Canine Endometrial Mesenchymal Stem Cells: Characterization and Functional Assessment for Cartilage Repair
Abstract
1. Introduction
2. Results
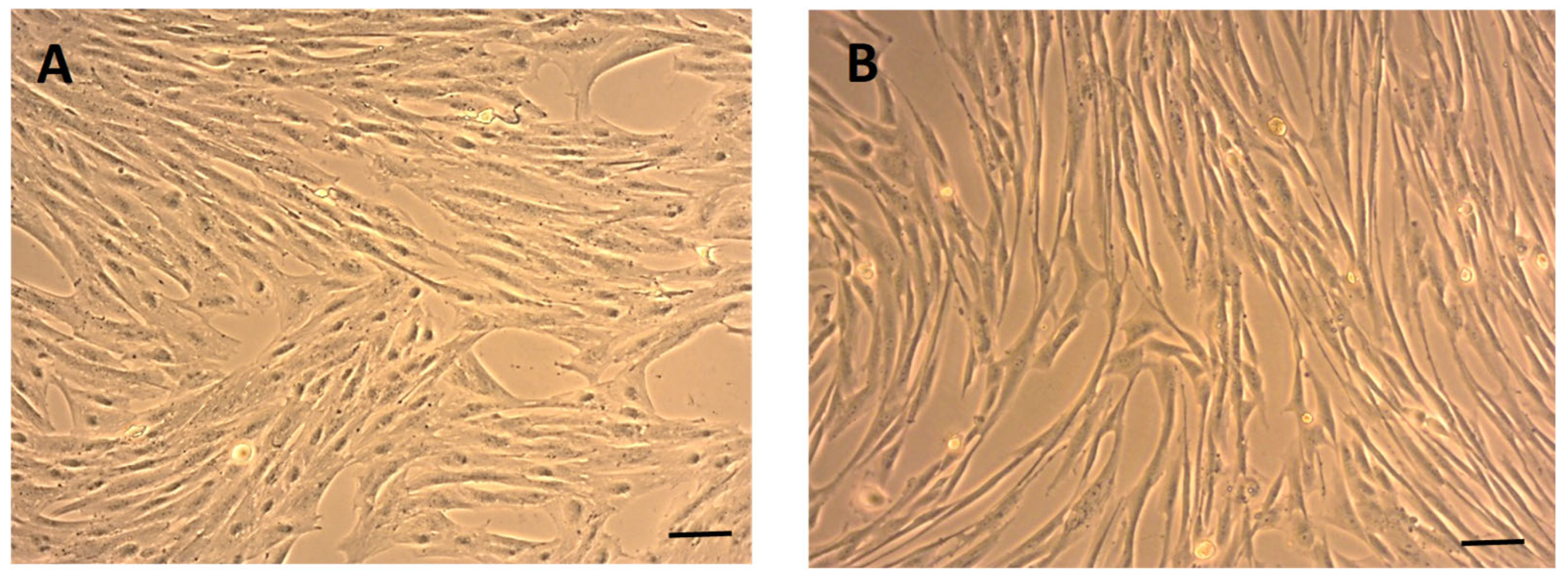
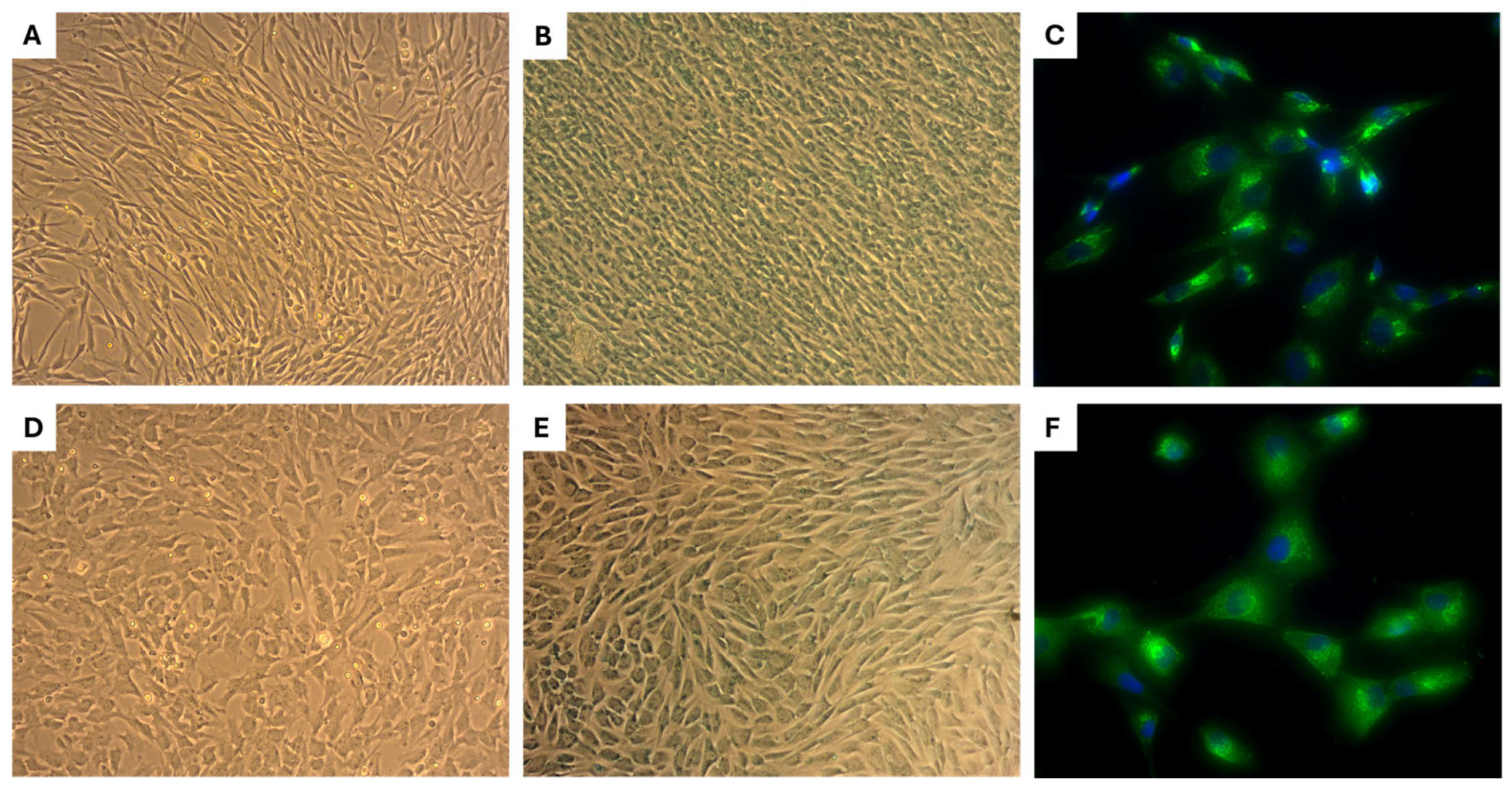
2.1. Morphology of eMSCs
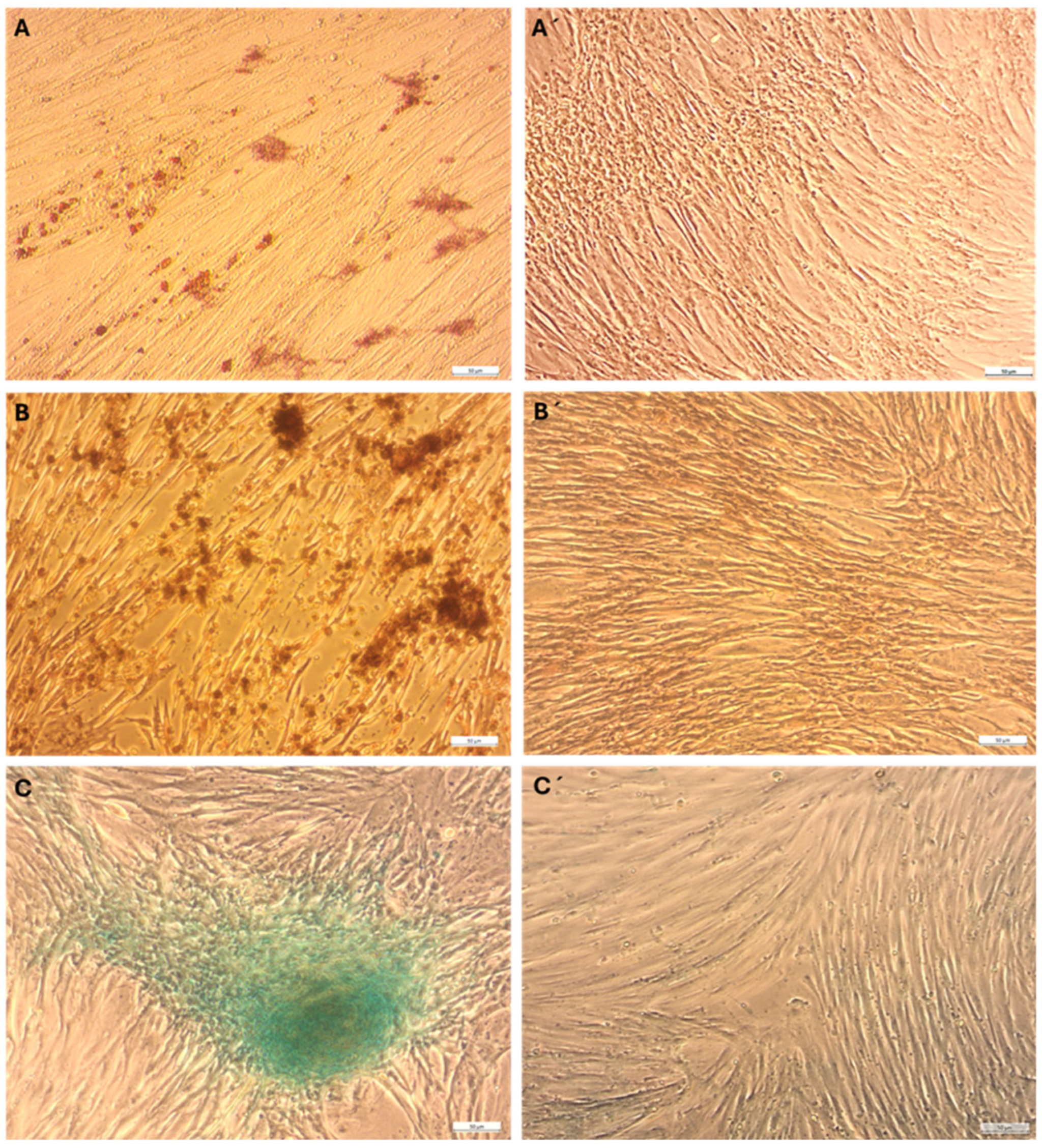
2.2. Multilineage Differentiation
2.3. Flow Cytometry Analysis of eMSC Surface Marker Expression
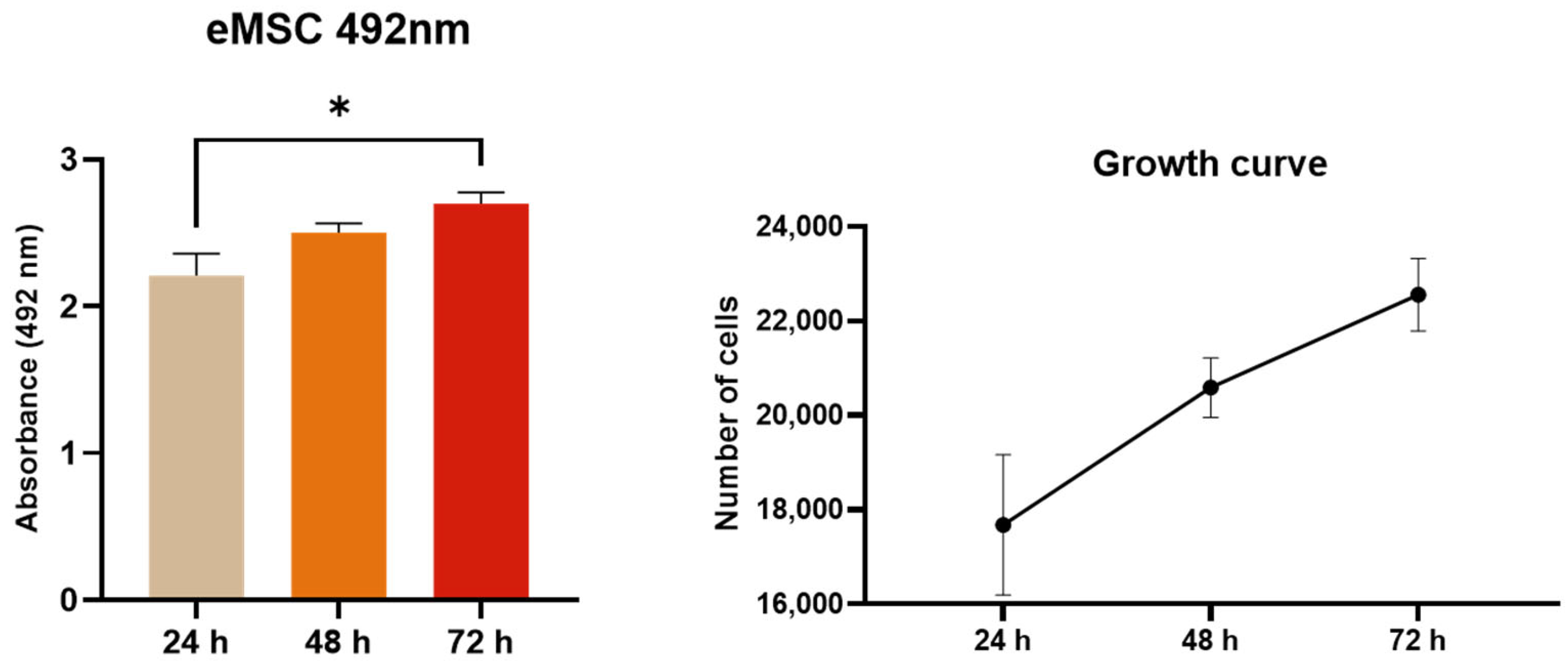
2.4. XTT Assay
2.5. Morphology, Alcian Blue Staining, and Collagen II Expression of Primary Chondrocytes
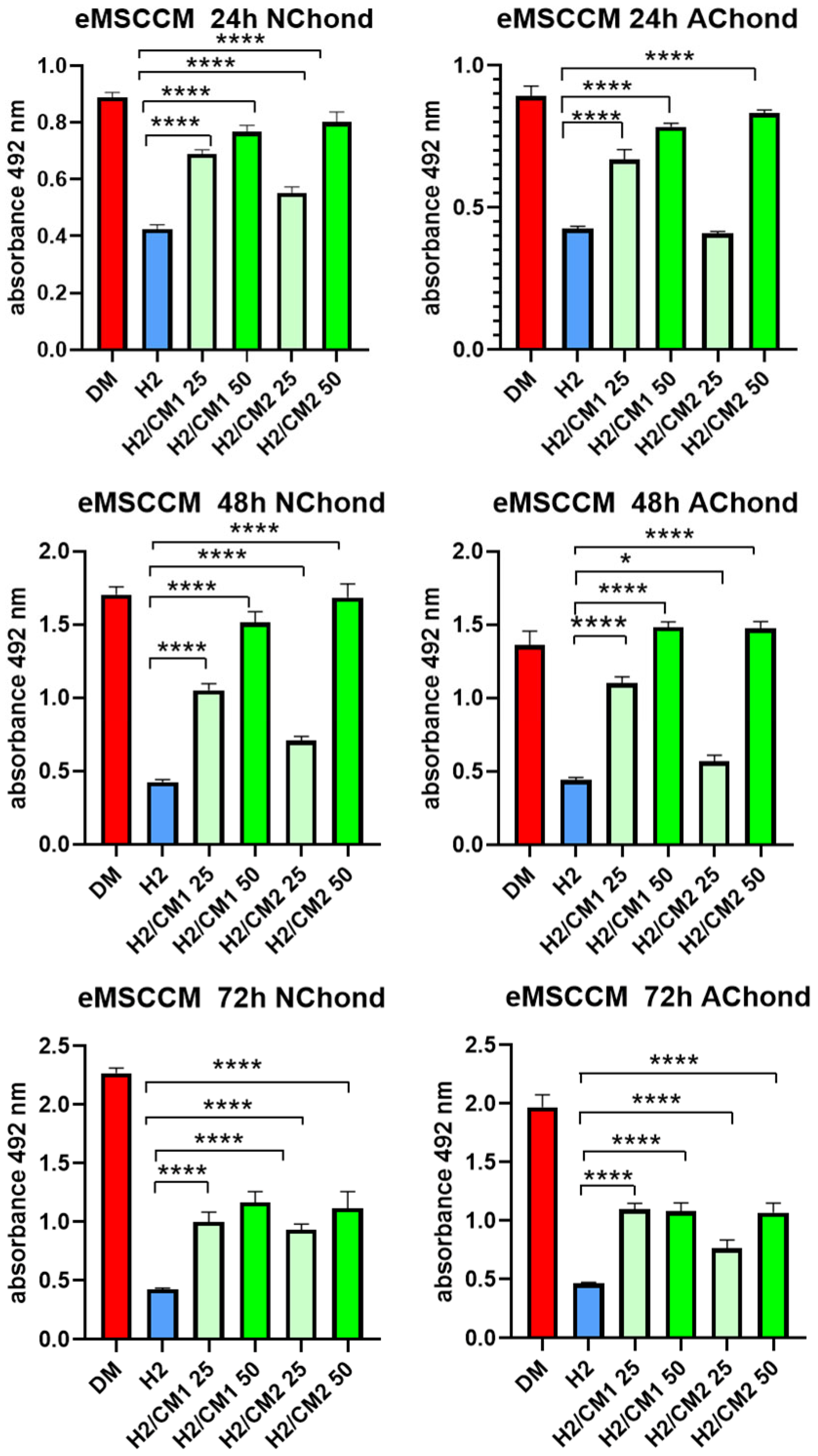
2.6. Evaluation of Cytoprotective Properties of eMSCCM on Stressed Chondrocytes
3. Discussion
4. Materials and Methods
4.1. Animal Selection and Ethical Approval
4.2. Hormonal Status Assessment: Anesthesia and Surgical
4.3. Anesthesia and Surgery
4.4. Sample Collection
4.5. Isolation of eMSCs
4.6. Cell Passaging
4.7. Morphology and Metabolic Activity of eMSCs
4.8. Multilineage Differentiation
4.9. Flow Cytometry
4.10. Isolation of Conditioned Medium from Endometrial MSC (eMSCCM)
4.11. Primary Cultures of Canine Chondrocytes
4.12. Histochemical and Immunocytochemical Staining of Chondrocytes
4.13. Functional In Vitro Analyses: Cytoprotective Effect of eMSCCM on Stressed Chondrocytes
4.13.1. Oxidative Stress Induction in Primary Canine Chondrocyte Cultures
4.13.2. XTT Assay
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fan, X.-L.; Zhang, Y.; Li, X.; Fu, Q.-L. Mechanisms Underlying the Protective Effects of Mesenchymal Stem Cell-Based Therapy. Cell. Mol. Life Sci. CMLS 2020, 77, 2771–2794. [Google Scholar] [CrossRef]
- Gargett, C.E.; Schwab, K.E.; Deane, J.A. Endometrial Stem/Progenitor Cells: The First 10 Years. Hum. Reprod. Update 2016, 22, 137–163. [Google Scholar] [CrossRef]
- Gargett, C.E.; Hapangama, D. Endometrial Stem/Progenitor Cells: Prospects and Challenges. J. Pers. Med. 2022, 12, 1466. [Google Scholar] [CrossRef]
- Momeni, J.; Naserzadeh, E.; Sepehrinezhad, A.; Ahmadabad, R.A.; Negah, S.S. Human Endometrial Regenerative Cells for Neurological Disorders: Hype or Hope? Int. J. Stem Cells 2024, 17, 224–235. [Google Scholar] [CrossRef]
- Bausyte, R.; Vaigauskaite-Mazeikiene, B.; Borutinskaite, V.; Valatkaite, E.; Besusparis, J.; Valkiuniene, R.B.; Kazenaite, E.; Ramasauskaite, D.; Navakauskiene, R. Human Endometrium-Derived Mesenchymal Stem/Stromal Cells Application in Endometrial-Factor Induced Infertility. Front. Cell Dev. Biol. 2023, 11, 1227487. [Google Scholar] [CrossRef] [PubMed]
- Phyo, H.; Aburza, A.; Mellanby, K.; Esteves, C.L. Characterization of Canine Adipose- and Endometrium-Derived Mesenchymal Stem/Stromal Cells and Response to Lipopolysaccharide. Front. Vet. Sci. 2023, 10, 1180760. [Google Scholar] [CrossRef] [PubMed]
- Franklin, S.P.; Stoker, A.M.; Murphy, S.M.; Kowaleski, M.P.; Gillick, M.; Kim, S.E.; Karlin, M.; Cross, A.; Cook, J.L. Outcomes Associated with Osteochondral Allograft Transplantation in Dogs. Front. Vet. Sci. 2021, 8, 759610. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, A.; Mizuno, M.; Mochizuki, M.; Sekiya, I. Mesenchymal Stem Cells for Cartilage Regeneration in Dogs. World J. Stem Cells 2019, 11, 254–269. [Google Scholar] [CrossRef]
- Yuan, Q.; Zhang, Y.; Chen, Q. Mesenchymal Stem Cell-Derived Extracellular Vesicles: Potential Therapeutics as MSC Trophic Mediators in Regenerative Medicine. Anat. Rec. 2020, 303, 1735–1742. [Google Scholar] [CrossRef]
- Costela-Ruiz, V.J.; Melguizo-Rodríguez, L.; Bellotti, C.; Illescas-Montes, R.; Stanco, D.; Arciola, C.R.; Lucarelli, E. Different Sources of Mesenchymal Stem Cells for Tissue Regeneration: A Guide to Identifying the Most Favorable One in Orthopedics and Dentistry Applications. Int. J. Mol. Sci. 2022, 23, 6356. [Google Scholar] [CrossRef]
- Tempest, N.; Maclean, A.; Hapangama, D.K. Endometrial Stem Cell Markers: Current Concepts and Unresolved Questions. Int. J. Mol. Sci. 2018, 19, 3240. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.C.; Krause, D.S.; Deans, R.J.; Keating, A.; Prockop, D.J.; Horwitz, E.M. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Wagner, W.; Horn, P.; Castoldi, M.; Diehlmann, A.; Bork, S.; Saffrich, R.; Benes, V.; Blake, J.; Pfister, S.; Eckstein, V.; et al. Replicative Senescence of Mesenchymal Stem Cells: A Continuous and Organized Process. PLoS ONE 2008, 3, e2213. [Google Scholar] [CrossRef] [PubMed]
- Weng, Z.; Wang, Y.; Ouchi, T.; Liu, H.; Qiao, X.; Wu, C.; Zhao, Z.; Li, L.; Li, B. Mesenchymal Stem/Stromal Cell Senescence: Hallmarks, Mechanisms, and Combating Strategies. Stem Cells Transl. Med. 2022, 11, 356–371. [Google Scholar] [CrossRef]
- Sattar, M.A.; Lingens, L.F.; Guillaume, V.G.J.; Goetzl, R.; Beier, J.P.; Ruhl, T. Association between Donor Age and Osteogenic Potential of Human Adipose Stem Cells in Bone Tissue Engineering. Curr. Issues Mol. Biol. 2024, 46, 1424–1436. [Google Scholar] [CrossRef] [PubMed]
- Al-Azab, M.; Safi, M.; Idiiatullina, E.; Al-Shaebi, F.; Zaky, M.Y. Aging of Mesenchymal Stem Cell: Machinery, Markers, and Strategies of Fighting. Cell. Mol. Biol. Lett. 2022, 27, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.-W.; Gao, Z.-L.; Mei, H.; Li, Y.-L.; Wang, Y. Differentiation of Human Mesenchymal Stem Cells: The Potential Mechanism for Estrogen-Induced Preferential Osteoblast versus Adipocyte Differentiation. Am. J. Med. Sci. 2011, 341, 460–468. [Google Scholar] [CrossRef]
- Tofiño-Vian, M.; Guillén, M.I.; Pérez Del Caz, M.D.; Castejón, M.A.; Alcaraz, M.J. Extracellular Vesicles from Adipose-Derived Mesenchymal Stem Cells Downregulate Senescence Features in Osteoarthritic Osteoblasts. Oxidative Med. Cell. Longev. 2017, 2017, 7197598. [Google Scholar] [CrossRef]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In Vitro Chondrogenesis of Bone Marrow-Derived Mesenchymal Progenitor Cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef]
- Cox-York, K.A.; Erickson, C.B.; Pereira, R.I.; Bessesen, D.H.; Van Pelt, R.E. Region-Specific Effects of Oestradiol on Adipose-Derived Stem Cell Differentiation in Post-Menopausal Women. J. Cell. Mol. Med. 2017, 21, 677–684. [Google Scholar] [CrossRef]
- Lai, F.; Wang, J.; Tang, H.; Bian, X.; Lu, K.; He, G.; Huang, P.; Liu, J.; Zhou, M.; Liu, J.; et al. Adipogenic Differentiation Was Inhibited by Downregulation of PPARγ Signaling Pathway in Aging Tendon Stem/Progenitor Cells. J. Orthop. Surg. 2021, 16, 614. [Google Scholar] [CrossRef]
- Storck, K.; Ell, J.; Regn, S.; Rittler-Ungetüm, B.; Mayer, H.; Schantz, T.; Müller, D.; Buchberger, M. Optimization of in Vitro Cultivation Strategies for Human Adipocyte Derived Stem Cells. Adipocyte 2015, 4, 181–187. [Google Scholar] [CrossRef]
- De Cesaris, V.; Grolli, S.; Bresciani, C.; Conti, V.; Basini, G.; Parmigiani, E.; Bigliardi, E. Isolation, Proliferation and Characterization of Endometrial Canine Stem Cells. Reprod. Domest. Anim. Zuchthyg. 2017, 52, 235–242. [Google Scholar] [CrossRef]
- Morawska-Kozłowska, M.; Pitas, M.; Zhalniarovich, Y. Mesenchymal Stem Cells in Veterinary Medicine—Still Untapped Potential. Animals 2025, 15, 1175. [Google Scholar] [CrossRef]
- Cousins, F.L.; Filby, C.E.; Gargett, C.E. Endometrial Stem/Progenitor Cells—Their Role in Endometrial Repair and Regeneration. Front. Reprod. Health 2022, 3, 811537. [Google Scholar] [CrossRef] [PubMed]
- Szukiewicz, D.; Stangret, A.; Ruiz-Ruiz, C.; Olivares, E.G.; Soriţău, O.; Suşman, S.; Szewczyk, G. Estrogen- and Progesterone (P4)-Mediated Epigenetic Modifications of Endometrial Stromal Cells (EnSCs) and/or Mesenchymal Stem/Stromal Cells (MSCs) in the Etiopathogenesis of Endometriosis. Stem Cell Rev. Rep. 2021, 17, 1174–1193. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Liu, C.; Xie, Z.; Song, P.; Zhao, R.C.H.; Guo, L.; Liu, Z.; Wu, Y. Epigenetic Dysregulation in Mesenchymal Stem Cell Aging and Spontaneous Differentiation. PLoS ONE 2011, 6, e20526. [Google Scholar] [CrossRef]
- Huang, M.; Wu, Q.; Jiang, Z.-H. Epigenetic Alterations under Oxidative Stress in Stem Cells. Oxidative Med. Cell. Longev. 2022, 2022, 6439097. [Google Scholar] [CrossRef] [PubMed]
- Campioni, D.; Rizzo, R.; Stignani, M.; Melchiorri, L.; Ferrari, L.; Moretti, S.; Russo, A.; Bagnara, G.P.; Bonsi, L.; Alviano, F.; et al. A Decreased Positivity for CD90 on Human Mesenchymal Stromal Cells (MSCs) Is Associated with a Loss of Immunosuppressive Activity by MSCs. Cytometry B Clin. Cytom. 2009, 76, 225–230. [Google Scholar] [CrossRef]
- Estrada, J.C.; Albo, C.; Benguría, A.; Dopazo, A.; López-Romero, P.; Carrera-Quintanar, L.; Roche, E.; Clemente, E.P.; Enríquez, J.A.; Bernad, A.; et al. Culture of Human Mesenchymal Stem Cells at Low Oxygen Tension Improves Growth and Genetic Stability by Activating Glycolysis. Cell Death Differ. 2012, 19, 743–755. [Google Scholar] [CrossRef]
- Guest, D.J.; Dudhia, J.; Smith, R.K.W.; Roberts, S.J.; Conzemius, M.; Innes, J.F.; Fortier, L.A.; Meeson, R.L. Position Statement: Minimal Criteria for Reporting Veterinary and Animal Medicine Research for Mesenchymal Stromal/Stem Cells in Orthopedic Applications. Front. Vet. Sci. 2022, 9, 817041. [Google Scholar] [CrossRef]
- Maličev, E.; Jazbec, K. An Overview of Mesenchymal Stem Cell Heterogeneity and Concentration. Pharmaceuticals 2024, 17, 350. [Google Scholar] [CrossRef] [PubMed]
- Payr, S.; Schuseil, T.; Unger, M.; Seeliger, C.; Tiefenboeck, T.; Balmayor, E.R.; van Griensven, M. Effect of Donor Age and 3D-Cultivation on Osteogenic Differentiation Capacity of Adipose-Derived Mesenchymal Stem Cells. Sci. Rep. 2020, 10, 10408. [Google Scholar] [CrossRef]
- Liu, L.; Luo, P.; Yang, M.; Wang, J.; Hou, W.; Xu, P. The Role of Oxidative Stress in the Development of Knee Osteoarthritis: A Comprehensive Research Review. Front. Mol. Biosci. 2022, 9, 1001212. [Google Scholar] [CrossRef]
- Bolduc, J.A.; Collins, J.A.; Loeser, R.F. Reactive Oxygen Species, Aging and Articular Cartilage Homeostasis. Free Radic. Biol. Med. 2019, 132, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yahaya, B.H.; Ng, W.H.; Yusoff, N.M.; Lin, J. Conditioned Medium of Human Menstrual Blood-Derived Endometrial Stem Cells Protects Against MPP+-Induced Cytotoxicity in Vitro. Front. Mol. Neurosci. 2019, 12, 80. [Google Scholar] [CrossRef]
- Sahraei, S.S.; Kowsari, A.; Asl, F.D.; Sheykhhasan, M.; Naserpoor, L.; Sheikholeslami, A. Evaluating the Effect of Conditioned Medium from Endometrial Stem Cells on Endometriosis-Derived Endometrial Stem Cells. Anat. Cell Biol. 2022, 55, 100–108. [Google Scholar] [CrossRef]
- Sun, C.; Hao, J.; Qin, H.; Zhu, Y.; Li, X.; Zhang, B.; Qin, Y.; Li, G.; Wang, H.; Wang, H. Endometrial Regenerative Cell-Derived Conditioned Medium Alleviates Experimental Colitis. Stem Cells Int. 2022, 2022, 7842296. [Google Scholar] [CrossRef]
- Xu, S.; Liu, C.; Ji, H.-L. Concise Review: Therapeutic Potential of the Mesenchymal Stem Cell Derived Secretome and Extracellular Vesicles for Radiation-Induced Lung Injury: Progress and Hypotheses. Stem Cells Transl. Med. 2019, 8, 344–354. [Google Scholar] [CrossRef]
- Marinaro, F.; Gómez-Serrano, M.; Jorge, I.; Silla-Castro, J.; Vázquez, J.; Sánchez-Margallo, F.; Blazquez, R.; López, E.; Álvarez Pérez, V.; Casado, J. Unraveling the Molecular Signature of Extracellular Vesicles from Endometrial-Derived Mesenchymal Stem Cells: Potential Modulatory Effects and Therapeutic Applications. Front. Bioeng. Biotechnol. 2019, 7, 431. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of Mesenchymal Stem Cells in Immunomodulation: Pathological and Therapeutic Implications. Nat. Immunol. 2014, 15, 1009–1016. [Google Scholar] [CrossRef] [PubMed]
- Vizoso, F.J.; Eiro, N.; Cid, S.; Schneider, J.; Perez-Fernandez, R. Mesenchymal Stem Cell Secretome: Toward Cell-Free Therapeutic Strategies in Regenerative Medicine. Int. J. Mol. Sci. 2017, 18, 1852. [Google Scholar] [CrossRef] [PubMed]
- Vassilieva, I.; Kosheverova, V.; Vitte, M.; Kamentseva, R.; Shatrova, A.; Tsupkina, N.; Skvortsova, E.; Borodkina, A.; Tolkunova, E.; Nikolsky, N.; et al. Paracrine Senescence of Human Endometrial Mesenchymal Stem Cells: A Role for the Insulin-like Growth Factor Binding Protein 3. Aging 2020, 12, 1987–2004. [Google Scholar] [CrossRef]
- Hudakova, N.; Mudronova, D.; Marcincakova, D.; Slovinska, L.; Majerova, P.; Maloveska, M.; Petrouskova, P.; Humenik, F.; Cizkova, D. The Role of Primed and Non-Primed MSC-Derived Conditioned Media in Neuroregeneration. Front. Mol. Neurosci. 2023, 16, 1241432. [Google Scholar] [CrossRef]
- Vozar, J.; Hudakova, N.; Nosalova, N.; Huniadi, M.; Marcincakova, D.; Hornak, S.; Hornakova, L.; Majerova, P.; Cizkova, D. Impact of Eggshell Membrane on Metabolism and Cell Adhesion in Oxidatively Stressed Canine Chondrocytes. Front. Vet. Sci. 2025, 11, 1517349. [Google Scholar] [CrossRef] [PubMed]
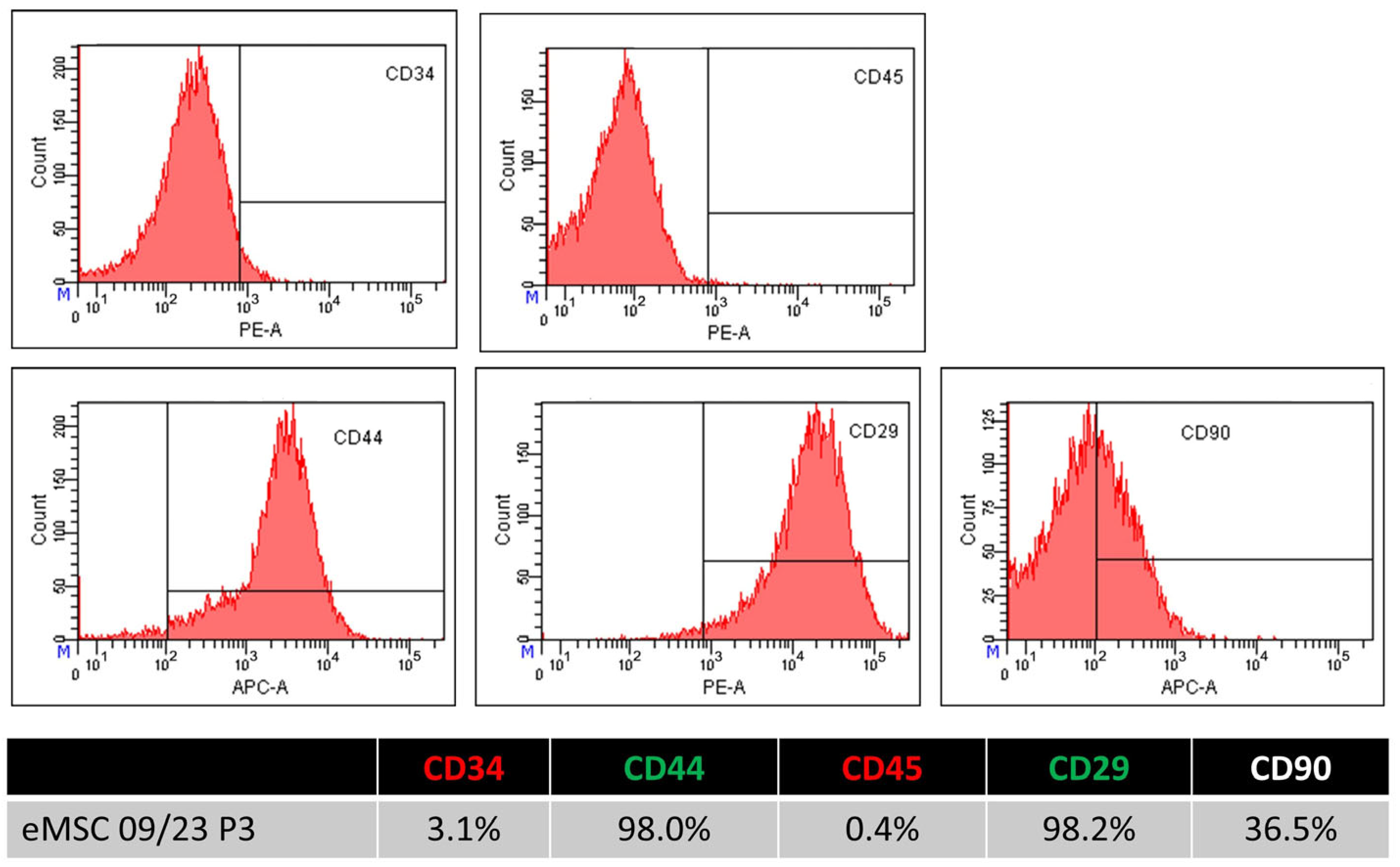

| Passage 3 | CD34 | CD45 | CD29 | CD44 | CD90 |
|---|---|---|---|---|---|
| eMSC 01 | 0.00 | 5.30 | 97.80 | 96.00 | 11.60 |
| eMSC 02 | 5.30 | 3.20 | 98.40 | 94.90 | 5.00 |
| eMSC 03 | 3.10 | 0.40 | 98.20 | 98.00 | 36.50 |
| Average | 2.80 | 2.97 | 98.13 | 96.30 | 17.70 |
| STDEV | 2.381 | 2.198 | 0.273 | 1.405 | 14.858 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vikartovska, Z.; Maloveska, M.; Nosalova, N.; Hornakova, L.; Huniadi, M.; Hudakova, N.; Hornak, S.; Kalinaj, B.; Kubatka, P.; Cizkova, D. Canine Endometrial Mesenchymal Stem Cells: Characterization and Functional Assessment for Cartilage Repair. Int. J. Mol. Sci. 2025, 26, 8091. https://doi.org/10.3390/ijms26168091
Vikartovska Z, Maloveska M, Nosalova N, Hornakova L, Huniadi M, Hudakova N, Hornak S, Kalinaj B, Kubatka P, Cizkova D. Canine Endometrial Mesenchymal Stem Cells: Characterization and Functional Assessment for Cartilage Repair. International Journal of Molecular Sciences. 2025; 26(16):8091. https://doi.org/10.3390/ijms26168091
Chicago/Turabian StyleVikartovska, Zuzana, Marcela Maloveska, Natalia Nosalova, Lubica Hornakova, Mykhailo Huniadi, Nikola Hudakova, Slavomir Hornak, Blazej Kalinaj, Peter Kubatka, and Dasa Cizkova. 2025. "Canine Endometrial Mesenchymal Stem Cells: Characterization and Functional Assessment for Cartilage Repair" International Journal of Molecular Sciences 26, no. 16: 8091. https://doi.org/10.3390/ijms26168091
APA StyleVikartovska, Z., Maloveska, M., Nosalova, N., Hornakova, L., Huniadi, M., Hudakova, N., Hornak, S., Kalinaj, B., Kubatka, P., & Cizkova, D. (2025). Canine Endometrial Mesenchymal Stem Cells: Characterization and Functional Assessment for Cartilage Repair. International Journal of Molecular Sciences, 26(16), 8091. https://doi.org/10.3390/ijms26168091







