Post-Translational Modifications of Lipoproteins: Emerging Players Linking Inflammation and Cardiovascular Disease in Rheumatoid Arthritis—A Narrative Review
Abstract
1. Introduction
Study Rationale and Aims
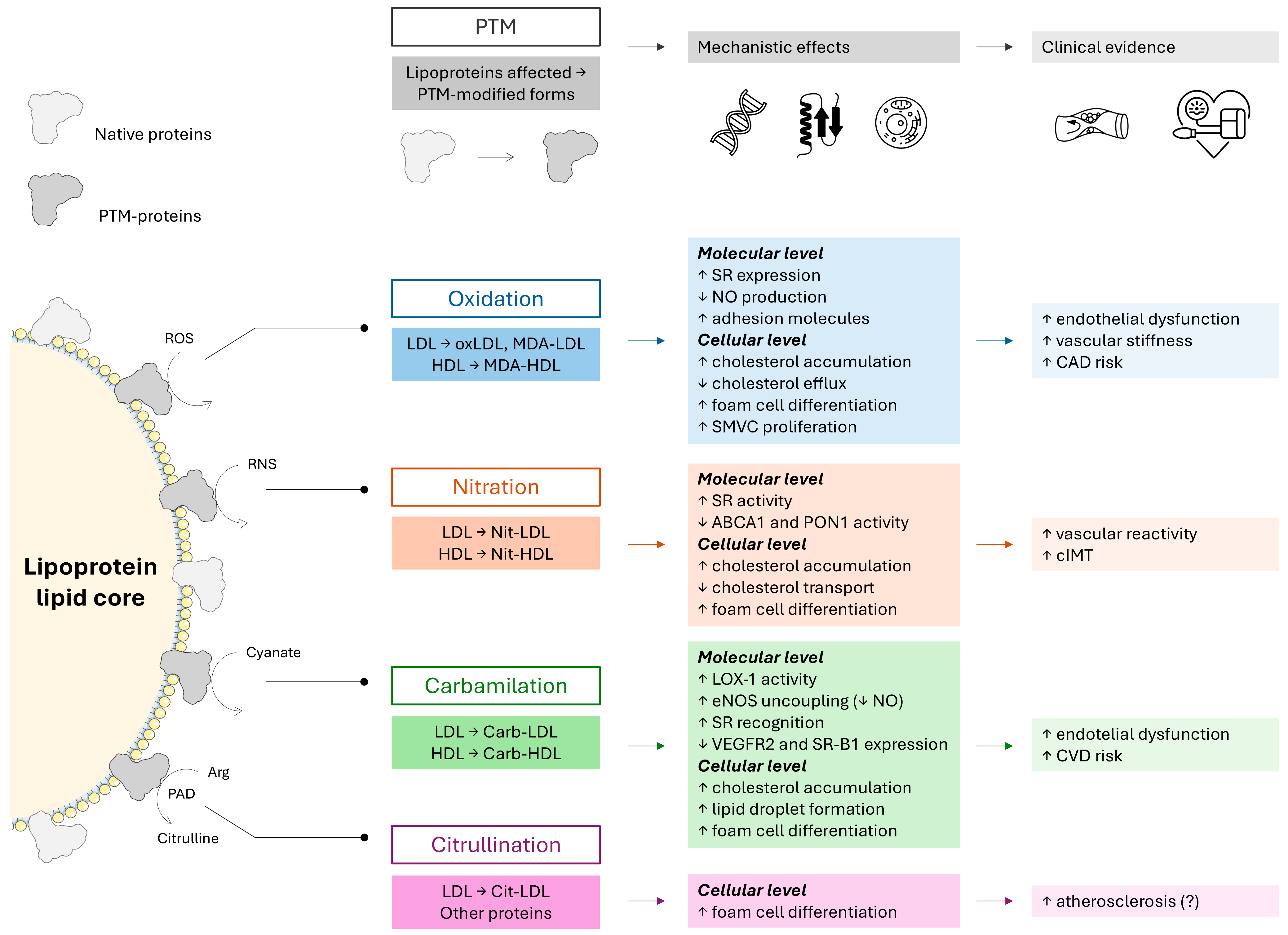
2. Emerging Evidence for Post-Translational Modifications of Lipoproteins in Cardiovascular Diseases and Rheumatoid Arthritis
2.1. Oxidation of Lipoproteins
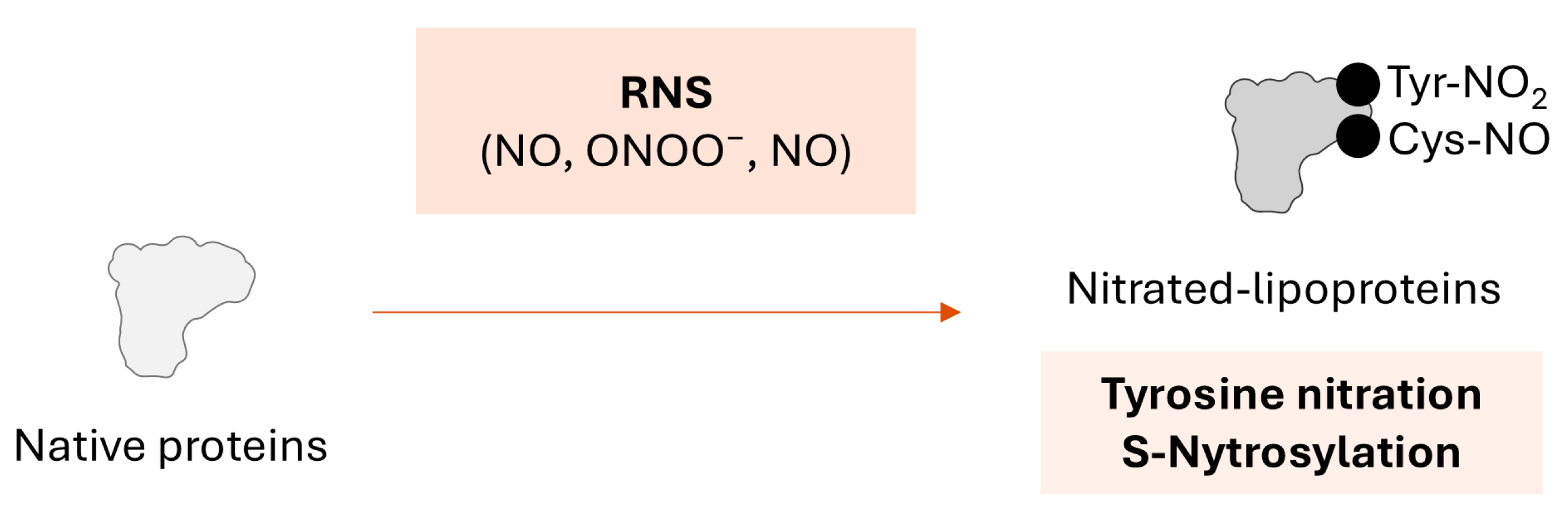
2.2. Nitration of Lipoproteins
2.3. Carbamylation of Lipoproteins
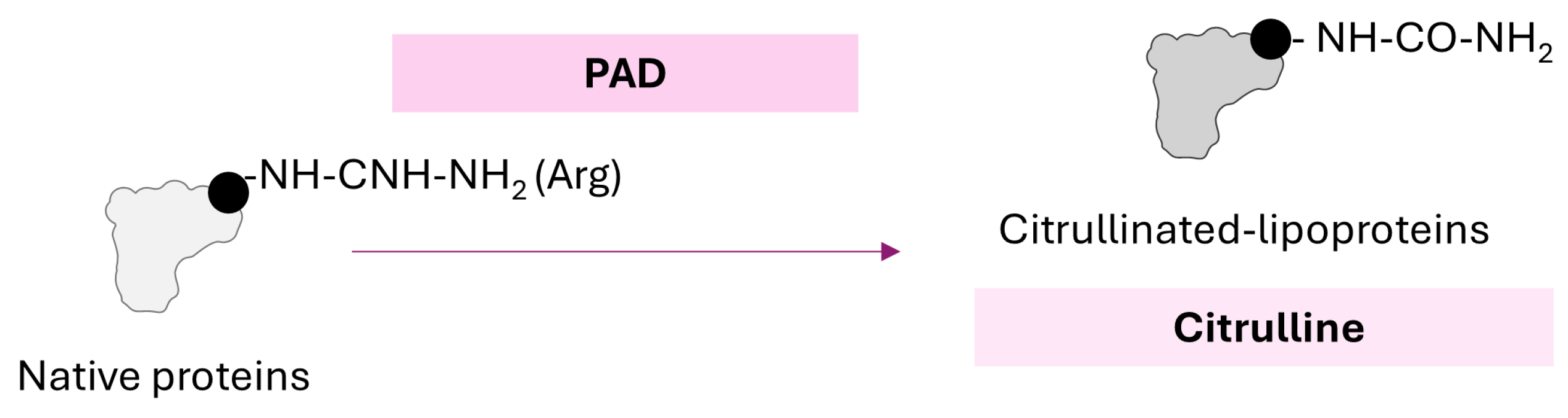
2.4. Citrullination of Lipoproteins
3. Humoral Immune Responses Against PTMs of Lipoproteins
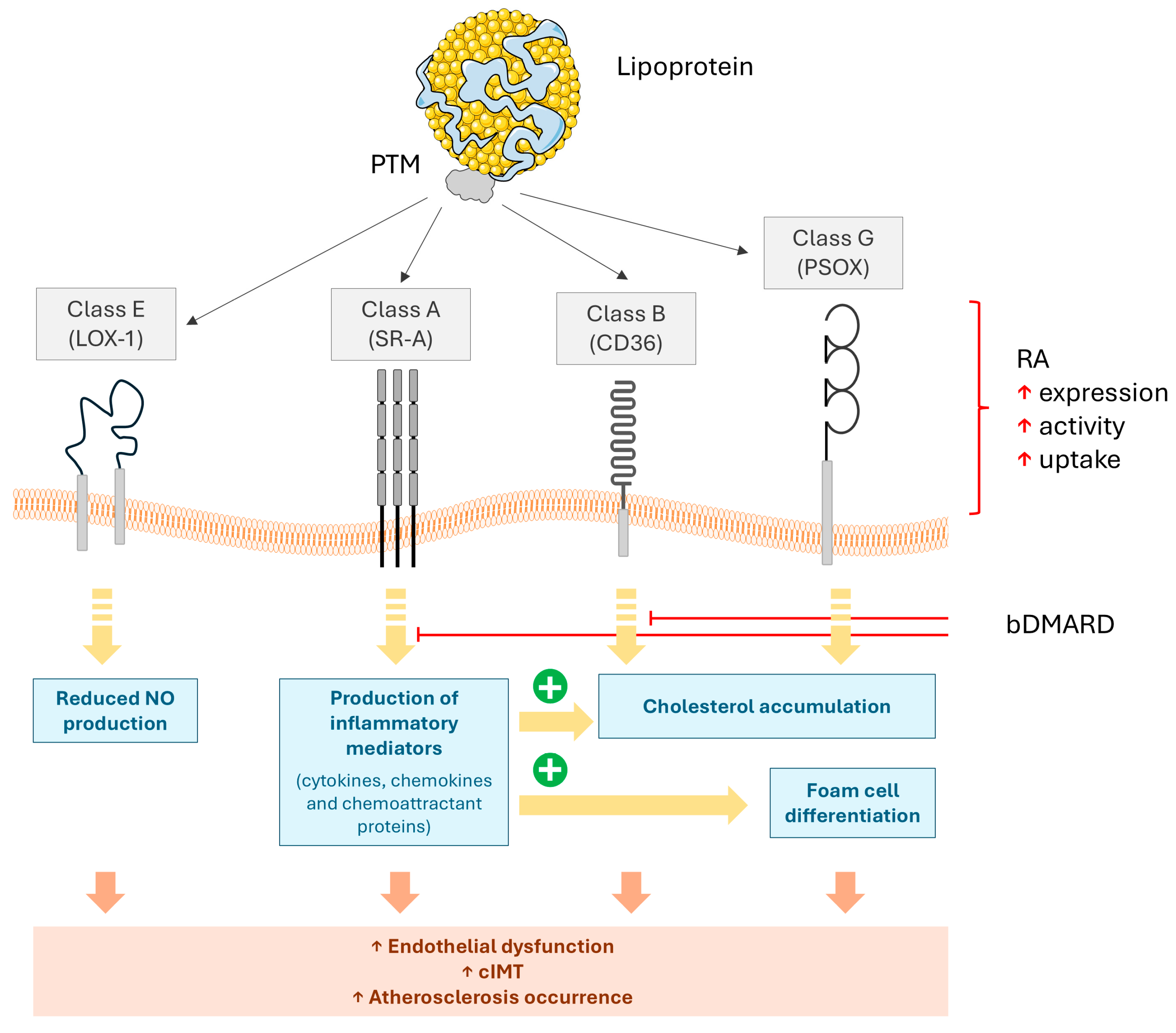
4. The Synergistic Effects of Scavenger Receptors and PTMs of LDL on Atherosclerosis
5. The Impacts of Disease-Modifying Anti-Rheumatic Drugs (DMARDs) on the Interplay Between Inflammation and Lipoprotein PTMs
6. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gravallese, E.M.; Firestein, G.S. Rheumatoid Arthritis—Common Origins, Divergent Mechanisms. N. Engl. J. Med. 2023, 388, 529–542. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid arthritis. Nat. Rev. Dis. Primers 2018, 4, 18001. [Google Scholar] [CrossRef]
- Avina-Zubieta, J.A.; Thomas, J.; Sadatsafavi, M.; Lehman, A.J.; Lacaille, D. Risk of incident cardiovascular events in patients with rheumatoid arthritis: A meta-analysis of observational studies. Ann. Rheum. Dis. 2012, 71, 1524–1529. [Google Scholar] [CrossRef]
- Aviña-Zubieta, J.A.; Choi, H.K.; Sadatsafavi, M.; Etminan, M.; Esdaile, J.M.; Lacaille, D. Risk of cardiovascular mortality in patients with rheumatoid arthritis: A meta-analysis of observational studies. Arthritis Care Res. 2008, 59, 1690–1697. [Google Scholar] [CrossRef]
- Sokka, T.; Abelson, B.; Pincus, T. Mortality in rheumatoid arthritis: 2008 update. Clin. Exp. Rheumatol. 2008, 26 (Suppl. S51), S35–S61. [Google Scholar]
- Agca, R.; Hopman, L.; Laan, K.J.C.; van Halm, V.P.; Peters, M.J.L.; Smulders, Y.M.; Dekker, J.M.; Nijpels, G.; Stehouwer, C.D.A.; Voskuyl, A.E.; et al. Cardiovascular Event Risk in Rheumatoid Arthritis Compared with Type 2 Diabetes: A 15-year Longitudinal Study. J. Rheumatol. 2020, 47, 316–324. [Google Scholar] [CrossRef]
- Conrad, N.; Verbeke, G.; Molenberghs, G.; Goetschalckx, L.; Callender, T.; Cambridge, G.; Mason, J.C.; Rahimi, K.; McMurray, J.J.V.; Verbakel, J.Y. Autoimmune diseases and cardiovascular risk: A population-based study on 19 autoimmune diseases and 12 cardiovascular diseases in 22 million individuals in the UK. Lancet 2022, 400, 733–743. [Google Scholar] [CrossRef]
- Xu, C.; Wang, X.; Mu, R.; Yang, L.; Zhang, Y.; Han, S.; Li, X.; Wang, Y.; Wang, G.; Zhu, P.; et al. Societal Costs of Rheumatoid Arthritis in China: A Hospital-Based Cross-Sectional Study. Arthritis Care Res. 2014, 66, 523–531. [Google Scholar] [CrossRef]
- Agca, R.; Heslinga, S.C.; Rollefstad, S.; Heslinga, M.; McInnes, I.B.; Peters, M.J.L.; Kvien, T.K.; Dougados, M.; Radner, H.; Atzeni, F.; et al. EULAR recommendations for cardiovascular disease risk management in patients with rheumatoid arthritis and other forms of inflammatory joint disorders: 2015/2016 update. Ann. Rheum. Dis. 2017, 76, 17–28. [Google Scholar] [CrossRef]
- del Rincon, I.D.; Williams, K.; Stern, M.P.; Freeman, G.L.; Escalante, A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001, 44, 2737–2745. [Google Scholar] [CrossRef]
- Crowson, C.S.; Rollefstad, S.; Ikdahl, E.; Kitas, G.D.; van Riel, P.L.C.M.; Gabriel, S.E.; Matteson, E.L.; Kvien, T.K.; Douglas, K.; Sandoo, A.; et al. Impact of risk factors associated with cardiovascular outcomes in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2018, 77, 48–54. [Google Scholar] [CrossRef]
- Sewell, J.; Hussain, S.M.; Wang, Y.; Wluka, A.E.; Lim, Y.Z.; Carrington, M.J.; Samaras, K.; Cicuttini, F.M. Association between arthritis and cardiovascular risk factors in community-based adults: An opportunity to target cardiovascular risk. BMC Cardiovasc. Disord. 2022, 22, 232. [Google Scholar] [CrossRef]
- D’Agostino, R.B., Sr.; Vasan, R.S.; Pencina, M.J.; Wolf, P.A.; Cobain, M.; Massaro, J.M.; Kannel, W.B. General cardiovascular risk profile for use in primary care: The Framingham Heart Study. Circulation 2008, 117, 743–753. [Google Scholar] [CrossRef]
- Conroy, R.M.; Pyorala, K.; Fitzgerald, A.P.; Sans, S.; Menotti, A.; De Backer, G.; De Bacquer, D.; Ducimetiere, P.; Jousilahti, P.; Keil, U.; et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: The SCORE project. Eur. Heart J. 2003, 24, 987–1003. [Google Scholar] [CrossRef]
- Arts, E.E.; Popa, C.; Den Broeder, A.A.; Semb, A.G.; Toms, T.; Kitas, G.D.; van Riel, P.L.; Fransen, J. Performance of four current risk algorithms in predicting cardiovascular events in patients with early rheumatoid arthritis. Ann. Rheum. Dis. 2015, 74, 668–674. [Google Scholar] [CrossRef]
- Arts, E.E.; Popa, C.D.; Den Broeder, A.A.; Donders, R.; Sandoo, A.; Toms, T.; Rollefstad, S.; Ikdahl, E.; Semb, A.G.; Kitas, G.D.; et al. Prediction of cardiovascular risk in rheumatoid arthritis: Performance of original and adapted SCORE algorithms. Ann. Rheum. Dis. 2016, 75, 674–680. [Google Scholar] [CrossRef]
- Gomez-Vaquero, C.; Corrales, A.; Zacarias, A.; Rueda-Gotor, J.; Blanco, R.; Gonzalez-Juanatey, C.; Llorca, J.; Gonzalez-Gay, M.A. SCORE and REGICOR function charts underestimate the cardiovascular risk in Spanish patients with rheumatoid arthritis. Arthritis Res. Ther. 2013, 15, R91. [Google Scholar] [CrossRef]
- Chang, P.Y.; Chen, Y.J.; Chang, F.H.; Lu, J.; Huang, W.H.; Yang, T.C.; Lee, Y.T.; Chang, S.F.; Lu, S.C.; Chen, C.H. Aspirin protects human coronary artery endothelial cells against atherogenic electronegative LDL via an epigenetic mechanism: A novel cytoprotective role of aspirin in acute myocardial infarction. Cardiovasc. Res. 2013, 99, 137–145. [Google Scholar] [CrossRef][Green Version]
- Solomon, D.H.; Greenberg, J.; Curtis, J.R.; Liu, M.; Farkouh, M.E.; Tsao, P.; Kremer, J.M.; Etzel, C.J. Derivation and internal validation of an expanded cardiovascular risk prediction score for rheumatoid arthritis: A Consortium of Rheumatology Researchers of North America Registry Study. Arthritis Rheumatol. 2015, 67, 1995–2003. [Google Scholar] [CrossRef] [PubMed]
- Wahlin, B.; Innala, L.; Magnusson, S.; Moller, B.; Smedby, T.; Rantapaa-Dahlqvist, S.; Wallberg-Jonsson, S. Performance of the Expanded Cardiovascular Risk Prediction Score for Rheumatoid Arthritis Is Not Superior to the ACC/AHA Risk Calculator. J. Rheumatol. 2019, 46, 130–137. [Google Scholar] [CrossRef]
- Crowson, C.S.; Gabriel, S.E.; Semb, A.G.; van Riel, P.L.C.M.; Karpouzas, G.; Dessein, P.H.; Hitchon, C.; Pascual-Ramos, V.; Kitas, G.D.; Trans-Atlantic Cardiovascular Consortium for Rheumatoid Arthritis. Rheumatoid arthritis-specific cardiovascular risk scores are not superior to general risk scores: A validation analysis of patients from seven countries. Rheumatology 2017, 56, 1102–1110. [Google Scholar] [CrossRef]
- Sysojev, A.Ö.; Alfredsson, L.; Klareskog, L.; the Swedish Rheumatology Quality Register Biobank Group; Silberberg, G.N.; Saevarsdottir, S.; Padyukov, L.; Magnusson, P.K.E.; Askling, J.; Westerlind, H. Minor Genetic Overlap Among Rheumatoid Arthritis, Myocardial Infarction, and Myocardial Infarction Risk Determinants. Arthritis Rheumatol. 2024, 76, 1344–1352. [Google Scholar] [CrossRef]
- Guo, Y.; Chung, W.; Shan, Z.; Zhu, Z.; Costenbader, K.H.; Liang, L. Genome-Wide Assessment of Shared Genetic Architecture Between Rheumatoid Arthritis and Cardiovascular Diseases. J. Am. Heart Assoc. 2023, 12, e030211. [Google Scholar] [CrossRef]
- Yuan, S.; Carter, P.; Mason, A.M.; Yang, F.; Burgess, S.; Larsson, S.C. Genetic Liability to Rheumatoid Arthritis in Relation to Coronary Artery Disease and Stroke Risk. Arthritis Rheumatol. 2022, 74, 1638–1647. [Google Scholar] [CrossRef]
- Furman, D.; Campisi, J.; Verdin, E.; Carrera-Bastos, P.; Targ, S.; Franceschi, C.; Ferrucci, L.; Gilroy, D.W.; Fasano, A.; Miller, G.W.; et al. Chronic inflammation in the etiology of disease across the life span. Nat. Med. 2019, 25, 1822–1832. [Google Scholar] [CrossRef]
- Carubbi, F.; Alunno, A.; Gerli, R.; Giacomelli, R. Post-Translational Modifications of Proteins: Novel Insights in the Autoimmune Response in Rheumatoid Arthritis. Cells 2019, 8, 657. [Google Scholar] [CrossRef]
- Ucci, F.M.; Recalchi, S.; Barbati, C.; Manganelli, V.; Capozzi, A.; Riitano, G.; Buoncuore, G.; Garofalo, T.; Ceccarelli, F.; Spinelli, F.R.; et al. Citrullinated and carbamylated proteins in extracellular microvesicles from plasma of patients with rheumatoid arthritis. Rheumatology 2023, 62, 2312–2319. [Google Scholar] [CrossRef]
- Rodríguez-Carrio, J.; Alperi-López, M.; López, P.; Pérez-Álvarez, Á.I.; Gil-Serret, M.; Amigó, N.; Ulloa, C.; Benavente, L.; Ballina-García, F.J.; Suárez, A. GlycA Levels during the Earliest Stages of Rheumatoid Arthritis: Potential Use as a Biomarker of Subclinical Cardiovascular Disease. J. Clin. Med. 2020, 9, 2472. [Google Scholar] [CrossRef]
- Gyebrovszki, B.; Ács, A.; Szabó, D.; Auer, F.; Novozánszki, S.; Rojkovich, B.; Magyar, A.; Hudecz, F.; Vékey, K.; Drahos, L.; et al. The Role of IgG Fc Region N-Glycosylation in the Pathomechanism of Rheumatoid Arthritis. Int. J. Mol. Sci. 2022, 23, 5828. [Google Scholar] [CrossRef]
- Iuliano, L.; Mauriello, A.; Sbarigia, E.; Spagnoli, L.G.; Violi, F. Radiolabeled native low-density lipoprotein injected into patients with carotid stenosis accumulates in macrophages of atherosclerotic plaque: Effect of vitamin E supplementation. Circulation 2000, 101, 1249–1254. [Google Scholar] [CrossRef]
- Ansell, B.J.; Watson, K.E.; Fogelman, A.M.; Navab, M.; Fonarow, G.C. High-Density Lipoprotein Function: Recent Advances. J. Am. Coll. Cardiol. 2005, 46, 1792–1798. [Google Scholar] [CrossRef]
- Myasoedova, E.; Crowson, C.S.; Kremers, H.M.; Roger, V.L.; Fitz-Gibbon, P.D.; Therneau, T.M.; Gabriel, S.E. Lipid paradox in rheumatoid arthritis: The impact of serum lipid measures and systemic inflammation on the risk of cardiovascular disease. Ann. Rheum. Dis. 2011, 70, 482–487. [Google Scholar] [CrossRef]
- Robertson, J.; Peters, M.J.; McInnes, I.B.; Sattar, N. Changes in lipid levels with inflammation and therapy in RA: A maturing paradigm. Nat. Rev. Rheumatol. 2013, 9, 513–523. [Google Scholar] [CrossRef]
- Choy, E.; Ganeshalingam, K.; Semb, A.G.; Szekanecz, Z.; Nurmohamed, M. Cardiovascular risk in rheumatoid arthritis: Recent advances in the understanding of the pivotal role of inflammation, risk predictors and the impact of treatment. Rheumatology 2014, 53, 2143–2154. [Google Scholar] [CrossRef]
- Fernández-Friera, L.; Fuster, V.; López-Melgar, B.; Oliva, B.; García-Ruiz, J.M.; Mendiguren, J.; Bueno, H.; Pocock, S.; Ibáñez, B.; Fernández-Ortiz, A.; et al. Normal LDL-Cholesterol Levels Are Associated With Subclinical Atherosclerosis in the Absence of Risk Factors. J. Am. Coll. Cardiol. 2017, 70, 2979–2991. [Google Scholar] [CrossRef]
- Lee, C.K.; Liao, C.W.; Meng, S.W.; Wu, W.K.; Chiang, J.Y.; Wu, M.S. Lipids and Lipoproteins in Health and Disease: Focus on Targeting Atherosclerosis. Biomedicines 2021, 9, 985. [Google Scholar] [CrossRef]
- Wu, Z.; Jankowski, V.; Jankowski, J. Irreversible post-translational modifications—Emerging cardiovascular risk factors. Mol. Asp. Med. 2022, 86, 101010. [Google Scholar] [CrossRef]
- Pruijn, G.J.M. Citrullination and Carbamylation in the Pathophysiology of Rheumatoid Arthritis. Front. Immunol. 2015, 6, 192. [Google Scholar] [CrossRef]
- Sokolove, J.; Brennan, M.J.; Sharpe, O.; Lahey, L.J.; Kao, A.H.; Krishnan, E.; Edmundowicz, D.; Lepus, C.M.; Wasko, M.C.; Robinson, W.H. Brief report: Citrullination within the atherosclerotic plaque: A potential target for the anti-citrullinated protein antibody response in rheumatoid arthritis. Arthritis Rheum. 2013, 65, 1719–1724. [Google Scholar] [CrossRef]
- Lushchak, V.I.; Lushchak, O. Interplay between reactive oxygen and nitrogen species in living organisms. Chem. Biol. Interact. 2021, 349, 109680. [Google Scholar] [CrossRef]
- Chung, H.S.; Wang, S.B.; Venkatraman, V.; Murray, C.I.; Van Eyk, J.E. Cysteine oxidative posttranslational modifications: Emerging regulation in the cardiovascular system. Circ. Res. 2013, 112, 382–392. [Google Scholar] [CrossRef]
- Anjo, S.; He, Z.; Hussain, Z.; Farooq, A.; McIntyre, A.; Laughton, C.; Carvalho, A.; Finelli, M. Protein Oxidative Modifications in Neurodegenerative Diseases: From Advances in Detection and Modelling to Their Use as Disease Biomarkers. Antioxidants 2024, 13, 681. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xu, C.; Wang, Z.; Han, Z.; Xia, Q.; Wei, S.; Sun, Q.; Liu, S. Effect of MDA-mediated oxidation on the protein structure and digestive properties of golden pomfret. Food Chem. 2024, 443, 138563. [Google Scholar] [CrossRef]
- Yoshida, H.; Quehenberger, O.; Kondratenko, N.; Green, S.; Steinberg, D. Minimally oxidized low-density lipoprotein increases expression of scavenger receptor A, CD36, and macrosialin in resident mouse peritoneal macrophages. Arter. Thromb. Vasc. Biol. 1998, 18, 794–802. [Google Scholar] [CrossRef]
- Jiang, H.; Zhou, Y.; Nabavi, S.M.; Sahebkar, A.; Little, P.J.; Xu, S.; Weng, J.; Ge, J. Mechanisms of Oxidized LDL-Mediated Endothelial Dysfunction and Its Consequences for the Development of Atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 925923. [Google Scholar] [CrossRef] [PubMed]
- Nishi, K.; Itabe, H.; Uno, M.; Kitazato, K.T.; Horiguchi, H.; Shinno, K.; Nagahiro, S. Oxidized LDL in Carotid Plaques and Plasma Associates with Plaque Instability. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 1649–1654. [Google Scholar] [CrossRef]
- Brinkley, T.E.; Nicklas, B.J.; Kanaya, A.M.; Satterfield, S.; Lakatta, E.G.; Simonsick, E.M.; Sutton-Tyrrell, K.; Kritchevsky, S.B. Plasma oxidized low-density lipoprotein levels and arterial stiffness in older adults: The health, aging, and body composition study. Hypertension 2009, 53, 846–852. [Google Scholar] [CrossRef]
- Tsimikas, S.; Brilakis, E.S.; Miller, E.R.; McConnell, J.P.; Lennon, R.J.; Kornman, K.S.; Witztum, J.L.; Berger, P.B. Oxidized phospholipids, Lp(a) lipoprotein, and coronary artery disease. N. Engl. J. Med. 2005, 353, 46–57. [Google Scholar] [CrossRef]
- Meisinger, C.; Baumert, J.; Khuseyinova, N.; Loewel, H.; Koenig, W. Plasma oxidized low-density lipoprotein, a strong predictor for acute coronary heart disease events in apparently healthy, middle-aged men from the general population. Circulation 2005, 112, 651–657. [Google Scholar] [CrossRef]
- Levitan, I.; Volkov, S.; Subbaiah, P.V. Oxidized LDL: Diversity, patterns of recognition, and pathophysiology. Antioxid. Redox Signal. 2010, 13, 39–75. [Google Scholar] [CrossRef]
- Itabe, H.; Obama, T. The Oxidized Lipoproteins In Vivo: Its Diversity and Behavior in the Human Circulation. Int. J. Mol. Sci. 2023, 24, 5747. [Google Scholar] [CrossRef]
- Tsimikas, S.; Witztum, J.L. Measuring Circulating Oxidized Low-Density Lipoprotein to Evaluate Coronary Risk. Circulation 2001, 103, 1930–1932. [Google Scholar] [CrossRef]
- Nowak, B.; Madej, M.; Łuczak, A.; Małecki, R.; Wiland, P. Disease Activity, Oxidized-LDL Fraction and Anti-Oxidized LDL Antibodies Influence Cardiovascular Risk in Rheumatoid Arthritis. Adv. Clin. Exp. Med. 2016, 25, 43–50. [Google Scholar] [CrossRef]
- Fernández-Ortiz, A.M.; Ortiz, A.M.; Pérez, S.; Toledano, E.; Abásolo, L.; González-Gay, M.A.; Castañeda, S.; González-Álvaro, I. Effects of disease activity on lipoprotein levels in patients with early arthritis: Can oxidized LDL cholesterol explain the lipid paradox theory? Arthritis Res. Ther. 2020, 22, 213. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Lee, C.K.; Lee, E.Y.; Park, S.Y.; Cho, Y.S.; Yoo, B.; Moon, H.B. Serum oxidized low-density lipoproteins in rheumatoid arthritis. Rheumatol. Int. 2004, 24, 230–233. [Google Scholar] [CrossRef]
- Ajeganova, S.; de Faire, U.; Jogestrand, T.; Frostegård, J.; Hafström, I. Carotid atherosclerosis, disease measures, oxidized low-density lipoproteins, and atheroprotective natural antibodies for cardiovascular disease in early rheumatoid arthritis—An inception cohort study. J. Rheumatol. 2012, 39, 1146–1154. [Google Scholar] [CrossRef]
- Wang, J.; Hu, B.; Meng, Y.; Zhang, C.; Li, K.; Hui, C. The level of malondialdehyde-modified LDL and LDL immune complexes in patients with rheumatoid arthritis. Clin. Biochem. 2009, 42, 1352–1357. [Google Scholar] [CrossRef]
- Farina, C.J.; Davidson, M.H.; Shah, P.K.; Stark, C.; Lu, W.; Shirodaria, C.; Wright, T.; Antoniades, C.A.; Nilsson, J.; Mehta, N.N. Inhibition of oxidized low-density lipoprotein with orticumab inhibits coronary inflammation and reduces residual inflammatory risk in psoriasis: A pilot randomized, double-blind placebo-controlled trial. Cardiovasc. Res. 2024, 120, 678–680. [Google Scholar] [CrossRef]
- Vivekanandan-Giri, A.; Slocum, J.L.; Byun, J.; Tang, C.; Sands, R.L.; Gillespie, B.W.; Heinecke, J.W.; Saran, R.; Kaplan, M.J.; Pennathur, S. High density lipoprotein is targeted for oxidation by myeloperoxidase in rheumatoid arthritis. Ann. Rheum. Dis. 2013, 72, 1725–1731. [Google Scholar] [CrossRef]
- Morgantini, C.; Meriwether, D.; Baldi, S.; Venturi, E.; Pinnola, S.; Wagner, A.C.; Fogelman, A.M.; Ferrannini, E.; Natali, A.; Reddy, S.T. HDL lipid composition is profoundly altered in patients with type 2 diabetes and atherosclerotic vascular disease. Nutr. Metab. Cardiovasc. Dis. 2014, 24, 594–599. [Google Scholar] [CrossRef]
- Feng, J.; Wang, Y.; Li, W.; Zhao, Y.; Liu, Y.; Yao, X.; Liu, S.; Yu, P.; Li, R. High levels of oxidized fatty acids in HDL impair the antioxidant function of HDL in patients with diabetes. Front. Endocrinol. 2022, 13, 993193. [Google Scholar] [CrossRef] [PubMed]
- Martínez-López, D.; Camafeita, E.; Cedó, L.; Roldan-Montero, R.; Jorge, I.; García-Marqués, F.; Gómez-Serrano, M.; Bonzon-Kulichenko, E.; Blanco-Vaca, F.; Blanco-Colio, L.M.; et al. APOA1 oxidation is associated to dysfunctional high-density lipoproteins in human abdominal aortic aneurysm. eBioMedicine 2019, 43, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Ischiropoulos, H. Protein tyrosine nitration. Redox Biochem. Chem. 2024, 8, 100030. [Google Scholar] [CrossRef]
- Bakillah, A.; Tedla, F.; Ayoub, I.; John, D.; Norin, A.J.; Hussain, M.M.; Brown, C. Plasma Nitration of High-Density and Low-Density Lipoproteins in Chronic Kidney Disease Patients Receiving Kidney Transplants. Mediat. Inflamm. 2015, 2015, 352356. [Google Scholar] [CrossRef]
- Adedayo, A.; Eluwole, A.; Tedla, F.; Kremer, A.; Mastrogiovanni, N.; Khan, M.; Rosenberg, C.; Dreizen, P.; Rosa, J.L.; Salciccioli, L.; et al. Association between nitrated lipoproteins and vascular function in type 2 diabetes. FBL 2021, 26, 644–663. [Google Scholar] [CrossRef]
- Podrez, E.A.; Febbraio, M.; Sheibani, N.; Schmitt, D.; Silverstein, R.L.; Hajjar, D.P.; Cohen, P.A.; Frazier, W.A.; Hoff, H.F.; Hazen, S.L. Macrophage scavenger receptor CD36 is the major receptor for LDL modified by monocyte-generated reactive nitrogen species. J. Clin. Investig. 2000, 105, 1095–1108. [Google Scholar] [CrossRef]
- Luoma, J.S.; Ylä-Herttuala, S. Expression of inducible nitric oxide synthase in macrophages and smooth muscle cells in various types of human atherosclerotic lesions. Virchows Arch. 1999, 434, 561–568. [Google Scholar] [CrossRef]
- Shishehbor, M.H.; Aviles, R.J.; Brennan, M.L.; Fu, X.; Goormastic, M.; Pearce, G.L.; Gokce, N.; Keaney, J.F., Jr.; Penn, M.S.; Sprecher, D.L.; et al. Association of nitrotyrosine levels with cardiovascular disease and modulation by statin therapy. JAMA 2003, 289, 1675–1680. [Google Scholar] [CrossRef]
- Leeuwenburgh, C.; Hardy, M.M.; Hazen, S.L.; Wagner, P.; Oh-ishi, S.; Steinbrecher, U.P.; Heinecke, J.W. Reactive nitrogen intermediates promote low density lipoprotein oxidation in human atherosclerotic intima. J. Biol. Chem. 1997, 272, 1433–1436. [Google Scholar] [CrossRef]
- Griffiths, H.R.; Aldred, S.; Dale, C.; Nakano, E.; Kitas, G.D.; Grant, M.G.; Nugent, D.; Taiwo, F.A.; Li, L.; Powers, H.J. Homocysteine from endothelial cells promotes LDL nitration and scavenger receptor uptake. Free Radic. Biol. Med. 2006, 40, 488–500. [Google Scholar] [CrossRef] [PubMed]
- Jakubiak, G.K.; Cieślar, G.; Stanek, A. Nitrotyrosine, Nitrated Lipoproteins, and Cardiovascular Dysfunction in Patients with Type 2 Diabetes: What Do We Know and What Remains to Be Explained? Antioxidants 2022, 11, 856. [Google Scholar] [CrossRef] [PubMed]
- Verbrugge, F.H.; Tang, W.H.; Hazen, S.L. Protein carbamylation and cardiovascular disease. Kidney Int. 2015, 88, 474–478. [Google Scholar] [CrossRef]
- Wang, Z.; Nicholls, S.J.; Rodriguez, E.R.; Kummu, O.; Hörkkö, S.; Barnard, J.; Reynolds, W.F.; Topol, E.J.; DiDonato, J.A.; Hazen, S.L. Protein carbamylation links inflammation, smoking, uremia and atherogenesis. Nat. Med. 2007, 13, 1176–1184. [Google Scholar] [CrossRef]
- Manganelli, V.; Recalchi, S.; Capozzi, A.; Riitano, G.; Mattei, V.; Longo, A.; Di Franco, M.; Alessandri, C.; Bombardieri, M.; Valesini, G.; et al. Autophagy induces protein carbamylation in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Rheumatology 2018, 57, 2032–2041. [Google Scholar] [CrossRef] [PubMed]
- Apostolov, E.O.; Shah, S.V.; Ok, E.; Basnakian, A.G. Quantification of carbamylated LDL in human sera by a new sandwich ELISA. Clin. Chem. 2005, 51, 719–728. [Google Scholar] [CrossRef] [PubMed]
- Speer, T.; Owala, F.O.; Holy, E.W.; Zewinger, S.; Frenzel, F.L.; Stahli, B.E.; Razavi, M.; Triem, S.; Cvija, H.; Rohrer, L.; et al. Carbamylated low-density lipoprotein induces endothelial dysfunction. Eur. Heart J. 2014, 35, 3021–3032. [Google Scholar] [CrossRef]
- Apostolov, E.O.; Basnakian, A.G.; Yin, X.; Ok, E.; Shah, S.V. Modified LDLs induce proliferation-mediated death of human vascular endothelial cells through MAPK pathway. Am. J. Physiol. Heart Circ. Physiol. 2007, 292, H1836–H1846. [Google Scholar] [CrossRef]
- Apostolov, E.O.; Shah, S.V.; Ray, D.; Basnakian, A.G. Scavenger receptors of endothelial cells mediate the uptake and cellular proatherogenic effects of carbamylated LDL. Arter. Thromb. Vasc. Biol. 2009, 29, 1622–1630. [Google Scholar] [CrossRef]
- Sun, J.T.; Yang, K.; Lu, L.; Zhu, Z.B.; Zhu, J.Z.; Ni, J.W.; Han, H.; Chen, N.; Zhang, R.Y. Increased carbamylation level of HDL in end-stage renal disease: Carbamylated-HDL attenuated endothelial cell function. Am. J. Physiol. Renal. Physiol. 2016, 310, F511–F517. [Google Scholar] [CrossRef]
- Tan, J.T.; Prosser, H.C.; Vanags, L.Z.; Monger, S.A.; Ng, M.K.; Bursill, C.A. High-density lipoproteins augment hypoxia-induced angiogenesis via regulation of post-translational modulation of hypoxia-inducible factor 1alpha. FASEB J. 2014, 28, 206–217. [Google Scholar] [CrossRef]
- Holzer, M.; Gauster, M.; Pfeifer, T.; Wadsack, C.; Fauler, G.; Stiegler, P.; Koefeler, H.; Beubler, E.; Schuligoi, R.; Heinemann, A.; et al. Protein carbamylation renders high-density lipoprotein dysfunctional. Antioxid. Redox Signal. 2011, 14, 2337–2346. [Google Scholar] [CrossRef]
- Hirano, K.; Yamashita, S.; Nakagawa, Y.; Ohya, T.; Matsuura, F.; Tsukamoto, K.; Okamoto, Y.; Matsuyama, A.; Matsumoto, K.; Miyagawa, J.; et al. Expression of human scavenger receptor class B type I in cultured human monocyte-derived macrophages and atherosclerotic lesions. Circ. Res. 1999, 85, 108–116. [Google Scholar] [CrossRef]
- Khan, A.A.; Alsahli, M.A.; Rahmani, A.H. Myeloperoxidase as an Active Disease Biomarker: Recent Biochemical and Pathological Perspectives. Med. Sci. 2018, 6, 33. [Google Scholar] [CrossRef]
- Charles-Schoeman, C.; Lee, Y.Y.; Grijalva, V.; Amjadi, S.; FitzGerald, J.; Ranganath, V.K.; Taylor, M.; McMahon, M.; Paulus, H.E.; Reddy, S.T. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann. Rheum. Dis. 2012, 71, 1157–1162. [Google Scholar] [CrossRef]
- Kumar, A.P.; Reynolds, W.F. Statins downregulate myeloperoxidase gene expression in macrophages. Biochem. Biophys. Res. Commun. 2005, 331, 442–451. [Google Scholar] [CrossRef]
- Shi, J.; van Veelen, P.A.; Mahler, M.; Janssen, G.M.; Drijfhout, J.W.; Huizinga, T.W.; Toes, R.E.; Trouw, L.A. Carbamylation and antibodies against carbamylated proteins in autoimmunity and other pathologies. Autoimmun. Rev. 2014, 13, 225–230. [Google Scholar] [CrossRef]
- Verheul, M.K.; Yee, A.; Seaman, A.; Janssen, G.M.; van Veelen, P.A.; Drijfhout, J.W.; Toes, R.E.M.; Mahler, M.; Trouw, L.A. Identification of carbamylated alpha 1 anti-trypsin (A1AT) as an antigenic target of anti-CarP antibodies in patients with rheumatoid arthritis. J. Autoimmun. 2017, 80, 77–84. [Google Scholar] [CrossRef]
- Gyorgy, B.; Toth, E.; Tarcsa, E.; Falus, A.; Buzas, E.I. Citrullination: A posttranslational modification in health and disease. Int. J. Biochem. Cell Biol. 2006, 38, 1662–1677. [Google Scholar] [CrossRef]
- Vossenaar, E.R.; Zendman, A.J.; van Venrooij, W.J.; Pruijn, G.J. PAD, a growing family of citrullinating enzymes: Genes, features and involvement in disease. Bioessays 2003, 25, 1106–1118. [Google Scholar] [CrossRef]
- Shen, S.; Wang, X.; Lv, H.; Shi, Y.; Xiao, L. PADI4 mediates autophagy and participates in the role of ganoderic acid A monomers in delaying the senescence of Alzheimer’s cells through the Akt/mTOR pathway. Biosci. Biotechnol. Biochem. 2021, 85, 1818–1829. [Google Scholar] [CrossRef]
- Sorice, M.; Iannuccelli, C.; Manganelli, V.; Capozzi, A.; Alessandri, C.; Lococo, E.; Garofalo, T.; Di Franco, M.; Bombardieri, M.; Nerviani, A.; et al. Autophagy generates citrullinated peptides in human synoviocytes: A possible trigger for anti-citrullinated peptide antibodies. Rheumatology 2016, 55, 1374–1385. [Google Scholar] [CrossRef]
- Ireland, J.M.; Unanue, E.R. Processing of proteins in autophagy vesicles of antigen-presenting cells generates citrullinated peptides recognized by the immune system. Autophagy 2012, 8, 429–430. [Google Scholar] [CrossRef]
- Derksen, V.; Huizinga, T.W.J.; van der Woude, D. The role of autoantibodies in the pathophysiology of rheumatoid arthritis. Semin. Immunopathol. 2017, 39, 437–446. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef]
- Aggarwal, R.; Liao, K.; Nair, R.; Ringold, S.; Costenbader, K.H. Anti-citrullinated peptide antibody assays and their role in the diagnosis of rheumatoid arthritis. Arthritis Rheum. 2009, 61, 1472–1483. [Google Scholar] [CrossRef]
- Spinelli, F.R.; Pecani, A.; Conti, F.; Mancini, R.; Alessandri, C.; Valesini, G. Post-translational modifications in rheumatoid arthritis and atherosclerosis: Focus on citrullination and carbamylation. J. Int. Med. Res. 2016, 44 (Suppl. S1), 81–84. [Google Scholar] [CrossRef]
- Westerlind, H.; Rönnelid, J.; Hansson, M.; Alfredsson, L.; Mathsson-Alm, L.; Serre, G.; Cornillet, M.; Holmdahl, R.; Jakobsson, P.J.; Skriner, K.; et al. Anti-Citrullinated Protein Antibody Specificities, Rheumatoid Factor Isotypes, and Incident Cardiovascular Events in Patients With Rheumatoid Arthritis. Arthritis Rheumatol. 2020, 72, 1658–1667. [Google Scholar] [CrossRef]
- Arts, E.E.; Fransen, J.; Den Broeder, A.A.; van Riel, P.; Popa, C.D. Low disease activity (DAS28≤3.2) reduces the risk of first cardiovascular event in rheumatoid arthritis: A time-dependent Cox regression analysis in a large cohort study. Ann. Rheum. Dis. 2017, 76, 1693–1699. [Google Scholar] [CrossRef]
- Barbarroja, N.; Pérez-Sanchez, C.; Ruiz-Limon, P.; Castro-Villegas, C.; Aguirre, M.A.; Carretero, R.; Segui, P.; Jimenez-Gomez, Y.; Sanna, M.; Rodriguez-Ariza, A.; et al. Anticyclic citrullinated protein antibodies are implicated in the development of cardiovascular disease in rheumatoid arthritis. Arter. Thromb. Vasc. Biol. 2014, 34, 2706–2716. [Google Scholar] [CrossRef]
- Rajamohan, A.; Heit, B.; Cairns, E.; Barra, L. Citrullinated and homocitrullinated low- density lipoprotein in rheumatoid arthritis. Scand. J. Rheumatol. 2021, 50, 343–350. [Google Scholar] [CrossRef]
- Ross, R. Atherosclerosis—An Inflammatory Disease. N. Engl. J. Med. 1999, 340, 115–126. [Google Scholar] [CrossRef]
- Pattarabanjird, T.; Li, C.; McNamara, C. B Cells in Atherosclerosis: Mechanisms and Potential Clinical Applications. JACC Basic Transl. Sci. 2021, 6, 546–563. [Google Scholar] [CrossRef]
- Schioppo, T.; Ubiali, T.; Ingegnoli, F.; Bollati, V.; Caporali, R. The role of extracellular vesicles in rheumatoid arthritis: A systematic review. Clin. Rheumatol. 2021, 40, 3481–3497. [Google Scholar] [CrossRef]
- Alghamdi, M.; Alamry, S.A.; Bahlas, S.M.; Uversky, V.N.; Redwan, E.M. Circulating extracellular vesicles and rheumatoid arthritis: A proteomic analysis. Cell Mol. Life Sci. 2021, 79, 25. [Google Scholar] [CrossRef]
- Riitano, G.; Recalchi, S.; Capozzi, A.; Manganelli, V.; Misasi, R.; Garofalo, T.; Sorice, M.; Longo, A. The Role of Autophagy as a Trigger of Post-Translational Modifications of Proteins and Extracellular Vesicles in the Pathogenesis of Rheumatoid Arthritis. Int. J. Mol. Sci. 2023, 24, 12764. [Google Scholar] [CrossRef]
- Arntz, O.J.; Pieters, B.C.H.; Thurlings, R.M.; Wenink, M.H.; van Lent, P.L.E.M.; Koenders, M.I.; van den Hoogen, F.H.J.; van der Kraan, P.M.; van de Loo, F.A.J. Rheumatoid Arthritis Patients With Circulating Extracellular Vesicles Positive for IgM Rheumatoid Factor Have Higher Disease Activity. Front. Immunol. 2018, 9, 2388. [Google Scholar] [CrossRef]
- Shi, J.; van de Stadt, L.A.; Levarht, E.W.; Huizinga, T.W.; Toes, R.E.; Trouw, L.A.; van Schaardenburg, D. Anti-carbamylated protein antibodies are present in arthralgia patients and predict the development of rheumatoid arthritis. Arthritis Rheum. 2013, 65, 911–915. [Google Scholar] [CrossRef]
- El Hawary, M.S.; Hassan, S.A.; SA, E.L.; Khalil, N.M. Anti-Carbamylated Protein Antibodies, Tumour Necrosis Factor Alpha and Insulin Resistance in Egyptian Patients With Rheumatoid Arthritis and Systemic Lupus Erythematosus. Reumatol. Clin. (Engl. Ed.) 2021, 18, 469–474. [Google Scholar] [CrossRef]
- Humphreys, J.H.; Verheul, M.K.; Barton, A.; MacGregor, A.J.; Lunt, M.; Toes, R.E.; Symmons, D.P.; Trouw, L.A.; Verstappen, S.M. Anticarbamylated protein antibodies are associated with long-term disability and increased disease activity in patients with early inflammatory arthritis: Results from the Norfolk Arthritis Register. Ann. Rheum. Dis. 2016, 75, 1139–1144. [Google Scholar] [CrossRef]
- Shi, J.; Knevel, R.; Suwannalai, P.; van der Linden, M.P.; Janssen, G.M.; van Veelen, P.A.; Levarht, N.E.; van der Helm-van Mil, A.H.; Cerami, A.; Huizinga, T.W.; et al. Autoantibodies recognizing carbamylated proteins are present in sera of patients with rheumatoid arthritis and predict joint damage. Proc. Natl. Acad. Sci. USA 2011, 108, 17372–17377. [Google Scholar] [CrossRef]
- Truchetet, M.E.; Dublanc, S.; Barnetche, T.; Vittecoq, O.; Mariette, X.; Richez, C.; Blanco, P.; Mahler, M.; Contin-Bordes, C.; Schaeverbeke, T.; et al. Association of the Presence of Anti-Carbamylated Protein Antibodies in Early Arthritis With a Poorer Clinical and Radiologic Outcome: Data From the French ESPOIR Cohort. Arthritis Rheumatol. 2017, 69, 2292–2302. [Google Scholar] [CrossRef]
- Vidal-Bralo, L.; Perez-Pampin, E.; Regueiro, C.; Montes, A.; Varela, R.; Boveda, M.D.; Gomez-Reino, J.J.; Gonzalez, A. Anti-carbamylated protein autoantibodies associated with mortality in Spanish rheumatoid arthritis patients. PLoS ONE 2017, 12, e0180144. [Google Scholar] [CrossRef]
- Spinelli, F.R.; Pecani, A.; Ciciarello, F.; Colasanti, T.; Di Franco, M.; Miranda, F.; Conti, F.; Valesini, G.; Alessandri, C. Association between antibodies to carbamylated proteins and subclinical atherosclerosis in rheumatoid arthritis patients. BMC Musculoskelet. Disord. 2017, 18, 214. [Google Scholar] [CrossRef]
- Binder, C.J.; Silverman, G.J. Natural antibodies and the autoimmunity of atherosclerosis. Springer Semin. Immunopathol. 2005, 26, 385–404. [Google Scholar] [CrossRef]
- Jones, P.W.; Mallat, Z.; Nus, M. T-Cell/B-Cell Interactions in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2024, 44, 1502–1511. [Google Scholar] [CrossRef]
- Porsch, F.; Binder, C.J. Impact of B-Cell-Targeted Therapies on Cardiovascular Disease. Arter. Thromb. Vasc. Biol. 2019, 39, 1705–1714. [Google Scholar] [CrossRef] [PubMed]
- Porsch, F.; Mallat, Z.; Binder, C.J. Humoral immunity in atherosclerosis and myocardial infarction: From B cells to antibodies. Cardiovasc. Res. 2021, 117, 2544–2562. [Google Scholar] [CrossRef] [PubMed]
- Lorenzo, C.; Delgado, P.; Busse, C.E.; Sanz-Bravo, A.; Martos-Folgado, I.; Bonzon-Kulichenko, E.; Ferrarini, A.; Gonzalez-Valdes, I.B.; Mur, S.M.; Roldán-Montero, R.; et al. ALDH4A1 is an atherosclerosis auto-antigen targeted by protective antibodies. Nature 2021, 589, 287–292. [Google Scholar] [CrossRef] [PubMed]
- Lourida, E.S.; Georgiadis, A.N.; Papavasiliou, E.C.; Papathanasiou, A.I.; Drosos, A.A.; Tselepis, A.D. Patients with early rheumatoid arthritis exhibit elevated autoantibody titers against mildly oxidized low-density lipoprotein and exhibit decreased activity of the lipoprotein-associated phospholipase A2. Arthritis Res. Ther. 2007, 9, R19. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Youssef, M.; Mosaad, Y.M. Antibodies against oxidized low-density lipoprotein are associated with subclinical atherosclerosis in recent-onset rheumatoid arthritis. Clin. Rheumatol. 2010, 29, 1237–1243. [Google Scholar] [CrossRef]
- Cvetkovic, J.T.; Wållberg-Jonsson, S.; Ahmed, E.; Rantapää-Dahlqvist, S.; Lefvert, A.K. Increased levels of autoantibodies against copper-oxidized low density lipoprotein, malondialdehyde-modified low density lipoprotein and cardiolipin in patients with rheumatoid arthritis. Rheumatology 2002, 41, 988–995. [Google Scholar] [CrossRef]
- Ames, P.R.; Delgado Alves, J.; Lopez, L.R.; Gentile, F.; Margarita, A.; Pizzella, L.; Batuca, J.; Scenna, G.; Brancaccio, V.; Matsuura, E. Antibodies against beta2-glycoprotein I complexed with an oxidised lipoprotein relate to intima thickening of carotid arteries in primary antiphospholipid syndrome. Clin. Dev. Immunol. 2006, 13, 1–9. [Google Scholar] [PubMed]
- Lopez, L.R.; Salazar-Paramo, M.; Palafox-Sanchez, C.; Hurley, B.L.; Matsuura, E.; Garcia-De La Torre, I. Oxidized low-density lipoprotein and beta2-glycoprotein I in patients with systemic lupus erythematosus and increased carotid intima-media thickness: Implications in autoimmune-mediated atherosclerosis. Lupus 2006, 15, 80–86. [Google Scholar] [CrossRef]
- Deroissart, J.; Binder, C.J.; Porsch, F. Role of Antibodies and Their Specificities in Atherosclerotic Cardiovascular Disease. Arter. Thromb. Vasc. Biol. 2024, 44, 2154–2168. [Google Scholar] [CrossRef] [PubMed]
- Witztum, J.L.; Lichtman, A.H. The Influence of Innate and Adaptive Immune Responses on Atherosclerosis. Annu. Rev. Pathol. Mech. Dis. 2014, 9, 73–102. [Google Scholar] [CrossRef] [PubMed]
- Tsimikas, S.; Miyanohara, A.; Hartvigsen, K.; Merki, E.; Shaw, P.X.; Chou, M.Y.; Pattison, J.; Torzewski, M.; Sollors, J.; Friedmann, T.; et al. Human oxidation-specific antibodies reduce foam cell formation and atherosclerosis progression. J. Am. Coll. Cardiol. 2011, 58, 1715–1727. [Google Scholar] [CrossRef]
- Taleb, A.; Willeit, P.; Amir, S.; Perkmann, T.; Kozma, M.O.; Watzenböck, M.L.; Binder, C.J.; Witztum, J.L.; Tsimikas, S. High immunoglobulin-M levels to oxidation-specific epitopes are associated with lower risk of acute myocardial infarction. J. Lipid Res. 2023, 64, 100391. [Google Scholar] [CrossRef]
- Rahman, M.; Sing, S.; Golabkesh, Z.; Fiskesund, R.; Gustafsson, T.; Jogestrand, T.; Frostegård, A.G.; Hafström, I.; Liu, A.; Frostegård, J. IgM antibodies against malondialdehyde and phosphorylcholine are together strong protection markers for atherosclerosis in systemic lupus erythematosus: Regulation and underlying mechanisms. Clin. Immunol. 2016, 166–167, 27–37. [Google Scholar] [CrossRef]
- Ajeganova, S.; Andersson, M.L.E.; Frostegård, J.; Hafström, I. Higher levels of anti-phosphorylcholine autoantibodies in early rheumatoid arthritis indicate lower risk of incident cardiovascular events. Arthritis Res. Ther. 2021, 23, 201. [Google Scholar] [CrossRef]
- Harrison, J.; Newland, S.A.; Jiang, W.; Giakomidi, D.; Zhao, X.; Clement, M.; Masters, L.; Corovic, A.; Zhang, X.; Drago, F.; et al. Marginal zone B cells produce ‘natural’ atheroprotective IgM antibodies in a T cell–dependent manner. Cardiovasc. Res. 2024, 120, 318–328. [Google Scholar] [CrossRef]
- Karpouzas, G.A.; Ormseth, S.R.; Ronda, N.; Hernandez, E.; Budoff, M.J. Lipoprotein oxidation may underlie the paradoxical association of low cholesterol with coronary atherosclerotic risk in rheumatoid arthritis. J. Autoimmun. 2022, 129, 102815. [Google Scholar] [CrossRef]
- Cinoku, I.; Mavragani, C.P.; Tellis, C.C.; Nezos, A.; Tselepis, A.D.; Moutsopoulos, H.M. Autoantibodies to ox-LDL in Sjögren’s syndrome: Are they atheroprotective? Clin. Exp. Rheumatol. 2018, 36 (Suppl. S112), 61–67. [Google Scholar]
- Rodríguez-Carrio, J.; Cerro-Pardo, I.; Lindholt, J.S.; Bonzon-Kulichenko, E.; Martínez-López, D.; Roldán-Montero, R.; Escolà-Gil, J.C.; Michel, J.B.; Blanco-Colio, L.M.; Vázquez, J.; et al. Malondialdehyde-modified HDL particles elicit a specific IgG response in abdominal aortic aneurysm. Free. Radic. Biol. Med. 2021, 174, 171–181. [Google Scholar] [CrossRef]
- Zhang, L.; Li, P.; Li, Y.; Qu, W.; Shi, Y.; Zhang, T.; Chen, Y. The role of immunoglobins in atherosclerosis development; friends or foe? Mol. Cell. Biochem. 2025, 480, 2737–2747. [Google Scholar] [CrossRef]
- Atzeni, F.; Rodríguez-Carrio, J.; Popa, C.D.; Nurmohamed, M.T.; Szűcs, G.; Szekanecz, Z. Cardiovascular effects of approved drugs for rheumatoid arthritis. Nat. Rev. Rheumatol. 2021, 17, 270–290. [Google Scholar] [CrossRef]
- Atzeni, F.; Maiani, S.; Corda, M.; Rodríguez-Carrio, J. Diagnosis and management of cardiovascular risk in rheumatoid arthritis: Main challenges and research agenda. Expert Rev. Clin. Immunol. 2023, 19, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Carrio, J.; Alperi-López, M.; López, P.; Pérez-Álvarez, Á.I.; Robinson, G.A.; Alonso-Castro, S.; Amigo-Grau, N.; Atzeni, F.; Suárez, A. Humoral responses against HDL are linked to lipoprotein traits, atherosclerosis, inflammation and pathogenic pathways during early arthritis stages. Rheumatology 2023, 62, 2898–2907. [Google Scholar] [CrossRef]
- Finckh, A.; Courvoisier, D.S.; Pagano, S.; Bas, S.; Chevallier-Ruggeri, P.; Hochstrasser, D.; Roux-Lombard, P.; Gabay, C.; Vuilleumier, N. Evaluation of cardiovascular risk in patients with rheumatoid arthritis: Do cardiovascular biomarkers offer added predictive ability over established clinical risk scores? Arthritis Care Res. 2012, 64, 817–825. [Google Scholar] [CrossRef] [PubMed]
- Rubini Gimenez, M.; Pagano, S.; Virzi, J.; Montecucco, F.; Twerenbold, R.; Reichlin, T.; Wildi, K.; Grueter, D.; Jaeger, C.; Haaf, P.; et al. Diagnostic and prognostic value of autoantibodies anti-apolipoprotein A-1 and anti-phosphorylcholine in acute non-ST elevation myocardial infarction. Eur. J. Clin. Investig. 2015, 45, 369–379. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. Receptor-mediated endocytosis: Insights from the lipoprotein receptor system. Proc. Natl. Acad. Sci. USA 1979, 76, 3330–3337. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Goldstein, J.L.; Krieger, M.; Ho, Y.K.; Anderson, R.G. Reversible accumulation of cholesteryl esters in macrophages incubated with acetylated lipoproteins. J. Cell Biol. 1979, 82, 597–613. [Google Scholar] [CrossRef]
- Suzuki, H.; Kurihara, Y.; Takeya, M.; Kamada, N.; Kataoka, M.; Jishage, K.; Ueda, O.; Sakaguchi, H.; Higashi, T.; Suzuki, T.; et al. A role for macrophage scavenger receptors in atherosclerosis and susceptibility to infection. Nature 1997, 386, 292–296. [Google Scholar] [CrossRef]
- Yu, X.; Guo, C.; Fisher, P.B.; Subjeck, J.R.; Wang, X.Y. Scavenger Receptors: Emerging Roles in Cancer Biology and Immunology. Adv. Cancer Res. 2015, 128, 309–364. [Google Scholar]
- Cornejo, F.; Vruwink, M.; Metz, C.; Muñoz, P.; Salgado, N.; Poblete, J.; Andrés, M.E.; Eugenín, J.; von Bernhardi, R. Scavenger Receptor-A deficiency impairs immune response of microglia and astrocytes potentiating Alzheimer’s disease pathophysiology. Brain Behav. Immun. 2018, 69, 336–350. [Google Scholar] [CrossRef] [PubMed]
- Handberg, A.; Levin, K.; Højlund, K.; Beck-Nielsen, H. Identification of the oxidized low-density lipoprotein scavenger receptor CD36 in plasma: A novel marker of insulin resistance. Circulation 2006, 114, 1169–1176. [Google Scholar] [CrossRef]
- Ramirez-Ortiz, Z.G.; Pendergraft, W.F., 3rd; Prasad, A.; Byrne, M.H.; Iram, T.; Blanchette, C.J.; Luster, A.D.; Hacohen, N.; El Khoury, J.; Means, T.K. The scavenger receptor SCARF1 mediates the clearance of apoptotic cells and prevents autoimmunity. Nat. Immunol. 2013, 14, 917–926. [Google Scholar] [CrossRef] [PubMed]
- Kzhyshkowska, J.; Neyen, C.; Gordon, S. Role of macrophage scavenger receptors in atherosclerosis. Immunobiology 2012, 217, 492–502. [Google Scholar] [CrossRef]
- de Winther, M.P.J.; van Dijk, K.W.; Havekes, L.M.; Hofker, M.H. Macrophage Scavenger Receptor Class A. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 290–297. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, J.; Cui, W.; Silverstein, R.L. CD36, a signaling receptor and fatty acid transporter that regulates immune cell metabolism and fate. J. Exp. Med. 2022, 219, e20211314. [Google Scholar] [CrossRef]
- Akhmedov, A.; Sawamura, T.; Chen, C.-H.; Kraler, S.; Vdovenko, D.; Lüscher, T.F. Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1): A crucial driver of atherosclerotic cardiovascular disease. Eur. Heart J. 2020, 42, 1797–1807. [Google Scholar] [CrossRef]
- Grundy, S.M. Scavenger Receptor B-1 Emerges as Anti-atherogenic Candidate. Cell Metab. 2016, 23, 755–757. [Google Scholar] [CrossRef]
- Huang, L.; Chambliss, K.L.; Gao, X.; Yuhanna, I.S.; Behling-Kelly, E.; Bergaya, S.; Ahmed, M.; Michaely, P.; Luby-Phelps, K.; Darehshouri, A.; et al. SR-B1 drives endothelial cell LDL transcytosis via DOCK4 to promote atherosclerosis. Nature 2019, 569, 565–569. [Google Scholar] [CrossRef]
- Mineo, C. Lipoprotein receptor signalling in atherosclerosis. Cardiovasc. Res. 2019, 116, 1254–1274. [Google Scholar] [CrossRef]
- Hu, F.; Jiang, X.; Guo, C.; Li, Y.; Chen, S.; Zhang, W.; Du, Y.; Wang, P.; Zheng, X.; Fang, X.; et al. Scavenger receptor-A is a biomarker and effector of rheumatoid arthritis: A large-scale multicenter study. Nat. Commun. 2020, 11, 1911. [Google Scholar] [CrossRef]
- Hashizume, M.; Mihara, M. Blockade of IL-6 and TNF-α inhibited oxLDL-induced production of MCP-1 via scavenger receptor induction. Eur. J. Pharmacol. 2012, 689, 249–254. [Google Scholar] [CrossRef]
- Wen, W.; He, M.; Liang, X.; Gao, S.S.; Zhou, J.; Yuan, Z.Y. Accelerated transformation of macrophage-derived foam cells in the presence of collagen-induced arthritis mice serum is associated with dyslipidemia. Autoimmunity 2016, 49, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Bañuelos, E.; Martín-Márquez, B.T.; Martínez-García, E.A.; Figueroa-Sanchez, M.; Nuñez-Atahualpa, L.; Rocha-Muñoz, A.D.; Sánchez-Hernández, P.E.; Navarro-Hernandez, R.E.; Madrigal-Ruiz, P.M.; Saldaña-Millan, A.A.; et al. Low levels of CD36 in peripheral blood monocytes in subclinical atherosclerosis in rheumatoid arthritis: A cross-sectional study in a Mexican population. BioMed Res. Int. 2014, 2014, 736786. [Google Scholar] [CrossRef]
- Ishikawa, M.; Ito, H.; Akiyoshi, M.; Kume, N.; Yoshitomi, H.; Mitsuoka, H.; Tanida, S.; Murata, K.; Shibuya, H.; Kasahara, T.; et al. Lectin-like oxidized low-density lipoprotein receptor 1 signal is a potent biomarker and therapeutic target for human rheumatoid arthritis. Arthritis Rheum. 2012, 64, 1024–1034. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, T.; Akagi, M.; Hoshikawa, H.; Chen, M.; Yasuda, T.; Mukai, S.; Ohsawa, K.; Masaki, T.; Nakamura, T.; Sawamura, T. Lectin-like oxidized low-density lipoprotein receptor 1 mediates leukocyte infiltration and articular cartilage destruction in rat zymosan-induced arthritis. Arthritis Rheum. 2002, 46, 2486–2494. [Google Scholar] [CrossRef]
- Akhmedov, A.; Crucet, M.; Simic, B.; Kraler, S.; Bonetti, N.R.; Ospelt, C.; Distler, O.; Ciurea, A.; Liberale, L.; Jauhiainen, M.; et al. TNFα induces endothelial dysfunction in rheumatoid arthritis via LOX-1 and arginase 2: Reversal by monoclonal TNFα antibodies. Cardiovasc. Res. 2022, 118, 254–266. [Google Scholar] [CrossRef]
- Voloshyna, I.; Modayil, S.; Littlefield, M.J.; Belilos, E.; Belostocki, K.; Bonetti, L.; Rosenblum, G.; Carsons, S.E.; Reiss, A.B. Plasma from rheumatoid arthritis patients promotes pro-atherogenic cholesterol transport gene expression in THP-1 human macrophages. Exp. Biol. Med. 2013, 238, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- van Lieshout, A.W.; Popa, C.; Meyer-Wentrup, F.; Lemmers, H.L.; Stalenhoef, A.F.; Adema, G.J.; van Riel, P.L.; van Tits, L.J.; Radstake, T.R. Circulating CXCL16 is not related to circulating oxLDL in patients with rheumatoid arthritis. Biochem. Biophys. Res. Commun. 2007, 355, 392–397. [Google Scholar] [CrossRef]
- Riitano, G.; Capozzi, A.; Recalchi, S.; Caissutti, D.; Longo, A.; Mattei, V.; Conti, F.; Misasi, R.; Garofalo, T.; Sorice, M.; et al. Anti-β2-GPI Antibodies Induce Endothelial Cell Expression of Tissue Factor by LRP6 Signal Transduction Pathway Involving Lipid Rafts. Cells 2022, 11, 1288. [Google Scholar] [CrossRef]
- Riitano, G.; Capozzi, A.; Recalchi, S.; Augusto, M.; Conti, F.; Misasi, R.; Garofalo, T.; Sorice, M.; Manganelli, V. Role of Lipid Rafts on LRP8 Signaling Triggered by Anti-β2-GPI Antibodies in Endothelial Cells. Biomedicines 2023, 11, 3135. [Google Scholar] [CrossRef] [PubMed]
- Shen, C.; Lu, Y.; Zhang, B.; Figueiredo, D.; Bean, J.; Jung, J.; Wu, H.; Barik, A.; Yin, D.M.; Xiong, W.C.; et al. Antibodies against low-density lipoprotein receptor-related protein 4 induce myasthenia gravis. J. Clin. Investig. 2013, 123, 5190–5202. [Google Scholar] [CrossRef] [PubMed]
- Zehra, S.; Ghouri, M.; Jafari, H.; Saleem, S.; Fatima, S.; Azhar, A. Single nucleotide polymorphisms (rs3736228 and rs4988321) in low-density lipoprotein receptor-related protein-5 gene with predisposition to rheumatoid arthritis. Gene 2023, 851, 147025. [Google Scholar] [CrossRef]
- Bernardes, M.; Durães, C.; Oliveira, A.; Martins, M.J.; Lucas, R.; Costa, L.; Pereira, J.G.; Ramos, I.; Machado, J.C.; Simões-Ventura, F. LRP5 gene polymorphisms and radiographic joint damage in rheumatoid arthritis patients. Osteoporos. Int. 2018, 29, 2355–2368. [Google Scholar] [CrossRef]
- Ooka, S.; Matsui, T.; Nishioka, K.; Kato, T. Autoantibodies to low-density-lipoprotein-receptor-related protein 2 (LRP2) in systemic autoimmune diseases. Arthritis Res. Ther. 2003, 5, R174–R180. [Google Scholar] [CrossRef]
- van Vollenhoven, R.F.; Fleischmann, R.; Cohen, S.; Lee, E.B.; García Meijide, J.A.; Wagner, S.; Forejtova, S.; Zwillich, S.H.; Gruben, D.; Koncz, T.; et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N. Engl. J. Med. 2012, 367, 508–519. [Google Scholar] [CrossRef]
- Choi, H.K.; Hernán, M.A.; Seeger, J.D.; Robins, J.M.; Wolfe, F. Methotrexate and mortality in patients with rheumatoid arthritis: A prospective study. Lancet 2002, 359, 1173–1177. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Pradhan, A.; MacFadyen, J.G.; Solomon, D.H.; Zaharris, E.; Mam, V.; Hasan, A.; Rosenberg, Y.; Iturriaga, E.; et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N. Engl. J. Med. 2018, 380, 752–762. [Google Scholar] [CrossRef]
- Roubille, C.; Richer, V.; Starnino, T.; McCourt, C.; McFarlane, A.; Fleming, P.; Siu, S.; Kraft, J.; Lynde, C.; Pope, J.; et al. The effects of tumour necrosis factor inhibitors, methotrexate, non-steroidal anti-inflammatory drugs and corticosteroids on cardiovascular events in rheumatoid arthritis, psoriasis and psoriatic arthritis: A systematic review and meta-analysis. Ann. Rheum. Dis. 2015, 74, 480–489. [Google Scholar] [CrossRef]
- Popa, C.; van Tits, L.J.H.; Barrera, P.; Lemmers, H.L.M.; van den Hoogen, F.H.J.; van Riel, P.L.C.M.; Radstake, T.R.D.J.; Netea, M.G.; Roest, M.; Stalenhoef, A.F.H. Anti-inflammatory therapy with tumour necrosis factor alpha inhibitors improves high-density lipoprotein cholesterol antioxidative capacity in rheumatoid arthritis patients. Ann. Rheum. Dis. 2009, 68, 868–872. [Google Scholar] [CrossRef]
- O’Neill, F.; Charakida, M.; Topham, E.; McLoughlin, E.; Patel, N.; Sutill, E.; Kay, C.W.M.; D’Aiuto, F.; Landmesser, U.; Taylor, P.C.; et al. Anti-inflammatory treatment improves high-density lipoprotein function in rheumatoid arthritis. Heart 2017, 103, 766–773. [Google Scholar] [CrossRef]
- Schiff, M.H.; Kremer, J.M.; Jahreis, A.; Vernon, E.; Isaacs, J.D.; van Vollenhoven, R.F. Integrated safety in tocilizumab clinical trials. Arthritis Res. Ther. 2011, 13, R141. [Google Scholar] [CrossRef]
- Strang, A.C.; Bisoendial, R.J.; Kootte, R.S.; Schulte, D.M.; Dallinga-Thie, G.M.; Levels, J.H.M.; Kok, M.; Vos, K.; Tas, S.W.; Tietge, U.J.F.; et al. Pro-atherogenic lipid changes and decreased hepatic LDL receptor expression by tocilizumab in rheumatoid arthritis. Atherosclerosis 2013, 229, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Giles, J.T.; Sattar, N.; Gabriel, S.; Ridker, P.M.; Gay, S.; Warne, C.; Musselman, D.; Brockwell, L.; Shittu, E.; Klearman, M.; et al. Cardiovascular Safety of Tocilizumab Versus Etanercept in Rheumatoid Arthritis: A Randomized Controlled Trial. Arthritis Rheumatol. 2020, 72, 31–40. [Google Scholar] [CrossRef] [PubMed]
- McInnes, I.B.; Thompson, L.; Giles, J.T.; Bathon, J.M.; Salmon, J.E.; Beaulieu, A.D.; Codding, C.E.; Carlson, T.H.; Delles, C.; Lee, J.S.; et al. Effect of interleukin-6 receptor blockade on surrogates of vascular risk in rheumatoid arthritis: MEASURE, a randomised, placebo-controlled study. Ann. Rheum. Dis. 2015, 74, 694–702. [Google Scholar] [CrossRef]
- Pierini, F.S.; Botta, E.; Soriano, E.R.; Martin, M.; Boero, L.; Meroño, T.; Saez, M.S.; Lozano Chiappe, E.; Cerda, O.; Citera, G.; et al. Effect of Tocilizumab on LDL and HDL Characteristics in Patients with Rheumatoid Arthritis. An Observational Study. Rheumatol. Ther. 2021, 8, 803–815. [Google Scholar] [CrossRef] [PubMed]
- Ytterberg, S.R.; Bhatt, D.L.; Mikuls, T.R.; Koch, G.G.; Fleischmann, R.; Rivas, J.L.; Germino, R.; Menon, S.; Sun, Y.; Wang, C.; et al. Cardiovascular and Cancer Risk with Tofacitinib in Rheumatoid Arthritis. N. Engl. J. Med. 2022, 386, 316–326. [Google Scholar] [CrossRef]
- Liu, C.; Kieltyka, J.; Fleischmann, R.; Gadina, M.; O’Shea, J.J. A Decade of JAK Inhibitors: What Have We Learned and What May Be the Future? Arthritis Rheumatol. 2021, 73, 2166–2178. [Google Scholar] [CrossRef]
- Feng, W.; Yang, K.; Ju, M.; Wang, T.; Xiao, R. Epigenetic Regulation of Cholesterol and Oxysterol Homeostasis. Nutr. Rev. 2025, nuaf082. [Google Scholar] [CrossRef]
- Buratta, S.; Tancini, B.; Sagini, K.; Delo, F.; Chiaradia, E.; Urbanelli, L.; Emiliani, C. Lysosomal Exocytosis, Exosome Release and Secretory Autophagy: The Autophagic- and Endo-Lysosomal Systems Go Extracellular. Int. J. Mol. Sci. 2020, 21, 2576. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Li, J.; Kang, R.; Tang, D. Interplay Between Lipid Metabolism and Autophagy. Front. Cell Dev. Biol. 2020, 8, 431. [Google Scholar] [CrossRef]
- Iqbal, T.; Salma, U.; Fatima, M.; Khan, M.N.; Farooq, S.; Abrar, M.; Tasleem, M.; Afzal, A. Cell membrane coated polymeric nanocarriers: A novel drug delivery approach for the targeted therapy of rheumatoid arthritis. Agrobiol. Rec. 2024, 15, 91–102. [Google Scholar] [CrossRef]
- Wani, W.Y.; Boyer-Guittaut, M.; Dodson, M.; Chatham, J.; Darley-Usmar, V.; Zhang, J. Regulation of autophagy by protein post-translational modification. Lab. Investig. 2015, 95, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Shu, F.; Xiao, H.; Li, Q.N.; Ren, X.S.; Liu, Z.G.; Hu, B.W.; Wang, H.S.; Wang, H.; Jiang, G.M. Epigenetic and post-translational modifications in autophagy: Biological functions and therapeutic targets. Signal. Transduct. Target. Ther. 2023, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Langer, H.F.; Haubner, R.; Pichler, B.J.; Gawaz, M. Radionuclide imaging: A molecular key to the atherosclerotic plaque. J. Am. Coll. Cardiol. 2008, 52, 1–12. [Google Scholar] [CrossRef]
- Li, D.; Patel, A.R.; Klibanov, A.L.; Kramer, C.M.; Ruiz, M.; Kang, B.Y.; Mehta, J.L.; Beller, G.A.; Glover, D.K.; Meyer, C.H. Molecular imaging of atherosclerotic plaques targeted to oxidized LDL receptor LOX-1 by SPECT/CT and magnetic resonance. Circ. Cardiovasc. Imaging 2010, 3, 464–472. [Google Scholar] [CrossRef]
- Sasaki, T.; Kobayashi, K.; Kita, S.; Kojima, K.; Hirano, H.; Shen, L.; Takenaka, F.; Kumon, H.; Matsuura, E. In vivo distribution of single chain variable fragment (scFv) against atherothrombotic oxidized LDL/β2-glycoprotein I complexes into atherosclerotic plaques of WHHL rabbits: Implication for clinical PET imaging. Autoimmun. Rev. 2017, 16, 159–167. [Google Scholar] [CrossRef]




| Lipoprotein PTMs in Human | PTM in RA Patients | Lipoprotein PTMs in RA Patients | Specific Humoral Responses | |
|---|---|---|---|---|
| Oxidation | Ox-LDL MDA-LDL MDA-HDL | Collagen | Ox-LDL MDA-LDL | IgM anti-Ox-LDL IgG anti-Ox-LDL IgG anti-MDA-LDL IgG anti-MDA-HDL |
| Nitration | Nit-LDL Nit-HDL | Nil | Nit-LDL among RA with CVD Nit-HDL among RA with CVD | Nil |
| Carbamylation | Carb-LDL Carb-HDL | Vimentin Alpha-Enolase Collagen | Unclear | IgG anti-Carb-LDL |
| Citrullination | Unclear | Vimentin Alpha-Enolase Collagen | Nil | Nil |
| Acetylation | Nil | Vimentin | Nil | Nil |
| Domains | Research Priorities |
|---|---|
| Methodological |
|
| Basic/translational |
|
| Clinical |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Rodríguez-Carrio, J.; Xie, Y.; Hu, F.; Chong, W.M.; Hou, H.W.; Dalan, R.; Leong, K.P. Post-Translational Modifications of Lipoproteins: Emerging Players Linking Inflammation and Cardiovascular Disease in Rheumatoid Arthritis—A Narrative Review. Int. J. Mol. Sci. 2025, 26, 8514. https://doi.org/10.3390/ijms26178514
Xu C, Rodríguez-Carrio J, Xie Y, Hu F, Chong WM, Hou HW, Dalan R, Leong KP. Post-Translational Modifications of Lipoproteins: Emerging Players Linking Inflammation and Cardiovascular Disease in Rheumatoid Arthritis—A Narrative Review. International Journal of Molecular Sciences. 2025; 26(17):8514. https://doi.org/10.3390/ijms26178514
Chicago/Turabian StyleXu, Chuanhui, Javier Rodríguez-Carrio, Yang Xie, Fanlei Hu, Wei Ming Chong, Han Wei Hou, Rinkoo Dalan, and Khai Pang Leong. 2025. "Post-Translational Modifications of Lipoproteins: Emerging Players Linking Inflammation and Cardiovascular Disease in Rheumatoid Arthritis—A Narrative Review" International Journal of Molecular Sciences 26, no. 17: 8514. https://doi.org/10.3390/ijms26178514
APA StyleXu, C., Rodríguez-Carrio, J., Xie, Y., Hu, F., Chong, W. M., Hou, H. W., Dalan, R., & Leong, K. P. (2025). Post-Translational Modifications of Lipoproteins: Emerging Players Linking Inflammation and Cardiovascular Disease in Rheumatoid Arthritis—A Narrative Review. International Journal of Molecular Sciences, 26(17), 8514. https://doi.org/10.3390/ijms26178514






