Insights into Complex Compounds of Ampicillin: Potentiometric and Spectroscopic Studies
Abstract
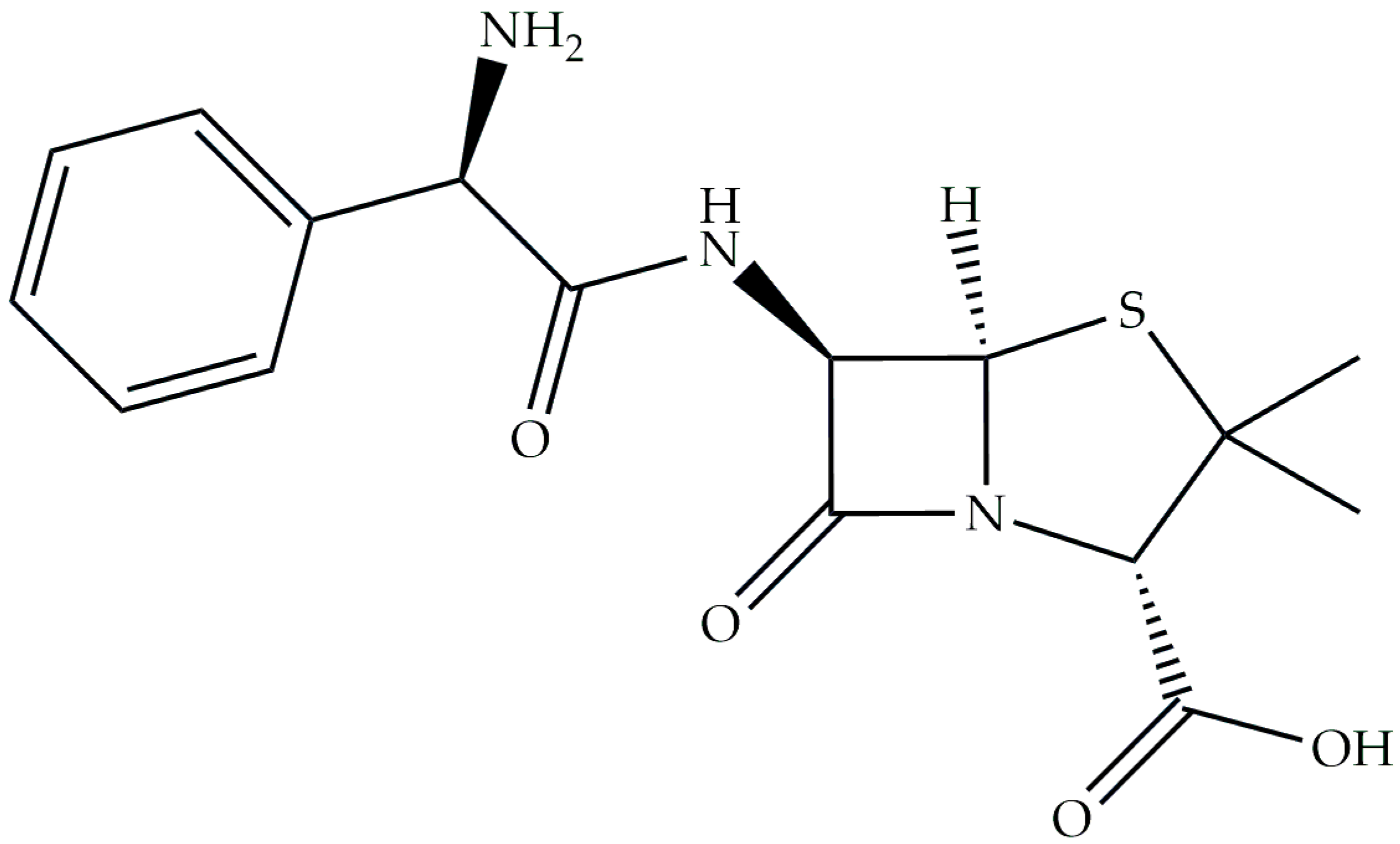
1. Introduction
2. Results and Discussion
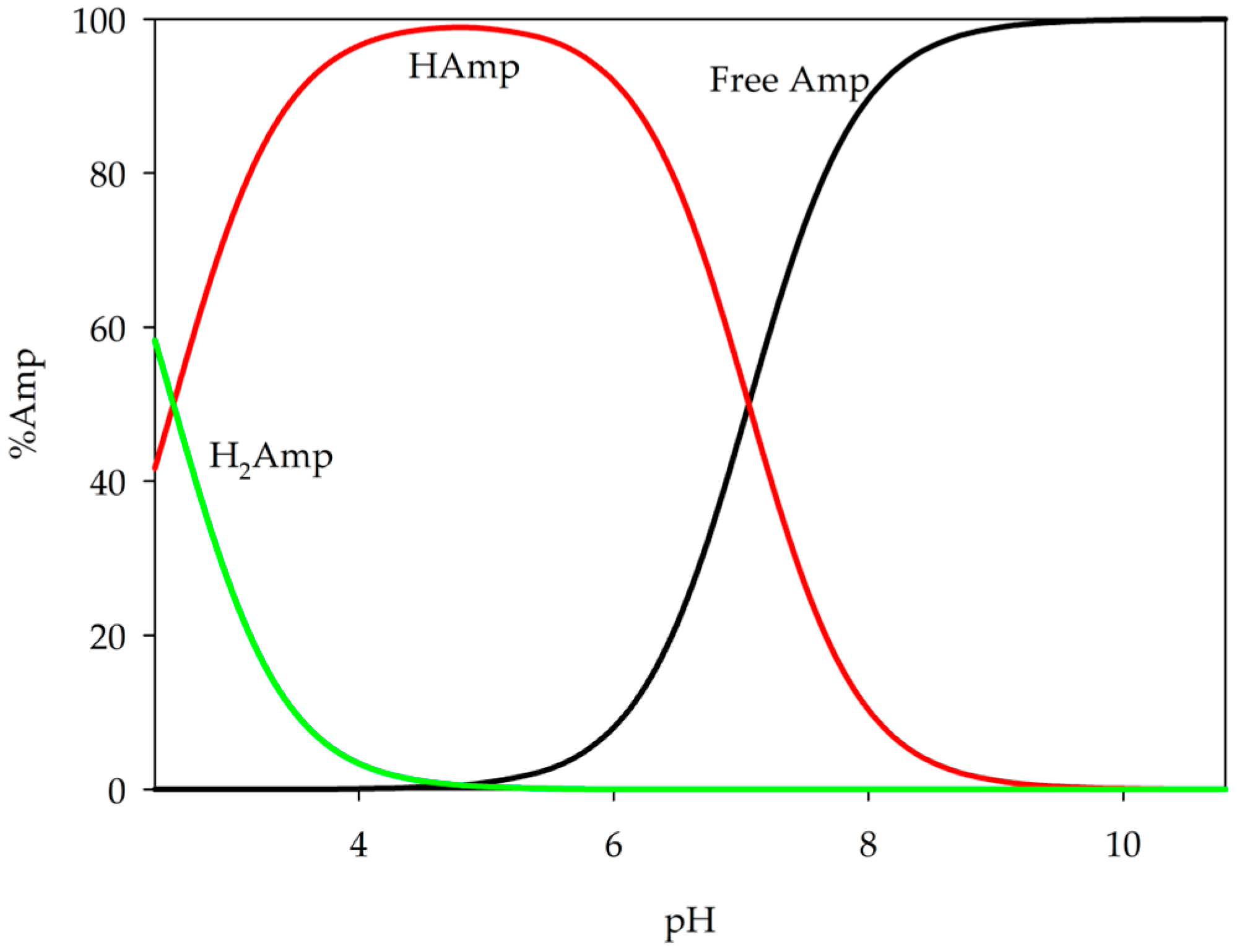
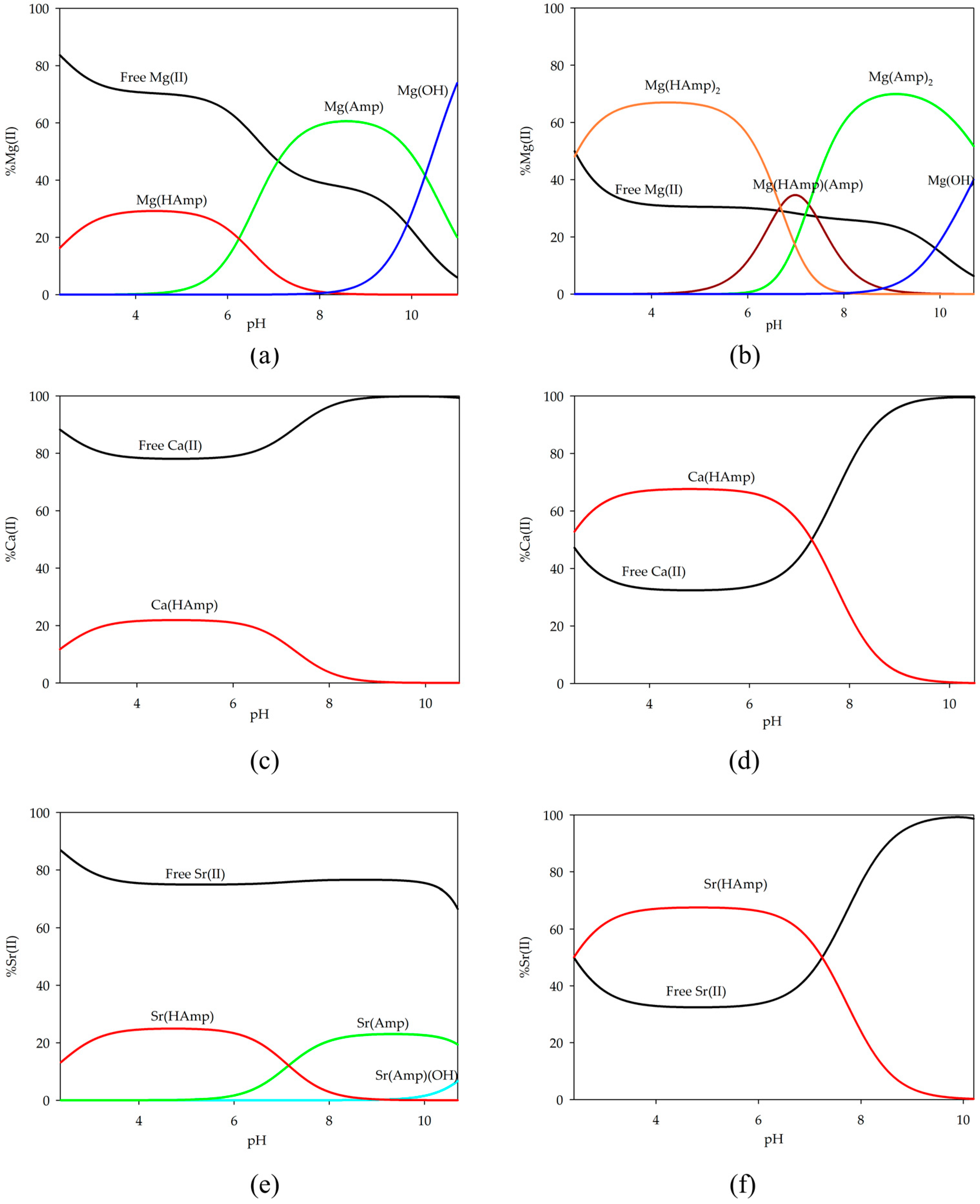
2.1. Equilibrium Study of S-Block Elements
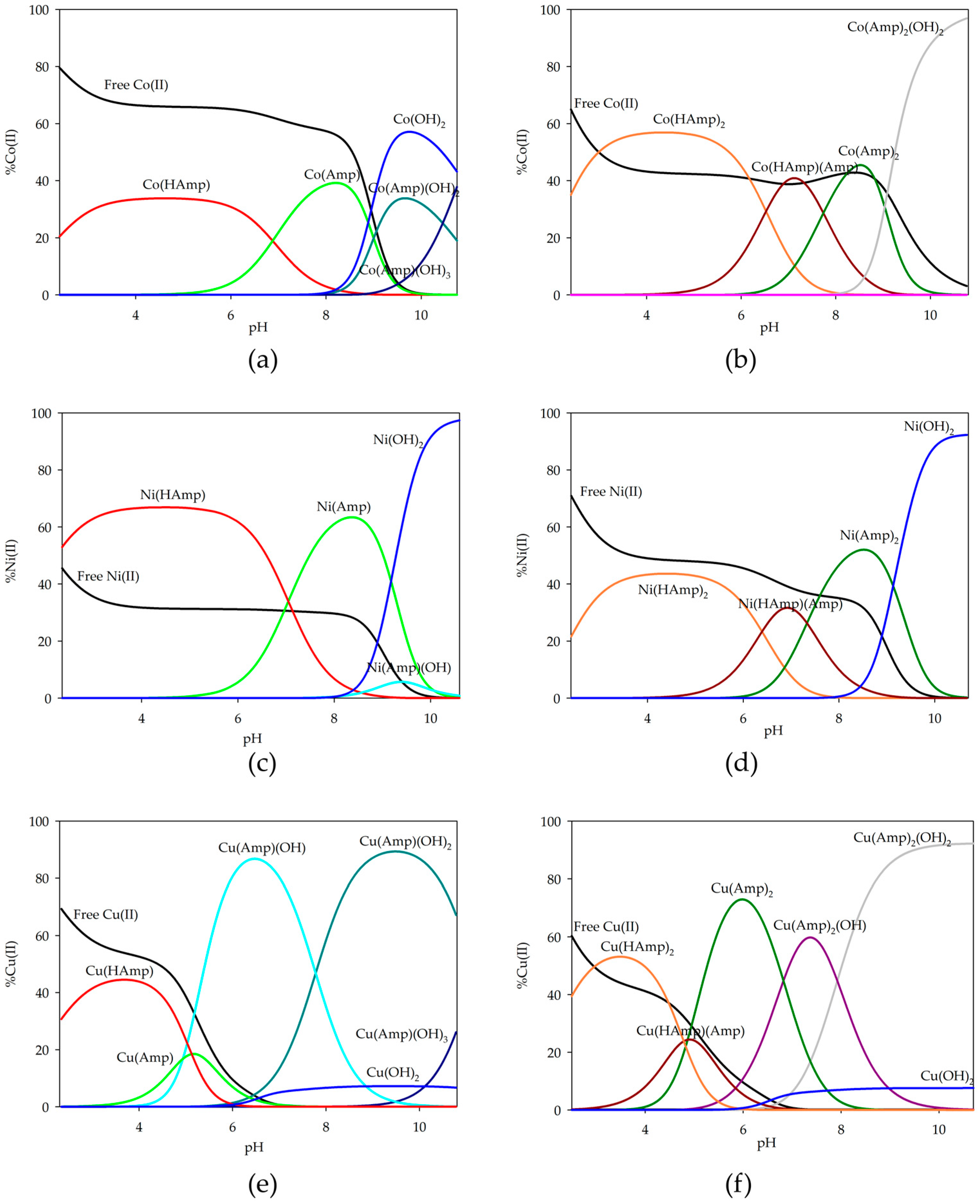
2.2. Equilibrium Study of D-Block Elements
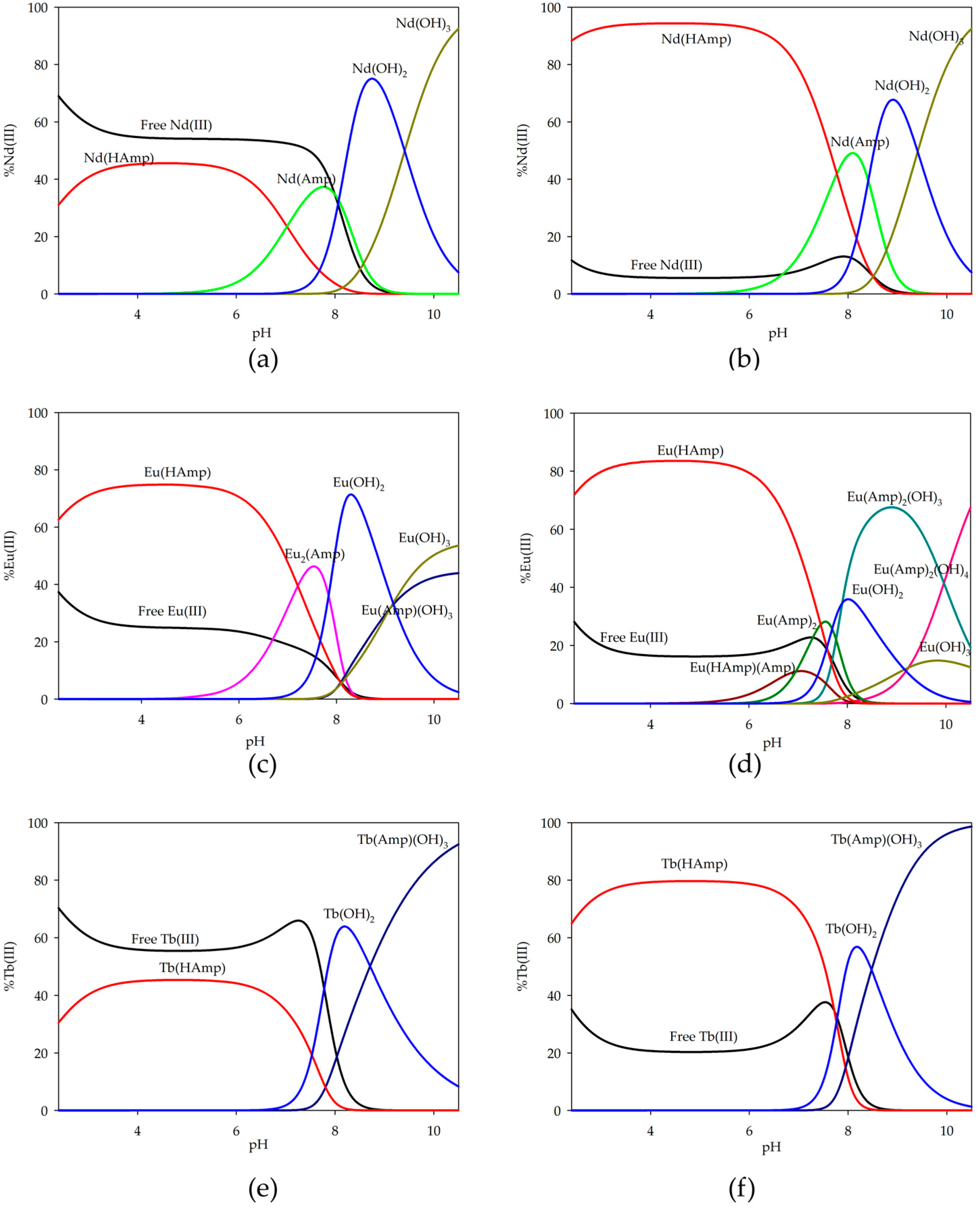
2.3. Equilibrium Study of F-Block Elements
2.4. UV–Vis Spectroscopy
2.5. EPR Spectroscopy
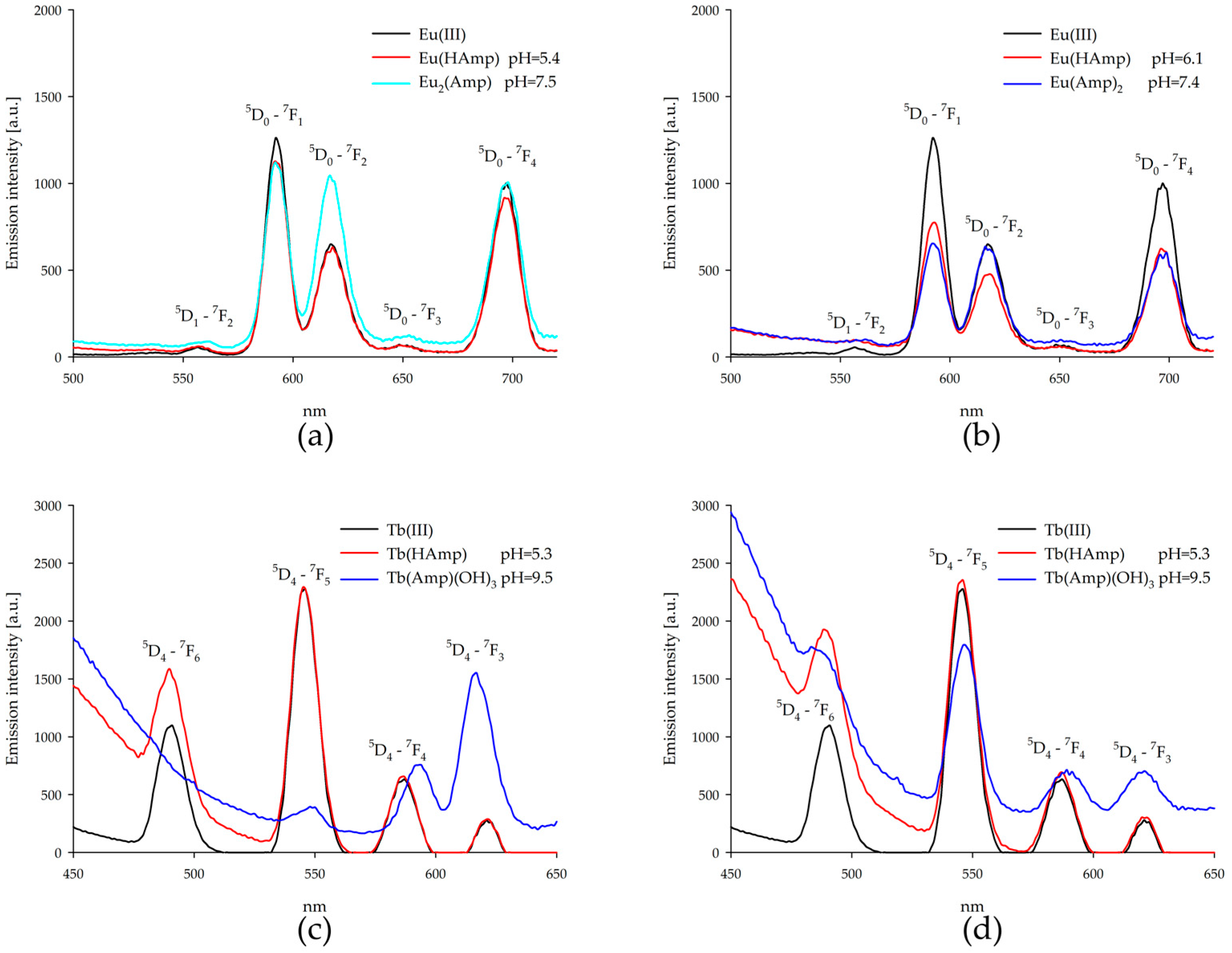
2.6. Luminescence Spectroscopy
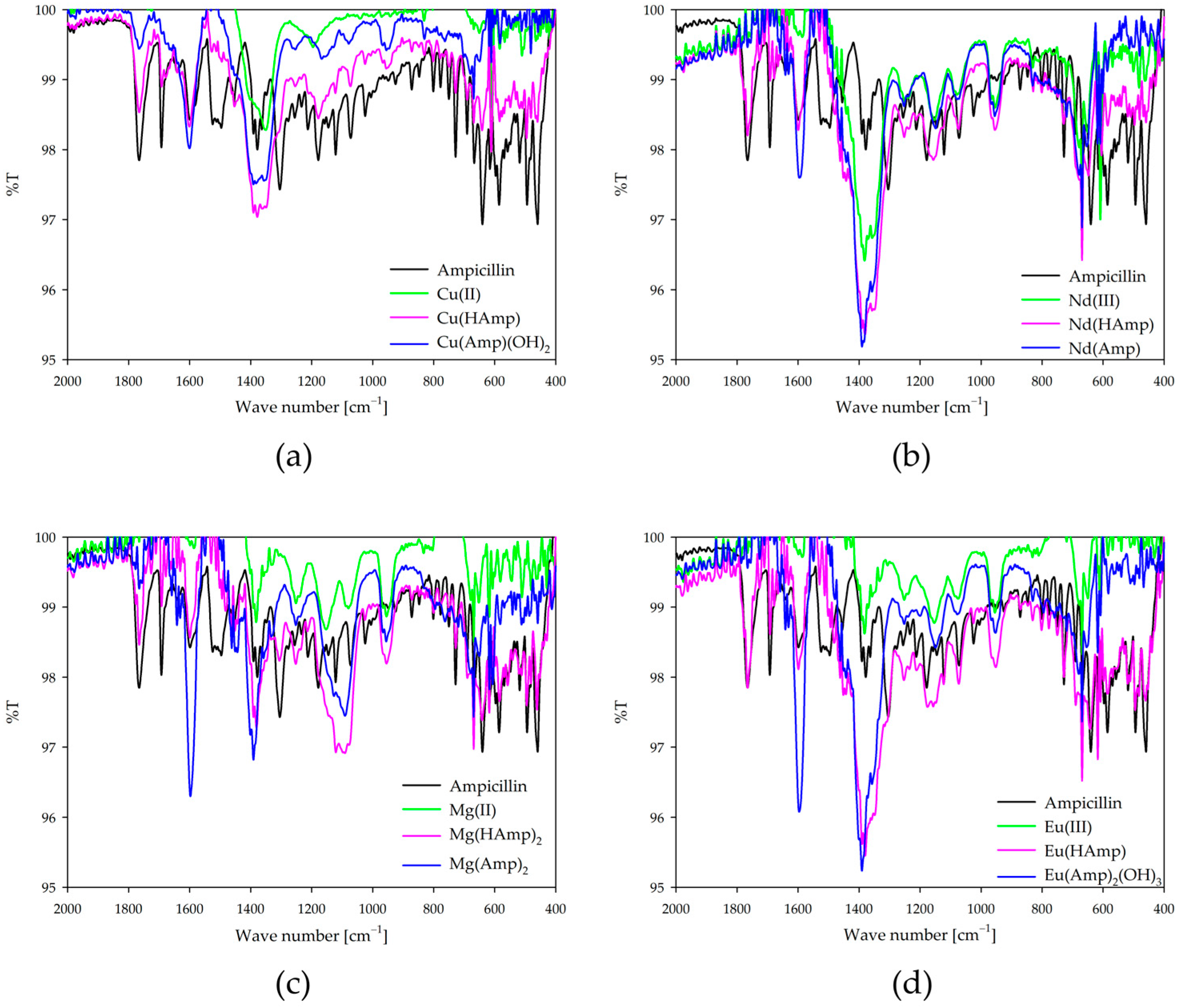
2.7. IR Spectroscopy
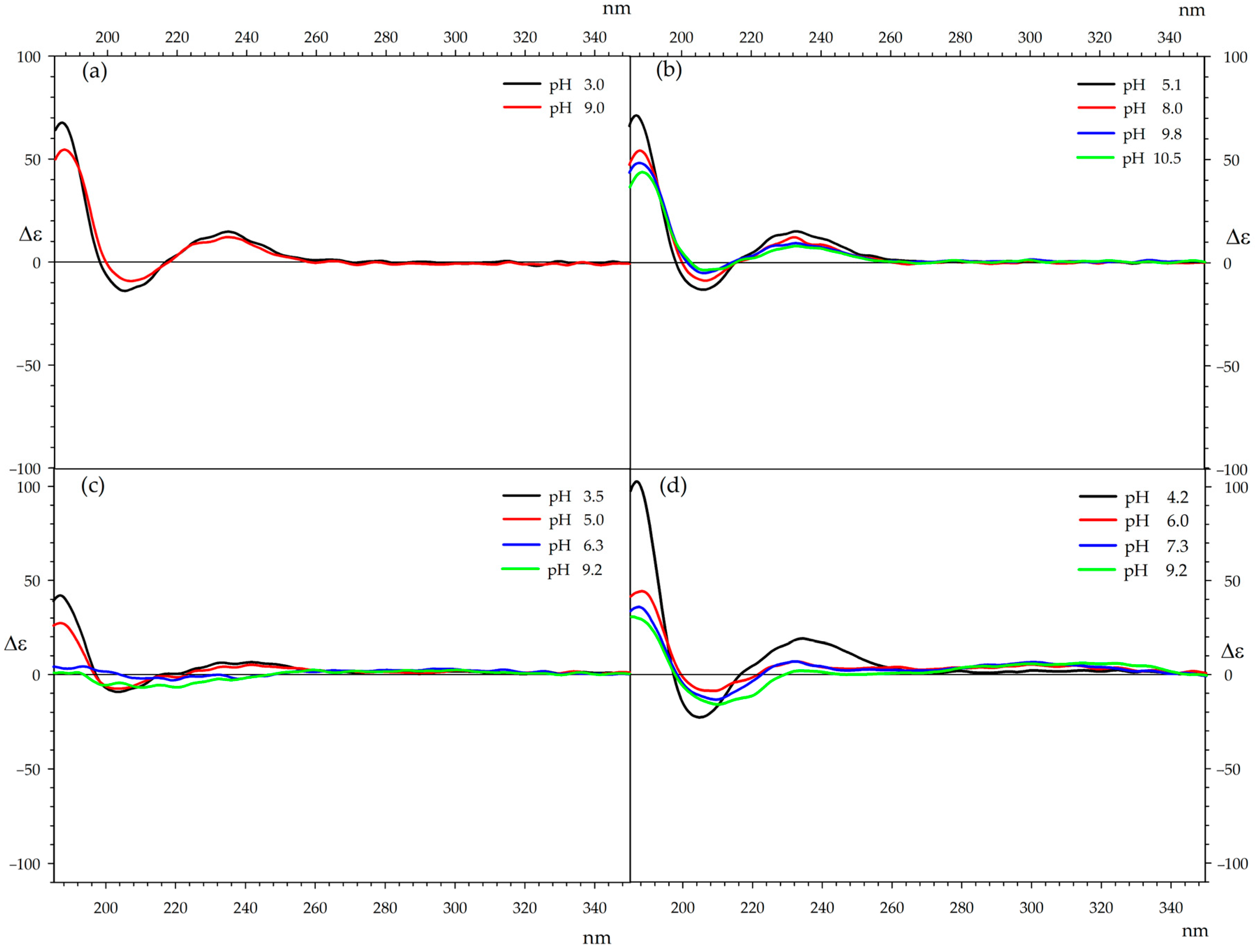
2.8. CD Spectroscopy
3. Materials and Methods
3.1. Materials
3.2. Equilibrium Studies
3.3. UV–Vis Spectroscopy
3.4. EPR Spectroscopy
3.5. Luminescence Spectroscopy
3.6. Infrared Spectroscopy (FT-IR)
3.7. CD Spectroscopy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Amp | Ampicillin |
References
- Lima, L.M.; Silva, B.N.M.D.; Barbosa, G.; Barreiro, E.J. β-Lactam antibiotics: An overview from a medicinal chemistry perspective. Eur. J. Med. Chem. 2020, 208, 112829. [Google Scholar] [CrossRef]
- Muteeb, G.; Rehman, M.T.; Shahwan, M.; Aatif, M. Origin of antibiotics and antibiotic resistance, and their impacts on drug development: A narrative review. Pharmaceuticals 2023, 16, 1615. [Google Scholar] [CrossRef]
- Fernandes, R.; Amador, P.; Prudêncio, C. β-Lactams: Chemical structure, mode of action and mechanisms of resistance. Rev. Med. Microbiol. 2013, 24, 7–17. [Google Scholar] [CrossRef]
- Bush, K. β-Lactam antibiotics. In Antibiotic and Chemotherapy, 9th ed.; Finch, R.G., Greenwood, D., Norrby, S.R., Whitley, R.J., Eds.; Elsevier: London, UK, 2010; pp. 200–225. [Google Scholar] [CrossRef]
- Peechakara, B.V.; Basit, H.; Gupta, M. Ampicillin. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. Available online: http://www.ncbi.nlm.nih.gov/books/NBK519569/ (accessed on 10 June 2025).
- Kwan, K.C.; Rogers, J.D. Pharmacokinetics of β-Lactam Antibiotics. In Antibiotics; Demain, A.L., Solomon, N.A., Eds.; Springer: Berlin/Heidelberg, Germany, 1983; pp. 247–370. [Google Scholar] [CrossRef]
- Waxman, D.J.; Strominger, J.L. Penicillin-binding proteins and the mechanism of action of beta-lactam antibiotics. Annu. Rev. Biochem. 1983, 52, 825–869. [Google Scholar] [CrossRef]
- Kong, K.; Schneper, L.; Mathee, K. Beta-lactam antibiotics: From antibiosis to resistance and bacteriology. APMIS 2010, 118, 1–36. [Google Scholar] [CrossRef]
- Shah, K.J.; Cherabuddi, K.; Shultz, J.; Borgert, S.; Ramphal, R.; Klinker, K.P. Ampicillin for the treatment of complicated urinary tract infections caused by vancomycin–resistant Enterococcus spp. (VRE): A single-center university hospital experience. Int. J. Antimicrob. Agents 2018, 51, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Bunnell, K.; Duong, A.; Ringsred, T.; Mian, A.; Bhathena, S. Aminopenicillins for treatment of ampicillin-resistant enterococcal urinary tract infections. Am. J. Health-Syst. Pharm. 2022, 79, 1056–1065. [Google Scholar] [CrossRef] [PubMed]
- Suleyman, G.; Zervos, M.J. Safety and efficacy of commonly used antimicrobial agents in the treatment of enterococcal infections: A review. Expert. Opin. Drug Saf. 2016, 15, 153–167. [Google Scholar] [CrossRef]
- Miller, W.R.; Munita, J.M.; Arias, C.A. Mechanisms of antibiotic resistance in enterococci. Expert. Rev. Anti Infect. Ther. 2014, 12, 1221–1236. [Google Scholar] [CrossRef] [PubMed]
- Nadgir, C.A.; Biswas, D.A. Antibiotic resistance and its impact on disease management. Cureus 2023, 15, e38251. [Google Scholar] [CrossRef]
- McEwen, S.A.; Collignon, P.J. Antimicrobial resistance: A One Health perspective. Microbiol. Spectr. 2018, 6, 2. [Google Scholar] [CrossRef]
- Li, M.; Liu, Q.; Teng, Y.; Ou, L.; Xi, Y.; Chen, S.; Duan, G. The resistance mechanism of Escherichia coli induced by ampicillin in laboratory. Infect. Drug Resist. 2019, 12, 2853–2863. [Google Scholar] [CrossRef]
- Türkyılmaz, O.; Darcan, C. Resistance mechanism of Escherichia coli strains with different ampicillin resistance levels. Appl. Microbiol. Biotechnol. 2024, 108, 5. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global burden of bacterial antimicrobial resistance in 2019: A systematic analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations; HM Government and Wellcome Trust: London, UK, 2016. [Google Scholar]
- Holmes, A.H.; Moore, L.S.; Sundsfjord, A.; Steinbakk, M.; Regmi, S.; Karkey, A.; Guerin, P.J.; Piddock, L.J. Understanding the mechanisms and drivers of antimicrobial resistance. Lancet 2016, 387, 176–187. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, K. The global burden of antimicrobial resistance. Nat. Med. 2022. [Google Scholar] [CrossRef] [PubMed]
- Salam, A.; Al-Amin, Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial resistance: A growing serious threat for global public health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef]
- Mdarhri, H.A.; Benmessaoud, R.; Yacoubi, H.; Seffar, L.; Assimi, H.G.; Hamam, M.; Boussettine, R.; Filali-Ansari, N.; Lahlou, F.A.; Diawara, I.; et al. Alternatives therapeutic approaches to conventional antibiotics: Advantages, limitations and potential application in medicine. Antibiotics 2022, 11, 1826. [Google Scholar] [CrossRef]
- Martirosov, D.M.; Lodise, T.P. Emerging trends in epidemiology and management of infections caused by carbapenem-resistant Enterobacteriaceae. Diagn. Microbiol. Infect. Dis. 2016, 85, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Mohd Sazlly Lim, S.; Sime, F.B.; Roberts, J.A. Multidrug-resistant Acinetobacter baumannii infections: Current evidence on treatment options and the role of pharmacokinetics/pharmacodynamics in dose optimisation. Int. J. Antimicrob. Agents 2019, 53, 726–745. [Google Scholar] [CrossRef]
- Singh, G.; Rana, A.; Smriti. Decoding antimicrobial resistance: Unraveling molecular mechanisms and targeted strategies. Arch. Microbiol. 2024, 206, 280. [Google Scholar] [CrossRef]
- Gauba, A.; Rahman, K.M. Evaluation of antibiotic resistance mechanisms in Gram-negative bacteria. Antibiotics 2023, 12, 1590. [Google Scholar] [CrossRef]
- Zabiszak, M.; Frymark, J.; Ogawa, K.; Skrobańska, M.; Nowak, M.; Jastrzab, R.; Kaczmarek, M.T. Complexes of β-lactam antibiotics and their Schiff-base derivatives as a weapon in the fight against bacterial resistance. Coord. Chem. Rev. 2023, 493, 215326. [Google Scholar] [CrossRef]
- Alekseev, V.G. Metal complexes of penicillins and cephalosporins (Review). Pharm. Chem. J. 2012, 45, 679–697. [Google Scholar] [CrossRef]
- Abd El Wahed, M.G.; Ayad, M. Stability constants of Cu2+, Fe3+ ↦ Zr4+ chelates of ampicillin, dopamine and α-methyl L-dopa in aqueous medium. Anal. Lett. 1984, 17, 819–836. [Google Scholar] [CrossRef]
- Alekseev, V.G.; Shcherbakova, E.E.; Yakubovich, Y.Y.; Vorob’ev, N.V.; Larin, S.V.; Shigina, O.Y. Interaction of ampicillin with Mn(II), Co(II), and Ni(II) ions. Russ. J. Gen. Chem. 2006, 76, 321–324. [Google Scholar] [CrossRef]
- Moustakas, M. The role of metal ions in biology, biochemistry and medicine. Materials 2021, 14, 549. [Google Scholar] [CrossRef]
- Frei, A. Metal complexes as a promising source for new antibiotics. Chem. Sci. 2020, 11, 2627–2639. [Google Scholar] [CrossRef]
- Božić Cvijan, B.; Korać Jačić, J.; Bajčetić, M. The impact of copper ions on the activity of antibiotic drugs. Molecules 2023, 28, 5133. [Google Scholar] [CrossRef]
- Pavlić, A.; Gobin, I.; Begić, G.; Tota, M.; Abram, M.; Špalj, S. The effect of nickel ions on the susceptibility of bacteria to ciprofloxacin and ampicillin. Folia Microbiol. 2022, 67, 649–657. [Google Scholar] [CrossRef]
- Karaky, N.; Tang, S.; Ramalingam, P.; Kirby, A.; McBain, A.J.; Banks, C.E.; Whitehead, K.A. Multidrug-resistant Escherichia coli remains susceptible to metal ions and graphene-based compounds. Antibiotics 2024, 13, 381. [Google Scholar] [CrossRef]
- Gawrońska, M.; Kowalik, M.; Makowski, M. Recent advances in medicinal chemistry of ampicillin: Derivatives, metal complexes, and sensing approaches. TrAC Trends Anal. Chem. 2022, 155, 116691. [Google Scholar] [CrossRef]
- Alderighi, L.; Gans, P.; Ienco, A.; Peters, D.; Sabatini, A.; Vacca, A. Hyperquad simulation and speciation (HySS): A utility program for the investigation of equilibria involving soluble and partially soluble species. Coord. Chem. Rev. 1999, 184, 311–318. [Google Scholar] [CrossRef]
- Zabiszak, M.; Frymark, J.; Grajewski, J.; Jastrzab, R. Spectroscopic studies of lanthanide(III) complexes with L-malic acid in binary systems. Int. J. Mol. Sci. 2024, 25, 9210. [Google Scholar] [CrossRef]
- Zabiszak, M.; Frymark, J.; Nowak, M.; Grajewski, J.; Stachowiak, K.; Kaczmarek, M.T.; Jastrzab, R. Influence of d-electron divalent metal ions in complex formation with L-tartaric and L-malic acids. Molecules 2021, 26, 5290. [Google Scholar] [CrossRef]
- Peisach, J.; Blumberg, W.E. Structural implications derived from the analysis of electron paramagnetic resonance spectra of natural and artificial copper proteins. Arch. Biochem. Biophys. 1974, 165, 691–708. [Google Scholar] [CrossRef]
- Eliseeva, S.V.; Bünzli, J.-C.G. Lanthanide Luminescence for Functional Materials and Bio-Sciences. Chem. Soc. Rev. 2010, 39, 189–227. [Google Scholar] [CrossRef]
- Bünzli, J.-C.G. Lanthanide Luminescence: From Fundamental Concepts to Photonic Applications. Chem. Rev. 2010, 110, 2729–2755. [Google Scholar] [CrossRef]
- McAfee, L.L. Infrared and Raman Spectra of Inorganic and Coordination Compounds. Part A: Theory and Applications in Inorganic Chemistry; Part B: Application in Coordination, Organometallic, and Bioinorganic Chemistry. J. Chem. Educ. 2000, 77, 1122–1124. [Google Scholar] [CrossRef]
- Baraldi, C.; Tinti, A.; Ottani, S.; Gamberini, M.C. Characterization of polymorphic ampicillin forms. J. Pharm. Biomed. Anal. 2014, 100, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Al-Khodir, F.A.I.; Refat, M.S. Spectroscopic elaboration and structural characterizations of new Fe(III), Pd(II), and Au(III) ampicillin complexes: Metal-antibiotic ligational behaviors. J. Pharm. Innov. 2015, 10, 335–347. [Google Scholar] [CrossRef]
- Abbas, A.M.; Fisal, S.R.; Orabi, A.S. Novel β-lactam antibiotic derivative and its complexes: DFT, frontier energy levels, DNA interaction, docking, physicochemical and antimicrobial properties. J. Mol. Struct. 2020, 1218, 128487. [Google Scholar] [CrossRef]
- Frymark, J.; Zabiszak, M.; Grajewski, J.; Hnatejko, Z.; Kołodynska, D.; Kaczmarek, M.T. Jastrzab, Excess of polyamine as a factor influencing the mode of coordination in the Eu(III)/α-hydroxy acid/spermine system. Polyhedron 2022, 221, 115853. [Google Scholar] [CrossRef]
- Zabiszak, M.; Nowak, M.; Gabryel, M.; Ogawa, K.; Kaczmarek, M.T.; Hnatejko, Z.; Jastrzab, R. New coordination compounds of citric acid and polyamines with lanthanide ions—Potential application in monitoring the treatment of cancer diseases. J. Inorg. Biochem. 2019, 198, 110715. [Google Scholar] [CrossRef]
- Glasoe, P.K.; Long, F.A. Use of glass electrodes to measure acidities in deuterium oxide. J. Phys. Chem. 1960, 64, 188–190. [Google Scholar] [CrossRef]
- Spałek, T.; Pietrzyk, P.; Sojka, Z. Application of the Genetic Algorithm Joint with the Powell Method to Nonlinear Least-Squares Fitting of Powder EPR Spectra. J. Chem. Inf. Model. 2005, 45, 18–29. [Google Scholar] [CrossRef]


| Species | Mg(II) | Ca(II) | Sr(II) | ||||
|---|---|---|---|---|---|---|---|
| logβ | logKe | logβ | logKe | logβ | logKe | ||
| 1:1 | M(HAmp) | 9.85(5) | 2.75 | 10.00(2) | 2.90 | 9.99(1) | 2.89 |
| M(Amp) | 3.60(2) | 3.60 | - | - | 2.60(7) | 2.60 | |
| M(Amp)(OH) | - | - | - | - | −8.56(5) | 2.60 | |
| 1:2 | M(HAmp) | - | - | 10.00(2) | 2.90 | 9.99(1) | 2.89 |
| M(HAmp)(HAmp) | 20.91(4) | 6.72 | - | - | - | - | |
| M(HAmp)(Amp) | 14.23(5) | 7.13 | - | - | - | - | |
| M(Amp)2 | 6.96(4) | 3.36 | - | - | - | - | |
| Species | Co(II) | Ni(II) | Cu(II) | ||||
|---|---|---|---|---|---|---|---|
| logβ | logKe | logβ | logKe | logβ | logKe | ||
| 1:1 | M(HAmp) | 9.99(6) | 2.90 | 10.91(2) | 3.82 | 10.36(4) | 3.26 |
| M(Amp) | 3.12(6) | 3.12 | 3.86(1) | 3.86 | 5.23(5) | 5.23 | |
| M(Amp)(OH) | - | - | −6.21(1) | 3.70 | 0.29(2) | 8.83 | |
| M(Amp)(OH)2 | −14.88(8) | 12.66 | - | - | −7.48(3) | 5.99 | |
| M(Amp)(OH)3 | −25.33(4) | 3.32 | - | - | −18.67(4) | 2.58 | |
| 1:2 | M(HAmp)(HAmp) | 20.49(4) | 6.30 | 19.99(3) | 5.80 | 20.54(2) | 6.35 |
| M(HAmp)(Amp) | 13.96(4) | 6.86 | 13.60(3) | 6.50 | 15.75(4) | 8.66 | |
| M(Amp)2 | 6.22(5) | 3.10 | 6.27(2) | 2.41 | 10.89(2) | 5.65 | |
| M(Amp)2(OH) | - | - | - | - | 4.06(2) | 6.95 | |
| M(Amp)2(OH)2 | −11.84(3) | 9.47 | - | - | −3.85(2) | 5.85 | |
| Species | Nd(III) | Eu(III) | Tb(III) | ||||
|---|---|---|---|---|---|---|---|
| logβ | logKe | logβ | logKe | logβ | logKe | ||
| 1:1 | M(HAmp) | 10.82(2) | 3.72 | 10.76(4) | 3.66 | 10.45(2) | 3.18 |
| M(Amp) | 3.42(2) | 3.42 | - | - | - | - | |
| M(Amp)(OH)3 | - | - | −21.11(4) | 20.20 | −21.06(1) | 20.24 | |
| M2(Amp) | - | - | 7.47(3) | 7.47 | - | - | |
| 1:2 | M(HAmp) | 10.82(2) | 3.72 | 10.76(4) | 3.66 | 10.45(2) | 3.35 |
| M(Amp) | 3.42(2) | 3.42 | - | - | - | - | |
| M(HAmp)(Amp) | - | - | 13.45(5) | 13.45 | - | - | |
| M(Amp)2 | - | - | 6.50(6) | 6.50 | - | - | |
| M(Amp)(OH)3 | - | - | - | - | −21.06(1) | 20.24 | |
| M(Amp)2(OH)3 | - | - | −16.81(5) | 4.22 | - | - | |
| M(Amp)2(OH)4 | - | - | −26.77(4) | 3.81 | - | - | |
| Species | pH | A | λmax (nm) | ε (dm3·mol−1·cm−1) | ||
|---|---|---|---|---|---|---|
| 1:1 | Co(II) | M(HAmp) | 5.3 | 0.0096 | 517 | 9.6 |
| M(Amp) | 7.9 | 0.0084 | 510 | 8.4 | ||
| Ni(II) | M(HAmp) | 5.3 | 0.0056 0.0023 | 670 905 | 5.6 2.3 | |
| M(Amp) | 8.1 | 0.0036 0.0016 | 675 900 | 3.6 1.6 | ||
| Cu(II) | M(HAmp) | 3.6 | 0.018 | 775 | 18 | |
| M(Amp) | 4.9 | 0.055 | 710 | 55 | ||
| M(Amp)(OH) | 6.4 | 0.13 | 610 | 130 | ||
| 1:2 | Co(II) | M(HAmp)(HAmp) | 5.1 | 0.012 | 510 | 12 |
| M(HAmp)(Amp) | 7.0 | 0.011 | 510 | 11 | ||
| M(Amp)2 | 8.1 | 0.020 | 490 | 20 | ||
| Ni(II) | M(HAmp)(HAmp) | 5.2 | 0.0052 0.0022 | 700 890 | 5.2 2.2 | |
| M(HAmp)(Amp) | 6.1 | 0.0045 0.0021 | 710 894 | 4.5 2.1 | ||
| M(Amp)2 | 8.7 | 0.0031 0.0019 | 644 900 | 3.1 1.9 | ||
| Cu(II) | M(HAmp)(Amp) | 4.2 | 0.044 | 725 | 44 | |
| M(Amp)2 | 5.6 | 0.13 | 630 | 130 | ||
| Metal/Ampicillin (1:1 Ratio) | Metal/Ampicillin (1:2 Ratio) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mg(II) | Ca(II) | Sr(II) | Mg(II) | Ca(II) | Sr(II) | ||||||
| pH | ∆ε (nm) | pH | ∆ε (nm) | pH | ∆ε (nm) | pH | ∆ε (nm) | pH | ∆ε (nm) | pH | ∆ε (nm) |
| 4.3 | 13.7 (233) | 5.0 | 12.0 (233) | 5.0 | 12.5 (232) | 4.5 | 25.6 (233) | 5.0 | 26.4 (233) | 5.0 | 25.0 (233) |
| −12.1 (207) | −11.3 (206) | −12.2 (207) | −23.5 (208) | −23.8 (207) | −23.1 (207) | ||||||
| 64.8 (187) | 59.5 (187) | 59.6 (187) | 126.7 (187) | 127.0 (187) | 125.5 (187) | ||||||
| 8.8 | 11.3 (234) | 9.0 | 11.8 (233) | 7.0 | 25.1 (234) | ||||||
| −8.9 (206) | −7.6 (206) | −19.6 (207) | |||||||||
| 49.7 (188) | 46.0 (189) | 115 (187) | |||||||||
| 9.0 | 24.1 (233) | ||||||||||
| −15.3 (207) | |||||||||||
| 102.1 (188) | |||||||||||
| Metal/Ampicillin (1:1 Ratio) | Metal/Ampicillin (1:2 Ratio) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Co(II) | Ni(II) | Cu(II) | Co(II) | Ni(II) | Cu(II) | ||||||
| pH | ∆ε (nm) | pH | ∆ε (nm) | pH | ∆ε (nm) | pH | ∆ε (nm) | pH | ∆ε (nm) | pH | ∆ε (nm) |
| 5.1 | 15.2 (233) | 4.5 | 15.7 (233) | 3.5 | 6.5 (242) | 5.0 | 26.8 (233) | 4.4 | 27.0 (233) | 4.2 | 19.2 (235) |
| −13.0 (207) | −15.0 (207) | −9.2 (204) | −23.7 (207) | −21.0 (206) | −22.7 (205) | ||||||
| 71.4 (187) | 77.6 (187) | 42.0 (187) | 126.1 (187) | 128.2 (187) | 102.7 (187) | ||||||
| 8.0 | 12.3 (233) | 8.3 | 15.5 (233) | 5.0 | 5.1 (242) | 7.1 | 23.8 (233) | 6.8 | 25.7 (232) | 6.0 | 6.9 (232) |
| −8.7 (207) | −7.3 (207) | −7.5 (203) | −19.3 (206) | −19.4 (206) | −8.8 (209) | ||||||
| 54.4 (188) | 57.2 (188) | 27.3 (187) | 112.4 (188) | 118.3 (187) | 44.3 (189) | ||||||
| 9.8 | 9.4 (233) | 6.3 | 3.0 (294) | 8.5 | 19.0 (233) | 8.2 | 23.7 (233) | 7.3 | 6.6 (305) 7.0 (232) | ||
| −5.0 (207) | −2.3 (238) −3.1 (220) | −11.3 (207) | −14.1 (207) | −13.3 (210) | |||||||
| 48.3 (188) | 4.3 (194) | 88.7 (188) | 98.7 (188) | 36.0 (188) | |||||||
| 10.5 | 8.2 (233) | 9.2 | 2.6 (301) 2.6 (260) | 10.0 | 17.9 (233) | 9.2 | 6.2 (314) 2.2 (234) | ||||
| −3.4 (207) | −6.5 (221) −7.0 (210) −5.5 (200) | −7.7 (208) | −15.7 (210) | ||||||||
| 44.0 (189) | 84.1 (189) | 30.9 (186) | |||||||||
| Metal/Ampicillin (1:1 Ratio) | Metal/Ampicillin (1:2 Ratio) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Nd(III) | Eu(III) | Tb(III) | Nd(III) | Eu(III) | Tb(III) | ||||||
| pH | ∆ε (nm) | pH | ∆ε (nm) | pH | ∆ε (nm) | pH | ∆ε (nm) | pH | ∆ε (nm) | pH | ∆ε (nm) |
| 5.0 | 10.8 (236) | 5.0 | 13.3 (236) | 5.0 | 15.9 (236) | 5.0 | 25.8 (235) | 2.4 | 23.3 (232) | 5.0 | 28.4 (235) |
| −9.8 (204) | −13.5 (207) | −14.0 (206) | −24.8 (206) | −27.9 (206) | −26.0 (206) | ||||||
| 57.1 (187) | 74.3 (187) | 80.2 (187) | 131.6 (187) | 123.3 (187) | 137.3 (188) | ||||||
| 7.7 | 10.7 (235) | 7.5 | 13.5 (235) | 9.2 | 13.8 (236) | 8.1 | 22.9 (235) | 5.0 | 26.7 (233) | 9.2 | 24.7 (235) |
| −8.4 (205) | −10.6 (205) | −11.1 (206) | −17.0 (207) | −23.1 (206) | −19.3 (206) | ||||||
| 48.6 (187) | 66.4 (187) | 60.4 (188) | 104.0 (188) | 127.1 (187) | 108.7 (189) | ||||||
| 10.0 | 11.9 (236) | 7.4 | 23.9 (235) | ||||||||
| −8.8 (206) | −19.2 (207) | ||||||||||
| 54.9 (188) | 112.9 (188) | ||||||||||
| 9.0 | 22.9 (234) | ||||||||||
| −16.2 (207) | |||||||||||
| 101.4 (188) | |||||||||||
| 10.0 | 20.9 (235) | ||||||||||
| −15.7 (207) | |||||||||||
| 91.2 (189) | |||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frymark, J.; Zabiszak, M.; Grajewski, J.; Tylkowski, B.; Jastrzab, R. Insights into Complex Compounds of Ampicillin: Potentiometric and Spectroscopic Studies. Int. J. Mol. Sci. 2025, 26, 7605. https://doi.org/10.3390/ijms26157605
Frymark J, Zabiszak M, Grajewski J, Tylkowski B, Jastrzab R. Insights into Complex Compounds of Ampicillin: Potentiometric and Spectroscopic Studies. International Journal of Molecular Sciences. 2025; 26(15):7605. https://doi.org/10.3390/ijms26157605
Chicago/Turabian StyleFrymark, Justyna, Michał Zabiszak, Jakub Grajewski, Bartosz Tylkowski, and Renata Jastrzab. 2025. "Insights into Complex Compounds of Ampicillin: Potentiometric and Spectroscopic Studies" International Journal of Molecular Sciences 26, no. 15: 7605. https://doi.org/10.3390/ijms26157605
APA StyleFrymark, J., Zabiszak, M., Grajewski, J., Tylkowski, B., & Jastrzab, R. (2025). Insights into Complex Compounds of Ampicillin: Potentiometric and Spectroscopic Studies. International Journal of Molecular Sciences, 26(15), 7605. https://doi.org/10.3390/ijms26157605







