AMPK-Targeting Effects of (−)-Epicatechin Gallate from Hibiscus sabdariffa Linne Leaves on Dual Modulation of Hepatic Lipid Accumulation and Glycogen Synthesis in an In Vitro Oleic Acid Model
Abstract
1. Introduction
2. Results
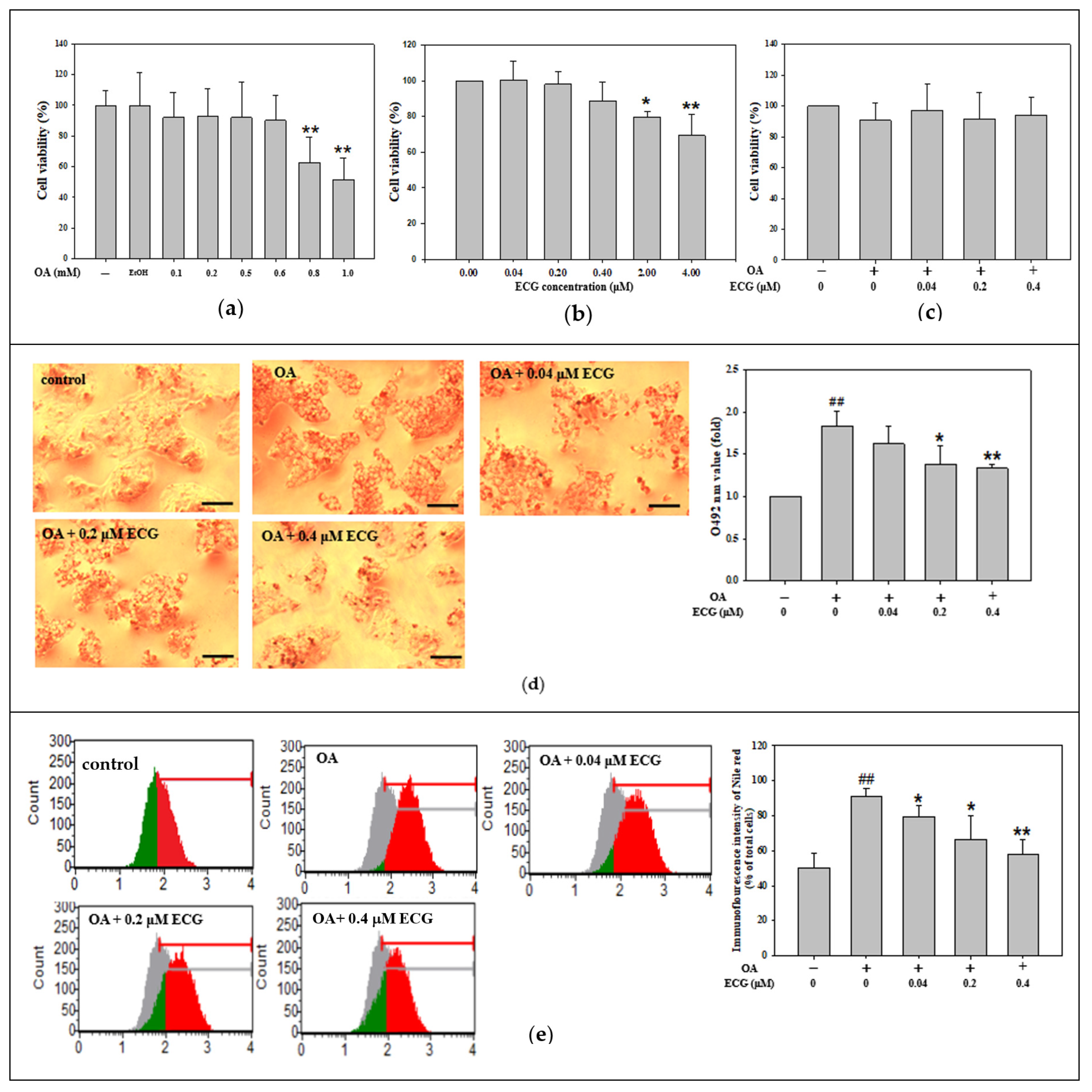
2.1. Noncytotoxic Doses of ECG Abolished the OA-Stimulated Cellular Lipid Accumulation
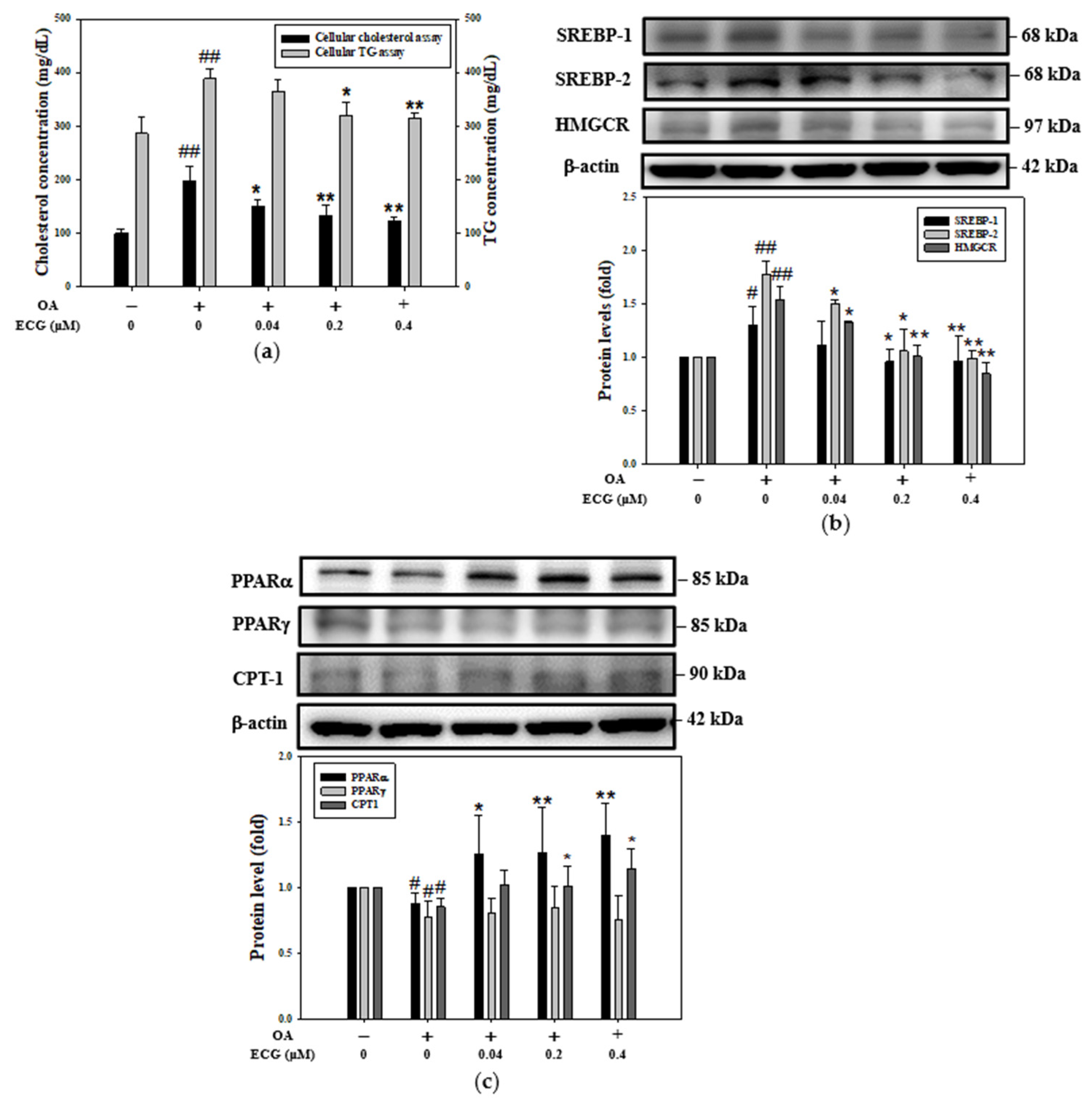
2.2. ECG Inhibited the OA-Induced Increase in Intracellular Lipid Content, Involving Regulation of Lipogenesis and Lipolysis
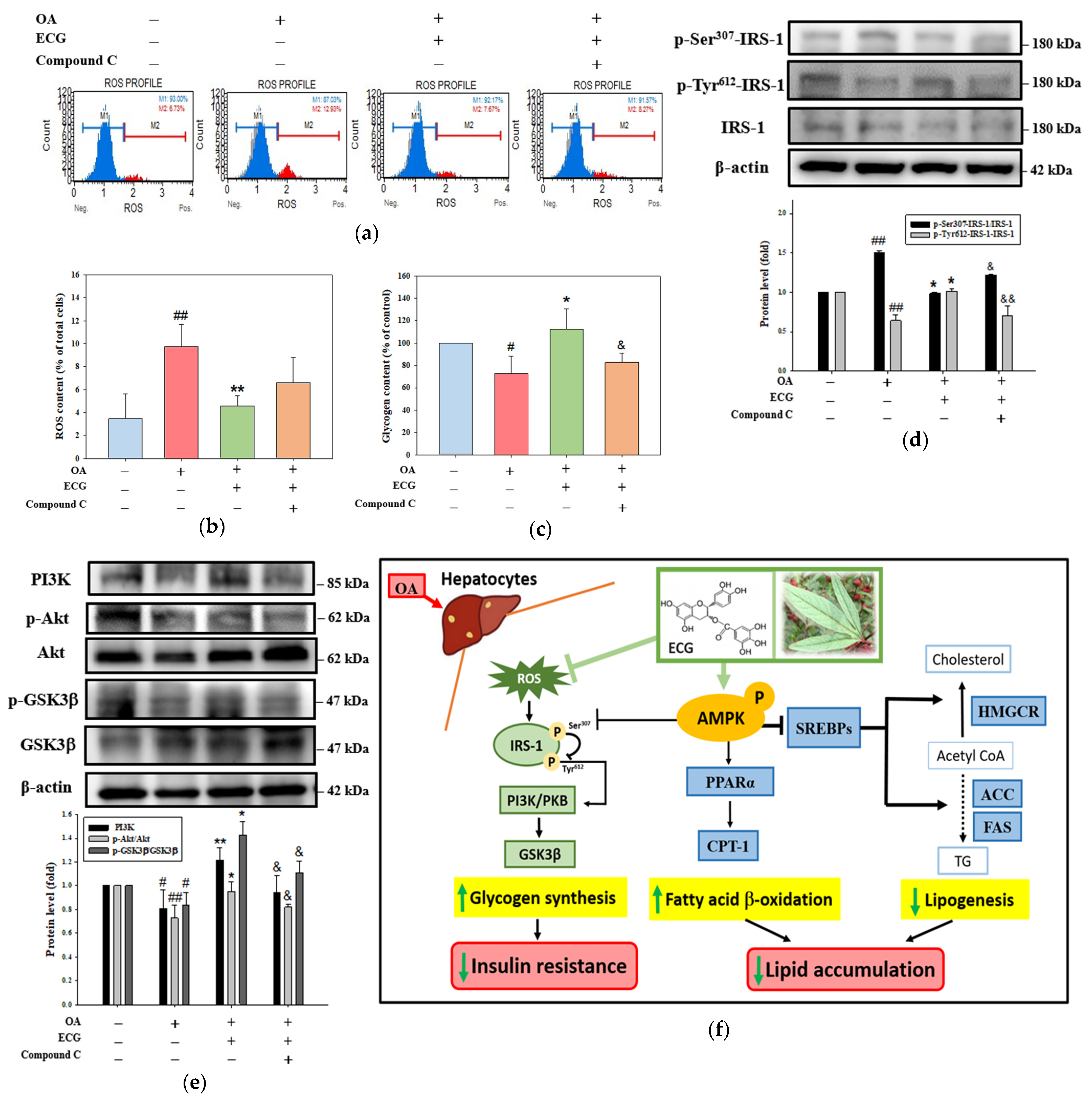
2.3. AMPK Is Essential for the Influence of ECG on the OA-Mediated Lipid Dysmetabolism
2.4. AMPK Is Essential for the Effect of ECG on Lipogenesis and FA β-Oxidation in the OA-Treated Cells
2.5. AMPK Is Partially Responsible for the Impact of ECG on Glycogen Synthesis and Insulin Signaling in the OA-Treated Cells
3. Discussion
4. Materials and Methods
4.1. Extraction, Identification, and Flavonoid Compound Isolation of Polyphenolic Extract of Hibiscus Leaves
4.2. Cell Culture, Treatment, and Viability Assay
4.3. Lipid Accumulation Assay
4.4. Cellular Lipid Content Analysis
4.5. Western Blotting
4.6. SwissTarget Prediction Analysis
4.7. AMPK Inhibition Test
4.8. Cellular ROS Measurement
4.9. Glycogen Synthesis Assay
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MASLD | metabolic dysfunction-associated steatotic liver disease |
| MASH | metabolic dysfunction-associated steatohepatitis |
| ECM | extracellular matrix |
| FA | fatty acid |
| SREBPs | sterol regulatory element-binding proteins |
| ACC | acetyl-CoA carboxylase |
| FAS | fatty acid synthase |
| TG | triglyceride |
| HMGCR | 3-hydroxy-3-methylglutaryl-CoA reductase |
| LDL | low-density lipoprotein |
| PPARs | peroxisome proliferator-activated receptors |
| CPT1 | carnitine palmitoyltransferase I |
| AMPK | adenosine monophosphate-activated protein kinase |
| PI3K | phosphatidyl inositol 3 kinase |
| PKB | protein kinase B |
| GSK-3β | glycogen synthase kinase-3beta |
| ECG | (−)-epicatechin gallate |
| SDS-PAGE | sodium dodecyl sulfate-polyacrylamide gel electrophoresis |
| TBS | tris-buffered saline |
| ECL | enhanced chemiluminescence |
References
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD Development and therapeutic strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Rinella, M.E.; Lazarus, J.V.; Ratziu, V.; Francque, S.M.; Sanyal, A.J.; Kanwal, F.; Romero, D.; Abdelmalek, M.F.; Anstee, Q.M.; Arab, J.P.; et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology 2023, 78, 1966–1986. [Google Scholar] [CrossRef]
- Lekakis, V.; Papatheodoridis, G.V. Natural history of metabolic dysfunction-associated steatotic liver disease. Eur. J. Intern. Med. 2024, 122, 3–10. [Google Scholar] [CrossRef]
- Armandi, A.; Bugianesi, E. Natural history of NASH. Liver Int. 2021, 41, 78–82. [Google Scholar] [CrossRef]
- Wells, R.G. Cellular sources of extracellular matrix in hepatic fibrosis. Clin. Liver Dis. 2008, 12, 759–768. [Google Scholar] [CrossRef] [PubMed]
- Shimano, H. Sterol regulatory element-binding proteins (SREBPs): Transcriptional regulators of lipid synthetic genes. Prog. Lipid Res. 2001, 40, 439–452. [Google Scholar] [CrossRef] [PubMed]
- Sung, Y.Y.; Son, E.; Im, G.; Kim, D.S. Herbal Combination of Phyllostachys pubescens and Scutellaria baicalensis Inhibits Adipogenesis and Promotes Browning via AMPK Activation in 3T3-L1 Adipocytes. Plants 2020, 9, 1422. [Google Scholar] [CrossRef]
- Lee, C.H.; Olson, P.; Evans, R.M. Minireview: Lipid metabolism, metabolic diseases, and peroxisome proliferator-activated receptors. Endocrinology 2003, 144, 2201–2207. [Google Scholar] [CrossRef]
- Postic, C.; Girard, J. Contribution of de novo fatty acid synthesis to hepatic steatosis and insulin resistance: Lessons from genetically engineered mice. J. Clin. Investig. 2008, 118, 829–838. [Google Scholar] [CrossRef]
- Au-Yeung, K.K.W.; Shang, Y.; Wijerathne, C.U.B.; Madduma Hewage, S.; Siow, Y.L.; O, K. Acute Kidney Injury Induces Oxidative Stress and Hepatic Lipid Accumulation through AMPK Signaling Pathway. Antioxidants 2023, 12, 883. [Google Scholar] [CrossRef]
- Zhou, G.; Myers, R.; Li, Y.; Chen, Y.; Shen, X.; Fenyk-Melody, J.; Wu, M.; Ventre, J.; Doebber, T.; Fujii, N.; et al. Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Investig. 2001, 108, 1167–1174. [Google Scholar] [CrossRef]
- Kumashiro, N.; Erion, D.M.; Zhang, D.; Kahn, M.; Beddow, S.A.; Chu, X.; Still, C.D.; Gerhard, G.S.; Han, X.; Dziura, J.; et al. Cellular mechanism of insulin resistance in nonalcoholic fatty liver disease. Proc. Natl. Acad. Sci. USA 2011, 108, 16381–16385. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Lin, J.K. Epigallocatechin gallate (EGCG) attenuates high glucose-induced insulin signaling blockade in human hepG2 hepatoma cells. Mol. Nutr. Food Res. 2008, 52, 930–939. [Google Scholar] [CrossRef]
- Thirone, A.C.; Huang, C.; Klip, A. Tissue-specific roles of IRS proteins in insulin signaling and glucose transport. Trends Endocrinol. Metab. 2006, 17, 72–78. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.V.; Accili, D. Hormonal regulation of hepatic glucose production in health and disease. Cell Metab. 2011, 14, 9–19. [Google Scholar] [CrossRef]
- Liu, C.H.; Zeng, Q.M.; Hu, T.Y.; Huang, Y.; Song, Y.; Guan, H.; Rockey, D.C.; Tang, H.; Li, S. Resmetirom and thyroid hormone receptor-targeted treatment for metabolic dysfunction-associated steatotic liver disease (MASLD). Portal Hypertens. Cirrhosis 2025, 4, 66–78. [Google Scholar] [CrossRef]
- Zhang, X.; Lau, H.C.H.; Yu, J. Pharmacological treatment for metabolic dysfunction–associated steatotic liver disease and related disorders: Current and emerging therapeutic options. Pharmacol. Rev. 2025, 77, 100018. [Google Scholar] [CrossRef]
- Gosain, S.; Ircchiaya, R.; Sharma, P.C.; Thareja, S.; Kalra, A.; Deep, A.; Bhardwaj, T.R. Hypolipidemic effect of ethanolic extract from the leaves of Hibiscus sabdariffa L. in hyperlipidemic rats. Acta. Pol. Pharm. 2010, 67, 179–184. Available online: https://pubmed.ncbi.nlm.nih.gov/20369795/ (accessed on 16 June 2025).
- Chen, J.H.; Wang, C.J.; Wang, C.P.; Sheu, J.Y.; Lin, C.L.; Lin, H.H. Hibiscus sabdariffa leaf polyphenolic extract inhibits LDL oxidation and foam cell formation involving up-regulation of LXRα/ABCA1 pathway. Food Chem. 2013, 141, 397–406. [Google Scholar] [CrossRef]
- Chen, J.H.; Lee, M.S.; Wang, C.P.; Hsu, C.C.; Lin, H.H. Autophagic effects of Hibiscus sabdariffa leaf polyphenols and epicatechin gallate (ECG) against oxidized LDL-induced injury of human endothelial cells. Eur. J. Nutr. 2017, 56, 1963–1981. [Google Scholar] [CrossRef] [PubMed]
- Chou, C.C.; Wang, C.P.; Chen, J.H.; Lin, H.H. Anti-Atherosclerotic Effect of Hibiscus Leaf Polyphenols against Tumor Necrosis Factor-alpha-Induced Abnormal Vascular Smooth Muscle Cell Migration and Proliferation. Antioxidants 2019, 8, 620. [Google Scholar] [CrossRef]
- Zhen, J.; Villani, T.S.; Guo, Y.; Qi, Y.; Chin, K.; Pan, M.H.; Ho, C.T.; Simon, J.E.; Wu, Q. Phytochemistry, antioxidant capacity, total phenolic content and anti-inflammatory activity of Hibiscus sabdariffa leaves. Food Chem. 2016, 190, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Cho, K.N.; Sukhthankar, M.; Lee, S.H.; Yoon, J.H.; Baek, S.J. Green tea catechin (−)-epicatechin gallate induces tumour suppressor protein ATF3 via EGR-1 activation. Eur. J. Cancer 2007, 43, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Miura, Y.; Chiba, T.; Tomita, I.; Koizumi, H.; Miura, S.; Umegaki, K.; Hara, Y.; Ikeda, M.; Tomita, T. Tea catechins prevent the development of atherosclerosis in apoprotein E-defcient mice. J. Nutr. 2001, 131, 27–32. [Google Scholar] [CrossRef]
- Wu, X.; Zhang, G.; Hu, X.; Pan, J.; Liao, Y.; Ding, H. Inhibitory effect of epicatechin gallate on protein glycation. Food Res. Int. 2019, 122, 230–240. [Google Scholar] [CrossRef]
- Wang, W.; Le, T.; Wang, W.W.; Yin, J.F.; Jiang, H.Y. The Effects of Structure and Oxidative Polymerization on Antioxidant Activity of Catechins and Polymers. Foods 2023, 12, 4207. [Google Scholar] [CrossRef]
- Guo, X.; Long, P.; Meng, Q.; Ho, C.T.; Zhang, L. An emerging strategy for evaluating the grades of Keemun black tea by combinatory liquid chromatography-Orbitrap mass spectrometry-based untargeted metabolomics and inhibition effects on alpha-glucosidase and alpha-amylase. Food Chem. 2018, 246, 74–81. [Google Scholar] [CrossRef]
- Deng, Y.T.; Chang, T.W.; Lee, M.S.; Lin, J.K. Suppression of free fatty acid induced insulin resistance by phytopolyphenols in C2C12 mouse skeletal muscle cells. J. Agric. Food Chem. 2012, 60, 1059–1066. [Google Scholar] [CrossRef]
- Zhou, C.; Tan, Y.; Xu, B.; Wang, Y.; Cheang, W.S. 3,4′,5-Trimethoxy-trans-stilbene Alleviates Endothelial Dysfunction in Diabetic and Obese Mice via Activation of the AMPK/SIRT1/eNOS Pathway. Antioxidants 2022, 11, 1286. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, Y.Y.; Nie, Y.Q.; Zhou, Y.J.; Cao, C.Y.; Xu, L. Association between metabolic syndrome and the development of non-alcoholic fatty liver disease. Exp. Ther. Med. 2013, 6, 77–84. [Google Scholar] [CrossRef]
- Povero, D.; Feldstein, A.E. Novel molecular mechanisms in the development of nonalcoholic steatohepatitis. Diabetes Metab. J. 2016, 40, 1–11. [Google Scholar] [CrossRef]
- Gomez-Lechon, M.J.; Donato, M.T.; Martinez-Romero, A.; Jimenez, N.; Castell, J.V.; O’Connor, J.E. A human hepatocellular In Vitro model to investigate steatosis. Chem. Biol. Interact. 2007, 165, 106–116. [Google Scholar] [CrossRef]
- Zeng, L.; Tang, W.; Yin, J.; Feng, L.; Li, Y.; Yao, X.; Zhou, B. Alisol A 24-Acetate Prevents Hepatic Steatosis and Metabolic Disorders in HepG2 Cells. Cell Physiol. Biochem. 2016, 40, 453–464. [Google Scholar] [CrossRef]
- Moravcová, A.; Červinková, Z.; Kučera, O.; Mezera, V.; Rychtrmoc, D.; Lotková, H. The effect of oleic and palmitic acid on induction of steatosis and cytotoxicity on rat hepatocytes in primary culture. Physiol. Res. 2015, 64, 627–636. [Google Scholar] [CrossRef] [PubMed]
- Ricchi, M.; Odoardi, M.R.; Carulli, L.; Anzivino, C.; Ballestri, S.; Pinetti, A.; Fantoni, L.I.; Marra, F.; Bertolotti, M.; Banni, S.; et al. Differential effect of oleic and palmitic acid on lipid accumulation and apoptosis in cultured hepatocytes. J. Gastroenterol. Hepatol. 2009, 24, 830–840. [Google Scholar] [CrossRef]
- Rafiei, H.; Omidian, K.; Bandy, B. Dietary Polyphenols Protect Against Oleic Acid-Induced Steatosis in an In Vitro Model of NAFLD by Modulating Lipid Metabolism and Improving Mitochondrial Function. Nutrients 2019, 11, 541. [Google Scholar] [CrossRef]
- Musolino, V.; Macrì, R.; Cardamone, A.; Serra, M.; Coppoletta, A.R.; Tucci, L.; Maiuolo, J.; Lupia, C.; Scarano, F.; Carresi, C.; et al. Nocellara Del Belice (Olea europaea L. Cultivar): Leaf Extract Concentrated in Phenolic Compounds and Its Anti-Inflammatory and Radical Scavenging Activity. Plants 2022, 12, 27. [Google Scholar] [CrossRef]
- Chang, J.J.; Hsu, M.J.; Huang, H.P.; Chung, D.J.; Chang, Y.C.; Wang, C.J. Mulberry anthocyanins inhibit oleic acid induced lipid accumulation by reduction of lipogenesis and promotion of hepatic lipid clearance. J. Agric. Food Chem. 2013, 61, 6069–6676. [Google Scholar] [CrossRef] [PubMed]
- Służały, P.; Paśko, P.; Galanty, A. Natural Products as Hepatoprotective Agents-A Comprehensive Review of Clinical Trials. Plants 2024, 13, 1985. [Google Scholar] [CrossRef] [PubMed]
- Weerawatanakorn, M.; Kamchonemenukool, S.; Koh, Y.C.; Pan, M.H. Exploring Phytochemical Mechanisms in the Prevention of Cholesterol Dysregulation: A Review. J. Agric. Food Chem. 2014, 72, 6833–6849. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Hong, H.Q.; Zhao, L.; Cai, Y.P.; Qin, Y. (−)-Epicatechin-3-gallate (a polyphenol from green tea) potentiates doxorubicin-induced apoptosis in H9C2 cardiomyocytes. Biotechnol. Lett. 2015, 37, 1937–1943. [Google Scholar] [CrossRef] [PubMed]
- Chan, F.L.; Choi, H.L.; Chen, Z.Y.; Chan, P.S.; Huang, Y. Induction of apoptosis in prostate cancer cell lines by a flavonoid, baicalin. Cancer Lett. 2000, 160, 219–228. [Google Scholar] [CrossRef]
- Chen, Q.; Ganapathy, S.; Singh, K.P.; Shankar, S.; Srivastava, R.K. Resveratrol induces growth arrest and apoptosis through activation of FOXO transcription factors in prostate cancer cells. PLoS ONE 2010, 5, e15288. [Google Scholar] [CrossRef] [PubMed]
- Gurusamy, N.; Lekli, I.; Mukherjee, S.; Ray, D.; Ahsan, M.K.; Gherghiceanu, M.; Popescu, L.M.; Das, D.K. Cardioprotection by resveratrol: A novel mechanism via autophagy involving the mTORC2 pathway. Cardiovasc. Res. 2010, 86, 103–112. [Google Scholar] [CrossRef]
- Chen, C.; Liu, Q.; Liu, L.; Hu, Y.Y.; Feng, Q. Potential Biological Effects of (-)-Epigallocatechin-3-gallate on the Treatment of Nonalcoholic Fatty Liver Disease. Mol. Nutr. Food Res. 2018, 62, 1700483. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Yun, J.P.; Yang, Y.N.; Hua, F.; Zhang, X.W.; Lin, H.; Lv, X.X.; Li, K.; Zhang, P.C.; Hu, Z.W. A novel ECG analog 4-(S)-(2,4,6-trimethylthiobenzyl)-epigallocatechin gallate selectively induces apoptosis of B16-F10 melanoma via activation of autophagy and ROS. Sci. Rep. 2017, 7, 42194. [Google Scholar] [CrossRef]
- Ku, H.C.; Chang, H.H.; Liu, H.C.; Hsiao, C.H.; Lee, M.J.; Hu, Y.J.; Hung, P.F.; Liu, C.W.; Kao, Y.H. Green tea (-) epigallocatechin gallate inhibits insulin stimulation of 3T3-L1 preadipocyte mitogenesis via the 67-kDa laminin receptor pathway. Am. J. Physiol. Cell Physiol. 2009, 297, C121–C132. [Google Scholar] [CrossRef]
- Gan, L.; Meng, Z.J.; Xiong, R.B.; Guo, J.Q.; Lu, X.C.; Zheng, Z.W.; Deng, Y.P.; Luo, B.D.; Zou, F.; Li, H. Green tea polyphenol epigallocatechin-3-gallate ameliorates insulin resistance in non-alcoholic fatty liver disease mice. Acta Pharmacol. Sin. 2015, 36, 597–605. [Google Scholar] [CrossRef]
- Yang, C.S.; Zhang, J.; Zhang, L.; Huang, J.; Wang, Y. Mechanisms of body weight reduction and metabolic syndrome alleviation by tea. Mol. Nutr. Food Res. 2016, 60, 160–174. [Google Scholar] [CrossRef]
- Guo, Y.; Zhang, Y.; Hong, K.; Luo, F.; Gu, Q.; Lu, N.; Bai, A. AMPK inhibition blocks ROS-NFκB signaling and attenuates endotoxemia-induced liver injury. PLoS ONE 2014, 9, e86881. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, Y.; Lu, S.; Hu, C.; Zhong, W.; Chai, Y. Beneficial effects of paeoniflorin on osteoporosis induced by high-carbohydrate, high-fat diet-associated hyperlipidemia In Vivo. Biochem. Biophys. Res. Commun. 2018, 498, 981–987. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.; Wang, Z.; Chen, J.; Zhao, M.; Guo, C.; Wang, T.; Li, R.; Zhang, H.; Ma, X.; et al. Preclinical evidence construction for epigallocatechin-3-gallate against non-alcoholic fatty liver disease: A meta-analysis and machine learning study. Phytomedicine 2025, 142, 156651. [Google Scholar] [CrossRef] [PubMed]
- Xie, C.; Chen, Z.; Zhang, C.; Xu, X.; Jin, J.; Zhan, W.; Han, T.; Wang, J. Dihydromyricetin ameliorates oleic acid-induced lipid accumulation in L02 and HepG2 cells by inhibiting lipogenesis and oxidative stress. Life Sci. 2016, 157, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.Y.; Huang, C.N.; Chan, K.C.; Yang, Y.S.; Peng, C.H.; Wang, C.J. Mulberry leaf polyphenols possess antiatherogenesis effect via inhibiting LDL oxidation and foam cell formation. J. Agric. Food Chem. 2011, 59, 1985–1995. [Google Scholar] [CrossRef]
- Yu, P.R.; Tseng, C.Y.; Hsu, C.C.; Chen, J.H.; Lin, H.H. In Vitro and In Vivo protective potential of quercetin-3-glucuronide against lipopolysaccharide-induced pulmonary injury through dual activation of nuclear factor-erythroid 2 related factor 2 and autophagy. Arch. Toxicol. 2024, 98, 1415–1436. [Google Scholar] [CrossRef]
- Lin, C.L.; Ying, T.H.; Yang, S.F.; Lin, C.L.; Chiou, H.L.; Hsieh, Y.H. Magnolin targeting of the JNK/Sp1/MMP15 signaling axis suppresses cervical cancer microenvironment and metastasis via microbiota modulation. Cancer Lett. 2024, 583, 216584. [Google Scholar] [CrossRef]
- Zhang, L.; Li, H.X.; Pan, W.S.; Khan, F.U.; Qian, C.; Qi-Li, F.R.; Xu, X. Novel hepatoprotective role of Leonurine hydrochloride against experimental non-alcoholic steatohepatitis mediated via AMPK/SREBP1 signaling pathway. Biomed. Pharmacother. 2019, 110, 571–581. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, H.-H.; Wu, P.-T.; Liang, Y.-H.; Lee, M.-S.; Chen, J.-H. AMPK-Targeting Effects of (−)-Epicatechin Gallate from Hibiscus sabdariffa Linne Leaves on Dual Modulation of Hepatic Lipid Accumulation and Glycogen Synthesis in an In Vitro Oleic Acid Model. Int. J. Mol. Sci. 2025, 26, 7612. https://doi.org/10.3390/ijms26157612
Lin H-H, Wu P-T, Liang Y-H, Lee M-S, Chen J-H. AMPK-Targeting Effects of (−)-Epicatechin Gallate from Hibiscus sabdariffa Linne Leaves on Dual Modulation of Hepatic Lipid Accumulation and Glycogen Synthesis in an In Vitro Oleic Acid Model. International Journal of Molecular Sciences. 2025; 26(15):7612. https://doi.org/10.3390/ijms26157612
Chicago/Turabian StyleLin, Hui-Hsuan, Pei-Tzu Wu, Yu-Hsuan Liang, Ming-Shih Lee, and Jing-Hsien Chen. 2025. "AMPK-Targeting Effects of (−)-Epicatechin Gallate from Hibiscus sabdariffa Linne Leaves on Dual Modulation of Hepatic Lipid Accumulation and Glycogen Synthesis in an In Vitro Oleic Acid Model" International Journal of Molecular Sciences 26, no. 15: 7612. https://doi.org/10.3390/ijms26157612
APA StyleLin, H.-H., Wu, P.-T., Liang, Y.-H., Lee, M.-S., & Chen, J.-H. (2025). AMPK-Targeting Effects of (−)-Epicatechin Gallate from Hibiscus sabdariffa Linne Leaves on Dual Modulation of Hepatic Lipid Accumulation and Glycogen Synthesis in an In Vitro Oleic Acid Model. International Journal of Molecular Sciences, 26(15), 7612. https://doi.org/10.3390/ijms26157612





