Exogenous Gibberellic Acid (GA3) and Benzylaminopurine Enhance the Antioxidant Properties of Vaccinium corymbosum L. ‘Biloxi’ Fruits Without Affecting Yield
Abstract
1. Introduction
2. Results
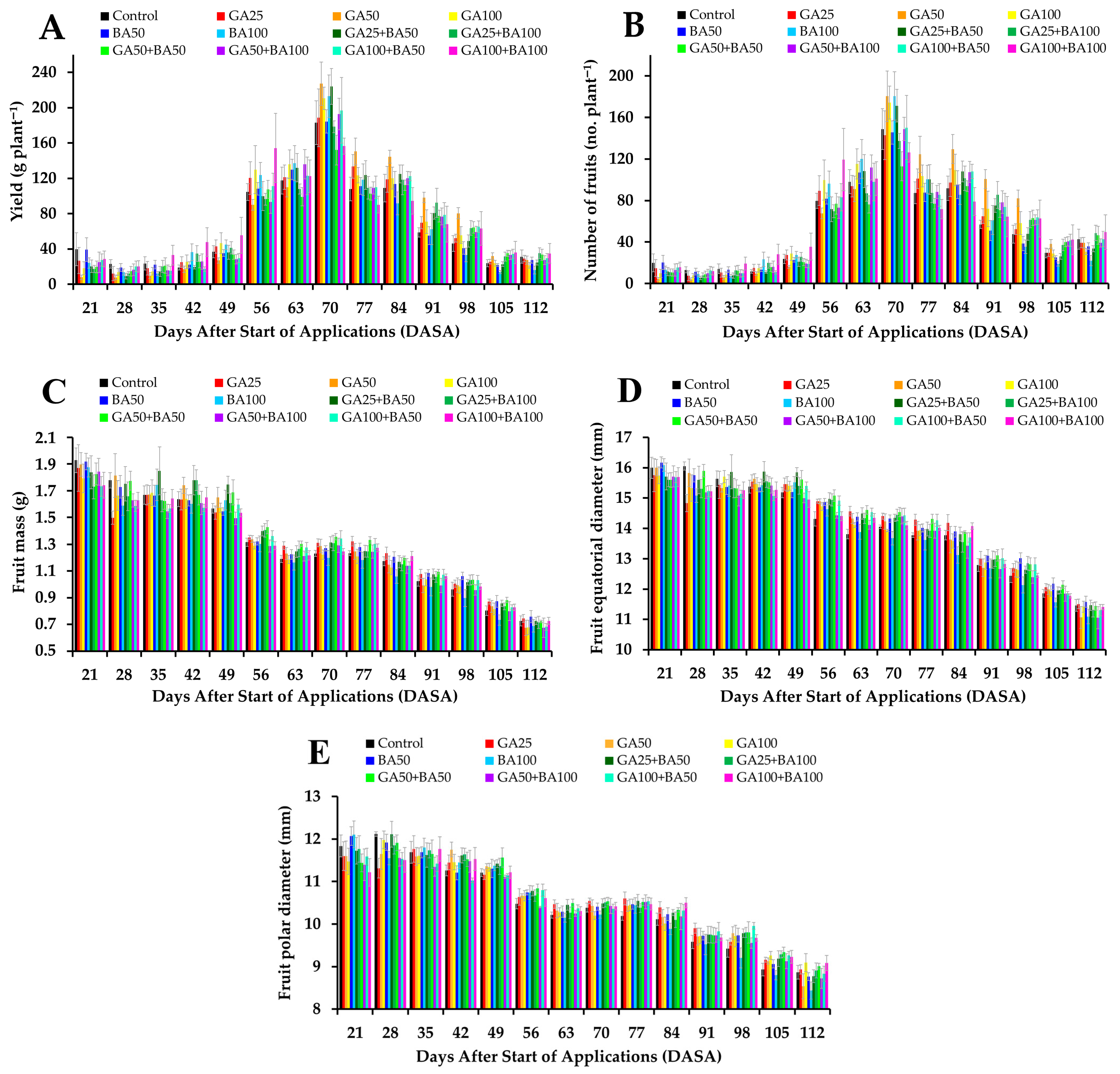
2.1. Productive and Physical Characteristics of Fruits
2.2. Antioxidant Composition of Fruits
2.3. Chemical Characteristics of Fruits
3. Discussion
3.1. Productive and Physical Characteristics of Fruits
3.2. Chemical and Antioxidant Characteristics of Fruits
4. Materials and Methods
4.1. Site Characterization
4.2. Plant Material
4.3. Experimental Design and Treatments
4.4. Evaluations
4.4.1. Productive and Physical Characteristics of Fruits
- Yield (g plant−1): all fruits collected from the three plants of each treatment replicate were weighed on an electronic scale with accuracy of 0.01 g. Subsequently, the average was calculated to determine yield per plant.
- Number of fruits (no. plant−1): all fruits collected from the three plants of each treatment replicate were counted. Subsequently, the average was calculated to determine the number of fruits per plant.
- Fruit mass (g): obtained through the quotient between yield and number of fruits per plant.
- Fruit equatorial diameter and polar diameter (mm): obtained by the average of 20 fruits randomly collected per replicate individually measured with a digital caliper with precision of 0.01 mm (Mitutoyo, Neuss, Germany, model 500-196-30).
4.4.2. Chemical and Antioxidant Characteristics of Fruits
- pH: determined in 10 mL of the concentrated juice with a pH meter (Hanna, Smithfield, RI, USA, model pH 21).
- Soluble solids (SS): determined with three drops of the concentrated juice using a digital refractometer with automatic temperature compensation (Asko, Oslo, Norway, model RHB32), previously calibrated with deionized water. The quantification was performed in triplicate and the average data expressed in °Brix.
- Titratable acidity (TA): determined by potentiometric volumetry, where the concentrated juice (10 mL) was diluted in 100 mL of deionized water and this mixture was titrated with 0.1 N NaOH solution until reaching pH of 8.2. The results were expressed as % citric acid.
- SS/TA ratio: maturity index obtained by the soluble solids to titratable acidity ratio.
- Soluble sugars: extraction was performed according to methodology proposed by Garcia et al. [83], where 100 mg of the plant material was dissolved in 1 mL of 80% ethanol; the mixture was incubated in water bath at 80 °C for 15 min and centrifuged at 12,000 rpm at 25 °C for 15 min. The plant material was submitted to three extractions and the supernatants were combined and equalized with deionized water to a final volume of 3 mL. Quantification was performed by the phenol-sulfuric method [84], where a 10 µL aliquot of the extract was incorporated into 490 µL of deionized water, 0.5 mL of 5% phenol and 2.5 mL of concentrated sulfuric acid. The solution was homogenized, cooled to room temperature for 5 min and read on a UV-Vis spectrophotometer (Bel Engineering®, Monza, Italy, model UV-M51) at 490 nm. The concentration of soluble sugars was calculated using an anhydrous glucose calibration curve (y = 0.0183x + 0.0719, R2 = 0.9998) and expressed in milligrams of glucose equivalent per gram of fresh mass.
- Total phenols: equivalent to the concentration of phenolic compounds in the sample, quantified according to the Folin-Ciocalteau method, with adaptations [85]. The plant material (100 mg) was dissolved in 5 mL of 50% acetone and the mixture was vortexed for 30 s, submitted to ultrasonic bath for 20 s and centrifuged at 5000 rpm for 10 min. Two extractions were performed and the supernatants were combined. An aliquot of 0.5 mL of the extract was incorporated into 0.5 mL of deionized water, 0.5 mL of Folin-Ciocalteau reagent (1:4) and 2.5 mL of 4% Na2CO3. After homogenization and remaining in the dark and at room temperature for one hour, reading was performed on a UV-Vis spectrophotometer at 725 nm. The concentration of total phenols was calculated using the gallic acid calibration curve (y = 0.0265x + 0.0032, R2 = 0.9955) and expressed in milligrams of gallic acid equivalent per gram of fresh mass.
- Flavonoids: the plant material (100 mg) was dissolved in 4 mL of acidified methanol (85:15, 70% methanol: 10% acetic acid); the mixture was vortexed for 30 s, submitted to ultrasonic bath for 30 min and subsequently incorporated into 1 mL of 5% aluminum chloride. After homogenization and remaining in the dark and at room temperature for 30 min, centrifugation was performed at 7830 rpm at 5 °C for 22 min and the extracted supernatant was read in a UV-Vis spectrophotometer at 425 nm [86,87]. The concentration of total flavonoids was determined by the rutin calibration curve (y = 0.0026x − 0.0009, R2 = 0.9999) and the results were expressed in milligrams of rutin equivalent per gram of fresh mass.
- Anthocyanins: determination by differential pH method, with adaptations [88]. For extraction, 300 mg of plant material was diluted in 13 mL of extracting solution (99:1; MeOH: 1 N HCl), remaining at rest for 24 h at 4 °C. After this period, the mixture was sonicated for 60 min, centrifuged at 5000 rpm for 10 min and, finally, the supernatant was separated from the plant material. The extract was dissolved separately in two buffer solutions, one at pH 1.0 (KCl, 0.025 M) and the other at pH 4.5 (CH3COONa, 0.40 M), in the proportion of 1.0 mL of extract to 3.0 mL of each solution. The absorbance of each dilution was measured in a UV-Vis spectrophotometer at 520 and 700 nm, using the extracting solution as a blank. Quantification was performed using the following formula: anthocyanin pigment (mg/mL) = A × MW × DF/(ε × I). Where A = (A520 nm − A700 nm) pH 1.0 − (A520 nm − A700 nm) pH 4.5; MW (molecular weight) = 449.2 g/mol of cyanidin-3-glucoside; DF = dilution factor; ε (extinction coefficient, in mol/L of cyanidin-3-glucoside) = 26,900 molar; I = cuvette thickness in cm (1.0). Results were expressed in milligram equivalents of cyanidin-3-glucoside per gram of fresh mass.
- Antioxidant activity: determination according to the method that evaluates the scavenging capacity of 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals, with adaptations [89,90]. The plant material (200 mg) was dissolved in 5 mL of acidified methanol (80:19:1, methanol:deionized water:acetic acid). This mixture was vortexed for 10 s, submitted to ultrasonic bath for 15 min and centrifuged at 2000 rpm at 5 °C for 10 min, and the supernatant was subsequently extracted. An aliquot of 500 μL of the supernatant was incorporated into 3 mL of concentrated ethanol and 300 μL of the DPPH solution (2 × 10−4 g mL−1). After homogenization and remaining in the dark and at room temperature for 40 min, reading was performed, together with the blank, in a UV-Vis spectrophotometer at 517 nm. Results were expressed in % of reduced DPPH, using the following formula: % reduced DPPH = (Blank Absorbance − Sample Absorbance)/Blank Absorbance × 100.
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Spinardi, A.; Cola, G.; Gardana, C.S.; Mignani, I. Variation of Anthocyanin Content and Profile Throughout Fruit Development and Ripening of Highbush Blueberry Cultivars Grown at Two Different Altitudes. Front. Plant Sci. 2019, 10, 1045. [Google Scholar] [CrossRef]
- Xie, G.; Xu, X.; Zhou, X.; Liu, Y.; Zhao, Z. Changes in Phenolic Profiles and Antioxidant Activity in Rabbiteye Blueberries during Ripening. Int. J. Food Prop. 2019, 22, 320–329. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Wei, Z.; Yin, B.; Man, C.; Jiang, Y. Enhancement of Functional Characteristics of Blueberry Juice Fermented by Lactobacillus plantarum. LWT 2021, 139, 110590. [Google Scholar] [CrossRef]
- Das, P.R.; Darwish, A.G.; Ismail, A.; Haikal, A.M.; Gajjar, P.; Balasubramani, S.P.; Sheikh, M.B.; Tsolova, V.; Soliman, K.F.A.; Sherif, S.M.; et al. Diversity in Blueberry Genotypes and Developmental Stages Enables Discrepancy in the Bioactive Compounds, Metabolites, and Cytotoxicity. Food Chem. 2022, 374, 131632. [Google Scholar] [CrossRef]
- Huang, W.; Yan, Z.; Li, D.; Ma, Y.; Zhou, J.; Sui, Z. Antioxidant and Anti-Inflammatory Effects of Blueberry Anthocyanins on High Glucose-Induced Human Retinal Capillary Endothelial Cells. Oxid. Med. Cell Longev. 2018, 2018, 1862462. [Google Scholar] [CrossRef]
- Jiao, X.; Wang, Y.; Lin, Y.; Lang, Y.; Li, E.; Zhang, X.; Zhang, Q.; Feng, Y.; Meng, X.; Li, B. Blueberry Polyphenols Extract as a Potential Prebiotic with Anti-Obesity Effects on C57BL/6 J Mice by Modulating the Gut Microbiota. J. Nutr. Biochem. 2019, 64, 88–100. [Google Scholar] [CrossRef]
- Wood, E.; Hein, S.; Heiss, C.; Williams, C.; Rodriguez-Mateos, A. Blueberries and Cardiovascular Disease Prevention. Food Funct. 2019, 10, 7621–7633. [Google Scholar] [CrossRef]
- Tian, J.-L.; Liao, X.-J.; Wang, Y.-H.; Si, X.; Shu, C.; Gong, E.-S.; Xie, X.; Ran, X.-L.; Li, B. Identification of Cyanidin-3-Arabinoside Extracted from Blueberry as a Selective Protein Tyrosine Phosphatase 1B Inhibitor. J. Agric. Food Chem. 2019, 67, 13624–13634, Erratum in J. Agric. Food Chem. 2022, 70, 2060. [Google Scholar] [CrossRef]
- Rodriguez-Saona, C.; Vincent, C.; Isaacs, R. Blueberry IPM: Past Successes and Future Challenges. Annu. Rev. Entomol. 2019, 64, 95–114. [Google Scholar] [CrossRef]
- FAO. Food and Agriculture Organization of the United Nations (FAO) Statistics Division. Available online: http://www.fao.org/faostat/en/#home (accessed on 4 February 2025).
- Mohd Ali, N.; Yeap, S.K.; Ho, W.Y.; Beh, B.K.; Tan, S.W.; Tan, S.G. The Promising Future of Chia, Salvia hispanica L. J. Biomed. Biotechnol. 2012, 2012, 171956. [Google Scholar] [CrossRef]
- Carroll, J.L.; Orr, S.T.; Retano, A.; Gregory, A.D.; Lukas, S.B.; Bryla, D.R. Weather-Based Scheduling and Pulse Drip Irrigation Increase Growth and Production of Northern Highbush Blueberry. HortScience 2024, 59, 571–577. [Google Scholar] [CrossRef]
- Zhang, Q.; Zang, H.; Guo, X.; Li, S.; Xin, X.; Li, Y. A Systematic Study on Composition and Antioxidant of 6 Varieties of Highbush Blueberries by 3 Soil Matrixes in China. Food Chem. 2025, 472, 142974. [Google Scholar] [CrossRef]
- Min, Z.; Jiang, L.; Zhao, Y.; Wang, X.; Liu, Q.; Zhang, Y. Effects of 24-Epibrassinolide on the Postharvest Quality and Antioxidant Activities of Blueberry Fruits. N. Z. J. Crop Hortic. Sci. 2025, 53, 53–66. [Google Scholar] [CrossRef]
- Makarov, S.S.; Vinogradova, V.S.; Khanbabaeva, O.E.; Makarova, T.A.; Chudetsky, A.I.; Sokolkina, A.I. Prospects for Enhanced Growth and Yield of Blueberry (Vaccinium angustifolium Ait.) Using Organomineral Fertilizers for Reclamation of Disturbed Forest Lands in European Part of Russia. Agronomy 2024, 14, 1498. [Google Scholar] [CrossRef]
- Duan, Y.; Tarafdar, A.; Chaurasia, D.; Singh, A.; Bhargava, P.C.; Yang, J.; Li, Z.; Ni, X.; Tian, Y.; Li, H.; et al. Blueberry Fruit Valorization and Valuable Constituents: A Review. Int. J. Food Microbiol. 2022, 381, 109890. [Google Scholar] [CrossRef]
- Simpson, M.; Parks, S.E.; Morris, S.; Joyce, D. Use of Thinners Can Increase the Fruit Size of Blueberries in an Evergreen System. N. Z. J. Crop Hortic. Sci. 2023, 51, 188–197. [Google Scholar] [CrossRef]
- Bons, H.K.; Kaur, M. Role of Plant Growth Regulators in Improving Fruit Set, Quality and Yield of Fruit Crops: A Review. J. Hortic. Sci. Biotechnol. 2020, 95, 137–146. [Google Scholar] [CrossRef]
- Dong, Y.; Wu, Y.; Zhang, Z.; Wang, S.; Cheng, J.; Gao, Y.; Wang, W.; Ma, N.; Wang, Y. Transcriptomic Analysis Reveals GA3 Is Involved in Regulating Flavonoid Metabolism in Grape Development for Facility Cultivation. Mol. Genet. Genom. 2023, 298, 845–855. [Google Scholar] [CrossRef]
- Kapłan, M.; Najda, A.; Klimek, K.; Borowy, A. Effect of Gibberellic Acid (GA3) Inflorescence Application on Content of Bioactive Compounds and Antioxidant Potential of Grape (Vitis L.) ‘Einset Seedless’ Berries. S. Afr. J. Enol. Vitic. 2018, 40, 1–10. [Google Scholar] [CrossRef]
- Mesbah Uddin, A.S.M.; Gomasta, J.; Islam, T.; Islam, M.; Kayesh, E.; Karim, M.R. Gibberellic Acid Spray Modulates Fruiting, Yield, Quality, and Shelf Life of Rambutan (Nephelium lappaceum L.). J. Hortic. Res. 2024, 32, 51–66. [Google Scholar] [CrossRef]
- Khalil, H.A. Improved Yield, Fruit Quality, and Shelf Life in ‘Flame Seedless’ Grapevine with Pre-Harvest Foliar Applications of Forchlorfenuron, Gibberellic Acid, and Abscisic Acid. J. Hortic. Res. 2020, 28, 77–86. [Google Scholar] [CrossRef]
- Xu, Y.; Hou, X.; Feng, J.; Khalil-Ur-Rehman, M.; Tao, J. Transcriptome Sequencing Analyses Reveals Mechanisms of Eliminated Russet by Applying GA3 and CPPU on ‘Shine Muscat’ Grape. Sci. Hortic. 2019, 250, 94–103. [Google Scholar] [CrossRef]
- Mosa, W.F.A.; Abd EL-Megeed, N.A.; Ali, M.M.; Abada, H.S.; Ali, H.M.; Siddiqui, M.H.; Sas-Paszt, L. Preharvest Foliar Applications of Citric Acid, Gibberellic Acid and Humic Acid Improve Growth and Fruit Quality of ‘Le Conte’ Pear (Pyrus communis L.). Horticulturae 2022, 8, 507. [Google Scholar] [CrossRef]
- Çolak, A.M. Effect of Melatonin and Gibberellic Acid Foliar Application on the Yield and Quality of Jumbo Blackberry Species. Saudi J. Biol. Sci. 2018, 25, 1242–1246. [Google Scholar] [CrossRef]
- Aremu, A.O.; Fawole, O.A.; Makunga, N.P.; Masondo, N.A.; Moyo, M.; Buthelezi, N.M.D.; Amoo, S.O.; Spíchal, L.; Doležal, K. Applications of Cytokinins in Horticultural Fruit Crops: Trends and Future Prospects. Biomolecules 2020, 10, 1222. [Google Scholar] [CrossRef]
- Hussain, S.; Chang, J.; Li, J.; Chen, L.; Ahmad, S.; Song, Z.; Zhang, B.; Chen, X. Multifunctional Role of Cytokinin in Horticultural Crops. Int. J. Mol. Sci. 2025, 26, 1037. [Google Scholar] [CrossRef]
- Marchioretto, L.D.R.; De Rossi, A.; do Amaral, L.O.; de Souza Ribeiro, A.M.A. Efficacy and Mode of Action of Blossom Thinners on ‘Fuji More’ Apple Trees. Sci. Hortic. 2019, 246, 634–642. [Google Scholar] [CrossRef]
- Dong, Y.; Song, M.; Liu, X.; Tian, R.; Zhang, L.; Gan, L. Effects of Exogenous KT and BA on Fruit Quality in Strawberry (Fragaria vesca). J. Hortic. Sci. Biotechnol. 2022, 97, 236–243. [Google Scholar] [CrossRef]
- Koron, D.; Stopar, M. Effect of Thinners on Yield, Fruit Size and Ripening Time of Highbush Blueberry. Acta Hortic. 2006, 715, 273–278. [Google Scholar] [CrossRef]
- Milić, B.; Tarlanović, J.; Keserović, Z.; Magazin, N.; Miodragović, M.; Popara, G. Bioregulators Can Improve Fruit Size, Yield and Plant Growth of Northern Highbush Blueberry (Vaccinium corymbosum L.). Sci. Hortic. 2018, 235, 214–220. [Google Scholar] [CrossRef]
- Sun, Y.; Hou, Z.; Su, S.; Yaun, J. Effects of ABA, GA3 and NAA on Fruit Development and Anthocyanin Accumulation in Blueberry. J. South China Agric. Univ. 2013, 34, 6–11. [Google Scholar]
- Hu, L.; Wang, X.; Liu, H.; Wu, Y.; Wu, W.; Lyu, L.; Li, W. Mechanisms of Exogenous GA3 Induced Inhibition of Seed Development in Southern Highbush Blueberry (Vaccinium darrowii). Sci. Hortic. 2023, 322, 112430. [Google Scholar] [CrossRef]
- Pérez-León, M.I.; González-Fuentes, J.A.; Valdez-Aguilar, L.A.; Benavides-Mendoza, A.; Alvarado-Camarillo, D.; Castillo-Chacón, C.E. Effect of Glutamic Acid and 6-Benzylaminopurine on Flower Bud Biostimulation, Fruit Quality and Antioxidant Activity in Blueberry. Plants 2023, 12, 2363. [Google Scholar] [CrossRef]
- Zang, Y.-X.; Chun, I.-J.; Zhang, L.-L.; Hong, S.-B.; Zheng, W.-W.; Xu, K. Effect of Gibberellic Acid Application on Plant Growth Attributes, Return Bloom, and Fruit Quality of Rabbiteye Blueberry. Sci. Hortic. 2016, 200, 13–18. [Google Scholar] [CrossRef]
- NeSmith, D.S. Response of Rabbiteye Blueberry (Vaccinium ashei Reade) to the Growth Regulators CPPU and Gibberellic Acid. HortScience 2002, 37, 666–668. [Google Scholar] [CrossRef]
- Cartagena, J.R.; Matta, F.B.; Spiers, J.M. Chemical Fruit Thinning of Vaccinium ashei Reade. J. Am. Soc. Hortic. Sci. 1994, 119, 1133–1136. [Google Scholar] [CrossRef]
- USENIK, V. Physicochemical Changes of Sweet Cherry Fruits Related to Application of Gibberellic Acid. Food Chem. 2005, 90, 663–671. [Google Scholar] [CrossRef]
- Milović, M.; Kevrešan, Ž.; Mastilović, J.; Kovač, R.; Kalajdžić, J.; Magazin, N.; Bajić, A.; Milić, B.; Barać, G.; Keserović, Z. Could an Early Treatment with GA and BA Impact Prolonged Cold Storage and Shelf Life of Apricot? Horticulturae 2022, 8, 1220. [Google Scholar] [CrossRef]
- Canli, F.A.; Orhan, H. Effects of Preharvest Gibberellic Acid Applications on Fruit Quality of ‘0900 Ziraat’ Sweet Cherry. Horttechnology 2009, 19, 127–129. [Google Scholar] [CrossRef]
- Wang, X.; Wu, Y.; Hu, L.; Zhang, C.; Wu, W.; Li, W.; Fang, J. Elucidation of the Mechanism Underlying Seedless Blueberry Formation after GA3 Treatment Based on the Phenotype, Physiology, Metabolism and Transcriptome. Sci. Hortic. 2023, 311, 111781. [Google Scholar] [CrossRef]
- Kumar Shivandu, S.; Singh, D.; Kumar, G.; Sharma, I.; Garg, J. Plant Growth Regulators: Key Drivers of Fruit Crop Productivity. In Fruit Crops Science; IntechOpen: London, UK, 2025. [Google Scholar]
- Milić, B.; Tarlanović, J.; Keserović, Z.; Zorić, L.; Blagojević, B.; Magazin, N. The Growth of Apple Central Fruits as Affected by Thinning with NAA, BA and Naphthenic Acids. Erwerbs-Obstbau 2017, 59, 185–193. [Google Scholar] [CrossRef]
- Wismer, P.T.; Proctor, J.T.A.; Elfving, D.C. Benzyladenine Affects Cell Division and Cell Size during Apple Fruit Thinning. J. Am. Soc. Hortic. Sci. 1995, 120, 802–807. [Google Scholar] [CrossRef]
- Williamson, J.G.; NeSmith, D.S. Effects of CPPU Applications on Southern Highbush Blueberries. HortScience 2007, 42, 1612–1615. [Google Scholar] [CrossRef]
- Lee, S.; Jung, E.S.; Do, S.-G.; Jung, G.; Song, G.; Song, J.; Lee, C.H. Correlation between Species-Specific Metabolite Profiles and Bioactivities of Blueberries (Vaccinium Spp.). J. Agric. Food Chem. 2014, 62, 2126–2133. [Google Scholar] [CrossRef]
- Li, D.; Li, B.; Ma, Y.; Sun, X.; Lin, Y.; Meng, X. Polyphenols, Anthocyanins, and Flavonoids Contents and the Antioxidant Capacity of Various Cultivars of Highbush and Half-High Blueberries. J. Food Compos. Anal. 2017, 62, 84–93. [Google Scholar] [CrossRef]
- Ehlenfeldt, M.K.; Prior, R.L. Oxygen Radical Absorbance Capacity (ORAC) and Phenolic and Anthocyanin Concentrations in Fruit and Leaf Tissues of Highbush Blueberry. J. Agric. Food Chem. 2001, 49, 2222–2227. [Google Scholar] [CrossRef]
- Prior, R.L.; Cao, G.; Martin, A.; Sofic, E.; McEwen, J.; O’Brien, C.; Lischner, N.; Ehlenfeldt, M.; Kalt, W.; Krewer, G.; et al. Antioxidant Capacity As Influenced by Total Phenolic and Anthocyanin Content, Maturity, and Variety of Vaccinium Species. J. Agric. Food Chem. 1998, 46, 2686–2693. [Google Scholar] [CrossRef]
- González-Villagra, J.; Reyes-Díaz, M.; Alberdi, M.; Mora, M.L.; Ulloa-Inostroza, E.M.; Ribera-Fonseca, A.E. Impact of Cold-Storage and UV-C Irradiation Postharvest Treatments on Quality and Antioxidant Properties of Fruits from Blueberry Cultivars Grown in Southern Chile. J. Soil. Sci. Plant Nutr. 2020, 20, 1751–1758. [Google Scholar] [CrossRef]
- Alam, M.d.A.; Islam, P.; Subhan, N.; Rahman, M.d.M.; Khan, F.; Burrows, G.E.; Nahar, L.; Sarker, S.D. Potential Health Benefits of Anthocyanins in Oxidative Stress Related Disorders. Phytochem. Rev. 2021, 20, 705–749. [Google Scholar] [CrossRef]
- Wang, L.; Yang, S.; Ni, J.; Teng, Y.; Bai, S. Advances of Anthocyanin Synthesis Regulated by Plant Growth Regulators in Fruit Trees. Sci. Hortic. 2023, 307, 111476. [Google Scholar] [CrossRef]
- Montero, T.; Mollá, E.; Martín-Cabrejas, M.A.; López-Andréu, F.J. Effects of Gibberellic Acid (GA3) on Strawberry PAL (Phenylalanine Ammonia-Lyase) and TAL (Tyrosine Ammonia-Lyase) Enzyme Activities. J. Sci. Food Agric. 1998, 77, 230–234. [Google Scholar] [CrossRef]
- Zhang, D.; Xu, X.; Zhang, Z.; Jiang, G.; Feng, L.; Duan, X.; Jiang, Y. 6-Benzylaminopurine Improves the Quality of Harvested Litchi Fruit. Postharvest Biol. Technol. 2018, 143, 137–142. [Google Scholar] [CrossRef]
- Zahid, N.; Alowaiesh, B.F.; Masood, N.; Ahmad, K.S.; Khalid, S.; Khalid, M.S.; Maqbool, M.; Awan, S.I.; Imtiaz, Z. Multi-Locational Study on Plant Growth Regulators to Minimize Pre-Mature Fruit Drop and Maximize Postharvest Quality of Apples. Cogent Food Agric. 2024, 10, 2300178. [Google Scholar] [CrossRef]
- Angami, T.; Assumi, S.R.; Kalita, H.; Bhagawati, K.; Chandra, A.; Alone, R.A. Springtime Foliar Application of Plant Bio-Regulators on off Season Strawberry Production under Mid Hill Condition. J. Exp. Biol. Agric. Sci. 2020, 8, 544–550. [Google Scholar] [CrossRef]
- Xie, S.; Liu, Y.; Chen, H.; Yang, B.; Ge, M.; Zhang, Z. Effects of Gibberellin Applications before Flowering on the Phenotype, Ripening, and Flavonoid Compounds of Syrah Grape Berries. J. Sci. Food Agric. 2022, 102, 6100–6111. [Google Scholar] [CrossRef]
- Teszlák, P.; Gaál, K.; Pour Nikfardjam, M.S. Influence of Grapevine Flower Treatment with Gibberellic Acid (GA3) on Polyphenol Content of Vitis vinifera L. Wine. Anal. Chim. Acta 2005, 543, 275–281. [Google Scholar] [CrossRef]
- Asgari, F.; Kalateh Jari, S.; Motesharezadeh, B.; Ghanbari Jahromi, M.; Weisany, W. Application of Benzylaminopurine with Methyl Jasmonate and Epibrassinolide Improved Growth and Physio-Biochemical Attributes of Strawberry (Fragaria × ananassa Cv. ‘Albion’). Appl. Fruit Sci. 2024, 66, 453–463. [Google Scholar] [CrossRef]
- Barać, G.; Mastilović, J.; Kevrešan, Ž.; Milić, B.; Kovač, R.; Milović, M.; Kalajdžić, J.; Bajić, A.; Magazin, N.; Keserović, Z. Effects of Plant Growth Regulators on Plum (Prunus domestica L.) Grown on Two Rootstocks at Harvest and at the Postharvest Period. Horticulturae 2022, 8, 621. [Google Scholar] [CrossRef]
- Murcia, G.; Fontana, A.; Pontin, M.; Baraldi, R.; Bertazza, G.; Piccoli, P.N. ABA and GA3 Regulate the Synthesis of Primary and Secondary Metabolites Related to Alleviation from Biotic and Abiotic Stresses in Grapevine. Phytochemistry 2017, 135, 34–52. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Liao, Y.; Lin, K.; Wu, W.; Duan, L.; Wang, P.; Xiao, X.; Zhang, T.; Chen, X.; Wang, J.; et al. Cytokinin Promotes Anthocyanin Biosynthesis via Regulating Sugar Accumulation and MYB113 Expression in Eucalyptus. Tree Physiol. 2024, 44, tpad154. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Duan, Y.; Wei, Z.; Wu, Y.; Zhang, C.; Wu, W.; Lyu, L.; Li, W. Integrated Physiological and Metabolomic Analyses Reveal the Differences in the Fruit Quality of the Blueberry Cultivated in Three Soilless Substrates. Foods 2022, 11, 3965. [Google Scholar] [CrossRef]
- Ahmed, W.; Tahir, F.M.; Rajwana, I.A.; Raza, S.A.; Asad, H.U. Comparative Evaluation of Plant Growth Regulators for Preventing Premature Fruit Drop and Improving Fruit Quality Parameters in ‘Dusehri’ Mango. Int. J. Fruit Sci. 2012, 12, 372–389. [Google Scholar] [CrossRef]
- Anjum, N. Effect of Gibberellic Acid on Berry Yield and Quality Attributes of Grapes Cv. Sultanina. Pure Appl. Biol. 2020, 9, 1319–1324. [Google Scholar] [CrossRef]
- Canli, F.A.; Pektas, M.; Ercisli, S. Benzyladenine and Gibberellin Applications Improve Fruit Weight and Delay Maturity of Sweet Cherry. Erwerbs-Obstbau 2015, 57, 71–75. [Google Scholar] [CrossRef]
- Yang, D.; Li, Z.; Li, J.; Chen, J.; Wang, J.; Jing, X.; Guan, X. Effect of Pre-Flowering Gibberellic Acid Applications on Tartaric Acid Content in Grape Berries. Sci. Hortic. 2024, 325, 112659. [Google Scholar] [CrossRef]
- Li, X.; Cai, Z.; Liu, X.; Wu, Y.; Han, Z.; Yang, G.; Li, S.; Xie, Z.; Liu, L.; Li, B. Effects of Gibberellic Acid on Soluble Sugar Content, Organic Acid Composition, Endogenous Hormone Levels, and Carbon Sink Strength in Shine Muscat Grapes during Berry Development Stage. Horticulturae 2024, 10, 346. [Google Scholar] [CrossRef]
- Patel, J.S.; Dhruve, J.J.; Motka, G.N.; Patel, A.D. Influence of Plant Growth Regulators and Boron on Nutritional Quality and Shelflife of Aonla Fruit. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2533–2540. [Google Scholar] [CrossRef]
- Li, L.; Li, D.; Luo, Z.; Huang, X.; Li, X. Proteomic Response and Quality Maintenance in Postharvest Fruit of Strawberry (Fragaria × ananassa) to Exogenous Cytokinin. Sci. Rep. 2016, 6, 27094. [Google Scholar] [CrossRef]
- Jáuregui-Riquelme, F.; Kremer-Morales, M.S.; Alcalde, J.A.; Pérez-Donoso, A.G. Pre-Anthesis CPPU Treatment Modifies Quality and Susceptibility to Post-Harvest Berry Cracking of Vitis vinifera Cv. ‘Thompson Seedless’. J. Plant Growth Regul. 2017, 36, 413–423. [Google Scholar] [CrossRef]
- Domingos, S.; Nobrega, H.; Raposo, A.; Cardoso, V.; Soares, I.; Ramalho, J.C.; Leitão, A.E.; Oliveira, C.M.; Goulao, L.F. Light Management and Gibberellic Acid Spraying as Thinning Methods in Seedless Table Grapes (Vitis vinifera L.): Cultivar Responses and Effects on the Fruit Quality. Sci. Hortic. 2016, 201, 68–77. [Google Scholar] [CrossRef]
- Bezerra, J.B.N.; de Jesus, P.R.R.; Souza, I.D.; Bezerra, W.C.; Martins, G.C.S.B.; Ribeiro, V.G. Plant Regulators on the Growth, Quality and Production of ‘Tommy Atkins’ Mango Fruits. Rev. Bras. Frutic. 2021, 43, e546. [Google Scholar] [CrossRef]
- Beaudry, R. Blueberry Quality Characteristics and How They Can Be Optimized. Annu. Rep. Mich. State Hortic. Soc. 1992, 122, 140–145. [Google Scholar]
- IŞÇI, B. Yield and Quality of Sultani Grapes (Vitis vinifera L.) Treated with 28-Homobrassinolide and Gibberellic Acid. Appl. Ecol. Environ. Res. 2019, 17, 12441–12450. [Google Scholar] [CrossRef]
- Milić, B.M.; Mastilović, J.S.; Kevrešan, Ž.S.; Kovač, R.; Bajić, A.R.; Keserović, Z.Ž.; Magazin, N.P.; Milović, M.Đ.; Kalajdžić, J.D.; Barać, G.N. Consequences of NAA, BA and GA3 Treatment in Early Fruit Development Phase on Postharvest Properties of Apricot Cv. NS4. Acta Sci. Pol. Hortorum Cultus 2022, 21, 49–59. [Google Scholar] [CrossRef]
- Canli, F.A.; Sahin, M.; Temurtas, N.; Pektas, M. Improving Fruit Quality of Apricot by Means of Preharvest Benzyladenine and Benzyladenine Plus Gibberellin Applications. Horttechnology 2014, 24, 424–427. [Google Scholar] [CrossRef]
- Spiers, J.M.; Stringer, S.J.; Draper, A.D.; Gupton, C.L. “Biloxi” Southern Highbush Blueberry. Acta Hortic. 2002, 574, 153–155. [Google Scholar] [CrossRef]
- Rossi, M. Mapa Pedológico Do Estado de São Paulo: Revisado e Ampliado; Instituto Florestal: São Paulo, Brazil, 2017; Volume 1. [Google Scholar]
- Rolim, G.d.S.; de Camargo, M.B.P.; Lania, D.G.; de Moraes, J.F.L. Classificação Climática de Köppen e de Thornthwaite e Sua Aplicabilidade Na Determinação de Zonas Agroclimáticas Para o Estado de São Paulo. Bragantia 2007, 66, 711–720. [Google Scholar] [CrossRef]
- Pratap-Singh, A.; Shojaei, M.; Singh, A.; Ye, Y.; Mandal, R.; Yan, Y.; Pico, J.; Gerbrandt, E.M.; Castellarin, S.D. Effects of Pulsed Light on the Postharvest Quality and Shelf-Life of Highbush Blueberries (Cv. Draper). Appl. Food Res. 2023, 3, 100273. [Google Scholar] [CrossRef]
- Instituto Adolfo Lutz. Métodos Físico-Químicos Para Análise de Alimentos; Zenebon, O., Pascuet, N.S., Tiglea, P., Eds.; Instituto Adolfo Lutz: São Paulo, Brazil, 2008. [Google Scholar]
- Garcia, I.S.; Souza, A.; Barbedo, C.J.; Dietrich, S.M.C.; Figueiredo-Ribeiro, R.C.L. Changes in Soluble Carbohydrates during Storage of Caesalpinia echinata LAM. (Brazilwood) Seeds, an Endangered Leguminous Tree from the Brazilian Atlantic Forest. Braz. J. Biol. 2006, 66, 739–745. [Google Scholar] [CrossRef] [PubMed]
- DuBois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric Method for Determination of Sugars and Related Substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of Total Phenols and Other Oxidation Substrates and Antioxidants by Means of Folin-Ciocalteu Reagent. Methods Enzym. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Awad, M.A.; de Jager, A.; Van Westing, L.M. Flavonoid and Chlorogenic Acid Levels in Apple Fruit: Characterisation of Variation. Sci. Hortic. 2000, 83, 249–263. [Google Scholar] [CrossRef]
- dos Santos, M.D.; Blatt, C.T.T. Teor de Flavonoides e Fenóis Totais Em Folhas de Pyrostegia venusta Miers. de Mata e de Cerrado. Rev. Bras. De Botânica 1998, 21, 135–140. [Google Scholar] [CrossRef]
- Lee, J.; Durst, R.W.; Wrolstad, R.E.; Eisele, T.; Giusti, M.M.; Hach, J.; Hofsommer, H.; Koswig, S.; Krueger, D.A.; Kupina, S.; et al. Determination of Total Monomeric Anthocyanin Pigment Content of Fruit Juices, Beverages, Natural Colorants, and Wines by the PH Differential Method: Collaborative Study. J. AOAC Int. 2005, 88, 1269–1278. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT-Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Rossetto, M.R.M.; Vianello, F.; Rocha, S.A.; Lima, G.P.P. Antioxidant Substances and Pesticide in Parts of Beet Organic and Conventional Manure. Afr. J. Plant Sci. 2009, 3, 245–253. [Google Scholar]
| Treatments | Total Phenols (mg g−1) | Flavonoids (mg g−1) | Anthocyanins (mg g−1) | Antioxidant Activity (%) |
|---|---|---|---|---|
| Control | 2.95 ± 0.09 b | 2.00 ± 0.03 b | 1.35 ± 0.01 b | 89.64 ± 0.28 b |
| GA25 | 3.16 ± 0.12 b | 1.96 ± 0.03 b | 1.31 ± 0.04 b | 88.17 ± 0.26 c |
| GA50 | 2.83 ± 0.13 b | 2.16 ± 0.08 a | 1.26 ± 0.06 b | 89.91 ± 0.45 a |
| GA100 | 3.09 ± 0.16 b | 1.85 ± 0.06 b | 1.47 ± 0.05 a | 90.52 ± 0.21 a |
| BA50 | 3.08 ± 0.12 b | 2.12 ± 0.06 a | 1.51 ± 0.03 a | 89.04 ± 0.26 b |
| BA100 | 3.05 ± 0.12 b | 2.18 ± 0.03 a | 1.35 ± 0.05 b | 87.72 ± 0.35 c |
| GA25 + BA50 | 2.79 ± 0.13 b | 1.90 ± 0.08 b | 1.51 ± 0.07 a | 87.52 ± 0350 c |
| GA25 + BA100 | 3.39 ± 0.13 a | 2.02 ± 0.04 b | 1.45 ± 0.02 a | 89.08 ± 0.13 b |
| GA50 + BA50 | 3.16 ± 0.20 b | 1.87 ± 0.06 b | 1.32 ± 0.05 b | 89.35 ± 0.10 b |
| GA50 + BA100 | 3.51 ± 0.11 a | 2.01 ± 0.02 b | 1.47 ± 0.04 a | 90.35 ± 0.11 a |
| GA100 + BA50 | 3.48 ± 0.06 a | 2.00 ± 0.10 b | 1.43 ± 0.09 a | 90.28 ± 0.58 a |
| GA100 + BA100 | 3.52 ± 0.13 a | 2.09 ± 0.01 a | 1.47 ± 0.03 a | 90.97 ± 0.16 a |
| p | 0.0167 * | 0.0053 n.s. | 0.0317 * | <0.0001 * |
| F | 2.47 | 2.94 | 2.20 | 10.90 |
| CV (%) | 10.84 | 7.04 | 9.11 | 0.85 |
| Beginning of the Yield Cycle (28 DASA) | ||||
|---|---|---|---|---|
| Treatments | Total Phenols (mg g−1) | Flavonoids (mg g−1) | Anthocyanins (mg g−1) | Antioxidant Activity (%) |
| Control | 3.45 ± 0.05 b | 2.27 ± 0.02 b | 1.19 ± 0.02 b | 90.76 ± 0.19 b |
| GA100 | 3.74 ± 0.11 a | 2.48 ± 0.11 b | 1.40 ± 0.06 a | 90.38 ± 0.17 b |
| BA100 | 4.00 ± 0.08 a | 2.70 ± 0.10 a | 1.36 ± 0.04 a | 90.78 ± 0.29 b |
| GA25 + BA50 | 3.95 ± 0.09 a | 2.49 ± 0.10 b | 1.35 ± 0.03 a | 89.84 ± 0.29 b |
| GA100 + BA50 | 3.78 ± 0.06 a | 2.82 ± 0.10 a | 1.47 ± 0.06 a | 90.33 ± 0.10 b |
| GA100 + BA100 | 3.92 ± 0.03 a | 2.31 ± 0.10 b | 1.34 ± 0.02 a | 91.94 ± 0.23 a |
| p | 0.0012 * | 0.0124 * | 0.0180 * | <0.0001 * |
| F | 6.28 | 3.91 | 3.57 | 9.63 |
| CV (%) | 4.68 | 9.65 | 8.28 | 0.56 |
| Treatments | Soluble Sugars (mg g−1) | SS (°Brix) | TA (% C.A.) | SS/TA Ratio | pH |
|---|---|---|---|---|---|
| Control | 75.88 ± 2.09 b | 10.20 ± 0.18 b | 0.64 ± 0.01 b | 16.06 ± 0.26 a | 3.22 ± 0.03 a |
| GA25 | 68.85 ± 3.14 b | 10.05 ± 0.07 b | 0.71 ± 0.02 b | 14.22 ± 0.45 b | 3.19 ± 0.02 a |
| GA50 | 70.39 ± 2.43 b | 9.80 ± 0.18 b | 0.70 ± 0.03 b | 14.13 ± 0.80 b | 3.22 ± 0.02 a |
| GA100 | 81.50 ± 0.69 a | 10.35 ± 0.10 a | 0.67 ± 0.02 b | 15.41 ± 0.42 a | 3.23 ± 0.03 a |
| BA50 | 70.35 ± 2.07 b | 9.75 ± 0.18 b | 0.75 ± 0.02 a | 12.97 ± 0.40 b | 3.21 ± 0.02 a |
| BA100 | 78.84 ± 2.28 a | 10.85 ± 0.18 a | 0.68 ± 0.02 b | 15.95 ± 0.49 a | 3.22 ± 0.01 a |
| GA25 + BA50 | 81.36 ± 1.98 a | 9.95 ± 0.03 b | 0.63 ± 0.03 b | 15.86 ± 0.73 a | 3.22 ± 0.03 a |
| GA25 + BA100 | 86.14 ± 1.85 a | 9.95 ± 0.03 b | 0.76 ± 0.02 a | 13.13 ± 0.37 b | 3.19 ± 0.02 a |
| GA50 + BA50 | 83.88 ± 2.93 a | 10.73 ± 0.10 a | 0.65 ± 0.02 b | 16.65 ± 0.66 a | 3.25 ± 0.02 a |
| GA50 + BA100 | 82.92 ± 1.81 a | 10.45 ± 0.14 a | 0.77 ± 0.03 a | 13.65 ± 0.62 b | 3.17 ± 0.02 a |
| GA100 + BA50 | 78.53 ± 1.97 a | 10.85 ± 0.03 a | 0.77 ± 0.01 a | 14.03 ± 0.22 b | 3.16 ± 0.01 a |
| GA100 + BA100 | 83.90 ± 2.27 a | 10.63 ± 0.17 a | 0.71 ± 0.00 b | 15.05 ± 0.31 a | 3.22 ± 0.01 a |
| p | <0.0001 * | <0.0001 * | 0.0001 * | 0.0002 * | 0.1952 n.s. |
| F | 5.52 | 7.09 | 4.52 | 4.31 | 1.43 |
| CV (%) | 7.18 | 3.29 | 7.71 | 8.99 | 1.47 |
| Beginning of the Yield Cycle (28 DASA) | |||||
|---|---|---|---|---|---|
| Treatments | Soluble Sugars (mg g−1) | SS (°Brix) | TA (% C.A.) | SS/TA Ratio | pH |
| Control | 103.49 ± 0.94 b | 13.04 ± 0.15 b | 1.17 ± 0.03 b | 11.15 ± 0.35 b | 2.78 ± 0.02 a |
| GA100 | 113.56 ± 1.40 a | 13.00 ± 0.37 b | 1.22 ± 0.02 a | 10.64 ± 0.31 b | 2.79 ± 0.02 a |
| BA100 | 115.58 ± 3.66 a | 14.08 ± 0.20 a | 1.26 ± 0.04 a | 11.25 ± 0.39 b | 2.81 ± 0.02 a |
| GA25 + BA50 | 121.89 ± 3.80 a | 12.44 ± 0.36 b | 1.27 ± 0.04 a | 9.81 ± 0.41 b | 2.73 ± 0.03 a |
| GA100 + BA50 | 115.07 ± 2.66 a | 13.56 ± 0.30 a | 1.28 ± 0.03 a | 10.68 ± 0.51 b | 2.79 ± 0.04 a |
| GA100 + BA100 | 115.23 ± 2.72 a | 13.56 ± 0.13 a | 1.08 ± 0.02 b | 12.61 ± 0.26 a | 2.86 ± 0.03 a |
| p | 0.0043 * | 0.0135 * | 0.0053 * | 0.0102 * | 0.1381 n.s. |
| F | 4.91 | 3.83 | 4.71 | 4.08 | 1.91 |
| CV (%) | 5.27 | 4.92 | 6.58 | 9.35 | 2.38 |
| Months | Average Temperature (°C) | Average Relative Air Humidity (%) | Rainfall (mm) |
|---|---|---|---|
| July | 19.76 | 69.64 | 0.00 |
| August | 23.08 | 60.76 | 13.50 |
| September | 26.65 | 58.29 | 37.00 |
| October | 26.11 | 73.21 | 221.30 |
| November | 27.47 | 66.25 | 72.50 |
| Average | 24.61 | 65.63 | - |
| Total rainfall | - | - | 344.30 |
| Treatment | GA3 (mg L−1) | BA (mg L−1) | Identification |
|---|---|---|---|
| T1 * | - | - | Control |
| T2 | 25 | - | GA25 |
| T3 | 50 | - | GA50 |
| T4 | 100 | - | GA100 |
| T5 | - | 50 | BA50 |
| T6 | - | 100 | BA100 |
| T7 | 25 | 50 | GA25 + BA50 |
| T8 | 25 | 100 | GA25 + BA100 |
| T9 | 50 | 50 | GA50 + BA50 |
| T10 | 50 | 100 | GA50 + BA100 |
| T11 | 100 | 50 | GA100 + BA50 |
| T12 | 100 | 100 | GA100 + BA100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodrigues, L.S.; Cardoso, C.P.; Savazaki, E.T.; Kim, S.C.R.; Mimi, C.O.; De-la-Cruz-Chacón, I.; Ferreira, G. Exogenous Gibberellic Acid (GA3) and Benzylaminopurine Enhance the Antioxidant Properties of Vaccinium corymbosum L. ‘Biloxi’ Fruits Without Affecting Yield. Int. J. Mol. Sci. 2025, 26, 7984. https://doi.org/10.3390/ijms26167984
Rodrigues LS, Cardoso CP, Savazaki ET, Kim SCR, Mimi CO, De-la-Cruz-Chacón I, Ferreira G. Exogenous Gibberellic Acid (GA3) and Benzylaminopurine Enhance the Antioxidant Properties of Vaccinium corymbosum L. ‘Biloxi’ Fruits Without Affecting Yield. International Journal of Molecular Sciences. 2025; 26(16):7984. https://doi.org/10.3390/ijms26167984
Chicago/Turabian StyleRodrigues, Larissa Silva, Caroline Pardine Cardoso, Edson Tadashi Savazaki, Stephane Catarine Rosa Kim, Carolina Ovile Mimi, Iván De-la-Cruz-Chacón, and Gisela Ferreira. 2025. "Exogenous Gibberellic Acid (GA3) and Benzylaminopurine Enhance the Antioxidant Properties of Vaccinium corymbosum L. ‘Biloxi’ Fruits Without Affecting Yield" International Journal of Molecular Sciences 26, no. 16: 7984. https://doi.org/10.3390/ijms26167984
APA StyleRodrigues, L. S., Cardoso, C. P., Savazaki, E. T., Kim, S. C. R., Mimi, C. O., De-la-Cruz-Chacón, I., & Ferreira, G. (2025). Exogenous Gibberellic Acid (GA3) and Benzylaminopurine Enhance the Antioxidant Properties of Vaccinium corymbosum L. ‘Biloxi’ Fruits Without Affecting Yield. International Journal of Molecular Sciences, 26(16), 7984. https://doi.org/10.3390/ijms26167984







