Peripheral Nerve Decellularisation Protocol for Allogeneic Transplantation: From Tissue Procurement to Banking
Abstract
1. Introduction
2. Results
2.1. Microbiological Testing
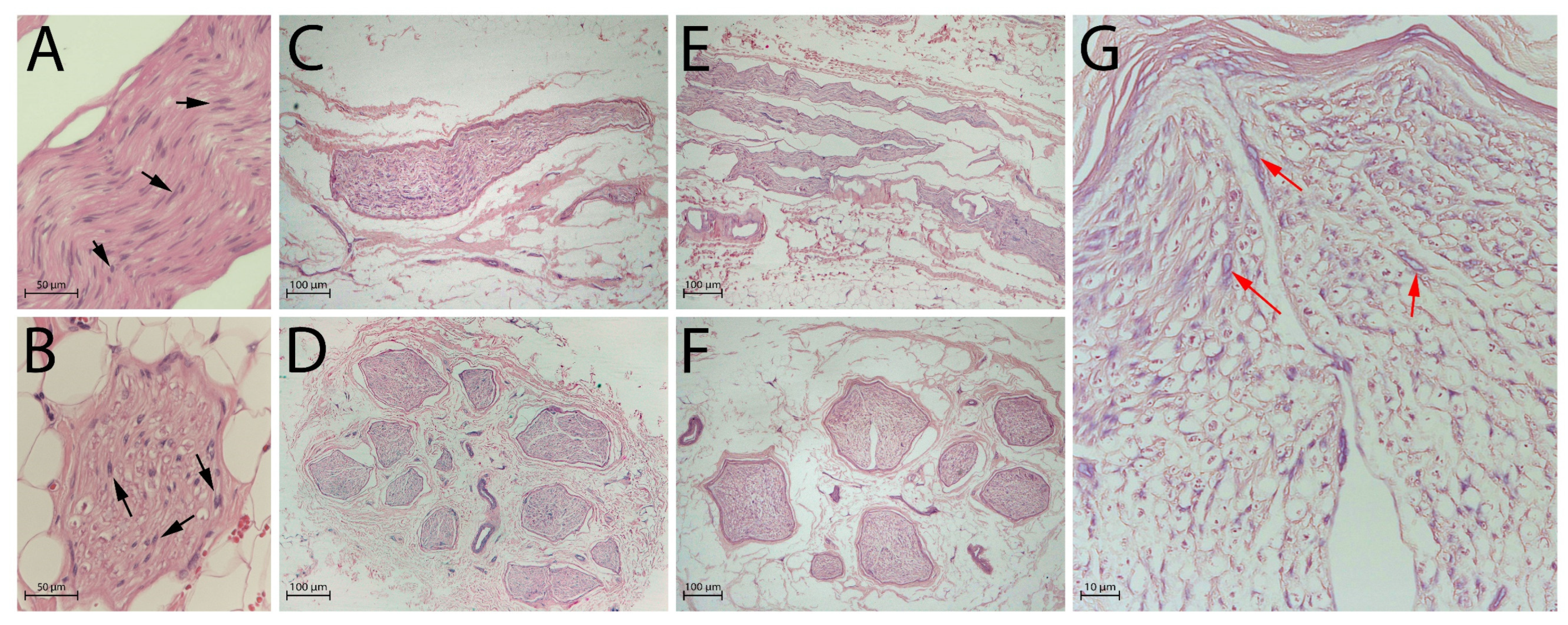
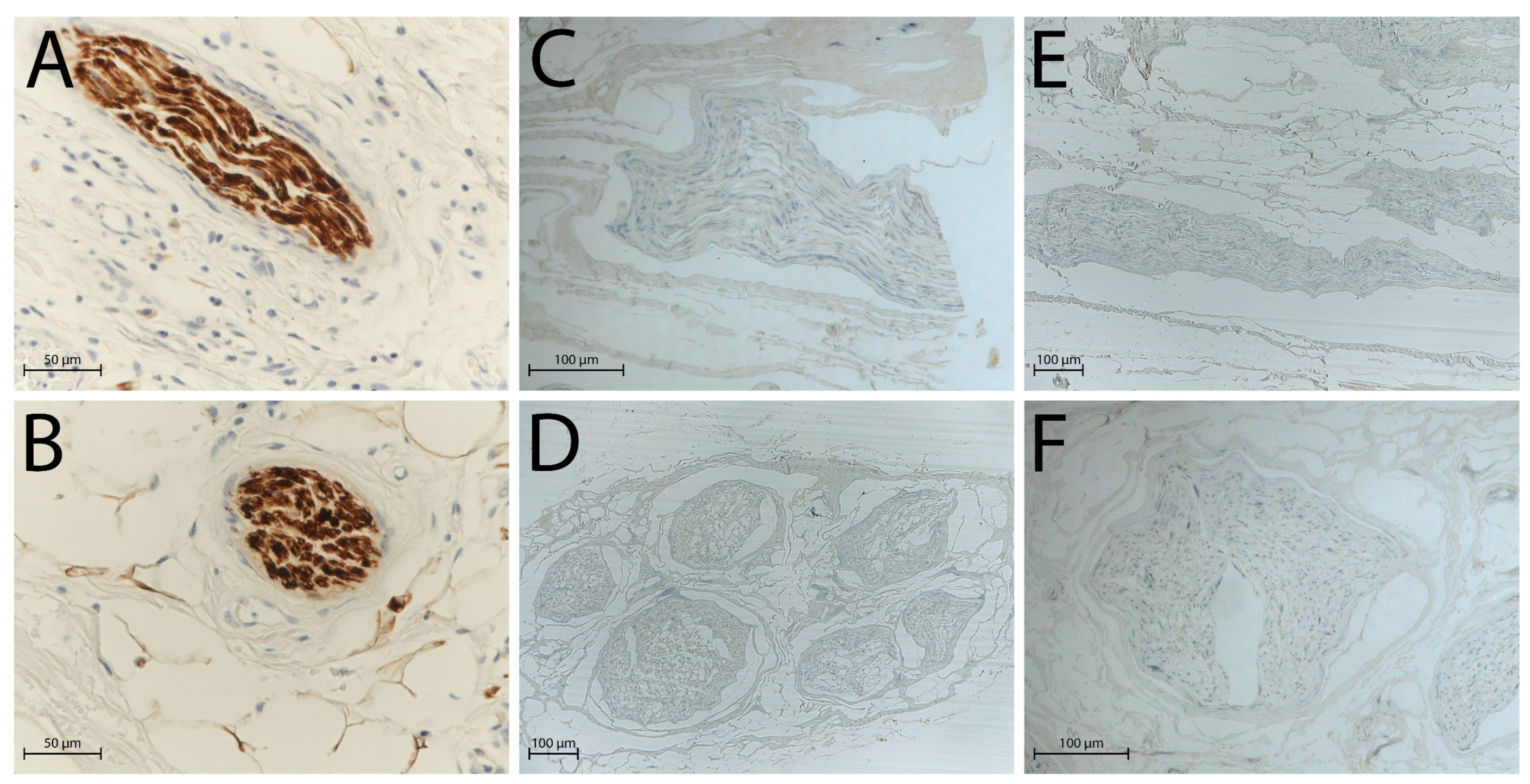
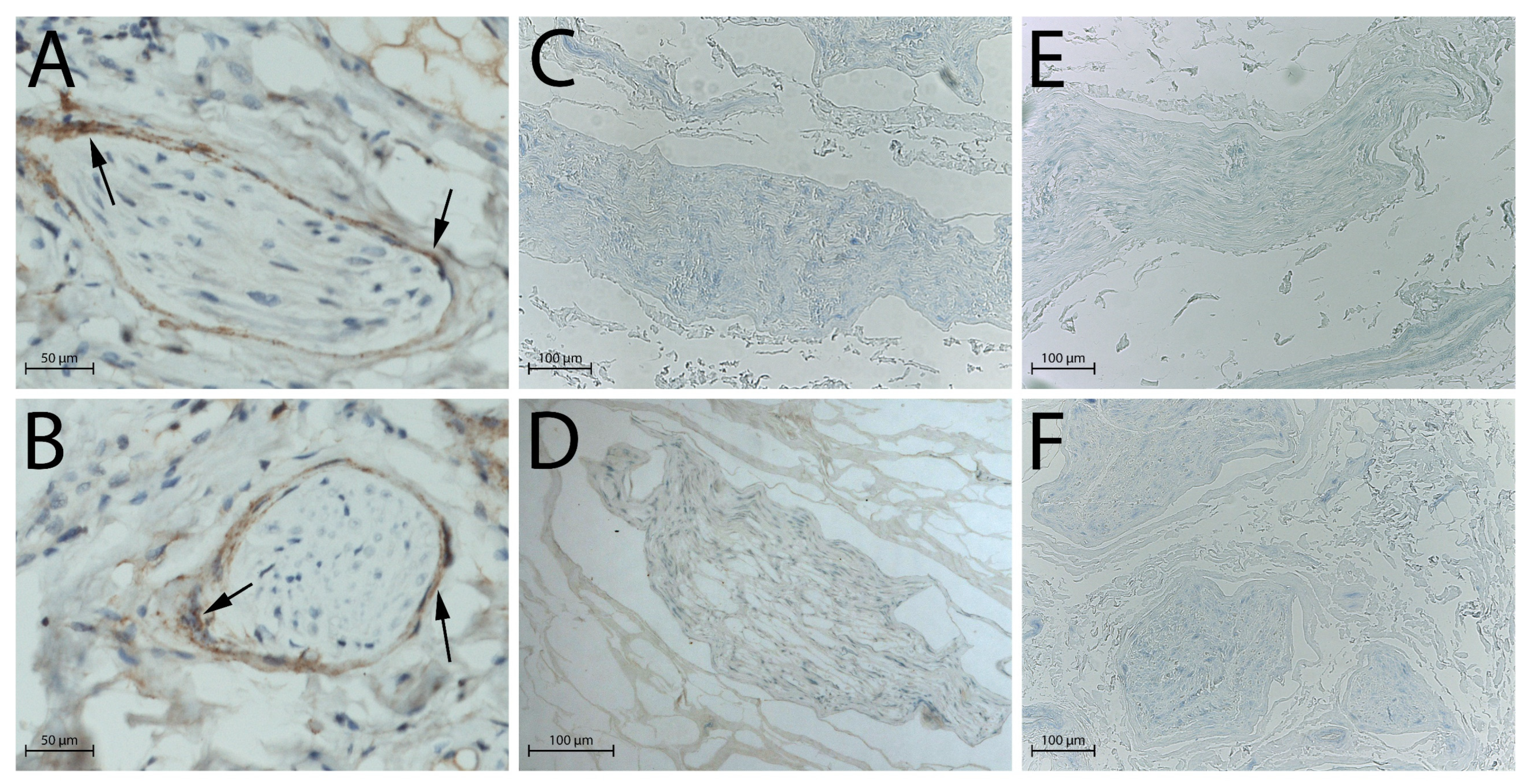
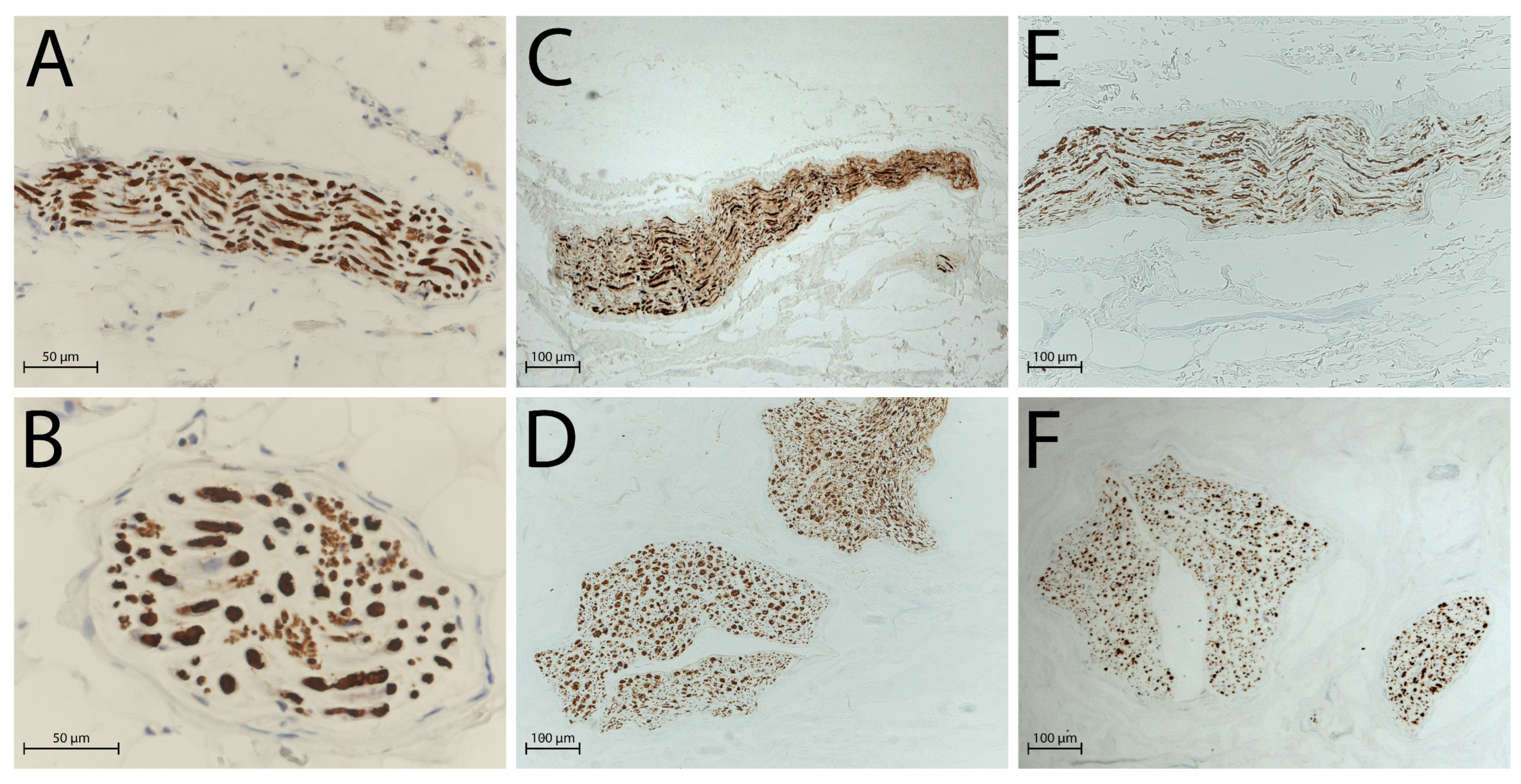
2.2. Histological Analysis
2.3. DAPI Staining
3. Discussion
4. Materials and Methods
4.1. Preparation of P-Nerve and D-Nerve Sterile Solutions
4.2. Donor Selection and Tissue Collection
4.3. Decellularisation Protocol
- −
- Samples and the D-Nerve solution are thawed at 4 °C overnight.
- −
- The day after, inside an ISO Class A cleanroom environment, the P-Nerve solution is discarded, and thawed nerves are placed on a sterile stainless-steel board and cut with a sterile scalpel blade in segments of required dimensions.
- −
- All the segments are inserted in a sterile polypropylene/polyethylene (PP/PE) container (Agricons Ricerche, Padua, Italy), immersed in 50 mL of sterile saline solution, and agitated with the incubator shaker MD3000 (PBI S.r.l., Milan, Italy) for 10 min at RT. This washing step is repeated three times with fresh sterile saline solution.
- −
- Each segment is inserted in a sterile 50 mL tube, immersed in about 30 mL of D-Nerve solution, and undergoes five cycles of agitation/sonication at RT. Agitation steps are performed with the MD3000 incubator shaker (PBI-International, Milan, Italy) for 27 min, while ultrasound irradiation is carried out by the Sonica 3200L ultrasonic bath (SOLTEC S.r.l., Milan, Italy), featuring a stainless steel tank and autoclavable accessories, at the frequency of 39 kHz ± 1 kHz with SWEEP SYSTEM Technology for 3 min. Specifically, the SWEEP SYSTEM technology adjusts the amplitude of the electrical signal with an oscillating frequency of 39 kHz ± 1 kHz, enhancing ultrasonic cavitation for superior performance [61].
- −
- Three further washing steps (10 min each) with sterile saline are needed to conclude the decellularisation protocol.
- −
4.4. Microbiological Tests
4.5. Histological Analysis
4.6. DAPI Fluorescence
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANA | Acellular nerve allograft |
| DAPI | 4′,6-diamidino-2-phenylindole |
| ECM | Extracellular Matrix |
| EDQM | European Directorate for the Quality of Medicines & HealthCare |
| EMA | Epithelial Membrane Antigen |
| FDA | Food and Drug Administration |
| GMP | Good Manufacturing Practice |
| GTP | Good Tissue Practice |
| H&E | Haematoxylin and Eosin |
| HPF | High-Powered Field |
| NF | Neurofilament |
| PP/PE | Polypropylene/Polyethylene |
| REACH | Registration, Evaluation, Authorization, and Restriction of Chemicals |
| SoHO | Substances of Human Origin |
| RT | Room Temperature |
| TCTPs | Tissue and Cells Therapies and Products |
References
- Li, C.; Liu, S.Y.; Pi, W.; Zhang, P.X. Cortical plasticity and nerve regeneration after peripheral nerve injury. Neural Regen. Res. 2021, 16, 1518–1523. [Google Scholar] [CrossRef]
- Sachanandani, N.F.; Pothula, A.; Tung, T.H. Nerve Gaps. Plast. Reconstr. Surg. 2014, 133, 313–319. [Google Scholar] [CrossRef]
- Kornfeld, T.; Vogt, P.M.; Radtke, C. Nerve grafting for peripheral nerve injuries with extended defect sizes. Wien. Med. Wochenschr. 2019, 169, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Ray, W.Z.; Mackinnon, S.E. Management of nerve gaps: Autografts, allografts, nerve transfers, and end-to-side neurorrhaphy. Exp. Neurol. 2010, 223, 77–85. [Google Scholar] [CrossRef]
- Kallio, P.K.; Vastamäki, M. An Analysis of the Results of Late Reconstruction of 132 Median Nerves. J. Hand Surg. 1993, 18, 97–105. [Google Scholar] [CrossRef]
- Lee, S.K.; Wolfe, S.W. Peripheral Nerve Injury and Repair. J. Am. Acad. Orthop. Surg. 2000, 8, 243–252. [Google Scholar] [CrossRef]
- Wood, M.B. Peroneal nerve repair. Surgical results. Clin. Orthop. Relat. Res. 1991, 267, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Houshyar, S.; Bhattacharyya, A.; Shanks, R. Peripheral Nerve Conduit: Materials and Structures. ACS Chem. Neurosci. 2019, 10, 3349–3365. [Google Scholar] [CrossRef]
- Geuna, S.; Tos, P.; Titolo, P.; Ciclamini, D.; Beningo, T.; Battiston, B. Update on nerve repair by biological tubulization. J. Brachial Plex. Peripher. Nerve Inj. 2014, 9, 3. [Google Scholar] [CrossRef]
- Tos, P.; Colzani, G.; Ciclamini, D.; Titolo, P.; Pugliese, P.; Artiaco, S. Clinical Applications of End-to-Side Neurorrhaphy: An Update. BioMed Res. Int. 2014, 2014, 646128. [Google Scholar] [CrossRef]
- Siemionow, M.; Sonmez, E. Nerve Allograft Transplantation: A Review. J. Reconstr. Microsurg. 2007, 23, 511–520. [Google Scholar] [CrossRef]
- Squintani, G.; Bonetti, B.; Paolin, A.; Vici, D.; Cogliati, E.; Murer, B.; Stevanato, G. Nerve regeneration across cryopreserved allografts from cadaveric donors: A novel approach for peripheral nerve reconstruction. J. Neurosurg. 2013, 119, 907–913. [Google Scholar] [CrossRef]
- Axogen. Avance Nerve Graft. Available online: https://axogeninc.eu/avance-nerve-graft/ (accessed on 28 May 2025).
- Gut, G.; Marowska, J.; Jastrzebska, A.; Olender, E.; Kamiński, A. Structural mechanical properties of radiation-sterilized human Bone-Tendon-Bone grafts preserved by different methods. Cell Tissue Bank. 2016, 17, 277–287. [Google Scholar] [CrossRef]
- Harrell, C.R.; Djonov, V.; Fellabaum, C.; Volarevic, V. Risks of Using Sterilization by Gamma Radiation: The Other Side of the Coin. Int. J. Med. Sci. 2018, 15, 274–279. [Google Scholar] [CrossRef]
- Nguyen, H.; Morgan, D.A.F.; Forwood, M.R. Sterilization of allograft bone: Effects of gamma irradiation on allograft biology and biomechanics. Cell Tissue Bank. 2007, 8, 93–105. [Google Scholar] [CrossRef]
- Hopf, A.; Al-Bayati, L.; Schaefer, D.J.; Kalbermatten, D.F.; Guzman, R.; Madduri, S. Optimized Decellularization Protocol for Large Peripheral Nerve Segments: Towards Personalized Nerve Bioengineering. Bioengineering 2022, 9, 412. [Google Scholar] [CrossRef] [PubMed]
- McCrary, M.W.; Vaughn, N.E.; Hlavac, N.; Song, Y.H.; Wachs, R.A.; Schmidt, C.E. Novel Sodium Deoxycholate-Based Chemical Decellularization Method for Peripheral Nerve. Tissue Eng. Part C Methods 2020, 26, 23–36. [Google Scholar] [CrossRef] [PubMed]
- Vasudevan, S.; Huang, J.B.; Botterman, B.; Matloub, H.S.M.; Keefer, E.; Cheng, J.M. Detergent-free Decellularized Nerve Grafts for Long-gap Peripheral Nerve Reconstruction. Plast. Reconstr. Surg. Glob. Open 2014, 2, e201. [Google Scholar] [CrossRef] [PubMed]
- Lovati, A.B.; D’aRrrigo, D.; Odella, S.; Tos, P.; Geuna, S.; Raimondo, S. Nerve Repair Using Decellularized Nerve Grafts in Rat Models. A Review of the Literature. Front. Cell. Neurosci. 2018, 12, 427. [Google Scholar] [CrossRef]
- Shin, Y.H.; Park, S.Y.; Kim, J.K. Comparison of systematically combined detergent and nuclease-based decellularization methods for acellular nerve graft: An ex vivo characterization and in vivo evaluation. J. Tissue Eng. Regen. Med. 2019, 13, 1241–1252. [Google Scholar] [CrossRef]
- Bae, J.Y.; Park, S.Y.; Shin, Y.H.; Choi, S.W.; Kim, J.K. Preparation of human decellularized peripheral nerve allograft using amphoteric detergent and nuclease. Neural Regen. Res. 2021, 16, 1890–1896. [Google Scholar] [CrossRef]
- Nieto-Nicolau, N.; López-Chicón, P.; Fariñas, O.; Bolívar, S.; Udina, E.; Navarro, X.; Casaroli-Marano, R.; Vilarrodona, A. Effective decellularization of human nerve matrix for regenerative medicine with a novel protocol. Cell Tissue Res. 2021, 384, 167–177. [Google Scholar] [CrossRef]
- Bolognesi, F.; Fazio, N.; Boriani, F.; Fabbri, V.P.; Gravina, D.; Pedrini, F.A.; Zini, N.; Greco, M.; Paolucci, M.; Re, M.C.; et al. Validation of a Cleanroom Compliant Sonication-Based Decellularization Technique: A New Concept in Nerve Allograft Production. Int. J. Mol. Sci. 2022, 23, 1530. [Google Scholar] [CrossRef] [PubMed]
- Boriani, F.; Fazio, N.; Fotia, C.; Savarino, L.; Aldini, N.N.; Martini, L.; Zini, N.; Bernardini, M.; Baldini, N. A novel technique for decellularization of allogenic nerves and in vivo study of their use for peripheral nerve reconstruction. J. Biomed. Mater. Res. Part A 2017, 105, 2228–2240. [Google Scholar] [CrossRef] [PubMed]
- European Directorate for the Quality of Medicines & HealthCare. 5th Edition of the Guide to the Quality and Safety of Tissues and Cells for Human Application. Available online: https://www.edqm.eu/en/-/edqm-releases-new-edition-of-the-tissue-and-cells-guide-providing-state-of-the-art-guidance-for-healthcare-professionals-1 (accessed on 28 May 2025).
- Italian National Transplant Center. Linee Guida per Il Prelievo, la Processazione e la Distribuzione di Tessuti a Scopo di Trapianto. Available online: https://www.trapianti.salute.gov.it/trapianti/archivioProtocolliCnt.jsp?lingua=italiano&anno=2013&btnCerca=cerca (accessed on 28 May 2025).
- Whitlock, E.L.; Tuffaha, S.H.; Luciano, J.P.; Yan, Y.; Hunter, D.A.; Magill, C.K.; Moore, A.M.; Tong, A.Y.; Mackinnon, S.E.; Borschel, G.H. Processed allografts and type I collagen conduits for repair of peripheral nerve gaps. Muscle Nerve 2009, 39, 787–799. [Google Scholar] [CrossRef]
- Osawa, T.; Tohyama, K.; Ide, C. Allogeneic nerve grafts in the rat, with special reference to the role of Schwann cell basal laminae in nerve regeneration. J. Neurocytol. 1990, 19, 833–849. [Google Scholar] [CrossRef]
- Hudson, T.W.; Zawko, S.; Deister, C.; Lundy, S.; Hu, C.Y.; Lee, K.; Schmidt, C.E. Optimized Acellular Nerve Graft Is Immunologically Tolerated and Supports Regeneration. Tissue Eng. 2004, 10, 1641–1651. [Google Scholar] [CrossRef]
- Seddon, A.M.; Curnow, P.; Booth, P.J. Membrane proteins, lipids and detergents: Not just a soap opera. Biochim. Biophys. Acta (BBA)-Biomembr. 2004, 1666, 105–117. [Google Scholar] [CrossRef]
- Regulations in the European Union for the Use of Triton X-100 in the Pharmaceutical Industry. Available online: https://www.bdo.com/insights/industries/life-sciences/regulations-in-the-european-union-for-the-use-of-triton-x-100-in-the-pharmaceutical-industry (accessed on 28 May 2025).
- de Luca, A.C.; Lacour, S.P.; Raffoul, W.; di Summa, P.G. Extracellular matrix components in peripheral nerve repair: How to affect neural cellular response and nerve regeneration. Neural Regen. Res. 2014, 9, 1943–1948. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Chen, M.; Liu, N. Interactions between Schwann cell and extracellular matrix in peripheral nerve regeneration. Front. Neurol. 2024, 15, 1372168. [Google Scholar] [CrossRef]
- Akram, R.; Anwar, H.; Javed, M.S.; Rasul, A.; Imran, A.; Malik, S.A.; Raza, C.; Khan, I.U.; Sajid, F.; Iman, T.; et al. Axonal Regeneration: Underlying Molecular Mechanisms and Potential Therapeutic Targets. Biomedicines 2022, 10, 3186. [Google Scholar] [CrossRef]
- Bunge, R.P. The role of the Schwann cell in trophic support and regeneration. J. Neurol. 1994, 242, S19–S21. [Google Scholar] [CrossRef]
- Huang, Y.Y.; Xu, X.L.; Huang, X.J.; Liu, J.H.; Qi, J.; Zhu, S.; Zhu, Z.W.; He, B.; Zhu, Q.T.; Xu, Y.B.; et al. Various changes in cryopreserved acellular nerve allografts at −80 °C. Neural Regen. Res. 2018, 13, 1643–1649. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Zhang, G.; Hou, B.; Song, E.; Wen, J.; Ba, Y.; Zhu, D.; Wang, G.; Qin, F. Effects of ECM proteins (laminin, fibronectin, and type IV collagen) on the biological behavior of Schwann cells and their roles in the process of remyelination after peripheral nerve injury. Front. Bioeng. Biotechnol. 2023, 11, 1133718. [Google Scholar] [CrossRef]
- Huang, W.-H.; Ding, S.-L.; Zhao, X.-Y.; Li, K.; Guo, H.-T.; Zhang, M.-Z.; Gu, Q. Collagen for neural tissue engineering: Materials, strategies, and challenges. Mater. Today Bio 2023, 20, 100639. [Google Scholar] [CrossRef]
- Su, M.; Soomro, S.H.; Jie, J.; Fu, H. Effects of the extracellular matrix on myelin development and regeneration in the central nervous system. Tissue Cell 2021, 69, 101444. [Google Scholar] [CrossRef] [PubMed]
- Tusnim, J.; Budharaju, K.; Grasman, J.M. Fabrication of ECM protein coated hollow collagen channels to study peripheral nerve regeneration. Sci. Rep. 2024, 14, 16096. [Google Scholar] [CrossRef] [PubMed]
- EuroGTP II. Available online: http://goodtissuepractices.eu/ (accessed on 6 August 2025).
- EuroGTP II Interactive Assessment Tool. Available online: https://tool.goodtissuepractices.site/ (accessed on 6 August 2025).
- Groves, M.L.; McKeon, R.; Werner, E.; Nagarsheth, M.; Meador, W.; English, A.W. Axon regeneration in peripheral nerves is enhanced by proteoglycan degradation. Exp. Neurol. 2005, 195, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Krekoski, C.A.; Neubauer, D.; Zuo, J.; Muir, D. Axonal regeneration into acellular nerve grafts is enhanced by degradation of chondroitin sulfate proteoglycan. J. Neurosci. 2001, 21, 6206–6213. [Google Scholar] [CrossRef] [PubMed]
- Neubauer, D.; Graham, J.B.; Muir, D. Chondroitinase treatment increases the effective length of acellular nerve grafts. Exp. Neurol. 2007, 207, 163–170. [Google Scholar] [CrossRef]
- The Avance Method. Available online: https://www.axogeninc.com/wp-content/uploads/2021/08/The-Avance-Methods-Brochure-MKTG-0245-R00.pdf (accessed on 10 July 2025).
- Leckenby, J.I.; Furrer, C.; Haug, L.; Juon Personeni, B.; Vogelin, E. A Retrospective Case Series Reporting the Outcomes of Avance Nerve Allografts in the Treatment of Peripheral Nerve Injuries. Plast. Reconstr. Surg. 2020, 145, 368e–381e. [Google Scholar] [CrossRef]
- Peters, B.R.; Wood, M.D.; Hunter, D.A.; Mackinnon, S.E. Acellular Nerve Allografts in Major Peripheral Nerve Repairs: An Analysis of Cases Presenting With Limited Recovery. Hand 2023, 18, 236–243. [Google Scholar] [CrossRef]
- Zuo, K.J.; Borschel, G.H. A Retrospective Case Series Reporting the Outcomes of Avance Nerve Allografts in the Treatment of Peripheral Nerve Injuries. Plast. Reconstr. Surg. 2021, 147, 350e–351e. [Google Scholar] [CrossRef]
- Mathot, F.; Rbia, N.; Thaler, R.; Bishop, A.T.; van Wijnen, A.J.; Shin, A.Y. Introducing human adipose-derived mesenchymal stem cells to Avance(®) nerve grafts and NeuraGen(®) nerve guides. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 1473–1481. [Google Scholar] [CrossRef] [PubMed]
- Ansaripour, A.; Thompson, A.; Styron, J.F.; Javanbakht, M. Cost-effectiveness analysis of Avance((R)) allograft for the treatment of peripheral nerve injuries in the USA. J. Comp. Eff. Res. 2024, 13, e230113. [Google Scholar] [CrossRef]
- Boriani, F.; Savarino, L.; Fazio, N.; Pedrini, F.A.; Fini, M.; Nicoli Aldini, N.; Martini, L.; Zini, N.; Bernardini, M.; Bolognesi, F.; et al. Auto-Allo Graft Parallel Juxtaposition for Improved Neuroregeneration in Peripheral Nerve Reconstruction Based on Acellular Nerve Allografts. Ann. Plast. Surg. 2019, 83, 318–325. [Google Scholar] [CrossRef]
- Hernandez-Fuentes, M.P.; Salama, A. In vitro assays for immune monitoring in transplantation. Methods Mol. Biol. 2006, 333, 269–290. [Google Scholar] [CrossRef] [PubMed]
- Al-Qurayshi, Z.; Wafa, E.I.; Rossi Meyer, M.K.; Owen, S.; Salem, A.K. Tissue Engineering the Pinna: Comparison and Characterization of Human Decellularized Auricular Biological Scaffolds. ACS Appl. Bio Mater. 2021, 4, 7234–7242. [Google Scholar] [CrossRef] [PubMed]
- Pusnik, L.; Radochova, B.; Janacek, J.; Saudek, F.; Sersa, I.; Cvetko, E.; Umek, N.; Snoj, Z. Fascicle differentiation of upper extremity nerves on high-resolution ultrasound with multimodal microscopic verification. Sci. Rep. 2025, 15, 557. [Google Scholar] [CrossRef]
- Yuan, X.; Li, W.; Yan, Q.; Ou, Y.; Long, Q.; Zhang, P. Biomarkers of mature neuronal differentiation and related diseases. Future Sci. OA 2024, 10, 2410146. [Google Scholar] [CrossRef]
- EU Tissue Establishment List. Available online: https://webgate.ec.europa.eu/eucoding/reports/te/index.xhtml (accessed on 11 June 2025).
- Musculoskeletal Tissue Bank of IRCCS Istituto Ortopedico Rizzoli. Available online: https://btm.ior.it/en (accessed on 11 June 2025).
- Ruffilli, A.; Barile, F.; Fiore, M.; Manzetti, M.; Viroli, G.; Mazzotti, A.; Govoni, M.; De Franceschi, L.; Dallari, D.; Faldini, C. Allogenic bone grafts and postoperative surgical site infection: Are positive intraoperative swab cultures predictive for a higher infectious risk? Cell Tissue Bank. 2023, 24, 627–637. [Google Scholar] [CrossRef] [PubMed]
- Sweep System Technology. Available online: https://www.soltec.it/d0/en/node/507 (accessed on 24 June 2025).
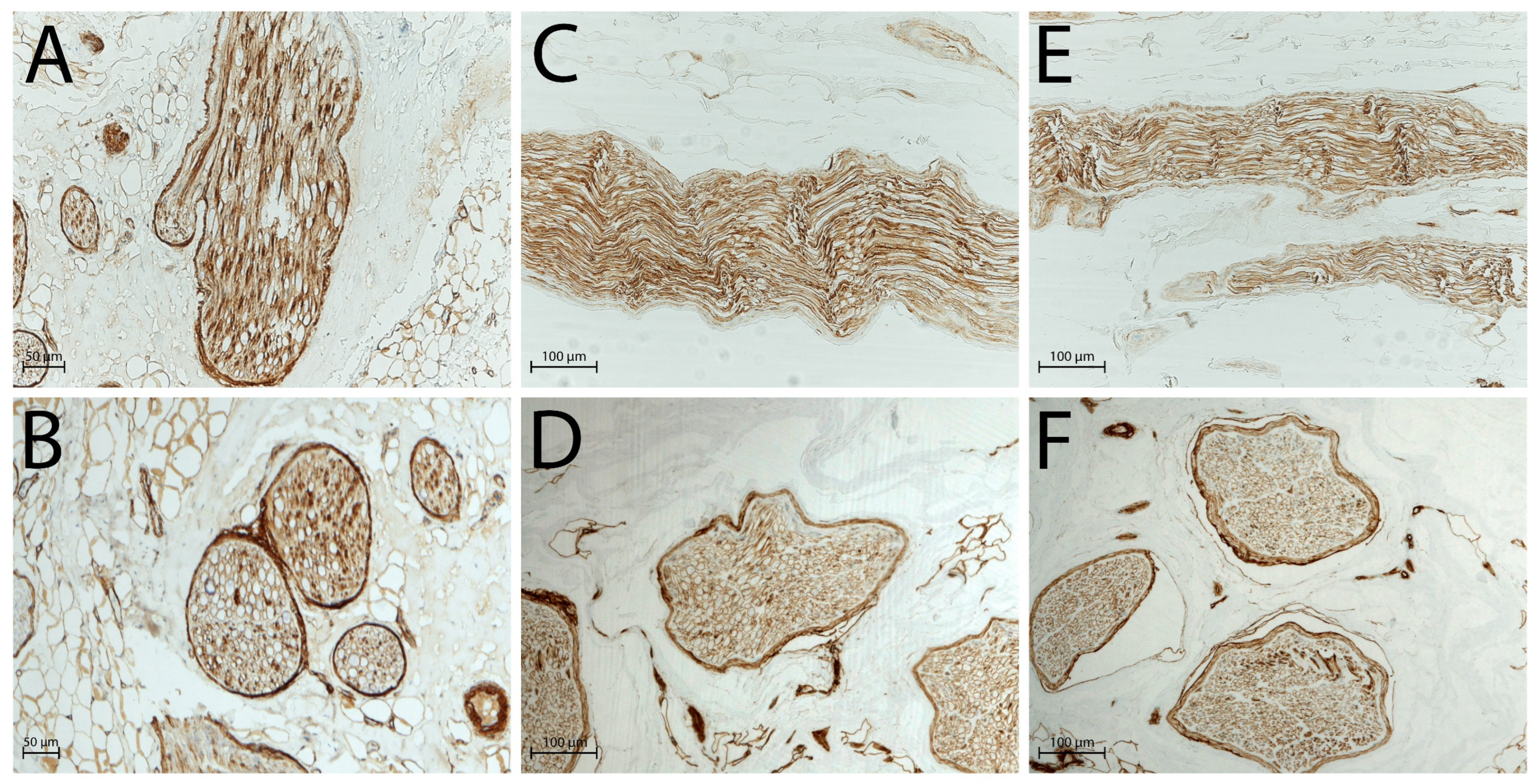
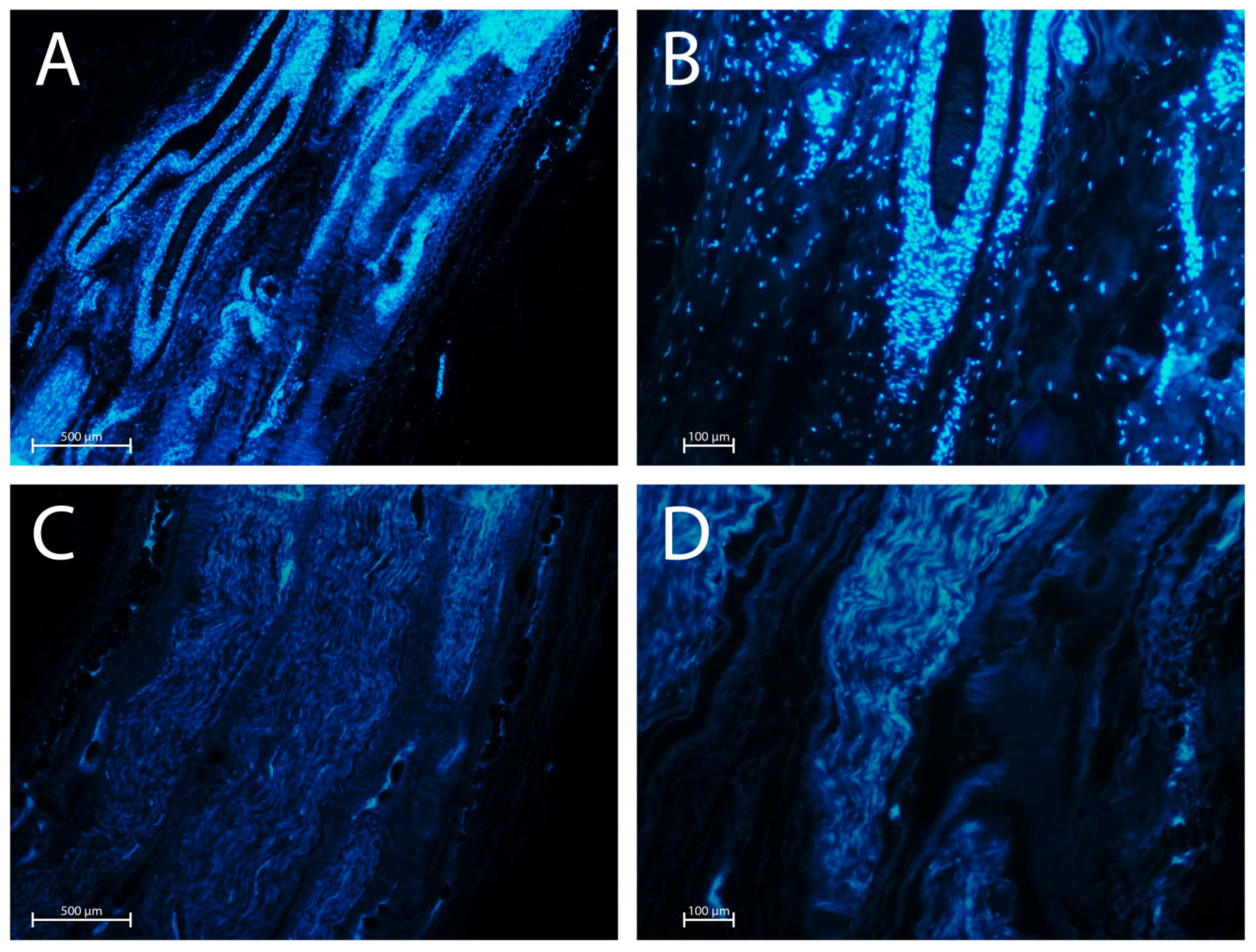

| Sample N. | Global Nerve Preservation | Nuclear Status | Intact Schwann Cells | Intact Perineural Cells | Axonal Network Preservation | ECM Preservation |
|---|---|---|---|---|---|---|
| 1 | well | degenerated | absent | absent | moderate | well |
| 2 | well | degenerated | absent | absent | moderate | well |
| 3 | well | degenerated | absent | absent | moderate | well |
| Sample N. | Global Nerve Preservation | Nuclear Status | Intact Schwann Cells | Intact Perineural Cells | Axonal Network Preservation | ECM Preservation |
|---|---|---|---|---|---|---|
| 1 | well | degenerated | absent | absent | moderate | well |
| 2 | well | degenerated | absent | absent | moderate | well |
| 3 | well | degenerated | absent | absent | moderate | well |
| Decellularisation Protocol | Chemical Reagents | Enzymatic Treatment | Physical Treatment | Duration | Regulatory Compliance | Clinical Readiness Level |
|---|---|---|---|---|---|---|
| Our protocol | Tween 20 SB3-10 SDS | / | Sonication, washing | ∼5 days | Approved by competent authorities | Moderate/high (GMP, EDQM, national approval) |
| Avance® | SB-10 SB-16 NaCl Sodium phosphate buffer Phosphate buffer | CSPGs | Washing, terminal irradiation | >3 days | FDA certified | High (commercially available) |
| Bae et al. [22] | Triton X-100 NaCl Sodium deoxycholate | / | Washing | ∼6 days | Research use only | Low |
| CHAPS NaCl | DNase RNase A | Washing | ∼5 days | Research use only | Low | |
| Nieto-Nicolau et al. [23] | Triton X-100 SB3-10 SB3-16 Tris-EDTA | DNase | Washing | ∼7 days | Research use only | Low |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Govoni, M.; Vivarelli, L.; Fazio, N.; Bolognesi, F.; Fabbri, V.P.; Maso, A.; Storni, E.; Querzoli, G.; Contartese, D.; Pagani, S.; et al. Peripheral Nerve Decellularisation Protocol for Allogeneic Transplantation: From Tissue Procurement to Banking. Int. J. Mol. Sci. 2025, 26, 7937. https://doi.org/10.3390/ijms26167937
Govoni M, Vivarelli L, Fazio N, Bolognesi F, Fabbri VP, Maso A, Storni E, Querzoli G, Contartese D, Pagani S, et al. Peripheral Nerve Decellularisation Protocol for Allogeneic Transplantation: From Tissue Procurement to Banking. International Journal of Molecular Sciences. 2025; 26(16):7937. https://doi.org/10.3390/ijms26167937
Chicago/Turabian StyleGovoni, Marco, Leonardo Vivarelli, Nicola Fazio, Federico Bolognesi, Viscardo Paolo Fabbri, Alessandra Maso, Elisa Storni, Giulia Querzoli, Deyanira Contartese, Stefania Pagani, and et al. 2025. "Peripheral Nerve Decellularisation Protocol for Allogeneic Transplantation: From Tissue Procurement to Banking" International Journal of Molecular Sciences 26, no. 16: 7937. https://doi.org/10.3390/ijms26167937
APA StyleGovoni, M., Vivarelli, L., Fazio, N., Bolognesi, F., Fabbri, V. P., Maso, A., Storni, E., Querzoli, G., Contartese, D., Pagani, S., Cavazza, L., Pluchino, M., De Franceschi, L., Giavaresi, G., & Dallari, D. (2025). Peripheral Nerve Decellularisation Protocol for Allogeneic Transplantation: From Tissue Procurement to Banking. International Journal of Molecular Sciences, 26(16), 7937. https://doi.org/10.3390/ijms26167937










