Protein Marker-Dependent Drug Discovery Targeting Breast Cancer Stem Cells
Abstract
1. Introduction
1.1. Breast Cancer Subtypes and BCSC
1.2. Cancer Stem Cells
2. Common Protein Markers of Breast Cancer Stem Cells
2.1. CD44+/CD24− Are Classic Markers of BCSCs, but What Are They Exactly?
2.2. CD133, Also Known as Prominin-1, Is a Transmembrane Glycoprotein Commonly Used as a Stem Cell Marker Due to Its Role in Suppressing Differentiation
2.3. EpCAM Is a Transmembrane Glycoprotein Expressed in Most Epithelial Cells, and It Mediates Cell–Cell Adhesion Independent of Ca2+, Which Is Unique and Different from Typical Epithelial Cadherins
2.4. CXCR1, or C–X–C Motif Chemokine Receptor 1, Is One of the Receptors for Interleukin-8
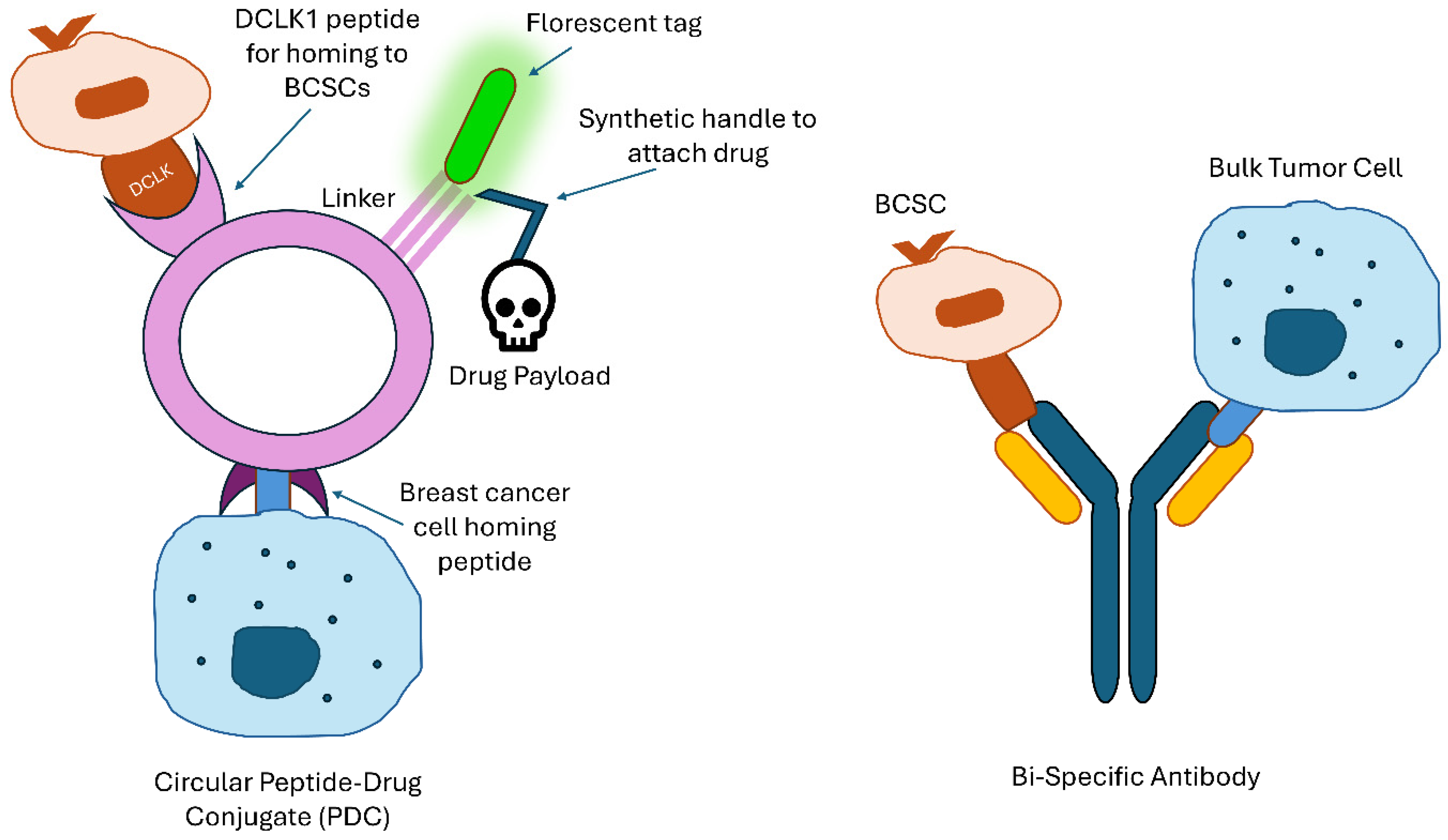
2.5. DCLK1
2.6. ALDH1
2.7. ABC Efflux Factors
3. Current Strategies for Targeting Common BCSC Marker Molecules
3.1. Small Molecule Inhibitors
3.2. Drug Delivery
3.3. Immunotherapy
3.4. DCLK1
3.5. ALDH1
3.6. ABC Efflux Factors
4. BCSC Targets in Signaling Pathways
5. Challenges of the Existing Marker-Dependent Drug Discoveries Against Breast Cancer Stem Cells
6. Future Directions in Targeting Breast Cancer Stem Cells
6.1. Circadian Therapy
6.2. CTCs vs. CSCs
6.3. Infradian Rhythms and Therapy
6.4. Artificial Intelligence and Computer-Aided Drug Design
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Parkin, D.M.; Pineros, M.; Znaor, A.; Bray, F. Cancer statistics for the year 2020: An overview. Int. J. Cancer 2021, 149, 778–789. [Google Scholar] [CrossRef]
- Caswell-Jin, J.L.; Sun, L.P.; Munoz, D.; Lu, Y.; Li, Y.; Huang, H.; Hampton, J.M.; Song, J.; Jayasekera, J.; Schechter, C.; et al. Analysis of Breast Cancer Mortality in the US—1975 to 2019. JAMA 2024, 331, 233–241. [Google Scholar] [CrossRef]
- Courtney, D.; Davey, M.G.; Moloney, B.M.; Barry, M.K.; Sweeney, K.; McLaughlin, R.P.; Malone, C.M.; Lowery, A.J.; Kerin, M.J. Breast cancer recurrence: Factors impacting occurrence and survival. Ir. J. Med. Sci. 2022, 191, 2501–2510. [Google Scholar] [CrossRef]
- Conde, I.; Ribeiro, A.S.; Paredes, J. Breast Cancer Stem Cell Membrane Biomarkers: Therapy Targeting and Clinical Implications. Cells 2022, 11, 934. [Google Scholar] [CrossRef]
- Nguyen, L.V.; Vanner, R.; Dirks, P.; Eaves, C.J. Cancer stem cells: An evolving concept. Nat. Rev. Cancer 2012, 12, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Reya, T.; Morrison, S.J.; Clarke, M.F.; Weissman, I.L. Stem cells, cancer, and cancer stem cells. Nature 2001, 414, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Vargo-Gogola, T.; Rosen, J.M. Modelling breast cancer: One size does not fit all. Nat. Rev. Cancer 2007, 7, 659–672. [Google Scholar] [CrossRef] [PubMed]
- Sorlie, T.; Tibshirani, R.; Parker, J.; Hastie, T.; Marron, J.S.; Nobel, A.; Deng, S.; Johnsen, H.; Pesich, R.; Geisler, S.; et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc. Natl. Acad. Sci. USA 2003, 100, 8418–8423. [Google Scholar] [CrossRef]
- Bianchini, G.; Balko, J.M.; Mayer, I.A.; Sanders, M.E.; Gianni, L. Triple-negative breast cancer: Challenges and opportunities of a heterogeneous disease. Nat. Rev. Clin. Oncol. 2016, 13, 674–690. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Colaprico, A.; Silva, T.C.; Chen, J.; An, H.; Ban, Y.; Huang, H.; Wang, L.; James, J.L.; Balko, J.M.; et al. Multi-omics analysis identifies therapeutic vulnerabilities in triple-negative breast cancer subtypes. Nat. Commun. 2021, 12, 6276. [Google Scholar] [CrossRef]
- Ricardo, S.; Vieira, A.F.; Gerhard, R.; Leitao, D.; Pinto, R.; Cameselle-Teijeiro, J.F.; Milanezi, F.; Schmitt, F.; Paredes, J. Breast cancer stem cell markers CD44, CD24 and ALDH1: Expression distribution within intrinsic molecular subtype. J. Clin. Pathol. 2011, 64, 937–946. [Google Scholar] [CrossRef]
- Yamamoto, M.; Taguchi, Y.; Ito-Kureha, T.; Semba, K.; Yamaguchi, N.; Inoue, J. NF-kappaB non-cell-autonomously regulates cancer stem cell populations in the basal-like breast cancer subtype. Nat. Commun. 2013, 4, 2299. [Google Scholar] [CrossRef] [PubMed]
- Paula, A.D.C.; Lopes, C. Implications of Different Cancer Stem Cell Phenotypes in Breast Cancer. Anticancer Res. 2017, 37, 2173–2183. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Ye, Y.; Yearsley, K.; Jones, S.; Barsky, S.H. The lymphovascular embolus of inflammatory breast cancer expresses a stem cell-like phenotype. Am. J. Pathol. 2008, 173, 561–574. [Google Scholar] [CrossRef] [PubMed]
- Neumeister, V.; Agarwal, S.; Bordeaux, J.; Camp, R.L.; Rimm, D.L. In situ identification of putative cancer stem cells by multiplexing ALDH1, CD44, and cytokeratin identifies breast cancer patients with poor prognosis. Am. J. Pathol. 2010, 176, 2131–2138. [Google Scholar] [CrossRef]
- Landeros, N.; Castillo, I.; Perez-Castro, R. Preclinical and Clinical Trials of New Treatment Strategies Targeting Cancer Stem Cells in Subtypes of Breast Cancer. Cells 2023, 12, 720. [Google Scholar] [CrossRef]
- Fillmore, C.M.; Kuperwasser, C. Human breast cancer cell lines contain stem-like cells that self-renew, give rise to phenotypically diverse progeny and survive chemotherapy. Breast Cancer Res. 2008, 10, R25. [Google Scholar] [CrossRef]
- Al-Hajj, M.; Wicha, M.S.; Benito-Hernandez, A.; Morrison, S.J.; Clarke, M.F. Prospective identification of tumorigenic breast cancer cells. Proc. Natl. Acad. Sci. USA 2003, 100, 3983–3988. [Google Scholar] [CrossRef]
- Ray, S.K.; Mukherjee, S. Breast cancer stem cells as novel biomarkers. Clin. Chim. Acta 2024, 557, 117855. [Google Scholar] [CrossRef]
- Wang, C.; Xu, K.; Wang, R.; Han, X.; Tang, J.; Guan, X. Heterogeneity of BCSCs contributes to the metastatic organotropism of breast cancer. J. Exp. Clin. Cancer Res. 2021, 40, 370. [Google Scholar] [CrossRef]
- Fischer, K.R.; Durrans, A.; Lee, S.; Sheng, J.; Li, F.; Wong, S.T.; Choi, H.; El Rayes, T.; Ryu, S.; Troeger, J.; et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 2015, 527, 472–476. [Google Scholar] [CrossRef]
- Ye, X.; Brabletz, T.; Kang, Y.; Longmore, G.D.; Nieto, M.A.; Stanger, B.Z.; Yang, J.; Weinberg, R.A. Upholding a role for EMT in breast cancer metastasis. Nature 2017, 547, E1–E3. [Google Scholar] [CrossRef]
- Korkaya, H.; Paulson, A.; Iovino, F.; Wicha, M.S. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene 2008, 27, 6120–6130. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Onder, T.; Jiang, G.; Tao, K.; Kuperwasser, C.; Weinberg, R.; Lander, E. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009, 138, 645–659. [Google Scholar] [CrossRef]
- Qi, D.; Liu, Y.; Li, J.; Huang, J.H.; Hu, X.; Wu, E. Salinomycin as a potent anticancer stem cell agent: State of the art and future directions. Med. Res. Rev. 2022, 42, 1037–1063. [Google Scholar] [CrossRef] [PubMed]
- Mokbel, K. GD2 in Breast Cancer: A Potential Biomarker and Therapeutic Target. Cancer Genom. Proteom. 2024, 21, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Zhou, Y.; Xie, T.; Yuan, Y.; Li, H.; Shi, W.; Zheng, L.; Li, X.; Zhang, W. Tumor microenvironment of cancer stem cells: Perspectives on cancer stem cell targeting. Genes Dis. 2024, 11, 101043. [Google Scholar] [CrossRef]
- Romaniuk-Drapala, A.; Toton, E.; Taube, M.; Idzik, M.; Rubis, B.; Lisiak, N. Breast Cancer Stem Cells and Tumor Heterogeneity: Characteristics and Therapeutic Strategies. Cancers 2024, 16, 2481. [Google Scholar] [CrossRef]
- Ali, K.; Nabeel, M.; Mohsin, F.; Iqtedar, M.; Islam, M.; Rasool, M.F.; Hashmi, F.K.; Hussain, S.A.; Saeed, H. Recent developments in targeting breast cancer stem cells (BCSCs): A descriptive review of therapeutic strategies and emerging therapies. Med. Oncol. 2024, 41, 112. [Google Scholar] [CrossRef]
- Pupa, S.M.; Ligorio, F.; Cancila, V.; Franceschini, A.; Tripodo, C.; Vernieri, C.; Castagnoli, L. HER2 Signaling and Breast Cancer Stem Cells: The Bridge behind HER2-Positive Breast Cancer Aggressiveness and Therapy Refractoriness. Cancers 2021, 13, 4778. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.Y.; Jeon, S.E.; Choi, J.H.; Lee, C.J.; Jang, T.Y.; Yun, H.J.; Lee, Y.; Kim, P.; Cho, S.H.; et al. DCLK1 promotes colorectal cancer stemness and aggressiveness via the XRCC5/COX2 axis. Theranostics 2022, 12, 5258–5271. [Google Scholar] [CrossRef]
- Shimoda, M.; Ota, M.; Okada, Y. Isolation of Cancer Stem Cells by Side Population Method. Methods Mol. Biol. 2018, 1692, 49–59. [Google Scholar] [CrossRef]
- Ogino, S.; Nishida, N.; Umemoto, R.; Suzuki, M.; Takeda, M.; Terasawa, H.; Kitayama, J.; Matsumoto, M.; Hayasaka, H.; Miyasaka, M.; et al. Two-state conformations in the hyaluronan-binding domain regulate CD44 adhesiveness under flow condition. Structure 2010, 18, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Velasco-Velazquez, M.A.; Popov, V.M.; Lisanti, M.P.; Pestell, R.G. The role of breast cancer stem cells in metastasis and therapeutic implications. Am. J. Pathol. 2011, 179, 2–11. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Apple, S.; Zhao, H.; Song, J.; Lee, M.; Luo, W.; Wu, X.; Chung, D.; Pietras, R.J.; Chang, H.R. CD24 Expression and differential resistance to chemotherapy in triple-negative breast cancer. Oncotarget 2017, 8, 38294–38308. [Google Scholar] [CrossRef] [PubMed]
- Pleskac, P.; Fargeas, C.A.; Veselska, R.; Corbeil, D.; Skoda, J. Emerging roles of prominin-1 (CD133) in the dynamics of plasma membrane architecture and cell signaling pathways in health and disease. Cell. Mol. Biol. Lett. 2024, 29, 41. [Google Scholar] [CrossRef]
- Brandolini, L.; Cristiano, L.; Fidoamore, A.; De Pizzol, M.; Di Giacomo, E.; Florio, T.M.; Confalone, G.; Galante, A.; Cinque, B.; Benedetti, E.; et al. Targeting CXCR1 on breast cancer stem cells: Signaling pathways and clinical application modelling. Oncotarget 2015, 6, 43375–43394. [Google Scholar] [CrossRef]
- Westphalen, C.B.; Quante, M.; Wang, T.C. Functional implication of Dclk1 and Dclk1-expressing cells in cancer. Small GTPases 2017, 8, 164–171. [Google Scholar] [CrossRef]
- Lu, Q.; Feng, H.; Chen, H.; Weygant, N.; Du, J.; Yan, Z.; Cao, Z. Role of DCLK1 in oncogenic signaling (Review). Int. J. Oncol. 2022, 61, 137. [Google Scholar] [CrossRef]
- Liu, H.; Wen, T.; Zhou, Y.; Fan, X.; Du, T.; Gao, T.; Li, L.; Liu, J.; Yang, L.; Yao, J.; et al. DCLK1 Plays a Metastatic-Promoting Role in Human Breast Cancer Cells. BioMed Res. Int. 2019, 2019, 1061979. [Google Scholar] [CrossRef]
- Middelhoff, M.; Westphalen, C.B.; Hayakawa, Y.; Yan, K.S.; Gershon, M.D.; Wang, T.C.; Quante, M. Dclk1-expressing tuft cells: Critical modulators of the intestinal niche? Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G285–G299. [Google Scholar] [CrossRef]
- Liu, H.; Yan, R.; Xiao, Z.; Huang, X.; Yao, J.; Liu, J.; An, G.; Ge, Y. Targeting DCLK1 attenuates tumor stemness and evokes antitumor immunity in triple-negative breast cancer by inhibiting IL-6/STAT3 signaling. Breast Cancer Res. 2023, 25, 43. [Google Scholar] [CrossRef]
- Wang, J.; Wang, S.; Zhou, J.; Qian, Q. miR-424-5p regulates cell proliferation, migration and invasion by targeting doublecortin-like kinase 1 in basal-like breast cancer. Biomed. Pharmacother. 2018, 102, 147–152. [Google Scholar] [CrossRef]
- Liu, Y.H.; Tsang, J.Y.; Ni, Y.B.; Hlaing, T.; Chan, S.K.; Chan, K.F.; Ko, C.W.; Mujtaba, S.S.; Tse, G.M. Doublecortin-like kinase 1 expression associates with breast cancer with neuroendocrine differentiation. Oncotarget 2016, 7, 1464–1476. [Google Scholar] [CrossRef] [PubMed]
- Qiao, S.; Zhao, Y.; Geng, S.; Li, Y.; Hou, X.; Liu, Y.; Lin, F.H.; Yao, L.; Tian, W. A novel double-targeted nondrug delivery system for targeting cancer stem cells. Int. J. Nanomed. 2016, 11, 6667–6678. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Sheng, D.; Wang, D.; Ma, W.; Deng, Q.; Deng, L.; Liu, S. Identification of cancer-type specific expression patterns for active aldehyde dehydrogenase (ALDH) isoforms in ALDEFLUOR assay. Cell Biol. Toxicol. 2019, 35, 161–177. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Qiang, J.; Deng, Q.; Xia, J.; Deng, L.; Zhou, L.; Wang, D.; He, X.; Liu, Y.; Zhao, B.; et al. ALDH1A1 Activity in Tumor-Initiating Cells Remodels Myeloid-Derived Suppressor Cells to Promote Breast Cancer Progression. Cancer Res. 2021, 81, 5919–5934. [Google Scholar] [CrossRef]
- Nguyen, A.L.; Facey, C.O.B.; Boman, B.M. The Significance of Aldehyde Dehydrogenase 1 in Cancers. Int. J. Mol. Sci. 2024, 26, 251. [Google Scholar] [CrossRef]
- Poturnajova, M.; Kozovska, Z.; Matuskova, M. Aldehyde dehydrogenase 1A1 and 1A3 isoforms—Mechanism of activation and regulation in cancer. Cell. Signal. 2021, 87, 110120. [Google Scholar] [CrossRef]
- Panigoro, S.S.; Kurnia, D.; Kurnia, A.; Haryono, S.J.; Albar, Z.A. ALDH1 Cancer Stem Cell Marker as a Prognostic Factor in Triple-Negative Breast Cancer. Int. J. Surg. Oncol. 2020, 2020, 7863243. [Google Scholar] [CrossRef]
- Sabatier, R.; Charafe-Jauffret, E.; Pierga, J.Y.; Cure, H.; Lambaudie, E.; Genre, D.; Houvenaeghel, G.; Viens, P.; Ginestier, C.; Bertucci, F.; et al. Stem Cells Inhibition by Bevacizumab in Combination with Neoadjuvant Chemotherapy for Breast Cancer. J. Clin. Med. 2019, 8, 612. [Google Scholar] [CrossRef]
- Shatsky, R.A.; Batra-Sharma, H.; Helsten, T.; Schwab, R.B.; Pittman, E.I.; Pu, M.; Weihe, E.; Ghia, E.M.; Rassenti, L.Z.; Molinolo, A.; et al. A phase 1b study of zilovertamab in combination with paclitaxel for locally advanced/unresectable or metastatic Her2-negative breast cancer. Breast Cancer Res. 2024, 26, 32. [Google Scholar] [CrossRef]
- Schott, A.F.; Landis, M.D.; Dontu, G.; Griffith, K.A.; Layman, R.M.; Krop, I.; Paskett, L.A.; Wong, H.; Dobrolecki, L.E.; Lewis, M.T.; et al. Preclinical and clinical studies of gamma secretase inhibitors with docetaxel on human breast tumors. Clin. Cancer Res. 2013, 19, 1512–1524. [Google Scholar] [CrossRef] [PubMed]
- Theodoulou, F.L.; Kerr, I.D. ABC transporter research: Going strong 40 years on. Biochem. Soc. Trans. 2015, 43, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Modi, A.; Roy, D.; Sharma, S.; Vishnoi, J.R.; Pareek, P.; Elhence, P.; Sharma, P.; Purohit, P. ABC transporters in breast cancer: Their roles in multidrug resistance and beyond. J. Drug Target. 2022, 30, 927–947. [Google Scholar] [CrossRef] [PubMed]
- Chiang, Y.F.; Huang, K.C.; Chen, H.Y.; Hamdy, N.M.; Huang, T.C.; Chang, H.Y.; Shieh, T.M.; Huang, Y.J.; Hsia, S.M. Hinokitiol Inhibits Breast Cancer Cells In Vitro Stemness-Progression and Self-Renewal with Apoptosis and Autophagy Modulation via the CD44/Nanog/SOX2/Oct4 Pathway. Int. J. Mol. Sci. 2024, 25, 3904. [Google Scholar] [CrossRef]
- Chanmee, T.; Ontong, P.; Kimata, K.; Itano, N. Key Roles of Hyaluronan and Its CD44 Receptor in the Stemness and Survival of Cancer Stem Cells. Front. Oncol. 2015, 5, 180. [Google Scholar] [CrossRef]
- Goldstein, L.J.; Perez, R.P.; Yardley, D.; Han, L.K.; Reuben, J.M.; Gao, H.; McCanna, S.; Butler, B.; Ruffini, P.A.; Liu, Y.; et al. A window-of-opportunity trial of the CXCR1/2 inhibitor reparixin in operable HER-2-negative breast cancer. Breast Cancer Res. 2020, 22, 4. [Google Scholar] [CrossRef]
- Goldstein, L.J.; Mansutti, M.; Levy, C.; Chang, J.C.; Henry, S.; Fernandez-Perez, I.; Prausova, J.; Staroslawska, E.; Viale, G.; Butler, B.; et al. A randomized, placebo-controlled phase 2 study of paclitaxel in combination with reparixin compared to paclitaxel alone as front-line therapy for metastatic triple-negative breast cancer (fRida). Breast Cancer Res. Treat. 2021, 190, 265–275. [Google Scholar] [CrossRef]
- Gener, P.; Gouveia, L.P.; Sabat, G.R.; de Sousa Rafael, D.F.; Fort, N.B.; Arranja, A.; Fernandez, Y.; Prieto, R.M.; Ortega, J.S.; Arango, D.; et al. Fluorescent CSC models evidence that targeted nanomedicines improve treatment sensitivity of breast and colon cancer stem cells. Nanomedicine 2015, 11, 1883–1892. [Google Scholar] [CrossRef]
- Han, N.K.; Shin, D.H.; Kim, J.S.; Weon, K.Y.; Jang, C.Y.; Kim, J.S. Hyaluronan-conjugated liposomes encapsulating gemcitabine for breast cancer stem cells. Int. J. Nanomed. 2016, 11, 1413–1425. [Google Scholar] [CrossRef]
- Kesharwani, P.; Chadar, R.; Sheikh, A.; Rizg, W.Y.; Safhi, A.Y. CD44-Targeted Nanocarrier for Cancer Therapy. Front. Pharmacol. 2021, 12, 800481. [Google Scholar] [CrossRef]
- Riechelmann, H.; Sauter, A.; Golze, W.; Hanft, G.; Schroen, C.; Hoermann, K.; Erhardt, T.; Gronau, S. Phase I trial with the CD44v6-targeting immunoconjugate bivatuzumab mertansine in head and neck squamous cell carcinoma. Oral Oncol. 2008, 44, 823–829. [Google Scholar] [CrossRef] [PubMed]
- Rupp, U.; Schoendorf-Holland, E.; Eichbaum, M.; Schuetz, F.; Lauschner, I.; Schmidt, P.; Staab, A.; Hanft, G.; Huober, J.; Sinn, H.P.; et al. Safety and pharmacokinetics of bivatuzumab mertansine in patients with CD44v6-positive metastatic breast cancer: Final results of a phase I study. Anticancer Drugs 2007, 18, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Segues, A.; Huang, S.; Sijts, A.; Berraondo, P.; Zaiss, D.M. Opportunities and challenges of bi-specific antibodies. Int. Rev. Cell Mol. Biol. 2022, 369, 45–70. [Google Scholar] [CrossRef] [PubMed]
- Surowka, M.; Klein, C. A pivotal decade for bispecific antibodies? MAbs 2024, 16, 2321635. [Google Scholar] [CrossRef]
- Linke, R.; Klein, A.; Seimetz, D. Catumaxomab: Clinical development and future directions. MAbs 2010, 2, 129–136. [Google Scholar] [CrossRef]
- Schmidt, M.; Ruttinger, D.; Sebastian, M.; Hanusch, C.A.; Marschner, N.; Baeuerle, P.A.; Wolf, A.; Goppel, G.; Oruzio, D.; Schlimok, G.; et al. Phase IB study of the EpCAM antibody adecatumumab combined with docetaxel in patients with EpCAM-positive relapsed or refractory advanced-stage breast cancer. Ann. Oncol. 2012, 23, 2306–2313. [Google Scholar] [CrossRef]
- Marei, H.E.; Bedair, K.; Hasan, A.; Al-Mansoori, L.; Caratelli, S.; Sconocchia, G.; Gaiba, A.; Cenciarelli, C. Current status and innovative developments of CAR-T-cell therapy for the treatment of breast cancer. Cancer Cell Int. 2025, 25, 3. [Google Scholar] [CrossRef]
- Patel, K.K.; Tariveranmoshabad, M.; Kadu, S.; Shobaki, N.; June, C. From concept to cure: The evolution of CAR-T cell therapy. Mol. Ther. J. Am. Soc. Gene Ther. 2025, 33, 2123–2140. [Google Scholar] [CrossRef]
- Li, D.; Guo, X.; Yang, K.; Yang, Y.; Zhou, W.; Huang, Y.; Liang, X.; Su, J.; Jiang, L.; Li, J.; et al. EpCAM-targeting CAR-T cell immunotherapy is safe and efficacious for epithelial tumors. Sci. Adv. 2023, 9, eadg9721. [Google Scholar] [CrossRef]
- Cordova, A.; Woodrick, J.; Grindrod, S.; Zhang, L.; Saygideger-Kont, Y.; Wang, K.; DeVito, S.; Daniele, S.G.; Paige, M.; Brown, M.L. Aminopeptidase P Mediated Targeting for Breast Tissue Specific Conjugate Delivery. Bioconjugate Chem. 2016, 27, 1981–1990. [Google Scholar] [CrossRef]
- Kong, Y.; Jung, M.; Wang, K.; Grindrod, S.; Velena, A.; Lee, S.A.; Dakshanamurthy, S.; Yang, Y.; Miessau, M.; Zheng, C.; et al. Histone deacetylase cytoplasmic trapping by a novel fluorescent HDAC inhibitor. Mol. Cancer Ther. 2011, 10, 1591–1599. [Google Scholar] [CrossRef] [PubMed]
- Zeng, M.; Wu, B.; Wei, W.; Jiang, Z.; Li, P.; Quan, Y.; Hu, X. Disulfiram: A novel repurposed drug for cancer therapy. Chin. Med. J. 2024, 137, 1389–1398. [Google Scholar] [CrossRef] [PubMed]
- Wei, Y.; Li, Y.; Chen, Y.; Liu, P.; Huang, S.; Zhang, Y.; Sun, Y.; Wu, Z.; Hu, M.; Wu, Q.; et al. ALDH1: A potential therapeutic target for cancer stem cells in solid tumors. Front. Oncol. 2022, 12, 1026278. [Google Scholar] [CrossRef] [PubMed]
- Bu, J.; Zhang, Y.; Wu, S.; Li, H.; Sun, L.; Liu, Y.; Zhu, X.; Qiao, X.; Ma, Q.; Liu, C.; et al. KK-LC-1 as a therapeutic target to eliminate ALDH(+) stem cells in triple negative breast cancer. Nat. Commun. 2023, 14, 2602. [Google Scholar] [CrossRef]
- Okamoto, A.; Funakoshi, Y.; Oe, M.; Takai, R.; Suto, H.; Nagatani, Y.; Nishimura, M.; Imamura, Y.; Kunihisa, T.; Kiyota, N.; et al. Identification of Breast Cancer Stem Cells Using a Newly Developed Long-acting Fluorescence Probe, C5S-A, Targeting ALDH1A1. Anticancer. Res. 2022, 42, 1199–1205. [Google Scholar] [CrossRef]
- Mohan, A.; Raj Rajan, R.; Mohan, G.; Kollenchery Puthenveettil, P.; Maliekal, T.T. Markers and Reporters to Reveal the Hierarchy in Heterogeneous Cancer Stem Cells. Front. Cell Dev. Biol. 2021, 9, 668851. [Google Scholar] [CrossRef]
- Salinas-Jazmin, N.; Rosas-Cruz, A.; Velasco-Velazquez, M. Reporter gene systems for the identification and characterization of cancer stem cells. World J. Stem Cells 2021, 13, 861–876. [Google Scholar] [CrossRef]
- Mohan, A.; Raj, R.R.; Mohan, G.; P, P.K.; Maliekal, T.T. Reporters of Cancer Stem Cells as a Tool for Drug Discovery. Front. Oncol. 2021, 11, 669250. [Google Scholar] [CrossRef]
- Kumar, A.; Jaitak, V. Natural products as multidrug resistance modulators in cancer. Eur. J. Med. Chem. 2019, 176, 268–291. [Google Scholar] [CrossRef]
- He, J.; Fortunati, E.; Liu, D.X.; Li, Y. Pleiotropic Roles of ABC Transporters in Breast Cancer. Int. J. Mol. Sci. 2021, 22, 3199. [Google Scholar] [CrossRef]
- Wu, S.; Fu, L. Tyrosine kinase inhibitors enhanced the efficacy of conventional chemotherapeutic agent in multidrug resistant cancer cells. Mol. Cancer 2018, 17, 25. [Google Scholar] [CrossRef] [PubMed]
- Lobo, N.A.; Shimono, Y.; Qian, D.; Clarke, M.F. The biology of cancer stem cells. Annu. Rev. Cell Dev. Biol. 2007, 23, 675–699. [Google Scholar] [CrossRef] [PubMed]
- Diamond, J.R.; Becerra, C.; Richards, D.; Mita, A.; Osborne, C.; O’Shaughnessy, J.; Zhang, C.; Henner, R.; Kapoun, A.M.; Xu, L.; et al. Phase Ib clinical trial of the anti-frizzled antibody vantictumab (OMP-18R5) plus paclitaxel in patients with locally advanced or metastatic HER2-negative breast cancer. Breast Cancer Res. Treat. 2020, 184, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Zhu, Y.; Wu, Z.; Cui, C.; Cai, F. Anticancer Mechanisms of Salinomycin in Breast Cancer and Its Clinical Applications. Front. Oncol. 2021, 11, 654428. [Google Scholar] [CrossRef]
- Curtin, J.C.; Lorenzi, M.V. Drug discovery approaches to target Wnt signaling in cancer stem cells. Oncotarget 2010, 1, 563–577. [Google Scholar] [CrossRef]
- Takebe, N.; Harris, P.J.; Warren, R.Q.; Ivy, S.P. Targeting cancer stem cells by inhibiting Wnt, Notch, and Hedgehog pathways. Nat. Rev. Clin. Oncol. 2011, 8, 97–106. [Google Scholar] [CrossRef]
- Londono-Joshi, A.I.; Arend, R.C.; Aristizabal, L.; Lu, W.; Samant, R.S.; Metge, B.J.; Hidalgo, B.; Grizzle, W.E.; Conner, M.; Forero-Torres, A.; et al. Effect of niclosamide on basal-like breast cancers. Mol. Cancer Ther. 2014, 13, 800–811. [Google Scholar] [CrossRef]
- Burock, S.; Daum, S.; Keilholz, U.; Neumann, K.; Walther, W.; Stein, U. Phase II trial to investigate the safety and efficacy of orally applied niclosamide in patients with metachronous or sychronous metastases of a colorectal cancer progressing after therapy: The NIKOLO trial. BMC Cancer 2018, 18, 297. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, S.; Jung, E.; Shin, J.; Kim, Y.J.; Kim, J.Y.; Sessler, J.L.; Seo, J.H.; Kim, J.S. A dual-action niclosamide-based prodrug that targets cancer stem cells and inhibits TNBC metastasis. Proc. Natl. Acad. Sci. USA 2023, 120, e2304081120. [Google Scholar] [CrossRef]
- Suman, S.; Das, T.P.; Damodaran, C. Silencing NOTCH signaling causes growth arrest in both breast cancer stem cells and breast cancer cells. Br. J. Cancer 2013, 109, 2587–2596. [Google Scholar] [CrossRef]
- Harrison, H.; Farnie, G.; Brennan, K.R.; Clarke, R.B. Breast cancer stem cells: Something out of notching? Cancer Res. 2010, 70, 8973–8976. [Google Scholar] [CrossRef]
- Harrison, H.; Farnie, G.; Howell, S.J.; Rock, R.E.; Stylianou, S.; Brennan, K.R.; Bundred, N.J.; Clarke, R.B. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 2010, 70, 709–718. [Google Scholar] [CrossRef]
- McCaw, T.R.; Inga, E.; Chen, H.; Jaskula-Sztul, R.; Dudeja, V.; Bibb, J.A.; Ren, B.; Rose, J.B. Gamma Secretase Inhibitors in Cancer: A Current Perspective on Clinical Performance. Oncologist 2021, 26, e608–e621. [Google Scholar] [CrossRef] [PubMed]
- Ran, Y.; Hossain, F.; Pannuti, A.; Lessard, C.B.; Ladd, G.Z.; Jung, J.I.; Minter, L.M.; Osborne, B.A.; Miele, L.; Golde, T.E. gamma-Secretase inhibitors in cancer clinical trials are pharmacologically and functionally distinct. EMBO Mol. Med. 2017, 9, 950–966. [Google Scholar] [CrossRef] [PubMed]
- Daniel, D.B.; Rudin, C.M.; Hart, L.; Spigel, D.R.; Edelman, M.J.; Goldschmidt, J.; Bordoni, R.; Glisson, B.; Burns, T.F.; Dowlati, A.; et al. Results of a randomized, placebo-controlled, phase 2 study of tarextumab (TRXT, anti-Notch2/3) in combination with etoposide and platinum (EP) in patients (pts) with untreated extensive-stage small-cell lung cancer (ED-SCLC). Ann. Oncol. 2017, 28, v540. [Google Scholar] [CrossRef]
- Lee, J.Y.; Shin, D.H.; Kim, J.S. Anticancer Effect of Metformin in Herceptin-Conjugated Liposome for Breast Cancer. Pharmaceutics 2019, 12, 11. [Google Scholar] [CrossRef]
- Morio, K.; Kurata, Y.; Kawaguchi-Sakita, N.; Shiroshita, A.; Kataoka, Y. Efficacy of Metformin in Patients With Breast Cancer Receiving Chemotherapy or Endocrine Therapy: Systematic Review and Meta-analysis. Ann. Pharmacother. 2022, 56, 245–255. [Google Scholar] [CrossRef]
- Sigafoos, A.N.; Paradise, B.D.; Fernandez-Zapico, M.E. Hedgehog/GLI Signaling Pathway: Transduction, Regulation, and Implications for Disease. Cancers 2021, 13, 3410. [Google Scholar] [CrossRef]
- Rubin, L.L.; de Sauvage, F.J. Targeting the Hedgehog pathway in cancer. Nat. Rev. Drug Discov. 2006, 5, 1026–1033. [Google Scholar] [CrossRef]
- Ramaswamy, B.; Lu, Y.; Teng, K.Y.; Nuovo, G.; Li, X.; Shapiro, C.L.; Majumder, S. Hedgehog signaling is a novel therapeutic target in tamoxifen-resistant breast cancer aberrantly activated by PI3K/AKT pathway. Cancer Res. 2012, 72, 5048–5059. [Google Scholar] [CrossRef]
- Ruiz-Borrego, M.; Jimenez, B.; Antolin, S.; Garcia-Saenz, J.A.; Corral, J.; Jerez, Y.; Trigo, J.; Urruticoechea, A.; Colom, H.; Gonzalo, N.; et al. A phase Ib study of sonidegib (LDE225), an oral small molecule inhibitor of smoothened or Hedgehog pathway, in combination with docetaxel in triple negative advanced breast cancer patients: GEICAM/2012-12 (EDALINE) study. Investig. New Drugs 2019, 37, 98–108. [Google Scholar] [CrossRef]
- Tsao, A.N.; Chuang, Y.S.; Lin, Y.C.; Su, Y.; Chao, T.C. Dinaciclib inhibits the stemness of two subtypes of human breast cancer cells by targeting the FoxM1 and Hedgehog signaling pathway. Oncol. Rep. 2022, 47, 105. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, H.E.; Li, H.; Shipitsin, M.; Gelman, R.; Polyak, K. Heterogeneity for stem cell-related markers according to tumor subtype and histologic stage in breast cancer. Clin. Cancer Res. 2010, 16, 876–887. [Google Scholar] [CrossRef] [PubMed]
- Lynce, F.; Stevens, L.E.; Li, Z.; Brock, J.E.; Gulvady, A.; Huang, Y.; Nakhlis, F.; Patel, A.; Force, J.M.; Haddad, T.C.; et al. TBCRC 039: A phase II study of preoperative ruxolitinib with or without paclitaxel for triple-negative inflammatory breast cancer. Breast Cancer Res. 2024, 26, 20. [Google Scholar] [CrossRef] [PubMed]
- Blanchard, Z.; Brown, E.A.; Ghazaryan, A.; Welm, A.L. PDX models for functional precision oncology and discovery science. Nat. Rev. Cancer 2025, 25, 153–166. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Guan, Z.; Cai, G.; Nie, Y.; Zhang, C.; Luo, W.; Liu, J. Patient-derived organoids: A promising tool for breast cancer research. Front. Oncol. 2024, 14, 1350935. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, Y.; Gao, J.; Zhang, Q.; Sun, L.; Ma, Q.; Qiao, X.; Li, X.; Liu, J.; Bu, J.; et al. A highly potent small-molecule antagonist of exportin-1 selectively eliminates CD44(+)CD24(−) enriched breast cancer stem-like cells. Drug Resist. Updat. 2023, 66, 100903. [Google Scholar] [CrossRef]
- Wang, Y.; Narasimamurthy, R.; Qu, M.; Shi, N.; Guo, H.; Xue, Y.; Barker, N. Circadian regulation of cancer stem cells and the tumor microenvironment during metastasis. Nat. Cancer 2024, 5, 546–556. [Google Scholar] [CrossRef]
- Finger, A.M.; Kramer, A. Mammalian circadian systems: Organization and modern life challenges. Acta Physiol. 2021, 231, e13548. [Google Scholar] [CrossRef] [PubMed]
- Matsunaga, N.; Ogino, T.; Hara, Y.; Tanaka, T.; Koyanagi, S.; Ohdo, S. Optimized Dosing Schedule Based on Circadian Dynamics of Mouse Breast Cancer Stem Cells Improves the Antitumor Effects of Aldehyde Dehydrogenase Inhibitor. Cancer Res. 2018, 78, 3698–3708. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.; Nagai, Y.; Wu, Q.; Hovsepyan, A.; Mkhitaryan, S.; Wang, J.; Karapetyan, G.; Kamenecka, T.; Solt, L.A.; Cope, J.; et al. Advancing Clinical Response Against Glioblastoma: Evaluating SHP1705 CRY2 Activator Efficacy in Preclinical Models and Safety in Phase I Trials. Neuro Oncol. 2025; Online ahead of print. [Google Scholar] [CrossRef]
- Diamantopoulou, Z.; Castro-Giner, F.; Schwab, F.D.; Foerster, C.; Saini, M.; Budinjas, S.; Strittmatter, K.; Krol, I.; Seifert, B.; Heinzelmann-Schwarz, V.; et al. The metastatic spread of breast cancer accelerates during sleep. Nature 2022, 607, 156–162. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, K.; Massague, J. Targeting metastatic cancer. Nat. Med. 2021, 27, 34–44. [Google Scholar] [CrossRef]
- Dittmar, T.; Heyder, C.; Gloria-Maercker, E.; Hatzmann, W.; Zanker, K.S. Adhesion molecules and chemokines: The navigation system for circulating tumor (stem) cells to metastasize in an organ-specific manner. Clin. Exp. Metastasis 2008, 25, 11–32. [Google Scholar] [CrossRef]
- Mrozik, K.M.; Blaschuk, O.W.; Cheong, C.M.; Zannettino, A.C.W.; Vandyke, K. N-cadherin in cancer metastasis, its emerging role in haematological malignancies and potential as a therapeutic target in cancer. BMC Cancer 2018, 18, 939. [Google Scholar] [CrossRef]
- Loreth, D.; Schuette, M.; Zinke, J.; Mohme, M.; Piffko, A.; Schneegans, S.; Stadler, J.; Janning, M.; Loges, S.; Joosse, S.A.; et al. CD74 and CD44 Expression on CTCs in Cancer Patients with Brain Metastasis. Int. J. Mol. Sci. 2021, 22, 6993. [Google Scholar] [CrossRef]
- Castro-Giner, F.; Aceto, N. Tracking cancer progression: From circulating tumor cells to metastasis. Genome Med. 2020, 12, 31. [Google Scholar] [CrossRef]
- Juratli, M.A.; Sarimollaoglu, M.; Nedosekin, D.A.; Melerzanov, A.V.; Zharov, V.P.; Galanzha, E.I. Dynamic Fluctuation of Circulating Tumor Cells during Cancer Progression. Cancers 2014, 6, 128–142. [Google Scholar] [CrossRef]
- Williams, A.L.; Fitzgerald, J.E.; Ivich, F.; Sontag, E.D.; Niedre, M. Short-Term Circulating Tumor Cell Dynamics in Mouse Xenograft Models and Implications for Liquid Biopsy. Front. Oncol. 2020, 10, 601085. [Google Scholar] [CrossRef]
- Zhu, X.; Suo, Y.; Fu, Y.; Zhang, F.; Ding, N.; Pang, K.; Xie, C.; Weng, X.; Tian, M.; He, H.; et al. In vivo flow cytometry reveals a circadian rhythm of circulating tumor cells. Light. Sci. Appl. 2021, 10, 110. [Google Scholar] [CrossRef]
- Paiva, B.; Paino, T.; Sayagues, J.M.; Garayoa, M.; San-Segundo, L.; Martin, M.; Mota, I.; Sanchez, M.L.; Barcena, P.; Aires-Mejia, I.; et al. Detailed characterization of multiple myeloma circulating tumor cells shows unique phenotypic, cytogenetic, functional, and circadian distribution profile. Blood 2013, 122, 3591–3598. [Google Scholar] [CrossRef] [PubMed]
- Bornes, L.; van Winden, L.J.; Geurts, V.C.M.; de Bruijn, B.; Azarang, L.; Lanfermeijer, M.; Caruso, M.; Proost, N.; Boeije, M.; Lohuis, J.O.; et al. The oestrous cycle stage affects mammary tumour sensitivity to chemotherapy. Nature 2025, 637, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Heer, E.; Harper, A.; Escandor, N.; Sung, H.; McCormack, V.; Fidler-Benaoudia, M.M. Global burden and trends in premenopausal and postmenopausal breast cancer: A population-based study. Lancet Glob. Health 2020, 8, e1027–e1037. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, Q.H.; Lukowski, S.W.; Chiu, H.S.; Senabouth, A.; Bruxner, T.J.C.; Christ, A.N.; Palpant, N.J.; Powell, J.E. Single-cell RNA-seq of human induced pluripotent stem cells reveals cellular heterogeneity and cell state transitions between subpopulations. Genome Res. 2018, 28, 1053–1066. [Google Scholar] [CrossRef]
- Li, Z.; Napolitano, A.; Fedele, M.; Gao, X.; Napolitano, F. AI identifies potent inducers of breast cancer stem cell differentiation based on adversarial learning from gene expression data. Brief. Bioinform. 2024, 25, bbae207. [Google Scholar] [CrossRef]
- Fetse, J.; Kandel, S.; Mamani, U.F.; Cheng, K. Recent advances in the development of therapeutic peptides. Trends Pharmacol. Sci. 2023, 44, 425–441. [Google Scholar] [CrossRef]
- Shukla, S.P.; Raymond, A.; Rustagi, V.; Kedika, S.R.; Tran, O.; Wang, L.; Guo, B.; Udugamasooriya, D.G. A novel peptidomimetic therapeutic for selective suppression of lung cancer stem cells over non-stem cancer cells. Bioorganic Chem. 2021, 116, 105340. [Google Scholar] [CrossRef]


| Marker | Description | Role in BCSCs | References |
|---|---|---|---|
| CD44 | Cell-surface glycoprotein involved in cell adhesion and migration. EMT requires an isoform switch from CD44v to CD44s. | High CD44+/Low CD24− phenotype marks BCSCs; linked to tumorigenicity and metastasis. | [4,18] |
| CD24 | Surface protein containing sialic acid sugars that is involved in adhesion and co-stimulatory signaling to immune cells (like CD4+ T cells). | High CD44+/Low CD24− indicates BCSCs with enhanced invasive capabilities, as opposed to the ALDH1+ BCSCs. | [4,18] |
| ALDH1 (endogenous marker) | Enzyme catalyzing oxidation of aldehydes and other important physiologic and toxicological functions. | High ALDH1 activity identifies BCSCs, especially the proliferative epithelial subtype associated with increased self-renewal and drug resistance. | [4,20] |
| CD133 | Transmembrane glycoprotein associated with stemness as it suppresses differentiation, though its precise mechanism is unclear. | Marker for BCSCs with tumor-initiating capacity; overall, it has been associated with poor survival and prognosis. | [28] |
| EpCAM | An adhesion molecule located on the basolateral membrane of cells. | Helps BCSCs with invasion and metastasis, marker for isolating circulating tumor cells (CTCs) enriched with BCSC properties. | [4] |
| CXCR1 | Chemokine receptor facilitating migration and invasion via the IL-8 axis. | Regulates BCSC activity; targeted by Reparixin in trials to reduce BCSC populations. | [26,29] |
| HER2 | Receptor tyrosine kinase promoting cell growth and differentiation, and its downstream signaling pathway participates in crosstalk with other signaling pathways. | Overexpressed in HER2+ BCSCs; correlates with aggressive tumors by interacting with signaling pathways and increasing stemness features. | [4,30] |
| DCLK1 | A CSC marker that is overexpressed in many types of cancer, including breast cancer | Overexpression of DCLK1 in tumor cells will likely contribute to stemness and self-renewal. | [31] |
| ABC Efflux Factors | CSCs express a high proportion of ABC transporters | The basis of a flow cytometry method to identify CSC | [32] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, A.V.; Kong, Y.; Wang, K.; Brown, M.L.; Mu, D. Protein Marker-Dependent Drug Discovery Targeting Breast Cancer Stem Cells. Int. J. Mol. Sci. 2025, 26, 7935. https://doi.org/10.3390/ijms26167935
Huang AV, Kong Y, Wang K, Brown ML, Mu D. Protein Marker-Dependent Drug Discovery Targeting Breast Cancer Stem Cells. International Journal of Molecular Sciences. 2025; 26(16):7935. https://doi.org/10.3390/ijms26167935
Chicago/Turabian StyleHuang, Ashley V., Yali Kong, Kan Wang, Milton L. Brown, and David Mu. 2025. "Protein Marker-Dependent Drug Discovery Targeting Breast Cancer Stem Cells" International Journal of Molecular Sciences 26, no. 16: 7935. https://doi.org/10.3390/ijms26167935
APA StyleHuang, A. V., Kong, Y., Wang, K., Brown, M. L., & Mu, D. (2025). Protein Marker-Dependent Drug Discovery Targeting Breast Cancer Stem Cells. International Journal of Molecular Sciences, 26(16), 7935. https://doi.org/10.3390/ijms26167935







