SERS- and SEIRA-Based Characterization and Sensing of Highly Selective Bradykinin B2 Receptor Antagonists
Abstract
1. Introduction
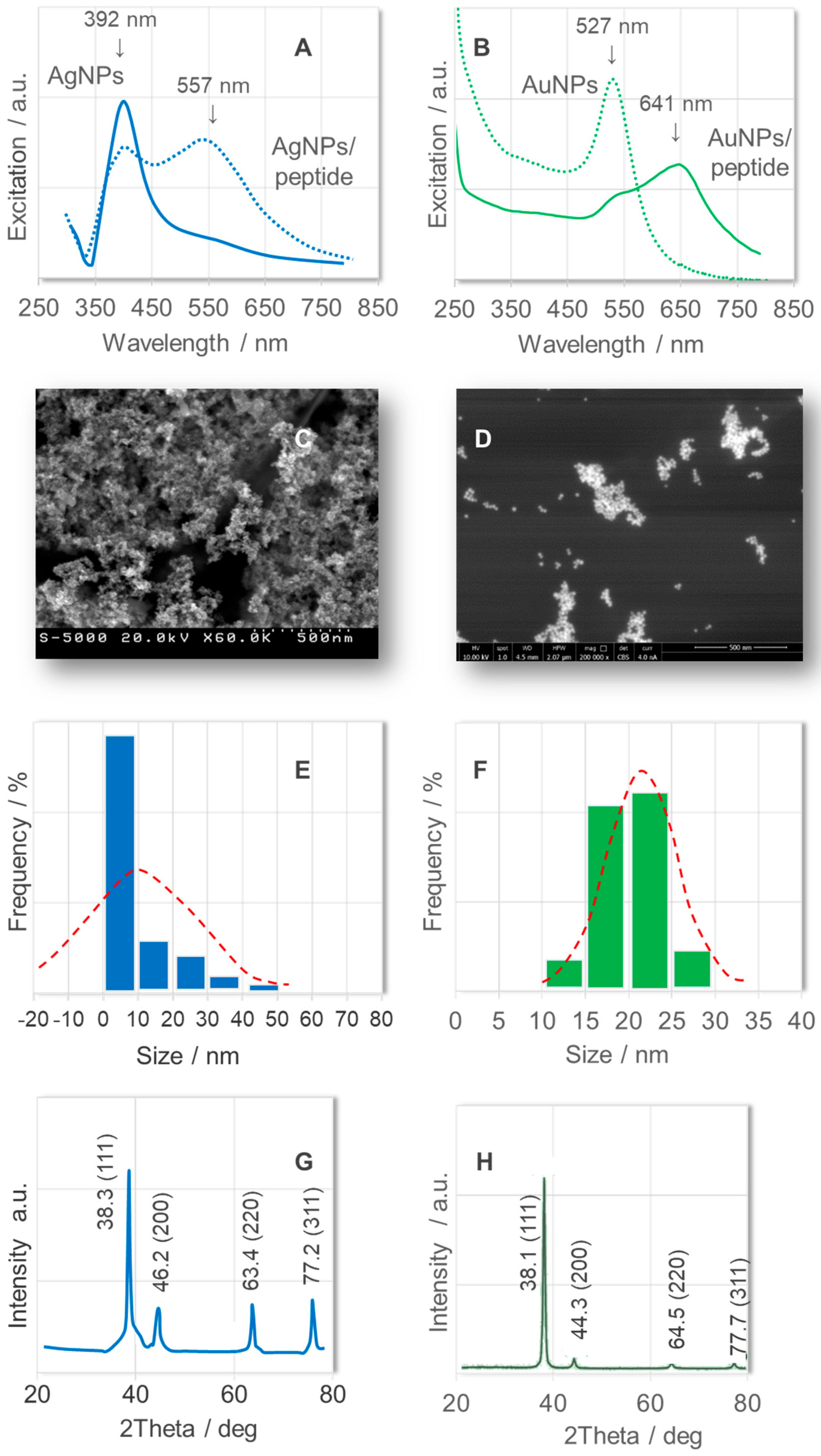
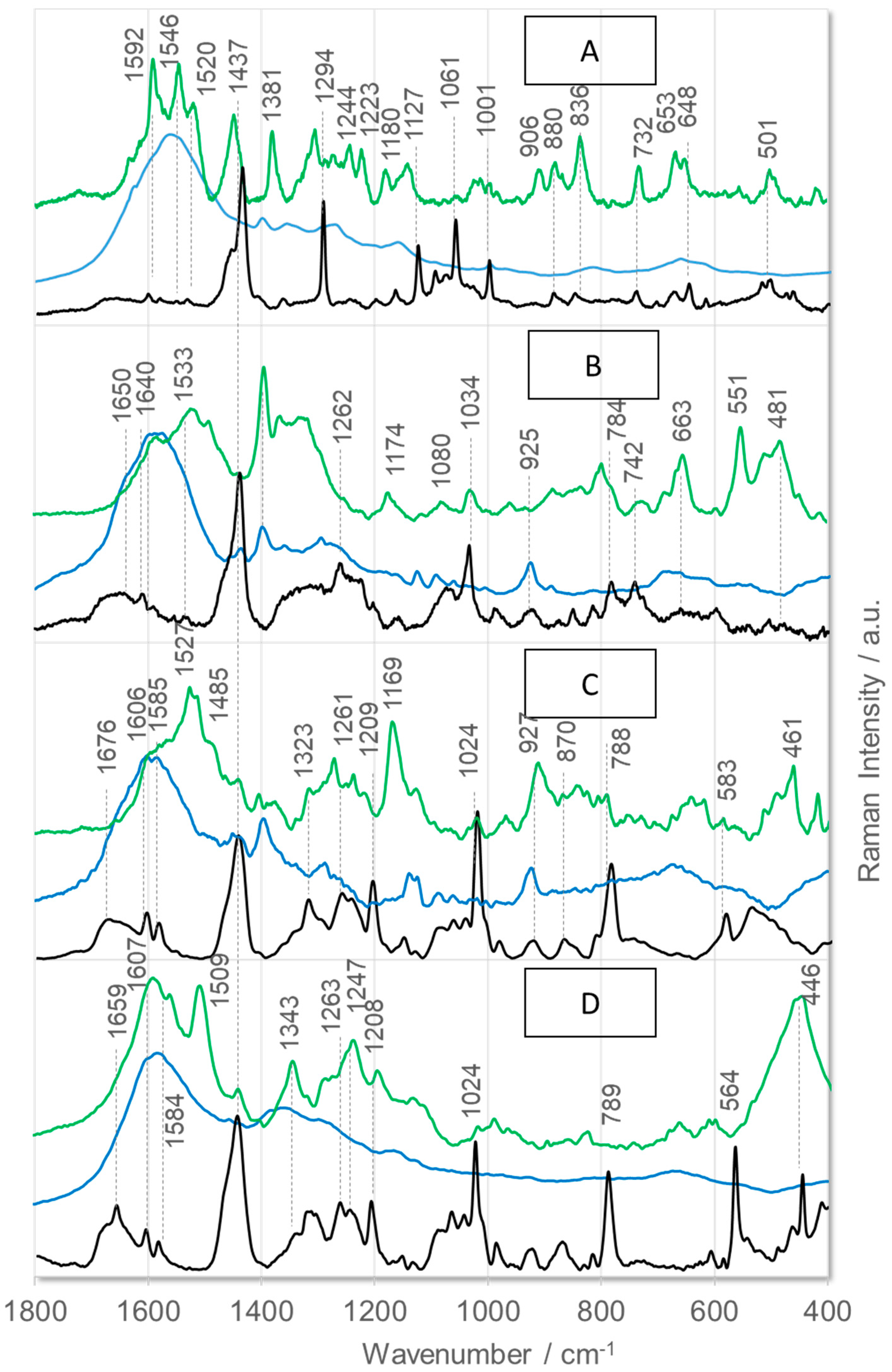
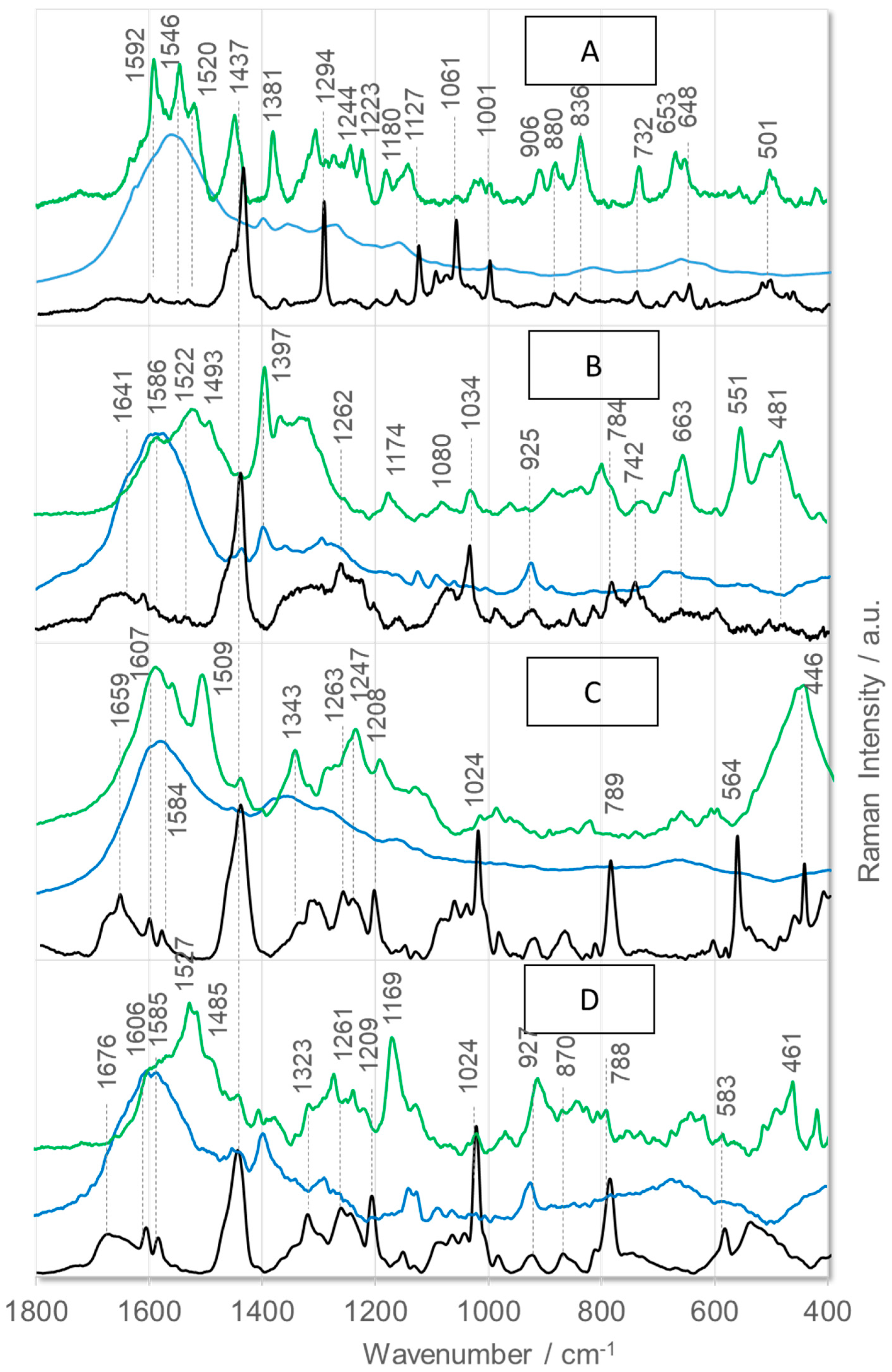
2. Results and Discussion
- -
- Phe: at 1604 cm−1 [ν8a], 1584 cm−1 [ν8b], 1204 cm−1 [ν7a], 1030 cm−1 [ν18a], 1002 cm−1 [ν12], and 619 cm−1 [ν6b] [62];
- -
- Phe(5F): at 1662 cm−1 [ν8a], 1622 cm−1 [ν8b], 1552/1514 cm−1 [ν(CC)], 1174 cm−1 [ν(CF)], 1020 cm−1 [ν(CF)], 782 cm−1 [ρt(CF)], and 655 cm−1 [ρt(CCCC)] [63];
- -
- Thi: at 1409 cm−1 [ν(C=CH) + ν(CC)+ρr(CH)], 1363 cm−1 [ρr(CH) + ν(C=C)], 1036 cm−1 [ν(CC) + ρr(CH)], 986 cm−1 [ρr(CH2)], 850 and 817 cm−1 [ν(CC) + δ(ring)], and 667 cm−1 [ρw(CH)] [45];
- -
- Arg: 1574 cm−1 [ρs(NH2)], 1466–1218 cm−1 [different CH2 group vibrations], 1176 and 1135 cm−1 [ρr(NH2)], 1085 and 1068 cm−1 [ν(CN)], 997 and 971 cm−1 [ρr(CH2)], 900–850 cm−1 [ν(CC)], 848 cm−1 [ρw(NH)], 793 cm−1 [ρr(NH)], 746 cm−1 [ρr(CH2)], and 524 cm−1 [ρt(NH2)] [64];
- -
- Pro: 1470 cm−1 [δ(CH2) + imide-II], 1438 cm−1 [imide-II + δ(CH2)], 1317 cm−1 [δ(CCαH)], 1286 cm−1 [ρr/t(CH2)], 1263, 1237, 1178, and 1102 cm−1 [δ(CH2) and/or δ(NH)], 983 and 950 cm−1 [ν(CC) + ν(CCN)], 850 cm−1 [ρr(CβH2], 780 cm−1 [ρr(CγH2], 530 cm−1 [δ(CCN)], and 480 cm−1 [pyrrolidine deformations] [65,66];
- -
- Tetrahydroisoquinoline: 1602, 1582, 1460, 1440, 1220, 1196, 1025, 724, 590, 510, and 440 cm−1 [67];
- -
- Amide bonds of disordered structures: 1660 m−1 [amide-I], 1544 cm−1 [amide-II], 1240 cm−1 [amide-III], and 644 cm−1 [amide-V] [68].
3. Materials and Methods
3.1. Synthesis of Bradykinin Antagonists
3.2. Synthesis of Colloidal Silver Nanoparticles (AgNPs)
3.3. Synthesis of Colloidal Gold Nanoparticles (AuNPs)
3.4. Sample Preparation for SERS and SEIRA Measurements
3.5. Raman and Surface-Enhanced Raman Spectroscopy (SERS) Measurements
3.6. Attenuated Total Reflection Infrared Spectroscopy (ATR-FTIR) and Surface-Enhanced Infrared Spectroscopy (SEIRA) Measurements
3.7. Scanning Electron Microscopy (SEM) Imaging
3.8. Dynamic Light Scattering (DLS) Measurements
3.9. X-Ray Powder Diffraction (XRD) Pattern Measurements
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ATR-FTIR | Attenuated total reflection Fourier transform infrared spectroscopy |
| AuNPs | Gold nanoparticles |
| BK | Bradykinin |
| FWHM | Full width at half maximum |
| GPCR | G-protein-coupled superfamily |
| Hyp | L-hydroxyproline |
| Igl | 2-indanylglycine |
| Oic | L-octahydroindole-2-carboxylic acid |
| Phe(5F) | D-pentafluorophenylalanine |
| SEIRA | Surface-enhanced infrared spectroscopy |
| SERS | Surface-enhanced Raman scattering |
| SEM | Scanning electron microscopy |
| D-Tic | D-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid |
| Thi | L-thienylalanine |
| TERS | Tip-enhanced Raman scattering |
| AgNPs | Silver nanoparticles |
| SCLC | Small cell lung cancer |
| UV-Vis | Excitation spectroscopy |
References
- Global Cancer Burden Growing, Amidst Mounting Need for Services. Available online: https://www.who.int/news/item/01-02-2024-global-cancer-burden-growing--amidst-mounting-need-for-services (accessed on 1 February 2024).
- Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/cancer (accessed on 3 February 2025).
- Yang, Y.; Sun, L.; Liu, X.; Liu, W.; Zhang, Z.; Zhou, X.; Zhao, X.; Zheng, R.; Zhang, Y.; Guo, W.; et al. Neurotransmitters: Impressive regulators of tumor progression. Biomed. Pharmacother. 2024, 176, 116844. [Google Scholar] [CrossRef] [PubMed]
- Jayachandran, P.; Battaglin, F.; Strelez, C.; Lenz, A.; Algaze, S.; Soni, S.; Lo, J.H.; Yang, Y.; Millstein, J.; Zhang, W.; et al. Breast cancer and neurotransmitters: Emerging insights on mechanisms and therapeutic directions. Oncogene 2023, 42, 627–637. [Google Scholar] [CrossRef]
- Xiao, L.; Li, X.; Fang, C.; Yu, Y.; Chen, T. Neurotransmitters: Promising immune modulators in the tumor microenvironment. Front. Immunol. Sec. Cancer Immun. Immunother. 2023, 14, 1118637. [Google Scholar] [CrossRef]
- de Visser, K.E.; Joyce, J.A. The evolving tumor microenvironment: From cancer initiation to metastatic outgrowth. Cancer Cell 2023, 41, 374–403. [Google Scholar] [CrossRef] [PubMed]
- Yu, S.; Huang, Y.; Hu, Y. Cancer-nervous system crosstalk: From biological mechanism to therapeutic opportunities. Mol. Cancer 2025, 24, 133. [Google Scholar] [CrossRef]
- Devi, L.A.; Fricker, L.D. Transmitters and Peptides: Basic Principles. In Neuroscience in the 21st Century; Pfaff, D.W., Ed.; Springer: New York, NY, USA, 2013; pp. 1487–1503. [Google Scholar]
- Jensen, R.T.; Moody, T.W. Bombesin-related peptides and neurotensin: Effects on cancer growth/proliferation and cellular signaling in cancer. In Handbook of Biologically Active Peptides; Kastin, A.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2006; pp. 429–434. [Google Scholar]
- Ananias, H.J.; van den Heuvel, M.C.; Helfrich, W.; de Jong, I.J. Expression of the Gastrin-Releasing Peptide Receptor, the Prostate Stem Cell Antigen and the Prostate-Specific Membrane Antigen in Lymph Node and Bone Metastases of Prostate Cancer. Prostate 2009, 69, 1101–1108. [Google Scholar] [CrossRef]
- Alifano, M.; Souazé, F.; Dupouy, S.; Camilleri-Broet, S.; Younes, M.; Ahmed-Zaid, S.M.; Takahashi, T.; Cancellieri, A.; Damiani, S.; Boaron, M.; et al. Neurotensin Receptor 1 Determines the Outcome of Non–Small Cell Lung Cancer. Clin. Cancer Res. 2010, 16, 4401–4410. [Google Scholar] [CrossRef] [PubMed]
- Mattei, J.; Achcar, R.D.; Cano, C.H.; Macedo, B.R.; Meurer, L.; Batlle, B.S.; Groshong, S.D.; Kulczynski, J.M.; Roesler, R.; Dal Lango, L.; et al. Gastrin-releasing peptide receptor expression in lung cancer. Arch. Pathol. Lab. Med. 2014, 138, 98–104. [Google Scholar] [CrossRef]
- Reubi, J.C.; Waser, B.; Friess, H.; Buchler, M.; Laissue, J. Neurotensin receptors: A new marker for human ductal pancreatic adenocarcinoma. Gut 1998, 42, 546–550. [Google Scholar] [CrossRef]
- Zhang, M.; Chen, T.; Lu, X.; Lan, X.; Chen, Z.; Lu, S. G protein-coupled receptors (GPCRs): Advances in structures, mechanisms and drug discovery. Signal Transduct. Target Ther. 2024, 9, 88. [Google Scholar] [CrossRef]
- Bar-Shavit, B.; Maoz, M.; Kancharla, A.; Kumar Nag, J.; Agranovich, D.; Grisaru-Granovsky, S.; Uziely, B. G Protein-Coupled Receptors in Cancer. Int. J. Mol. Sci. 2016, 17, 1320. [Google Scholar] [CrossRef]
- Ryder, N.M.; Guha, S.; Hines, O.J.; Reber, H.A.; Rozengurt, E. G protein-coupled receptor signaling in human ductal pancreatic cancer cells: Neurotensin responsiveness and mitogenic stimulation. J. Cell Physiol. 2001, 186, 53–64. [Google Scholar] [CrossRef]
- Mankoff, D.A.; Link, J.A.; Linden, H.A.; Sundararajan, L.; Krohn, K.A. Tumor Receptor Imaging. J. Nucl. Med. 2008, 49, 149S–163S. [Google Scholar] [CrossRef] [PubMed]
- Okarvi, S.M. Peptide-based radiopharmaceuticals and cytotoxic conjugates: Potential tools against cancer. Cancer Treat. Rev. 2008, 34, 13–26. [Google Scholar] [CrossRef]
- Golias, C.; Charalabopoulos, A.; Stagikas, D.; Charalabopoulos, K.; Batistatou, A. The kinin system—Bradykinin: Biological effects and clinical implications. Multiple role of the kinin system—Bradykinin. Hippokratia 2007, 11, 124–128. [Google Scholar]
- Stewart, J.M.; Gera, L. Bradykinin and cancer. In Handbook of Biologically Active Peptides; Academic Press: New York, NY, USA, 2006; Volume 63, pp. 443–446. [Google Scholar]
- Deepak, K.; Roy, P.K.; Kola, P.; Mukherjee, B.; Mandal, M. An overview of kinin mediated events in cancer progression and therapeutic applications. Biochim. Biophys. Acta-Rev. Cancer 2022, 1877, 188807. [Google Scholar] [CrossRef]
- Skidgel, R.A. Histamine, Bradykinin, and Their Antagonists. In Goodman & Gilman’s: The Pharmacological Basis of Therapeutics, 13th ed.; Brunton, L.L., Hilal-Dandan, R., Knollmann, B.C., Eds.; McGraw-Hill Education: Berkshire, UK, 2017. [Google Scholar]
- Stewart, J.M. Bradykinin Antagonists as Anti-Cancer Agents. Curr. Pharmac. Design 2003, 9, 2036–2042. [Google Scholar] [CrossRef]
- Stewart, J.M.; Gera, L.; Chan, D.C.; York, E.J.; Simkeviciene, V.; Bunn, P.A., Jr.; Taraseviciene-Stewart, L. Combination cancer chemotherapy with one compound: Pluripotent bradykinin antagonists. Peptides 2005, 26, 1288–1291. [Google Scholar] [CrossRef]
- Hock, F.J.; Wirth, K.; Albus, U.; Linz, W.; Gerhards, H.J.; Wiemer, G.; Henke, S.; Breipohl, G.; Konig, W.; Knolle, J.; et al. Hoe 140 a new potent and long acting bradykinin-antagonist: In vitro studies. Br. J. Pharmacol. 1991, 102, 769–773. [Google Scholar] [CrossRef] [PubMed]
- Griesbacher, T.; Kolbitsch, C.; Tiran, B.; Lembeck, F. Effects of the bradykinin antagonist, icatibant (Hoe 140), on pancreas and liver functions during and after caerulein-induced pancreatitis in rats. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1995, 352, 557–564. [Google Scholar] [CrossRef] [PubMed]
- Gras, J. Icatibant for hereditary angioedema. Drugs Today 2009, 45, 855–864. [Google Scholar] [CrossRef] [PubMed]
- Introduction to NPC 349 Peptide. Available online: https://ontosight.ai/glossary/term/introduction-to-npc-349-peptide--67a276b2c445bf945af12999 (accessed on 17 August 2025).
- Kindgen-Milles, D.; Klement, W. Pain and inflammation evoked in human skin by bradykinin receptor antagonists. Eur. J. Pharmacol. 1992, 218, 183–185. [Google Scholar] [CrossRef] [PubMed]
- Lehmberg, J.; Beck, J.; Baethmann, A.; Uhl, E. Influence of the bradykinin B1/B2-receptor-antagonist B 9430 on the cerebral microcirculation and outcome of gerbils from global cerebral ischemia. Acta Neurochir. Suppl. 2000, 76, 39–41. [Google Scholar]
- Kiew, L.-V.; Chang, C.-Y.; Huang, S.-Y.; Wang, P.-W.; Heh, C.-H.; Liu, C.-T.; Cheng, C.-H.; Lu, Y.-X.; Chen, Y.-C.; Huang, Y.-X.; et al. Development of flexible electrochemical impedance spectroscopy-basedbiosensing platform for rapid screening of SARS-CoV-2 inhibitors. Biosens Bioelectron. 2021, 183, 113213. [Google Scholar] [CrossRef] [PubMed]
- Schnaider, L.; Ghosh, M.; Bychenko, D.; Grigoriants, I.; Ya’ari, S.; Antsel, T.S.; Matalon, S.; Sarig, R.; Brosh, T.; Pilo, R.; et al. Enhanced Nanoassembly-Incorporated Antibacterial Composite Materials. ACS Appl. Mater. Interf. 2019, 11, 21334−21342. [Google Scholar] [CrossRef]
- Gera, L.; Stewart, J.M. Novel highly potent bradykinin antagonists containing pentafluorophenylalanine. Pept. Front. Pept. Sci. 2002, 5, 730–732. [Google Scholar]
- Kim, M.; Choi, Y.S.; Jeong, D.H. SERS detection of dopamine using metal-chelated Ag nanoshell. RSC Adv. 2024, 14, 14214. [Google Scholar] [CrossRef]
- Ciallaa, D.; Polloka, S.; Steinbrücker, C.; Weber, K.; Popp, J. SERS-based detection of biomolecules. Nanophotonics 2014, 3, 383–411. [Google Scholar] [CrossRef]
- Lussier, F.; Brulé, T.; Bourque, M.J.; Ducrot, C.; Trudeau, L.E.; Masson, J.F. Dynamic SERS nanosensor for neurotransmitter sensing near neurons. Faraday Disc. 2017, 205, 387–407. [Google Scholar] [CrossRef]
- Fleger, Y.; Rosenbluh, M. Surface Plasmons and Surface Enhanced Raman Spectra of Aggregated and Alloyed Gold-Silver Nanoparticles. Int. J. Optics 2009, 2009, 475941. [Google Scholar] [CrossRef]
- Wang, H.-L.; You, E.-M.; Panneerselvam, R.; Ding, S.-Y.; Tian, Z.-Q. Advances of surface-enhanced Raman and IR spectroscopies: From nano/microstructures to macro-optical design. Light Sci. Appl. 2021, 10, 161. [Google Scholar] [CrossRef]
- Święch, D.; Ozaki, Y.; Kim, Y.; Proniewicz, E. Surface- and tip-enhanced Raman scattering of bradykinin onto the colloidal suspended Ag surface. Phys. Chem. Chem. Phys. 2015, 17, 17140–17149. [Google Scholar] [CrossRef]
- Proniewicz, E.; Ignatjev, I.; Niaura, G.; Sobolewski, D.; Prahl, A.; Proniewicz, L.M. Role of Phe-D5 isotopically labeled analogues of bradykinin on elucidation of its adsorption mode on Ag, Au, and Cu electrodes. Surface enhanced Raman spectroscopy studies. J. Raman Spectrosc. 2013, 44, 1096–1104. [Google Scholar] [CrossRef]
- Proniewicz, E.; Skołuba, D.; Ignatjev, I.; Niaura, G.; Sobolewski, D.; Prahl, A.; Proniewicz, L.M. Influence of applied potential on bradykinin adsorption onto Ag, Au, and Cu electrodes. J. Raman Spectrosc. 2013, 44, 655–664. [Google Scholar] [CrossRef]
- Proniewicz, E.; Skołuba, D.; Kudelski, A.; Sobolewski, D.; Kim, Y.; Prahl, A.; Proniewicz, L.M. B2 Bradykinin Receptor Antagonists: Adsorption Mechanism onto Electrochemically Roughened Ag Substrate. J. Raman Spectrosc. 2012, 44, 205–211. [Google Scholar] [CrossRef]
- Sobolewski, D.; Proniewicz, E.; Skołuba, D.; Prahl, A.; Ozaki, Y.; Kim, Y.; Proniewicz, L.M. Characterization of Adsorption Mode of New B2 Bradykinin Receptor Antagonists onto Colloidal Ag Substrate. J. Raman Spectrosc. 2012, 44, 212–218. [Google Scholar] [CrossRef]
- Święch, D.; Tanabe, I.; Vantasin, S.; Sobolewski, D.; Ozaki, Y.; Prahl, A.; Maćkowski, S.; Proniewicz, E. Tip-enhanced Raman spectroscopy of bradykinin and its B2 receptor antagonists onto colloidal suspended Ag nanowires. Phys. Chem. Chem. Phys. 2015, 17, 22882–22892. [Google Scholar] [CrossRef] [PubMed]
- Proniewicz, E.; Burnat, G.; Domin, H.; Iłowska, E.; Roman, A.; Prahl, A. Spectroscopic characterization and in vitro studies of biological activity of bradykinin derivatives. Sci. Rep. 2022, 12, 19015. [Google Scholar] [CrossRef]
- Baalousha, M. Aggregation and disaggregation of iron oxide nanoparticles: Influence of particle concentration, pH and natural organic matter. Sci. Total Environ. 2009, 407, 2093–2101. [Google Scholar] [CrossRef] [PubMed]
- Behrens, M.A.; Franze’n, A.; Carlert, S.; Skantze, U.; Lindfors, L.; Olsson, U. On the Ostwald ripening of crystalline and amorphous nanoparticles. Soft Matt. 2025, 21, 2349. [Google Scholar] [CrossRef] [PubMed]
- Podstawka, E.; Proniewicz, L.M. The orientation of BN-related peptides adsorbed at a SERS-active silver nanoparticles: Comparison with a silver electrode surface. J. Phys. Chem. B 2009, 113, 4978–4985. [Google Scholar] [CrossRef]
- Proniewicz, E.; Ozaki, Y.; Kim, Y.; Proniewicz, L.M. Adsorption Mode of Neurotensin Family Peptides Adsorbed onto a Colloidal Silver Surface: SERS Studies. J. Raman Spectrosc. 2013, 44, 355–361. [Google Scholar] [CrossRef]
- Moskovits, M.; DiLella, D.P.; Maynard, K.J. Surface Raman spectroscopy of a number of cyclic aromatic molecules adsorbed on silver: Selection rules and molecular reorientation. Langmuir 1988, 4, 67–76. [Google Scholar] [CrossRef]
- Gao, X.; Davies, J.P.; Weaver, M.J. Test of surface selection rules for surface-enhanced Raman scattering: The orientation of adsorbed benzene and monosubstituted benzenes on gold. J. Phys. Chem. 1990, 94, 6858–6864. [Google Scholar] [CrossRef]
- Pemberton, J.E.; Bryant, M.A.; Sobocinski, R.L.; Joa, S.L. A simple method for determination of orientation of adsorbed organics of low symmetry using surface-enhanced Raman scattering. J. Phys. Chem. 1992, 96, 3776–3782. [Google Scholar] [CrossRef]
- Carron, K.T.; Hurley, L.G. Axial and azimuthal angle determination with surface-enhanced Raman spectroscopy: Thiophenol on copper, silver, and gold metal surfaces. J. Phys. Chem. 1991, 95, 9979–9984. [Google Scholar] [CrossRef]
- Moskovits, M.; Suh, J.S. Surface selection rules for surface-enhanced Raman spectroscopy: Calculations and application to the surface-enhanced Raman spectrum of phthalazine on silver. J. Phys. Chem. 1984, 88, 5526–5530. [Google Scholar] [CrossRef]
- Moskovits, M.; Suh, J.S. The geometry of several molecular ions adsorbed on the surface of colloidal silver. J. Phys. Chem. 1984, 88, 1293–1298. [Google Scholar] [CrossRef]
- Moskvits, M. Surface-Enhanced Spectroscopy. Rev. Mod. Phys. 1985, 57, 783–826. [Google Scholar] [CrossRef]
- Gao, X.; Weaver, M.J. Surface-enhanced Raman spectroscopy as a probe of adsorbate-surface bonding: Benzene and monosubstituted benzenes adsorbed at gold electrodes. J. Phys. Chem. 1985, 89, 5040–5046. [Google Scholar] [CrossRef]
- Podstawka, E. Effect of amino acids modifications on non-adsorbed and adsorbed on electrochemically roughened silver surface molecular structure of bombesin 6-14 fragments. J. Raman Spectrosc. 2008, 39, 1290–1305. [Google Scholar] [CrossRef]
- Domin, H.; Piergies, N.; Święch, D.; Pięta, E.; Kim, Y.; Proniewicz, E. SERS characterization of neuropeptide Y and its C-terminal fragments onto colloidal gold nanoparticles surface. Coll. Surf. B 2017, 149, 80–88. [Google Scholar] [CrossRef]
- Podstawka, E.; Sikorska, E.; Proniewicz, L.M.; Lammek, B. Raman and Surface-enhanced Raman spectroscopy investigation of vasopressin analogues containing 1-aminocyclohexane-1-carboxylic acid residue. Biopolymers 2006, 83, 193–203. [Google Scholar] [CrossRef] [PubMed]
- Ignatjev, I.; Proniewicz, E.; Proniewicz, L.M.; Niaura, G. Effect of Potential on Temperature-Dependent SERS Spectra of Neuromedin B on Cu Electrode. Phys. Chem. Chem. Phys. 2013, 15, 807–815. [Google Scholar] [CrossRef]
- Podstawka, E.; Kudelski, A.; Proniewicz, L.M. Adsorption mechanism of physiologically active L-phenylalanine phosphonodipeptide analogues: Comparison of colloidal silver and macroscopic silver substrates. Surf. Sci. 2007, 601, 4971–4983. [Google Scholar] [CrossRef]
- Rao, P.V.R.; Srishailam, K.; Ravindranath, L.; Reddy, B.V.; Rao, G.R. Structural and vibrational properties of pentabromophenol and pentafluorophenol: A spectroscopic investigation using density functional theory. J. Mol. Struct. 2019, 1180, 665–675. [Google Scholar] [CrossRef]
- Aliaga, A.E.; Garrido, C.; Leyton, P.; Diaz, G.; Gomez-Jeria, J.S.; Aguayo, T.; Clavijo, E.; Campos Vallette, M.M.; Sanchez-Cortes, S. SERS and theoretical studies of arginine. Spectrochim. Acta A 2010, 76, 458–463. [Google Scholar] [CrossRef]
- Cárcamo, J.J.; Aliaga, A.E.; Clavijo, E.; Garrido, C.; Gómez-Jeria, J.S.; Campos-Vallette, M.M. Proline and hydroxyproline deposited on silver nanoparticles. A Raman, SERS and theoretical studies. J. Raman Spectrosc. 2012, 43, 750–755. [Google Scholar] [CrossRef]
- Podstawka, E.; Ozaki, Y.; Proniewicz, L.M. Part I: Surface enhanced Raman spectroscopy investigation of amino acids and their homodipeptides adsorbed on colloidal silver. Appl. Spectrosc. 2004, 58, 570–580. [Google Scholar] [CrossRef]
- Chemical Book, 1,2,3,4-TETRAHYDROISOQ UINOLINE(91-21-4) Raman. Available online: https://www.chemicalbook.com/SpectrumEN_91-21-4_Raman.htm (accessed on 17 August 2025).
- Podstawka, E.; Ozaki, Y.; Proniewicz, L.M. Structures and bonding on colloidal silver surface of the various length carboxyl terminal fragments of bombesin. Langmuir 2008, 24, 10807–10816. [Google Scholar] [CrossRef] [PubMed]
- Podstawka, E.; Borszowska, R.; Grabowska, M.; Drąg, M.; Kafarski, P.; Proniewicz, L.M. Investigation of Molecular Structure and Adsorption Mechanism of Phosphonodipeptides by infrared, Raman, and surface enhanced Raman spectroscopy. Surf. Sci. 2005, 599, 207–220. [Google Scholar] [CrossRef]
- Wang, S.S. p-Alkoxybenzyl Alcohol Resin and p-Alkoxybenzyloxycarbonylhydrazide Resin for Solid Phase Synthesis of Protected Peptide Fragments. J. Am. Chem. Soc. 1973, 95, 1328–1333. [Google Scholar] [CrossRef] [PubMed]
| Peptide | Peptide Sequence | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| Native BK | – | Arg | Pro | Pro | Gly | Phe | Ser | Pro | Phe | Arg |
| [D-Arg0,Hyp3,Thi5,D-Phe7,Thi8]BK | D-Arg | Arg | Pro | Hyp | Gly | Thi | Ser | D-Phe | Thi | Arg |
| [D-Arg0,Hyp3,Thi5,D-Tic7,Oic8]BK | D-Arg | Arg | Pro | Hyp | Gly | Thi | Ser | D-Tic | Oic | Arg |
| [D-Arg0,Hyp3,Igl5,D-Phe(5F)7,Oic8]BK | D-Arg | Arg | Pro | Hyp | Gly | L-Igl | Ser | D-Phe(5F) | Oic | Arg |
| [D-Arg0,Hyp3,Igl5,D-Igl7,Oic8]BK | D-Arg | Arg | Pro | Hyp | Gly | L-Igl | Ser | D-Igl | Oic | Arg |
| Hyp L-hydroxyproline | 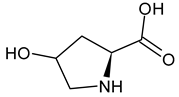 | |||||||||
| Thi L-thienylalanine | 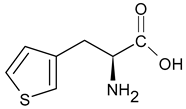 | |||||||||
| D-Tic D-1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid | 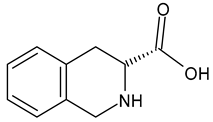 | |||||||||
| Igl 2-indanylglycine |  | |||||||||
| Oic L-octahydroindole-2-carboxylic acid |  | |||||||||
| Assignment | Wavenumber/cm−1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [D-Arg0,Hyp3, Thi5, D-Phe7,Thi8]BK | [D-Arg0,Hyp3,Thi5, D-Tic7,Oic8]BK | [D-Arg0,Hyp3,Igl5,D-Phe(5F)7,Oic8]BK | [D-Arg0,Hyp3,Igl5, D-Igl7,Oic8]BK | |||||||||
| Raman | SERS Ag | SERS Au | Raman | SERS Ag | SERS Au | Raman | SERS Ag | SERS Au | Raman | SERS Ag | SERS Au | |
| Phe(F5) [ν8a] | 1682 | |||||||||||
| Amide I/Arg νas(C=N) | 1660 | 1565 | 1546 | 1663 | 1586 | 1522 | 1659 | 1585 | 1591 | 1676 | 1586 | 1564 |
| Phenyl ring [ν8a] | 1603 | 1592 | 1610 | 1583 | 1607 | 1560 | 1606 | 1598 | ||||
| Phenyl ring [ν8a] | 1584 | 1590 | 1584 | 1585 | ||||||||
| νas(COO−) | 1534 | 1520 | 1533 | 1522 | 1509 | 1527 | ||||||
| δ(CH2) + Imide II/Arg ρasb(CH2)/a-ring ρs(CH2) | 1458 | 1456 | 1449 | 1461 | 1457 | 1490 | 1464 | 1455 | 1468 | 1456 | 1485 | |
| δ(CH2) and Imide II | 1437 | 1438 | 1438 | 1445 | 1442 | 1446 | 1437 | 1444 | ||||
| νs(COO−) | 1381 | 1397 | 1393 | 1397 | 1404 | |||||||
| Arg ρb(CH2)/Thi δ(CH) + ν(C=C) and a-ring combination | 1366 | 1352 | 1343 | 1352 | 1364 | 1343 | 1352 | 1343 | 1343 | 1352 | 1367 | |
| a-ring ν(CN), δ(CCαH) | 1305 | 1322 | 1322 | 1315 | 1323 | |||||||
| a-ring [ν(CN) + ρb(CCH) + ρr/t(CH2)]/Imide II | 1294 | 1284 | 1276 | 1296 | 1296 | 1294 | 1290 | |||||
| Thi ρr(CH)/a-ring ρt(CH2) | 1262 | 1283 | 1263 | 1261 | 1272 | |||||||
| Amide III | 1248 | 1244 | 1246 | 1247 | 1239 | 1248 | 1237 | |||||
| Phenyl ring [ν7a] | 1201 | 1223 | 1201 | 1208 | 1198 | 1209 | 1215 | |||||
| Arg ρr(NH2)/a-ring ρt(CH2) | 1167 | 1166 | 1180 | 1158 | 1174 | 1154 | 1166 | 1153 | 1139 | 1169 | ||
| a-ring ρt(CH2) | 1127 | 1123 | 1123 | 1134 | 1123 | 1127 | ||||||
| ν(CN) | 1097 | 1096 | 1087 | 1080 | 1088 | 1088 | 1087 | |||||
| Arg ν(CC) + ν(NC)/a-ring ν(CC) | 1061 | 1074 | 1066 | 1066 | ||||||||
| Igl ν(CC) + ν(NC) | 1045 | 1044 | ||||||||||
| Thi ν(CC) + ρr(CH)/Arg guanidino group/δ(a-ring) + ρt(CH2)/Phe [ν18a] | 1013 | 1034 | 1034 | 1024 | 1024 | 1024 | 1020 | |||||
| Phe [ν12] | 1001 | 996 | ||||||||||
| Igl | 1011 | 1011 | ||||||||||
| ρr(CH2) | 982 | 990 | 959 | 987 | 988 | 987 | 991 | |||||
| Arg ν(CC)/a-ring ρr(CH) /ρw(CH2) + γ(ring)/[ν(CCOO–)], | 906 | 925 | 925 | 924 | 928 | 925 | 912 | |||||
| Thi δ(ring) + ν(CS)/a-ring ν(CCC) | 888 | 880 | 883 | 870 | 870 | |||||||
| Thi δ(ring) + ν(CC)/Arg ν(CC)/a-ring (ρr(CβH2) | 850 | 836 | 851 | 833 | 824 | |||||||
| Thi δ(ring) + ν(CC)/a-ring breath | 811 | 817 | 811 | 817 | 829 | |||||||
| a-ring (ρr(CγH2) | 784 | 789 | 788 | |||||||||
| Thi δ(ring) + ν(CS)/a-ring ρr(CH2) + ρb(CNH) | 741 | 732 | 742 | 742 | ||||||||
| Thi ring ρw(CH) | 673 | 673 | 653 | 662 | 674 | 663 | 675 | 664 | 673 | 666 | ||
| ρb(COO)/Amide V | 649 | 648 | 599 | 653 | 648 | 607 | 585 | 606 | ||||
| Arg ρt(NH2)/a-ring δ(CCN)/Phe(F5) | 505 | 501 | 506 | 551 | 564 | 542 | ||||||
| γ(ring) | 465 | 481 | 445 | 446 | 461 | |||||||
| Assignment | Wavenumber/cm−1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [D-Arg0,Hyp3,Thi5,D-Phe7,Thi8]BK | [D-Arg0,Hyp3,Thi5,D-Tic7,Oic8]BK | [D-Arg0,Hyp3,Igl5,D-Phe(5F)7,Oic8]BK | [D-Arg0,Hyp3,Igl5,D-Igl7,Oic8]BK | |||||||||
| ATR-FTIR | SEIRA Ag | SEIRA Au | ATR-FTIR | SEIRA Ag | SEIRA Au | ATR-FTIR | SEIRA Ag | SEIRA Au | ATR-FTIR | SEIRA Ag | SEIRA Au | |
| ν(C=O) | 1729 | 1729 | 1750 | 1728 | 1750 | 1724 | 1735 | |||||
| Amide I [ν(C=O)]/Arg νas(C=N), δ(NH2) | 1647 | 1660 | 1660 1640 | 1632 | 1665 | 1631 | 1638 | 1664 | 1658 1626 | 1648 | 1653 | 1659 1620 |
| νas(COO−) | 1580 | 1579 | 1580 | 1572 | ||||||||
| Amide II [ν(NC) + ρipb(NH)] | 1537 | 1533 | 1533 | 1545 1530 | 1548 1533 | 1545 | 1551 | 1548 | 1548 | 1555 | ||
| Imide II/Arg ρas b(CH2) | 1470 | 1467 | 1456 | 1470 | 1457 | 1471 | 1465 | |||||
| a-ring ρs(CH2) | 1456 | 1433 | 1457 | 1447 | 1447 | 1450 | 1450 | 1452 | 1452 | |||
| νs(COO−) | 1397 | 1396 | 1396 | 1398 | 1396 | 1393 | ||||||
| ρ(CH2) | 1368 | 1339 | 1367 | 1339 | 1365 | 1364 | ||||||
| Amide III | 1251 | 1251 | 1251 | 1251 | 1251 | 1252 | 1251 | 1261 | 1251 | 1251 | 1259 | 1251 |
| ρr(CH2), ν(CC) | 1202 | 1197 | 1196 | 1197 | 1203 | |||||||
| ν(CN) | 1068 | 1078 | 1050 | 1068 | 1074 | 1067 | 1068 | 1078 | 1058 | 1068 | 1078 | 1080 |
| ρr(CH2) | 976 | 977 | 956 | |||||||||
| Thi δ(ring) + ν(CC)/Arg ν(CC), δ(NH)/a-ring ν(CC) | 842 | 841 | 843 | 842 | 840 | 842 | 842 | |||||
| δ(NH) | 788 | 757 | 788 | 789 | ||||||||
| Skeletal | 719 | 709 | 718 | 721 | ||||||||
| Amide | 651 | 638 | 638 | 684 | 639 | 679 | 637 | |||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Proniewicz, E.; Prahl, A. SERS- and SEIRA-Based Characterization and Sensing of Highly Selective Bradykinin B2 Receptor Antagonists. Int. J. Mol. Sci. 2025, 26, 8089. https://doi.org/10.3390/ijms26168089
Proniewicz E, Prahl A. SERS- and SEIRA-Based Characterization and Sensing of Highly Selective Bradykinin B2 Receptor Antagonists. International Journal of Molecular Sciences. 2025; 26(16):8089. https://doi.org/10.3390/ijms26168089
Chicago/Turabian StyleProniewicz, Edyta, and Adam Prahl. 2025. "SERS- and SEIRA-Based Characterization and Sensing of Highly Selective Bradykinin B2 Receptor Antagonists" International Journal of Molecular Sciences 26, no. 16: 8089. https://doi.org/10.3390/ijms26168089
APA StyleProniewicz, E., & Prahl, A. (2025). SERS- and SEIRA-Based Characterization and Sensing of Highly Selective Bradykinin B2 Receptor Antagonists. International Journal of Molecular Sciences, 26(16), 8089. https://doi.org/10.3390/ijms26168089







