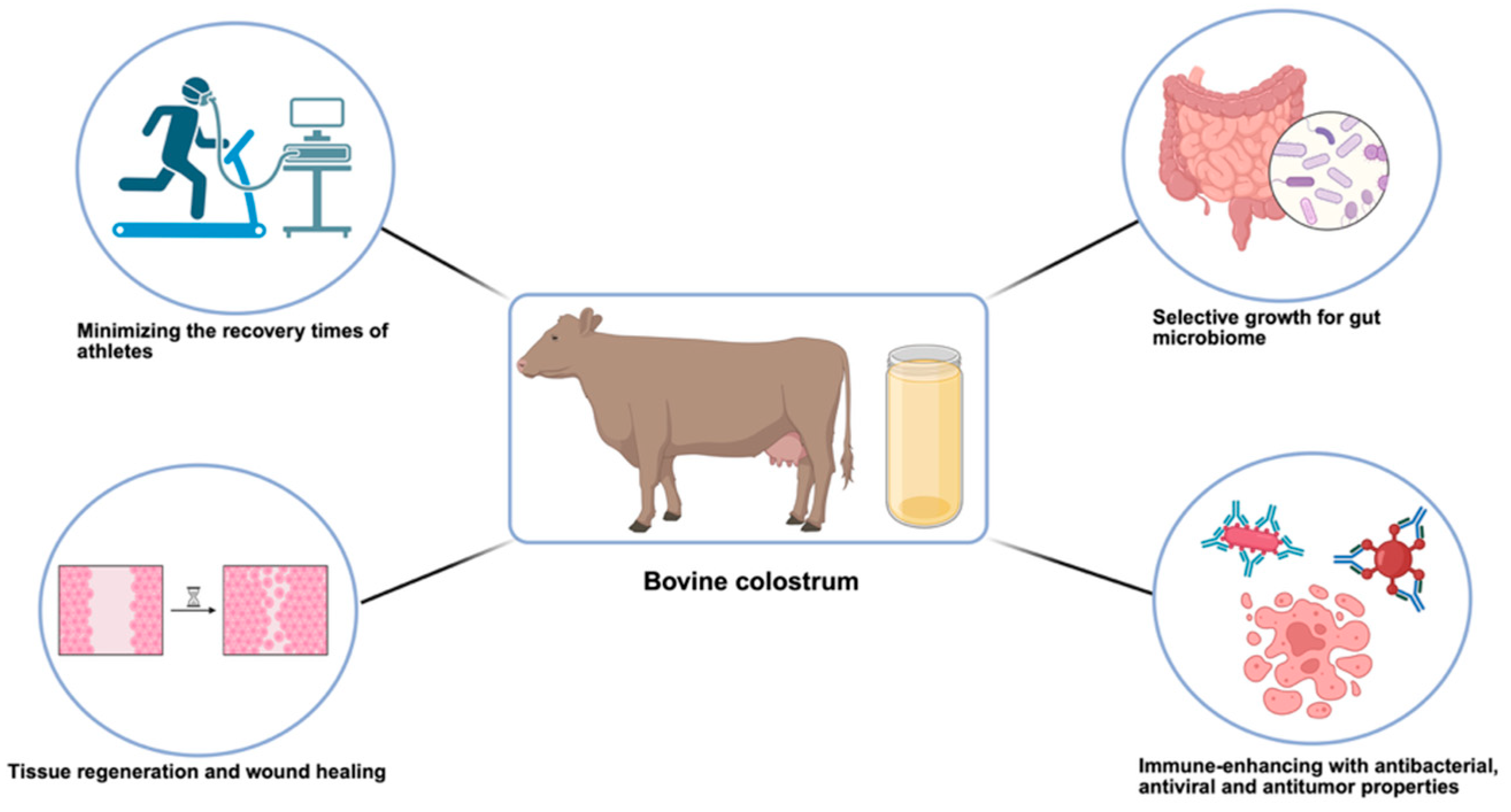
Exploring the Therapeutic Potential of Bovine Colostrum for Cancer Therapies
Abstract
1. Introduction
| Component | Health Implication | Concentration | Reference |
|---|---|---|---|
| Lactoferrin | Supports immune system, has antimicrobial properties, and may aid in iron regulation and gut health. | 0.82 mg/mL | [2,5,6] |
| Immunoglobulins | Help protect the body by identifying and neutralizing pathogens, playing a key role in immune defense. | IgG: 46.40 g/L IgM: 6.77 g/L IgA: 5.86 g/L | [7] |
| Exosomes | Support immune development, carry regulatory molecules like miRNAs, and may serve as biomarkers for health and disease. | - | [8] |
| Oligosaccharides | Support gut health, promote beneficial microbes, and enhance immune protection in early life. | -- | [5,9] |
| Lactoperoxidase | Supports immune defense by exhibiting antimicrobial activity against bacteria, viruses, and fungi. | - | [2,3] |
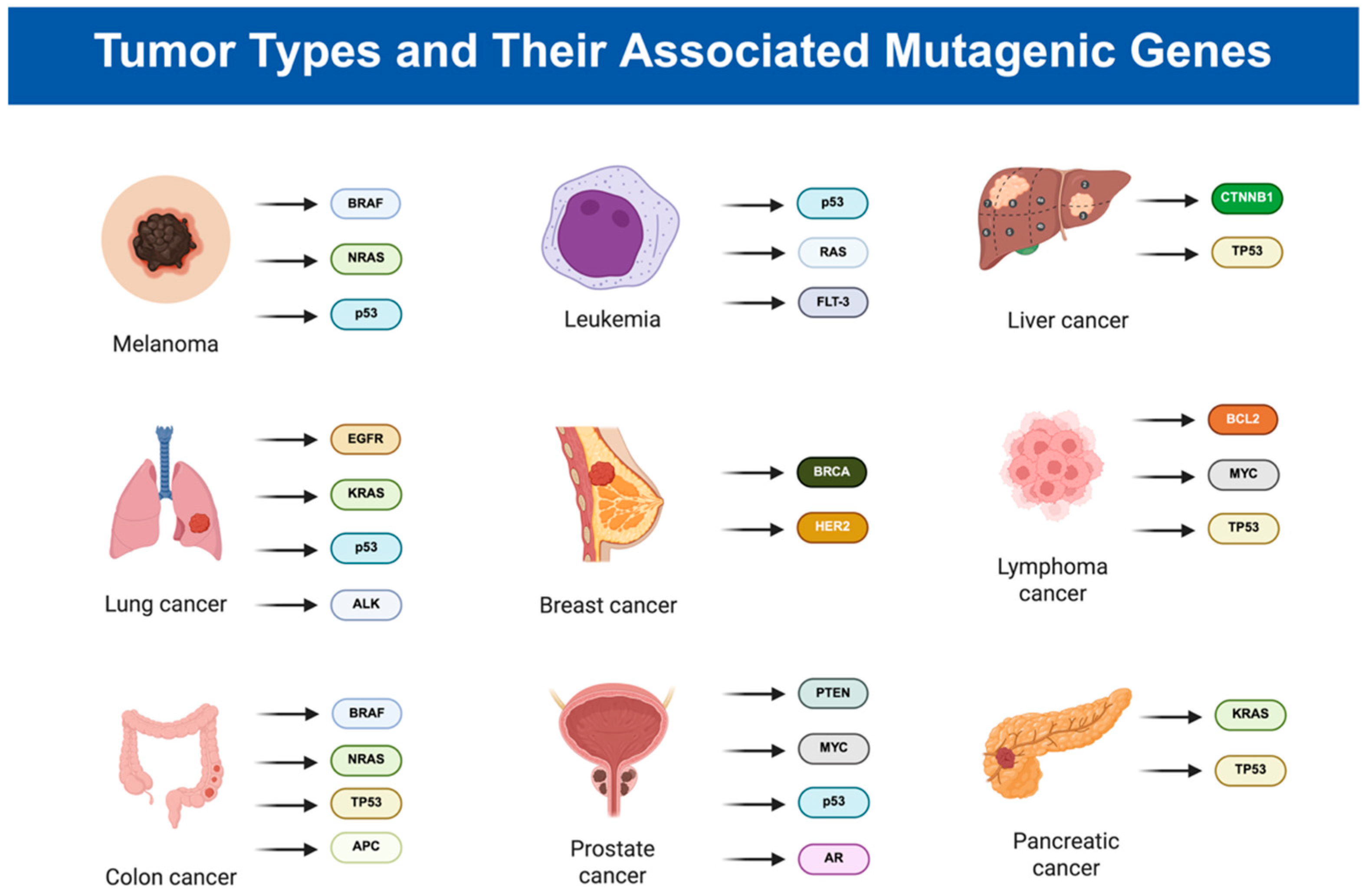
2. Exploring Cancer: Mechanisms, Types, and Statistics
3. Bovine Colostrum as Therapeutic Agent
4. Future Perspective
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Arslan, A.; Kaplan, M.; Duman, H.; Bayraktar, A.; Ertürk, M.; Henrick, B.M.; Frese, S.A.; Karav, S. Bovine Colostrum and Its Potential for Human Health and Nutrition. Front. Nutr. 2021, 8, 651721. [Google Scholar] [CrossRef]
- Yalçıntaş, Y.M.; Duman, H.; Rocha, J.M.; Bartkiene, E.; Karav, S.; Ozogul, F. Role of Bovine Colostrum against Various Diseases. Food Biosci. 2024, 61, 104818. [Google Scholar] [CrossRef]
- Eker, F.; Akdaşçi, E.; Duman, H.; Yalçıntaş, Y.M.; Canbolat, A.A.; Kalkan, A.E.; Karav, S.; Šamec, D. Antimicrobial Properties of Colostrum and Milk. Antibiotics 2024, 13, 251. [Google Scholar] [CrossRef]
- Yalçıntaş, Y.M.; Duman, H.; López, J.M.M.; Portocarrero, A.C.M.; Lombardo, M.; Khallouki, F.; Koch, W.; Bordiga, M.; El-Seedi, H.; Raposo, A.; et al. Revealing the Potency of Growth Factors in Bovine Colostrum. Nutrients 2024, 16, 2359. [Google Scholar] [CrossRef]
- Karav, S. Selective Deglycosylation of Lactoferrin to Understand Glycans’ Contribution to Antimicrobial Activity of Lactoferrin. Cell. Mol. Biol. (Noisy-Le-Grand) 2018, 64, 52–57. [Google Scholar] [CrossRef] [PubMed]
- Niaz, B.; Saeed, F.; Ahmed, A.; Imran, M.; Maan, A.A.; Khan, M.K.I.; Tufail, T.; Anjum, F.M.; Hussain, S.; Suleria, H.A.R. Lactoferrin (LF): A Natural Antimicrobial Protein. Int. J. Food Prop. 2019, 22, 1626–1641. [Google Scholar] [CrossRef]
- Batista da Silva Galdino, A.; do Nascimento Rangel, A.H.; Buttar, H.S.; Sales Lima Nascimento, M.; Cristina Gavioli, E.; Oliveira, R.D.P.; Cavalcanti Sales, D.; Urbano, S.A.; Anaya, K. Bovine Colostrum: Benefits for the Human Respiratory System and Potential Contributions for Clinical Management of COVID-19. Food Agric. Immunol. 2021, 32, 143–162. [Google Scholar] [CrossRef]
- Samuel, M.; Chisanga, D.; Liem, M.; Keerthikumar, S.; Anand, S.; Ang, C.-S.; Adda, C.G.; Versteegen, E.; Jois, M.; Mathivanan, S. Bovine Milk-Derived Exosomes from Colostrum Are Enriched with Proteins Implicated in Immune Response and Growth. Sci. Rep. 2017, 7, 5933. [Google Scholar] [CrossRef]
- Karav, S.; German, J.B.; Rouquié, C.; Le Parc, A.; Barile, D. Studying Lactoferrin N-Glycosylation. Int. J. Mol. Sci. 2017, 18, 870. [Google Scholar] [CrossRef]
- Yalçıntaş, Y.M.; Baydemir, B.; Duman, H.; Eker, F.; Bayraktar Biçen, A.; Ertürk, M.; Karav, S. Exploring the Impact of Colostrum Supplementation on Athletes: A Comprehensive Analysis of Clinical Trials and Diverse Properties. Front. Immunol. 2024, 15, 1395437. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer Treatment and Survivorship Statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef]
- Michor, F.; Iwasa, Y.; Nowak, M.A. Dynamics of Cancer Progression. Nat. Rev. Cancer 2004, 4, 197–205. [Google Scholar] [CrossRef]
- Wang, L. Early Diagnosis of Breast Cancer. Sensors 2017, 17, 1572. [Google Scholar] [CrossRef] [PubMed]
- Boffetta, P.; Nyberg, F. Contribution of Environmental Factors to Cancer Risk. Br. Med. Bull. 2003, 68, 71–94. [Google Scholar] [CrossRef] [PubMed]
- Landi, S. Genetic Predisposition and Environmental Risk Factors to Pancreatic Cancer: A Review of the Literature. Mutat. Res./Rev. Mutat. Res. 2009, 681, 299–307. [Google Scholar] [CrossRef] [PubMed]
- Futreal, P.A.; Coin, L.; Marshall, M.; Down, T.; Hubbard, T.; Wooster, R.; Rahman, N.; Stratton, M.R. A Census of Human Cancer Genes. Nat. Rev. Cancer 2004, 4, 177–183. [Google Scholar] [CrossRef]
- Weinberg, R.A. How Cancer Arises. Sci. Am. 1996, 275, 62–70. [Google Scholar] [CrossRef]
- Pedraza-Fariña, L.G. Mechanisms of Oncogenic Cooperation in Cancer Initiation and Metastasis. Yale J. Biol. Med. 2006, 79, 95–103. [Google Scholar]
- Alsayed, A.R.; Hasoun, L.Z.; Khader, H.A.; Basheti, I.A.; Permana, A.D. Bovine Colostrum Treatment of Specific Cancer Types: Current Evidence and Future Opportunities. Molecules 2022, 27, 8641. [Google Scholar] [CrossRef]
- Artym, J.; Zimecki, M. Colostrum Proteins in Protection against Therapy-Induced Injuries in Cancer Chemo- and Radiotherapy: A Comprehensive Review. Biomedicines 2023, 11, 114. [Google Scholar] [CrossRef]
- Torghabe, S.Y.; Alavi, P.; Rostami, S.; Davies, N.M.; Kesharwani, P.; Karav, S.; Sahebkar, A. Modulation of the Ubiquitin-Proteasome System by Curcumin: Therapeutic Implications in Cancer. Pathol. Res. Pract. 2025, 265, 155741. [Google Scholar] [CrossRef] [PubMed]
- Orrantia-Borunda, E.; Anchondo-Nuñez, P.; Acuña-Aguilar, L.E.; Gómez-Valles, F.O.; Ramírez-Valdespino, C.A. Subtypes of Breast Cancer. In Breast Cancer; Mayrovitz, H.N., Ed.; Exon Publications: Brisbane, Australia, 2022; ISBN 978-0-6453320-3-2. [Google Scholar]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA A Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Benson, A.B.; Bekaii-Saab, T.; Chan, E.; Chen, Y.-J.; Choti, M.A.; Cooper, H.S.; Engstrom, P.F.; Enzinger, P.C.; Fakih, M.G.; Fuchs, C.S.; et al. Rectal Cancer. J. Natl. Compr. Cancer Netw. 2012, 10, 1528–1564. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.T.; Duong, H.-Q. The Molecular Characteristics of Colorectal Cancer: Implications for Diagnosis and Therapy (Review). Oncol. Lett. 2018, 16, 9–18. [Google Scholar] [CrossRef]
- Common Cancer Types—NCI. Available online: https://www.cancer.gov/types/common-cancers (accessed on 21 December 2024).
- Papavassiliou, K.A.; Sofianidi, A.A.; Gogou, V.A.; Anagnostopoulos, N.; Papavassiliou, A.G. P53 and Rb Aberrations in Small Cell Lung Cancer (SCLC): From Molecular Mechanisms to Therapeutic Modulation. Int. J. Mol. Sci. 2024, 25, 2479. [Google Scholar] [CrossRef]
- D’Angelo, A.; Sobhani, N.; Chapman, R.; Bagby, S.; Bortoletti, C.; Traversini, M.; Ferrari, K.; Voltolini, L.; Darlow, J.; Roviello, G. Focus on ROS1-Positive Non-Small Cell Lung Cancer (NSCLC): Crizotinib, Resistance Mechanisms and the Newer Generation of Targeted Therapies. Cancers 2020, 12, 3293. [Google Scholar] [CrossRef]
- Noronha, E.P.; Marques, L.V.C.; Andrade, F.G.; Thuler, L.C.S.; Terra-Granado, E.; Pombo-de-Oliveira, M.S.; Brazilian Collaborative Study Group of Acute Leukemia; da Paz Zampier, C.; da Conceição Barbosa, T.; Chagas Neto, P.; et al. The Profile of Immunophenotype and Genotype Aberrations in Subsets of Pediatric T-Cell Acute Lymphoblastic Leukemia. Front. Oncol. 2019, 9, 316. [Google Scholar] [CrossRef]
- Daver, N.; Schlenk, R.F.; Russell, N.H.; Levis, M.J. Targeting FLT3 Mutations in AML: Review of Current Knowledge and Evidence. Leukemia 2019, 33, 299–312. [Google Scholar] [CrossRef]
- Litwin, M.S.; Tan, H.-J. The Diagnosis and Treatment of Prostate Cancer: A Review. JAMA 2017, 317, 2532–2542. [Google Scholar] [CrossRef]
- Klap, J.; Schmid, M.; Loughlin, K.R. The Relationship between Total Testosterone Levels and Prostate Cancer: A Review of the Continuing Controversy. J. Urol. 2015, 193, 403–413. [Google Scholar] [CrossRef]
- Sia, D.; Villanueva, A.; Friedman, S.L.; Llovet, J.M. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology 2017, 152, 745–761. [Google Scholar] [CrossRef]
- Guo, J.; Jiang, X.; Lian, J.; Li, H.; Zhang, F.; Xie, J.; Deng, J.; Hou, X.; Du, Z.; Hao, E. Evaluation of the Effect of GSK-3β on Liver Cancer Based on the PI3K/AKT Pathway. Front. Cell Dev. Biol. 2024, 12, 1431423. [Google Scholar] [CrossRef]
- Luo, J. KRAS Mutation in Pancreatic Cancer. Semin. Oncol. 2021, 48, 10–18. [Google Scholar] [CrossRef]
- Moore, A.; Donahue, T. Pancreatic Cancer. JAMA 2019, 322, 1426. [Google Scholar] [CrossRef] [PubMed]
- Fisher, S.G.; Fisher, R.I. The Epidemiology of Non-Hodgkin’s Lymphoma. Oncogene 2004, 23, 6524–6534. [Google Scholar] [CrossRef] [PubMed]
- Gebauer, N.; Bernard, V.; Gebauer, W.; Thorns, C.; Feller, A.C.; Merz, H. TP53 Mutations Are Frequent Events in Double-Hit B-Cell Lymphomas with MYC and BCL2 but Not MYC and BCL6 Translocations. Leuk. Lymphoma 2015, 56, 179–185. [Google Scholar] [CrossRef]
- Kaplan, M.; Arslan, A.; Duman, H.; Karyelioğlu, M.; Baydemir, B.; Günar, B.B.; Alkan, M.; Bayraktar, A.; Tosun, H.İ.; Ertürk, M.; et al. Production of Bovine Colostrum for Human Consumption to Improve Health. Front. Pharmacol. 2021, 12, 796824. [Google Scholar] [CrossRef] [PubMed]
- Ulfman, L.H.; Leusen, J.H.W.; Savelkoul, H.F.J.; Warner, J.O.; van Neerven, R.J.J. Effects of Bovine Immunoglobulins on Immune Function, Allergy, and Infection. Front. Nutr. 2018, 5, 52. [Google Scholar] [CrossRef]
- Polidori, P.; Rapaccetti, R.; Klimanova, Y.; Zhang, J.-J.; Santini, G.; Vincenzetti, S. Nutritional Parameters in Colostrum of Different Mammalian Species. Beverages 2022, 8, 54. [Google Scholar] [CrossRef]
- Sokołowska, A.; Bednarz, R.; Pacewicz, M.; Georgiades, J.A.; Wilusz, T.; Polanowski, A. Colostrum from Different Mammalian Species—A Rich Source of Colostrinin. Int. Dairy J. 2008, 18, 204–209. [Google Scholar] [CrossRef]
- Karav, S.; Parc, A.L.; Leite Nobrega de Moura Bell, J.M.; Frese, S.A.; Kirmiz, N.; Block, D.E.; Barile, D.; Mills, D.A. Oligosaccharides Released from Milk Glycoproteins Are Selective Growth Substrates for Infant-Associated Bifidobacteria. Appl. Environ. Microbiol. 2016, 82, 12. [Google Scholar] [CrossRef]
- Bunyatratchata, A.; Parc, A.L.; de Moura Bell, J.M.L.N.; Cohen, J.L.; Duman, H.; Arslan, A.; Kaplan, M.; Barile, D.; Karav, S. Release of Bifidogenic N-Glycans from Native Bovine Colostrum Proteins by an Endo-β-N-Acetylglucosaminidase. Enzym. Microb. Technol. 2023, 162, 110138. [Google Scholar] [CrossRef]
- Bruno-Barcena, J.M.; Azcarate-Peril, M.A. Galacto-Oligosaccharides and Colorectal Cancer: Feeding Our Intestinal Probiome. J. Funct. Foods 2015, 12, 92–108. [Google Scholar] [CrossRef] [PubMed]
- Qamar, T.R.; Iqbal, S.; Syed, F.; Nasir, M.; Rehman, H.; Iqbal, M.A.; Liu, R.H. Impact of Novel Prebiotic Galacto-Oligosaccharides on Various Biomarkers of Colorectal Cancer in Wister Rats. Int. J. Mol. Sci. 2017, 18, 1785. [Google Scholar] [CrossRef] [PubMed]
- Tan, H.; Chen, W.; Liu, Q.; Yang, G.; Li, K. Pectin Oligosaccharides Ameliorate Colon Cancer by Regulating Oxidative Stress- and Inflammation-Activated Signaling Pathways. Front. Immunol. 2018, 9, 1504. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Li, H.; Qiao, J.; Yang, Y.; Wang, Y.; Liu, W.; Han, B. Potential Analysis and Preparation of Chitosan Oligosaccharides as Oral Nutritional Supplements of Cancer Adjuvant Therapy. Int. J. Mol. Sci. 2019, 20, 920. [Google Scholar] [CrossRef]
- Christiansen, M.N.; Chik, J.; Lee, L.; Anugraham, M.; Abrahams, J.L.; Packer, N.H. Cell Surface Protein Glycosylation in Cancer. Proteom. Syst. Biol. 2014, 14, 525–546. [Google Scholar] [CrossRef]
- Osumi, D.; Takahashi, M.; Miyoshi, E.; Yokoe, S.; Lee, S.H.; Noda, K.; Nakamori, S.; Gu, J.; Ikeda, Y.; Kuroki, Y.; et al. Core Fucosylation of E-Cadherin Enhances Cell–Cell Adhesion in Human Colon Carcinoma WiDr Cells. Cancer Sci. 2009, 100, 888–895. [Google Scholar] [CrossRef]
- Bresalier, R.S.; Niv, Y.; Byrd, J.C.; Duh, Q.Y.; Toribara, N.W.; Rockwell, R.W.; Dahiya, R.; Kim, Y.S. Mucin Production by Human Colonic Carcinoma Cells Correlates with Their Metastatic Potential in Animal Models of Colon Cancer Metastasis. J. Clin. Invest. 1991, 87, 1037–1045. [Google Scholar] [CrossRef]
- Debruyne, E.N.; Delanghe, J.R. Diagnosing and Monitoring Hepatocellular Carcinoma with Alpha-Fetoprotein: New Aspects and Applications. Clin. Chim. Acta 2008, 395, 19–26. [Google Scholar] [CrossRef]
- Coşkun, N.; Sarıtaş, S.; Jaouhari, Y.; Bordiga, M.; Karav, S. The Impact of Freeze Drying on Bioactivity and Physical Properties of Food Products. Appl. Sci. 2024, 14, 9183. [Google Scholar] [CrossRef]
- Farziyan, M.A.; Moradian, F.; Rafiei, A.R. Anticancer Effect of Bovine Lactoferrin on Human Esophagus Cancer Cell Line. Res. Mol. Med. 2016, 4, 18–23. [Google Scholar] [CrossRef]
- Abu-Serie, M.M.; El-Fakharany, E.M. Efficiency of Novel Nanocombinations of Bovine Milk Proteins (Lactoperoxidase and Lactoferrin) for Combating Different Human Cancer Cell Lines. Sci. Rep. 2017, 7, 16769. [Google Scholar] [CrossRef]
- Sugihara, Y.; Zuo, X.; Takata, T.; Jin, S.; Miyauti, M.; Isikado, A.; Imanaka, H.; Tatsuka, M.; Qi, G.; Shimamoto, F. Inhibition of DMH-DSS-induced Colorectal Cancer by Liposomal Bovine Lactoferrin in Rats. Oncol. Lett. 2017, 14, 5688–5694. [Google Scholar] [CrossRef] [PubMed]
- Gasser, M.; Lissner, R.; Nawalaniec, K.; Hsiao, L.-L.; Waaga-Gasser, A.M. KMP01D Demonstrates Beneficial Anti-Inflammatory Effects on Immune Cells: An Ex Vivo Preclinical Study of Patients With Colorectal Cancer. Front. Immunol. 2020, 11, 684. [Google Scholar] [CrossRef]
- Tung, Y.-T.; Chen, H.-L.; Yen, C.-C.; Lee, P.-Y.; Tsai, H.-C.; Lin, M.-F.; Chen, C.-M. Bovine Lactoferrin Inhibits Lung Cancer Growth through Suppression of Both Inflammation and Expression of Vascular Endothelial Growth Factor. J. Dairy Sci. 2013, 96, 2095–2106. [Google Scholar] [CrossRef]
- Amiri, F.; Moradian, F.; Rafiei, A. Anticancer Effect of Lactoferrin on Gastric Cancer Cell Line AGS. Res. Mol. Med. 2015, 3, 11–16. [Google Scholar] [CrossRef]
- Shahzad, M.M.K.; Felder, M.; Ludwig, K.; Galder, H.R.V.; Anderson, M.L.; Kim, J.; Cook, M.E.; Kapur, A.K.; Patankar, M.S. Trans10,Cis12 Conjugated Linoleic Acid Inhibits Proliferation and Migration of Ovarian Cancer Cells by Inducing ER Stress, Autophagy, and Modulation of Src. PLoS ONE 2018, 13, e0189524. [Google Scholar] [CrossRef]
- Kandimalla, R.; Wallen, M.; Jeyabalan, J.; Moholkar, D.N.; Spencer, W.; Gupta, R.C.; Aqil, F. Abstract 7235: Exosome-Mediated Delivery of siRNA against NRF2 for Treatment of Lung Cancer. Cancer Res. 2024, 84, 7235. [Google Scholar] [CrossRef]
- Scott, J.L.; Gupta, R.C.; Aqil, F.; Jeyabalan, J.; Schultz, D.J. Exosomal Delivery Enhances the Antiproliferative Effects of Acid-Hydrolyzed Apiaceae Spice Extracts in Breast Cancer Cells. Foods 2024, 13, 2811. [Google Scholar] [CrossRef]
- Tikhonov, S.; Chernukha, I.; Dunchenko, N. Comparative Evaluation Antimicrobial and Antitumor Activities of Natural Colostrum Peptide and Its Synthesized Analogue. Food Sci. Appl. Biotechnol. 2024, 7, 333–343. [Google Scholar] [CrossRef]
- Kumar, D.N.; Chaudhuri, A.; Shiromani, U.; Kumar, D.; Agrawal, A.K. An Investigation of In Vitro Anti-Cancer Efficacy of Dihydroartemisinin-Loaded Bovine Milk Exosomes Against Triple-Negative Breast Cancer. AAPS J. 2024, 26, 91. [Google Scholar] [CrossRef]
- Kandimalla, R.; Aqil, F.; Alhakeem, S.S.; Jeyabalan, J.; Tyagi, N.; Agrawal, A.; Yan, J.; Spencer, W.; Bondada, S.; Gupta, R.C. Targeted Oral Delivery of Paclitaxel Using Colostrum-Derived Exosomes. Cancers 2021, 13, 3700. [Google Scholar] [CrossRef] [PubMed]
- Samuel, M.; Fonseka, P.; Sanwlani, R.; Gangoda, L.; Chee, S.H.; Keerthikumar, S.; Spurling, A.; Chitti, S.V.; Zanker, D.; Ang, C.-S.; et al. Oral Administration of Bovine Milk-Derived Extracellular Vesicles Induces Senescence in the Primary Tumor but Accelerates Cancer Metastasis. Nat. Commun. 2021, 12, 3950. [Google Scholar] [CrossRef]
- Munagala, R.; Aqil, F.; Jeyabalan, J.; Kandimalla, R.; Wallen, M.; Tyagi, N.; Wilcher, S.; Yan, J.; Schultz, D.J.; Spencer, W.; et al. Exosome-Mediated Delivery of RNA and DNA for Gene Therapy. Cancer Lett. 2021, 505, 58–72. [Google Scholar] [CrossRef] [PubMed]
- Pullan, J.; Dailey, K.; Bhallamudi, S.; Feng, L.; Alhalhooly, L.; Froberg, J.; Osborn, J.; Sarkar, K.; Molden, T.; Sathish, V.; et al. Modified Bovine Milk Exosomes for Doxorubicin Delivery to Triple-Negative Breast Cancer Cells. ACS Appl. Bio Mater. 2022, 5, 2163–2175. [Google Scholar] [CrossRef]
- Rodriguez-Ochoa, N.; Cortes-Reynosa, P.; Rodriguez-Rojas, K.; de la Garza, M.; Salazar, E.P. Bovine Holo-Lactoferrin Inhibits Migration and Invasion in MDA-MB-231 Breast Cancer Cells. Mol. Biol. Rep. 2023, 50, 193–201. [Google Scholar] [CrossRef]
- Kaplan, M.; Baktıroğlu, M.; Kalkan, A.E.; Canbolat, A.A.; Lombardo, M.; Raposo, A.; de Brito Alves, J.L.; Witkowska, A.M.; Karav, S. Lactoferrin: A Promising Therapeutic Molecule against Human Papillomavirus. Nutrients 2024, 16, 3073. [Google Scholar] [CrossRef] [PubMed]
- Akdaşçi, E.; Eker, F.; Duman, H.; Singh, P.; Bechelany, M.; Karav, S. Lactoferrin as a Versatile Agent in Nanoparticle Applications: From Therapeutics to Agriculture. Nanomaterials 2024, 14, 2018. [Google Scholar] [CrossRef]
- Eker, F.; Duman, H.; Ertürk, M.; Karav, S. The Potential of Lactoferrin as Antiviral and Immune-Modulating Agent in Viral Infectious Diseases. Front. Immunol. 2024, 15, 1402135. [Google Scholar] [CrossRef]
- Timofeeva, A.M.; Paramonik, A.P.; Sedykh, S.S.; Nevinsky, G.A. Milk Exosomes: Next-Generation Agents for Delivery of Anticancer Drugs and Therapeutic Nucleic Acids. Int. J. Mol. Sci. 2023, 24, 10194. [Google Scholar] [CrossRef] [PubMed]
- Gurunathan, S.; Kang, M.-H.; Kim, J.-H. A Comprehensive Review on Factors Influences Biogenesis, Functions, Therapeutic and Clinical Implications of Exosomes. Int. J. Nanomed. 2021, 16, 1281–1312. [Google Scholar] [CrossRef] [PubMed]
- del Pozo-Acebo, L.; López de las Hazas, M.-C.; Tomé-Carneiro, J.; Gil-Cabrerizo, P.; San-Cristobal, R.; Busto, R.; García-Ruiz, A.; Dávalos, A. Bovine Milk-Derived Exosomes as a Drug Delivery Vehicle for miRNA-Based Therapy. Int. J. Mol. Sci. 2021, 22, 1105. [Google Scholar] [CrossRef] [PubMed]
- Jabłońska, M.; Sawicki, T.; Żulewska, J.; Staniewska, K.; Łobacz, A.; Przybyłowicz, K.E. The Role of Bovine Milk-Derived Exosomes in Human Health and Disease. Molecules 2024, 29, 5835. [Google Scholar] [CrossRef]
- Pontoppidan, P.E.; Shen, R.L.; Cilieborg, M.S.; Jiang, P.; Kissow, H.; Petersen, B.L.; Thymann, T.; Heilmann, C.; Müller, K.; Sangild, P.T. Bovine Colostrum Modulates Myeloablative Chemotherapy–Induced Gut Toxicity in Piglets1, 2, 3. J. Nutr. 2015, 145, 1472–1480. [Google Scholar] [CrossRef]
- Rathe, M.; De Pietri, S.; Wehner, P.S.; Frandsen, T.L.; Grell, K.; Schmiegelow, K.; Sangild, P.T.; Husby, S.; Müller, K. Bovine Colostrum Against Chemotherapy-Induced Gastrointestinal Toxicity in Children with Acute Lymphoblastic Leukemia: A Randomized, Double-Blind, Placebo-Controlled Trial. J. Parenter. Enter. Nutr. 2020, 44, 337–347. [Google Scholar] [CrossRef]
- Çelebì, A.; Dörtbudak, M.B.; Keskinrüzgar, A.; Yüksel, H. The Therapeutic Effect of Bovine Colostrum on 5-Fluorouracil-Induced Oral Mucositis in Rats. J. Stomatol. Oral Maxillofac. Surg. 2022, 123, e682–e686. [Google Scholar] [CrossRef]
- Alsudani, A.A. Study Of The Potential Effect Of Bovine Colostrum On Some Physiological Parameters In Male Rats Receiving Etoposide Therapy. IJASR 2024, 07, 214–222. [Google Scholar] [CrossRef]
- Shen, R.L.; Pontoppidan, P.E.L.; Rathe, M.; Jiang, P.; Hansen, C.F.; Buddington, R.K.; Heegaard, P.M.H.; Müller, K.; Sangild, P.T. Milk Diets Influence Doxorubicin-Induced Intestinal Toxicity in Piglets. Am. J. Physiol.-Gastrointest. Liver Physiol. 2016, 311, G324–G333. [Google Scholar] [CrossRef] [PubMed]
- Bateman, E.; Weaver, E.; Klein, G.; Wignall, A.; Wozniak, B.; Plews, E.; Mayo, B.; White, I.; Keefe, D. Serum-Derived Bovine Immunoglobulin/Protein Isolate in the Alleviation of Chemotherapy-Induced Mucositis. Support. Care Cancer 2016, 24, 377–385. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jones, A.W.; Thatcher, R.; March, D.S.; Davison, G. Influence of 4 Weeks of Bovine Colostrum Supplementation on Neutrophil and Mucosal Immune Responses to Prolonged Cycling. Scand. J. Med. Sci. Sports 2015, 25, 788–796. [Google Scholar] [CrossRef] [PubMed]
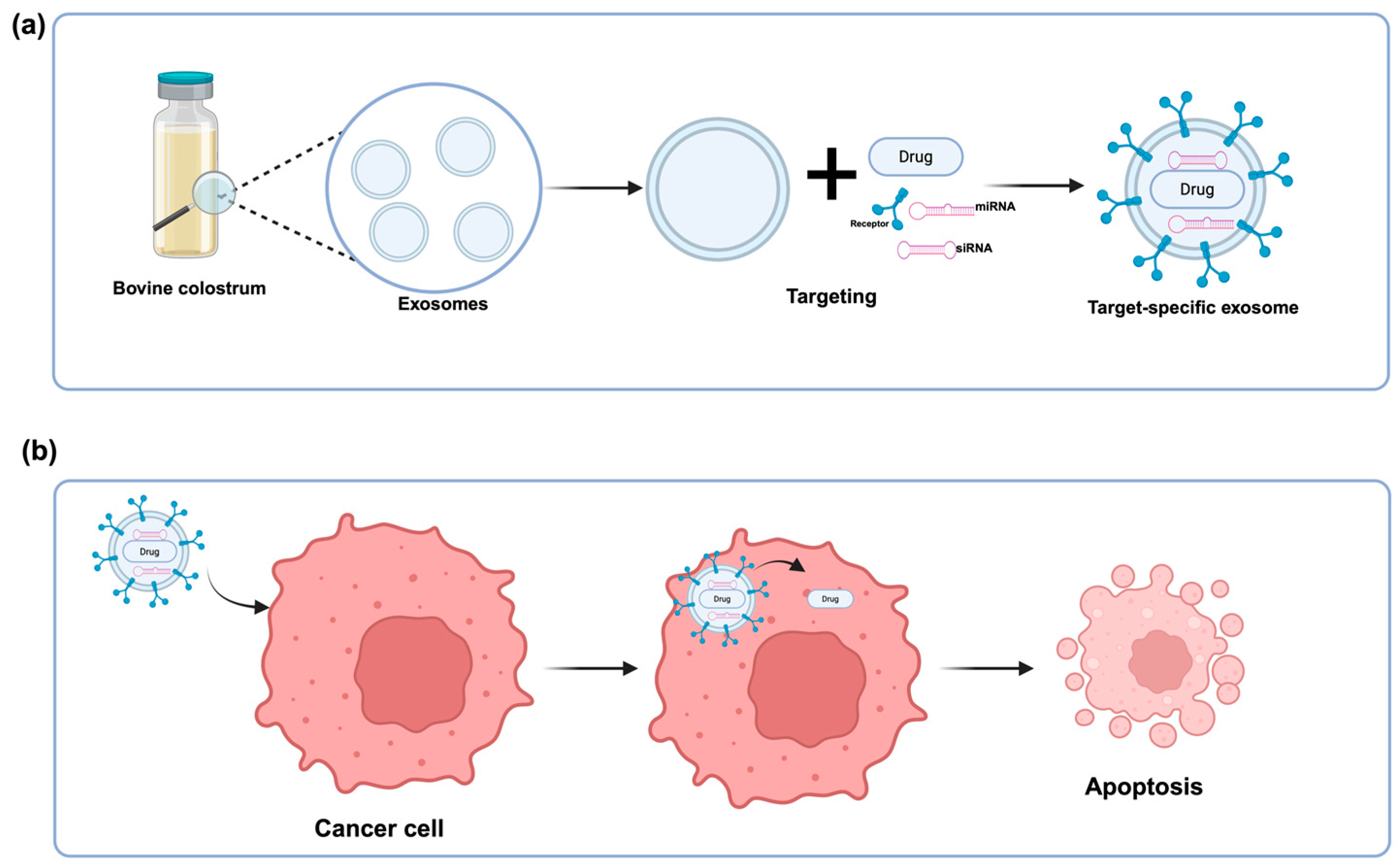
| Cancer Type | Study Design | Target Group | Supplement and TARGET | Dose and Duration | Effect | Reference |
|---|---|---|---|---|---|---|
| Esophagus cancer | in vitro experimental study | Esophageal cancer cell line KYSE-30 | Lactoferrin from bovine colostrum | 500 μg/mL for 20 h | Lactoferrin has blocked the growth of tumor cells. | [54] |
| Tumor cells | in vitro comparative study | Human cancer cell lines HepG-2, Caco-2, MCF-7 and PC-3 | Nanoparticle-based Lactoperoxidase and Lactoferrin | 315–1388 μg/mL for 72 h | Nanoparticle-based compounds have inhibited tumor development and growth. | [55] |
| Lung cancer | in vivo experimental study | 5-week-old F344 rats (weighing 70–90 g) | Liposomal bovine lactoferrin | ≥10 μg/mL for 8 weeks | Tumor development and uncontrolled proliferation have been inhibited. | [56] |
| Colorectal cancer | ex vivo observational study | Tumor-derived cells from CRC patients | KMP01D | 10 mg KMP01D and 0.025 μg vitamin D3/5 for 24 h | Demonstrated anti-inflammatory effects in immune cells from CRC patients by reducing inflammatory cytokines and enhancing apoptosis. | [57] |
| Lung cancer | combined in vitro and in vivo experimental study | Human lung adenocarcinoma cell lines A549 | Lactoferrin | 15 to approximately 1 mg/mL for 48 h | Inhibited lung cancer by reducing the expression of vascular endothelial growth factor (VEGF) and inflammatory cytokines (TNF-α, IL-4, IL-6, IL-10), thereby limiting tumor growth and inflammation in both cell and animal models. | [58] |
| Gastric cancer | in vitro experimental study | Stomach cancer cell line, HEK-293 and HFF | Lactoferrin | 500 µg/mL for 20, 36, 48 h | Lactoferrin inhibited stomach tumor cells. | [59] |
| Ovarian cancer | in vitro mechanistic study | Ovarian cancer cell lines SKOV-3 and A2780 | Bovine serum | The treatment duration varied and was terminated at 24, 48, 72, and 96 h. | Inhibited the proliferation, migration, and invasion of ovarian cancer cells. | [60] |
| Lung cancer | in vitro and in vivo mechanistic study | A549 lung cancer cells in vitro and immunocompromised mice bearing subcutaneous and orthotopic lung tumors | siNRF2 delivered via bovine colostrum-derived exosome–polyethyleneimine matrix (EPM) | 20 μg | Significant reduction of tumor growth. | [61] |
| Breast cancer | in vitro experimental study | T-47D, MDA-MB-231, and BT-474 breast cancer cells | Bovine milk exosomes | 500 µg/mL for 68–70 h | Drug delivery was achieved through bovine exosomes, leading to the inhibition of cancer growth. | [62] |
| Tumor cells | in vitro evaluation | C6 rat glioblastoma cell line. | Bovine colostrum peptide | 312.7 ± 3.5 mg/mL for 48 h | Reduced tumor cell population by 50% after 48 h. | [63] |
| Breast cancer | in vitro experimental study | The MDA-MB-231 (MD Anderson Metastatic Breast Cancer) cell lines | Bovine milk-derived exosomes loaded with dihydroartemisinin | 24, 48, and 72 h | Enhanced its anticancer activity, including cytotoxicity, ROS generation, and inhibition of migration, improving its efficacy against cancer cells. | [64] |
| Lung cancer | in vitro and in vivo experimental designs | Human lung cancer cell lines A549 and mice bearing subcutaneous and orthotopic lung tumors | Bovine colostrum-derived exosomes and paclitaxel | 6 mg/kg (low dose) or 4 → 8 mg/kg (escalated dose) for 7 weeks | Significantly inhibited lung cancer growth and reduced toxicity compared to conventional treatments | [65] |
| Colorectal and breast cancer | in vitro and in vivo experimental designs | 4T1.2, LIM1215 and MCF7 cells | Bovine milk-derived extracellular vesicles | 100 µg/mL in vitro (for 48–72 h) and 25 mg/kg orally in vivo | Components of bovine colostrum reduced primary tumor growth in colorectal and breast cancer but accelerated metastasis, with timing of administration influencing these effects. | [66] |
| Many cancer types | in vitro and in vivo experimental designs | Lung: H1299, A549, H522; pancreatic: Panc-1, MiaPaCa-2; breast: MDA-MB-231 cell lines | Bovine colostrum-derived exosome–polyethyleneimine matrices (EPM) | 0.01–20 μg for 48 h | Targeting KRAS and p53 inhibited lung tumor growth, reduced KRAS expression, restored p53 expression, and enhanced chemo-sensitization to paclitaxel. | [67] |
| Breast cancer | in vitro experimental design | Four triple-negative breast cancer (TNBC) cell lines (MDA-MB-231, MDA-MB-468, HCC1806, HCC1937) | Bovine milk-derived exosomes | 20 μM for 48 h | Reported a 50% reduction in cell viability. | [68] |
| Breast cancer | in vitro experimental design | Human breast cancer cells MDA-MB-231 and MCF-7 | Bovine Apo- and Holo- lactoferrin | Durations ranging from 20 min to 48 h | Inhibited migration and invasion, and modulated expression of epithelial and mesenchymal proteins. | [69] |
| Disease | Study Design | Supplement | Effect | Reference |
|---|---|---|---|---|
| Gut toxicity | in vivo experimental design, orally fed daily for 10–11 days. | Bovine colostrum | Reduce gut toxicity during chemotherapy by preserving intestinal function and reducing inflammation. | [77] |
| Gastrointestinal Toxicity | A randomized, double-Blind, Placebo-Controlled, daily oral supplementation for 4 weeks during induction therapy. | Bovine colostrum | Reduced the severity of oral mucositis compared to placebo in cancer patients. | [78] |
| Oral mucositis | in vivo experimental design, 5 days orally by gavage (either pre- or post-OM induction, depending on group) | Bovine colostrum | No significant effect of bovine colostrum on the healing of oral mucositis was observed. | [79] |
| Chemotherapy-induced physiological parameters | in vivo experimental design, orally administered daily for 4 weeks at doses of 500, 1000, or 1500 IU/kg | Bovine colostrum | Colostrum improved physiological, immune, and circulatory functions while reducing the negative effects of Etoposide. | [80] |
| Intestinal toxicity | in vivo experimental design, orally fed for 5 days | Bovine colostrum | Reduced gastrointestinal toxicity and inflammation. | [81] |
| Gastrointestinal mucositis | in vivo experimental design, 250 or 500 mg/kg Bovine colostrum-derived immunoglobulin gavaged twice daily for 10 days | Bovine serum-derived immunoglobulin | Reduced the incidence, severity, and duration of irinotecan-induced mucositis and gastrointestinal damage. | [82] |
| Neutropenia | in vivo experimental design, 20 g/day COL supplementation for 4 weeks | Bovine colostrum | Increase absolute neutrophil counts in patients with ALL undergoing chemotherapy. | [83] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yalçıntaş, Y.M.; Bechelany, M.; Karav, S. Exploring the Therapeutic Potential of Bovine Colostrum for Cancer Therapies. Int. J. Mol. Sci. 2025, 26, 7936. https://doi.org/10.3390/ijms26167936
Yalçıntaş YM, Bechelany M, Karav S. Exploring the Therapeutic Potential of Bovine Colostrum for Cancer Therapies. International Journal of Molecular Sciences. 2025; 26(16):7936. https://doi.org/10.3390/ijms26167936
Chicago/Turabian StyleYalçıntaş, Yalçın Mert, Mikhael Bechelany, and Sercan Karav. 2025. "Exploring the Therapeutic Potential of Bovine Colostrum for Cancer Therapies" International Journal of Molecular Sciences 26, no. 16: 7936. https://doi.org/10.3390/ijms26167936
APA StyleYalçıntaş, Y. M., Bechelany, M., & Karav, S. (2025). Exploring the Therapeutic Potential of Bovine Colostrum for Cancer Therapies. International Journal of Molecular Sciences, 26(16), 7936. https://doi.org/10.3390/ijms26167936









