Melatonin-Producing Bacillus aerius EH2-5 Enhances Glycine max Plants Salinity Tolerance Through Physiological, Biochemical, and Molecular Modulation
Abstract
1. Introduction
2. Result
2.1. Identification of Chosen Bacterial Strain
2.2. Plant Growth-Promoting Traits of EH2-5
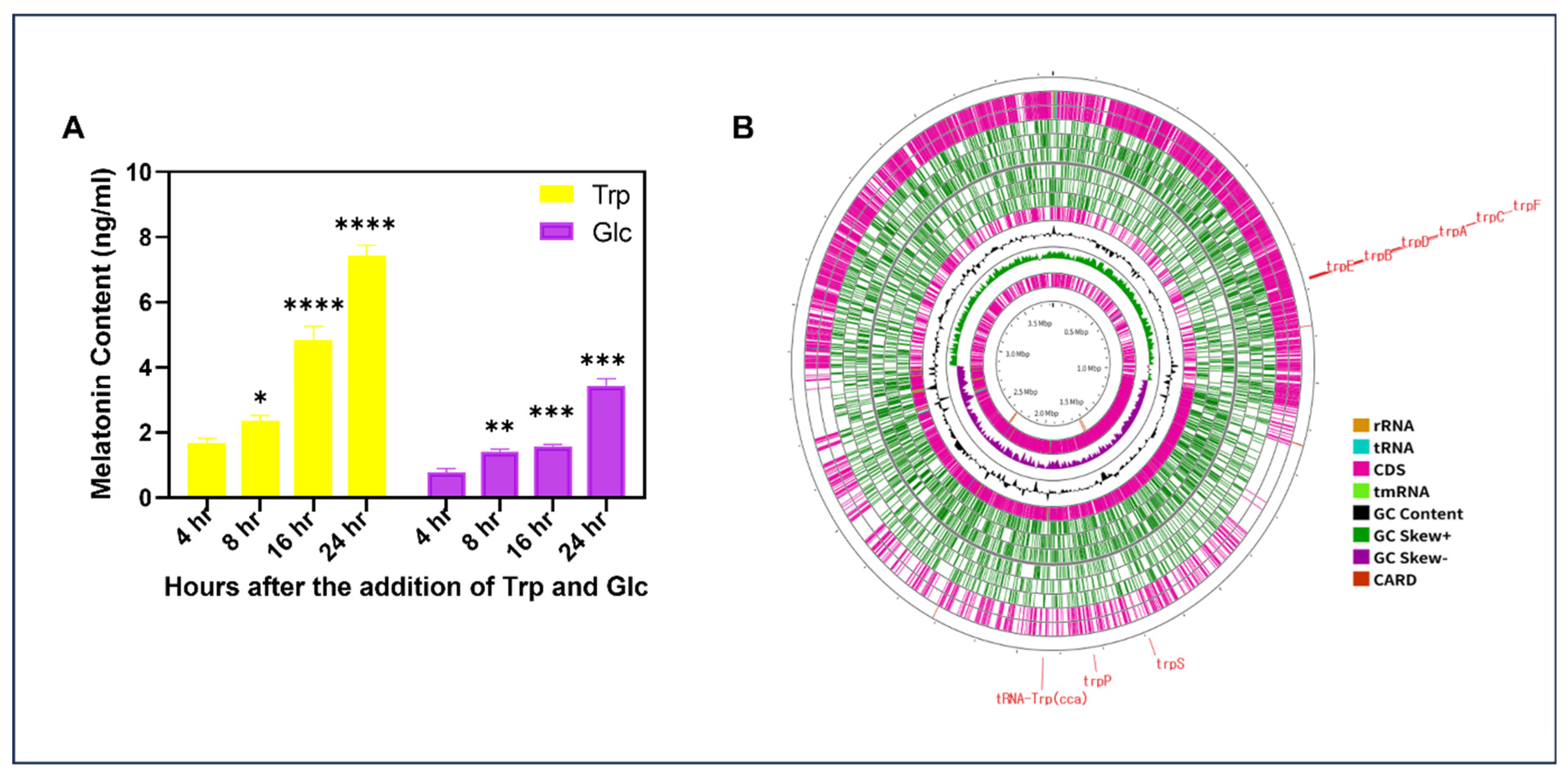
2.3. Melatonin Producing Ability of EH2-5
2.4. WGS Information of Strain EH2-5
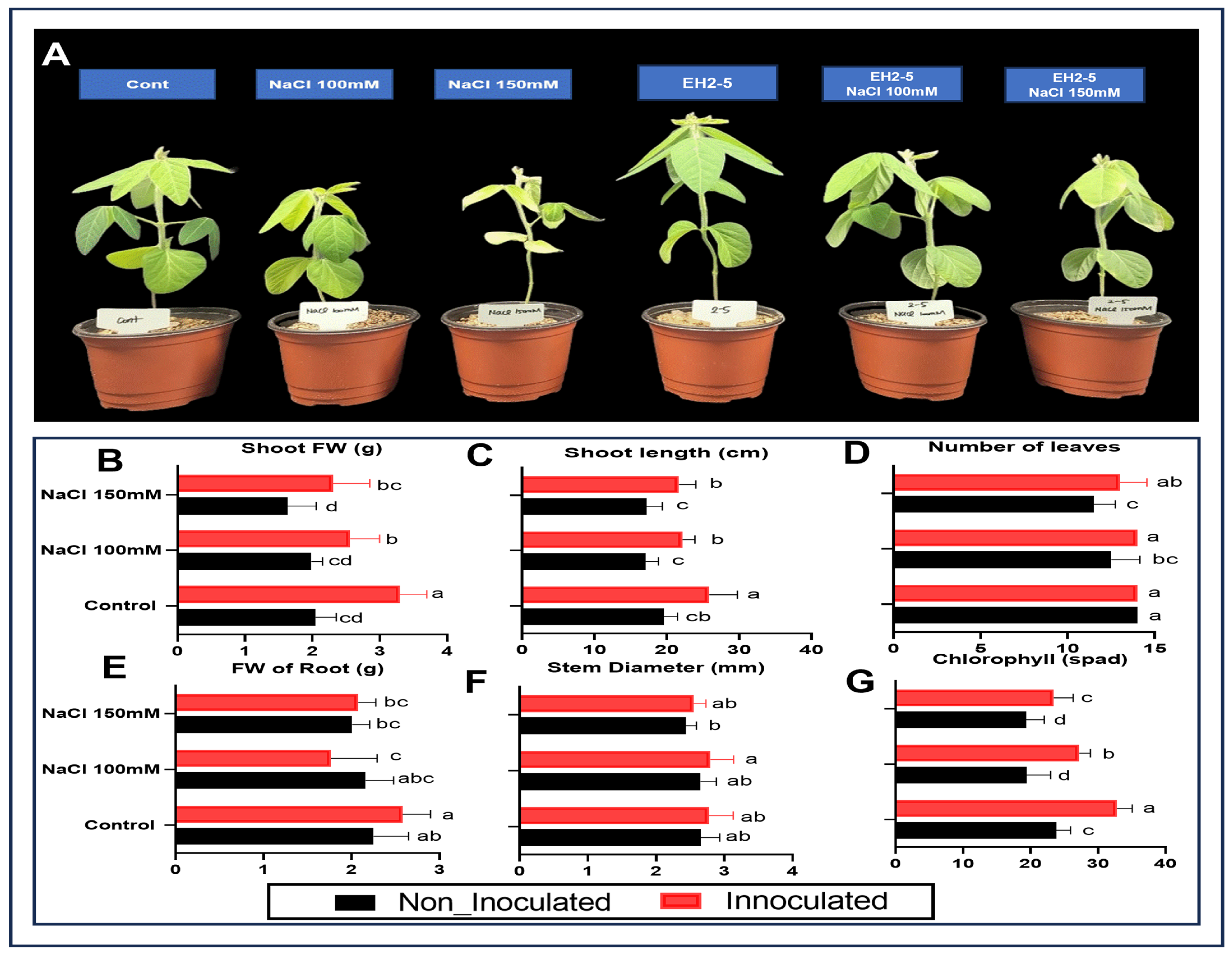
2.5. EH2-5 Improves Plant Growth and Development
2.6. EH2-5 Mitigates Salt Stress via Modulating of ROS with Antioxidants
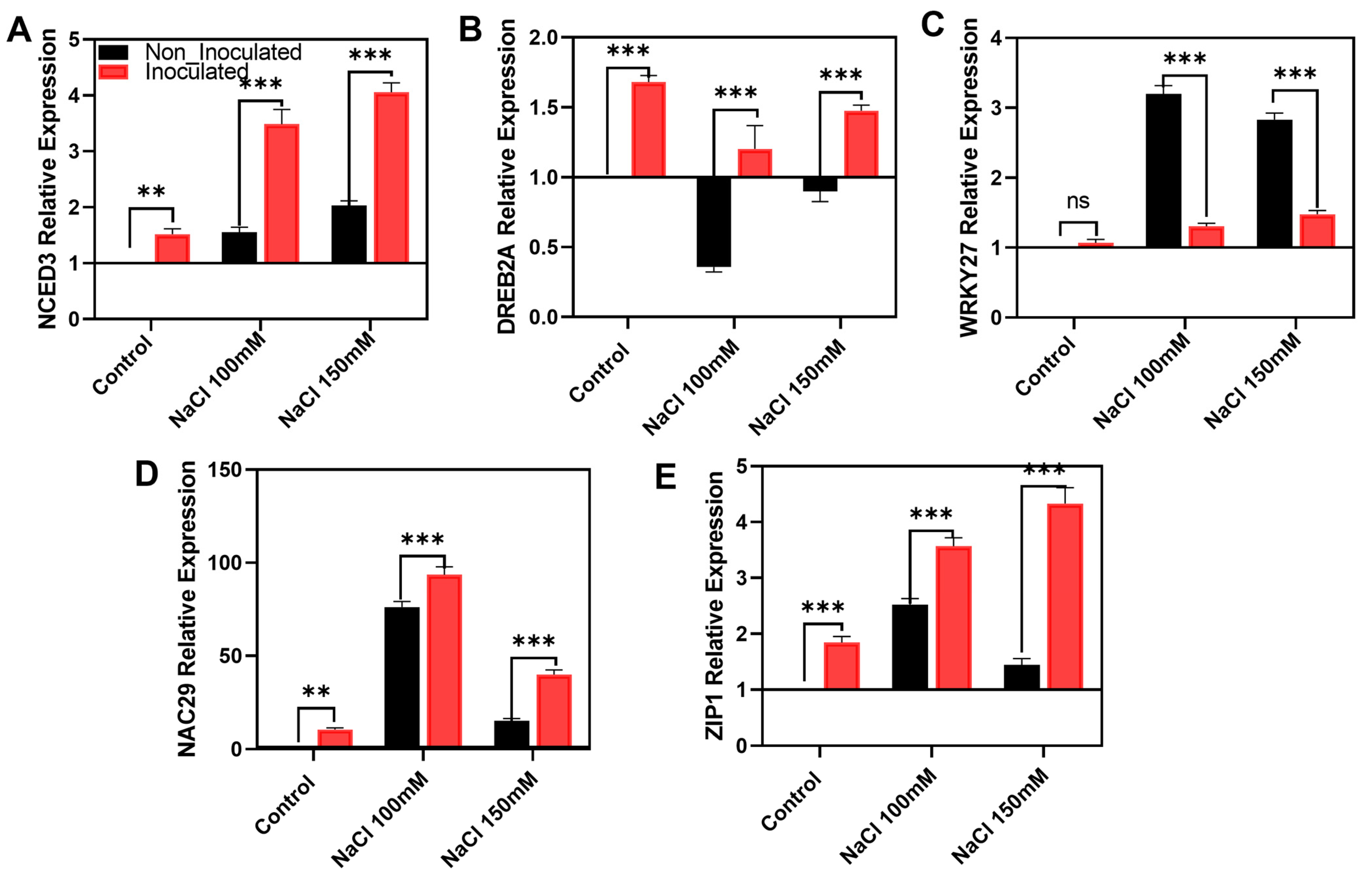
2.7. Expression of Salinity-Responsive Genes During the Bacillus aerius EH2-5 Application
3. Discussion
3.1. WGS of EH2-5
3.2. EH2-5′S Melatonin Producing Ability and Its Potential Ability as Biofertilizer
3.3. Effects of Melatonin-Producing Microbes on Plants: Antioxidants and Salinity-Related Gene Expression
3.4. Prospects and Challenges of Using Engineered Melatonin-Producing Microbiomes in Agriculture
4. Methods
4.1. Isolation of Plant Growth-Promoting Bacteria
4.2. Whole Genome Sequencing of EH2-5
4.3. Quantification of Organic Acids, Free Amino Acids in EH2-5 Cultures
4.4. Quantification of Melatonin in EH2-5 Cultures
4.5. Plant Materials and Experiments
4.5.1. NaCl and EH2-5 Treatment to Soybean Plants
4.5.2. Analysis of Antioxidant Enzymes
4.5.3. Quantitative Real-Time PCR
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
References
- Akter, S.E.; Reza, M.S.; Adhikary, S.; Alam, M.M.; Chowdhury, S.M.A.; Mondal, M.M.A. Effect of Salinity Stress on Plant Morphology and Yield Attributes of Soybean Genotypes. ISSRA J. Agric. Life Sci. 2024, 3, 7–12. [Google Scholar]
- Feng, C.; Gao, H.; Zhou, Y.; Jing, Y.; Li, S.; Yan, Z.; Xu, K.; Zhou, F.; Zhang, W.; Yang, X. Unfolding molecular switches for salt stress resilience in soybean: Recent advances and prospects for salt-tolerant smart plant production. Front. Plant Sci. 2023, 14, 1162014. [Google Scholar] [CrossRef]
- Balasubramaniam, T.; Shen, G.; Esmaeili, N.; Zhang, H. Plants’ response mechanisms to salinity stress. Plants 2023, 12, 2253. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Sun, B.; Xu, X.; Chen, H.; Zou, L.; Chen, G.; Cao, B.; Chen, C.; Lei, J. Overexpression of AtEDT1/HDG11 in Chinese kale (Brassica oleracea var. alboglabra) enhances drought and osmotic stress tolerance. Front. Plant Sci. 2016, 7, 1285. [Google Scholar]
- Xiao, F.; Zhou, H. Plant salt response: Perception, signaling, and tolerance. Front. Plant Sci. 2023, 13, 1053699. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; De Smet, I. Localised ABA signalling mediates root growth plasticity. Trends Plant Sci. 2013, 18, 533–535. [Google Scholar] [CrossRef]
- Smythers, A.L.; Bhatnagar, N.; Ha, C.; Majumdar, P.; McConnell, E.W.; Mohanasundaram, B.; Hicks, L.M.; Pandey, S. Abscisic acid-controlled redox proteome of Arabidopsis and its regulation by heterotrimeric Gβ protein. New Phytol. 2022, 236, 447–463. [Google Scholar] [CrossRef]
- Lerner, A.B.; Case, J.D.; Takahashi, Y. Isolation of melatonin and 5-methoxyindole-3-acetic acid from bovine pineal glands. J. Biol. Chem. 1960, 235, 1992–1997. [Google Scholar] [CrossRef]
- Afzal, A. Melatonin as a multifunctional modulator: Emerging insights into its role in health, reproductive efficiency, and productive performance in livestock. Front. Physiol. 2024, 15, 1501334. [Google Scholar] [CrossRef]
- Arnao, M.B.; Hernández-Ruiz, J. The physiological function of melatonin in plants. Plant Signal. Behav. 2006, 1, 89–95. [Google Scholar] [CrossRef]
- Salehi, B.; Sharopov, F.; Fokou, P.V.T.; Kobylinska, A.; Jonge, L.d.; Tadio, K.; Sharifi-Rad, J.; Posmyk, M.M.; Martorell, M.; Martins, N. Melatonin in medicinal and food plants: Occurrence, bioavailability, and health potential for humans. Cells 2019, 8, 681. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Li, Q.-T.; Chu, Y.-N.; Reiter, R.J.; Yu, X.-M.; Zhu, D.-H.; Zhang, W.-K.; Ma, B.; Lin, Q.; Zhang, J.-S. Melatonin enhances plant growth and abiotic stress tolerance in soybean plants. J. Exp. Bot. 2015, 66, 695–707. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.Y.; Byeon, Y.; Back, K. Melatonin as a signal molecule triggering defense responses against pathogen attack in Arabidopsis and tobacco. J. Pineal Res. 2014, 57, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Thakur, N.; Mann, N.A.; Umar, A. Melatonin as plant growth regulator in sustainable agriculture. Sci. Hortic. 2024, 323, 112421. [Google Scholar] [CrossRef]
- Ahmad, I.; Song, X.; Hussein Ibrahim, M.E.; Jamal, Y.; Younas, M.U.; Zhu, G.; Zhou, G.; Adam Ali, A.Y. The role of melatonin in plant growth and metabolism, and its interplay with nitric oxide and auxin in plants under different types of abiotic stress. Front. Plant Sci. 2023, 14, 1108507. [Google Scholar] [CrossRef]
- Yan, F.; Zhao, H.; Wu, L.; Huang, Z.; Niu, Y.; Qi, B.; Zhang, L.; Fan, S.; Ding, Y.; Li, G. Basic cognition of melatonin regulation of plant growth under salt stress: A meta-analysis. Antioxidants 2022, 11, 1610. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, L.; Li, H.; Zhang, S.; Fu, X.; Zhai, X.; Yang, N.; Shen, J.; Li, R.; Li, D. Exogenous melatonin promotes the salt tolerance by removing active oxygen and maintaining ion balance in wheat (Triticum aestivum L.). Front. Plant Sci. 2022, 12, 787062. [Google Scholar] [CrossRef]
- Tahjib-Ul-Arif, M.; Zahan, I.; Hossain, M.S.; Imran, S.; Hasanuzzaman, M.; Dawood, M.F.; Dawood, A.F.; Asaduzzaman, M.; Rhaman, M.S.; Souri, Z. Melatonin-mediated ionic homeostasis in plants: Mitigating nutrient deficiency and salinity stress. Discov. Plants 2025, 2, 143. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Gaurav, A.K.; Srivastava, S.; Verma, J.P. Plant growth-promoting bacteria: Biological tools for the mitigation of salinity stress in plants. Front. Microbiol. 2020, 11, 1216. [Google Scholar] [CrossRef]
- Tsotetsi, T.; Nephali, L.; Malebe, M.; Tugizimana, F. Bacillus for plant growth promotion and stress resilience: What have we learned? Plants 2022, 11, 2482. [Google Scholar] [CrossRef]
- Petkova, M.; Marcheva, M.; Petrova, A.-L.; Slavova, V.; Shilev, S. Plant Growth-Promoting and Biocontrol Characteristics of Four Bacillus Strains and Evaluation of Their Effects on Wheat (Tr. aestivum L.). Int. J. Plant Biol. 2024, 16, 1. [Google Scholar] [CrossRef]
- Lee, E.Y.; Hong, S.H. Plant growth-promoting ability by the newly isolated bacterium Bacillus aerius MH1RS1 from indigenous plant in sand dune. J. Korean Soc. Environ. Eng. 2013, 35, 687–693. [Google Scholar] [CrossRef]
- Chowhan, L.B.; Mir, M.I.; Sabra, M.A.; El-Habbab, A.A.; Kumar, B.K. Plant growth promoting and antagonistic traits of bacteria isolated from forest soil samples. Iran. J. Microbiol. 2023, 15, 278. [Google Scholar] [CrossRef] [PubMed]
- Reyad, A.M.; Radwan, T.E.; Hemida, K.A.; Abo Al-Qassem, N.; Ali, R. Salt tolerant endophytic bacteria from Carthamus tinctorius and their role in plant salt tolerance improvement. Inter. J. Curr. Sci. Res. 2017, 3, 1467–1488. [Google Scholar]
- Kwon, E.H.; Adhikari, A.; Khan, A.L.; Do, E.; Methela, N.J.; Lee, C.Y.; Kang, S.M.; Ku, K.M.; Yun, B.W.; Lee, I.J. Microbial Melatonin Production Improves Plant Metabolic Function in Short-Term Climate-Induced Stresses. J. Pineal Res. 2025, 77, e70052. [Google Scholar] [CrossRef] [PubMed]
- Kwon, E.H.; Adhikari, A.; Imran, M.; Hussain, A.; Gam, H.J.; Woo, J.I.; Jeon, J.R.; Lee, D.S.; Lee, C.Y.; Lay, L. Novel melatonin-producing Bacillus safensis EH143 mitigates salt and cadmium stress in soybean. J. Pineal Res. 2024, 76, e12957. [Google Scholar] [CrossRef]
- Rasheed, A.; Raza, A.; Jie, H.; Mahmood, A.; Ma, Y.; Zhao, L.; Xing, H.; Li, L.; Hassan, M.U.; Qari, S.H. Molecular tools and their applications in developing salt-tolerant soybean (Glycine max L.) cultivars. Bioengineering 2022, 9, 495. [Google Scholar] [CrossRef]
- Wang, C.; Li, Y.; Li, M.; Zhang, K.; Ma, W.; Zheng, L.; Xu, H.; Cui, B.; Liu, R.; Yang, Y. Functional assembly of root-associated microbial consortia improves nutrient efficiency and yield in soybean. J. Integr. Plant Biol. 2021, 63, 1021–1035. [Google Scholar] [CrossRef]
- Ren, J.; Ye, J.; Yin, L.; Li, G.; Deng, X.; Wang, S. Exogenous melatonin improves salt tolerance by mitigating osmotic, ion, and oxidative stresses in maize seedlings. Agronomy 2020, 10, 663. [Google Scholar] [CrossRef]
- Zeng, W.; Mostafa, S.; Lu, Z.; Jin, B. Melatonin-mediated abiotic stress tolerance in plants. Front. Plant Sci. 2022, 13, 847175. [Google Scholar] [CrossRef]
- Susin, M.F.; Baldini, R.L.; Gueiros-Filho, F.; Gomes, S.L. GroES/GroEL and DnaK/DnaJ have distinct roles in stress responses and during cell cycle progression in Caulobacter crescentus. J. Bacteriol. 2006, 188, 8044–8053. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.P.; Bron, S.; Venema, G.; Maarten van Dijl, J. Chaperone-like activities of the CsaA protein of Bacillus subtilis. Microbiology 2000, 146, 77–88. [Google Scholar] [CrossRef] [PubMed]
- Petersohn, A.; Brigulla, M.; Haas, S.; Hoheisel, J.r.D.; Völker, U.; Hecker, M. Global analysis of the general stress response of Bacillus subtilis. J. Bacteriol. 2001, 183, 5617–5631. [Google Scholar] [CrossRef] [PubMed]
- Maliandi, M.V.; Busi, M.V.; Turowski, V.R.; Leaden, L.; Araya, A.; Gomez-Casati, D.F. The mitochondrial protein frataxin is essential for heme biosynthesis in plants. FEBS J. 2011, 278, 470–481. [Google Scholar] [CrossRef]
- Ilangumaran, G.; Schwinghamer, T.D.; Smith, D.L. Rhizobacteria from root nodules of an indigenous legume enhance salinity stress tolerance in soybean. Front. Sustain. Food Syst. 2021, 4, 617978. [Google Scholar] [CrossRef]
- Lin, T.; Haider, F.U.; Liu, T.; Li, S.; Zhang, P.; Zhao, C.; Li, X. Salt Tolerance Induced by Plant Growth-Promoting Rhizobacteria Is Associated with Modulations of the Photosynthetic Characteristics, Antioxidant System, and Rhizosphere Microbial Diversity in Soybean (Glycine max (L.) Merr.). Agronomy 2025, 15, 341. [Google Scholar] [CrossRef]
- Morcillo-Parra, M.Á.; Beltran, G.; Mas, A.; Torija, M.-J. Effect of several nutrients and environmental conditions on intracellular melatonin synthesis in Saccharomyces cerevisiae. Microorganisms 2020, 8, 853. [Google Scholar] [CrossRef]
- Tan, D.-X.; Manchester, L.C.; Esteban-Zubero, E.; Zhou, Z.; Reiter, R.J. Melatonin as a potent and inducible endogenous antioxidant: Synthesis and metabolism. Molecules 2015, 20, 18886–18906. [Google Scholar] [CrossRef]
- Arnao, M.B.; Giraldo-Acosta, M.; Castejón-Castillejo, A.; Losada-Lorán, M.; Sánchez-Herrerías, P.; El Mihyaoui, A.; Cano, A.; Hernández-Ruiz, J. Melatonin from microorganisms, algae, and plants as possible alternatives to synthetic melatonin. Metabolites 2023, 13, 72. [Google Scholar] [CrossRef]
- Wong, R.K.; Yang, C.; Song, G.-H.; Wong, J.; Ho, K.-Y. Melatonin regulation as a possible mechanism for probiotic (VSL# 3) in irritable bowel syndrome: A randomized double-blinded placebo study. Dig. Dis. Sci. 2015, 60, 186–194. [Google Scholar]
- Jofre, M.F.; Mammana, S.B.; Appiolaza, M.L.; Silva, M.F.; Gomez, F.J.V.; Cohen, A.C. Melatonin production by rhizobacteria native strains: Towards sustainable plant growth promotion strategies. Physiol. Plant. 2023, 175, e13852. [Google Scholar] [CrossRef]
- Lu, X.; Min, W.; Shi, Y.; Tian, L.; Li, P.; Ma, T.; Zhang, Y.; Luo, C. Exogenous melatonin alleviates alkaline stress by removing reactive oxygen species and promoting antioxidant defence in rice seedlings. Front. Plant Sci. 2022, 13, 849553. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Y.-E.; Zhao, Y.-Q.; Ding, C.-B.; Liao, J.-Q.; Hu, C.; Zhou, L.-J.; Zhang, Z.-W.; Yuan, S.; Yuan, M. Exogenous melatonin alleviates oxidative damages and protects photosystem II in maize seedlings under drought stress. Front. Plant Sci. 2019, 10, 677. [Google Scholar] [CrossRef] [PubMed]
- Puértolas, J.; Castro-Valdecantos, P.; Chen, M.; Zhang, J.; Dodd, I.C. Drought-Induced Abscisic Acid Accumulation in Soybean Roots Depends on NCED Gene Expression More Than Shoot-to-Root ABA Transport. Plant Cell Environ. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Zhang, J.; Fan, C.; Liu, B.; Kong, F.; Li, H. Molecular Regulatory Network of Soybean Responses to Abiotic Stress. Plant Cell Environ. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Wang, Q.-Y.; Cheng, X.-G.; Xu, Z.-S.; Li, L.-C.; Ye, X.-G.; Xia, L.-Q.; Ma, Y.-Z. GmDREB2, a soybean DRE-binding transcription factor, conferred drought and high-salt tolerance in transgenic plants. Biochem. Biophys. Res. Commun. 2007, 353, 299–305. [Google Scholar] [CrossRef]
- Wang, F.; Chen, H.W.; Li, Q.T.; Wei, W.; Li, W.; Zhang, W.K.; Ma, B.; Bi, Y.D.; Lai, Y.C.; Liu, X.L. Gm WRKY 27 interacts with Gm MYB 174 to reduce expression of Gm NAC 29 for stress tolerance in soybean plants. Plant J. 2015, 83, 224–236. [Google Scholar] [CrossRef]
- Yaghoubian, I.; Modarres-Sanavy, S.A.M.; Smith, D.L. Plant growth promoting microorganisms (PGPM) as an eco-friendly option to mitigate water deficit in soybean (Glycine max L.): Growth, physio-biochemical properties and oil content. Plant Physiol. Biochem. 2022, 191, 55–66. [Google Scholar] [CrossRef]
- Adhikari, A.; Kwon, E.-H.; Khan, M.A.; Shaffique, S.; Kang, S.-M.; Lee, I.-J. Enhanced use of chemical fertilizers and mitigation of heavy metal toxicity using biochar and the soil fungus Bipolaris maydis AF7 in rice: Genomic and metabolomic perspectives. Ecotoxicol. Environ. Saf. 2024, 271, 115938. [Google Scholar] [CrossRef]
- Kazerooni, E.A.; Maharachchikumbura, S.S.; Adhikari, A.; Al-Sadi, A.M.; Kang, S.-M.; Kim, L.-R.; Lee, I.-J. Rhizospheric Bacillus amyloliquefaciens protects Capsicum annuum cv. Geumsugangsan from multiple abiotic stresses via multifarious plant growth-promoting attributes. Front. Plant Sci. 2021, 12, 669693. [Google Scholar] [CrossRef]
- Tariq, A.; Guo, S.; Farhat, F.; Shen, X. Engineering synthetic microbial communities: Diversity and applications in soil for plant resilience. Agronomy 2025, 15, 513. [Google Scholar] [CrossRef]
- Kang, S.-M.; Shahzad, R.; Bilal, S.; Khan, A.L.; Park, Y.-G.; Lee, K.-E.; Asaf, S.; Khan, M.A.; Lee, I.-J. Indole-3-acetic-acid and ACC deaminase producing Leclercia adecarboxylata MO1 improves Solanum lycopersicum L. growth and salinity stress tolerance by endogenous secondary metabolites regulation. BMC Microbiol. 2019, 19, 80. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants: Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Khan, M.A.; Sahile, A.A.; Jan, R.; Asaf, S.; Hamayun, M.; Imran, M.; Adhikari, A.; Kang, S.-M.; Kim, K.-M.; Lee, I.-J. Halotolerant bacteria mitigate the effects of salinity stress on soybean growth by regulating secondary metabolites and molecular responses. BMC Plant Biol. 2021, 21, 176. [Google Scholar] [CrossRef]
- Halo, B.A.; Khan, A.L.; Waqas, M.; Al-Harrasi, A.; Hussain, J.; Ali, L.; Adnan, M.; Lee, I.-J. Endophytic bacteria (Sphingomonas sp. LK11) and gibberellin can improve Solanum lycopersicum growth and oxidative stress under salinity. J. Plant Interact. 2015, 10, 117–125. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef]
- Zhang, J.; Kirkham, M. Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol. 1994, 35, 785–791. [Google Scholar] [CrossRef]



| PGP Trait-Related Genes | ||
|---|---|---|
| PGP Iron Genes | ||
| Locus tag | Gene | Product |
| AAHJMGKC_03269 | yfhA_1 | Putative siderophore transport system permease protein YfhA |
| AAHJMGKC_03698 | yfiY_1 | Putative siderophore-binding lipoprotein YfiY |
| AAHJMGKC_03792 | yusV_1 | Putative siderophore transport system ATP-binding protein YusV |
| AAHJMGKC_03835 | yusV_2 | Putative siderophore transport system ATP-binding protein YusV |
| AAHJMGKC_03868 | yfiY_2 | Putative siderophore-binding lipoprotein YfiY |
| AAHJMGKC_03869 | yfiZ | Putative siderophore transport system permease protein YfiZ |
| AAHJMGKC_03870 | yfhA_2 | Putative siderophore transport system permease protein YfhA |
| PGP Zinc Genes | ||
| Locus tag | Gene | Product |
| AAHJMGKC_00039 | znuA | High-affinity zinc uptake system binding-protein ZnuA |
| AAHJMGKC_00646 | znuC | High-affinity zinc uptake system ATP-binding protein ZnuC |
| AAHJMGKC_00647 | znuB | High-affinity zinc uptake system membrane protein ZnuB |
| AAHJMGKC_00648 | zur | Zinc-specific metallo-regulatory protein |
| AAHJMGKC_01314 | putative zinc protease | |
| AAHJMGKC_01620 | zosA | Zinc-transporting ATPase |
| AAHJMGKC_02153 | Zinc-type alcohol dehydrogenase-like protein | |
| AAHJMGKC_02961 | ftsH | ATP-dependent zinc metalloprotease FtsH |
| AAHJMGKC_03775 | cadA | Cadmium, zinc and cobalt-transporting ATPase |
| AAHJMGKC_03856 | sufU | Zinc-dependent sulfurtransferase SufU |
| PGP Nitrogen Genes | ||
| Locus tag | Gene | Product |
| AAMGKC_02753 | ptsN | Nitrogen regulatory protein |
| AAHJMGKC_03243 | aspA_1 | Aspartate ammonia-lyase |
| AAHJMGKC_03494 | nrgB | Nitrogen regulatory PII-like protein |
| AAHJMGKC_037 | aspA_2 | Aspartate ammonia-lyase |
| Tryptophan-related Genes | ||
| PGP Tryptophan Genes | ||
| Locus tag | Gene | Product |
| AAHJMGKC_00025 | tspO | Tryptophan-rich protein TspO |
| AAHJMGKC_00896 | trpB | Tryptophan synthase beta chain |
| AAHJMGKC_00897 | trpA | Tryptophan synthase alpha chain |
| AAHJMGKC_01846 | trpS | Tryptophan--tRNA ligase |
| AAHJMGKC_01971 | trpP | Putative tryptophan transport protein |
| Salinity Stress-related Genes | ||
| Locus tag | Gene | Product |
| AAHJMGKC_00045 | dps | General stress protein 20U |
| AAHJMGKC_00050 | yceD_1 | General stress protein 16U |
| AAHJMGKC_00606 | hemW | Heme chaperone HemW |
| AAHJMGKC_00609 | dnaK | Chaperone protein DnaK |
| AAHJMGKC_00610 | dnaJ | Chaperone protein DnaJ |
| AAHJMGKC_00962 | cspD | Cold shock protein CspD |
| AAHJMGKC_01038 | yocK | General stress protein 16O |
| AAHJMGKC_01058 | csaA | Putative chaperone CsaA |
| AAHJMGKC_01232 | fra | Intracellular iron chaperone frataxin |
| AAHJMGKC_01663 | ykoL | Stress response protein Yk |
| AAHJMGKC_01943 | ydaD_1 | General stress protein 39 |
| AAHJMGKC_01990 | yhaX | Stress response protein YhaX |
| AAHJMGKC_02003 | nhaX | Stress response protein NhaX |
| AAHJMGKC_02053 | cspB | Cold shock protein CspB |
| AAHJMGKC_02185 | yvgO | Stress response protein YvgO |
| AAHJMGKC_02205 | yfkM | General stress protein 18 |
| AAHJMGKC_02209 | treA | Trehalose-6-phosphate hydrolase |
| AAHJMGKC_02210 | treP | PTS system trehalose-specific EIIBC component |
| AAHJMGKC_02241 | yflT_1 | General stress protein 17M |
| AAHJMGKC_02254 | yocM | Salt stress-responsive protein YocM |
| AAHJMGKC_02294 | srkA | Stress response kinase A |
| AAHJMGKC_02498 | cspC | Cold shock protein CspC |
| AAHJMGKC_02569 | ydaG | General stress protein 26 |
| AAHJMGKC_02570 | ydaD_2 | General stress protein 39 |
| AAHJMGKC_02706 | yceD_2 | General stress protein 16U |
| AAHJMGKC_02707 | yceD_3 | General stress protein 16U |
| AAHJMGKC_02708 | yceC | Stress response protein SCP2 |
| AAHJMGKC_02794 | surA | Chaperone SurA |
| AAHJMGKC_02980 | ctc | General stress protein CTC |
| AAHJMGKC_03344 | yflT_2 | General stress protein 17M |
| AAHJMGKC_03483 | yciC | Putative metal chaperone YciC |
| AAHJMGKC_03610 | fliS | Flagellar secretion chaperone FliS |
| AAHJMGKC_03763 | copZ | Copper chaperone CopZ |
| AAHJMGKC_03970 | yugI | General stress protein 13 |
| Unclassified_Genes | ||
| Locus tag | Gene | Product |
| AAHJMGKC_00171 | tpx | Thiol peroxidase |
| AAHJMGKC_00523 | dhA | Glutathione-independent formaldehyde dehydrogenase |
| AAHJMGKC_00657 | sodA | |
| Superoxide dismutase [Mn] | ||
| AAHJMGKC_00679 | gloC | Hydroxyacylglutathione hydrolase GloC |
| AAHJMGKC_00782 | gloB_1 | Hydroxyacylglutathione hydrolase |
| AAHJMGKC_01332 | proS_1 | Proline--tRNA ligase |
| AAHJMGKC_01728 | ggt | Glutathione hydrolase proenzyme |
| AAHJMGKC_01822 | kefB | Glutathione-regulated potassium-efflux system protein KefB |
| AAHJMGKC_01849 | gsiC | Glutathione transport system permease protein GsiC |
| AAHJMGKC_02115 | gsiD_1 | Glutathione transport system permease protein GsiD |
| AAHJMGKC_02292 | opuE | Osmoregulated proline transporter OpuE |
| AAHJMGKC_02485 | cpo | Non-heme chloroperoxidase |
| AAHJMGKC_02603 | kefG | Glutathione-regulated potassium-efflux system ancillary protein KefG |
| AAHJMGKC_02660 | putR_1 | Proline-responsive transcriptional activator PutR |
| AAHJMGKC_02662 | putB | Proline dehydrogenase 2 |
| AAHJMGKC_02778 | proS_2 | Proline--tRNA ligase |
| AAHJMGKC_02782 | gloB_2 | Hydroxyacylglutathione hydrolase |
| AAHJMGKC_03122 | gloB_3 | Hydroxyacylglutathione hydrolase |
| AAHJMGKC_03301 | gloA | Lactoylglutathione lyase |
| AAHJMGKC_03376 | Putative heme-dependent peroxidase | |
| AAHJMGKC_03717 | gsiD_2 | Glutathione transport system permease protein GsiD |
| AAHJMGKC_03915 | putR_2 | Proline-responsive transcriptional activator PutR |
| Treatment | Detail |
|---|---|
| Cont | Irrigated with sterile distilled water only |
| NaCl 100 mM | Irrigated with 100 mM NaCl solution |
| NaCl 150 mM | Irrigated with 150 mM NaCl solution |
| EH2-5 | Irrigated with EH2-5 broth culture |
| EH2-5 + NaCl 100 mM | Irrigated with 100 mM NaCl and EH2-5 broth culture |
| EH2-5 + NaCl 150 mM | Irrigated with 150 mM NaCl and EH2-5 broth culture |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, E.-H.; Ahmad, S.; Lee, I.-J. Melatonin-Producing Bacillus aerius EH2-5 Enhances Glycine max Plants Salinity Tolerance Through Physiological, Biochemical, and Molecular Modulation. Int. J. Mol. Sci. 2025, 26, 7834. https://doi.org/10.3390/ijms26167834
Kwon E-H, Ahmad S, Lee I-J. Melatonin-Producing Bacillus aerius EH2-5 Enhances Glycine max Plants Salinity Tolerance Through Physiological, Biochemical, and Molecular Modulation. International Journal of Molecular Sciences. 2025; 26(16):7834. https://doi.org/10.3390/ijms26167834
Chicago/Turabian StyleKwon, Eun-Hae, Suhaib Ahmad, and In-Jung Lee. 2025. "Melatonin-Producing Bacillus aerius EH2-5 Enhances Glycine max Plants Salinity Tolerance Through Physiological, Biochemical, and Molecular Modulation" International Journal of Molecular Sciences 26, no. 16: 7834. https://doi.org/10.3390/ijms26167834
APA StyleKwon, E.-H., Ahmad, S., & Lee, I.-J. (2025). Melatonin-Producing Bacillus aerius EH2-5 Enhances Glycine max Plants Salinity Tolerance Through Physiological, Biochemical, and Molecular Modulation. International Journal of Molecular Sciences, 26(16), 7834. https://doi.org/10.3390/ijms26167834







