, B.E., Scott, J.P., Eds.; Plenum New-York: New York, NY, USA, 1971; pp. 11–42. [Google Scholar]
- Brain, P.F. The adaptiveness of house mouse aggression. In House Mouse Aggression: A Model for Understanding the Evolution of Social Behavior; Brain, P.F., Mainardi, D., Parmigiani, S., Eds.; Harwood Academic Publishers: Chur, Switzerland, 1979; pp. 1–21. [Google Scholar]
- Parmigiani, S.; Brain, P.F. Effects of residence, aggressive experience and intruder familiarity on attack shown by male mice. Behav. Proc. 1983, 8, 45–47. [Google Scholar] [CrossRef]
- Andrade, M.L.; Kamal, K.B.H.; Brain, P.F. Effects of positive and negative fighting experience on behaviour in adult male mice. In House Mouse Aggression: A Model for Understanding the Evolution of Social Behavior; Brain, P.F., Mainardi, D., Parmigiani, S., Eds.; Harwood Academic Publishers GmbH: Chur, Switzerland, 1987; pp. 223–232. [Google Scholar]
- Benton, D.; Brain, P.F. The role of opioid mechanisms in social interaction and attachment. In Endorphins, Opiates and Behavioural Processes; Rodgers, R.J., Cooper, S.J., Eds.; John Wiley and Sons Ltd.: London, UK, 1988; pp. 217–235. [Google Scholar]
- Brain, P.F.; Kamal, K.B.H. Effects of prior social experience on individual aggressiveness in laboratory rodents. Rass. Psicol. 1989, 6, 37–43. [Google Scholar]
- Caramaschi, D.; de Boer, S.F.; de Vries, H.; Koolhaas, J.M. Development of violence in mice through repeated victory along with changes in prefrontal cortex neurochemistry. Behav. Brain Res. 2008, 189, 263–272. [Google Scholar] [CrossRef]
- Coppens, C.M.; de Boer, S.F.; Buwalda, B.; Koolhaas, J.M. Aggression and aspects of impulsivity in wild-type rats. Aggress. Behav. 2014, 40, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, N.N. The sensory contact model for the study of aggressive and submissive behaviors in male mice. Aggress. Behav. 1991, 17, 285–291. [Google Scholar] [CrossRef]
- Kudryavtseva, N.N. An experimental approach to the study of learned aggression. Aggress. Behav. 2000, 26, 241–256. [Google Scholar] [CrossRef]
- Kudryavtseva, N.N. Psychopathology of repeated aggression: A neurobiological aspect. In Perspectives on the Psychology of Aggression; Morgan, J.P., Ed.; NOVA Science Publishers, Inc.: New York, NY, USA, 2006; pp. 35–64. [Google Scholar]
- Kudryavtseva, N.N. Positive fighting experience, addiction-like state, and relapse: Retrospective analysis of experimental studies. Aggress. Viol. Behav. 2020, 52, 101403. [Google Scholar] [CrossRef]
- Smagin, D.A.; Boyarskikh, U.A.; Bondar, N.P.; Filipenko, M.L.; Kudryavtseva, N.N. Reduction of serotonergic gene expression in the midbrain raphe nuclei under positive fighting experience. Adv. Biosci. Biotechnol. 2013, 4, 36–44. [Google Scholar] [CrossRef]
- Smagin, D.A.; Park, J.-H.; Michurina, T.V.; Peunova, N.; Glass, Z.; Sayed, K.; Enikolopov, G. Altered hippocampal neurogenesis and amygdalar neuronal activity in adult mice with repeated experience of aggression. Front. Neurosci. 2015, 9, 443. [Google Scholar] [CrossRef]
- Smagin, D.A.; Galyamina, A.G.; Kovalenko, I.L.; Kudryavtseva, N.N. Altered expression of genes associated with major neurotransmitter systems in the reward-related brain regions of mice with positive fighting experience. Int. J. Mol. Sci. 2022, 23, 13644. [Google Scholar] [CrossRef]
- Kudryavtseva, N.N.; Smagin, D.A.; Kovalenko, I.L.; Vishnivetskaya, G.B. Repeated positive fighting experience in male inbred mice. Nat. Protoc. 2014, 9, 2705–2717. [Google Scholar] [CrossRef]
- Kudryavtseva, N.N.; Kovalenko, I.L.; Smagin, D.A.; Galyamina, A.G.; Babenko, V.N. Abnormal social behaviors and dysfunction of autism-related genes associated with daily agonistic interactions in mice. In Molecular-Genetic and Statistical Techniques for Behavioral and Neural Research; Gerlai, R.T., Ed.; Academic Press: San Diego, CA, USA, 2018; pp. 309–344. [Google Scholar]
- Babenko, V.N.; Smagin, D.A.; Kovalenko, I.L.; Galyamina, A.G.; Kudryavtseva, N.N. Differentially expressed genes of the Slc6a family as markers of altered brain neurotransmitter system function in pathological states in mice. Neurosci. Behav. Physiol. 2020, 50, 199–209. [Google Scholar] [CrossRef]
- Babenko, V.; Redina, O.; Smagin, D.; Kovalenko, I.; Galyamina, A.; Babenko, R.; Kudryavtseva, N. Dorsal striatum transcriptome profile profound shift in repeated aggression mouse model converged to networks of 12 transcription factors after fighting deprivation. Genes 2021, 13, 21. [Google Scholar] [CrossRef]
- Babenko, V.; Redina, O.; Smagin, D.; Kovalenko, I.; Galyamina, A.; Kudryavtseva, N. Elucidation of the landscape of alternatively spliced genes and features in the dorsal striatum of aggressive/aggression-deprived mice in the model of chronic social conflicts. Genes 2023, 14, 599. [Google Scholar] [CrossRef]
- Redina, O.; Babenko, V.; Smagin, D.; Kovalenko, I.; Galyamina, A.; Efimov, V.; Kudryavtseva, N. Gene expression changes in the ventral tegmental area of male mice with alternative social behavior experience in chronic agonistic interactions. Int. J. Mol. Sci. 2020, 21, 6599. [Google Scholar] [CrossRef] [PubMed]
- Redina, O.E.; Babenko, V.N.; Smagin, D.A.; Kovalenko, I.L.; Galyamina, A.G.; Kudryavtseva, N.N. Correlation of expression changes between genes controlling 5-HT synthesis and genes Crh and Trh in the midbrain raphe nuclei of chronically aggressive and defeated male mice. Genes 2021, 12, 1811. [Google Scholar] [CrossRef] [PubMed]
- Redina, O.E.; Babenko, V.N.; Smagin, D.A.; Kovalenko, I.L.; Galyamina, A.G.; Efimov, V.M.; Kudryavtseva, N.N. Effects of positive fighting experience and its subsequent deprivation on the expression profile of mouse hippocampal genes associated with neurogenesis. Int. J. Mol. Sci. 2023, 24, 3040. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, N.N. Straub tail, the deprivation effect and addiction to aggression. In Motivation of Health Behavior; O’Neal, P.W., Ed.; NOVA Science Publishers: New York, NY, USA, 2007; pp. 97–110. [Google Scholar]
- Kudryavtseva, N.N.; Smagin, D.A.; Bondar, N.P. Modeling fighting deprivation effect in mouse repeated aggression paradigm. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1472–1478. [Google Scholar] [CrossRef]
- Ibrahim, M.K.; Hassanein, N.M.A.; Ahmed, H.M.S. Psychopharmacological assessment of the sensory contact model as a possible model of mania. J. Glob. Biosci. 2016, 5, 3725–3741. [Google Scholar]
- Miczek, K.A.; Faccidomo, S.P.; Fish, E.W.; DeBold, J.F. Neurochemistry and molecular neurobiology of aggressive behavior. In Handbook of Neurochemistry and Molecular Neurobiology: Behavioral Neurochemistry, Neuroendocrinology and Molecular Neurobiology; Lajtha, A., Blaustein, J.D., Eds.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 285–336. [Google Scholar]
- Bazov, I.; Sarkisyan, D.; Kononenko, O.; Watanabe, H.; Yakovleva, T.; Hansson, A.C.; Sommer, W.H.; Spanagel, R.; Bakalkin, G. Dynorphin and kappa-opioid receptor dysregulation in the dopaminergic reward system of human alcoholics. Mol. Neurobiol. 2018, 55, 7049–7061. [Google Scholar] [CrossRef]
- Covington, H.E., 3rd; Newman, E.L.; Leonard, M.Z.; Miczek, K.A. Translational models of adaptive and excessive fighting: An emerging role for neural circuits in pathological aggression. F1000Research 2019, 8, 963. [Google Scholar] [CrossRef] [PubMed]
- Haller, J. Studies into abnormal aggression in humans and rodents: Methodological and translational aspects. Neurosci. Biobehav. Rev. 2017, 76, 77–86. [Google Scholar] [CrossRef] [PubMed]
- Moeller, S.J.; London, E.D.; Northoff, G. Neuroimaging markers of glutamatergic and GABAergic systems in drug addiction: Relationships to resting-state functional connectivity. Neurosci. Biobehav. Rev. 2016, 61, 35–52. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Arias, M.; Navarrete, F.; Daza-Losada, M.; Navarro, D.; Aguilar, M.A.; Berbel, P.; Minarro, J.; Manzanares, J. CB1 cannabinoid receptor-mediated aggressive behavior. Neuropharmacology 2013, 75, 172–180. [Google Scholar] [CrossRef]
- Filipenko, M.L.; Alekseyenko, O.V.; Beilina, A.G.; Kamynina, T.P.; Kudryavtseva, N.N. Increase of tyrosine hydroxylase and dopamine transporter mRNA levels in ventral tegmental area of male mice under influence of repeated aggression experience. Brain Res. Mol. Brain Res. 2001, 96, 77–81. [Google Scholar] [CrossRef]
- Flanigan, M.E.; Russo, S.J. Recent advances in the study of aggression. Neuropsychopharmacology 2019, 44, 241–244. [Google Scholar] [CrossRef]
- Aleyasin, H.; Flanigan, M.E.; Russo, S.J. Neurocircuitry of aggression and aggression seeking behavior: Nose poking into brain circuitry controlling aggression. Curr. Opin. Neurobiol. 2018, 49, 184–191. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Lin, D. Functions of medial hypothalamic and mesolimbic dopamine circuitries in aggression. Curr. Opinion Behav. Sci. 2018, 24, 104–112. [Google Scholar] [CrossRef]
- Van Erp, A.M.; Miczek, K.A. Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. J. Neurosci. 2000, 20, 9320–9325. [Google Scholar] [CrossRef]
- Van Erp, A.M.M.; Miczek, K.A. Increased accumbal dopamine during daily alcohol consumption and subsequent aggressive behavior in rats. Psychopharmacology 2007, 191, 679–688. [Google Scholar] [CrossRef]
- Couppis, M.H.; Kennedy, C.H. The rewarding effect of aggression is reduced by nucleus accumbens dopamine receptor antagonism in mice. Psychopharmacology 2008, 197, 449–456. [Google Scholar] [CrossRef]
- Golden, S.A.; Heshmati, M.; Flanigan, M.; Christoffel, D.J.; Guise, K.; Pfau, M.L.; Aleyasin, H.; Menard, C.; Zhang, H.; Hodes, G.E.; et al. Basal forebrain projections to the lateral habenula modulate aggression reward. Nature 2016, 534, 688–692. [Google Scholar] [CrossRef]
- Trapnell, C.; Hendrickson, D.G.; Sauvageau, M.; Goff, L.; Rinn, J.L.; Pachter, L. Differential analysis of gene regulation at transcript resolution with RNA-seq. Nat. Biotechnol. 2013, 31, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Svenningsson, P.; Nishi, A.; Fisone, G.; Girault, J.-A.; Nairn, A.C.; Greengard, P. DARPP-32: An integrator of neurotransmission. Annu. Rev. Pharmacol. Toxicol. 2004, 44, 269–296. [Google Scholar] [CrossRef] [PubMed]
- Kudriavtseva, N.N.; Bakshtanovskaia, I.V. The neurochemical control of aggression and submission. Zh. Vyssh. Nerv. Deiat. Im. I P Pavlov. 1991, 41, 459–466. [Google Scholar]
- Bondar, N.P.; Boyarskikh, U.A.; Kovalenko, I.L.; Filipenko, M.L.; Kudryavtseva, N.N. Molecular implications of repeated aggression: Th, Dat1, Snca and Bdnf gene expression in the VTA of victorious male mice. PLoS ONE 2009, 4, e4190. [Google Scholar] [CrossRef]
- Kudryavtseva, N.N.; Avgustinovich, D.F.; Bondar, N.P.; Tenditnik, M.V.; Kovalenko, I.L. An experimental approach for the study of psychotropic drug effects under simulated clinical conditions. Curr. Drug Met. 2008, 9, 352–360. [Google Scholar] [CrossRef]
- Kudryavtseva, N.N.; Lipina, T.V.; Koryakina, L.A. Effects of haloperidol on communicative and aggressive behavior in male mice with different experiences of aggression. Pharmacol. Biochem. Behav. 1999, 63, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Bondar’, N.P.; Kudriavtseva, N.N. Effect of the D1-receptor antagonist SCH-23390 on the individual and aggressive behavior in male mice with various aggression experience. Ross. Fiziol. Zh. Im. I.M. Sechenova 2003, 89, 992–1000. [Google Scholar]
- Kudriavtseva, N.N.; Dolgov, V.V.; Avgustinovich, D.F.; Alekseenko, O.V.; Lipina, T.V.; Koriakina, L.A. Modifying effect of the repeated experience of agonistic confrontations on effect of naltrexone in male mice. Ross. Fiziol. Zh. Im. I. M. Sechenova 2001, 87, 227–238. [Google Scholar] [PubMed]
- Lipina, T.V.; Avgustinovich, D.F.; Koriakina, L.A.; Alekseenko, O.V.; Kudriavtseva, N.N. Differences in the effects of naltrexone on the communicative and aggressive behaviors of subjects with different experiences of social conquests. Eksp. Klin. Farmakol. 1998, 61, 13–18. [Google Scholar]
- Bondar, N.P.; Smagin, D.A.; Kudryavtseva, N.N. Effects of single and chronic naltrexone treatment on agonistic behavior of male mice with repeated experience of aggression. Psychopharmacol. Biol. Narcol. 2011, 11, 2688–2700. [Google Scholar]
- Kudryavtseva, N.N.; Gerrits, M.A.; Avgustinovich, D.F.; Tenditnik, M.V.; van Ree, J.M. Modulation of anxiety-related behaviors by mu- and kappa-opioid receptor agonists depends on the social status of mice. Peptides 2004, 25, 1355–1363. [Google Scholar] [CrossRef]
- Kudryavtseva, N.N.; Gerrits, M.A.; Avgustinovich, D.F.; Tenditnik, M.V.; Van Ree, J.M. Anxiety and ethanol consumption in victorious and defeated mice; effect of kappa-opioid receptor activation. Eur. Neuropsychopharmacol. 2006, 16, 504–511. [Google Scholar] [CrossRef] [PubMed]
- Goloshchapov, A.V.; Filipenko, M.L.; Bondar, N.P.; Kudryavtseva, N.M.; Van Ree, J.M. Decrease of kappa-opioid receptor mRNA level in ventral tegmental area of male mice after repeated experience of aggression. Brain. Res. Mol. Brain Res. 2005, 135, 290–292. [Google Scholar] [CrossRef]
- Stefano, G.B.; Goumon, Y.; Casares, F.; Cadet, P.; Fricchione, G.L.; Rialas, C.; Bianchi, E. Endogenous morphine. Trends Neurosci. 2000, 23, 436–442. [Google Scholar] [CrossRef]
- Neri, C.; Guarna, M.; Bianchi, E.; Sonetti, D.; Matteucci, G.; Stefano, G.B. Endogenous morphine and codeine in the brain of nonhuman primate. Med. Sci. Monit. 2004, 10, MS1–MS5. [Google Scholar]
- Kream, R.M.; Stefano, G.B.; Ptáček, R. Psychiatric implications of endogenous morphine: Up-to-date review. Folia Biol. 2010, 56, 231–241. [Google Scholar] [CrossRef]
- Tordjman, S.; Carlier, M.; Cohen, D.; Cesselin, F.; Bourgoin, S.; Colas-Linhart, N.; Petiet, A.; Perez-Diaz, F.; Hamon, M.; Roubertoux, P.L. Aggression and the three opioid families (endorphins, enkephalins, and dynorphins) in mice. Behav. Genet. 2003, 33, 529–536. [Google Scholar] [CrossRef] [PubMed]
- Kulikov, A.V.; Kozlachkova, E.Y.; Kudryavtseva, N.N.; Popova, N.K. Correlation between tryptophan hydroxylase activity in the brain and predisposition to pinch-induced catalepsy in mice. Pharmacol. Biochem. Behav. 1995, 50, 431–435. [Google Scholar] [CrossRef] [PubMed]
- Amstislavskaya, T.G.; Kudryavtseva, N.N. Effect of repeated experience of victory and defeat in daily agonistic confrontations on brain tryptophan hydroxylase activity. FEBS Lett. 1997, 406, 106–108. [Google Scholar] [CrossRef] [PubMed]
- Kudryavtseva, N.N.; Smagin, D.A.; Kovalenko, I.L.; Galyamina, A.G.; Vishnivetskaya, G.B.; Babenko, V.N.; Orlov, Y.L. Serotonergic genes in the development of anxiety/depression-like state and pathology of aggressive behavior in male mice: RNA-seq data. Mol. Biol. 2017, 51, 288–300. [Google Scholar] [CrossRef]
- Bondar, N.P.; Kudriavtseva, N.N. Effect of buspirone on aggressive and anxiety behavior of male mice with various aggressive experience. Eksp. Klin. Farmakol. 2003, 66, 12–16. [Google Scholar]
- Brown, G.L.; Ebert, M.H.; Goyer, P.F.; Jimerson, D.C.; Klein, W.J.; Bunney, W.E.; Goodwin, F.K. Aggression, suicide, and serotonin: Relationships to CSF amine metabolites. Am. J. Psychiatry. 1982, 139, 741–746. [Google Scholar] [CrossRef]
- Coccaro, E.F. Impulsive aggression and central serotonergic system function in humans: An example of a dimensional brain-behavior relationship. Int. Clin. Psychopharmacol. 1992, 7, 3–12. [Google Scholar] [CrossRef]
- Glick, A.R. The role of serotonin in impulsive aggression, suicide, and homicide in adolescents and adults: A literature review. Int. J. Adolesc. Med. Health 2015, 27, 143–150. [Google Scholar] [CrossRef]
- Ferrari, P.F.; Palanza, P.; Parmigiani, S.; de Almeida, R.M.M.; Miczek, K.A. Serotonin and aggressive behavior in rodents and nonhuman primates: Predispositions and plasticity. Eur. J. Pharmacol. 2005, 526, 259–273. [Google Scholar] [CrossRef] [PubMed]
- de Boer, S.F. Animal models of excessive aggression: Implications for human aggression and violence. Curr. Opin. Psychol. 2018, 19, 81–87. [Google Scholar] [CrossRef] [PubMed]
- De Boer, S.F.; Buwalda, B.; Koolhaas, J.M. Untangling the neurobiology of coping styles in rodents: Towards neural mechanisms underlying individual differences in disease susceptibility. Neurosci. Biobehav. Rev. 2017, 74, 401–422. [Google Scholar] [CrossRef]
- Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; (DSM-5TM); American Psychiatric Association: Washington, DC, USA, 2013.
- Golden, S.A.; Shaham, Y. Aggression addiction and relapse: A new frontier in psychiatry. Neuropsychopharmacology 2018, 43, 224–225. [Google Scholar] [CrossRef]
- Kudryavtseva, N.N. Use of the “partition” test in behavioral and pharmacological experiments. Neurosci. Behav. Physiol. 2003, 33, 461–471. [Google Scholar] [CrossRef]
- The Allen Mouse Brain Atlas. Available online: http://mouse.brain-map.org/static/atlas (accessed on 24 April 2021).
- Robison, A.J.; Nestler, E.J. Transcriptional and epigenetic mechanisms of addiction. Nat. Rev. Neurosci. 2011, 12, 623–637. [Google Scholar] [CrossRef]
- Bayassi-Jakowicka, M.; Lietzau, G.; Czuba, E.; Patrone, C.; Kowiański, P. More than addiction—The nucleus accumbens contribution to development of mental disorders and neurodegenerative diseases. Int. J. Mol. Sci. 2022, 23, 2618. [Google Scholar] [CrossRef] [PubMed]
- Olsen, C.M. Natural rewards, neuroplasticity, and non-drug addictions. Neuropharmacology 2011, 61, 1109–1122. [Google Scholar] [CrossRef]
- Meredith, G.E.; Pennartz, C.M.; Groenewegen, H.J. The cellular framework for chemical signalling in the nucleus accumbens. Prog. Brain Res. 1993, 99, 3–24. [Google Scholar] [CrossRef]
- Golden, S.A.; Jin, M.; Heins, C.; Venniro, M.; Michaelides, M.; Shaham, Y. Nucleus accumbens Drd1-expressing neurons control aggression self-administration and aggression seeking in mice. J. Neurosci. 2019, 39, 2482–2496. [Google Scholar] [CrossRef]
- Yu, Q.; Teixeira, C.M.; Mahadevia, D.; Huang, Y.; Balsam, D.; Mann, J.J.; Gingrich, J.A.; Ansorge, M.S. Dopamine and serotonin signaling during two sensitive developmental periods differentially impact adult aggressive and affective behaviors in mice. Mol. Psychiatry 2014, 19, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Miczek, K.A.; Takahashi, A.; Gobrogge, K.L.; Hwa, L.S.; de Almeida, R.M. Escalated aggression in animal models: Shedding new light on mesocorticolimbic circuits. Curr. Opin. Behav. Sci. 2015, 3, 90–95. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for taxonomy-based analysis of pathways and genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef] [PubMed]
- Szklarczyk, D.; Kirsch, R.; Koutrouli, M.; Nastou, K.; Mehryary, F.; Hachilif, R.; Gable, A.L.; Fang, T.; Doncheva, N.T.; Pyysalo, S.; et al. The STRING database in 2023: Protein-protein association networks and functional enrichment analyses for any sequenced genome of interest. Nucleic Acids Res. 2023, 51, D638–D646. [Google Scholar] [CrossRef] [PubMed]







| Comparisons | Number of DEGs | |
|---|---|---|
| p Value < 0.05 | q Value < 0.05 | |
| Control vs. A20 | 2629 | 1200 |
| Control vs. AD | 1111 | 232 |
| A20 vs. AD | 2578 | 1338 |
| Variables | Adra2c | Drd1 | Drd2 | Drd3 | Oprk1 | Pdyn | Penk | Ppp1r1b |
|---|---|---|---|---|---|---|---|---|
| Adra2c | 1 | 0.965 | 0.935 | 0.851 | 0.966 | 0.958 | 0.945 | 0.974 |
| Drd1 | 0.965 | 1 | 0.983 | 0.947 | 0.991 | 0.990 | 0.983 | 0.993 |
| Drd2 | 0.935 | 0.983 | 1 | 0.975 | 0.990 | 0.993 | 0.995 | 0.987 |
| Drd3 | 0.851 | 0.947 | 0.975 | 1 | 0.944 | 0.948 | 0.959 | 0.945 |
| Oprk1 | 0.966 | 0.991 | 0.990 | 0.944 | 1 | 0.996 | 0.997 | 0.997 |
| Pdyn | 0.958 | 0.990 | 0.993 | 0.948 | 0.996 | 1 | 0.995 | 0.990 |
| Penk | 0.945 | 0.983 | 0.995 | 0.959 | 0.997 | 0.995 | 1 | 0.991 |
| Ppp1r1b | 0.974 | 0.993 | 0.987 | 0.945 | 0.997 | 0.990 | 0.991 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kudryavtseva, N.N.; Smagin, D.A.; Redina, O.E.; Kovalenko, I.L.; Galyamina, A.G.; Babenko, V.N. Neurotransmitter Genes in the Nucleus Accumbens That Are Involved in the Development of a Behavioral Pathology After Positive Fighting Experiences and Their Deprivation: A Conceptual Paradigm for Data Analysis. Int. J. Mol. Sci. 2025, 26, 8580. https://doi.org/10.3390/ijms26178580
Kudryavtseva NN, Smagin DA, Redina OE, Kovalenko IL, Galyamina AG, Babenko VN. Neurotransmitter Genes in the Nucleus Accumbens That Are Involved in the Development of a Behavioral Pathology After Positive Fighting Experiences and Their Deprivation: A Conceptual Paradigm for Data Analysis. International Journal of Molecular Sciences. 2025; 26(17):8580. https://doi.org/10.3390/ijms26178580
Chicago/Turabian StyleKudryavtseva, Natalia N., Dmitry A. Smagin, Olga E. Redina, Irina L. Kovalenko, Anna G. Galyamina, and Vladimir N. Babenko. 2025. "Neurotransmitter Genes in the Nucleus Accumbens That Are Involved in the Development of a Behavioral Pathology After Positive Fighting Experiences and Their Deprivation: A Conceptual Paradigm for Data Analysis" International Journal of Molecular Sciences 26, no. 17: 8580. https://doi.org/10.3390/ijms26178580
APA StyleKudryavtseva, N. N., Smagin, D. A., Redina, O. E., Kovalenko, I. L., Galyamina, A. G., & Babenko, V. N. (2025). Neurotransmitter Genes in the Nucleus Accumbens That Are Involved in the Development of a Behavioral Pathology After Positive Fighting Experiences and Their Deprivation: A Conceptual Paradigm for Data Analysis. International Journal of Molecular Sciences, 26(17), 8580. https://doi.org/10.3390/ijms26178580







